Abstract
In this editorial trend, we aim to collect and present recently available data about the characteristics of SARS-CoV-2 virus, severity, infection, replication, diagnosis, and current medications. In addition, we propose the role of nanomaterials in controlling and treating COVID-19 through their antiviral and antibacterial potential with suggested action mechanisms indicating the capability of interaction between these nanomaterials and SARS-CoV-2. These nanomaterials might be among the possible and most effective cures against coronavirus.
Keywords: Corona viruses, Nanomaterials, COVID-19, Nano-vaccines, Nanodrugs
Introduction
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a lethal beta-type virus belonging to a related group of viruses known as coronaviruses, leading to serious infections in the respiratory tract of humans (Fan et al. 2019; Sivakumar 2020; Yang and Wang 2020). Many human coronaviruses have been discovered such as SARS-CoV, HCoV NL63, HKU1, MERS-CoV, and SARS-CoV-2 causing COVID-19 disease (Su et al. 2016; Zhu et al. 2020). The name of coronaviruses was derived from Latin corona meaning crown, the name corresponds to their unique morphology due to the presence of viral spike proteins (Chafekar 2012; Lopez 2007). Coronaviruses have large spherical structures with an average diameter of 120 nm, and they have a lipid bilayer envelope of about 80 nm and 20 nm long spikes (Buzon et al. 2011; Fehr and Perlman 2015; Goldsmith et al. 2004; Yang and Wang 2020). The members of beta-coronavirus possess additional short-like protein called hemagglutinin esterase (HE) on their surfaces (Fehr and Perlman 2015). Interestingly, the structural proteins of the membrane, envelope, and the spike are anchored. In December 2019, COVID-19 emerged for the first time in China and has quickly-transmitted in the world (Angel-Korman et al. 2020). Recently, COVID-19 pandemic outbreak was recently-declared by the World Health Organization (WHO) (Angel-Korman et al. 2020).
Severity of COVID-19
Unlike SARS-CoV, COVID-19 transmission takes place during the prodromal period when the infected persons are mildly ill and carrying on with their common activities, facilitating spread of infection (Liu et al. 2020). Direct contact with infected individuals and sneezing or coughing (droplets spread) are the main causes of person-to-person transmission (Chan et al. 2020; Thompson 2020). According to WHO, individuals at risk include elderly (> 60 years old); cardiac, diabetic, chronic respiratory, cancer patients, and individuals in long-term care facilities (Kabir et al. 2020).
SARS-CoV-2 infection and replication
The virus relies on the ACE2 receptor not only for host cell entry but also for subsequent viral replication (Zhang et al. 2020b). High viral loads have been observed in the lower respiratory tract, proposing that the virus might have a higher affinity for the epithelium of the lower respiratory tract and indicating a need for repeat testing of the upper or lower respiratory tract samples in the setting of an initial negative result of nasopharyngeal or throat swab in a suspected case (Jansen et al. 2011; McCulloh et al. 2013; Rai et al. 2020). ACE2 receptors’ presence in the extrapulmonary tissues (heart, kidney, endothelium, and intestine) could also explain the multiorgan dysfunction observed in patients (Zhang et al. 2020a). SARS-CoV-2 is known to cause a delayed type I interferon response during the initial phases of infection (Frieman and Baric 2008). Infection and viral replication lead to activation of neutrophils, macrophages, and monocytes (Nikitina et al. 2018). Th1/Th17-induced specific antibodies are produced (Mitsdoerffer et al. 2010). RNA viruses such as SARS-CoV and MERS are recognized pathogen associated molecular patterns by endosomal RNA receptors, TLR7 and TLR3, and the cytosolic RNA sensor, RIG-I/MDA5 (Kell and Gale Jr 2015; Poeck et al. 2010; Takeuchi and Akira 2008).
Diagnosis of COVID-19
WHO has published diagnostic criteria that help identify patients requiring confirmation of COVID-19 through either PCR or antibody detection tests (Vos et al. 2019). These criteria include several epidemiological and clinical criteria that the patient has to fulfill in order to be either COVID-19 suspected or confirmed case (https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention/guidance-correctional-detention.html). History of patients infected with coronavirus disease 2019 (COVID-19) can include international travel to where COVID-19 is highly-prevalent. Fever, fatigue, cough, and breath shortness are the most common symptoms that can appear 2–14 days after exposure (Cascella et al. 2020).
Coronaviruses-derived illness can be investigated through many laboratory tests. Specific laboratory tests include serology for viral antigen, molecular testing, and viral culture (Kabir et al. 2020; Storch 2000). All these tests can be used to confirm infection with coronavirus. Non-specific laboratory findings in COVID-19 include lymphocytopenia, thrombocytopenia, elevated CRP, elevated ALT, AST, creatine kinase, D-dimer, and markers of cell damage, e.g., troponin, lactate dehydrogenase, interleukin-4, and procalcitonin (Lippi and Plebani 2020).
The chest x-ray remarks of a suspected coronavirus case can be similar to pneumonia (Jacobi et al. 2020). Severe cases of COVID-19 progressing to acute respiratory distress syndrome (ARDS) can show a typical “white-out” on chest x-ray (Venugopal et al. 2020). There are no specific echocardiography/ultrasound findings associated with coronavirus infection (Zheng et al. 2020). Non-specific echocardiographic findings can include left ventricular systolic dysfunction and pericardial effusion (Pérez-Casares et al. 2017). Chest CT scan findings in patients infected with coronavirus can include unilateral or bilateral pneumonia, mottling and ground glass opacity, focal or multifocal opacities, consolidation, and septal thickening with sub-pleural and lower lobe involvement more likely (Wu et al. 2020).
COVID-19 current medications
Treatment of coronavirus infection includes supportive measures and symptomatic management. No specific treatment is available. Given the emergence of the cases during the influenza season, all patients presenting with COVID-19 were given oral and intravenous antibiotics and oseltamivir (75 mg twice daily via oral route) empirically (Control and Prevention 2014).
Corticosteroids (methyl prednisolone 40–120 mg/day) were given as a combined regimen if severe community-acquired pneumonia was diagnosed (Chen et al. 2011). Oxygen support (e.g., via nasal cannula and invasive mechanical ventilation) was given to patients indicated by the severity of hypoxemia (Curley et al. 2015). Surgery is not indicated in the management of COVID-19. Investigational therapies include hydroxychloroquine alone or in combination with azithromycin, remdesivir, and lopinavir/ritonavir. Currently, avoiding virus exposure is the best way to prevent the infection (Organization 2020).
Nanomaterials as promising nano-vaccines and nano-drug
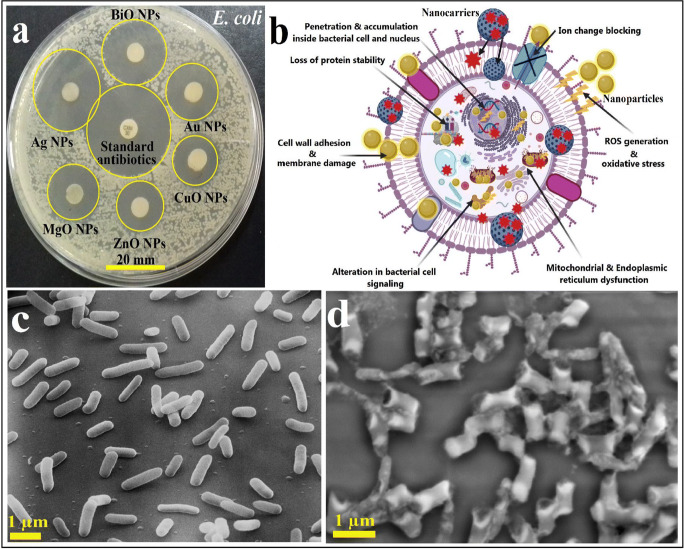
Nanotechnology through a large variety of nanomaterials (NMs) possessing multiple structures, properties, and characteristics can offer a revolutionary solution for the control and treatment of this disease. Many types of NMs showed very promising antiviral and antimicrobial activities which could be used as nano-vaccines to prevent microbial biofilm formation and the adsorption of microbes onto the surfaces and to control their transmission as shown in Fig. 1 (Abd Elkodous et al. 2019a, b; 2020; Chen and Liang 2020; El-Sayyad et al. 2020; Kim et al. 2020; Mohamed et al. 2020; Tran et al. 2020; Wong et al. 2020).
Fig. 1.
Antimicrobial potential of different synthesized NPs where (a) measuring the activity of different NPs by zone of inhibition (ZOI) method against E. coli bacterial cells, (b) suggested reaction mechanism of some nanoparticles (Ag and Au NPs) and drugs nanocarriers against bacterial cells ((I): NPs are firstly-interact with the external part of the pathogenic bacteria and change their membrane composition, and then they easily-enter bacterial cells because of their small nano-size; (II) NPs are quickly-diffused across bacterial cells and interacted with the main bacterial organelles and bio-molecules and increased cellular toxicity, loss of protein stability, and finally genotoxicity; (III) NPs generated active ROS inside bacterial cells and initiated bacterial cell disruption; and (VI) NPs have alternated the cellular sign system causing cell disorder. Also, NPs might serve as a vehicle to deliver their ions to the bacterial cytoplasm leading to a reduction in the proton motive force (PMF) which might decrease the bacterial cell pH to less than 3.5, and promoting the liberations of their ions), (c) normal E. coli growth without the effect of any synthesized NPs which appears as normal cell with a rigid cell wall and high cell count, and (d) malformed and distorted bacterial cell (E. coli) following the treatment of NPs suggesting the usage of NPs in fighting the secondary bacterial infection associated with viral diseases
The antimicrobial activity results of some green synthesized metal NPs (such as Ag NPs, and Au NPs) should be considered. The biogenic Au NPs mostly-displayed mild antibacterial activities against the highly-pathogenic bacteria (Aina et al. 2019; Elegbede et al. 2020). Sunday A. Ojo et al. (Ojo et al. 2016) investigated the antimicrobial potential of the green synthesized Au and Ag-Au alloy NPs, and the results indicated that they displayed growth inhibitions of 66.67–90.78% against strains of Aspergillus fumigatus and Aspergillus niger at a concentration of 200 μg/ml. In addition, Ag-Au NPs exhibited tremendous antifungal activities. An interesting result of nearly 100% inhibition of A. flavus growth after the treatment with Ag-Au NPs at a concentration of 150 μg/ml was reported (Lateef et al. 2016). The strong antifungal potential of Ag-Au NPs can be attributed to the adhesion on the microbial cell wall, subsequent suppression of fungal spores, then cytoplasmic content leakage, and finally microbial cell death (Elegbede et al. 2019).
The antimicrobial characteristics of some nanomaterials depend on their size, shape, surface features, and surface charge. As a result, their toxicological effects are controllable by the employed concentration (Sportelli et al. 2020). The toxic levels of NPs must be taken into consideration before designing any nano-drug, and the antimicrobial minimum inhibitory concentration (MIC) should be measured (Abdel Maksoud et al. 2020; El-Batal et al. 2020).
Many studies focusing on reaction mechanisms and cytotoxicity are needed before the development of reliable nano-vaccines and nano-drug (Abd Ellah et al. 2020).
Immune system–stimulating nano-vaccines could be easily-assembled through either encapsulation of viral parts within NMs or via decoration (covalent functionalization) on the surface of many suitable NMs such as virus-like nanoparticles (NPs), solid-lipid NPs, liposome-based NPs, and biodegradable polymer-based NPs (Dhakal and Renukaradhya 2019; Xu et al. 2020).
In addition, many types of NMs such as TiO2 NPs, ZnO NPs, and Cu NPs with very small concentrations could be employed to control the disease via surfaces’ coating and disinfection at hospitals and public places (Akinola et al. 2020; Xu et al. 2020). Moreover, self-assembled protein NPs obtained through recombinant technologies are considered on the most promising nano-vaccines against respiratory viruses (Scheerlinck and Greenwood 2008; Schneider-Ohrum and Ross 2012).
Christopher M. Coleman et al. reported a unique nano-vaccine based on lipid nanoparticles carrying SARS-CoV and MERS-CoV spike proteins without any viral proteins which effectively-induced antibody responses in mice (Coleman et al. 2014). Similarly, Hyo-Jung Park et al. developed two MERS-CoV vaccines from spike nanoparticles and spike protein-expressing recombinant adenovirus 5 (Park et al. 2017). Both vaccines triggered immune responses of Th1 and Th2.
Choi et al. (2017) reported another nano-vaccine for MERS-CoV in mice; the vaccine was developed from human Fc4-fused eS1–770 spike protein of the virus. After 10 days, they discovered large quantity of antibodies against MERS-CoV, while Ye V. Liu et al. tested vaccines based on virus-like particles (VLPs) similar in morphology and size to the wild SARS-CoV, comprising SARS spike protein conjugated with influenza M1 protein (Liu et al. 2011). Upon treatment, mice protection from death, reducing the lung infection and antibodies against the virus, were achieved.
Another study which showed the great promise of zirconia (ZrO2) NPs as antiviral candidates against H5N1 pathogenic influenza virus in mice was reported by Huo et al. (Huo et al. 2020). Their results revealed that ZrO2 NPs treatment resulted in a reduction in the virus replication, pro-inflammatory cytokines’ over expression, and less lung injury.
Reaction mechanisms of NMs against SARS-CoV-2
Indeed, NMs can be employed as either nano-vaccines to prevent viral infection and to control its spread or as nano-drugs for the treatment (Panda et al. 2020). Nano-vaccines are more effective than traditional vaccines because they are capable of stimulating the immune response (cell-mediated and humoral) of the body to form antibodies and to prevent it from spreading by blocking its entry to the host cell (Sekhon and Saluja 2011). In addition, nano-drugs can be used as nasal drops and can achieve effective targeting of a particular antigen for more effective treatment (Mehrabi et al. 2020).
The action mechanisms start when the NMs react with the virus through hydrogen bonding interactions and electrostatic attractions or when NMs enter the host cell through transient pores in its cell membrane to fight the virus inside (Verma et al. 2008).
The proposed action mechanisms of nano-vaccines and nano-drugs against SARS-CoV-2 can be classified into two categories, the action before virus entering to the host cell (nano-vaccines) and after entering it (nano-drugs). Firstly, several types of nano-vaccines such as virus-like NPs and polymeric NPs can effectively-block virus entry through blocking ACE2 cell receptor or virus spike protein through which it enters the host cell through endocytosis mediated by clathrin (Zhou et al. 2020).
SARS-CoV-2 spike protein-loaded NPs can be prepared to either block virus entry or activate the immune system to form antibodies against the virus. In addition, liposomes and nano-emulsion can dissolve the lipid bilayer envelope of the virus and destroy its structure, and inorganic NPs (Ag NPs) can effectively-initiate extracellular reactive-oxygen species (ROS) to kill the virus (El-Batal et al. 2018).
Secondly, after virus entry to the host cell, NMs can stimulate ROS and enhance lymphocyte proliferation, over expression of cytokines, and the formation of either neutralizing antibodies of opsonizing antibodies through Th1 or Th2 immune responses as shown in Fig. 2.
Fig. 2.
Proposed reaction mechanisms of various engineered NMs against SARS-CoV-2, action mechanisms outside host cell (I) blocking of virus entry through either binding with the virus spike protein or blocking its cell receptor (ACE2) by virus-like NPs, polymeric NPs, and SARS-CoV-2 spike protein NPs (has not been prepared yet); (II) dissolving the lipid bilayer of SARS-CoV-2 envelope by nano-emulsion, liposomes, and solid-lipid NPs; and (III) initiation of extracellular ROS by Ag NPs. Actions inside host cell include activation of transcription factors by receptor signaling as a response of viral RNA and either the formation of pro-inflammatory cytokines (IL-1B) and activation of Th2 pathway ending with the formation of neutralizing antibodies (IL-4, IL-5, and IL-13) or Th1 pathway activation and the formation of opsonizing antibodies (IgE, C4b, C3b, and IgG)
In addition, NPs such as TiO2, Ag, and Au can reduce the risk of high mortality among patients with COVID-19 due to hyperactivity of blood platelets which can lead to the formation of deadly blood clots (Akinola et al. 2020; Ojo et al. 2016). Moreover, serious complications such as heart attacks and strokes can be also suppressed (Elegbede et al. 2020). Coagulation of blood through lysis of blood clots can significantly-reduce the severity of COVID-19.
Conclusion
In this editorial trend, we covered most known data about coronavirus, properties, family identification, its severity, method of infection, and replication. In addition, we discussed its diagnosis and highlighted the currently-available medications to deal with its driven symptoms and effects. Finally, we proposed multiple nanomaterials that can be used as fighters against this disease, and we have classified them according to the mode of action into nano-vaccines and nano-drugs and revealed the way by which they can resist the virus. However, intensive efforts should be devoted for investigating the efficiency of these nanomaterials against viral samples as a step towards cure development. Importantly, with this high rate of dissemination of viruses and the frustrating slow drug development, there is an urgent need for developing new nanomedicines of high quality, safety, and availability to all countries at a reasonable cost.
Acknowledgments
All figures in this editorial trend were created with BioRender.com.
Authors’ contributions
(MAE, and GSE manuscript writing, draw figures and revision. MMA conducted manuscript moderation and revision).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Abd Elkodous, Email: mohamed.hamada.abdlekodous.xi@tut.jp.
Gharieb S. El-Sayyad, Email: Gharieb.Elsayyad@eaea.org.eg
Mohamed M. Abdel-Daim, Email: abdeldaim.m@vet.suez.edu.eg
References
- Abd Elkodous M, El-Sayyad GS, Abdelrahman IY, El-Bastawisy HS, Mohamed AE, Mosallam FM, Nasser HA, Gobara M, Baraka A, Elsayed MA, El-Batal AI. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf B: Biointerfaces. 2019;180:411–428. doi: 10.1016/j.colsurfb.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Abd Elkodous M, El-Sayyad GS, Nasser HA, Elshamy AA, Morsi M, Abdelrahman IY, Kodous AS, Mosallam FM, Gobara M, El-Batal AI. Engineered nanomaterials as potential candidates for HIV treatment: between opportunities and challenges. J Clust Sci. 2019;30:531–540. doi: 10.1007/s10876-019-01533-8. [DOI] [Google Scholar]
- Abd Elkodous M, El-Sayyad GS, Abdel Maksoud MIA, Abdelrahman IY, Mosallam FM, Gobara M, El-Batal AI. Fabrication of ultra-pure anisotropic zinc oxide nanoparticles via simple and cost-effective route: implications for UTI and EAC medications. Biol Trace Elem Res. 2020;196:297–317. doi: 10.1007/s12011-019-01894-1. [DOI] [PubMed] [Google Scholar]
- Abd Ellah NH, Gad SF, Muhammad K, Batiha GE, Hetta HF. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine. 2020;15:2085–2102. doi: 10.2217/nnm-2020-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Maksoud MIA, El-Sayyad GS, El-Khawaga AM, Abd Elkodous M, Abokhadra A, Elsayed MA, Gobara M, Soliman LI, El-Bahnasawy HH, Ashour AH (2020) Nanostructured Mg substituted Mn-Zn ferrites: A magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J Hazard Mater 399:123000 [DOI] [PubMed]
- Aina DA, Owolo O, Lateef A, Aina FO, Hakeem AS, Adeoye-Isijola M, Okon V, Asafa TB, Elegbede JA, Olukanni OD. Biomedical applications of Chasmanthera dependens stem extract mediated silver nanoparticles as antimicrobial, antioxidant, anticoagulant, thrombolytic, and larvicidal agents. Kar Inter J Mod Sci. 2019;5:2. [Google Scholar]
- Akinola P, Lateef A, Asafa T, Beukes L, Hakeem A, Irshad H. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon. 2020;6:e04610. doi: 10.1016/j.heliyon.2020.e04610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel-Korman A, Brosh T, Glick K, Leiba A. COVID-19, the kidney and hypertension. Harefuah. 2020;159:231–234. [PubMed] [Google Scholar]
- Buzon MJ, Seiss K, Weiss R, Brass AL, Rosenberg ES, Pereyra F, Xu GY, Lichterfeld M. Inhibition of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers. J Virol. 2011;85:9646–9650. doi: 10.1128/JVI.05327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19), StatPearls [Internet]. StatPearls Publishing [PubMed]
- Chafekar A (2012) Production of cytokines in human whole blood after incubation with the nucleocapsid protein of the NL63 coronavirus
- Chan JF-W, Yuan S, Kok K-H, Tos KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liang J (2020) An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C 110924 [DOI] [PMC free article] [PubMed]
- Chen Y, Li K, Pu H, Wu T (2011) Corticosteroids for pneumonia. Cochrane database of systematic reviews, Corticosteroids for pneumonia [DOI] [PubMed]
- Choi J, Kim M-G, Oh Y-K, Kim YB (2017) Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review. Expert Opin Ther Pat 27(6):721–731. 10.1080/13543776.2017.1281248 [DOI] [PubMed]
- Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, Glenn GM, Smith GE, Frieman MB. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Control CfD, Prevention (2014) Influenza antiviral medications: summary for clinicians
- Curley GF, Laffy JG, Zhang H, Slutsky AS. Noninvasive respiratory support for acute respiratory failure—high flow nasal cannula oxygen or non-invasive ventilation? J Thorac Dis. 2015;7:1092. doi: 10.3978/j.issn.2072-1439.2015.07.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal S, Renukaradhya GJ. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet Res. 2019;50:90. doi: 10.1186/s13567-019-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Batal AI, Mosallam FM, El-Sayyad GS. Synthesis of metallic silver nanoparticles by fluconazole drug and gamma rays to inhibit the growth of multidrug-resistant microbes. J Clust Sci. 2018;29:1003–1015. doi: 10.1007/s10876-018-1411-5. [DOI] [Google Scholar]
- El-Batal AI, Nada HG, El-Behery RR, Gobara M, El-Sayyad GS (2020) Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv 10:9274–9289 [DOI] [PMC free article] [PubMed]
- Elegbede JA, Lateef A, Azeez MA, Asafa TB, Yekeen TA, Oladipo IC, Hakeem AS, Beukes LS, Gueguim-Kana EB. Silver-gold alloy nanoparticles biofabricated by fungal xylanases exhibited potent biomedical and catalytic activities. Biotechnol Prog. 2019;35:e2829. doi: 10.1002/btpr.2829. [DOI] [PubMed] [Google Scholar]
- Elegbede JA, Lateef A, Azeez MA, Asafa TB, Yekeen TA, Oladipo IC, Aina DA, Beukes LS, Gueguim-Kana EB (2020) Biofabrication of gold nanoparticles using xylanases through valorization of corncob by Aspergillus niger and Trichoderma longibrachiatum: antimicrobial, antioxidant, anticoagulant and thrombolytic activities. Waste Biomass Valori 11:781–791
- El-Sayyad GS, Abd Elkodous M, El-Khawaga AM, Elsayed MA, El-Batal AI, Gobara M. Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment. RSC Adv. 2020;10:5241–5259. doi: 10.1039/C9RA10505K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhao K, Shi Z, Zhou P. Bat coronaviruses in China. Viruses. 2019;11:210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis, coronaviruses. Springer, pp 1–23 [DOI] [PMC free article] [PubMed]
- Frieman M, Baric R. Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation. Microbiol Mol Biol Rev. 2008;72:672–685. doi: 10.1128/MMBR.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith CS, Tatti KM, Ksiazek TG, Rollin PE, Comer JA, Lee WW, Rota PA, Bankamp B, Bellini WJ, Zaki SR. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10:320. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo C, Xiao J, Xiao K, Zou S, Wang M, Qi P, Liu T, Hu Y. Pre-treatment with zirconia nanoparticles reduces inflammation induced by the pathogenic H5N1 influenza virus. Int J Nanomedicine. 2020;15:661–674. doi: 10.2147/IJN.S221667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RR, Wieringa J, Koekkoek SM, Visser CE, Pajkrt D, Molenkamp R, de Jong MD, Schinkel J. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir MT, Uddin MS, Hossain MF, Abdulhakim JA, Alam MA, Ashraf GM, Bungau SG, Bin-Jumah MN, Abdel-Daim MM, Aleya L (2020) nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. Front Cell Dev Biol 8:616 [DOI] [PMC free article] [PubMed]
- Kell AM, Gale M., Jr RIG-I in RNA virus recognition. Virology. 2015;479:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yeom M, Lee T, Kim H-O, Na W, Kang A, Lim J-W, Park G, Park C, Song D (2020) Porous gold nanoparticles for attenuating infectivity of influenza a virus. J Nanobiotechnology 18:1–11 [DOI] [PMC free article] [PubMed]
- Lateef A, Ojo SA, Folarin BI, Gueguim-Kana EB, Beukes LS. Kolanut (Cola nitida) mediated synthesis of silver–gold alloy nanoparticles: antifungal, catalytic, larvicidal and thrombolytic applications. J Clust Sci. 2016;27:1561–1577. doi: 10.1007/s10876-016-1019-6. [DOI] [Google Scholar]
- Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. 20200198 [DOI] [PubMed]
- Liu YV, Massare MJ, Barnard DL, Kort T, Nathan M, Wang L, Smith G. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29:6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, Wang Z, Wang F, Liu L, Zhang Z (2020) Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis 26 [DOI] [PMC free article] [PubMed]
- Lopez LA (2007) Coronavirus envelope proteins: roles in subcellular trafficking and virus assembly. Arizona State University
- McCulloh RJ, Koster M, Chapin K. Respiratory viral testing: new frontiers in diagnostics and implications for antimicrobial stewardship. Virulence. 2013;4:1–2. doi: 10.4161/viru.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi M, Dounighi NM, Mohammadi M, Masoudi A. Nanoparticles and vaccine development. Pharm Nanotechnol. 2020;8:6–21. doi: 10.2174/2211738507666191024162042. [DOI] [PubMed] [Google Scholar]
- Mitsdoerffer M, Lee Y, Jäger A, Kim H-J, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HEA, Afridi S, Khalil AT, Ali M, Zohra T, Akhtar R, Ikram A, Shinwari ZK, Maaza M. Promising antiviral, antimicrobial and therapeutic properties of green nanoceria. Nanomedicine. 2020;15:467–488. doi: 10.2217/nnm-2019-0368. [DOI] [PubMed] [Google Scholar]
- Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci. 2018;19:2821. doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo SA, Lateef A, Azeez MA, Oladejo SM, Akinwale AS, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Gueguim-Kana EB. Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of Bacillus safensis LAU 13: antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Trans Nanobioscience. 2016;15:433–442. doi: 10.1109/TNB.2016.2559161. [DOI] [PubMed] [Google Scholar]
- Organization WH (2020) Coronavirus disease 2019 (COVID-19): situation report, 72
- Panda PK, Arul MN, Patel P, Verma SK, Luo W, Rubahn H-G, Mishra YK, Suar M, Ahuja R. Structure-based drug designing and immunoinformatics approach for SARS-CoV-2. Sci Adv. 2020;6:eabb8097. doi: 10.1126/sciadv.abb8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-J, Lee E-Y, Jung S, Ko HL, Lee S-M, Nam J-H (2017) Spike nanoparticle and recombinant adenovirus 5 vaccines induce specific antibodies against the Middle East respiratory syndrome coronavirus (MERS-CoV). Am Assoc Immnol
- Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr. 2017;5:79. doi: 10.3389/fped.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S, Barchet W. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Rai PK, Usmani Z, Thakur VK, Gupta VK, Mishra YK. Tackling COVID-19 pandemic through nanocoatings: confront and exactitude. Curr Opin Green Sustain Chem. 2020;3:100011. doi: 10.1016/j.crgsc.2020.100011. [DOI] [Google Scholar]
- Scheerlinck J-PY, Greenwood DLV. Virus-sized vaccine delivery systems. Drug Discov Today. 2008;13:882–887. doi: 10.1016/j.drudis.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Schneider-Ohrum K, Ross TM. Virus-like particles for antigen delivery at mucosal surfaces. In: Kozlowski PA, editor. Mucosal vaccines: modern concepts, strategies, and challenges. Berlin: Springer Berlin Heidelberg; 2012. pp. 53–73. [DOI] [PubMed] [Google Scholar]
- Sekhon BS, Saluja V. Nanovaccines-an overview. Int J Pharm Front Res. 2011;1:101–109. [Google Scholar]
- Sivakumar B. Educational evaluation survey on corona virus 19 (an awareness)–South India. Stud Ind Pl Nam. 2020;40:228–234. [Google Scholar]
- Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioffi N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials. 2020;10:802. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch GA. Diagnostic Virology. Clin Infect Dis. 2000;31:739–751. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20:280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DN, Vu NN, Nhan T, Bich NTT, Quang ML, To NB, Le Van P, Dang VQ (2020) Silver nanoparticles as potential antiviral agents against African swine fever virus. Materials Research Express
- Venugopal VK, Mahajan V, Rajan S, Agarwal VK, Rajan R, Syed S, Mahajan H (2020) A systematic meta-analysis of CT features of COVID-19: lessons from radiology. medRxiv
- Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos LM, Bruning AH, Reitsma JB, Schuurman R, Riezebos-Brilman A, Hoepelman AI, Oosterheert JJ. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis. 2019;69:1243–1253. doi: 10.1093/cid/ciz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Chan YS, Jeevanandam J, Pal K, Bechelany M, Abd Elkodous M, El-Sayyad GS. Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. J Clust Sci. 2020;31:367–389. doi: 10.1007/s10876-019-01651-3. [DOI] [Google Scholar]
- Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, Huang H, Li C. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Investig Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zheng J, Wu A (2020) Antibacterial applications of TiO 2 nanoparticles. TiO2 nanoparticles, 105-132
- Yang P, Wang X (2020) COVID-19: a new challenge for human beings. Cell Mol Immunol [DOI] [PMC free article] [PubMed]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020a) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med:1–5 [DOI] [PMC free article] [PubMed]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]