Abstract
The severe acute respiratory syndrome novel coronavirus-2 pandemic is affecting almost every country in the world. Even if the major symptoms of coronavirus disease-2019 are respiratory, different symptoms at presentation are now recognized. Venous thromboembolism has been reported in infected patients and few but increasing cases of arterial thrombosis have been described. We report a case of acute aortoiliac and lower limb artery occlusions in a patient presenting with severe coronavirus disease-2019 infection. The mechanism of the occlusion seemed to be distal embolization from a floating thrombus in the aortic arch caused by a major inflammatory state and virus infection. The patient underwent aortoiliac and lower limb artery mechanical thrombectomy, but required unilateral major amputation.
Keywords: COVID-19, Arterial occlusion, Inflammatory state, Coronavirus, Arterial thrombosis
The severe acute respiratory syndrome novel coronavirus-2 has spread to multiple countries rapidly during the last months. Although venous thromboembolism has been described as associated with severe coronavirus disease-2019 (COVID-19) cases,1 , 2 there are some but increasing reports of arterial thrombosis.3 We report a case of acute occlusion of the distal aorta in a patient with COVID-19 infection.
Case report
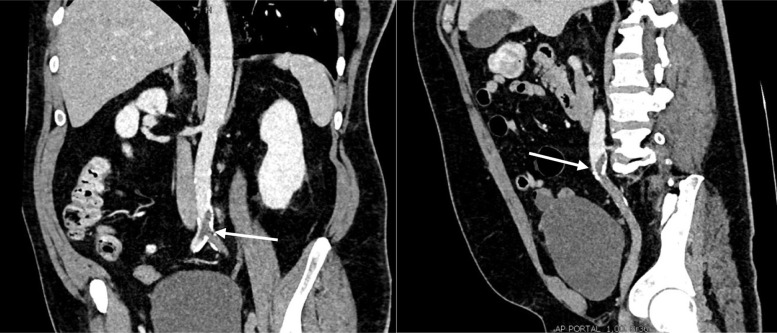
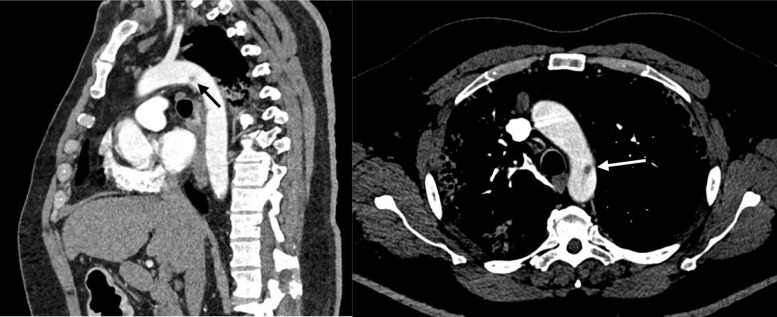
A 56-year-old male nonsmoker with risk factors of atherosclerosis (type 2 diabetes and arterial hypertension treated by angiotensin-converting enzyme inhibitor) and obesity (body mass index of 35 kg/m2) was admitted to the emergency department on April 4, 2020, because of acute muscle weakness and sensory loss of the left lower limb. After a rheumatologic consultation, he was treated for 3 days before admission with 80 mg of corticoids for acute lower back pain before, but no imaging had been performed before starting the treatment. He did not report any previous lower limb claudication nor arterial or venous thromboembolism. Blood analysis showed a high rate of C-reactive protein (235 mg/L), hyperleukocytosis (14.4 g/L), high fibrinogen (5.81 g/L), normal hemoglobin rate and platelet count, normal renal function and hepatic cytolysis (alanine aminotransferase of >3 times the upper limit of normal and an aspartate aminotransferase of >9 times the upper limit of normal). The d-dimer level was high (>20,000 μg/L) and the activated partial thromboplastin time (aPTT) was normal. There was no laboratory sign of disseminated intravascular coagulation. A thoracoabdominopelvic computed tomography (CT) scan was performed showing distal aorta and left iliac artery occlusions and calcifications of the aortic bifurcation (Fig 1 ). A small thrombus was found floating in the aortic arch (Fig 2 ), without calcification of the arterial wall. Thoracic images were typical of COVID-19 pulmonary lesions (reticular interlobular septa thickening within patchy ground-glass opacities (crazy paving4) (Fig 3 ). There was no pulmonary embolism.
Fig 1.
Infrarenal distal aortic and left iliac artery thrombotic occlusion on preoperative computed tomography (CT) scan (white arrows).
Fig 2.
Aortic arch thrombus (black and white arrows).
Fig 3.
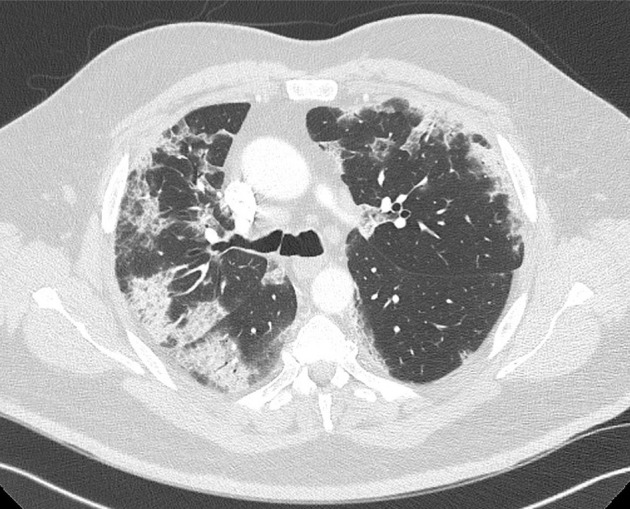
Thoracic preoperative computed tomography (CT) scan showing pulmonary lesions typical of coronavirus disease-2019 (COVID-19).
Even though at the time of admission, the polymerase chain reaction test by throat swab was negative, the patient was considered COVID positive owing to typical pulmonary images. Hydroxychloroquine, broad-spectrum antibiotics (macrolide and third-generation cephalosporin), and therapeutic unfractionated heparin (UFH) treatment (20,000 IU/12 hours by continuous infusion, with a goal of aPTT of 60-90) were initiated. Hydroxychloroquine was introduced for its antiviral effect. The patient was hospitalized in a COVID-positive unit. Surgery was undertaken on an emergent basis without any additional imaging of the infrainguinal arteries. Under general anesthesia, via a percutaneous right common femoral and open left common femoral approach, an occlusion balloon was placed in the right common iliac ostium to avoid a contralateral embolization and aortoiliac thrombectomy performed using embolectomy catheter (Fogarty, Edwards, Calif). Voluminous, fresh-appearing thrombi were removed (Fig 4 ). No iliac stenosis was found during intraoperative aortography. Covered kissing stents were placed to exclude residual thrombus in both common iliac arteries. Completion arteriography showed left popliteal occlusion, which was treated by open popliteal thrombectomy. Right lower limb arteriography was not performed. Fasciotomies were performed on the left side because of tight muscular compartment. Muscle edema was significant.
Fig 4.
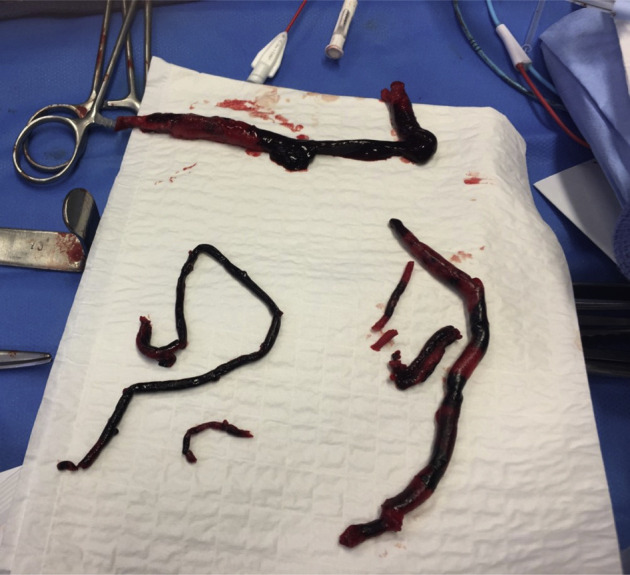
Thrombus extracted from the iliac and popliteal left arteries.
Thirteen minutes after the end of left limb revascularization, and before the patient regained consciousness in operative room, he showed signs of acute right limb ischemia with a cold and pale foot. We performed a right open femoral approach. Intraoperative arteriography of the right lower limb showed popliteal and distal leg artery thrombosis. A new thrombectomy was performed. All thrombi were removed from the popliteal and peroneal arteries. Subsequent histologic study showed a recent clot without signs of malignity.
His clinical condition deteriorated immediately after surgery because of a massive ischemia-reperfusion injury with renal failure and unstable hemodynamic state, requiring intubation. The patient was transferred to the intensive care unit (ICU). Hydroxychloroquine was interrupted at ICU admission. The UFH treatment had to be stopped because of a high aPTT (>180 seconds). A few hours later, the patient presented a new episode of right lower limb ischemia and concomitant signs of irreversible ischemia in the left foot. Concomitantly, aPTT was too low (20 seconds). A CT scan showed a satisfactory result of aortoiliac revascularization and a new thrombosis of the right popliteal artery extended to distal arteries. The iliofemoropopliteal left axis was patent except for an occlusion of the deep femoral artery. There was no patent artery below the left ankle. There was again no laboratory sign of disseminated intravascular coagulation and his platelet count was stable. Ten hours after the first right popliteal thrombectomy, a second right popliteal artery thrombectomy with fasciotomies were performed successfully. The patient was at this time diagnosed with COVID-19 by polymerase chain reaction-positive throat swab.
Indication for a third CT scan was retained because of deterioration of the hemodynamic state. Aortoiliac kissing stents and right popliteal artery were observed to be patent, but a recent small renal infarction with no evidence of renal thrombus was present on the right side. No other site of embolization was identified. A left transtibial amputation was undertaken after hemodynamic stabilization.
The clinical state and laboratory values of the patient improved after amputation. Subsequent coagulation tests and serologic testing showed lowered antithrombin (AT) value (21%), absence of anticardiolipin IgG, and anti-β2-glycoprotein I IgG antibodies. The last CT scan performed on May 1, 2020, to control vascular revascularization showed complete lysis of the thrombus in the thoracic aorta without any parietal atherosclerotic lesion as nidus.
At the time of this writing, the patient is still in ICU after 6 weeks but is now extubated and recovering. The patient gave his consent for publication.
Discussion
This case of severe proximal arterial thrombosis shows a link between COVID-19 infection and proximal arterial occlusions. The systemic inflammatory response caused by the virus induces a hypercoagulable state and an endothelial dysfunction.1, 2, 3 , 5, 6, 7 There was no pulmonary embolism in this case, although associated venous and arterial thrombosis have been previously reported.8 , 9 Aortoiliac occlusion may be due to an in situ thrombosis complicating calcifications in the common iliac arteries. But in our case, the presence of an aortic arch floating thrombus, the location of the occlusion at the aortic bifurcation, and the further onset of right renal infarction are rather in favor of embolic process from the aortic arch. In either case, the inflammatory response owing to COVID-19 infection may have induced aortic thrombus formation. There was no evidence in our case for either antiphospholipid syndrome, as recently reported in case of COVID-1910 or myeloproliferative syndrome. In our case, hydroxychloroquine was not introduced for its antithrombotic properties,11 but rather for its antiviral effect.12 , 13 Hydroxychloroquine was interrupted at ICU admission to avoid possible additional cardiac complications in an unstable patient.14 The patient presented a severe form of pulmonary injury and signs of inflammatory response. The d-dimer level and CRP rate were high at the time of this patient's admission. The inflammatory state activates the coagulation cascade and microthrombosis was described in many cases of COVID-19.15 Although a high d-dimer level has been associated with higher mortality in a few studies,16 , 17 our patient is still alive at time of writing. Unfortunately, laboratory values, including fibrin degradation products, serum ferritin, and procalcitonin, were not available. These laboratory parameters are not tested routinely in the ICU. Lupus anticoagulant, anticardiolipin IgM, and anti-beta-2 glycoprotein I IgM were not tested. Lupus anticoagulant testing was not possible because the patient was treated by UFH. And recent studies have shown that IgM detection are rather a second-line test for thrombotic risk stratification.18
We did not perform a thoracic endovascular repair. There is no consensus regarding management of mural thrombosis in aortic arch.19 Our experience leads us to prefer anticoagulation over endovascular exclusion of thrombus because the latter strategy may be associated with distal embolization.
The medical history of the patient can also explain the severity of the injuries. Diabetes, hypertension, and obesity have been described as risk factors of severe COVID-19 forms,17 , 20 but also as cardiovascular risk factors responsible of arterial disease. Prophylactic anticoagulation and antiplatelet therapy should be considered for patient with cardiovascular risk factors (both asymptomatic and symptomatic) and COVID-19, and should not be stopped during the inflammatory phase.
This case also reflects the difficulties to manage therapeutic anticoagulation with UFH because of such massive inflammatory reaction and because of a possible AT deficit in case of COVID-19.5 An AT deficit, which was present in our patient on subsequent analysis, might be due to liver dysfunction, consumption during clotting, and heparin treatment itself.21 UFH as well as low-molecular-weight heparin have anticoagulation effects only after binding to AT. It may be useful to know the level of AT in patients with COVID-19 with acute arterial occlusion to manage anticoagulation treatment. Heparin interruption was instituted because of a high aPTT, which may have contributed to the right arterial popliteal reocclusion without laboratory argument for disseminated intravascular coagulation or heparin induced platelet thrombopenia. During phase of systemic inflammatory response, the patient received higher doses of UFH (730 IU/kg/24 hours). He is still treated with UFH at time of submission, but at normal doses.
The patient presented with lower back pain for 3 days before vascular consultation. Retrospectively, lower back pain was the first sign of aortoiliac arterial occlusion. There was clearly a delayed diagnosis of acute lower limb ischemia. The medical risk of the pandemic is not limited to the respiratory complications of the infection, but also by the delayed diagnosis and treatment of other severe complications because of the multiple presentations of COVID-19.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Giuseppina L., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 April 29 doi: 10.1016/j.jvs.2020.04.483. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carotti M., Salaffi F., Sarzi-Puttini P., Agostini A., Borheresi A., Minorati D., et al. Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia: key points for radiologists. Radiol Med. 2020;125:636–646. doi: 10.1007/s11547-020-01237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H., Yang L., Liu R., Liu F., Wu K., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Berre A., Marteau V., Emmerich J., Zins M. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imaging. 2020;101:321–322. doi: 10.1016/j.diii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T.F., Lim W. What is the role of hydroxychloroquine in reducing thrombotic risk in patients with antiphospholipid antibodies? Hematol Am Soc Hematol Educ Program. 2016;2016:714–716. doi: 10.1182/asheducation-2016.1.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 15.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chayoua W., Kelchtermans H., Gris J.C., Moore G.W., Musiał J., Wahl D., et al. The (non-)sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J Thromb Haemost. 2020;18:169–179. doi: 10.1111/jth.14633. [DOI] [PubMed] [Google Scholar]
- 19.Tsilimparis N., Hanack U., Pisimisis G., Yousefi S., Wintzer C., Ruckert R.I. Thrombus in the non-aneurysmal, non-atherosclerotic descending thoracic aorta - an unusual source of arterial embolism. Eur J Vasc Endovasc Surg. 2011;41:450–457. doi: 10.1016/j.ejvs.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 March 31 doi: 10.1002/dmrr.3319. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cott E.M., Orlando C., Moore G.W., Cooper P.C., Meijer P., Marlar R. Recommendations for clinical laboratory testing for antithrombin deficiency; communication from the SSC of the ISTH. J Thromb Haemost. 2020;18:17–22. doi: 10.1111/jth.14648. [DOI] [PubMed] [Google Scholar]