Abstract
Transplant-associated thrombotic microangiopathy (TMA) in the post–organ transplantation setting occurs from a number of potential inciting factors, such as the use of calcineurin inhibitors, ischemic injury, infections, or antibody-mediated rejection leading to unchecked complement activation and end-organ damage. Delayed recognition of this condition can result in allograft loss. In this case description, we describe the first case of de novo TMA in a patient with polycystic kidney disease that occurred immediately after kidney transplantation. The diagnosis was made promptly on the basis of clinical and laboratory characteristics by a multidisciplinary team and confirmed through kidney biopsy, which showed acute TMA. The patient was successfully managed by replacing tacrolimus with belatacept, which targets cytotoxic T lymphocyte antigen 4, and use of eculizumab, a C5 inhibitor. Eculizumab treatment was discontinued after 3 months of complement inhibition on the patient’s request, and relapse of TMA has not been encountered after more than 1 year of follow-up.
Index Words: Thrombotic microangiopathy, kidney transplant, polycystic kidney disease, eculizumab, belatacept
Introduction
Transplant-associated thrombotic microangiopathy (TMA) is a rare complication encountered after kidney transplantation. Incidence rates are estimated at 0.8% to 15%, with allograft loss in up to 33% to 50% of cases.1,2 Key findings associated with this condition include thrombocytopenia, reduced haptoglobin and elevated lactate dehydrogenase (LDH) levels, and either lack of improvement or decline (once stabilized) in glomerular filtration rate, a set of laboratory findings that can arise from several other conditions in the usual posttransplantation course.
Calcineurin inhibitors (CNIs) are frequently implicated in transplant-associated TMA, especially in the early period (3-6 months) after transplantation.3,4 Mammalian target of rapamycin inhibitors have also been associated with TMA and only belatacept (cytotoxic T lymphocyte antigen 4–immunoglobulin fusion protein that inhibits T-cell function) exists as an alternative to these agents.5,6 Withdrawal of CNI treatment alone may not be sufficient to fully reverse the adverse effects of the alternative pathway of complement activation associated with this condition.2,3 To adequately reverse complement activation, eculizumab (C5 inhibitor) has been successfully used in a number of resistant cases of de novo transplant-associated TMA.7, 8, 9 We describe the successful use of eculizumab in a transplant recipient with kidney failure secondary to polycystic kidney disease (PKD) who developed de novo transplant-associated TMA in the immediate postsurgical setting.
Case Report
An unsensitized (panel-reactive antibody, 0%) 63-year-old woman of Korean ethnicity with kidney failure secondary to PKD underwent deceased donor kidney transplantation (HLA antigen match, 3/10). Donation was performed after cardiac death and Kidney Donor Profile Index was 51%. On the day of the transplantation she started her immunosuppressive regimen, which included 3 doses of thymoglobulin, tacrolimus, mycophenolate, and a rapid steroid taper. The patient experienced delayed graft function but also anemia and thrombocytopenia. Hematologic evaluation (Table 1) on postoperative day (POD) 2 revealed an elevated LDH level (1,359 IU/L), undetectable haptoglobin (<8 mg/dL), and the presence of schistocytes (2-5/high-power field) on review of the peripheral smear. These findings were concerning for posttransplantation TMA and tacrolimus treatment was discontinued on POD 3. Tacrolimus 12-hour trough levels decreased from 6.0 (POD 3) to 2.3 ng/mL (POD 4).
Table 1.
Laboratory Test Results During the Pre- and Postoperative Period
| Presurgery | POD 0 | POD 2 | POD 3 | POD 4 | POD 5 | POD 7 | POD 14 | POD 28 | 3 mo Later | |
|---|---|---|---|---|---|---|---|---|---|---|
| WBC, K/μL | 6.4 | 4.4 | 3.3 | 3.2 | 2.1 | 3.9 | 6 | 7.6 | 8.1 | 5.2 |
| Hemoglobin, g/dL | 12 | 10 | 8.1 | 7.5 | 6.4 | 8.5 | 8.6 | 6.8 | 10.1 | 9.9 |
| Platelets, K/μL | 154 | 107 | 56 | 40 | 32 | 26 | 65 | 145 | 239 | 167 |
| Creatinine, mg/dL | 5.59 | 5.88 | 6.49 | 7.27 | 8.54 | 5.48 | 7.83 | 7.72 | 1.54 | 1.30 |
| LDH, IU/L | 1,359 | 1,260 | 1,106 | 1,173 | 1,043 | 396 | 181 | 167 | ||
| Haptoglobin, mg/dL | <8 | <8 | <8 | <8 | <8 | 145 | 186 | 122 | ||
| CH50 (complement total), U/mL | 56 | <10 | <10 | |||||||
| Tacrolimus, ng/mL | 4.3 | 6 | 2.3 | |||||||
| Infectious workup | EBV IgG: positive; EBV IgM negative; CMV IgG positive; CMV IgM negative; HIV: negative | CMV DNA: negative; Parvo IgM: negative; EBV PCR: negative | BK virus DNA: negative | |||||||
| Autoimmune | Coombs: negative | Anti-PF4: negative | ||||||||
| Start of treatment | Eculizumab | Eculizumab | Eculizumab | Eculizumab | Eculizumab |
Abbreviations: Anti-PF4, anti–platelet factor 4 antibody; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; LDH, lactate dehydrogenase; PCR, polymerase chain reaction; POD, postoperative day; WBC, white blood cells.
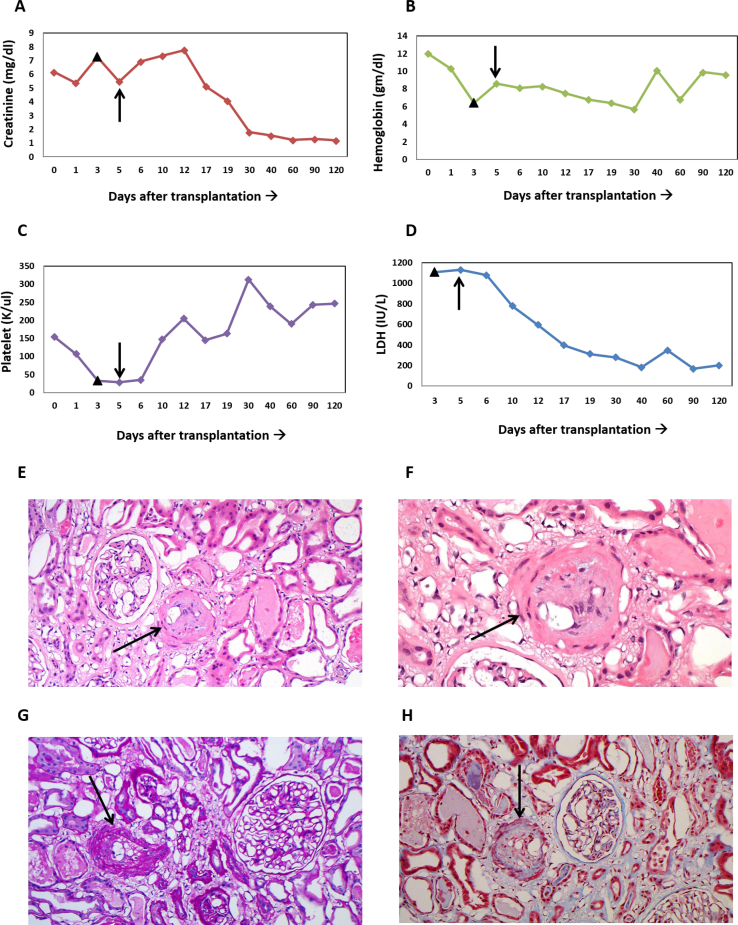
Despite these interventions, the patient’s serum creatinine and LDH levels remained elevated and hemoglobin level (nadir, 6.4 g/dL) and platelet count (nadir, 26 K/μL) continued to decline (Fig 1A). Kidney biopsy was not performed initially because of the degree of thrombocytopenia. The PLASMIC (platelet count, combined hemolysis variable, absence of active cancer, absence of stem-cell or solid-organ transplant, mean corpuscular volume, international normalized ratio, creatinine) score was 4, which is consistent with a low likelihood of thrombotic thrombocytopenic purpura, which was confirmed by ADAMTS13 (von Willebrand factor protease) activity of 55% (reference value ,>67%). Negative anti–platelet factor 4 antibody test results ruled out heparin-induced thrombocytopenia as a cause for thrombocytopenia. Coombs test was performed to investigate an autoimmune cause for the anemia and results were negative. The initial workup included testing for infections such as bacterial, Epstein-Barr virus, cytomegalovirus, and parvovirus. Epstein-Barr virus and cytomegalovirus polymerase chain reaction did not detect any viral reactivation. Serum protein electrophoresis did not detect monoclonal protein. After a comprehensive evaluation by the members of our multidisciplinary TMA team constituting experts from hematology, nephrology, and transplant surgery, a diagnosis of transplant-associated TMA was made in the absence of any other alternative diagnoses.
Figure 1.
Laboratory results and kidney biopsy findings are consistent with thrombotic microangiopathy involving the transplanted kidney. (A-D) Laboratory parameters show improvement after complement inhibition with eculizumab was initiated on postoperative day 5, with creatinine, hemoglobin, platelet, and lactate dehydrogenase (LDH) levels returning to normal range. Filled triangle marks the day of discontinuation of tacrolimus therapy, arrow marks the start of eculizumab therapy followed by the introduction of belatacept therapy on the following day. (E-F) Light microscopy of the kidney biopsy specimen (hematoxylin and eosin stain) is shown. Occluded arteriole (indicated by arrow) can be seen along with reactive endothelium and expanded subendothelial space. Red blood cell fragmentation can be seen beneath the endothelium. (G) Periodic acid–Schiff and (H) trichrome stain show similar findings.
On POD 5 (36 hours after discontinuation of tacrolimus therapy), eculizumab, 900 mg, was administered for 4 weekly doses followed by 1,200 mg every 2 weeks. On POD 7, hemoglobin level improved to 8.6 g/dL, LDH level decreased to 1,043 IU/L, and platelet count improved to 65 K/μL. After the second dose of eculizumab, urine output increased further and serum creatinine level decreased from 8.5 to 4.06 mg/dL. Total complement activity was sent before and after the first dose to assess complement inhibition.
After the improvement in platelet count, a kidney biopsy was pursued and confirmed a diagnosis of acute TMA with no evidence of rejection. Evaluation of glomeruli showed no evidence of necrosis, but arterioles showed reactive endothelium and expanded subendothelial space (1 arteriole with red blood cells and fibrin beneath the endothelium) with minimal mononuclear infiltrate in the interstitium (Fig 1E-H). C3 and C4d deposition was noted on intimal staining in the arteriole.
Prophylaxis against meningococcal infections was accomplished with penicillin VK (250 mg twice daily) for antibacterial prophylaxis, along with meningococcal group B and meningococcal (groups A, C, Y, and W-135) vaccines. In the absence of a CNI, immunosuppression was maintained with the aid of belatacept, while mycophenolate mofetil therapy was continued and low-dose prednisone was added (5 mg daily). The belatacept dosing protocol was based on the package insert: 10 mg/kg on days 1 and 5 and weeks 2, 4, 8, and 12, followed by 5 mg/kg monthly.10 By POD 30, serum creatinine and hemoglobin levels improved to 1.78 mg/dL and 9.6 g/dL, respectively, while both platelet count and LDH level normalized (Fig 1A).
The aHUS Genetic Panel (Machaon Diagnostics Inc) revealed a stop-loss variant (c.1708T>C, p.Ter570ArgextTer37) in exon 10, the last exon of the CFHR5 gene. No previously reported cases of transplant-associated TMA are associated with this variant. Three polymorphisms were also detected in the CFH gene (homozygous change in the promoter 2, homozygous silent variant in exon 13 [c.2016A>G, p.Gln672Gln], and homozygous missense variant in exon 18 [c.2808G>T, p.Glu936Asp]), which occur commonly in healthy individuals but are statistically enriched in patients with atypical hemolytic uremic syndrome (aHUS). There was no family history of TMA or consanguinity. Overall, this analysis was equivocal because no strong genetic risk for aHUS was identified.
Three months post–kidney transplantation, this patient continues on an immunosuppressive regimen of belatacept, mycophenolate mofetil, and prednisone with no ongoing evidence of TMA.
The patient discontinued eculizumab after 3 months of treatment and was followed up with clinical and laboratory assessment at monthly intervals. Laboratory monitoring has confirmed no TMA recurrence at 1 year posttransplantation.
Discussion
The development of hemolytic anemia and thrombocytopenia in the immediate post–kidney transplantation period should raise concern for a microangiopathic process. The diagnosis of posttransplantation TMA poses a diagnostic dilemma, especially in the immediate posttransplantation period in which a multitude of reasons, such as ischemia-reperfusion injury, use of CNIs, or antibody-mediated rejection, alone or together can cause delayed graft function.11,12 Early and accurate diagnosis of transplant-associated TMA facilitated by a TMA team followed by treatment with eculizumab, which is a recombinant C5 binding humanized antibody, can result in a quick TMA reversal, as seen in this case.7,8
Transplant-associated TMA results from endothelial damage due to unregulated complement activation and requires sufficient clinical-pathologic correlation.13 In a survey of kidney transplantation centers across Japan, 1.5% of patients exhibited evidence of TMA within 1 week of kidney transplantation.14 A kidney biopsy can be confirmatory in this setting but carries a high risk in a thrombocytopenic patient. In this case, TMA occurred in the immediate posttransplantation course and failed to respond to discontinuation of tacrolimus therapy, suggesting an alternative mechanism, and was only subsequently confirmed by kidney biopsy. An underlying predisposing genetic mutation can be identified in >30% of patients with transplant-associated TMA, including after solid-organ transplantation, and use of conventional therapies such as plasmapheresis can be ineffective in the absence of any quantitative defects in ADAMTS13 or inhibitors of complement pathway or antibody-mediated rejection.15,16 For this patient, loss of kidney function from PKD was the reason for kidney transplantation and possibly the presence of a CFHR5 mutation in the setting of surgery triggered the complement activation. In such cases, early use of eculizumab can reverse the complement activation and prevent further organ damage, leading to rapid recovery of hematologic and kidney function.17
There is no prevailing consensus on the duration of eculizumab therapy in cases of TMA following solid-organ transplantation.18 The presence of a predisposing genetic mutation can help assess the recurrence risk and duration of therapy.19 Often, genetic analysis reveals variants of unknown significance , as evident in this case in which a rare stop-loss variant (c.1708T>C) in exon 10 of the CFHR5 gene was identified, which has also been reported in a case of immunoglobulin A glomerulopathy and 3 healthy controls, with no cases of TMA identified in relation to this mutation.20 Additionally, there were also 3 variants in the CFH gene. The possibility cannot be excluded that these variants in combination with an inciting event can increase the probability of developing transplant-associated TMA, but this pathogenicity cannot be confirmed at this time given the lack of additional information. As the understanding of variants of unknown significance further evolves, caution is required in its interpretation because it does not necessarily implicate or preclude involvement in the pathogenesis.21,22 As genetic testing makes inroads into everyday clinical practice, the association of rare genetic variants can be laid bare.
The duration of complement inhibition with eculizumab for transplant-associated TMA is unclear. It has been proposed that patients with pathogenic complement regulatory mutations should continue eculizumab treatment indefinitely to prevent TMA relapse, although it might be safe to discontinue it after 6 to 12 months of treatment in patients with no genetic predisposition.23, 24, 25, 26 In this case, the genetic association with complement gene variants cannot be confirmed as pathogenic and eculizumab treatment was discontinued after 3 months as per the patient’s preference and was followed by close monitoring of kidney function and hemolysis parameters on a monthly basis. When complications of TMA have completely resolved, consideration can be made for discontinuation of therapy on a case-by-case basis, especially in the absence of any ongoing or predisposing triggers.9 If TMA recurs, reinitiating eculizumab therapy has been successful in suppressing complement activation.27
In summary, this case highlights the importance of prompt diagnosis of kidney transplant–associated TMA by a multidisciplinary TMA team and early initiation of eculizumab therapy to allow recovery of renal graft and hematologic parameters.28 Genetic testing should also be incorporated in the workup of TMA to identify any predisposition and guide the duration of therapy.
Article Information
Authors’ Full Names and Academic Degrees
Amandeep Godara, MD, Daniel R. Migliozzi, PharmD, Monika Pilichowska, MD, PhD, Nitender Goyal, MD, Cindy Varga, MD, and Craig E. Gordon MD, MS.
Support
None.
Financial Disclosure
Dr Gordon has received consulting fees and honoraria from Alexion, the manufacturer of eculizumab. The remaining authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received January 2, 2020. Evaluated by 3 external peer reviewers with editorial input from an Acting Editor-in-Chief (Editorial Board Member Michelle Rheault, MD). Accepted in revised form June 4, 2020. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
References
- 1.Schwimmer J., Nadasdy T.A., Spitalnik P.F., Kaplan K.L., Zand M.S. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41(2):471–479. doi: 10.1053/ajkd.2003.50058. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds J.C., Agodoa L.Y., Yuan C.M., Abbott K.C. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42(5):1058–1068. doi: 10.1016/j.ajkd.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Naesens M., Kuypers D.R., Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 4.Trimarchi H.M., Truong L.D., Brennan S., Gonzalez J.M., Suki W.N. FK506-associated thrombotic microangiopathy: report of two cases and review of the literature. Transplantation. 1999;67(4):539–544. doi: 10.1097/00007890-199902270-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nava F., Cappelli G., Mori G. Everolimus, cyclosporine, and thrombotic microangiopathy: clinical role and preventive tools in renal transplantation. Transplant Proc. 2014;46(7):2263–2268. doi: 10.1016/j.transproceed.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Vincenti F. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(26):2600–2601. doi: 10.1056/NEJMc1602859. [DOI] [PubMed] [Google Scholar]
- 7.Dedhia P., Govil A., Mogilishetty G., Alloway R.R., Woodle E.S., Abu Jawdeh B.G. Eculizumab and belatacept for de novo atypical hemolytic uremic syndrome associated with CFHR3-CFHR1 deletion in a kidney transplant recipient: a case report. Transplant Proc. 2017;49(1):188–192. doi: 10.1016/j.transproceed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T., Watarai Y., Futamura K. Efficacy of eculizumab therapy for atypical hemolytic uremic syndrome recurrence and antibody-mediated rejection progress after renal transplantation with preformed donor-specific antibodies: case report. Transplant Proc. 2017;49(1):159–162. doi: 10.1016/j.transproceed.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda T., Okumi M., Unagami K. Two cases of kidney transplantation-associated thrombotic microangiopathy successfully treated with eculizumab. Nephrology (Carlton) 2016;21(suppl 1):35–40. doi: 10.1111/nep.12768. [DOI] [PubMed] [Google Scholar]
- 10.FDA Belatacept prescribing information. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125288s070lbl.pdf. Accessed August 19, 2020.
- 11.Satoskar A.A., Pelletier R., Adams P. De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. Am J Transplant. 2010;10(8):1804–1811. doi: 10.1111/j.1600-6143.2010.03178.x. [DOI] [PubMed] [Google Scholar]
- 12.Siedlecki A., Irish W., Brennan D.C. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuber J., Le Quintrec M., Morris H., Fremeaux-Bacchi V., Loirat C., Legendre C. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando) 2013;27(4):117–125. doi: 10.1016/j.trre.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Satoh S., Saito K., Harada H., Okumi M., Saito M. Survey Committee for TA-TMA of the Japan Society for Transplantation Survey of thrombotic microangiopathy within 1 week after kidney transplantation between 2010 and 2015 in Japan. Clin Exp Nephrol. 2019;23(4):571–572. doi: 10.1007/s10157-018-1655-2. [DOI] [PubMed] [Google Scholar]
- 15.Ali M.N., Syed A., Bhandari S. Case series: hemolytic uremic syndrome--another cause of transplant dysfunction. Transplant Proc. 2013;45(9):3284–3288. doi: 10.1016/j.transproceed.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Le Quintrec M., Lionet A., Kamar N. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008;8(8):1694–1701. doi: 10.1111/j.1600-6143.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 17.Legendre C.M., Campistol J.M., Feldkamp T. Outcomes of patients with atypical haemolytic uraemic syndrome with native and transplanted kidneys treated with eculizumab: a pooled post hoc analysis. Transpl Int. 2017;30(12):1275–1283. doi: 10.1111/tri.13022. [DOI] [PubMed] [Google Scholar]
- 18.Abbas F., El Kossi M., Kim J.J., Sharma A., Halawa A. Thrombotic microangiopathy after renal transplantation: current insights in de novo and recurrent disease. World J Transplant. 2018;8(5):122–141. doi: 10.5500/wjt.v8.i5.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Quintrec M., Zuber J., Moulin B. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13(3):663–675. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- 20.Zhai Y.L., Meng S.J., Zhu L. Rare variants in the complement factor H-related protein 5 gene contribute to genetic susceptibility to IgA nephropathy. J Am Soc Nephrol. 2016;27(9):2894–2905. doi: 10.1681/ASN.2015010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman-Andrews L. The known unknown: the challenges of genetic variants of uncertain significance in clinical practice. J Law Biosci. 2017;4(3):648–657. doi: 10.1093/jlb/lsx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez Suarez M.L., Thongprayoon C., Mao M.A., Leeaphorn N., Bathini T., Cheungpasitporn W. Outcomes of kidney transplant patients with atypical hemolytic uremic syndrome treated with eculizumab: a systematic review and meta-analysis. J Clin Med. 2019, 919;8(7) doi: 10.3390/jcm8070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fakhouri F., Fila M., Provot F. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12(1):50–59. doi: 10.2215/CJN.06440616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodship T.H., Cook H.T., Fakhouri F. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91(3):539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Zuber J., Fakhouri F., Roumenina L.T., Loirat C., Fremeaux-Bacchi V. French Study Group for aHUS/C3G Collaborators Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 27.Macia M., de Alvaro Moreno F., Dutt T. Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J. 2017;10(3):310–319. doi: 10.1093/ckj/sfw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon C.E., Chitalia V.C., Sloan J.M. Thrombotic microangiopathy: a multidisciplinary team approach. Am J Kidney Dis. 2017;70(5):715–721. doi: 10.1053/j.ajkd.2017.05.017. [DOI] [PubMed] [Google Scholar]