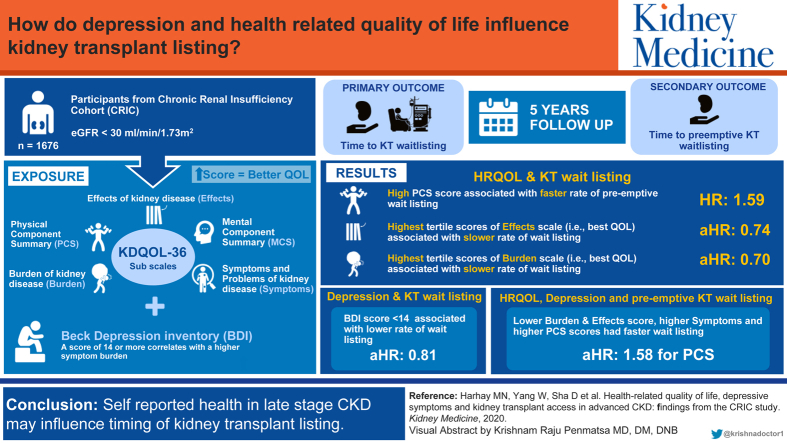
Abstract
Rationale & Objective
Among individuals with chronic kidney disease (CKD), poor self-reported health is associated with adverse outcomes including hospitalization and death. We sought to examine the association between health-related quality-of-life (HRQoL) and depressive symptoms in advanced CKD and subsequent access to the kidney transplant waiting list.
Study Design
Prospective cohort study.
Setting & Population
1,676 Chronic Renal Insufficiency Cohort (CRIC) study participants with estimated glomerular filtration rates ≤ 30 mL/min/1.73 m2 at study entry or during follow-up.
Exposures
HRQoL ascertained by 5 scales of the Kidney Disease Quality of Life-36 Survey (Physical Component Summary [PCS], Mental Component Summary, Symptoms, Burdens, and Effects), with higher scores indicating better HRQoL, and depressive symptoms ascertained using the Beck Depression Inventory.
Outcomes
Time to kidney transplant wait-listing and time to pre-emptive wait-listing.
Analytic Approach
Time-to-event analysis using Cox proportional hazards regression.
Results
During a median follow-up of 5.1 years, 652 (39%) participants were wait-listed, of whom 304 were preemptively wait-listed. Adjusted for demographics, comorbid conditions, estimated glomerular filtration rate slope, and cognitive function, participants with the highest scores on the Burden and Effects scales, respectively, had lower rates of wait-listing than those with the lowest scores on the Burden (wait-listing adjusted hazard ratio [aHR], 0.70; 95% CI, 0.57-0.85; P < 0.001) and Effects scales (wait-listing aHR, 0.74; 95% CI, 0.59-0.92; P = 0.007). Participants with fewer depressive symptoms (ie, Beck Depression Inventory score < 14) had lower wait-listing rates than those with more depressive symptoms (aHR, 0.81; 95% CI, 0.66-0.99; P = 0.04). Participants with lower Burden and Effects scale scores and those with higher Symptoms and PCS scores had higher pre-emptive wait-listing rates (aHR in highest tertile of PCS relative to lowest tertile, 1.58; 95% CI, 1.12-2.23; P = 0.01).
Limitations
Unmeasured confounders.
Conclusions
Self-reported health in late-stage CKD may influence the timing of kidney transplantation.
Index Words: Kidney Transplant, quality-of-life, wait-listing, depression
Visual abstract
Plain-Language Summary.
Compared with dialysis, kidney transplantation offers numerous benefits for people with kidney disease, including better health and longer life. We studied a large group of adults with advanced kidney disease. We examined whether having worse health-related quality of life and depressive symptoms was associated with being less likely to get on the waiting list for a kidney transplant. We found that people who reported the most negative effects and burdens of kidney disease had the fastest rates of wait-listing, and those with better physical function had higher rates of getting onto the waiting list before starting dialysis than those with low physical function.
A central priority of the recent Advancing American Kidney Health Initiative is to increase the rate of kidney transplantation as the first modality of kidney replacement therapy for people with end-stage kidney disease (ESKD) in the United States.1 Currently, <10% of people with ESKD in the United States are either placed on a waiting list or undergo transplantation before starting dialysis2 despite national policy that permits appropriate kidney transplantation candidates to begin accruing time on the waiting list when their estimated glomerular filtration rate (eGFR) reaches 20 mL/min/1.73 m2.3 Therefore, improving knowledge on which factors might promote or hinder early access to the kidney transplant waiting list is a public health priority.
Advanced chronic kidney disease (CKD) is associated with profoundly negative effects on health-related quality of life (HRQoL).4, 5, 6, 7 As CKD progresses, people may be burdened by more dietary and travel restrictions, decrements in physical and cognitive function,8,9 and increasing dependence on caregivers. Prior studies have shown that poor HRQoL is associated with higher risk for cardiovascular events and death among individuals with CKD,5 and that individuals with depression before starting dialysis are more likely to be hospitalized10 and die after starting dialysis.11 Therefore, although the desire to improve HRQoL may motivate many people with CKD to pursue kidney transplantation, existing evidence suggests that poor HRQoL could also be a barrier to achieving kidney transplantation. However, no studies to date have examined the association between self-reported health in advanced CKD and access to the kidney transplant waiting list.
The goal of this study was to evaluate whether, independent of traditional markers of disease burden, differences in HRQoL and depressive symptoms in advanced CKD influence the timing of subsequent kidney transplant wait-listing. Among participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study with stages 4-5 CKD, we assessed the independent association of HRQoL and depressive symptoms with time to wait-listing.
Methods
Study Population
The CRIC Study is an ongoing multicenter prospective study of risk factors for CKD progression and cardiovascular disease. The design and methods of the study and inclusion criteria for study participants have been described previously.12,13 Briefly, the CRIC Study recruited 3,939 participants aged 21 to 74 years with eGFRs between 20 and 70 mL/min/1.73 m2 from 2003 to 2008. Study participants completed extensive clinical evaluations at enrollment, including physical and laboratory assessments and questionnaires about medical history. During yearly re-evaluation visits, participants provided updated medical histories and underwent repeat laboratory and physical assessments. All participants provided informed consent. The study protocol was approved by the University of Pennsylvania Institutional Review Board (IRB Protocol 807882) and is in accordance with the Declaration of Helsinki.
The current analyses were restricted to participants with eGFR ≤ 30 mL/min/1.73 m2 at enrollment or during the course of CRIC Study follow-up. This cutoff was chosen to include individuals with stage 4 CKD, when guidelines suggest that patients should begin to be educated on options for kidney replacement therapy and referred for kidney transplantation evaluation.14 We estimated participants’ eGFR using the validated CRIC equation that includes serum creatinine level, serum cystatin C level, age, sex, and race.15
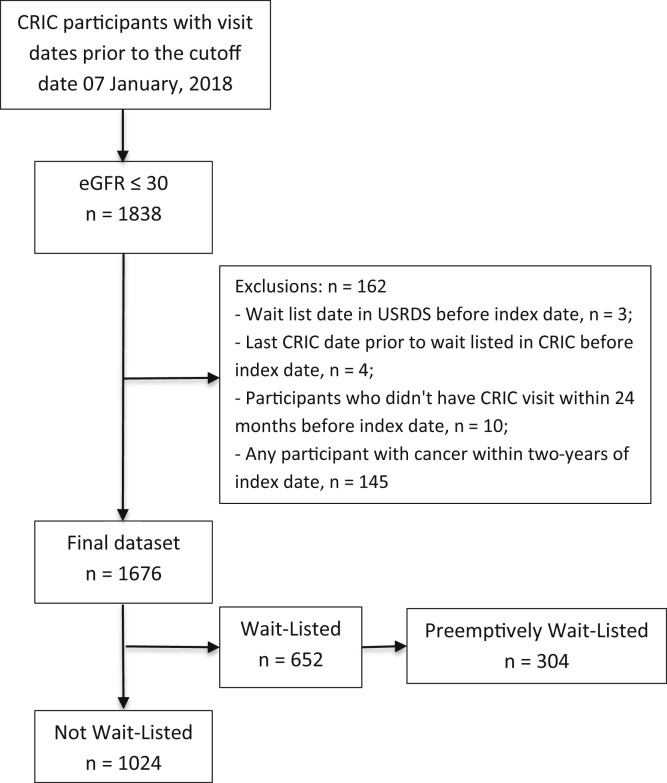
Participants contributed time to the current analysis from the calculated date of eGFR eligibility (ie, eGFR ≤ 30 mL/min/1.73 m2), defined as the index date, until they were wait-listed, died, or the end of the follow-up period in January 2018. We estimated index dates by assuming a linear decline in kidney function between annual CRIC visits. Individuals who were wait-listed or underwent transplantation before having a CRIC eGFR ≤ 30 mL/min/1.73 m2 and those who reported a new diagnosis of cancer were excluded from the cohort (Fig 1).
Figure 1.
Participant inclusion diagram. Abbreviations: CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; USRDS, US Renal Data System.
Primary Exposures: HRQoL Domains and Depressive Symptoms
The Kidney Disease Quality of Life-36 (KDQOL-36) is a measure of HRQoL that includes 2 generic scales from the 12-item Short Form Survey (SF-12) version 1 (Physical Component Summary [PCS] and Mental Component Summary [MCS]; 12 items total) and 3 kidney-specific scales (4-item Burden of Kidney Disease [Burden], 12-item Symptoms and Problems of Kidney Disease [Symptoms], and 8-item Effects of Kidney Disease [Effects]).16 The first item of the SF-12 asks participants to rate their overall health: “In general, would you say your health is: Excellent, Very Good, Good, Fair, or Poor.” The PCS is a measure of functional status that includes items about physical well-being, including activity limits and the ability to accomplish physical tasks. The MCS includes items that rate respondents’ emotional well-being, including levels of depression, anxiety, energy, and desire to participate in social activities. The Burden scale includes items about the extent to which CKD interferes with life and makes respondents feel like a burden on others. The Symptoms scale rates how bothered respondents are by symptoms of CKD (eg, nausea and shortness of breath). Finally, the Effects scale asks respondents how bothered they are by restrictions of CKD, including dependence on caregivers and the ability to travel.
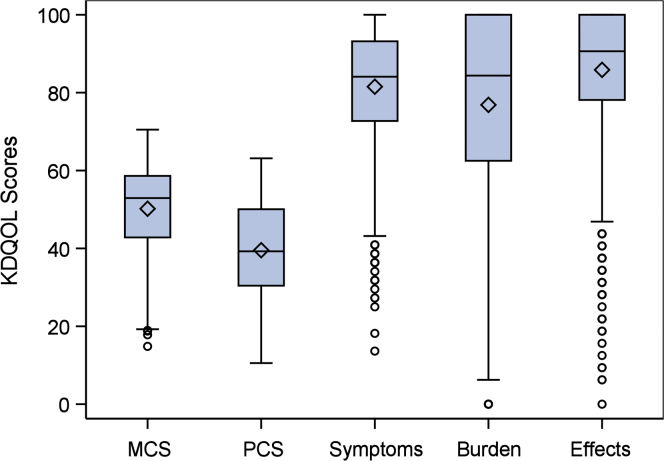
The SF-12 PCS and MCS are scored on a T-score metric (mean = 50, SD = 10, in the US general population). Raw values for the kidney-specific scale scores on each item are transformed to a 0 to 100 range, with higher scores indicating better HRQoL.17 CRIC participants completed the KDQOL-36 at study entry and then yearly thereafter. We included the KDQOL-36 measurement for each participant that was less than 12 months before the index date and closest in time to the index date. Given the non-normal distributions of HRQoL scores in the cohort (Fig 2), we analyzed KDQOL-36 subscale scores in tertiles, with the highest tertile indicating better HRQoL.
Figure 2.
Distribution of Kidney Disease Quality of Life (KDQOL) subscale scores in non–dialysis -dependent stage 4 chronic kidney disease (higher scores indicate better quality of life). Abbreviations: MCS, Mental Component Summary; PCS, Physical Component Summary.
The Beck Depression Inventory (BDI) is a screening instrument for depression that focuses on both cognitive and somatic symptoms of depression.18 The BDI has 21 items that measure respondents’ depressive symptoms in the prior week. Each question has 4 possible responses to indicate different levels of intensity. To score the test, each answer is assigned a value ranging from 0 to 3, with a minimum score of 0 and a maximum score of 63. CRIC participants completed the BDI at study entry and every 2 years thereafter. We included each participant’s BDI score that was less than 24 months before the index date and closest in time to the index date. We examined BDI cutoff scores of ≥11 and ≥14 because both thresholds have been described in the literature to screen for depression and related health outcomes in CKD and ESKD populations, respectively.19, 20, 21
Outcomes
Wait-listing, dialysis, and kidney transplantation dates were ascertained from CRIC medical event questionnaires, administered yearly to participants, and verified by linkage of CRIC data to the US Renal Data System (USRDS) data set. All-cause death was confirmed by report from next of kin, a review of hospital records if death occurred in the hospital, or through the Social Security Death Index.
Our primary outcome was time from the index date to wait-listing for a kidney transplant. Our secondary outcome was time from the index date to pre-emptive wait-listing for a kidney transplant. The USRDS data set provided exact dates of wait-listing, dialysis onset, and kidney transplantation through February 26, 2015. In cases for which wait-listing occurred after February 2015 (n = 379), we used the date of the first study visit at which the participant reported kidney transplant wait-listing as the event date. The follow-up period for the present study ended on January 7, 2018.
Analytic Strategy
Descriptive statistics were summarized as mean with standard deviation (SD) or median with interquartile range for continuous variables and as frequency and proportion for categorical variables. Continuous and categorical variables were compared using Kruskal-Wallis tests or χ2 test, as appropriate. All hypothesis tests were 2 sided, with a significance level of 0.05. We estimated wait-listing rates by KDQOL-36 scales, in tertiles, and by BDI threshold scores (ie, ≥11 and ≥14) using the Kaplan-Meier method and log-rank test to compare unadjusted survival curves. We then fit Cox proportional hazards models to evaluate the associations between HRQoL, depressive symptoms, and wait-listing for a kidney transplant.
We compared unadjusted models, models adjusted for sociodemographic characteristics (model 1), and models that were fully adjusted for clinically important covariates that may influence the likelihood of kidney transplant candidacy (model 2).5,8,22 Because we focused on estimating the effects of HRQoL and depressive symptoms on wait-listing (as opposed to the cumulative incidence of wait-listing23), participants were censored at death, study withdrawal, or end of study follow-up. Participants were additionally censored at the time of dialysis for the secondary outcome of pre-emptive wait-listing. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc).
Covariates
Models 1 and 2 included participant age, sex, race/ethnicity, income level, educational attainment, and insurance status immediately before the index date. Model 2 also included CRIC clinical site and the following variables, ascertained as close to the index date as possible: history of diabetes, hypertension, ischemic heart disease, peripheral arterial disease, chronic obstructive pulmonary disease, cognitive impairment (defined as Modified Mini-Mental State Examination score < 80),8 nephrology care, systolic and diastolic blood pressure, body mass index, tobacco use, alcohol use, recreational drug use, living alone, eGFR at index visit (mL/min/1.73 m2), eGFR slope from baseline to index visit (mL/min/1.73 m2 per year), 24-hour urinary protein excretion, serum albumin level, and glycated hemoglobin level.
Missing Data
Data were missing in <9% of participants for all variables with the exception of 24-hour urinary protein excretion (missing in 13% of participants). The following numbers of participants were missing values for the main exposure variables: KDQOL overall (n = 12), Burden (n = 8), Effects (n = 7), Symptoms/Problems (n = 6), SF-12 MCS (n = 29), SF-12 PCS (n = 29), and BDI (n = 76). Missing data were imputed using the fully conditional specification method of multiple imputation24,25 with 10 iterations. The final estimates were combined using Rubin’s formula.26
Sensitivity Analyses
There were 45 CRIC participants who reported dates for kidney transplantation but were missing dates of wait-listing in the CRIC data set. In the primary analyses, we used the date of kidney transplantation as the date of wait-listing for these participants because most (64%) were living donor recipients and kidney transplantation programs were not required to wait-list living donor candidates before kidney transplantation until September 2014.27 In sensitivity analysis, we censored these participants at the last CRIC visit date before the kidney transplantation date. Further, changes in the US kidney allocation system that occurred during our study period may have influenced wait-listing trends.28 Therefore, we examined whether post–kidney allocation system era (defined as post-2014) modified associations between HRQoL, depressive symptoms, and time to wait-listing. We tested era effects by including interaction terms in separate models that were adjusted for all other covariates. We tested interaction terms using Wald tests. Finally, we examined whether associations between HRQoL, depressive symptoms, and wait-listing were consistent in a subgroup that was younger (aged <65 years) at the index date.
Results
Baseline Characteristics
Among 3,939 participants enrolled in CRIC, 1,838 reached eGFR ≤ 30 mL/min/1.73 m2, and 1,676 met inclusion criteria for the study (Fig 1). Mean age of included participants was 59 years, 48% were women, and 46% were non-Hispanic Black. Average time from KDQOL-36 to the index date was 189 (SD, 223) days, and time from BDI to index date was 127 (SD, 145) days. Table 1 displays participant characteristics closest to the index date, stratified by responses to the overall health status question on the KDQOL-36 (cohort characteristics at CRIC study entry are displayed in Table S7). Figure 2 displays the cohort distributions of KDQOL-36 subscale scores. With respect to depressive symptoms, 505 (32%) participants had BDI scores ≥ 11 and 338 participants (21%) had scores ≥ 14.
Table 1.
Demographic and Clinical Characteristics of CRIC Participants with Stage 4 Chronic Kidney Disease, Stratified by Responses to the Overall Health Status Question on the KDQOL-36 Survey
| Characteristics at Index Visit | Overall (N = 1,664)a | Excellent/Very Good (N = 214) | Good (N = 620) | Fair (N = 631) | Poor (N = 199) |
|---|---|---|---|---|---|
| Age, y | 58.75 (11.44) | 58.78 (12.54) | 58.72 (11.92) | 59.31 (11.03) | 57.01 (9.75) |
| Female sex | 790 (47.5%) | 90 (42.1%) | 266 (42.9%) | 335 (53.1%) | 99 (49.7%) |
| Race-ethnicity category | |||||
| Hispanic | 300 (18.0%) | 14 (6.5%) | 107 (17.3%) | 119 (18.9%) | 60 (30.2%) |
| Non-Hispanic Black | 759 (45.6%) | 74 (34.6%) | 266 (42.9%) | 337 (53.4%) | 82 (41.2%) |
| Non-Hispanic White | 537 (32.3%) | 111 (51.9%) | 223 (36%) | 153 (24.2%) | 50 (25.1%) |
| Other | 68 (4.1%) | 15 (7%) | 24 (3.9%) | 22 (3.5%) | 7 (3.5%) |
| Annual Income | |||||
| ≤$20,000 | 661 (39.7%) | 39 (18.2%) | 212 (34.2%) | 295 (46.8%) | 115 (57.8%) |
| >$20,000 | 752 (45.2%) | 136 (63.6%) | 316 (51%) | 249 (39.5%) | 51 (25.6%) |
| Do not wish to answer | 251 (15.1%) | 39 (18.2%) | 92 (14.8%) | 87 (13.8%) | 33 (16.6%) |
| High school graduate | 1,205 (72.4%) | 195 (91.1%) | 476 (76.8%) | 419 (66.4%) | 115 (57.8%) |
| Insured | |||||
| No | 164 (10.7%) | 15 (7.2%) | 67 (11.5%) | 61 (10.7%) | 21 (11.9%) |
| Unknown/incomplete | 279 (18.2%) | 59 (28.4%) | 122 (20.9%) | 81 (14.2%) | 17 (9.6%) |
| Yes | 1,094 (71.2%) | 134 (64.4%) | 394 (67.6%) | 427 (75%) | 139 (78.5%) |
| Diabetes | 1,000 (60.1%) | 87 (40.7%) | 336 (54.2%) | 418 (66.2%) | 159 (79.9%) |
| Hypertension | 1,560 (93.8%) | 187 (87.4%) | 583 (94%) | 597 (94.6%) | 193 (97%) |
| Ischemic heart disease | 449 (27.0%) | 33 (15.4%) | 162 (26.1%) | 195 (30.9%) | 59 (29.6%) |
| History of COPD | 75 (4.6%) | 4 (1.9%) | 18 (2.9%) | 39 (6.3%) | 14 (7.1%) |
| Vascular disease | 175 (10.5%) | 5 (2.3%) | 58 (9.4%) | 78 (12.4%) | 34 (17.1%) |
| eGFR closest to index date | 31.43 (7.46) | 32.36 (6.70) | 31.73 (7.64) | 31.23 (7.40) | 30.13 (7.66) |
| Yearly change in eGFR before index date | −0.17 (1.01) | −0.29 (0.91) | −0.20 (1.00) | −0.16 (1.02) | −0.03 (1.06) |
| Body mass index, kg/m2 | 32.54 (8.11) | 29.94 (6.43) | 31.92 (7.56) | 33.51 (8.41) | 34.24 (9.46) |
| Systolic blood pressure, mm Hg | 133.78 (23.10) | 126.83 (19.22) | 131.80 (22.70) | 136.68 (24.46) | 138.27 (21.40) |
| Diastolic blood pressure, mm Hg | 70.94 (13.05) | 70.45 (12.80) | 70.87 (12.95) | 71.19 (13.58) | 70.91 (11.93) |
| 3MS score ≥ 80 | 1,377 (86.1%) | 186 (92.1%) | 519 (86.6%) | 519 (86.1%) | 153 (78.5%) |
| Live with others | 1,307 (78.7%) | 166 (77.6%) | 498 (80.3%) | 491 (78.1%) | 152 (76.8%) |
| Tobacco use | |||||
| Current smoker | 238 (14.3%) | 19 (8.9%) | 98 (15.8%) | 88 (13.9%) | 33 (16.6%) |
| Nonsmoker | 735 (44.2%) | 108 (50.5%) | 266 (42.9%) | 278 (44.1%) | 83 (41.7%) |
| Previous smoker | 691 (41.5%) | 87 (40.7%) | 256 (41.3%) | 265 (42%) | 83 (41.7%) |
| Consumes alcohol | 891 (53.5%) | 152 (71%) | 354 (57.1%) | 303 (48%) | 82 (41.2%) |
| Any illicit drug use | 541 (32.5%) | 66 (30.8%) | 186 (30%) | 223 (35.3%) | 66 (33.2%) |
| Recent visit to nephrologist | 1,440 (86.5%) | 187 (87.4%) | 541 (87.3%) | 545 (86.4%) | 167 (83.9%) |
| 24-h urinary protein, g/24 ha | 2.05 (3.11) | 1.26 (1.99) | 1.90 (2.94) | 2.20 (3.21) | 2.89 (3.96) |
| Serum albumin, g/dL | 3.78 (0.49) | 3.92 (0.42) | 3.80 (0.49) | 3.75 (0.49) | 3.65 (0.52) |
| Glycated hemoglobin, % | 6.91 (1.67) | 6.53 (1.59) | 6.76 (1.60) | 6.98 (1.66) | 7.55 (1.78) |
Note: Data for categorical variables expressed as number (percent); data for continuous variables expressed as median (interquartile range). Overall health question: In general, would you say your health is: Excellent, Very Good, Good, Fair, or Poor?
Abbreviations: 3MS, Modified Mini-Mental State Examination; COPD, chronic obstructive pulmonary disease; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); KDQOL-36, Kidney Disease Quality of Life-36.
Missing in 12 participants.
Association Between HRQoL Domains and Kidney Transplant Wait-Listing
During a median follow-up of 5.1 (interquartile range, 3.0-8.3) years, 652 (39%) participants were wait-listed for a kidney transplant and 547 (33%) died without wait-listing. Participants who scored in the highest tertile of the PCS, indicating those with the best physical health, had a higher unadjusted hazard of wait-listing than those in the lower 2 tertiles (log-rank P < 0.001; Fig S1). In the unadjusted Cox model, compared with participants in the lowest PCS score tertile, those in the highest tertile had a higher rate of wait-listing (hazard ratio [HR], 1.59; 95% confidence interval [CI], 1.30-1.93; P < 0.001). The association between higher PCS score and wait-listing was attenuated after adjustment for participant demographics and other covariates (Fig 3; Table S1).
Figure 3.
Adjusted associations between self-reported health assessments and (A) wait-listing and (B) pre-emptive wait-listing. Abbreviations: BDI, Beck Depression Inventory; CI, confidence interval; HR, hazard ratio; HRQOL, health-related quality of life; KDQOL, Kidney Disease Quality of Life; MCS, Mental Component Summary; PCS, Physical Component Summary; Ref, reference.
Participants who scored in the lower 2 tertiles of the Burden and Effects scales, indicating worse quality of life from the burdens and effects from CKD, respectively, had a higher unadjusted hazard of wait-listing than participants who scored in the highest tertiles of the Burden and Effects scales (Fig S1). In the fully adjusted model, compared with participants who scored in the lowest tertile of the Effects scale (ie, those most bothered by the effects of CKD), those in the highest tertile had a lower rate of wait-listing (model 2 adjusted HR [aHR], 0.74; 95% CI, 0.59-0.92; P = 0.007; Fig 3; Table S1). Compared with those who scored in the lowest tertile of the Burden scale (ie, those most burdened by CKD), participants in the highest tertile had a lower rate of wait-listing (model 2 aHR, 0.70; 95% CI, 0.57-0.85; P < 0.001). Other scales of the KDQOL-36 were not independently associated with differences in wait-listing.
Association Between Depressive Symptoms and Kidney Transplant Wait-Listing
Participants with fewer depressive symptoms, using either BDI score < 11 or BDI score < 14 as threshold scores, had similar unadjusted rates of wait-listing as those with more depressive symptoms (Fig 3; Table S1). After adjustment for demographics (model 1) and in the fully adjusted model, participants with BDI scores < 14 (ie, fewer depressive symptoms) had a lower rate of wait-listing than those with BDI scores ≥ 14 (model 2 aHR, 0.81; 95% CI, 0.66-0.99; P = 0.04). The BDI score threshold of <11 was not associated with differences in wait-listing in the fully adjusted model.
Association Between HRQoL, Depressive Symptoms, and Pre-emptive Wait-Listing
Among 652 participants who were wait-listed, 304 (47%) were wait-listed pre-emptively. In fully adjusted Cox models in which death and dialysis initiation were treated as censoring events, findings were similar with respect to Burden and Effects scale scores (Fig 3; Table S2). In addition, compared with participants in the lowest tertile of PCS scores (ie, lowest physical health), those in higher tertiles were more likely to be wait-listed pre-emptively (model 2 aHR for highest PCS tertile, 1.58; 95% CI, 1.12-2.23; P = 0.01). Further, compared with those who scored in the lowest tertile on the Symptoms scale (ie, those with the most symptoms and problems from CKD), participants in the middle tertile (ie, fewer symptoms) were more likely to be pre-emptively wait-listed (model 2 aHR, 1.40; 95% CI, 1.02-1.92; P = 0.04). Differences in other KDQOL scale scores and depressive symptoms were not associated with differences in pre-emptive wait-listing.
Results of Sensitivity Analyses
Results were similar in analyses of wait-listing and pre-emptive wait-listing in which participants with missing wait-list dates and non-missing kidney transplantation dates were censored at the last CRIC visit before kidney transplantation (Tables S3 and S4). There was no evidence of effect modification by pre– or post–kidney allocation system era on the association between KDQOL scale scores, depressive symptoms, and wait-listing (P > 0.05 for all interaction terms). Associations between HRQoL subscales and wait-listing and pre-emptive wait-listing were similar in the subgroup of CRIC participants that was younger than 65 years at the index date, but the association between BDI score ≥ 14 and wait-listing was attenuated and no longer statistically significant in unadjusted and adjusted models (aHR in model 2, 0.86; 95% CI, 0.69-1.07; P = 0.17; Tables S5 and S6).
Discussion
In a diverse and multicenter cohort with advanced CKD and detailed phenotyping, we found that those with fewer burdens, effects, and depressive symptoms had lower subsequent rates of kidney transplant wait-listing than those with more burdens, effects, and depressive symptoms, respectively. Pre-emptive wait-listing also occurred less rapidly among participants with fewer burdens and effects from CKD and occurred more rapidly among those with fewer CKD-related symptoms and better physical health. These robust associations were independent of age, cognitive function, eGFR slope, sociodemographic factors, and comorbid conditions. The results of our study underscore the importance of self-reported health on the path to kidney transplantation, and our findings could be harnessed to personalize care during the transition to ESKD and improve patient education about the benefits of kidney transplantation relative to dialysis.29, 30, 31
Individual experiences with late-stage CKD may differ substantially. For some individuals, CKD may fundamentally alter daily life, whereas other individuals may be largely asymptomatic and unaware of the severity of their disease until they become dialysis dependent.32,33 We found evidence that people with lower burdens and effects of CKD had lower rates of wait-listing and pre-emptive wait-listing for kidney transplantation than those with more burdens and effects, respectively, whereas other domains of HRQoL were not associated with differences in overall wait-listing rates. Further, although having more depressive symptoms was associated with faster wait-listing rates in the overall cohort, this association was not observed among those who were younger than 65 years at the index date or for the outcome of pre-emptive wait-listing. One potential explanation for the consistent associations we observed between the Burden and Effects subscale scores of the KDQOL-36 and wait-listing is that individuals who are burdened and bothered by the effects of advanced CKD may also be those who prioritize kidney transplantation the most to avoid dialysis and its punishing lifestyle, including limits on fluid and dietary intake, inability to work or travel, dependence on physicians and caregivers, and changes in physical appearance.6,34,35 However, more studies are needed to fully determine the influence of depressive symptoms and HRQoL on patient and physician decision making about the optimal modality of kidney replacement therapy in advanced CKD.
In contrast to our findings on the burdens and effects of CKD, we also observed that individuals with better physical health and fewer symptoms in late-stage CKD had higher rates of pre-emptive wait-listing than those with poor physical health and more symptoms, respectively. These findings may indicate that physical health and symptoms are useful proxies of disease severity in late-stage CKD. Prior work has also suggested that individuals with poor physical health or more symptoms from CKD may have difficulty completing the kidney transplantation evaluation process or may be deemed too frail by transplantation providers.36,37 Our findings that higher predialysis physical health and fewer symptoms were associated with pre-emptive but not overall wait-listing may be related to the changes in HRQoL that many people experience after initiating dialysis.38 Among those who report good physical health after starting dialysis, studies have shown higher rates of wait-listing, kidney transplantation. and posttransplantation survival.39, 40, 41 Interestingly, evidence suggests that among dialysis patients, self-reported overall health correlates poorly with self-reported physical health.42 Therefore, knowledge of several different domains of HRQoL may help providers better understand potential motivators for and barriers to pre-emptive kidney transplantation.
Numerous studies have highlighted the high prevalence and clinical implications of depression, poor physical function, frailty, and functional dependencies among patients receiving dialysis,20,43, 44, 45, 46, 47, 48 and KDQOL normative data have recently been published for US dialysis patients.49 Our findings are consistent with prior work establishing that, similar to dialysis patients, many individuals with advanced CKD report very poor psychosocial health.5 For example, we found that 1 in 5 individuals with late-stage CKD had a BDI score ≥ 14, indicating a substantial burden of depressive symptoms.19,20 Importantly, although our findings do not point to poor self-reported health as a universal barrier to kidney transplant wait-listing, prior studies suggest that worse HRQoL and depression increase the risk for adverse outcomes, such as hospitalization and death, among individuals with CKD.5,10,11 Studies are needed to learn whether targeted interventions, such as counseling and prehabilitation,50 can improve the transition to ESKD and access to kidney transplantation among individuals with poor self-reported health.
Our study has several strengths. To our knowledge, ours is the first study to assess the influence of psychosocial factors in advanced CKD on access to kidney transplantation. Our study design enabled us to adjust for several known confounders, including cognitive function, that are not typically available in registry data. Given the long follow-up period of the CRIC Study and linkage to the USRDS database, we were also able to confirm important outcomes.
However, our study was subject to certain limitations. For example, generalizability is a potential limitation because most CRIC sites are academic centers that are affiliated with kidney transplantation programs and may explain the overall high rate of wait-listing in this population. Nonetheless, our cohort characteristics mirror those of national dialysis cohorts such as the Dialysis Outcomes and Practice Patterns Study.40 There is also the possibility for type I error due to our examination of multiple health scales. Finally, although we adjusted for several known confounders including income and social support, there are likely to be other unmeasured confounders that influence wait-list eligibility (eg, severity of illness, timing of referral for kidney transplantation, availability of other laboratory results to qualify for wait-listing, and type of insurance coverage22) and are important in understanding differences in early access to kidney transplantation.
In summary, we found that in a large diverse cohort of individuals with late-stage CKD, those with the most effects, burdens, and depressive symptoms had the highest rates of subsequent wait-listing, whereas having better physical health and fewer symptoms was associated with more rapid pre-emptive wait-listing. Incorporating metrics of patient-reported health into predialysis CKD care may be useful to guide conversations about the potential benefits of kidney transplantation relative to dialysis and promote timely interventions to improve HRQoL regardless of kidney replacement therapy modality.
Article Information
CRIC Study Investigators
Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, Panduranga Rao, MD, and Mahboob Rahman, MD.
Authors’ Full Names and Academic Degrees
Meera Nair Harhay, MD, MSCE, Wei Yang, PhD, Daohang Sha, PhD, Jason Roy, PhD, Boyang Chai, MS, Michael J. Fischer, MD, L. Lee Hamm, MD, Peter D. Hart, MD, Chi-yuan Hsu, MD, Yonghong Huan, MD, Anne M. Huml, MD, Radhakrishna Reddy Kallem, MD, Manjula Kurella Tamura, MD, Anna C. Porter, MD, Ana C. Ricardo, MD, Anne Slaven, MSSA, Sylvia E. Rosas, MD, Raymond R. Townsend, MD, Peter P. Reese, MD, James P. Lash, MD, and Sanjeev Akkina, MD.
Authors’ Contributions
Research idea and study design: MNH, WY, JR, MF, LH, PH, C-yH, YH, AH, RRK, MKT, AP, AR, AS, SR, RT, PPR, JL, SA; data acquisition: MNH, PPR, JL, SA; data analysis/interpretation: MNH, WY, DS, JR, BC, PPR, JL, SA; supervision or mentorship: WY, PPR, JL, SA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland General Clinical Research Center M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/National Center for Research Resources UCSF-CTSI UL1 RR-024131. Dr Harhay is funded by the NIDDK K23DK105207 and R01DK124388 Awards. Dr Ricardo is funded by the NIDDK K23DK094829 Award. Dr Lash is funded by the NIDDK K24DK092290 and R01-DK072231-91 Awards. Dr Reese’s efforts were supported by National Institute of Allergy and Infectious Diseases K24AI146137. Funders of this study did not have any role in study design, analysis and interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received January 13, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor and the Editor-in-Chief. Accepted in revised form June 28, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: (A) Probability of Wait-Listing for KT by Tertile of Physical Health, (B) Effects of Kidney Disease, and (C) Burdens of Kidney Disease.
Table S1: Associations Between Self-reported Health Assessments and Time to Wait-Listing.
Table S2: Associations Between Self-reported Health Assessments and Pre-emptive Wait-Listing.
Table S3: Results of Sensitivity Analysis for Time to Wait-Listing in Which Individuals With Nonmissing Transplant Dates But Missing Wait-Listing Dates (N = 45) Are Censored at Their Last CRIC Visit Before Their Transplant Date.
Table S4: Results of Sensitivity Analysis for Time to Pre-emptive Wait-Listing in Which Individuals With Nonmissing Transplant Dates But Missing Wait-Listing Dates (N = 45) Are Censored at Their Last CRIC Visit Before Their Transplant Date.
Table S5: Results of Sensitivity Analysis for Time to Wait-Listing in the Subgroup of CRIC Participants Who Were Younger Than 65 Years at the Index Date.
Table S6: Results of Sensitivity Analysis for Time to Pre-emptive Wait-Listing in the Subgroup of CRIC Participants Who Were Younger Than 65 Years at the Index Date.
Table S7: Demographic and Clinical Characteristics of Study Participants at CRIC Study Entry, Stratified by Responses to the Overall Health Status Question∗ on the KDQOL-36 Survey.
Contributor Information
Meera Nair Harhay, Email: mnh52@drexel.edu.
CRIC Study Investigators:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, John W. Kusek, Panduranga Rao, and Mahboob Rahman
Supplementary Material
Figure S1, Tables S1-S7
References
- 1.National Kidney Foundation. The Advancing Kidney Health Initiative. 2019. National Kidney Foundation. https://www.kidney.org/advocacy/advancing-american-kidney-health-initiative. Accessed October 4, 2019.
- 2.Dudley C.R., Johnson R.J., Thomas H.L., Ravanan R., Ansell D. Factors that influence access to the national renal transplant waiting list. Transplantation. 2009;88(1):96–102. doi: 10.1097/TP.0b013e3181aa901a. [DOI] [PubMed] [Google Scholar]
- 3.Abecassis M., Bartlett S.T., Collins A.J. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3(2):471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorodetskaya I., Zenios S., McCulloch C.E. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–2808. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 5.Porter A.C., Lash J.P., Xie D. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujais S.K., Story K., Brouillette J. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlman R.L., Finkelstein F.O., Liu L. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45(4):658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Harhay M.N., Xie D., Zhang X. Cognitive impairment in non-dialysis-dependent CKD and the transition to dialysis: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2018;72(4):499–508. doi: 10.1053/j.ajkd.2018.02.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittiskulnam P., Sheshadri A., Johansen K.L. Consequences of CKD on functioning. Semin Nephrol. 2016;36(4):305–318. doi: 10.1016/j.semnephrol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowling C.B., Booth J.N., 3rd, Gutierrez O.M. Nondisease-specific problems and all-cause mortality among older adults with CKD: the REGARDS Study. Clin J Am Soc Nephrol. 2014;9(10):1737–1745. doi: 10.2215/CJN.00880114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar M.Z., Streja E., Sumida K. Pre-ESRD depression and post-ESRD mortality in patients with advanced CKD transitioning to dialysis. Clin J Am Soc Nephrol. 2017;12(9):1428–1437. doi: 10.2215/CJN.00570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7 suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplantation Network Minority Affairs Committee . Vol 2018. U.S. Department of Health & Human Services; Washington, DC: 2015. (Educational Guidance on Patient Referral to Kidney Transplantation). [Google Scholar]
- 15.Anderson A.H., Yang W., Hsu C.Y. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hays R.D., Kallich J.D., Mapes D.L., Coons S.J., Carter W.B. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 17.Hays R.D., Sherbourne C.D., Mazel R.M. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 18.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel P.L., Cukor D., Cohen S.D., Peterson R.A. Depression in end-stage renal disease patients: a critical review. Adv Chronic Kidney Dis. 2007;14(4):328–334. doi: 10.1053/j.ackd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S.D., Norris L., Acquaviva K., Peterson R.A., Kimmel P.L. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332–1342. doi: 10.2215/CJN.03951106. [DOI] [PubMed] [Google Scholar]
- 21.Hedayati S.S., Minhajuddin A.T., Toto R.D., Morris D.W., Rush A.J. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54(3):433–439. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King K.L., Husain S.A., Jin Z., Brennan C., Mohan S. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol. 2019;14(10):1500–1511. doi: 10.2215/CJN.03140319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4(3):287–295. doi: 10.6000/1929-6029.2015.04.03.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne J.A., White I.R., Carlin J.B. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin D.B. John Wiley & Sons; New York, NY: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 27.United Network for Organ Sharing. Reminder: Register All Candidates Before Transplant, Including Those Receiving Living Donor Organs. 2014. United Network of Organ Sharing. https://unos.org/news/reminder-register-candidates-transplant-including-receiving-living-donor-organs/. Accessed January 3, 2020.
- 28.Harhay M.N., Harhay M.O., Ranganna K. Association of the kidney allocation system with dialysis exposure before deceased donor kidney transplantation by preemptive wait-listing status. Clin Transplant. 2018;32(10) doi: 10.1111/ctr.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keown P. Improving quality of life--the new target for transplantation. Transplantation. 2001;72(12 suppl):S67–S74. [PubMed] [Google Scholar]
- 30.von der Lippe N., Waldum B., Brekke F.B., Amro A.A., Reisaeter A.V., Os I. From dialysis to transplantation: a 5-year longitudinal study on self-reported quality of life. BMC Nephrol. 2014;15:191. doi: 10.1186/1471-2369-15-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Painter P., Krasnoff J.B., Kuskowski M., Frassetto L., Johansen K. Effects of modality change on health-related quality of life. Hemodial Int. 2012;16(3):377–386. doi: 10.1111/j.1542-4758.2012.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal V., Jaar B.G., Frisby X.Y. Access to health care among adults evaluated for CKD: findings from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2012;59(3 suppl 2):S5–S15. doi: 10.1053/j.ajkd.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plantinga L.C., Boulware L.E., Coresh J. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mapes D.L., Lopes A.A., Satayathum S. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64(1):339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 35.von der Lippe N., Waldum-Grevbo B., Varberg Reisaeter A., Os I. Is HRQOL in dialysis associated with patient survival or graft function after kidney transplantation? BMC Nephrol. 2016;17:94. doi: 10.1186/s12882-016-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlam T., Ulrich E., Kent M., Malinzak L. Frailty testing pilot study: pros and pitfalls. J Clin Med Res. 2018;10(2):82–87. doi: 10.14740/jocmr3203w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugen C.E., Chu N.M., Ying H. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14(4):576–582. doi: 10.2215/CJN.12921118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto N.A., van Loon I.N., Boereboom F.T.J. Association of initiation of maintenance dialysis with functional status and caregiver burden. Clin J Am Soc Nephrol. 2019;14(7):1039–1047. doi: 10.2215/CJN.13131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reese P.P., Shults J., Bloom R.D. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. 2015;66(5):837–845. doi: 10.1053/j.ajkd.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szeifert L., Bragg-Gresham J.L., Thumma J. Psychosocial variables are associated with being wait-listed, but not with receiving a kidney transplant in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012;27(5):2107–2113. doi: 10.1093/ndt/gfr568. [DOI] [PubMed] [Google Scholar]
- 41.Harhay M.N., Hill A.S., Wang W. Measures of global health status on dialysis signal early rehospitalization risk after kidney transplantation. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen D.E., Lee A., Sibbel S., Benner D., Brunelli S.M., Tentori F. Use of the KDQOL-36 for assessment of health-related quality of life among dialysis patients in the United States. BMC Nephrol. 2019;20(1):112. doi: 10.1186/s12882-019-1295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook W.L., Jassal S.V. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73(11):1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 44.Anand S., Johansen K.L., Grimes B. Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: the Comprehensive Dialysis Study. Hemodial Int. 2013;17(1):50–58. doi: 10.1111/j.1542-4758.2012.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansen K.L., Chertow G.M., Jin C., Kutner N.G. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 46.Bao Y., Dalrymple L., Chertow G.M., Kaysen G.A., Johansen K.L. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kutner N.G., Zhang R., Huang Y., Johansen K.L. Depressed mood, usual activity level, and continued employment after starting dialysis. Clin J Am Soc Nephrol. 2010;5(11):2040–2045. doi: 10.2215/CJN.03980510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fried L.F., Biggs M.L., Shlipak M.G. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 49.Peipert J.D., Nair D., Klicko K., Schatell D.R., Hays R.D. Kidney Disease Quality of Life 36-Item Short Form Survey (KDQOL-36) normative values for the United States dialysis population and new single summary score. J Am Soc Nephrol. 2019;30(4):654–663. doi: 10.1681/ASN.2018100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheshadri A., Johansen K.L. Prehabilitation for the frail patient approaching ESRD. Semin Nephrol. 2017;37(2):159–172. doi: 10.1016/j.semnephrol.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Tables S1-S7