Abstract
Rationale & Objective
Since the change in erythropoiesis-stimulating agent (ESA) labeling and bundling of dialysis services in the United States, few studies have addressed the clinical importance of ESA hyporesponsiveness and none have considered health care resource use in this population. We aimed to further explore ESA hyporesponsiveness and its consequences.
Study Design
Retrospective observational cohort study.
Setting & Participants
US Renal Data System Medicare participants receiving dialysis with a minimum 6 months of continuous ESA use from 2012 to 2014.
Predictors
Erythropoietin resistance index (≥2.0 U/kg/wk/g/L) and ESA dose were used to identify ESA hyporesponders and hyporesponsive subgroups: isolated, intermittent, and chronic.
Outcomes
Associations between ESA responsiveness and mortality, cardiovascular hospitalization rates, and health care resource use were evaluated and compared across subgroups.
Analytical Approach
Baseline characteristics were compared using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Incidence rates of health care resource use were modeled using an unadjusted and adjusted generalized linear model.
Results
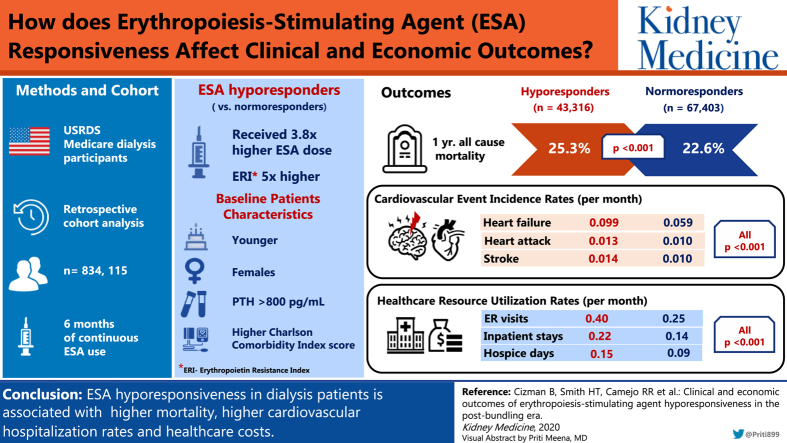
Of 834,115 dialysis patients in the CROWNWeb database, 38,891 ESA hyporesponders and 59,412 normoresponders met all inclusion criteria. Compared with normoresponders, hyporesponders were younger women, weighed less, and had longer durations of dialysis (all P < 0.001). Hyporesponders received 3.8-fold higher ESA doses (mean, 94,831 U/mo) and erythropoietin resistance index was almost 5 times higher than in normoresponders. Hyporesponders had lower hemoglobin levels and parathyroid hormone levels > 800 pg/mL, and iron deficiency was present in 26.5% versus 10.9% in normoresponders. One-year mortality was higher among hypo- compared with normoresponders (25.3% vs 22.6%). Hyporesponders also had significantly higher rates of hospitalization for cardiovascular events, emergency department visits, inpatient stays, home health agency visits, skilled nursing facility, and hospice days.
Limitations
Only US Medicare patients were included and different hyporesponder definitions may have influenced the results.
Conclusions
This study explored ESA hyporesponsiveness using new definitions and incorporated clinical and economic outcomes. It established that ESA-hyporesponsive dialysis patients had higher mortality, cardiovascular hospitalization rates, and health care costs as compared with ESA-normoresponsive patients.
Index Words: ESA hyporesponsiveness, erythropoietin, health care resource utilization, hemodialysis, USRDS Medicare, anemia of chronic kidney disease
Graphical abstract
Plain-Language Summary.
Few studies of chronic kidney disease anemia treatment have addressed the clinical importance of poor erythropoiesis-stimulating agent response (further called epoetins) and none have considered health care costs associated with this clinically challenging problem. This study explored epoetin hyporesponsiveness and its consequences in the US Medicare dialysis population from 2012 to 2014. Epoetin hyporesponders received on average almost 4 times higher epoetin doses per month, had lower hemoglobin values and higher parathyroid hormone levels and were more iron deficient as compared with normoresponders, and had higher 1-year mortality. Epoetin hyporesponders also had significantly higher rates of hospitalization for cardiovascular events, such as heart attack and congestive heart failure; emergency department visits; hospital inpatient stays; and home health agency visits.
Editorial, p. 526
Treatment of the anemia of chronic kidney disease with erythropoiesis-stimulating agents (ESAs) and iron has been well established since the 1990s.1 However, challenges remain with regard to the optimal ESA dose and hemoglobin targets due to the known increased risk for cardiovascular events, including stroke, myocardial infarction, thrombotic events, and death, as reported in seminal clinical trials a decade ago.2, 3, 4 Based on the evidence from these studies, in June 2011, the US Food and Drug Administration released a safety announcement and revised prescribing information recommending "more conservative dosing of ESA's and lower hemoglobin goals due to safety issues."5 Similarly, KDIGO (Kidney Disease: Improving Global Outcomes) issued guidelines in 2012 recommending that "ESA not be used to maintain hemoglobin concentration above 11.5 g/dL in adult patients with CKD [chronic kidney disease]."6 Based on these recommendations, ESAs should be used at the lowest dose possible to reduce the need for transfusions and minimize cardiovascular risks.
The guidance to minimize ESA dose poses a challenge for a group of patients, termed ESA hyporesponders (further called hyporesponders), who do not achieve target hemoglobin levels despite substantially increased ESA doses. These patients appear to be at greater risk for cardiovascular events and death.7, 8, 9, 10, 11 Although functional iron deficiency, secondary hyperparathyroidism, hospitalizations associated with acute events, and/or inflammation could explain about two-thirds of these occurrences, the cause of ESA hyporesponse is not completely understood in the remaining third.12 Treatment with pharmacologic agents to modify ESA hyporesponsivness has been partially successful.13 There is also no widely accepted definition for long-term ESA hyporesponse.10 Previous studies have used different approaches and thresholds to explore the impact of ESA hyporesponsiveness but have in general used variations on 2 approaches; a binary approach with hemoglobin level and ESA dose criteria8,10,14,15 and segmenting the cohort based on ESA response.16,17
Since 2011, a decrease in ESA use and an increase in iron use has been observed in dialysis patients in the United States, resulting in lower mean achieved hemoglobin levels. This change in clinical management has been partly driven by the concerns listed and also the adoption of a bundled payment system in the same year, which shifted the cost of ESAs to dialysis providers.6,18
The safety of exposure to higher doses of intravenous (IV) iron has also been assessed in several observational studies and clinical trials. The most recent results from the observational Dialysis Outcomes and Practice Patterns Study (DOPPS) and from the randomized Positive Impact of Endovascular Options for Treating Aneurysms Early (PIVOTAL) Study in United Kingdom's hemodialysis (HD) population suggest that use of IV iron in the range of up to 300 mg per month is not associated with increased cardiovascular risk.19,20 Thus, the changes in ESA and iron use and lower hemoglobin targets since 2011 may have altered the relationship between ESA responsiveness and associated outcomes.
This retrospective cohort analysis had 2 aims: first, to explore ESA hyporesponsivness and associated clinical characteristics and outcomes, namely all-cause mortality and major cardiovascular events; and second, to assess the health care resource use and costs associated with ESA hyporesponsiveness in the post-bundling era.
Methods
Study Design, Setting, and Participants
This study explored data from the US Renal Data System (USRDS) CROWNWeb clinical database from 2012 to 2014. All US patients with end-stage kidney disease (ESKD), regardless of insurance coverage and age, are included in the USRDS database. Information for health care resource use, such as hospitalizations, costs, and clinical services, is available for Medicare patients, except for those who newly develop ESKD at younger than 65 years and have private insurance.
Patients with ESKD included in this study were 18 years or older and were receiving dialysis; 97% were receiving HD. Patients had to have continuous ESA use (epoetin alfa or darbepoetin alfa) for the 6 months before the potential index date with no change in ESA drug during the last 2 months. Patients were also required to have a known route of ESA administration, type, total monthly dose, at least 1 postdialysis weight value, and at least 1 hemoglobin value within the same period. Patients were also required to have continuous Medicare Part A, B, and D eligibility during the baseline and study periods, during which Medicare was the primary payer.
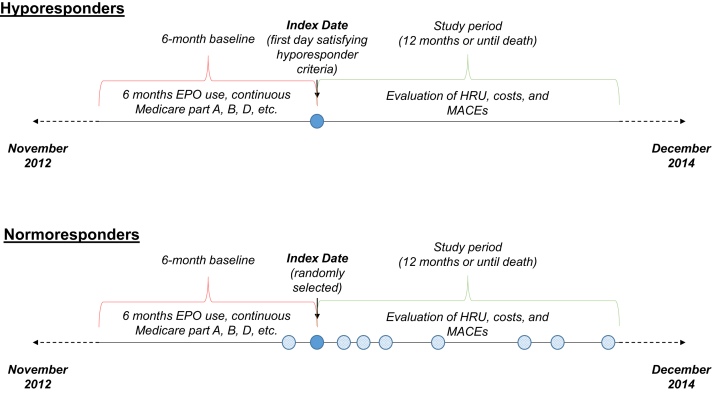
Building on the analyses in previous studies that explored acute versus chronic hyporesponse,10 further subgroup analyses were conducted to explore the effect of isolated ESA hyporesponse events in which patients may be hyporesponsive for a few months only compared with patients who have a large proportion of hyporesponsive months following their index date. To ensure enough follow-up time to classify hyporesponders into these subgroups, all patients included in the subgroup analysis and the comparator normoresponder cohort for this subgroup analysis were required to have at least 4 months of follow-up time after their index dates (Fig 1).
Figure 1.
Study design. Abbreviations: EPO, erythropoietin; HRU, health care resource use; MACE, major adverse cardiovascular event.
Exposure or Predictors
To define hyporesponsiveness in this study, 2 threshold approaches (ie, erythropoietin resistance index [ERI] and/or ESA dose) were combined. A conservative ERI ≥ 2.0 U/kg/wk/Hb [g/L] for epoetin alfa was selected to ensure that patients in the hyporesponder cohort were true hyporesponders, the impact of this threshold versus a lower threshold of 1.5 U/kg/wk/Hb [g/L] on cohort size was explored (Figs S1-S3). ESA hyporesponsiveness in this study was defined as follows: for epoetin alfa, ERI ≥ 2.0 U/kg/wk/Hb [g/L] and/or dose ≥ 450 U/kg per week; for darbepoetin, ERI ≥ 0.008 μg/kg/wk/Hb [g/L] and/or dose ≥ 1.5 μg/kg per week. The recombinant human erythropoietin dose for IV administration used a conversion multiplier factor of 1.5 for subcutaneous administration (ie, 1,000 units of epoetin alfa subcutaneous = 1,500 units of epoetin alfa IV).21,22
For each potential index date, ERI was calculated and ESA dose was evaluated for hyporesponsiveness. For patients with an ERI or ESA dose satisfying the listed hyporesponder definition, the earliest eligible date meeting the definition was defined as the index date. Patients were considered as controls (normoresponders) if their ERIs and/or ESA doses on all potential index dates in the same period were lower than the hyporesponder definition cutoffs. The index date for ESA normoresponders was randomly selected from all dates that met the inclusion criteria (Fig 1).
Within the subgroup analysis, hyporesponders were classified into 3 subgroups based on the pattern of hyporesponsive months during the study period. Isolated hyporesponders were defined as patients with up to 2 consecutive hyporesponsive months with no additional hyporesponsive months during the study period. Intermittent hyporesponders were defined as nonisolated hyporesponders with <75% of the study period with hyporesponsive months. Chronic hyporesponders were defined as nonisolated hyporesponders with most (≥75%) of the study period with hyporesponsive months. The 75% cutoff was selected as a reasonable threshold following clinical expert advice to define chronic versus intermittent hyporesponsivness.
Outcomes
Demographic characteristics, comorbid conditions, cardiovascular event–related hospitalizations, index date, type of ESA and route of administration, ERI, dialysis type, duration of dialysis, IV iron use, and history of blood transfusions were described and compared between the hypo- and normoresponders during the baseline period to index date. During the study period, which was the 12 months following the index date or the time to death if sooner, mortality, cardiovascular event–related hospitalization rates (identified as hospitalizations associated with at least 1 diagnosis for the related condition), health care resource use (including inpatient stays; outpatient, emergency department, and home health agency visits; and days in skilled nursing facilities and hospice), and associated costs per patient-month (in addition to other medical services and pharmacy costs) were evaluated and compared.
Hyporesponder subgroups were individually compared against normoresponders for all baseline characteristics and outcomes using the same methods as for the overall study population.
Analytical Approach
A quality check was performed on the key variables that were used in the analysis and data values that were considered extreme were excluded. For both the overall and subgroup analyses (Figs S1-S3), baseline characteristics were compared using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Incidence rates of health care resource use and rates of hospitalizations associated with adverse cardiovascular events, specifically stroke, myocardial infarction, thromboembolic events, and heart failure outcomes, were modeled using an unadjusted and adjusted generalized linear model with a log link and a quasi-Poisson distribution with overdispersion adjustment. Health care costs were modeled using an unadjusted and adjusted generalized linear model with a log link and a Tweedie distribution. Models were presented unadjusted and adjusted for age at index date, sex, race, region, index year, ESA drug, ESA route of administration, dialysis type, dialysis, cardiovascular event–related hospitalizations during the baseline period, all comorbid conditions with a prevalence > 5% during the baseline period, and Charlson Comorbidity Index score.
This study complied with all applicable laws regarding patient privacy. No direct patient contact or primary collection of individual human subject data occurred. Study results are presented as aggregate analyses that omit participant identification. New England Independent Review Board exemption was granted for this study on July 21, 2017.
Results
Clinical Outcomes
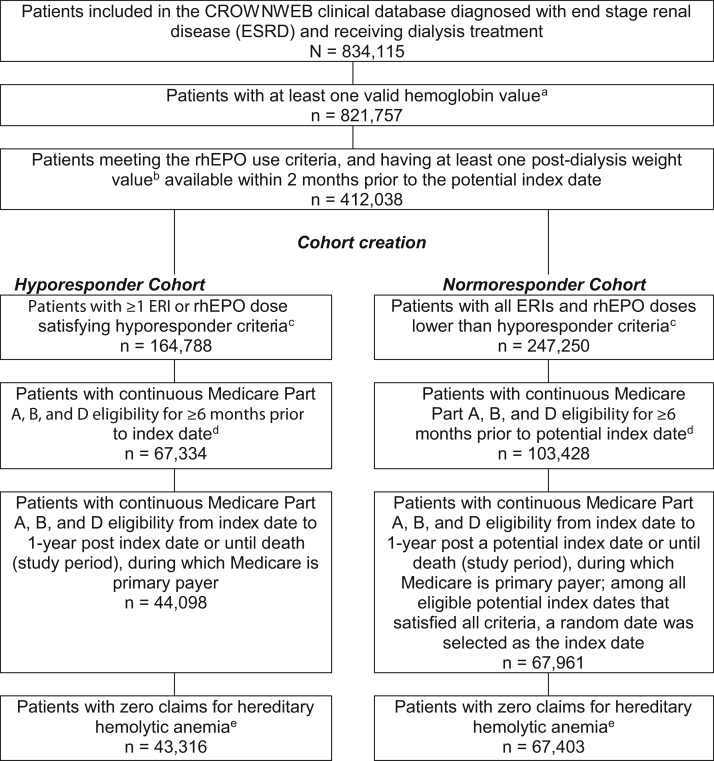
Of the 834,115 patients in the USRDS CROWNWeb clinical database with ESKD receiving dialysis treatment, 43,316 ESA hyporesponders and 67,403 normoresponders met all inclusion criteria. Numbers of patients meeting each inclusion criterion are included in Figure 2. Of the 43,316 hyporesponders, 38,891 had at least 4 months of post–index date follow-up and were included in subgroup analysis. Of these patients, 10,569 were classified as isolated hyporesponders, 19,347 were intermittent hyporesponders, and 8,975 were chronic hyporesponders. Of the 67,403 normoresponders, 59,412 had enough follow-up for inclusion in subgroup analysis. Inclusion criteria had similar effects on patient attrition for the hypo- and normoresponder cohorts following the cohort creation step in sample selection (26.3% of hyporesponders were selected following cohort creation vs 27.3% of normoresponders).
Figure 2.
Patient flow and cohort creation. aMonths with missing hemoglobin values or hemoglobin values < 0 g/dL or >20 g/dL were excluded from the analysis. The first date of each month with a valid hemoglobin value was defined as a potential index date. bMonths with missing weight values or weight values < 20 kg or >350 kg were excluded from the analysis. Monthly recombinant human erythropoietin (rhEPO) dose was calculated and converted to weekly dose. Hyporesponders were defined as described in the inclusion criteria. dPatients were excluded in this step mainly due to no Part D coverage, fewer than 6 months continuous eligibility before the index date, and Medicare was not the primary payer during the 6-month period. eHereditary hemolytic anemias were identified in the Medicare claims by International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 282. Abbreviation: ERI, erythropoietin resistance index.
Baseline demographic characteristics, comorbid conditions, and cardiovascular-related hospitalization during the baseline period are shown in Table 1. Compared with normoresponders, hyporesponders were minimally younger, weighed less, were more likely to be women, had a longer time receiving dialysis, and were more likely to have had at least 1 cardiovascular hospitalization during the baseline period (all P < 0.001). Charlson Comorbidity Index score was higher, and several individual comorbid conditions were more common among hyporesponders, including hypertension, heart failure, and coronary artery disease (all P < 0.001).
Table 1.
Patient Demographics
| Overall Cohort: Hyporesponder (N = 43,316) | Overall Cohort: Normoresponder (N = 67,403) | P | |
|---|---|---|---|
| Demographics on index date | |||
| Age as of index date, y | 62.3 ± 15.2 | 63.6 ± 14.4 | <0.001a |
| Female | 24,272 (56.0) | 33,194 (49.2) | <0.001a |
| Race | <0.001a | ||
| Black | 17,979 (41.5) | 25,022 (37.1) | |
| White | 22,225 (51.3) | 38,242 (56.7) | |
| Other/unknown | 3,112 (7.2) | 4,139 (6.1) | |
| Weight on index date, kg | 73.0 ± 20.2 | 83.4 ± 23.9 | <0.001a |
| Comorbid conditions during baseline period | |||
| Charlson Comorbidity Index score | 3.8 ± 2.2 | 3.2 ± 2.0 | <0.001a |
| Hypertension | 38,151 (88.1) | 54,544 (80.9) | <0.001a |
| Type 2 diabetes | 22,462 (51.9) | 36,277 (53.8) | <0.001a |
| Hyperlipidemia | 22,092 (51.0) | 32,365 (48.0) | <0.001a |
| Heart failure | 20,777 (48.0) | 24,412 (36.2) | <0.001a |
| Coronary heart/artery disease | 18,071 (41.7) | 23,643 (35.1) | <0.001a |
| Arrhythmia | 14,121 (32.6) | 17,064 (25.3) | <0.001a |
| Type 1 diabetes | 5,124 (11.8) | 7,855 (11.7) | 0.38 |
| Valvular heart disease | 1,305 (3.0) | 1,200 (1.8) | <0.001a |
| Cardiovascular event–related hospitalizations during baseline period | |||
| Hospitalization related to heart failure | 2,308 (5.3) | 1,931 (2.9) | <0.001a |
| Hospitalization related to thromboembolic event | 396 (0.9) | 219 (0.3) | <0.001a |
| Hospitalization related to stroke | 232 (0.5) | 205 (0.3) | <0.001a |
| Hospitalization related to myocardial infarction | 318 (0.7) | 270 (0.4) | <0.001a |
Note: Data for categorical variables expressed as number (percent); data for continuous variables expressed as mean ± standard deviation.
Statistically significant.
Dialysis-related treatment data and anemia-related laboratory values are shown in Table 2, with 97% of patients receiving HD and 3% receiving peritoneal dialysis, with no difference between the responder groups. Compared with normoresponders, hyporesponders received 3.8-fold higher IV epoetin doses. ERI was accordingly almost 5 times higher in the hypo- versus normoresponders group. Hyporesponders also had lower hemoglobin and transferrin saturation (TSAT) values and parathyroid hormone levels > 800 pg/mL (Table 2). Iron deficiency was more common in hyporesponders, with TSAT < 20% in 26.5% of hyporesponders versus 10.9% of normoresponders, and ferritin level < 200 ng/mL in 3.7% versus 2.1%, respectively (Table 2).
Table 2.
Dialysis-Related Outcomes, Treatment Characteristics, and ESA Hyporesponsiveness–Related Laboratory Values
| Hyporesponders (N = 43,316) | Normoresponders (N = 67,403) | |
|---|---|---|
| Outcomes | ||
| Died during study period | 10,944 (25.3) | 15,224 (22.6) |
| Time to death, mo | 5.4 ± 3.3 | 4.5 ± 3.2 |
| Treatment characteristics | ||
| Dialysis modality | ||
| Hemodialysis | 41,959 (96.9) | 65,434 (97.1) |
| Peritoneal dialysis | 1,341 (3.1) | 1,946 (2.9) |
| Time on dialysis at index date, y | 5.7 ± 5.1 | 4.9 ± 4.5 |
| Monthly epoetin alfa,a units | 94,831 ± 36,753 | 24,331 ± 17,364 |
| IV iron use 2 mo before index date | 32,451 (74.9) | 48,547 (72.0) |
| Blood transfusions during baseline period | 8,847 (20.4) | 4,281 (6.4) |
| Laboratory values | ||
| Hemoglobin, g/L | 10.4 [7.7-11.1] | 10.8 [10.3-11.3] |
| ERI, epoetin U/kg/wk/Hb [g/L] | 2.9 ± 1.2 | 0.6 ± 0.4 |
| Ferritin, ng/mL | 889 ± 518 | 897 ± 439 |
| TSAT, % | 27.7 ± 13.2 | 32.9 ± 14.0 |
| TSAT < 20% | 11,386 (26.5) | 7,273 (10.9) |
| Parathyroid hormone, pg/mL | 301.7 [178.1-511.6] | 279.3 [186.6-478.0] |
| Parathyroid hormone > 800 pg/mL | 2,354 (11.6) | 2,524 (8.9) |
Note: Data for categorical variables expressed as number (percent); data for continuous variables expressed as mean ± standard deviation or median [interquartile range].
Abbreviations:ERI, erythropoietin resistance index; ESA, erythropoiesis-stimulating agent; IV, intravenous; TSAT, transferrin saturation.
Average dose in the 2 months before the index date.
Similar proportions of hypo- and normoresponders used IV iron during the 2 months before the index date (74.9% vs 72%), but significantly more hyporesponders had at least 1 blood transfusion during the baseline period (20.4% vs 6.4%). Surprisingly, subcutaneous ESA use was more common among hyporesponders (with 13.1% vs 10.6% identified as receiving subcutaneous ESA alone and 1.1% vs 0.7% receiving a combination of subcutaneous and IV; all P < 0.001).
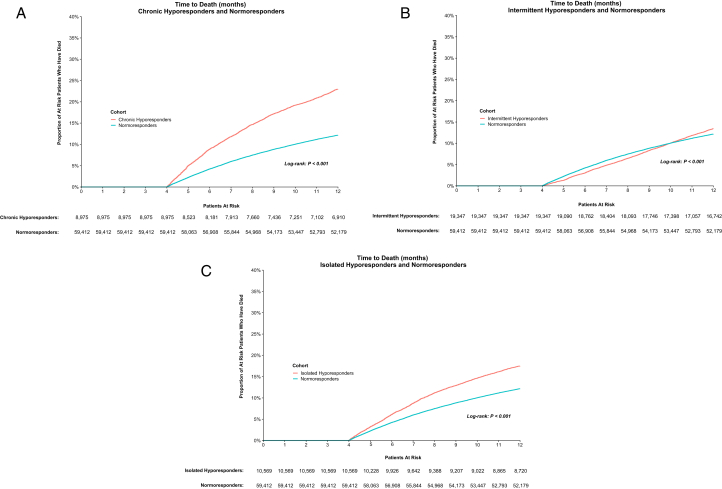
Mortality was significantly higher among hyporesponders compared with normoresponders in the 1 year following the index date (25.3% vs 22.6%; P < 0.001). In subgroup analysis, chronic hyporesponders had the highest proportion of death during the study period compared with intermittent hyporesponders, isolated hyporesponders, and normoresponders (23.0% vs 13.5% vs 17.5% vs 12.2%, respectively); the normoresponders being the subgroup cohort with a minimum 4-month follow-up.
Kaplan-Meier analysis showed that chronic and isolated hyporesponders had significantly increased risk for mortality as compared with normoresponders (both P for log-rank < 0.001). Intermittent hyporesponders had significantly different risk for mortality as compared with normoresponders (P for log-rank < 0.001), although the Kaplan-Meier curves crossed (Fig 3A-C). Consistent with the highest mortality rate among chronic hyporesponders during the study period, the mortality hazard ratio (HR) for chronic hyporesponders versus normoresponders was the highest among this subgroup (2.02 unadjusted; 1.73 adjusted, both P < 0.001). The mortality HR was also higher for isolated hyporesponders versus normoresponders (HR, 1.48 unadjusted; 1.18 adjusted; both P < 0.001).
Figure 3.
Time to death by hyporesponse cohort. (A) Chronic hyporesponders and normoresponders, (B) intermittent hyporesponders and normoresponders, and (C) isolated hyporesponders and normoresponders.
In both unadjusted and adjusted models, hyporesponders also had significantly higher rates of hospitalization for cardiovascular events, including heart failure, myocardial infarction, stroke, and thromboembolic events, compared with normoresponders (Table 3). Rates of these major cardiovascular events were highest among long-term hyporesponders and lowest among normoresponders. For all cohorts, heart failure–related hospitalizations per patient-month were the most common cardiovascular outcome.
Table 3.
Comparison of Cardiovascular Event Incidence and Regression Model Results
| HRU Rates, per patient-mo |
Incidence Rate Ratio (95% CI)a |
|||
|---|---|---|---|---|
| Hyporesponder (N = 43,316) | Normoresponder (N = 67,403) | Unadjusted Model | Adjusted Model | |
| Hospitalizations related to: | ||||
| Heart failure | 0.0992 | 0.0590 | 1.68 (1.64-1.72) | 1.39 (1.36-1.43) |
| Myocardial infarction | 0.0135 | 0.0101 | 1.34 (1.27-1.42) | 1.24 (1.17-1.31) |
| Stroke | 0.0144 | 0.0106 | 1.36 (1.30-1.43) | 1.28 (1.22-1.34) |
| Thromboembolic event | 0.0139 | 0.0084 | 1.66 (1.58-1.75) | 1.44 (1.36-1.51) |
| Mortalityb | 25.27% | 22.59% | ||
Note: P < 0.001 for all comparisons in both models.
Abbreviations:CI, confidence interval; HRU, health care resource use.
Hyporesponders versus normoresponders.
Proportion of patients who died during the study period.
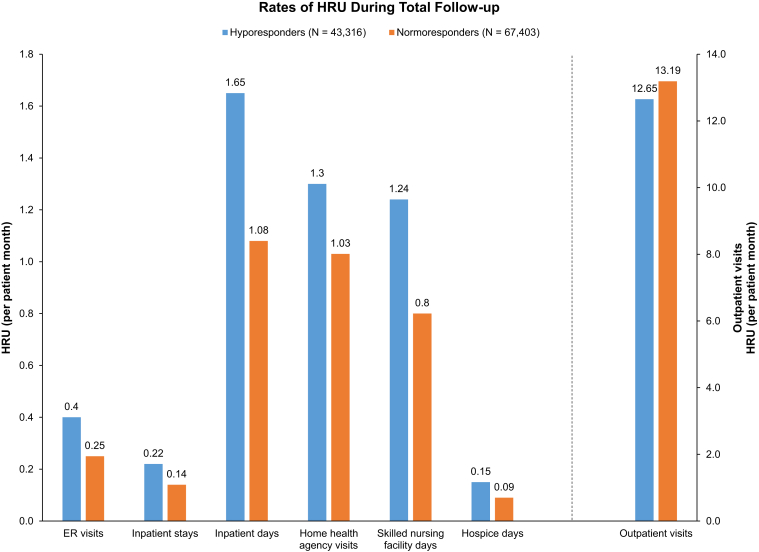
Health Care Resource Use
Hyporesponders had higher per-patient-month rates of emergency department visits, inpatient stays and days, home health agency visits, skilled nursing facility days, and hospice days (Table 4; Fig 4). In unadjusted and adjusted models, health care resource use per patient-month was higher for hyporesponders in all places of service, excluding outpatient visits in the unadjusted model.
Table 4.
Comparison of Incidence of Health-Related Use and Regression Analysis
| HRU Rates, per patient-mo |
Regression Modelsa |
|||
|---|---|---|---|---|
| Hyporesponder (N = 43,316) | Normoresponder (N = 67,403) | Unadjusted (95% CI) | Adjusted (95% CI) | |
| HRU rates, per patient-mo | ||||
| ED visits | 0.40 | 0.25 | 1.60 (1.58-1.63) | 1.36 (1.34-1.39) |
| Inpatient stays | 0.22 | 0.14 | 1.57 (1.55-1.60) | 1.57 (1.55-1.60) |
| Inpatient days | 1.65 | 1.08 | 1.53 (1.48-1.58) | 1.36 (1.32-1.41) |
| Outpatient visits | 12.65 | 13.19 | 0.96 (0.96-0.96) | 0.99 (0.98-0.99) |
| Home health agency | 1.30 | 1.03 | 1.27 (1.24-1.30) | 1.13 (1.10-1.15) |
| Skilled nursing facility days | 1.24 | 0.80 | 1.54 (1.45-1.64) | 1.42 (1.34-1.52) |
| Hospice days | 0.15 | 0.09 | 1.73 (1.50-1.98) | 1.54 (1.36-1.76) |
Note: P < 0.001 for all comparisons in both models.
Abbreviations: CI, confidence interval; ED, emergency department; HRU, health care resource use.
Hyporesponders versus normoresponders.
Figure 4.
Rate of health care resource use during total follow-up. ∗Outpatient visits likely reflect hemodialysis (HD) sessions (∼13 per month). Abbreviation: HRU, health care resource use.
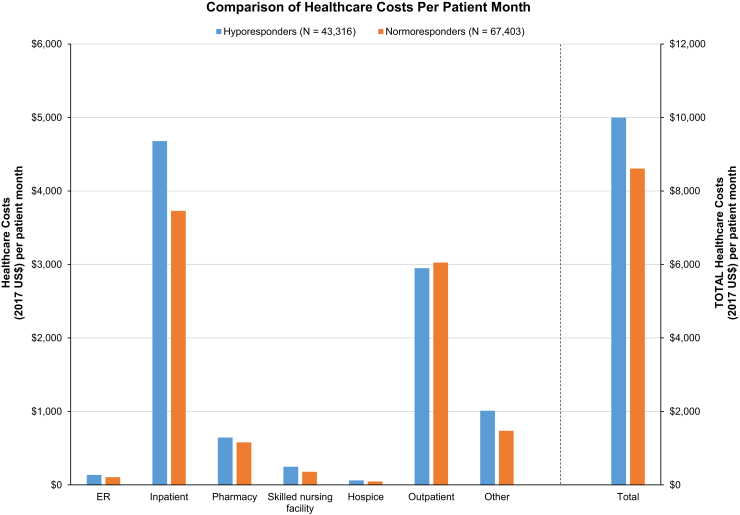
All hyporesponder subgroups had higher per-patient per-month total health care costs, medical costs, emergency department costs, inpatient costs, home health costs, skilled nursing facility costs, hospice costs, other costs, and pharmacy costs compared with normoresponders (all P < 0.001; Fig 5). Among the 3 hyporesponder subgroups, chronic hyporesponders had the highest health care resource use and costs among subgroups, except for outpatient resource use and costs most likely related to missed outpatient HD treatments. Normoresponders had slightly higher outpatient costs (P < 0.001), largely reflecting more outpatient HD sessions in their normal care setting.
Figure 5.
Comparison of health care costs (per patient-month).
Discussion
This retrospective cohort study of 2 different ESA-responsive populations introduces novel definitions of ESA hyporesponsive subgroups to examine different patterns of high ESA use. The most noticeable difference was the magnitude of the difference in monthly ESA dose (3.8 times more epoetin per month) and ERI (almost 5 times higher in hyporesponders). Hyporesponders experienced more iron deficiency based on TSAT percent and had more secondary hyperparathyroidism as determined by parathyroid hormone levels > 800 pg/mL, both factors that have been shown previously to be associated with ESA hyporesponsiveness.23 Both populations had high ferritin values (∼900 ng/mL) at baseline, representing values that are similar to the most recent DOPPS Practice Monitor data (data accessed on DOPPS DPM May 2019). Surprisingly, ferritin values were not very different between the 2 groups, suggesting that ferritin level may not be a good differentiating factor for ESA hyporesponsivness. Overall mortality rates in both populations were remarkably higher than has been recently reported in a retrospective study using DOPPS data24 and in the PIVOTAL Study.19 In addition, results of the latter study suggest that higher ESA dose per month and not more liberal IV iron use could explain higher cardiovascular events, but more definitive research on this is warranted. Furthermore, it would be of interest to explore ESA hyporesponsivenes in patients who were iron replete as per TSAT percentage and compare outcomes with normoresponders in future research.
No additional iron metabolism markers such as hepcidin or other biomarkers of inflammation were available in the database studied, so we could not assess the association between hepcidin level, inflammation, and ESA hyporesponsivness, as suggested by some authors.25
ESA hyporesponsiveness was associated with an increased incidence of cardiovascular hospitalizations, higher treatment costs, and higher health care resource use. Overall, ESA-hyporesponsive patients used more health care resources and were more costly to the health care system compared with normoresponders. The incremental burden of costs and of resource use were highest for long-term hyporesponders.
Surprisingly, subcutaneous ESA use was more common among hyporesponders, with 13.1% versus 10.6% identified as receiving subcutaneous ESA alone and 1.1% versus 0.7% receiving a combination of subcutaneous and IV (all P < 0.001). It could be hypothesized that hyporesponders may require supplementary ESA dosing in between dialysis sessions, but we are not able to confirm this from the data. Additionally, it could be that the conversion factor used for subcutaneous dosing in the ERI calculation affected this finding. The factor is based on data from meta-analysis of dosing in 2002 in which subcutaneous doses were 32% lower than IV.21
As noted, there is no widely accepted definition of ESA hyporesponse and studies to date have used different approaches to identify hyporesponders and evaluate the impact of hyporesponse on outcomes (Box 1). In clinical practice, hyporesponders are generally identified by the high ESA doses they require. In an effort to understand the effect of the definitions used in this study, we explored in sensitivity analysis how many patients would be reclassified as hyporesponders if the ERI cutoff was changed from 2 to 1.5 (further detail available in Figs S1-S3). Across all normoresponders in our sample, ∼20% had a month in which ERI was between 1.5 and 2. The reclassification would be lower than this due to the other eligibility criteria that are applied after the index month is chosen. It is unclear what effect this reclassification would have on the results. Although one could hypothesize that these less hyporesponsive patients might have better outcomes than the current cohort of hyporesponders and lessen the apparent effect of hyporesponse, it is also possible that these less hyporesponsive patients have worse outcomes than normoresponders and moving them out of the normoresponder cohort would improve the outcomes of that group, minimizing the change in relative difference between normo- and hyporesponders. In studies such as this, the definitions used will clearly affect the results, and a consistent approach to measuring hyporesponsiveness, including capturing the duration or transiency of hyporesponse episodes, that is translatable to clinical practice is needed.
Box 1. Approaches to Defining Hyporesponse.
Binary approach with Hb and ESA dose criteria: eg, Luo et al8 used 2 consecutive Hb measurements < 10 g/dL with contemporaneous ESA dose > 7,700 units per treatment and Hasegawa et al14 used a mean Hb level < 10 g/dL and mean ESA dose > 6,000 units per week, and in Sui et al,15 failure to achieve and maintain the target Hb level (110 g/L) at an ESA dose ≥ 300 IU/kg per week when administered subcutaneously. In Sibbel et al,10 this approach was developed further using a series of contiguous strata to define ESA hyporesponsiveness. Increasingly restrictive thresholds for ESA dose were required for strata characterized by higher Hb levels and patients identified using this Hb level by ESA dose strata were identified as ESA hyporesponders.
Segmenting the cohort based on ESA response: eg, Nishio et al16 divided the study cohort into tertiles of ESA hyporesponsivness, and in Minutolo et al,17 patients were classified, from lower to higher tertiles of ESA, as poor, intermediate, and good responders.
Abbreviations: ESA, erythropoiesis-stimulating agent; Hb, hemoglobin.
This study examined only Medicare patients due to data availability and the requirement to have hospitalization data for the analyses. Consequently, we could not explore differences in management and outcomes in a non-Medicare population. In addition, for patients younger than 65 years, we did not capture the initial 30 months of data because this study required Medicare to be the primary payer.
The inclusion criterion that resulted in the largest attrition of patients when defining the cohorts in the study was the requirement for 6 months of continuous enrollment in Medicare Parts A, B, and D during which Medicare is the primary payer. At this stage in sample selection, 59.0% and 58.1% of hyporesponders and normoresponders were removed from the sample, respectively. Although this is a large proportion of patients, the impact on each cohort is comparable.
As noted in the discussion, results are driven in part by the definitions used to define hyporesponse. To allow future comparisons and application to clinical practice, a consistent approach to identifying hyporesponsiveness specified in guidelines would be helpful. Furthermore, for our subgroup analysis, the definitions used were de novo for this study based on discussion with experts. Although we observed different types of hyporesponders, there is no consensus on the definitions of these subtypes and results may differ with different definitions.
Finally, in the subgroup analyses, to accommodate the attribution of chronic versus isolated, we were not able to include patients who died in the first 4 months. This may result in underestimation of the differences between the groups arising due to the variability in 4-month mortality across subgroups (ie, cohorts with higher death rates in the first 4 months would have higher costs had those patients been included).
Overall, this retrospective cohort study explored new definitions of ESA hyporesponsivness, clinical outcomes, and health care resource use of US Medicare dialysis patients in the post-bundled era from 2012 to 2014. The results confirm that ESA-hyporesponsive patients, on average using almost 4 times more recombinant human erythropoietin per month, have the highest mortality and higher cardiovascular hospitalization rates and health care resource use costs. ESA hyporesponsivness was associated with iron deficiency, as measured by TSAT < 20%; more severe secondary hyperparathyroidism; and in patients with longer dialysis vintage. The results of this study highlight the unmet need in the treatment of the ESA-hyporesponsive population. The kidney community is waiting for the results of large cardiovascular outcome trials with newer anemia investigational products, such as hypoxia-inducible factor prolyl hydroxylase inhibitors, to see whether these agents may provide an alternative option to treat this challenging population.
Article Information
Authors’ Full Names and Academic Degrees
Borut Cizman, MD, Helen T. Smith, MSc, Rodrigo Refoios Camejo, PhD, Linda Casillas, PhD, Harjeet Dhillon, MBBS, MRCP, Fan Mu, PhD, Eric Wu, PD, Jipan Xie, MD, PhD, Peter Zuckerman, BS, and Daniel Coyne, MD.
Authors’ Contributions
Research idea and study design: BC, HTS, LC, FM, EW, JX, PZ, DC; data acquisition: FM, EW, JX, PZ; data analysis/interpretation: BC, HTS, LC, RRC, HD, FM, EW, JX, PZ, DC; statistical analysis: FM, EW, JX, PZ. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author's own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding for this study (HO-17-17783) was provided by GlaxoSmithKline. The funder played a role in study design, data analysis/interpretation, and decision for publication.
Financial Disclosure
BC, HTS, RRC, LC, and HD are employees of and hold equity stock in GlaxoSmithKline (GSK). FM, EW, JX, and PZ are employees of Analysis Group, which was a paid consultant of GSK. DWC is a consultant and investigator for GSK and Fibrogen.
Acknowledgements
We thank Boris Gorsh for the initial protocol development and Yong Chen and Rebecca Lisle for input early in the study.
Peer Review
Received February 15, 2019. Evaluated by 3 external peer reviewers, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 26, 2019.
Footnotes
Complete author and article information provided before references.
Figure S1: Distributions of ERI at index date for epoetin alfa
Figure S2: Distributions of ERI at index date for darbepoetin
Figure S3: Distribution of highest ERI month among normoresponders- epoetin alfa.
Supplementary Material
Figures S1-S3.
References
- 1.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drueke T.B., Locatelli F., Clyne N. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 4.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 5.FDA. FDA Drug Safety Communication: modified dosing recommendations to improve the safety of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-modified-dosing-recommendations-improve-safe-use-erythropoiesis. 2011. Accessed December 11, 2019.
- 6.KDIGO clinical practice guideline for anemia in chronic kidney disease. 2012. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO-Anemia GL.pdf Accessed December 11, 2019.
- 7.Bonomini M., Del Vecchio L., Sirolli V., Locatelli F. New treatment approaches for the anemia of CKD. Am J Kidney Dis. 2016;67(1):133–142. doi: 10.1053/j.ajkd.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Luo J., Jensen D.E., Maroni B.J., Brunelli S.M. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68(5):763–771. doi: 10.1053/j.ajkd.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Saran R., Li Y., Robinson B. US Renal Data System 2014 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66(1 suppl 1):Svii. doi: 10.1053/j.ajkd.2015.05.001. S1-S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibbel S.P., Koro C.E., Brunelli S.M., Cobitz A.R. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. doi: 10.1186/s12882-015-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suttorp M.M., Hoekstra T., Rotmans J.I. Erythropoiesis-stimulating agent resistance and mortality in hemodialysis and peritoneal dialysis patients. BMC Nephrol. 2013;14:200. doi: 10.1186/1471-2369-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie I.A., Macdougall I.C., Richards S. Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: case-crossover study. Pharmacoepidemiol Drug Saf. 2015;24(4):414–426. doi: 10.1002/pds.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson D.W., Pascoe E.M., Badve S.V. A randomized, placebo-controlled trial of pentoxifylline on erythropoiesis-stimulating agent hyporesponsiveness in anemic patients with CKD: the Handling Erythropoietin Resistance With Oxpentifylline (HERO) trial. Am J Kidney Dis. 2015;65(1):49–57. doi: 10.1053/j.ajkd.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T., Zhao J., Fuller D.S. Erythropoietin hyporesponsiveness in dialysis patients: possible role of statins. Am J Nephrol. 2017;46(1):11–17. doi: 10.1159/000477217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui Z., Wang M., Zuo L. Statin therapy and erythropoiesis-stimulating agent hyporesponsiveness in patients with nondialysis chronic kidney disease: a retrospective study in Beijing, China. Medicine (Baltimore) 2019;98(2) doi: 10.1097/MD.0000000000013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishio A., Chhatkuli B.P., Ma J.Z., Kalantari K. Higher doses of erythropoietin-stimulating agents and hyporesponsiveness to their effects are associated with increased mortality among prevalent hemodialysis patients. Blood Purif. 2013;36(1):29–36. doi: 10.1159/000350583. [DOI] [PubMed] [Google Scholar]
- 17.Minutolo R., Conte G., Cianciaruso B. Hyporesponsiveness to erythropoiesis-stimulating agents and renal survival in non-dialysis CKD patients. Nephrol Dial Transplant. 2012;27(7):2880–2886. doi: 10.1093/ndt/gfs007. [DOI] [PubMed] [Google Scholar]
- 18.Locatelli F., Barany P., Covic A. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28(6):1346–1359. doi: 10.1093/ndt/gft033. [DOI] [PubMed] [Google Scholar]
- 19.Macdougall I.C., White C., Anker S.D. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 20.Robinson B.M., Larkina M., Bieber B. Evaluating the effectiveness of IV iron dosing for anemia management in common clinical practice: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) BMC Nephrol. 2017;18(1):330. doi: 10.1186/s12882-017-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besarab A., Reyes C.M., Hornberger J. Meta-analysis of subcutaneous versus intravenous epoetin in maintenance treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002;40(3):439–446. doi: 10.1053/ajkd.2002.34881. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman J.S., Reda D.J., Fye C.L. Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients. N Engl J Med. 1998;339(9):578–583. doi: 10.1056/NEJM199808273390902. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K., Lee G.H., Miller J.E. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53(5):823–834. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirnadel-Farrant H.A., Karaboyas A., Cizman B. Cardiovascular event rates among hemodialysis patients across geographical regions-a snapshot from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int Rep. 2019;4(6):864–872. doi: 10.1016/j.ekir.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malyszko J., Malyszko J.S., Mysliwiec M. Hyporesponsiveness to erythropoietin therapy in hemodialyzed patients: potential role of prohepcidin, hepcidin, and inflammation. Ren Fail. 2009;31(7):544–548. doi: 10.1080/08860220903082606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S3.