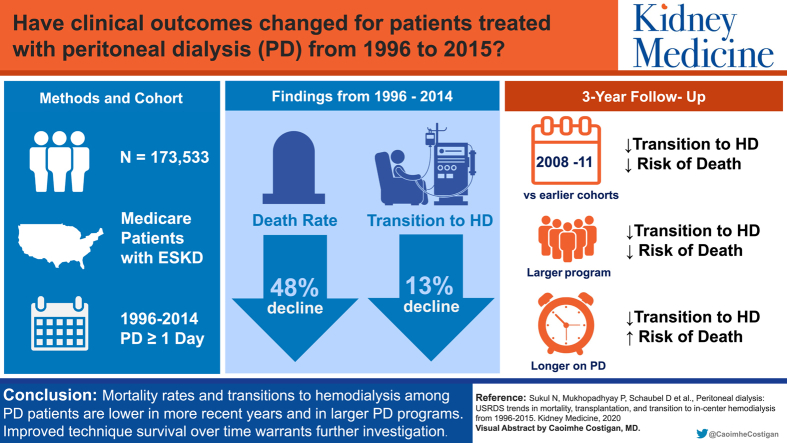
Abstract
Rationale & Objective
Transitions between dialysis modalities can be disruptive to care. Our goals were to evaluate rates of transition from peritoneal dialysis (PD) to in-center hemodialysis (HD), mortality, and transplantation among incident PD patients in the US Renal Data System from 1996 to 2015 and identify factors associated with these outcomes.
Study Design
Observational registry-based retrospective cohort study.
Setting & Participants
Medicare patients incident to end-stage renal disease (ESRD) from January 1, 1996, through December 31, 2011 (for adjusted analyses; through December 31, 2014, for unadjusted analyses), and treated with PD 1 or more days within 180 days of ESRD incidence (n = 173,533 for adjusted analyses; n = 219,787 for unadjusted analyses).
Exposure & Predictors
Exposure: 1 or more days of PD. Predictors: patient- and facility-level characteristics obtained from Centers for Medicare & Medicaid Services Form 2728 and other data sources.
Outcomes
Patients were followed up for 3 years until transition to in-center HD, death, or transplantation.
Analytical Approach
Multivariable Cox regression was used to estimate hazards over time and associations with predictors.
Results
Compared with earlier cohorts, recent incident PD patient cohorts had lower rates of death (48% decline) and transition to in-center HD (13% decline). Among many other findings, we found that: (1) rates of transition to in-center HD and death were lowest in the 2008 to 2011 cohort, (2) longer time receiving PD was associated with higher mortality risk but lower risk for transition to in-center HD, and (3) larger PD programs (≥25 vs ≤6 patients) displayed lower risks for death and transition to in-center HD.
Limitations
Data collected on Form 2728 are only at the time of ESRD incidence and do not provide information at the time of transition to in-center HD, death, or transplantation.
Conclusions
Rates of transition from PD to in-center HD and death rates for PD patients decreased over time and were lowest in PD programs with 25 or more patients. Implications of the observed improved technique survival warrant further investigation, focusing on modifiable factors of center-level performance to create opportunities for improved patient outcomes.
Index Words: Peritoneal dialysis, hemodialysis, mortality, transitions
Visual abstract
Plain-Language Summary.
Transitions in dialysis modality often represent a change in patients’ clinical status and can be disruptive to care. Our aim was to examine transitions among patients newly diagnosed with end-stage kidney disease who had just started peritoneal dialysis (PD), including transition to in-center hemodialysis (HD), as well as mortality and transplantation. We found lower rates of transition to in-center HD and death over time, an association between longer time receiving PD and mortality, and that larger PD units had lower risk for mortality. This is the largest epidemiologic study to date to evaluate patterns of transitions among new PD patients and will serve as a stepping stone in identifying modifiable factors to improve transitions when possible and mortality overall.
Editorial, p. 529
Although the number of patients receiving peritoneal dialysis (PD) has grown by 60% (from 30,861 to 49,489) from 1996 to 2015, the percentage of PD use among all dialysis patients declined from 14% to 10%.1 However, the updated Centers for Medicare & Medicaid Services (CMS) Prospective Payment System, put in place in 2011, financially incentivized greater PD use and is likely one of the reasons the percentage of PD increased from nearly 8.5% in 2011 to 10% in 2016. Transition from PD is a common reality, as prior observational studies of PD patients report that 34% to 52% of incident PD patients either transferred to hemodialysis (HD) or died while on PD by 3 years, depending on the country.2,3 More information is needed about the effect this increase in PD use has had on transitions from PD to in-center HD and on mortality while on PD.
Compared with in-center HD, PD has been shown to be more cost-effective,4,5 less technically demanding,6 and associated with better preservation of residual kidney function.7,8 It may be in both the provider’s and patient’s interest to increase PD use. Despite this, peritonitis remains an important risk factor for necessitating a switch to in-center HD, and the initial benefits of PD gradually disappear over time due to progressive loss of residual kidney function and reduced function of the peritoneal membrane, ultimately leading to insufficient clearance and ultrafiltration.9, 10, 11 This decreased clearance requires a more complex exchange regimen that can lead to decreased quality of life and patient burn out, eventually leading to a switch to in-center HD.11 Transition to in-center HD is often necessary (ie, during times of severe peritonitis) and, if done in a timely fashion, can improve patients’ overall outcome, but unplanned transitions from PD to in-center HD are associated with a higher number and longer duration of hospitalizations and higher risk for mortality, emphasizing the importance of anticipating the need for transition when possible.12,13
In this study, our goals were to focus on patterns of transition from PD to in-center HD and death but to also describe rates of kidney transplantation in PD patients from 1996 to 2015, hypothesizing that the rate of transition from PD to in-center HD and death would decline over the years due to hopefully more careful patient selection, while rates of transplantation would remain relatively stable. A complementary goal was to identify factors associated with these outcomes, hypothesizing that factors such as age, cohort year, PD program size, and rurality of the PD program would play important roles in these outcomes. Developing a better understanding of the patterns of and factors associated with transplantation may help increase the rate of this beneficial outcome. Our study represents an important first step toward developing methods to tailor preventive interventions aimed at improving clinical outcomes in the PD population.
Methods
Patients and Data Collection
Data were obtained from the US Renal Data System (USRDS). The study population included all dialysis patients incident to end-stage renal disease (ESRD) from January 1, 1996, through December 31, 2011 (for adjusted analyses; through December 31, 2014, for unadjusted analyses) and who were treated with PD for 1 or more days within 180 days of ESRD incidence (n = 173,533 for adjusted analyses; n = 219,787 for unadjusted analyses). Information for each patient’s dialysis modality and death was abstracted from the treatment history file, created using a combination of data from CMS Form 2728, Medicare claims, CMS-2746 Death Notification Form, and other ESRD data sources.1 We used demographic characteristics, first ESRD service date, prior transplantation date, initial treatment modality, primary cause of ESRD, comorbid conditions, multiple indicators of functional status, smoking history, and drug/alcohol dependence from CMS Form 2728 at the time of ESRD incidence. Completion of this form by a health care provider is required for all individuals in the United States having newly diagnosed ESRD.
We obtained facility-level characteristics from the CMS ESRD Facility Survey. Additionally, we calculated PD program size based on the number of PD patients treated in a dialysis facility as of December 31 of each year and categorized them into quartiles based on the distribution over all the years combined.
The number of prevalent PD patients in the United States by study year was calculated as those who were receiving PD as of December 31 for each year from 1996 to 2011.
Analyses
Annual cohorts of incident PD patients were followed up for up to 3 years (end of study period for adjusted and unadjusted analyses was December 31, 2015, and December 31, 2017, respectively) for 3 outcomes: (1) transition to in-center HD, defined as at least 30 consecutive days of in-center HD following the switch from PD to in-center HD; (2) death; and (3) transplantation. For unadjusted analyses, patients who were incident to ESRD in 2014 and starting PD anytime between January 1, 2015, and June 30, 2015 (within 180 days of ESRD initiation), had less than 3 years of follow-up time due to data availability. Time at risk was calculated as days from PD incidence until the outcome of interest, expressed in 100 patient-years (PY), or until censoring for loss to follow-up, recovery of kidney function, discontinuation of dialysis, or end of the 3-year follow-up. For mortality analyses, patients were followed up for 30 days after recovery of kidney function or discontinuation of dialysis before they were censored to avoid underestimation of death events. Patients were classified into annual cohorts based on their year of ESRD incidence; for each annual incident patient cohort from 1996 to 2014, we calculated temporal trends in the unadjusted rates of mortality, transplantation, and transition to in-center HD over a 3-year follow-up period, expressed as events per 100 PY at risk. These annual cohorts were then grouped into 4 multiyear cohorts (1996-1999, 2000-2003, 2004-2007, and 2008-2011). The 2012 to 2014 grouped cohort was omitted from analyses due to the unavailability of center-level factors.
We fit separate Cox regression models for each of the 3 outcomes, adjusted for the time when each cohort was incident to PD plus covariates of patient age, sex, race, ethnicity, 8 comorbid conditions (Table 1), functional status, tobacco use, alcohol/drug dependence, primary cause of ESRD, and facility-level factors (Table S1). To determine how outcome rates change during the 3-year follow-up period, we calculated average transition rates by dividing the 3-year follow-up time into 12 segments of 3 months and computing the cumulative hazard function (by taking the log of the survivor function) obtained from the regression model for each of the 12 segments. For each outcome and time period, we divided the increment in the cumulative hazard function by the length of time, thereby yielding the average transition rate for that period. These rates (per 100 PY at risk) are plotted by time receiving PD (months since starting PD). Each data point reflects the average transition rate of the preceding 3-month period. We also computed unadjusted transition rates. Hazard ratios (HRs) with 95% confidence intervals (CIs) were used to assess the associations of patient- and dialysis facility–level factors with each outcome.
Table 1.
Baseline Characteristics of PD Patients by Multiyear Cohort of ESRD Incidence
| Patient Characteristics | 1996-1999 (n = 46,546) | 2000-2003 (n = 42,883) | 2004-2007 (n = 39,665) | 2008-2011 (n = 44,124) | 2012-2014 (n = 45,877) |
|---|---|---|---|---|---|
| Age, y | 55 (18) | 55 (18) | 55 (18) | 56 (18) | 57 (17) |
| Race | |||||
| White | 32,471 (70%) | 30,275 (71%) | 28,746 (73%) | 31,336 (71%) | 32,220 (71%) |
| African American | 9,258 (20%) | 8,583 (20%) | 8,049 (20%) | 9,373 (21%) | 9,938 (22%) |
| Asian | 1,427 (3%) | 1,554 (4%) | 1,542 (4%) | 2,119 (5%) | 2,404 (5%) |
| Other/multiracial | 3,128 (7%) | 2,296 (5%) | 1,229 (3%) | 1,008 (2%) | 865 (2%) |
| Hispanic ethnicity | 4,944 (11%) | 5,045 (12%) | 4,586 (12%) | 5,584 (13%) | 5,903 (13%) |
| Women | 21,617 (47%) | 19,707 (46%) | 17,719 (45%) | 18,860 (43%) | 18,956 (42%) |
| Cause of ESRD | |||||
| Diabetes | 20,313 (44%) | 18,464 (43%) | 16,241 (41%) | 17,983 (41%) | 19,464 (43%) |
| Hypertension | 9,512 (20%) | 9,397 (22%) | 9,209 (23%) | 11,067 (25%) | 12,425 (27%) |
| Glomerulonephritis | 8,455 (18%) | 7,223 (17%) | 6,587 (17%) | 7,116 (16%) | 6,433 (14%) |
| Other cause | 6,269 (14%) | 6,069 (14%) | 5,957 (15%) | 6,326 (14%) | 5,922 (13%) |
| Unknown cause | 1,735 (4%) | 1,555 (4%) | 1,572 (4%) | 1,344 (3%) | 1,183 (3%) |
| Comorbid conditions | |||||
| CHF | 11,463 (25%) | 9,387 (22%) | 8,037 (20%) | 8,309 (19%) | 8,102 (18%) |
| COPD | 2,130 (5%) | 1,990 (5%) | 1,924 (5%) | 2,178 (5%) | 2,326 (5%) |
| CVA/TIA | 3,106 (7%) | 2,783 (6%) | 2,456 (6%) | 2,616 (6%) | 2,477 (5%) |
| Cardiovascular diseasea | 11,785 (25%) | 10,445 (24%) | 9,518 (24%) | 10,533 (24%) | 10,116 (22%) |
| PVD | 5,583 (12%) | 4,708 (11%) | 3,932 (10%) | 4,148 (9%) | 3,506 (8%) |
| Cancer | 1,559 (3%) | 1,765 (4%) | 1,924 (5%) | 2,221 (5%) | 2,291 (5%) |
| Diabetes | 25,498 (55%) | 23,596 (55%) | 21,044 (53%) | 22,103 (50%) | 24,503 (53%) |
| Hypertension | 35,570 (76%) | 34,804 (81%) | 33,739 (85%) | 38,674 (88%) | 40,622 (89%) |
| Immobilityb | 1.005 (2%) | 688 (2%) | 698 (2%) | 750 (2%) | 744 (2%) |
| Tobacco use | 2.625 (6%) | 2.304 (5%) | 2.431 (6%) | 2.696 (6%) | 2.689 (6%) |
| Alcohol/drug dependence | 535 (1%) | 375 (1%) | 391 (1%) | 439 (1%) | 393 (1%) |
Note: Not all categories add up to 100% (race and cause of ESRD) due to the small amount of missing data.
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; ESRD, end-stage renal disease; PD, peritoneal dialysis; PVD, peripheral vascular disease.
Atherosclerotic heart disease and other cardiac disease.
Inability to transfer or ambulate.
Of the 173,533 patients, 315 (0.2%) were excluded due to missing information at the patient or facility level, yielding 173,218 patients for analysis. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc).
Results
The size of the incident PD population declined from 12,730 in 1996 to a nadir of 9,507 patients in 2008, increasing thereafter to 16,819 patients (77% increase) in 2015. The prevalent PD population declined from 30,993 in 1996 to a nadir of 27,292 in 2000, increasing thereafter to 49,511 in 2015 (81% increase). The prevalence of PD (of all dialysis modalities) was 14% in 1996 and decreased to a nadir of 8% in 2004 to 2011 before increasing to 10% in 2014 to 2015.
Although baseline characteristics of incident PD patients were similar across the multiyear cohorts, the percentage of men and prevalence of hypertension increased slightly during the period and the prevalence of diabetes and congestive heart failure declined (Table 1). Mean age was 55 to 57 years, with a slight preponderance of men. Nearly half the incident PD patients had diabetes as a comorbid condition, and most had a history of hypertension.
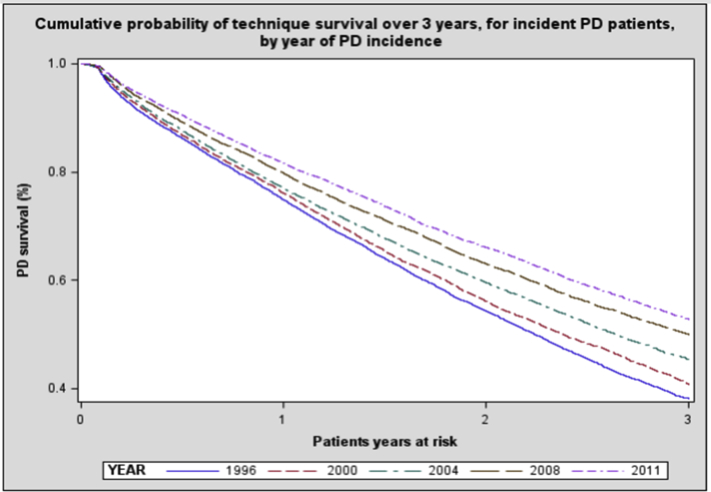
Figure 1 shows the cumulative probability of technique survival (defined as patients who did not die or transition to in-center HD) for incident PD patients during the 3-year follow-up period by year of PD incidence, demonstrating an increase in median survival from 2.2 years for the 1996 cohort to more than 3 years for the 2011 cohort. The probability of technique survival at 2 years increased from 54% in the 1996 cohort to 66% in the 2011 cohort.
Figure 1.
The cumulative probability of technique survival over 3 years, for incident peritoneal dialysis (PD) patients by year of PD incidence. Technique survival refers to PD patients who, at the indicated point in time, were still dialyzing with PD and had not died, undergone transplantation, or transitioned to in-center hemodialysis.
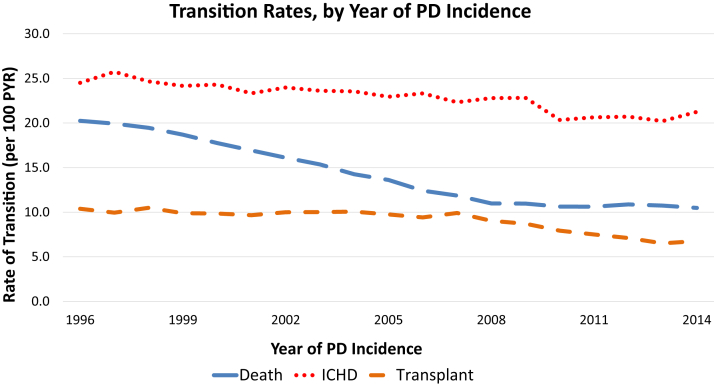
Unadjusted rates of transition from PD to in-center HD declined from 24.5 in 1996 to a nadir of 20.2 in 2013 (18% decrease) before increasing slightly to 21.3 in 2014, though with a notably steep decline from 2009 to 2010 (Fig 2). There was a generally linear decline in death rates, from 20.2 in 1996 to 11.0 in 2008 (46% decrease), followed by a shallower decline thereafter. Transplantation rates decreased overall from 10.4 in 1996 to 6.8 in 2014 (35% decrease), with the greatest decline from 2007 to 2013.
Figure 2.
Crude rates of outcomes, by year of peritoneal dialysis (PD) incidence. Annual cohorts of PD incident patients were followed up for up to 3 years for outcomes of transition to in-center hemodialysis (ICHD), death, and transplantation. Some patients in the 2014 cohort will have less than a 3-year follow-up depending on when they initiated PD. A transition to ICHD was defined as at least 30 consecutive days of ICHD following the switch from PD to ICHD. Time at risk was calculated as days from PD incidence until the outcome of interest occurred or until censoring for loss to follow-up, recovery of renal function, discontinuation of dialysis, or end of the 3-year follow-up. Patients were followed up for 30 days after recovery of renal function or discontinuation of dialysis before they were censored to avoid underestimation of death events. Abbreviation: PYR, patient-year.
Average Rates of Outcomes by Time Receiving PD
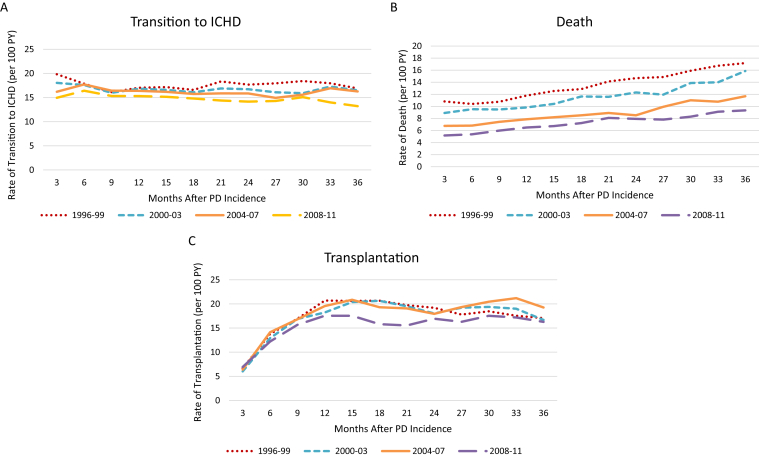
Figure 3A-C demonstrates adjusted rates of the 3 outcomes by time receiving PD and by calendar-year cohort. The 2008 to 2011 cohort had lower average rates than the earlier cohorts for all outcomes. In the 2008 to 2011 cohort, the adjusted rate of transition to in-center HD decreased by 12% from 0 to 36 months receiving PD, and the adjusted rate of mortality increased by 80% (Fig 3B). The average adjusted transplantation rate increased sharply in the first 12 months (Fig 3C), stabilizing thereafter. Unadjusted rates for each outcome (Fig S1a-c) showed similar trends, though the rate of increase in mortality rates was steeper in adjusted versus unadjusted analyses, principally because of age effect.
Figure 3.
(A-C) Adjusted rates of (A) transition to in-center hemodialysis (ICHD), (B) death, (C) and transplantation by months on peritoneal dialysis (PD) for grouped incident PD patient cohorts (1996-2011). To assess secular trends, patients were classified into multiyear cohorts (1996-1999, 2000-2003, 2004-2007, and 2008-2011) based on their year of end-stage renal disease (ESRD) incidence. Each Cox regression model was fit separately for each of the 3 outcomes and adjusted for the time when each cohort was incident to PD, plus covariates of patient age, sex, race, ethnicity, 8 comorbid conditions (Table 1), functional status, tobacco use, alcohol/drug dependence, primary cause of ESRD, and facility-level factors listed in Table S1. The 3-year follow-up time was divided into 12 segments of 3 months, and for each segment the cumulative hazard function obtained from the regression model was computed. For each outcome and each period, the increment in the cumulative hazard function was divided by the time to obtain the average transition rate. Each data point reflects the average transition rate of the preceding 3 months. Abbreviation: PY, patient-year.
Associations of Select Factors With Outcomes
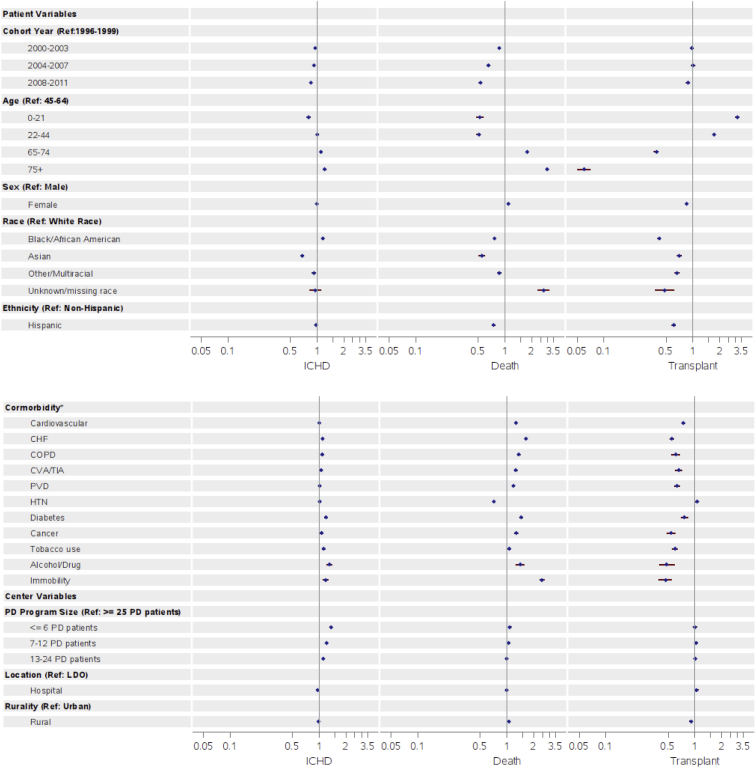
Figure 4 shows results from multivariable Cox models for transitions from PD to the 3 outcomes. HRs for mortality and transition from PD to in-center HD declined in more recent cohorts (compared with 1996-1999; HR, 0.53; 95% CI, 0.51-0.55 [for death] in 2008-2011; HR, 0.85; 95% CI, 0.82-0.87 [for transition to in-center HD] in 2008-2011). Progressively higher risks for transition to in-center HD were seen with increasingly smaller PD programs, with 36% higher risk for transition to in-center HD in PD programs treating 6 or fewer patients compared with 25 or more patients (HR, 1.36; 95% CI, 1.32-1.40; Table S1). Smaller PD programs also displayed slightly higher adjusted risks for mortality, with a 7% higher risk for mortality in PD programs treating 6 or fewer patients compared with 25 or more patients (HR, 1.07; 95% CI, 1.03-1.11; Table S1). Older age, African Americans (compared with patients of white race), diabetes as a comorbid condition, congestive heart failure, cancer, tobacco use, and alcohol/drug dependence had higher HRs for transitioning to in-center HD. Patients of Asian race had lower risk for transitioning to in-center HD than patients of white race (HR, 0.68; 95% CI, 0.65-0.71; Table S1). However, from 1996 to 2011, the elevated risk for transitioning from PD to in-center HD for older patients compared with younger patients attenuated in more recent years, as did the elevated risk in African Americans compared with patients of white race (Table S2a).
Figure 4.
Hazard ratios for (A) transition to in-center hemodialysis (ICHD), (B) death, and (C) transplantation by patient- and center-level variables. Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; HTN, hypertension; PD, peritoneal dialysis; PVD, peripheral vascular disease; Ref, reference.
Results were similar for the outcome of death, except that men, all non-white races (compared with white race), and Hispanics (compared with non-Hispanics) had lower risk for death (Fig 4). Patients treated in rural PD programs had higher risk for death than those in an urban PD facility (HR, 1.05; 95% CI, 1.02-1.08; Table S1).
Adjusted rates of transitions from PD to transplantation (Fig 4) were lower among older, non-white, female, and Hispanic patients (compared with non-Hispanics). Patients treated at an independent dialysis organization had higher rates of transplantation than those in large dialysis organizations (HR, 1.07; 95% CI, 1.02-1.11), and patients treated by facilities in rural locations had lower rates of transplantation than in urban locations (HR, 0.91; 95% CI, 0.87-0.94). The adjusted rates of transplantation progressively increased from 1996 to 2011 for patients 65 years or older and for African Americans (Table S2c).
Discussion
The size of the USRDS database and the length of follow-up make this the largest epidemiologic study to date to simultaneously evaluate patterns of mortality, transplantation, and transitions from PD to in-center HD among incident PD patients, and it has several novel findings. First, PD technique survival has improved during the study period. Second, from 1996 to 2014, there has been a decline in the rate of transition from PD to in-center HD and a large pronounced decline in the rate of death. Third, as time receiving PD increases, rates of transition to in-center HD decline slightly, while mortality rates increase, but both were lower in more recent years. Finally, we confirmed prior findings that older age, diabetes as a comorbid condition, and smaller PD programs were associated with higher hazards of transition to in-center HD and of death while on PD in this large, comprehensive, and contemporary population.
The most striking finding was the marked decline in mortality among incident PD patients. It is possible this may relate to improved PD therapy techniques and/or teaching. Alternatively, this could simply represent healthier patient selection rather than a comment on PD therapy itself given the lower prevalence of congestive heart failure, cardiovascular disease, peripheral vascular disease, and diabetes in the more recent patient cohorts. Mortality among incident HD patients showed similar patterns over time,1 possibly suggesting the effect of nondialysis factors in the improved mortality among dialysis patients in general.
Despite lower transition and mortality rates in more recent years, rates of mortality consistently increase as time on PD increases. Rates of mortality (per 100 PY) for incident patients starting on HD versus starting on PD in 2012 over the first 3 years of therapy decreased from 24.8 to 18.5 (25% decrease) and increased from 10.9 to 19.3 (78% increase), respectively.14 The increasing mortality rates with longer time on PD underscores the importance of identifying predictors of mortality so that providers can better counsel patients considering PD and increase their chances of positive outcomes.
The decline in transplantation rates among incident PD patients specifically from 2007 to 2014 and the decreased transplantation rates in the 2008 to 2011 cohort (compared with earlier cohorts) were notable despite the consistency in mean age of incident PD patients during the study period. The USRDS Annual Data Report demonstrated that transplantation rates decreased by 32% from 1996 to 2017,1 and although it does not distinguish between HD versus PD patients, most patients with ESRD are receiving HD (based on prevalence data). The declining rate of transplantation among patients with ESRD in general may simply be dependent on the growing number of needed kidneys among a possibly healthier ESRD population (given the declining mortality rate) mismatched against the number of kidneys available for transplantation.
The risk for transition from PD to HD described in the literature is variable, and there is not a standard way in which this is reported. At 6 months, transfer to HD ranges from 7% to 25%,15,16 with deaths at 8%,15 whereas at 1 year, transfer to HD ranges from 21% to 44%,16,17 whereas median PD survival time ranges from 1.6 to 3.7 years.12,15,18,19 Among PD patients who transitioned to HD, 20% to 25% transition within the first 6 months of PD therapy,20 with >40% transitioning by 12 months.16,20 Our data demonstrate that in the 2 earliest cohorts, rates of transition were highest within the first 3 months, decreasing thereafter, and in the 2 more recent cohorts, rates of transition to HD were slightly higher 3 to 6 months after PD initiation. Similar to our findings, Kolesnyk et al2 reported that transition is most common within the first 3 months of PD, whereas Guo and Mujais17 reported that transition rates were highest in the first 6 months of PD, highlighting this early period after starting PD as one of higher risk. With better understanding of the proportion of switches that occur as time receiving PD increases, we can potentially limit the clinical and psychological effects of unplanned transitions when possible.
Knowledge of why patients transfer will inform who may be at risk for this outcome. Reasons for transition have common themes, including peritonitis, loss of ultrafiltration, loss of PD adequacy, catheter-related problems, and patient choice.12,21 In a single-center Swiss study, the 2 most common reasons for cessation of PD or death from PD-related causes were peritonitis (38%) and psychosocial problems (23%), though catheter and psychosocial problems were more often responsible for cessation of PD or death by any cause within the first 6 months.18 Certain reasons for transfer are correlated with time receiving PD, such as decrease in residual kidney function,22 ultrafiltration failure,9,23 and sclerosing peritonitis.24
Confirming results of prior studies, we demonstrated that older patients16,18,25 and those with diabetes16 have a higher risk for transitioning and that comorbid conditions such as coronary artery disease and congestive heart failure were associated with mortality while on PD.16 Our findings are consistent with previous studies that have also shown an association between smaller PD program size and transfer to HD15,17,25, 26, 27, 28 and mortality.26 Data from a Baxter database have shown less catheter dysfunction, fewer infection problems, and less dialysis inadequacy among larger programs,29 and large centers with both established PD and HD programs may better manage short-term emergency transfers between the 2 modalities.11 Facility experience likely also relates to the number of new PD starts30 because studies have shown that risk for early transfer to HD13 and mortality7 was lower in centers initiating more new PD patients.
Understandably, not all shifts to HD occur predictably, such as transitions due to resistant peritonitis, an unpredictably nonfunctioning PD catheter, or sudden loss of residual kidney function. However, if we are better able to foresee who may require a transition for predictable reasons, there may be a higher chance that these patients could transition to HD before severe complications appear.21,31 If we can predict who might transfer to HD with long enough lead time, select patients could potentially be optimized for arteriovenous fistula placement. The high use of venous catheters is associated with increased risk for infection and poorer outcomes,32 underscoring the benefit of early recognition of a trajectory pointed toward transition to HD that can allow for appropriate vascular access planning.33 Despite certain limitations, studies have demonstrated that routine placement of prophylactic fistulas in PD patients is not successful or justifiable34, 35, 36, 37, 38 given their low use and high failure rate when their use is eventually attempted. Improving the ability to predict who may require a transition could enable more patients to benefit from the advantages of an established arteriovenous fistula close to the time of transition to HD, potentially saving them from a tunneled catheter.
Our analysis has limitations worth noting. Due to the addition and/or relabeling of comorbid conditions on CMS Form 2728 during the years of the study, analyses and covariate adjustments were limited to data consistently collected across all study years. Also, the data collected on Form 2728 are only at the time of ESRD incidence and do not provide information at the time of transition to in-center HD, death, or transplantation. Therefore, potential confounders not assessed by this study include comorbid conditions arising after ESRD incidence, residual kidney function, or other laboratory values at or just before the time of transition, such as levels of albumin or inflammatory markers. Additionally, some patients in the 2014 incident PD patient cohort in Fig 2 were followed up for less than 3 years due to data availability compared with the standard 3-year follow-up of the other cohorts, potentially affecting the observed rates. However, this study, based on comprehensive national data, is uniquely informative in its ability to assess long-term secular trends in the frequency of transitions and death. Next steps will include analyzing causes of transition and death to better discern between avoidable and unavoidable transitions.
In conclusion, although it is reassuring that rates of transition to in-center HD and mortality have declined in recent years, these 2 outcomes still affect a substantial number of patients, especially older patients, those with diabetes, and those dialyzing in smaller PD programs. Future work should include evaluation of hospitalization and mortality around the time of transition, which may allow for a more in-depth understanding of unplanned transitions. These results will hopefully serve as stepping stones to better predict situations in which patients will need a transition to HD and provide enough lead-time to systematically prepare patients mentally and physically.
Article Information
Authors’ Full Names and Academic Degrees
Nidhi Sukul, MD, Purna Mukhopadhyay, PhD, Douglas E. Schaubel, PhD, Jeffrey Pearson, MS, Marc Turenne, PhD, Rajiv Saran, MD, MRCP, MS, Bruce M. Robinson, MD, MS, and Ronald L. Pisoni, PhD, MS.
Authors’ Contributions
Research area and study design: BMR, RLP, RS, NS, DES, PM, JP, MT; data acquisition: PM; data analysis and interpretation: BMR, RLP, PM, NS; statistical analysis: PM; supervision or mentorship: BMR, RLP, RS, DES, JP, MT. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The data reported here have been supplied by the USRDS, which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through National Institutes of Health (NIH) contract HHSN276201400001C. This study was performed under the USRDS Coordinating Center contract with the NIH NIDDK; the work was conducted by the Coordinating Center, with the manuscript as originally submitted approved by NIDDK (NIDDK project officers are Kevin C. Abbott, MD, MPH, and Lawrence Y.C. Agodoa, MD). Except as indicated, the funder had no role in the study design; data collection, analysis, or interpretation; or writing of the report. At the time of writing this report, the USRDS Coordinating Center was located at the University of Michigan Kidney Epidemiology and Cost Center, in partnership with Arbor Research Collaborative for Health, Ann Arbor, MI. The USRDS director was Rajiv Saran, MD, MRCP, MS, Professor of Medicine and Epidemiology at the University of Michigan, and co-deputy directors were Vahakn Shahinian, MD, MS, Associate Professor of Medicine at the University of Michigan, and Bruce Robinson, MD. Dr Sukul is supported by MICHR U grant number: UL1TR002240.
Financial Disclosure
Drs Robinson, Pisoni, Turenne, Mukhopadhyay, and Pearson are employees of Arbor Research Collaborative for Health, which administers the Dialysis Outcomes and Practice Patterns Study. For details see https://www.dopps.org/AboutUs/Support.aspx. Drs Sukul, Saran, and Schaubel declare that they have no relevant financial interests.
Acknowledgements
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial support for this report.
Peer Review
Received December 16, 2019. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form June 28, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: Crude rates of transition to in-center HD, death, and transplantation by months on PD over 3-year follow-up, for grouped incident PD patient cohorts (1996-2011).
Table S1: Hazard ratios for (a) transition to in-center HD, (b) death, and (c) transplant by patient- and facility-level variables, among incident PD patients.
Table S2a: Hazard ratios for transition to in-center HD according to grouped cohort years of PD incidence by patient- and facility-level variables.
Table S2b: Hazard ratios for death according to grouped cohort years of PD incidence by patient- and facility-level variables.
Table S2c: Hazard ratios for transplant according to grouped cohort years of PD incidence by patient- and facility-level variables.
Supplementary Material
Figure S1, Tables S1-S2.
References
- 1.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2019. USRDS 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 2.Kolesnyk I., Dekker F.W., Boeschoten E.W., Krediet R.T. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30:170–177. doi: 10.3747/pdi.2008.00277. [DOI] [PubMed] [Google Scholar]
- 3.Van Biesen W., Vanholder R.C., Veys N., Dhondt A., Lameire N.H. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol. 2000;11:116–125. doi: 10.1681/ASN.V111116. [DOI] [PubMed] [Google Scholar]
- 4.Nayak Karopadi A., Mason G., Rettore E., Ronco C. The role of economies of scale in the cost of dialysis across the world: a macroeconomic perspective. Nephrol Dial Transplant. 2014;29:885–892. doi: 10.1093/ndt/gft528. [DOI] [PubMed] [Google Scholar]
- 5.Karopadi A.N., Mason G., Rettore E., Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra R., Devuyst O., Davies S.J., Johnson D.W. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen M.A., Hart A.A., Korevaar J.C., Dekker F.W., Boeschoten E.W., Krediet R.T. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 8.Moist L.M., Port F.K., Orzol S.M. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 9.Davies S.J., Bryan J., Phillips L., Russell G.I. Longitudinal changes in peritoneal kinetics: the effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant. 1996;11:498–506. [PubMed] [Google Scholar]
- 10.Selgas R., Munoz J., Cigarran S. Peritoneal functional parameters after five years on continuous ambulatory peritoneal dialysis (CAPD): the effect of late peritonitis. Perit Dial Int. 1989;9:329–332. [PubMed] [Google Scholar]
- 11.Van Biesen W., Davies S., Lameire N. An integrated approach to end-stage renal disease. Nephrol Dial Transplant. 2001;16(suppl 6):7–9. [PubMed] [Google Scholar]
- 12.Boissinot L., Landru I., Cardineau E., Zagdoun E., Ryckelycnk J.P., Lobbedez T. Is transition between peritoneal dialysis and hemodialysis really a gradual process? Perit Dial Int. 2013;33:391–397. doi: 10.3747/pdi.2011.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkart J. Transitions from PD are expected. Why not continue at home? Perit Dial Int. 2007;27:645–646. [PubMed] [Google Scholar]
- 14.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2018. USRDS 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 15.Bechade C., Guittet L., Evans D., Verger C., Ryckelynck J.P., Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant. 2014;29:2127–2135. doi: 10.1093/ndt/gft055. [DOI] [PubMed] [Google Scholar]
- 16.Chidambaram M., Bargman J.M., Quinn R.R., Austin P.C., Hux J.E., Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int. 2011;31:565–573. doi: 10.3747/pdi.2010.00096. [DOI] [PubMed] [Google Scholar]
- 17.Guo A., Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl. 2003;88:S3–S12. doi: 10.1046/j.1523-1755.2003.08801.x. [DOI] [PubMed] [Google Scholar]
- 18.Descoeudres B., Koller M.T., Garzoni D. Contribution of early failure to outcome on peritoneal dialysis. Perit Dial Int. 2008;28:259–267. [PubMed] [Google Scholar]
- 19.Le Registre de Dialyse Péritonéale de Langue Française (RDPLF) RDPLF; Pontoise, France: 2010. Causes and types of dropout (Metropolitan France)—2009 [French]http://www.rdplf.org/sorties/70-sor-tie-2009.html [Google Scholar]
- 20.Jaar B.G., Plantinga L.C., Crews D.C. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol. 2009;10(1):3. doi: 10.1186/1471-2369-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Biesen W., Dequidt C., Vijt D., Vanholder R., Lameire N. Analysis of the reasons for transfers between hemodialysis and peritoneal dialysis and their effect on survivals. Adv Perit Dial. 1998;14:90–94. [PubMed] [Google Scholar]
- 22.Davies S.J., Phillips L., Griffiths A.M., Russell L.H., Naish P.F., Russell G.I. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998;54:2207–2217. doi: 10.1046/j.1523-1755.1998.00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Heimburger O., Waniewski J., Werynski A., Tranaeus A., Lindholm B. Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int. 1990;38:495–506. doi: 10.1038/ki.1990.231. [DOI] [PubMed] [Google Scholar]
- 24.Campbell S., Clarke P., Hawley C., Wigan M., Butler J., Wall D. Sclerosing peritonitis: identification of diagnostic, clinical, and radiological features. Am J Kidney Dis. 1994;24:819–825. doi: 10.1016/s0272-6386(12)80677-9. [DOI] [PubMed] [Google Scholar]
- 25.Huisman R.M., Nieuwenhuizen M.G., Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in the Netherlands. Nephrol Dial Transplant. 2002;17:1655–1660. doi: 10.1093/ndt/17.9.1655. [DOI] [PubMed] [Google Scholar]
- 26.Schaubel D.E., Blake P.G., Fenton S.S. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int. 2001;60:1517–1524. doi: 10.1046/j.1523-1755.2001.00969.x. [DOI] [PubMed] [Google Scholar]
- 27.Plantinga L.C., Fink N.E., Finkelstein F.O., Powe N.R., Jaar B.G. Association of peritoneal dialysis clinic size with clinical outcomes. Perit Dial Int. 2009;29:285–291. [PMC free article] [PubMed] [Google Scholar]
- 28.Afolalu B., Troidle L., Osayimwen O., Bhargava J., Kitsen J., Finkelstein F.O. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int. 2009;29:292–296. [PubMed] [Google Scholar]
- 29.Mujais S., Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl. 2006;(103) doi: 10.1038/sj.ki.5001912. S21-S26. [DOI] [PubMed] [Google Scholar]
- 30.Lambie M., Davies S.J. Are peritoneal dialysis center characteristics a modifiable risk factor to improve peritoneal dialysis outcomes? Clin J Am Soc Nephrol. 2017;12:1032–1034. doi: 10.2215/CJN.05260517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panagoutsos S., Kantartzi K., Passadakis P. Timely transfer of peritoneal dialysis patients to hemodialysis improves survival rates. Clin Nephrol. 2006;65:43–47. doi: 10.5414/cnp65043. [DOI] [PubMed] [Google Scholar]
- 32.Ansell D., Feest T.G., Tomson C., Williams A.J., Warwick G. 9th Annual Report of the Renal Association. UK Renal Registry Report 2006. UK Renal Registry, Bristol, UK. Nephrol Dial Transplant. 2007;22(suppl 7) [Google Scholar]
- 33.Fluck R. Transitions in care: what is the role of peritoneal dialysis? Perit Dial Int. 2008;28:591–595. [PubMed] [Google Scholar]
- 34.Beckingham I.J., O’Rourke J.S., Bishop M.C., Blamey R.W. Are backup arteriovenous fistulae necessary for patients on continuous ambulatory peritoneal dialysis? Lancet. 1993;341(8857):1384–1386. doi: 10.1016/0140-6736(93)90951-c. [DOI] [PubMed] [Google Scholar]
- 35.Joffe P., Skov R., Olsen F. Do patients on continuous peritoneal dialysis need arterio-venous fistula? Perit Dial Int. 1986;6:193–195. [Google Scholar]
- 36.Chiarelli G., Beaulieu M., Cozzolino M. Vascular access planning in peritoneal dialysis patients. Perit Dial Int. 2008;28:585–590. [PubMed] [Google Scholar]
- 37.Farrington K., Brown A.L., Mathias M.T., Karim M.S., Cattell W.R., Baker L.R. Simultaneous creation of peritoneal and vascular access in patients commencing continuous ambulatory peritoneal dialysis. Nephron. 1991;59:323–325. doi: 10.1159/000186576. [DOI] [PubMed] [Google Scholar]
- 38.Chui A.K., Chiu E.Y., White E.A., Yumiba T. An investigation into the practice of concurrent chronic ambulatory peritoneal dialysis catheter insertion and arteriovenous fistula formation in patients needing dialysis. Hong Kong Med J. 2000;6:312–315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Tables S1-S2.