Abstract
Alport syndrome affects up to 60,000 people in the United States. The proposed reclassification of thin basement membrane nephropathy and some cases of focal segmental glomerulosclerosis as Alport syndrome could substantially increase the affected population. The reclassification scheme categorizes Alport syndrome as 3 distinct diseases of type IV collagen α3/4/5 based on a genetic evaluation: X-linked, autosomal, and digenic. This approach has the advantage of identifying patients at risk for progressive loss of kidney function. Furthermore, the shared molecular cause of Alport syndrome and thin basement membrane nephropathy arises from mutations in the COL4A3, COL4A4, and COL4A5 genes, which contribute to downstream pathophysiologic consequences, including chronic kidney inflammation. Recent evidence indicates that chronic inflammation and its regulation through anti-inflammatory nuclear factor erythroid 2–related factor 2 (Nrf2) and proinflammatory nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) transcription factors plays a central role in renal tubular and glomerular cell responses to injury. Crosstalk between the Nrf2 and NF-κB pathways is important in the regulation of inflammation in patients with chronic kidney disease; moreover, there is evidence that an insufficient Nrf2 response to inflammation contributes to disease progression. Given the association between type IV collagen abnormalities and chronic inflammation, there is renewed interest in targeted anti-inflammatory therapies in Alport syndrome and other forms of progressive chronic kidney disease.
Index Words: Alport syndrome, chronic kidney disease, chronic inflammation, COL4A3, COL4A4, COL4A5, Nrf2, NF-κB
Introduction
Alport syndrome is a hereditary kidney disease characterized by structural abnormalities and dysfunction in the glomerular basement membrane (GBM), as well as basement membranes of other tissues including the eye and ear. Patients with Alport syndrome often experience progressive loss of kidney function with distinctive ultrastructural changes in the GBM, sensorineural hearing loss, and variable ocular abnormalities.1 The GBM is the extracellular matrix (ECM) component of the selectively permeable glomerular filtration barrier, which serves as a critical barrier to the passage of blood cells and proteins from the blood to the urinary space.2,3 This sheet-like ECM is composed of 4 major macromolecules: laminin, type IV collagen, nidogen, and heparan sulfate proteoglycan (agrin). Type IV collagen is crucial for basement membrane stability4 and comprises ∼50% of the total GBM protein mass.3 There are 6 genetically distinct α1 to α6 type IV collagen chains that assemble to form 3 unique heterotrimers, α1α1α2, α3α4α5, and α5α5α6.3
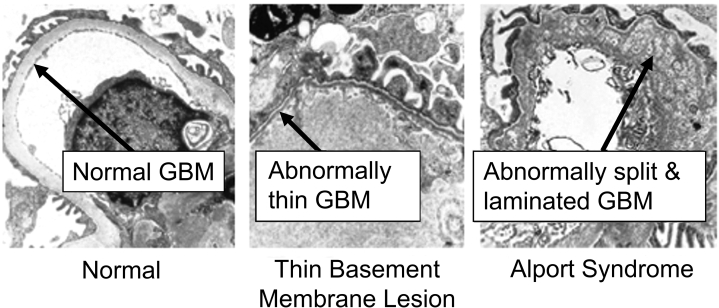
In Alport syndrome, mutations in the COL4A3, COL4A4, or COL4A5 genes result in defective type IV collagen α3, α4, or α5 chains, respectively.5,6 Histologic lesions, including GBM thinning and variable segmental GBM thickening and splitting, result from type IV collagen α-chain mutations and in many cases result in a basket weave appearance rather than the typical ribbon-like morphology when viewed on electron microscopy (Fig 1).7, 8, 9, 10, 11 These structural abnormalities result in a dysfunctional GBM leading to both hematuria and proteinuria, with downstream pathophysiologic consequences including chronic inflammation and eventual fibrosis.3,9 Due to the relationship between type IV collagen abnormalities and kidney inflammation and fibrosis, there is growing relevance for targeted anti-inflammatory therapies in Alport syndrome and other forms of progressive chronic kidney disease (CKD).
Figure 1.
COL4A3, COL4A4, and COL4A5 mutations cause dysfunctional glomerular basement membrane (GBM) in Alport syndrome.10,11 Kidney biopsy specimens from a patient with Alport syndrome, a patient without Alport syndrome, and a patient with a thin membrane lesion demonstrate that the GBM in patients with Alport syndrome is abnormally split and laminated compared with the GBM of patients with other conditions. In patients with a thin basement membrane lesion, which is now proposed to be considered as Alport syndrome, the GBM is abnormally thin. Images used with permission from UNC Kidney Center. www.unckidneycenter.org. Accessed April 20, 2020.
Update on the Genetics and Prevalence of Alport Syndrome
The prevalence of Alport syndrome is estimated at approximately 1 in 50,000 live births.12 Alport syndrome affects an estimated 30,000 to 60,000 persons in the United States.13 Based on the current classification scheme, Alport syndrome accounts for an estimated 3% of CKD in children and 0.2% of adults with end-stage kidney disease (ESKD) in the United States.13 Alport syndrome is the second most common monogenic cause of CKD after autosomal dominant polycystic kidney disease.9 However, based on a proposed reclassification by the Alport Syndrome Classification Working Group, the number of affected persons with Alport syndrome may be greater than currently estimated. The Working Group recommends reclassifying genetic diseases of type IV collagen as Alport syndrome, including thin basement membrane nephropathy (TBMN). Evidence suggests that a sizeable fraction of patients with otherwise uncharacterized CKD or those with focal segmental glomerulosclerosis (FSGS) diagnosed based on histology have genetic abnormalities in type IV collagen.11,14
Our understanding of the genetics of Alport syndrome has evolved in recent years.15 The prevailing view is that Alport syndrome is transmitted in an X-linked manner in most cases. Under this assumption, an affected father could not transmit the disease to his son.13 The X-linked mutation is seen in the COL4A5 gene.13 Pathogenic mutations in COL4A3 and COL4A4 can also cause Alport syndrome, and their transmission is autosomal.13
From analyses of the gnomAD and BRAVO databases, 2 large repositories of whole genome sequencing, Lanktree et al16 reported that the prevalence of truncating mutations per 100,000 individuals was 5 for COL4A5, 76 for COL4A4, and 115 for COL4A3. Truncating mutations are a fraction of the total mutations. Based on data from the Leiden Open Variation Database, it can be estimated that truncating mutations make up 22.8% of COL4A5, 43.3% of COL4A4, and 24.1% of COL4A3 mutations. Thus, the estimated prevalence of all mutations that could potentially lead to Alport syndrome per 100,000 individuals is 21.9 for COL4A5, 175 for COL4A4, and 477 for COL4A3.16, 17, 18, 19 Although the prevalence of COL4A4 and COL4A3 mutations is several times higher than the more widely recognized X-linked COL4A5 mutation, >15% of persons with autosomal dominant mutations may develop clinical manifestations of Alport syndrome.11,20, 21, 22, 23, 24 Taken together, persons carrying the COL4A4 and COL4A3 mutations may account for more cases of Alport syndrome than the X-linked variant despite X-linked Alport syndrome having 100% penetrance in men. Although autosomal dominant Alport syndrome is currently considered rare and X-linked Alport is still considered to account for most clinically apparent Alport syndrome cases by experts, with new sequencing technologies, the prevalence of all COL4A mutations (including what might be characterized as autosomal dominant Alport syndrome) in the population may be much higher than previously appreciated. Further studies are needed to ascertain the true prevalence of the autosomal dominant form of Alport syndrome and to better characterize the clinical implications of individual mutations.
Risk for Disease Progression in Alport Syndrome
The natural history of Alport syndrome is variable and is informed by genetics and environmental factors. The former are better studied and include large deletions, nonsense mutations, and small mutations affecting reading frames that result in lower or absent levels of functional protein and confer the highest risk for ESKD by age 30 years. The risk is lower with missense or splice site mutations.25
The estimated risk for ESKD in Alport syndrome varies with the mode of inheritance. For X-linked Alport syndrome, the clinical phenotype in women differs from that in men, who have a more severe presentation and universally develop ESKD: X-linked women have up to a 25% risk for ESKD, whereas X-linked men have a 100% risk.11,26 Under the reclassification scheme discussed later, women with X-linked inheritance, previously seen only as carriers, would have Alport syndrome diagnosed and be considered to have an appreciable risk for disease progression.11 Among persons with autosomal recessive inheritance, both sexes experience 100% risk for progression to ESKD.11,27 Among persons with autosomal dominant inheritance, the estimated risk for ESKD is ≥20% for those with risk factors for progression (proteinuria, FSGS, GBM thickening and lamellation, sensorineural hearing loss, evidence of progression in patient, or family genetic modifiers) and <1% in the absence of these risk factors.11 Digenic inheritance affecting COL4A3, COL4A4, and COL4A5 genes have a variable estimated risk for ESKD depending on the affected genetic state: up to 100% for COL4A3 and COL4A4 mutations in trans simulating autosomal recessive transmission, up to 20% for COL4A3 and COL4A4 mutations in cis simulating autosomal dominant transmission, and up to 100% of affected men for mutations in COL4A5 and either COL4A3 or COL4A4 in which the inheritance pattern does not simulate any Mendelian transmission (Table 1).11
Table 1.
New Classification Scheme Categorizes Genetic Diseases of COL4A3, COL4A4, and COL4A5 Into 3 Types of Alport Syndrome: X-linked, Autosomal, and Digenic
| Inheritance | Affected Gene(s) | Allelic State | Mutation Phenotype |
|---|---|---|---|
| X-linked | COL4A5 | Hemizygous (males) | NA |
| Heterozygous (females) | NA | ||
| Autosomal | COL4A3 or COL4A4 | Homozygous or compound heterozygous | Recessive |
| Heterozygous | Dominant | ||
| Digenic | COL4A3, COL4A4, and COL4A5 | Variable | |
Abbreviation: NA, not applicable.
Data from Kashtan et al.11
Progression to ESKD in affected persons follows predictable stages: (1) asymptomatic glomerular hematuria ranging from microscopic to gross hematuria, (2) microalbuminuria, and (3) declining glomerular filtration rate (GFR) and ultimately ESKD.28 There is a possibility that as COL4 mutations are identified more commonly in kidney disorders such as FSGS, progression may not be as predictable because some patients may present with primary proteinuric manifestations, as is the case with FSGS.29,30
It is important to note that the risk for progression to ESKD is based on current estimates. With greater frequency of genetic testing, our understanding of the risks for progression will improve as more individuals with autosomal dominant Alport syndrome are identified and are not either misdiagnosed or given a nonspecific diagnosis of CKD. Recent Expert Consensus Guidelines recommend genetic testing for confirmation of an Alport syndrome diagnosis.15 Genetic testing is more sensitive and specific than kidney biopsy and is recommended as the gold standard. In patients with suspected Alport syndrome, high-throughput–targeted next-generation sequencing technologies with a customized panel for testing all 3 Alport genes—COL4A3, COL4A4, and COL4A5—together can identify up to 95% of pathogenic COL4A variants.1,15 Toward that end, recent gene sequencing studies consistently demonstrate that variants in COL4A3, COL4A4, and COL4A5 commonly result in sporadic and familial adult FSGS, which should be identified as Alport syndrome based on the reclassification proposal.29,30
Extrarenal manifestations of Alport syndrome also mandate attention. Sensorineural hearing loss for high frequencies has been observed in patients with Alport syndrome, with the highest occurrence of 70% in male patients with X-linked Alport syndrome. Some patients develop lenticonus, which also occurs most frequently in X-linked affected men, up to 30% by the fourth decade of life, a time by which kidney failure, hearing loss, and retinopathy have already developed.1 However, a lack of sensorineural hearing loss should not diminish consideration of Alport syndrome in the differential diagnosis. Most women with progressive CKD due to X-linked Alport syndrome do not have hearing loss but still have a significant probability of progression to ESKD.26
Proposed Reclassification of Alport Syndrome
As referred to, the Alport Syndrome Classification Working Group recently proposed a reclassification scheme of genetic disorders of the type IV collagen α345 molecule.11 This proposed classification scheme is based on clinical and molecular genetic criteria instead of relying solely on histologic and clinical traits that are sex- and age-dependent or potentially modifiable by remote genetic variants11 or other exposures. By defining individuals genetically by identifying mutations affecting the type IV collagen α345 molecule as at risk for progressive kidney disease, the proposed scheme promotes surveillance of affected patients and early initiation of nephroprotective therapy.11
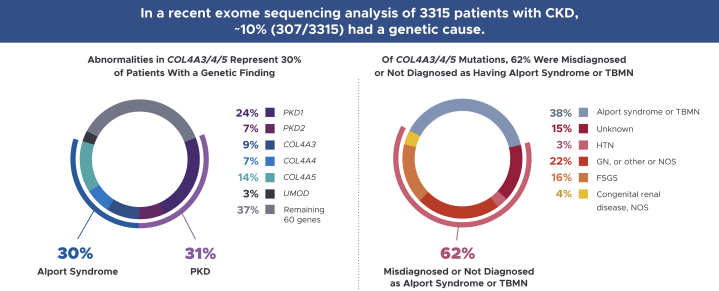
Results from genetic evaluations of patients with CKD suggest that the proposed reclassification would identify some patients for whom the cause of kidney disease is unknown or thought to be other more common conditions (eg, type 2 diabetes and hypertension) as being affected by Alport syndrome. For example, exome sequencing analysis in a cohort of 3,315 patients with CKD yielded a genetic diagnosis in ∼10% of cases (Fig 2).14 Among patients with a genetic diagnosis, ∼30% had COL4A3, COL4A4, or COL4A5 gene mutations.14 Notably, 56 of the 91 (62%) patients in this study with COL4A mutations did not have a clinical diagnosis of either Alport syndrome or TBMN.14 In a screening analysis for mutations in COL4A3, COL4A4, and COL4A5 in 101 unrelated patients with a positive history of hematuria—many of whom (77/101) also had a family history of hematuria, CKD, or both—80% (81/101) had COL4A mutations.31 Of these, ∼20% were autosomal dominant.31 In this study, the proportion of variations considered as possibly disease causing in patients with COL4A3 and COL4A4 mutations was higher than previously reported and prompted the suggestion that the frequency of autosomal Alport syndrome and particularly autosomal dominant disease was higher than previously believed.31
Figure 2.
COL4A3, COL4A4, and COL4A5 mutations represent 30% of patients with a genetic cause of chronic kidney disease (CKD).14 Alport syndrome and autosomal dominant polycystic kidney disease (ADPKD) were the most prevalent monogenetic causes of CKD detected in a recent exome sequencing analysis of patients with CKD. Notably, 56 of the 91 patients (62%) identified by mutational screening as having Alport syndrome did not have a clinical diagnosis of Alport syndrome. Abbreviations: FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; HTN, hypertension; NOS, not otherwise specified; PKD, polycystic kidney disease; TBMN, thin basement membrane nephropathy.
Unmet Needs in the Management of Alport Syndrome
The goal of kidney-related therapy in Alport syndrome is to delay progression to ESKD. Angiotensin-converting enzyme (ACE) inhibition has been shown to reduce proteinuria in patients with Alport syndrome, delay kidney failure in mice with Alport syndrome, and be of value in delaying kidney failure in humans. According to the European Alport Registry, data collected from several generations of Alport families (n = 283) during 2 decades demonstrate that early treatment with ACE inhibitors delayed kidney failure and enhanced life expectancy in a time-dependent manner, supporting the need for early diagnosis and nephroprotective treatment.32 A separate study of heterozygous carriers of X-linked Alport syndrome and a subgroup of patients with what has been referred to as TBMN due to heterozygous autosomal recessive Alport mutations (n = 234) also demonstrated that inhibition of the renin-angiotensin-aldosterone system (RAAS) significantly delayed the onset of ESKD in these subpopulations.33
Inhibition of the RAAS has been consistently shown to attenuate the loss of kidney function among patients with many glomerular diseases, with more pronounced benefits observed among patients with higher levels of proteinuria or albuminuria.34,35 Intensive lowering of systolic blood pressure, as tested in the Systolic Blood Pressure Intervention Trial (SPRINT), was shown to substantially reduce the incidence of death and cardiovascular events in patients with and without CKD, although there were too few events of CKD progression to determine whether lower systolic blood pressure targets resulted in higher or lower rates of progressive CKD or ESKD.36 The Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial demonstrated that more intensive blood pressure management in pediatric patients with CKD from any cause significantly prolonged the time until a 50% decline in GFR or progression to ESKD.37
However, despite treatment with RAAS inhibitors, there is progressive loss of kidney function in patients with Alport syndrome, as reflected by a mean decline of approximately −4 mL/min per year in estimated GFR at 72 weeks, as demonstrated by A Natural History Study to Observe Disease Progression, Standard of Care and Investigate Biomarkers in Alport Syndrome Patients (ATHENA), a noninterventional (observational) global multicenter study of 250 patients with Alport syndrome.38 In this regard, treatment with RAAS inhibitors slows but does not stop the progression to kidney failure in Alport syndrome and emphasizes the urgent need for the discovery of new treatment options.39
There are also a number of important disease management needs in addition to kidney-specific treatment. For instance, hearing aids are usually very effective for patients with concomitant hearing loss.40 As with any hereditary disease, psychosocial support for the patient and family is essential in the management of Alport syndrome.40 In a study examining the psychosocial impact of Alport syndrome, patients and family members experienced denial, chronic mental suffering, depressive symptomatology, and anxiety. Families with Alport syndrome should be encouraged to discuss their thoughts openly, and the empathy of the clinician as well as appropriate psychological support are critical to the process.41
Role of Chronic Inflammation and Metabolic Dysfunction in the Progression of Alport Syndrome
An important unmet need in Alport syndrome, in addition to the reclassification and management consideration discussed thus far, is to better understand emerging pathogenic mechanisms in the progression of the disease. Chronic inflammation and metabolic dysfunction have been identified as key pathogenic mechanisms in patients with a variety of kidney disorders, including TBMN (proposed to be Alport syndrome according to the reclassification).42 In a comprehensive pathway analysis of CKD that included human kidney biopsies of 157 diverse patients with CKD related to 9 different disorders including TBMN,11 chronic inflammation in both the tubulointerstitial and glomerular compartments and metabolic dysfunction were considered to be the final common pathways in the pathogenesis of CKD progression that correlate with reduced estimated GFR.42 This analysis demonstrated clear similarities in the inflammatory mechanisms involved in various forms of CKD, including TBMN.11,42 The anti-inflammatory nuclear factor erythroid 2–related factor 2 (Nrf2) pathway was identified as the pathogenic link between inflammatory and metabolic pathways in the progression of multiple types of CKD.42
Progression of CKD has been suggested to occur through a final common pathway of chronic inflammation leading to renal fibrosis.43 Acute inflammation is a natural immune response to kidney injury44 and plays a critical role in renal cell repair following damage. Chronic inflammation is a result of inadequate downregulation of proinflammatory gene transcription and signaling.44, 45, 46, 47, 48 Activation of inflammatory pathways may also interfere with the normal response of Nrf2-associated pathways to oxidative stress. For example, it has been shown that a subunit of the proinflammatory transcription factor, nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) p65 represses the Nrf2-antioxidant response element pathway at the transcriptional level.49
Role of Resident Kidney Cells in Chronic Inflammation
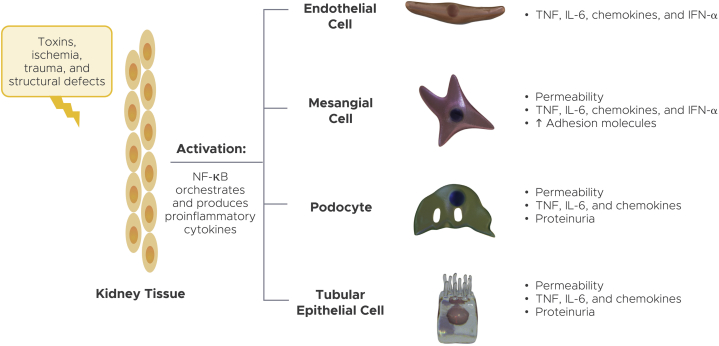
Although acute inflammation is marked by infiltrating white blood cells, including neutrophils, macrophages, dendritic cells, and T and B lymphocytes,50 chronic inflammation is characterized by activation of resident kidney cells that develop a proinflammatory response (Fig 3).51 These resident kidney cells include endothelial cells, mesangial cells, podocytes, and tubular epithelial cells, which proliferate and produce proinflammatory chemokines and cytokines that subsequently recruit macrophages to damaged tissue.
Figure 3.
Activation of resident kidney cells produces proinflammatory cytokines and chemokines.51 Chronic inflammation is evidenced by activation of resident kidney cells adapting a proinflammatory response. These resident cells include endothelial cells, mesangial cells, podocytes, and tubular epithelial cells. Abbreviations: IFN-ɑ, interferon α; IL-6, interleukin 6; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; TNF, tumor necrosis factor. Adapted by permission from Springer Nature. Nat Rev Immunol. The immune system and kidney disease: basic concepts and clinical implications. Kurts C, Panzer U, Anders HJ, Rees AJ. Copyright 2013.
This further perpetuates the cycle of chronic inflammation that leads to renal fibrosis.9,48,51 Typically, the endothelial cells of the glomerulus are uniquely adapted for selective filtration and permeability.52 Mesangial cells play a role in glomerular contraction, modulating the filtration coefficient—the permeability or conductivity of the filtration barrier denoted by the filtration coefficient—and the filtration surface area, which help regulate GFR.53 In the setting of oxidative stress and inflammation, dynamic and long-term actions are provoked, leading to GFR decline.53 These dynamic actions include the reactive oxygen species (ROS)-induced mesangial cell contraction and glomerular endothelial dysfunction due to decreased nitric oxide bioavailability, causing reduction of the filtration coefficient.53 Podocyte foot contraction and effacement are adaptive responses to inflammation and oxidative stress that may be instituted to prevent podocyte loss.54 As a consequence, the integrity of glomerular filtration is apparently reduced, owing to the denudation of the GBM54 and manifest by reduced GFR, some proportion of which appears to be reversibly recoverable with amelioration of the inflammatory signaling to mesangial cells and podocytes.55,56
In Alport syndrome, the altered composition of the GBM renders the glomerulus susceptible to damage caused by biomechanical stress, which induces proteinuria and damages the podocytes leading to increased albumin uptake by the proximal tubules.57 The glomerular damage further prompts a sustained inflammatory response leading to fibrosis.45,58 In CKD, myofibroblasts play a key role in the development of fibrosis; a variety of molecular signals and processes contribute to their formation and activation. Factors responsible for the injurious nature of myofibroblasts include the activation of endothelial cells by vascular endothelial cell growth factor released from injured interstitial fibroblasts, renal pericytes, fibrocytes, and tubular epithelial cells, which produce platelet-derived growth factor (PDGF) and transforming growth factor β (TGFβ).59, 60, 61 Pericytes subsequently detach from endothelial cells, proliferate, spread, and migrate into the interstitium. These events result in unstable vasculature, capillary loss, interstitial matrix expansion, and contraction of tissue architecture resulting in a phenotypic conversion of pericytes to myofibroblasts.59 Subsequently, myofibroblasts synthesize ECM components, leading to excessive collagen accumulation and fibrosis.45,58 The consequent fibrosis results in irreversible loss of kidney function.9,28,45,47,62, 63, 64
Proinflammatory Role of NF-κB
The transcription factor NF-κB is a regulator of proinflammatory genes that orchestrates hundreds of inflammatory cytokines and mediators.65 NF-κB promotes the expression of tumor necrosis factor α (TNF-α) and other cytokines that activate renal cells and recruit macrophages to damaged tissue, which contribute to progressive glomerulosclerosis.9,43,48,65,66 In mouse models of Alport syndrome, TNF-α messenger RNA expression was increased in the mesangium and podocytes of the COL4A3 mice glomeruli as the disease progressed, demonstrating that TNF-α contributed to the etiopathogenesis of progressive glomerulosclerosis.67
NF-κB also increases TGFβ expression, which promotes transformation of epithelial and mesangial cells into fibroblasts and myofibroblasts resulting in fibrosis.9,48 TGFβ1 and connective tissue growth factor, also known as cellular communication network factor 2, contribute to the altered glomerular structural and functional properties of the Alport syndrome GBM, which is characterized by irregular thickening, splitting, and increased permeability.9 TGFβ1 and platelet-derived growth factor are also important mediators of interstitial fibrosis, with TGFβ1 upregulation occurring in nearly every type of CKD and resulting in interstitial ECM accumulation, which contributes to functional loss.68
Additional proinflammatory cytokines that are activated in an X-linked Alport syndrome mouse model include interleukin 6 (IL-6) and IL-1β.69 There is evidence both in vitro (cultured podocytes) and in vivo (through hypertension induction in mice) that the altered GBM in mouse models of Alport syndrome induced the production of IL-6.70 Structural changes promoted by these mediators contribute to a progressive decline in GFR that is characteristic of Alport syndrome and other forms of CKD.71, 72, 73
Anti-Inflammatory Role of Nrf2
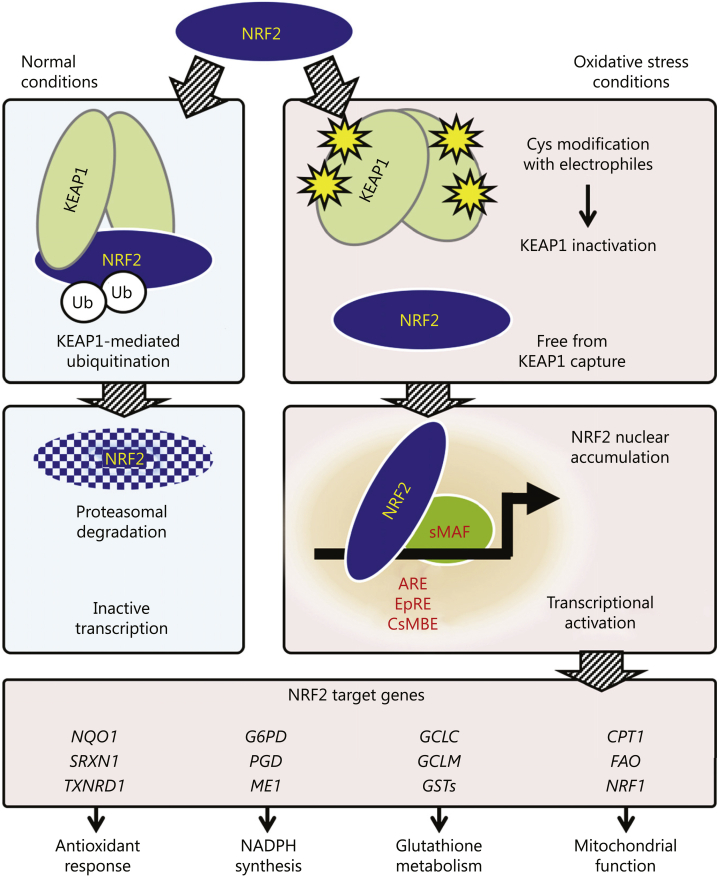
Nrf2 regulates the expression of specific genes that are involved in cytokine production and protection from oxidative damage that is triggered by injury and inflammation.74 A cytosolic inhibitor known as kelch-like ECH-associated protein 1 (Keap1) retains Nrf2 in the cytoplasm until disease triggers activate Nrf2 dissociation from Keap1, allowing nuclear translocation.74,75 As shown in Figure 4, under conditions of chronic inflammation and oxidative stress, electrophiles emerge and alter the conformation of Keap1 by directly adducting to the specific sensor cysteine residues in Keap1.76 Because this modification inactivates the interaction between Keap1 and Nrf2, Nrf2 avoids degradation in cells exposed to oxidative stress. Stabilized and newly synthesized Nrf2 translocates into the nucleus, where it activates transcription of its target genes by binding to specific recognition sequences, such as antioxidant response elements as Nrf2-small musculoaponeurotic fibrosarcoma heterodimers. Target genes of Nrf2 transcriptional regulation contain antioxidant response elements—the Nrf2 response elements—in their promoter region.76 As a result, Nrf2 induces gene transcription of anti-inflammatory and antioxidant mediators. Nrf2 also directly suppresses the expression of proinflammatory cytokine genes by binding to their promoters and inhibiting transcription.77 Extensive biochemical, biophysical, and structural analyses of the Keap1-Nrf2 interaction have demonstrated the central role of this system in protection from oxidative and electrophilic stress.78 Further, in vivo experiments have validated the functional relationship between Keap1 and Nrf2 by demonstrating that phenotypes of Keap1-null mice are attributable to the constitutive stabilization of Nrf2.79, 80, 81 Thus, the Keap1-Nrf2 system plays a key role in resolving inflammation by decreasing oxidative damage and inhibiting proinflammatory NF-κB signaling.82
Figure 4.
Molecular mechanisms of the nuclear factor erythroid 2–related factor 2 (Nrf2)-Keap1 (kelch-like ECH-associated protein 1) system.76 Nrf2 is a transcription factor that regulates the expression of hundreds of genes involved in the antioxidant response, metabolism and lipid regulation, and mitochondrial function. A cytosolic inhibitor, Keap1, retains Nrf2 in the cytoplasm until activated by disease triggers. Abbreviations: ARE, antioxidant response element; Cys, sensor cysteine residues; Ub, ubiquitin. Reproduced with permission. Nezu M, Suzuki N, Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45(6):473-483. Copyright © 2017 Karger Publishers, Basel, Switzerland.
Results from studies of experimental animals support the importance of Nrf2 in controlling inflammation in CKD. Nrf2-knockout mice fed a high-glucose diet rapidly develop diabetic nephropathy.83 Impaired Nrf2 signaling in conjunction with NF-κB activation has also been shown to promote inflammation and oxidative stress in a mouse 5/6 nephrectomy (remnant kidney) model.84 Studies in experimental animals have also indicated that Nrf2 activation may protect against fibrosis. Unilateral ureteral obstruction in mice results in a rapid nuclear accumulation of Nrf2, downregulation of Keap1, and induction of Nrf2-dependent genes, which prevent increased levels of ROS markers. However, longer-term obstruction results in a progressive reduction in nuclear Nrf2 and increased oxidative stress, inflammation, fibrosis, and tubular damage.85 In addition, examples from the animal kingdom imply that antioxidant defense mechanisms with enhanced Nrf2 expression have, over millennia, evolved to protect species during extreme environmental conditions.86
Results from multiple studies in patients have also indicated that Nrf2 is involved in kidney disease.83 Biopsy of kidney tissues from patients with diabetic nephropathy demonstrated high levels of glucose-induced ROS in mesangial cells and activation of Nrf2 and downstream genes.87 Immunohistochemical results from Jiang et al87 demonstrated that Nrf2 was expressed at low levels in normal glomeruli and was upregulated in glomeruli from patients with diabetic nephropathy. In contrast, an analysis of fasting plasma and urine samples from 120 healthy controls and 180 adult patients with stages 4-5 CKD showed activation of NF-κB and upregulation of proinflammatory and pro-oxidant messenger RNA and protein expression in patients with CKD, as well as downregulation of the Nrf2-associated antioxidant gene messenger RNA and protein expression.88
A separate study investigated the Nrf2 target gene NAD(P)H: quinone oxidoreductase 1 (NQO1) as a readout parameter for Nrf2 activity in monocytes of 63 patients with CKD compared with 16 healthy controls. This study revealed a 3- to 4-fold increase in NQO1 gene expression in patients with stages 1-5 CKD (n = 29), with less robust upregulation in stage 5 dialysis patients with advanced uremia (n = 34).89 These studies demonstrate how Nrf2 may be upregulated in response to ROS and damage in early stages of kidney disease but may become suppressed as the disease progresses and inflammation increases. In this regard, it is possible that variability in results with respect to Nrf2 expression in CKD may be related to the stage of disease.
Given that Nrf2 is a key regulator of the anti-inflammatory pathway in CKD and its activation can reduce the inflammatory cascade, prevent fibrosis, and restore kidney function, it is an attractive target for novel therapies.
Conclusions
Several observational studies suggest that disorders of type IV collagen, the collagen that is defective in Alport syndrome, may play a central role in CKD in a much larger proportion of the population than previously believed. More detailed evaluation of these variants of classic Alport syndrome are required to improve our understanding of the role of type IV collagen abnormalities in progressive CKD. Given the link between type IV collagen abnormalities and kidney inflammation and fibrosis, there is renewed interest in targeted anti-inflammatory therapies in Alport syndrome and other forms of progressive CKD. Human kidney biopsy samples suggest that the anti-inflammatory Nrf2 pathway is operative in Alport syndrome, as well as other conditions leading to progressive CKD, but that its activity may be insufficient to blunt chronic inflammation. Thus, therapeutic agents that favorably modify the Nrf2 pathway have the potential to attenuate, arrest, or reverse the expected loss of kidney function in adult and pediatric patients with Alport syndrome.
Article Information
Authors’ Full Names and Academic Degrees
Bradley A. Warady, MD, Rajiv Agarwal, MD, Sripal Bangalore, MD, MHA, Arlene Chapman, MD, Adeera Levin, MD, Peter Stenvinkel, MD, PhD, Robert D. Toto, MD, and Glenn M. Chertow, MD, MPH.
Support
Reata Pharmaceuticals, Inc. The authors made the final decision on the main points and conclusions of the manuscript.
Financial Disclosures
Dr Warady: Advisory Committee for Reata Pharmaceuticals, Inc; Medical Advisory Committee of the Alport Syndrome Foundation; Consultant for Bayer AG, Akebia Therapeutics, Relypsa, Inc, Amgen, and UpToDate, Inc; Research support from the National Institutes of Health (NIH) and Baxter Healthcare. Dr Agarwal: Consultant/Advisory Committee for Relypsa, Inc, Abbvie Inc, Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim International GmbH, Celgene Corp, a Bristol-Myers Squibb Company, Daiichi Sankyo Company, Ltd, Eli Lilly and Co, Gilead Sciences, Inc, GlaxoSmithKline plc, Johnson & Johnson Services, Inc, Merck & Co, Inc, Novartis International AG, Sandoz International GmbH, ZS Pharma Inc, Akebia Therapeutics Inc, Takeda Pharmaceutical Co Ltd, Sanofi SA, Reata Pharmaceuticals, Inc, Ironwood Pharmaceuticals, Inc, Otsuka America Pharmaceutical, Inc, Opko Health, Inc, and Bird Rock Bio, Inc. Grants from the NIH and Veterans Affairs. Dr Bangalore: Advisory Committee for Reata Pharmaceuticals, Inc. Dr Chapman: Advisory Committee for Reata Pharmaceuticals, Inc, Sanofi SA, and Otsuka America Pharmaceutical, Inc; Speakers Bureau for Otsuka America Pharmaceutical, Inc; Contributor to UpToDate, Inc. Dr Levin: Advisory Committee for Reata Pharmaceuticals, Inc; Grants from Otsuka America Pharmaceutical, Inc, AstraZeneca, and Boehringer Ingelheim International GmbH. Dr Stenvinkel: Scientific Advisory Boards for Reata Pharmaceuticals, Inc, AstraZeneca, and Baxter Healthcare. Dr Toto: Executive Committee for AstraZeneca, Amgen; Consultant for Bayer AG, Boehringer Ingelheim International GmbH; Data Monitoring Committee for IQVIA Inc, Reata Pharmaceuticals, Inc, Akebia Therapeutics, Inc; Advisory Board for Relypsa Inc, Medscape (WebMD LLC), and Reata Pharmaceuticals, Inc. Dr Chertow: Board of Directors, Satellite Healthcare, Inc; Consultant/Advisor: Akebia Therapeutics, Amgen, Ardelyx, Inc, AstraZeneca, Baxter Healthcare, CloudCath, Cricket Health, DiaMedica Therapeutics, Inc, Durect Corp, DxNow, Inc, Gilead Sciences, Inc, Miromatrix Medical, Inc, Outset Medical, Reata Pharmaceuticals, Inc, Sanifit, and Vertex Pharmaceuticals Inc.
Other Disclosures
The authors are part of the CKD Advisory Committee for Reata Pharmaceuticals, Inc. Reata is evaluating bardoxolone methyl, an Nrf2 activator, for the treatment of Alport syndrome in the phase 3 CARDINAL study (ClinicalTrials.gov Identifier: NCT03019185).
Acknowledgements
Editing, referencing, and graphic support were provided by Fallon Medica LLC with special thanks to Christine Park.
Peer Review
Received March 13, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form May 30, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Savige J., Gregory M., Gross O., Kashtan C., Ding J., Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;24(3):364–375. doi: 10.1681/ASN.2012020148. [DOI] [PubMed] [Google Scholar]
- 2.Miner J.H. The glomerular basement membrane. Exp Cell Res. 2012;318(9):973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh J.H., Miner J.H. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9(8):470–477. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 5.Hudson B.G. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15(10):2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 6.Kruegel J., Rubel D., Gross O. Alport syndrome--insights from basic and clinical research. Nat Rev Nephrol. 2013;9(3):170–178. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 7.Lin X., Suh J.H., Go G., Miner J.H. Feasibility of repairing glomerular basement membrane defects in Alport syndrome. J Am Soc Nephrol. 2014;25(4):687–692. doi: 10.1681/ASN.2013070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashtan C.E. Alport’s and other familial glomerular syndromes. In: Floege J., Johnson R.J., Feehally J., editors. Comprehensive Clinical Nephrology. 4th ed. Elsevier Saunders; St. Louis, MO: 2010. pp. 560–572. [Google Scholar]
- 9.Savige J. Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol. 2014;592(18):4013–4023. doi: 10.1113/jphysiol.2014.274449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.University of North Carolina (UNC) Alport syndrome. https://unckidneycenter.org/kidneyhealthlibrary/glomerular-disease/alport-syndrome/
- 11.Kashtan C.E., Ding J., Garosi G. Alport syndrome: a unified classification of genetic disorders of collagen IV alpha345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93(5):1045–1051. doi: 10.1016/j.kint.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Kelly Y.P., Wallis L., Patil A. Alport syndrome: no evidence of improved prognosis in modern era. J Clin Nephrol Ren Care. 2018;4(1):036. [Google Scholar]
- 13.National Organization for Rare Disorders Alport syndrome. https://rarediseases.org/rare-diseases/alport-syndrome/
- 14.Groopman E.E., Marasa M., Cameron-Christie S. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380(2):142–151. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savige J., Ariani F., Mari F. Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatr Nephrol. 2019;34(7):1175–1189. doi: 10.1007/s00467-018-3985-4. [DOI] [PubMed] [Google Scholar]
- 16.Lanktree M.B., Haghighi A., Guiard E. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol. 2018;29(10):2593–2600. doi: 10.1681/ASN.2018050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leiden Open Variation Database All transcript variants in gene COL4A3. https://databases.lovd.nl/shared/variants/COL4A3
- 18.Leiden Open Variation Database All transcript variants in gene COL4A4. https://databases.lovd.nl/shared/variants/COL4A4
- 19.Leiden Open Variation Database All transcript variants in gene COL4A5. https://databases.lovd.nl/shared/variants/COL4A5
- 20.van Paassen P., van Breda Vriesman P.J., van Rie H., Tervaert J.W. Signs and symptoms of thin basement membrane nephropathy: a prospective regional study on primary glomerular disease-the Limburg Renal Registry. Kidney Int. 2004;66(3):909–913. doi: 10.1111/j.1523-1755.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwhof C.M., de Heer F., de Leeuw P., van Breda Vriesman P.J. Thin GBM nephropathy: premature glomerular obsolescence is associated with hypertension and late onset renal failure. Kidney Int. 1997;51(5):1596–1601. doi: 10.1038/ki.1997.219. [DOI] [PubMed] [Google Scholar]
- 22.Pierides A., Voskarides K., Athanasiou Y. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24(9):2721–2729. doi: 10.1093/ndt/gfp158. [DOI] [PubMed] [Google Scholar]
- 23.Voskarides K., Damianou L., Neocleous V. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18(11):3004–3016. doi: 10.1681/ASN.2007040444. [DOI] [PubMed] [Google Scholar]
- 24.US Renal Data System (USRDS) 2018 USRDS Annual Data Report: executive summary. https://www.usrds.org/2018/download/v1_00_ExecSummary_18.pdf
- 25.Jais J.P., Knebelmann B., Giatras I. X-Linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J Am Soc Nephrol. 2000;11(4):649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 26.Jais J.P., Knebelmann B., Giatras I. X-Linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14(10):2603–2610. doi: 10.1097/01.asn.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 27.Savige J., Colville D., Rheault M. Alport syndrome in women and girls. Clin J Am Soc Nephrol. 2016;11(9):1713–1720. doi: 10.2215/CJN.00580116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashtan C.E. Long-term management of Alport syndrome in pediatric patients. Pediatr Health Med Ther. 2013;4:41–45. [Google Scholar]
- 29.Yao T., Udwan K., John R. Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol. 2019;14(2):213–223. doi: 10.2215/CJN.08750718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribouval O., Boyer O., Hummel A. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int. 2018;94(5):1013–1022. doi: 10.1016/j.kint.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Morinière V., Dahan K., Hilbert P. Improving mutation screening in familial hematuric nephropathies through next generation sequencing. J Am Soc Nephrol. 2014;25(12):2740–2751. doi: 10.1681/ASN.2013080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross O., Licht C., Anders H.J. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81(5):494–501. doi: 10.1038/ki.2011.407. [DOI] [PubMed] [Google Scholar]
- 33.Temme J., Peters F., Lange K. Incidence of renal failure and nephroprotection by RAAS inhibition in heterozygous carriers of X-chromosomal and autosomal recessive Alport mutations. Kidney Int. 2012;81(8):779–783. doi: 10.1038/ki.2011.452. [DOI] [PubMed] [Google Scholar]
- 34.Cook J., Daneman D., Spino M., Sochett E., Perlman K., Balfe J.W. Angiotensin converting enzyme inhibitor therapy to decrease microalbuminuria in normotensive children with insulin-dependent diabetes mellitus. J Pediatr. 1990;117(1, pt 1):39–45. doi: 10.1016/s0022-3476(05)82441-2. [DOI] [PubMed] [Google Scholar]
- 35.Moriyama T., Tsuruta Y., Kojima C. Beneficial effect of aliskiren combined with olmesartan in reducing urinary protein excretion in patients with chronic kidney disease. Int Urol Nephrol. 2012;44(3):841–845. doi: 10.1007/s11255-011-9991-0. [DOI] [PubMed] [Google Scholar]
- 36.Wright J.T., Jr., Williamson J.D., Whelton P.K., SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wühl E., Trivelli A., Picca S., ESCAPE Trial Group Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 38.Gross O, Appel G, Simon J, et al. Progression of chronic kidney disease in Alport syndrome: interim data from the ATHENA study. Poster FR-PO636 presented at: American Society of Nephrology (ASN Kidney Week) Conference; November 15-20, 2016; Chicago, IL.
- 39.Gross O., Kashtan C.E., Rheault M.N. Advances and unmet needs in genetic, basic and clinical science in Alport syndrome: report from the 2015 International Workshop on Alport Syndrome. Nephrol Dial Transplant. 2017;32(6):916–924. doi: 10.1093/ndt/gfw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson S., Bush J.S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2019. Alport syndrome.https://www.ncbi.nlm.nih.gov/books/NBK470419/ Published January 2019. Updated November 5, 2019. [Google Scholar]
- 41.Pajari H., Sinkkonen J. Psychosocial impact of an X-linked hereditary disease: a study of Alport syndrome patients and family members. Child Care Health Dev. 2000;26(3):239–250. doi: 10.1046/j.1365-2214.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 42.Martini S., Nair V., Keller B.J. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol. 2014;25(11):2559–2572. doi: 10.1681/ASN.2013080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv W., Booz G.W., Wang Y., Fan F., Roman R.J. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tecklenborg J., Clayton D., Siebert S., Coley S.M. The role of the immune system in kidney disease. Clin Exp Immunol. 2018;192(2):142–150. doi: 10.1111/cei.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10(9):493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 46.Schlondorff D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008;74(7):860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 47.Falke L.L., Gholizadeh S., Goldschmeding R., Kok R.J., Nguyen T.Q. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat Rev Nephrol. 2015;11(4):233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 48.Noone D., Licht C. An update on the pathomechanisms and future therapies of Alport syndrome. Pediatr Nephrol. 2013;28(7):1025–1036. doi: 10.1007/s00467-012-2272-z. [DOI] [PubMed] [Google Scholar]
- 49.Liu G.H., Qu J., Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783(5):713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Lee S.A., Noel S., Sadasivam M., Hamad A.R., Rabb H. Role of immune cells in acute kidney injury and repair. Nephron. 2017;137(4):282–286. doi: 10.1159/000477181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 52.Kitching A.R., Hutton H.L. The players: cells involved in glomerular disease. Clin J Am Soc Nephrol. 2016;11(9):1664–1674. doi: 10.2215/CJN.13791215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamawaki K., Kanda H., Shimazaki R. Nrf2 activator for the treatment of kidney diseases. Toxicol Appl Pharmacol. 2018;360:30–37. doi: 10.1016/j.taap.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 54.Trimarchi H. Podocyturia: what is in a name? J Transl Int Med. 2015;3(2):51–56. doi: 10.1515/jtim-2015-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badr K.F. Filtration function in glomerulonephritis. Kidney Int. 2005;68(4):1905–1919. doi: 10.1111/j.1523-1755.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang H.C., Fogo A.B. Mechanisms of disease reversal in FSGS. Adv Chronic Kidney Dis. 2014;21(5):442–447. doi: 10.1053/j.ackd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner M.C., Campos-Bilderback S.B., Chowdhury M. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol. 2016;27(2):482–494. doi: 10.1681/ASN.2014111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imig J.D., Ryan M.J. Immune and inflammatory role in renal disease. Comp Physiol. 2013;3(2):957–976. doi: 10.1002/cphy.c120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang F.C., Chou Y.H., Chen Y.T., Lin S.L. Novel insights into pericyte-myofibroblast transition and therapeutic targets in renal fibrosis. J Formos Med Assoc. 2012;111(11):589–598. doi: 10.1016/j.jfma.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Kanasaki K., Taduri G., Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne) 2013;4:7. doi: 10.3389/fendo.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrimpf C., Duffield J.S. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20(3):297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 62.Schelling J.R. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol. 2016;31(5):693–706. doi: 10.1007/s00467-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grgic I., Campanholle G., Bijol V. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 65.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q., Verma I.M. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 67.Ryu M., Mulay S.R., Miosge N., Gross O., Anders H.J. Tumour necrosis factor-α drives Alport glomerulosclerosis in mice by promoting podocyte apoptosis. J Pathol. 2012;226(1):120–131. doi: 10.1002/path.2979. [DOI] [PubMed] [Google Scholar]
- 68.Farris A.B., Colvin R.B. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21(3):289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koga T., Kai Y., Fukuda R. Mild electrical stimulation and heat shock ameliorates progressive proteinuria and renal inflammation in mouse model of Alport syndrome. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meehan D.T., Delimont D., Cheung L. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int. 2009;76(9):968–976. doi: 10.1038/ki.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghayur A., Margetts P.J. Transforming growth factor-beta and the glomerular filtration barrier. Kidney Res Clin Pract. 2013;32(1):3–10. doi: 10.1016/j.krcp.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loeffler I., Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol Dial Transplant. 2014;29(suppl 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 73.Kashtan C. Alport syndrome: facts and opinions. F1000Res. 2017;6:50. doi: 10.12688/f1000research.9636.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pedruzzi L.M., Stockler-Pinto M.B., Leite M., Jr., Mafra D. Nrf2-keap1 system versus NF-kappaB: the good and the evil in chronic kidney disease? Biochimie. 2012;94(12):2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 75.Choi B.H., Kang K.S., Kwak M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules. 2014;19(8):12727–12759. doi: 10.3390/molecules190812727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nezu M., Suzuki N., Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45(6):473–483. doi: 10.1159/000475890. [DOI] [PubMed] [Google Scholar]
- 77.Kobayashi E.H., Suzuki T., Funayama R. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki T., Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free Radic Biol Med. 2015;88(pt B):93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Taguchi K., Maher J.M., Suzuki T., Kawatani Y., Motohashi H., Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol. 2010;30(12):3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wakabayashi N., Itoh K., Wakabayashi J. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35(3):238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 82.Li W., Khor T.O., Xu C. Activation of Nrf2-antioxidant signaling attenuates NF kappa B-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76(11):1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Sawaf O., Clarner T., Fragoulis A. Nrf2 in health and disease: current and future clinical implications. Clin Sci (Lond) 2015;129(12):989–999. doi: 10.1042/CS20150436. [DOI] [PubMed] [Google Scholar]
- 84.Kim H.J., Vaziri N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298(3):F662–F671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 85.Rinaldi Tosi M.E., Bocanegra V., Manucha W., Gil Lorenzo A., Vallés P.G. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chaperones. 2011;16(1):57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stenvinkel P., Meyer C.J., Block G.A., Chertow G.M., Shiels P.G. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2–related factor 2—lessons from evolution, the animal kingdom and rare progeroid syndromes [published online ahead of print July 13, 2019]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfz120 [DOI] [PMC free article] [PubMed]
- 87.Jiang T., Huang Z., Lin Y., Zhang Z., Fang D., Zhang D.D. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(4):850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen D.Q., Cao G., Chen H. Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/β-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol. 2017;12:505–521. doi: 10.1016/j.redox.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen J., Rasmussen M., Dong Q.R. Expression of the Nrf2 target gene NQO1 is enhanced in mononuclear cells in human chronic kidney disease. Oxid Med Cell Longev. 2017;2017:9091879. doi: 10.1155/2017/9091879. [DOI] [PMC free article] [PubMed] [Google Scholar]