Abstract
Background:
In this retrospective study, we evaluated the patterns of postoperative recovery for patients who were initially paraplegic before the excision of thoracic spine meningiomas. We also determined how the various prognostic factors impacted outcomes.
Methods:
Twenty patients with paraplegia underwent surgical excision of thoracic spine meningiomas at 2016– 2019. Patients’ demographics, clinical, radiological data, operative details, histopathology, and postoperative complications were recorded; patients were reassessed at 6 months and 1 year postoperatively.
Results:
Fourteen patients improved postoperatively, becoming, ambulatory with/without assistance; only six remained paraplegic. Poor prognostic factors for postoperative motor recovery included larger tumor size, longer duration of preoperative symptoms/paraplegia, and greater severity of sensory loss.
Conclusion:
For 6/20 patients with thoracic meningiomas, poor postoperative recovery of motor function correlated with larger tumor size, longer duration of preoperative symptoms/paraplegia, and more severe sensory loss.
Keywords: Meningioma, Outcome, Paraplegic, Spinal, Surgery

INTRODUCTION
Spinal meningiomas are benign slow-growing tumors that usually present with back pain in 50% of cases. Clinical findings vary including sensorimotor deficits, radicular pain, and sphincter dysfunction.[5,9] This wide range of clinical findings may cause difficulties in timely diagnosis. Surgical excision is the cornerstone of treatment with favorable outcomes in most cases. Nevertheless, profound preoperative motor weakness has been suggested as a predictor associated with poor results.[2,6] The improvement in microsurgical techniques, neuroimaging technologies, and rehabilitation programs has further improved postoperative outcomes.[1,8] However, since some patients presented late with paraplegia, it is extremely difficult to predict the prognosis.
Here, we retrospectively analyzed the outcomes for 20 patients who were paraplegic before the resection of thoracic meningiomas. Our aim was to identify the poor prognostic factors negatively impacting postoperative motor recovery.
MATERIALS AND METHODS
After approval from the Institutional Review Board, we retrospectively analyzed 20 patients all of whom were paraplegic before the surgical resection of thoracic spine meningiomas (2016–2019). Patients were followed for at least 1 postoperative year. Preoperatively, multiple clinical (e.g., including genetic evaluation for Type 2 neurofibromatosis), radiological (e.g., MR studies documenting tumor location), and surgical parameters were recorded for all 20 patients.
Demographic and clinical data
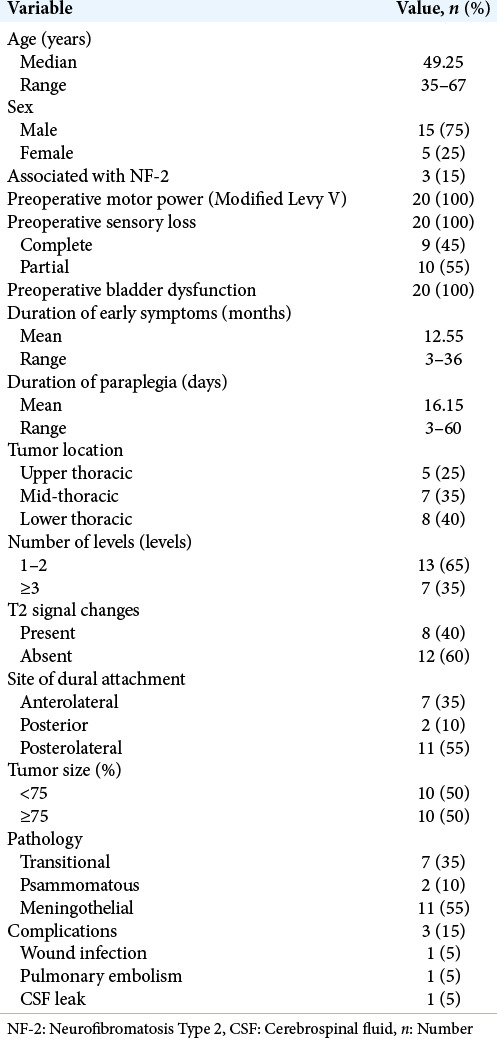
Patients averaged 49.25 years of age and 15 were female. The accompanying diagnosis of neurofibromatosis type 2 was established in 3 cases (15%). Initial symptoms were present for an average of 12.55 months, while the duration of preoperative paraplegia averaged 16.15 days (range 3–60 days). All patients presented with complete motor paraplegia and sphincter dysfunction. Sensory loss was partial in 11 cases (55%) and complete in the remainder (9 cases: 45%) [Table 1].
Table 1:
Demographic, clinical, radiological, and pathological data of the patients.
Radiological data for MR findings of thoracic meningiomas
Tumors were assigned three locations; five T1-T4 upper thoracic, seven T5-T8 mid-thoracic, and eight T9-T12 lower thoracic lesions. In 10 cases, tumors occupied more than 75% of the spinal canal. In addition, 13 tumors (65%) involved 1 or 2 levels, while 7 tumors (35%) extended over three levels or more levels. Dural attachments were posterior (2 cases), anterolateral (7 cases), and posterolateral (11 cases). On MR, in 8 cases, focal hyperintense intramedullary T2W signals were seen at the tumor levels [Table 1].
Following typical laminectomies, patients’ neurological motor outcomes were assessed up to 1 year postoperatively.
Statistical analysis
Data were coded and entered using the Statistical Package for the Social Sciences version 26 (IBM Corp., Armonk, NY, USA). Comparisons between quantitative variables were done using the nonparametric Mann–Whitney U-test. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is <5. P < 0.05 was considered as statistically significant.
RESULTS
Surgical and pathological data
Through midline laminectomies, all intradural extramedullary tumors were completely excised utilizing central debulking techniques followed by careful arachnoid dissection from the sites of dural attachment [Figure 1]. Postoperatively, the length of hospital stay ranged from 3 days to 21 days (mean 5.25 days). Histopathological examinations confirmed benign meningiomas in all 20 cases; lesions were categorized as transitional (7 cases), meningothelial (11 cases), and psammomatous (2 cases).
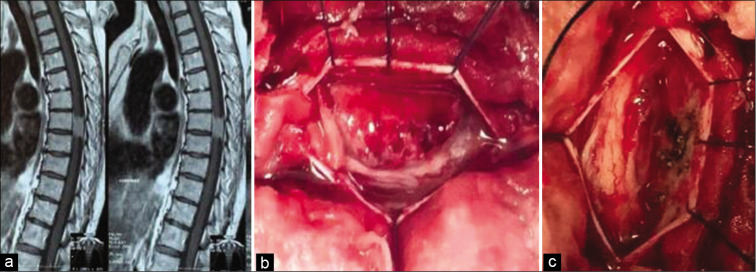
Figure 1:
(a) Sagittal view of MRI thoracic spine with contrast showing T7 meningioma (b) Intraoperative view showing a spinal meningioma compressing the spinal cord (c) Intraoperative view after complete excision of the lesion and coagulation of the dural attachment.
Postoperative complications
There were no mortalities, but three postoperative complications (15%); one superficial wound infection (i.e., resolved on antibiotics), one cerebrospinal fluid leak (i.e., treated with lumbar drain), and one pulmonary embolism (i.e., routinely managed).
Outcomes
Six months and 1 year postoperatively, eight patients fully recovered motor function; six could walk with assistance (Grade I Modified Levy Score), while six remained unchanged. Of interest, sensory function improved in 12 patients (60%), while 10 patients (50%) regained sphincter control.
Major predictors of poor outcomes
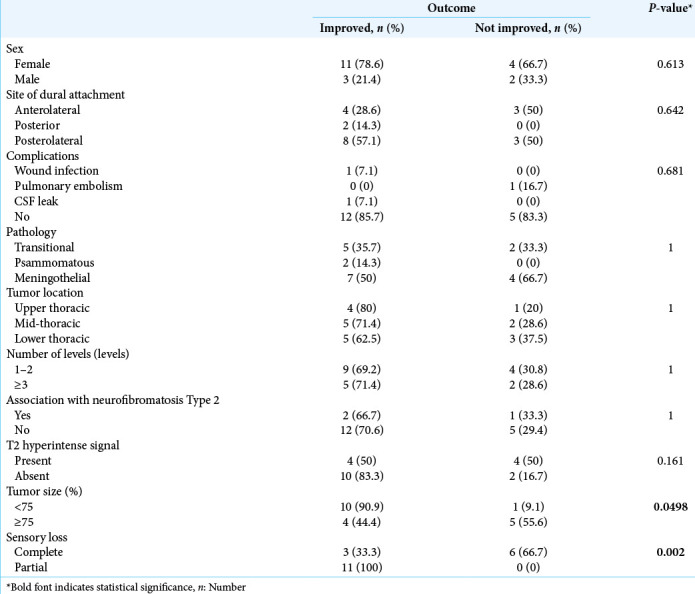
Poor prognostic factors for postoperative recovery of motor/ sensory function for patients with preoperative paraplegia attributed to spinal meningiomas included longer duration of preoperative paraplegia and sensory deficits, and larger tumor size [Table 2].
Table 2:
Prognostic factors for motor power improvement.
DISCUSSION
Spinal meningiomas account for 25–46% of all primary spinal cord tumors that most typically involve the thoracic spine in females. The duration of symptoms may vary from several months to years.[4,10]
Delays in diagnosis and surgery may lead to irreversible neurological injury.[2,11] We found a significant negative correlation between the preoperative duration and severity of motor and sensory deficits, and quality of outcomes; other reports do not support these findings.[2] Of interest, all patients who experienced recovery of motor function had surgery within 2 weeks following the onset of paraplegia, while partial rather than complete sensory loss was a positive prognostic sign. Larger tumors are typically associated with poor outcomes as validated in our series.[7] However, there was no significant correlation between the number of levels impacted by tumor and outcome.[7]
Prognostic factors: Motor deficit and preoperative MR findings
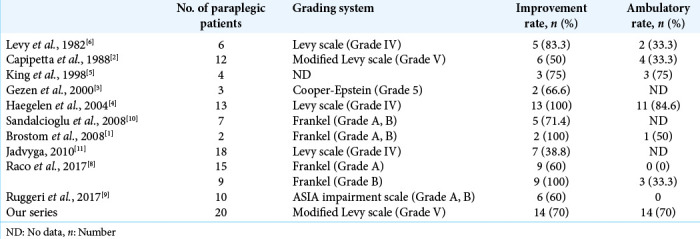
In our study, 70% of the patients improved after surgery (e.g., all having been operated upon within 2 weeks of the onset of paraplegia) and became ambulatory with or without assistance. Nevertheless, other authors have found that poor preoperative neurological status was an independent predictor for poor outcomes [Table 3].[2,3] Further, although Maiti et al. determined that preoperative MR T2 hyperintense cord signals were poor prognostic findings, this was not corroborated in our series.[7]
Table 3:
Review of literature.
Histopathology
In our study, all the spinal meningiomas were benign, meningothelial lesions (55%) (World Health Organization Grade I); this finding was in accordance with the literature.[8] Notably, we found no significant correlation between the histopathological type and the postoperative outcome.
CONCLUSION
About 70% of patients who are paraplegic for up to 2 weeks before resection of thoracic meningiomas may exhibit adequate delayed postoperative recovery.
Footnotes
How to cite this article: Ashry A, Mahmoud AT, Gabr M. Delayed recovery from paraplegia following resections of thoracic meningiomas. Surg Neurol Int 2020;11:321.
Contributor Information
Ahmed Ashry, Email: elashry10@yahoo.com.
Ayman Tarek Mahmoud, Email: aymantarek84@gmail.com.
Mohamed Gabr, Email: mgabr082@gmail.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Boström A, Bürgel U, Reinacher P, Krings T, Rohde V, Gilsbach JM, et al. A less invasive surgical concept for the resection of spinal meningiomas. Acta Neurochir (Wien) 2008;150:551–6. doi: 10.1007/s00701-008-1514-0. [DOI] [PubMed] [Google Scholar]
- 2.Ciappetta P, Domenicucci M, Raco A. Spinal meningiomas: Prognosis and recovery factors in 22 cases with severe motor deficits. Acta Neurol Scand. 1988;77:27–30. doi: 10.1111/j.1600-0404.1988.tb06969.x. [DOI] [PubMed] [Google Scholar]
- 3.Gezen F, Kahraman S, Canakci Z, Bedük A. Review of 36 cases of spinal cord meningioma. Spine (Phila Pa 1976) 2000;25:727–31. doi: 10.1097/00007632-200003150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Haegelen C, Morandi X, Riffaud L, Amlashi SF, Leray E, Brassier G. Results of spinal meningioma surgery in patients with severe preoperative neurological deficits. Eur Spine J. 2005;14:440–4. doi: 10.1007/s00586-004-0809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King AT, Sharr MM, Gullan RW, Bartlett JR. Spinal meningiomas: A 20-year review. Br J Neurosurg. 1998;12:521–6. doi: 10.1080/02688699844367. [DOI] [PubMed] [Google Scholar]
- 6.Levy WJ, Jr, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57:804–12. doi: 10.3171/jns.1982.57.6.0804. [DOI] [PubMed] [Google Scholar]
- 7.Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: Clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 8.Raco A, Pesce A, Toccaceli G, Domenicucci M, Miscusi M, Delfini R. Factors leading to a poor functional outcome in spinal meningioma surgery: Remarks on 173 cases. Neurosurgery. 2017;80:602–9. doi: 10.1093/neuros/nyw092. [DOI] [PubMed] [Google Scholar]
- 9.Ruggeri AG, Fazzolari B, Colistra D, Cappelletti M, Marotta N, Delfini R. Calcified spinal meningiomas. World Neurosurg. 2017;102:406–12. doi: 10.1016/j.wneu.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–41. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subačiūtė J. Spinal meningioma surgery: Predictive factors of outcome. Acta Med Litu. 2010;17:133–6. [Google Scholar]