Abstract
New Schiff bases {N'-(phenyl(pyridin-2-yl)methylene) isonicotinohydrazide (L1H), N1-(naphthalen-1-yl)-N2-(phenyl(pyridin-2-yl) methylidene) ethane-1,2-diamine (L2H), N-(6-chlorobenzo[d]thiazol-2-yl)-1-phenyl-1-(pyridin-2-yl) methanimine (L3H)}were synthesized by reaction of 2-benzoylpyridine with different amines (2-amino-6-chlorobenzothiazole, isonicotinohydrazide and N1-(naphthalen-1-yl)ethane-1,2-diamine) and characterized by 1H-NMR, 13C-NMR, IR mass spectroscopy and elemental analysis. The compounds were assayed by the disc diffusion method for anti-bacterial against five pathogenic bacteria species (Staphylococcus aureus, Micrococcus luteus, Staphylococcus pyogenes, Bacillus subtilis, and E. coli). All prepared Schiff bases showed good activity compared to positive control (streptomycin), Moreover the L3H showed the highest activity against S. aureus, and M. luteus than the other compounds and streptomycin. In additional molecular docking studies with 3-chymotrypsin-like protease (3CLpro), the essential enzyme for SARS-CoV-2 proliferation. The rest of compounds have shown promising results as 3CLpro inhibitors interacting with the active sites of the enzymes. Finally, DFT 's estimated electrostatic molecular potential results were used to illustrate the molecular docking findings. The DFT calculations showed that L3H has the highest dipole moment and electrophilicity index. Interestingly, L2H of the largest energy gap ∆E = 2.49 eV, there are several hydrophilic interactions that could facilitate the binding with the receptors. All of these parameters could be shared to significantly affect the protein sites of binding affinity with different extent.
Keywords: COVID 19, DFT calculations, Schiff bases, Molecular docking
1. Introduction
Novel coronavirus (COVID-19) has arisen as an infectious disease and spread rapidly throughout the world and is transmitted mainly through contact with contaminated saliva droplets or by nose discharge while Patients diagnosed with cough or sneeze. Human coronaviruses were first described in the mid-1960s [1, 2]. Coronaviruses belong to the Coronaviridae family, a family of single stranded enveloped- positive sense RNA viruses. In addition, the Coronaviridae family was divided into four genera: α, β, γ, and δ. Coronaviruses of ubiquitous and genera commonly infect mammals and humans while birds are primarily infected by the form and generations. That specification is in line with coronavirus phylogenetic analysis and genome structure [3]. Computational features of the novel coronavirus [4, 5] or more generally new testable theories for standard drugs involved. A virtual screening technique has recently been performed to identify the active site on the viral protease for the binding of many natural compounds by molecular docking and cell-based assays [6]. Our research group recently concentrated on determining the molecular geometry of synthesized materials by comparing the desired properties from experimental evaluation to estimated parameters from computational calculations [7].
Schiff bases have been reported to possess a wide range of biological properties such as being anti-tumor, antiviral, anti-bacterial, anti-fungal and anti-inflammatory etc. [8] Schiff bases containing N, S, and O atoms in structures show an important role in biological systems because they have unusual electronic properties [9].
2-phenylquinazoline-4(3)H-one Schiff bases are confirmed to have antiviral activity against certain strains of viruses, such as feline corona virus, influenza Viruses, and type 1 and type 2 herpes simplex viruses [10]. From published literature, the antiviral ability of these Schiff bases is evident and thus further focused work will help to discover and improve new potential lead compounds to use them as drug candidates.
Recently a novel SARS-CoV-2 virus properly originated from bat was reported, cause the severe acute respiratory syndrome, known as COVID-19 [11], [12], [13]. The enzyme 3-chymotrypsin-like protease (3CLpro) cleaves at least 11 sites on the polyproteins translated from the viral RNA of SARS-CoV-2. Thus, the compounds having ability to inhibit this enzyme can be considering as an effective therapeutic agent for COVID-19. This work describes the synthesis and spectroscopic characterization of new Schiff bases and evaluated as anti-bacterial, besides, the synthetic compounds have also been studied as 3CLpro inhibitors.
2. Experimental
2.1. Materials and methods
2-amino-6-chlorobenzothiazole, isonicotinohydrazide, N 1-(naphthalen-1-yl)ethane-1,2-diamine, 2-benzoylpyridine and solvents were obtained through Sigma-Aldrich, and used without any further purification. 1H and 13C NMR spectra were recorded on Bruker Avance 400 MHz NMR spectrometer in DMSO as deuterated solvent. The melting point was measured using SMP30 melting point apparatus. IR spectra were recorded on a Shimadzu FT-IR 8400 spectrophotometer using KBr discs in 400-4000 cm−1 range.
2.2. Preparation of Schiff bases
2.2.1. Preparation of N'-(phenyl(pyridin-2-yl)methylene)isonicotinohydrazide (L1H)
A colorless solution of 2-benzoylpyridine (0.730 g, 3.985 mmol) in EtOH (15mL) was added to an ethanol solution of isonicotinohydrazide (0.547g, 3.985 mmol) in (20 mL) with some drops of glacial acetic acid. The mixture was refluxed for 6 h, the formed solution was filtered and left aside to cool slowly, then the dark creamy ppt. was separated and recrystallized from hot ethanol and dried under vacuum.
L1H. Dark creamy powder. Yield: (1.16 g, 91%). M.p. 182-183°C. Anal. Calc. for C18H14N4O, (%):C, 71.51; H, 4.67; N, 18.53. Found C, 71.69; H, 4.53; N, 18.62. IR (KBr, cm−1): 3110m(NH), 3049m, 2858m, 1668(C=O), 1633s (C=N), 1554s(C=C), 1487w, 1407s, 1330s, 1135s, 995s, 844s, 744s.1H NMR (δ, ppm, DMSO-d 6): δ 11.53(s, 1H, NH), 8.92(d, J = 7.2 Hz, 2H, H15,16), 8.74 (dd, J = 7.4, 2.5 Hz, 1H, H1), 8.12 (dd, J = 7.4, 1.3 Hz, 1H, H3), 7.92(d, J = 7.2 Hz, 2H, H14,17), 7.71 (d, J = 7.6 Hz, 1H, H4), 7.62 (d, J = 7.5 Hz, 1H, H2), 7.39 (m, 5H, H8-12). 13C NMR (δ, ppm, DMSO-d 6): δ 169.53 (C=O), 161.92 (C6), 152.13(C5), 149.73(C1), 147.81(C15,16), 140.29(C7), 132.63(C3), 131.04(C8,12), 129.78(C10), 128.12(C9,11), 123.22 (C14,17), 119.39(C4). ESI-MS (m/z), calc. C18H14N4O, 302.116, found, 302.117.
2.2.2. Preparation of N1-(naphthalen-1-yl)-N2-(phenyl(pyridin-2-yl)methylidene) ethane-1,2-diamine (L2H)
L2H was prepared by a similar method to that of L1H using N 1-(naphthalen-1-yl)ethane-1,2-diamine in place of isonicotinohydrazide.
L2H. Light brown-yellow powder. Yield: (1.20 g, 86%). M.p. 167-168°C. Anal. Calc. for C24H21N3 (%):C, 82.02; H, 6.02; N, 11.96. Found C, 82.17; H, 6.23; N, 12.18. IR (KBr, cm−1): 3131m (NH), 3087m, 2945w(C-H), 1639s (C=N), 1529s(C=C), 1452s, 1311s, 1164w, 927m, 811s, 690s, 607m. 1H NMR (δ, ppm, DMSO-d 6): δ 8.72 (ddd, J = 6.7, 1.4 Hz, 1H, H2), 8.07 (ddd, J = 7.7, 1.7 Hz, 1H, H3), 7.98 (m, 4H, H2,4,14,15), 7.67 (m, 3H, H9,10,11), 7.54 (t, J = 7.7 Hz, 2H, H8,12), 7.43 (s, 1H, NH), 7.32 (d, J = 6.8 Hz, 2H, H19,22), 7.21 (d, J = 8.1 Hz, 1H, H14), 7.01(dd, J = 7.7 Hz, 2H, H20,21), 2.302 (s, 4H, NH-CH2), 2.295 (s, 2H, CH2C=N). 13C NMR (δ, ppm, DMSO-d 6): δ 165.28 (C6), 154.13(C13), 150.29(C5), 148.21(C1), 137.30(C7), 135.64(C17), 132.639(C3), 130.64(C18), 130.22(C8,12), 129.50(C10), 127.85(C9,11), 126.37(C16,19), 126.04(C15,22), 123.78(C20,21), 120.44(C4), 117.04(C14), 21.48 (CH2-N=C) 20.36 (CH2-NH). ESI-MS (m/z), calc. C24H21N3, 351.174, found, 351.176.
2.2.3. Preparation of N-(6-chlorobenzo[d]thiazol-2-yl)-1-phenyl-1-(pyridin-2-yl) methanimine (L3H)
L3H was prepared by a similar method to that of L1H using 2-amino-6-chlorobenzothiazole in place of isonicotinohydrazide.
L3H. brownish yellow powder. Yield: (0.93 g, 76%). M.p. 156-158°C. Anal. Calc. for C19H12ClN3S, (%):C, C, 65.23; H, 3.46; N, 12.01; S, 9.16. Found C, 65.15; H, 3.28; N, 12.31; S, 9.23. IR (KBr, cm−1): 3086m(CH), 1627s (C=N), 1542s(C=C), 1415s, 1315s, 1274s, 1141s, 1088s, 698s, 651m.1H NMR (δ, ppm, DMSO-d 6): δ 8.80 (dd, J = 7.8, 1.7 Hz, 1H, H1), 8.12 (s, 1H, H18), 7.98 (dd, J = 7.6, 1.5 Hz, 1H, H3), 7.72(d, J = 7.4 Hz, 2H, H4), 7.68(d, J = 7.6 Hz, 1H, H2), 7.56 (m, 4H, H8,12,15,16), 7.36(m, 3H, H9-11). 13C NMR (δ, ppm, DMSO-d 6): δ 171.78 (C13), 162.45 (C6), 153.40(C5), 148.92(C1), 147.81(C14), 141.55(C7), 134.07(C3), 133.65(C19), 131.73(C8,12), 128.96(C10), 128.03(C9,11), 126.56(15), 123.22(C16), 120.78(C18), 118.77(C4), 116.78(C17). ESI-MS (m/z), calc. C19H12ClN3S, 349.044, found, 349.033.
2.3. Antibacterial studies
The anti-bacterial activities of the complexes were tested by agar disc diffusion method originally described by Bauer [14] against five bacteria types, Staphylococcus aureus, Micrococcus luteus, Staphylococcus pyogenes, Bacillus subtilis, and E. coli in (50, 100, and 200 μg) of each compound compared with streptomycin as positive control.
Minimum inhibitory concentration (MIC) of the Schiff bases were tested in in Nutrient broth for bacteria by the two-fold serial dilution method [15]. The bacterial suspension was attuned with sterile saline to a concentration of 1*10−5 - 10−6 CFU. The tested compounds and standard control (Streptomycin) were prepared by two-fold serial dilution to obtain the essential concentrations of 200, 100, 50, 25, 12.5 and 6.25 μg/mL. The tubes were incubated in incubators at 37 °C. The MICs were recorded by visual observations after 24 h.
2.4. Optimization and molecular docking
The three-dimensional structures of the L1H, L2H, and L3H were generated and optimized by Gaussian 09 program package [16] software. The crystal structure of 3CLpro of SARS-CoV-2 was obtained from the Protein Data Bank database (PDB ID: 6Y2E). Molecular docking experiments were carried out using the AutoDock Vina tool plugin UCSF Chimera software (v 1.14), adopting the default values for the parameters, and a grid box (-16 × -24.0 × 17) Å was centered at (35, 65, 65) Å. In order to represents the real environment, water was added as a solvent, with accessible surface area of 14358.5. The predicted binding affinity score was explored utilizing the View Dock tool. The binding to active sites and images were processed by the UCSF Chimera [17], [18], [19].
3. Results and discussion
3.1. Synthesis of Schiff bases
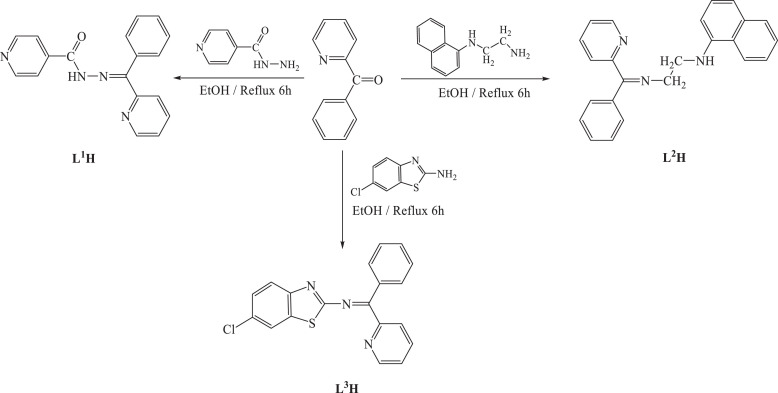
The Schiff bases have been synthesized by condensation of 2-benzoylpyridine with 2-amino-6-chlorobenzothiazole or isonicotinohydrazide or N 1-(naphthalen-1-yl)ethane-1,2-diamine to afford a dark creamy with L1H, light brown-yellow in L2H, and a brownish yellow with L3H ( Scheme 1 ). The prepared Schiff bases are stable in air and soluble in EtOH, DMSO and DMF. The compounds were characterized by using elemental analysis, 1H, 13C NMR, and IR techniques. All attempts to get crystals suitable for X-ray diffraction studies were unsuccessful.
Scheme 1.
Preparation of Schiff bases L1H, L2H and L3H
3.2. Characterization of Schiff bases
3.2.1. IR Spectra
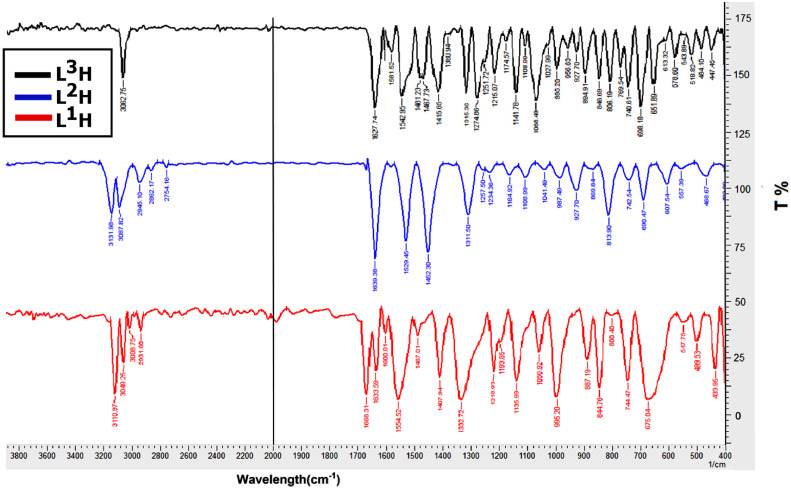
The IR spectra of the prepared Schiff bases (Fig. 1 ) displayed a strong band within the 1627-1639 cm−1 range for the azomethine group υ(C=N) [20], [21], [22]. And disappeared the υ(C=O) of 2-phenylpyridine, and υ(NH2)sy, asy stretching vibration of amine, indicates the formation of the proposed compounds. The υ(C=C) vibration of the aromatic rings showed within the 1529-1554 cm−1 range [22]. The spectra of L1H and L2H displayed a medium band at 3110cm−1 and 3131cm−1assigned to the υ(N-H). And the L1H spectrum appeared the υ(C=O) of isonicotine group at 1668cm−1 [23] Also the spectrum of L2H showed the stretching vibration of aliphatic group at 2945cm−1 [22]. Other vibrations are listed in experimental section.
Fig. 1.
IR spectra of the prepared Schiff bases
3.2.2. 1H and 13CNMR spectra
The 1H-NMR spectrum of L1H in DMSO-d6 displayed the NH proton at δ11.53 ppm as a singlet peak. And four doublet peaks at δ8.92ppm, δ7.92ppm, δ7.71ppm and δ7.62ppm, assigned to the H15,16, H14,17, H4, and H2, corresponding to 2,1,2,1 protons respectively. Also the spectrum showed two doublet of doublets peaks at δ8.74ppm, and δ8.12ppm for the H1 and H3, respectively. A multiplet peak was showed at δ7.39ppm assigned to protons in position 8-12 (phenyl ring) (Fig. SI1, supporting information).
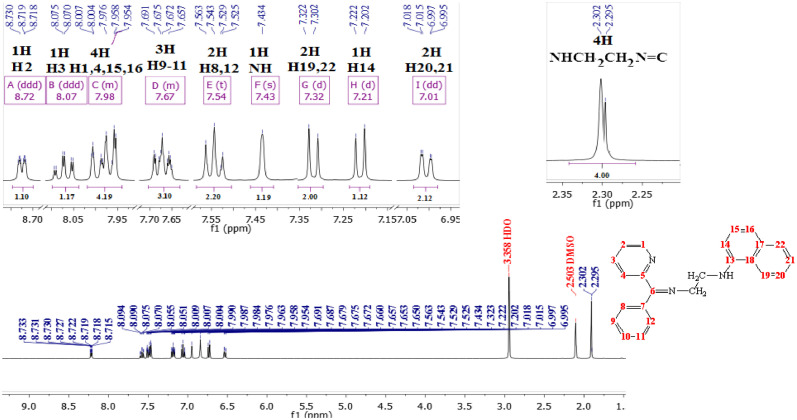
The 1H-NMR spectrum of L2H (Fig 2 ), clearly display the phenyl protons belong to the pyridyl, phenyl and naphthyl rings. A two doublet peaks displayed at δ7.21ppm and δ7.32ppm due to a H14 and H19,22, corresponding to one and two protons, respectively. And two doublet of doublet of doublets at δ8.72ppm, and δ8.07ppm for the H2 and H3, respectively. Also the spectrum displayed two multiplets peaks at δ7.98ppm and δ7.67ppm, due to the protons in position (1,4,15,16) and (9-11), respectively. The protons in position 20 and 21 appeared as doublet of doublets at δ7.01ppm (J HH = 7.8 Hz). And the H8,12 showed as triplet peak at δ7.54ppm with coupling constant to the neighboring protons (J HH = 7.7 Hz). The NH proton was showed at δ7.43 ppm as a singlet peak, whereas the methylene groups displayed as two singlet at δ2.302 and 2.295 ppm, due to the NH-CH 2 and CH 2N=C, respectively, corresponding to four protons.
Fig. 2.
1H NMR spectrum of the L2H in DMSO-d6
The 1H-NMR spectrum of L3H in DMSO-d6 showed two doublet of doublets peaks at δ8.80ppm, and δ7.98ppm for the H1 and H3, respectively. And two doublet peaks at δ7.72ppm, and δ7.56ppm, due to the H4 and H2, corresponding to one proton of each peak, respectively. Also the spectrum showed two multiplet peaks were showed at δ7.56ppm and δ7.36ppm due to protons in position (8,12,15,16) and (9-11). The H18 was showed as a singlet peak at δ8.12ppm (Fig. SI 3, supporting information).
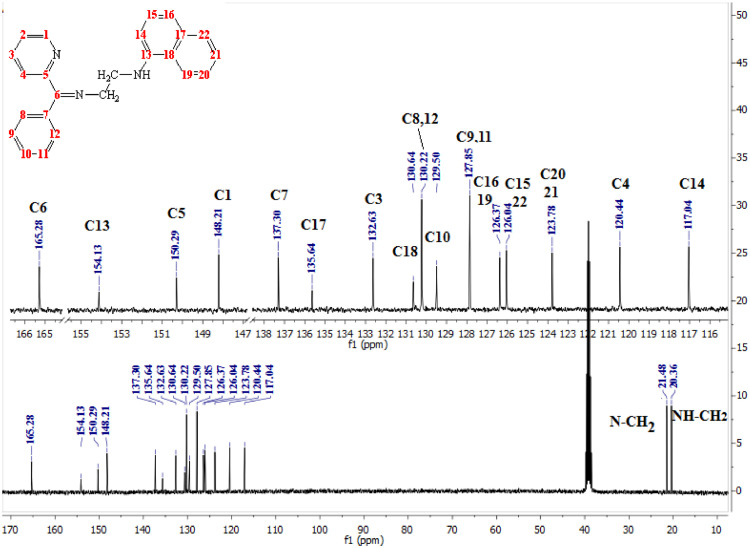
The above results were supported by 13C NMR and elemental analysis data (Fig. 3 ). The δC of the azomethine group (C6) of the new Schiff bases appeared at δ161.92ppm, 165.28ppm, and 162.45ppm for the L1H, L2H and L3H respectively. Whereas the C1 displayed at δ148.21ppm, 149.73ppm, and 148.92ppm, respectively. And that the methylene group of the L2H appeared at δ21.48ppm, 20.36ppm assigned to (CH2-N=C) and (CH2-NH) respectively. The spectrum of L1H displayed the chemical shift of carbonyl group at δ 169.53ppm. Other 13C chemical shifts are listed in experimental section(Fig. SI 2 and 4, supporting information).
Fig. 3.
13C NMR spectrum of the L2H in DMSO-d6
3.3. Antibacterial activity
Antibacterial activity of the Schiff bases (L1H, L2H and L3H) are summarized in Table 1 . The data were obtained against five types of the pathogen bacteria (Staphylococcus aureus, Micrococcus luteus, Staphylococcus pyogenes, Bacillus subtilis, and Escherichia coli in (50, 100, and 200 μg/Disc) of each compound compared with streptomycin as positive control. The diameter of the inhibitory zone (DIZ) was compared to that of streptomycin, which is the positive control. The compounds displayed good activity against the bacteria. The Schiff base L3H is more active for the tested bacteria compared with other Schiff bases (L1H, and L2H), whereas the L2H, exhibits less activity against all the tested bacteria. Also designate the increasing concentration of Schiff bases from 50 to 200 μg/disc, the inhibition effect is increased.
Table 1.
Antibacterial activity (diameter of inhibition zone (cm)) of Schiff bases against five four different bacterial species
| Comp. | Conc. μg/disc | Micrococcus luteus | Staphylococcus aureus | Staphylococcus pyogenes | Bacillus subtilis | Escherichia coli |
|---|---|---|---|---|---|---|
| L1H | 50 | 9 | 12 | 11 | 8 | 10 |
| 100 | 14 | 15 | 14 | 12 | 13 | |
| 200 | 19 | 19 | 18 | 17 | 17 | |
| L2H | 50 | 9 | 8 | 10 | 9 | 9 |
| 100 | 13 | 12 | 12 | 12 | 11 | |
| 200 | 16 | 17 | 15 | 17 | 14 | |
| L3H | 50 | 12 | 14 | 13 | 10 | 11 |
| 100 | 16 | 20 | 17 | 15 | 14 | |
| 200 | 19 | 24 | 18 | 19 | 18 | |
| Streptomycin | 100 | 22 | 19 | 21 | 19 | 23 |
The MICs are given in Table 2 . Schiff base L3H displayed the highest effective against the tested bacteria S. aureus, M. luteus, S. pyogenes, B. subtilis with MIC values of 6.25, 25, 25, and 25μg/mL, respectively, but less active against E. coli, with MIC value 100μg/mL. The antibacterial activity of L3H is more active than the other Schiff bases (L1H, and L2H), suggesting the L3H compound has a (Cl and S) atoms in the structure, these atoms possibly via enhanced membrane transport into the cell or some other mode of action [24, 25].
Table 2.
Minimum inhibitory concentration (MIC) in (μg/mL) of Schiff bases.
| Compounds | Micrococcus luteus | Staphylococcus aureus | Staphylococcus pyogenes | Bacillus subtilis | Escherichia coli |
|---|---|---|---|---|---|
| L1H | 50 | 25 | 100 | 50 | 100 |
| L2H | 25 | 50 | 50 | 100 | 50 |
| L3H | 25 | 12.5 | 25 | 25 | 100 |
| Streptomycin | 12.5 | 3.12 | 6.25 | 12.5 | 6.25 |
3.4. Theoretical studies
3.4.1. DFT calculations studies
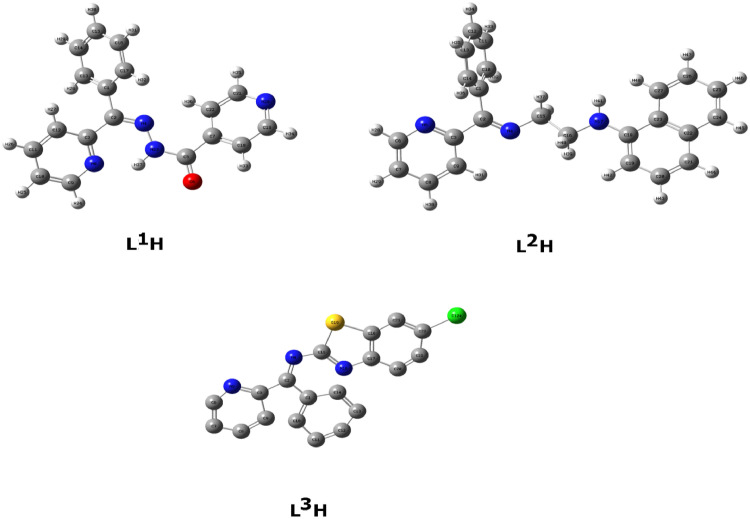
At B3LYP 6-311G (d,p) basis set, the theoretical DFT calculations were carried out in gas phase by DFT method. The results of the theoretical DFT calculations for all the compounds under investigation (Figs 4 –6 ) showed the non-planarity. Table 3 summarizes the approximate calculations of DFT for Electronic Energy, Heat Capacity, Entropy (S), Thermal Energy, polarizability, and dipole moment of L1H, L2H, and L3H.
Fig. 4.
Optimized geometrical structures of L1H, L2H, and L3H with atomic numbering.
Fig. 6.
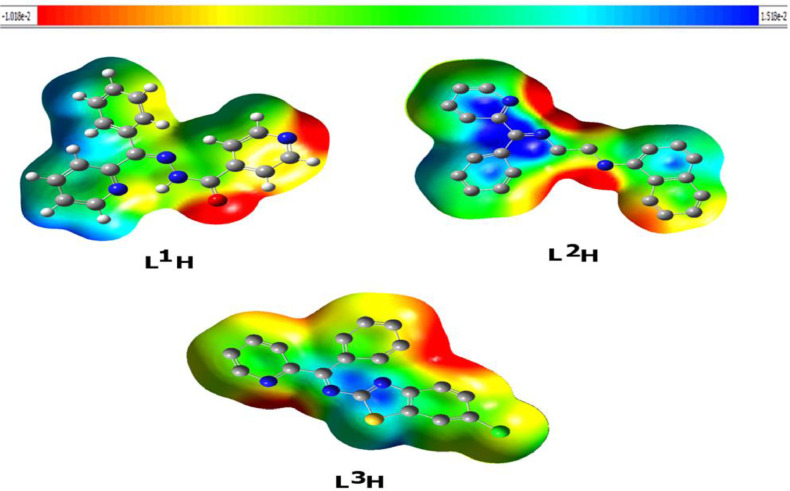
Molecular electrostatic potentials (MEP) of the L1H, L2H, and L3H compounds
Table 3.
Electronic Energy (Hartree/Particle), Heat Capacity (Cv), Entropy (S) (cal/mol-kelvin), Thermal Energy polarizability α (a.u.), and dipole moment (Debye) of L1H, L2H, and L3H
| Parameter | L1H | L2H | L3H |
|---|---|---|---|
| Electronic Energy | -988.49 | -1091.40 | -1745.44 |
| Total Dipole Moment | 7.99 | 2.59 | 3.33 |
| Polarizability (α) | 235.49 | 285.79 | 268.28 |
| E (Thermal) | 192.294 | 261.24 | 69.58 |
| Heat Capacity (Cv) | 69.839 | 86.79 | 67.89 |
| Entropy (S) | 142.655 | 166.53 | 134.04 |
DFT calculated data showed that the L1H, L2H, and L3H dipole moment are in the order of L2H < L3H < L1H. The high dipole moment L1H may demonstrate their binding pose within a specific target protein and its results from the predicted binding affinity to be discussed in the next molecular docking section. The polarizability of the materials depends on how a charge approach influences the resistance of the molecular system electron cloud. Additionally, it depends on the nature of the compounds and the scale of the molecular structure. Large-sized molecules are more polarizable compounds. L1H is the smallest in size and has the least polarizability (235.49 a.u.), but L2H with the greatest complexity is expected to have the greatest polarizability, (285.79 a.u.). Ultimately, heat capacity is the one with the most complex set of concepts and the richest set of consequences for protein folding and binding, of all the major thermodynamic variables calculated for proteins. It gives entropy and enthalpy a temperature dependency which will alter their signs and determine which of them will dominate. The heat capacity order is as follows: L3H < L1H < L2H. The unfolding protein typically has a positive Cp, which results in optimum stability and often cold denaturation [26].
3.4.2. Frontier molecular orbitals
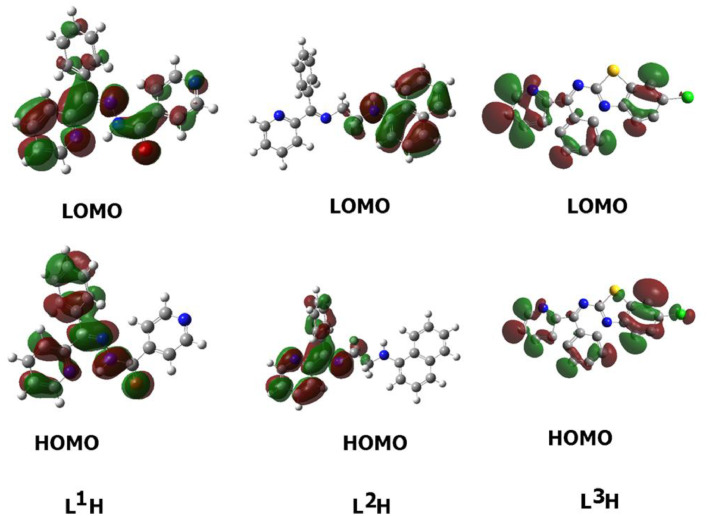
The frontier molecular orbitals (FMO) can provide objective qualitative information about the HOMO electrons being susceptible to transfer to the LUMO. In addition, HOMO and LUMO are very useful quantum chemical parameters to assess the molecules' reactivity and are used to measure other parameters, such as the descriptors for chemical reactivity. The energies of the studied compounds' HOMOs and LUMOs were measured using DFT method at the base set of B3LYP 6-311 G (d, p) and are tabled in Table 4 . The isodensity surface plots of HOMO and LUMO for L1H, L2H, and L3H are shown in Fig. 5.
Table 4.
Calculated EHOMO (EH), ELUMO (EL), energy band gap (EH – EL), chemical potential (μ), electronegativity (χ), global hardness (η), global softness (S), global electrophilicity index (ω) and softness (σ) for L1H, L2H, and L3H.
| Comp. | EH / eV | EL / eV | (EL-EH) /Ev | χ / eV | μ / eV | η / eV | S / eV−1 | ω / eV | σ / eV−1 |
|---|---|---|---|---|---|---|---|---|---|
| L1H | -6.6 | -6.16 | 0.44 | 6.38 | -6.38 | 0.22 | 0.11 | 92.51 | 2.27 |
| L2H | -7.32 | -4.83 | 2.49 | 6.075 | -3.78 | 1.245 | 0.6225 | 5.74 | 0.40 |
| L3H | -5.39 | -4.72 | 0.67 | 5.055 | -1.55 | 0.335 | 0.1675 | 3.56 | 1.49 |
Fig. 5.
HOMO and LUMO plots of the L1H, L2H, and L3H compounds
The results of the FMOs energy analysis revealed that the energies of HOMOs of L3H is higher compared with L1H and L2H. However, the destabilization of the LUMO level is found to be higher in L3H than the others. Consequently, the energy gap is in the order of L1H < L3H < L2H.
Recently, many reports showed that the FMOs have to be taken into consideration in investigation of the structure activity relationships [27], [28], [29]. The FMOs theory showed that the energy level of the HOMO and the LUMO are the most significant aspects that impact the bioactivities of small structural drugs. Mainly HOMOs that offer electrons, however, the LUMOs accept electrons. Obviously, the level of energy of HOMOs are different for all studied compounds. L3H showed the most lying HOMO than the other compounds and consequently it could be a better electron donor drug. Interestingly, L2H of the largest energy gap ∆E = 2.49 eV, there are several hydrophilic interactions that could facilitate the binding with the receptors. This suggests that such hydrophilic interactions considerably impact the binding affinity of such small drugs to the receptors. The HOMO of a certain drug and the LUMO with the adjacent residues could share the orbital interactions during the binding process.
3.4.3. Chemical reactivity descriptors
Calculations, such as the energy of the highest occupied molecular orbital, EHOMO, energy of the lowest unoccupied molecular orbital, ELUMO, obtain quantium chemical parameters of organic compounds. Additional parameters, such as separation energies (ΔE), absolute electro-negativities (v), chemical potentials (Pi), absolute hardness (g), absolute softness (r), global electrophilicity (x), global and softness (S) were calculated by Eqs. (1–3) [30, 31].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The inverse value of the global hardness is designed as the softness (σ) as follow:
| (6) |
3.4.5. Molecular electrostatic potential (MEP)
The molecular electrostatic potential (MEP) is important to quantify in order to validate the evidence regarding the reactivity of the compounds studied as inhibitors. Even though the MEP provides an indication of the molecular size and shape of both the positive, negative and neutral electrostatic potential. This may be a method for predicting relationships of physicochemical properties with the molecular structure of the drugs being investigated. In addition, the electrostatic molecular potential is a valuable method for estimating drug reactivity against electrophilic and nucleophilic attacks.
Under the same base sets, the molecular electrostatic potential of the L1H, L2H, and L3H is determined using the same process and is seen in Fig. 6. The maximum negative area within the MEP is the chosen electrophilic attack sites, indicated as red color. So, the negatively charged sites, and the reverse satiation for the blue regions, should draw an attacking electrophile. It is apparent that the molecular size and shape as well as the orientation of the negative, positive, and neutral electrostatic potential differed by product due to the type of atoms and their electronic existence. The difference in mapping the electrostatic potential around the compound may be primarily responsible for variation of its binding receptor affinities.
3.4.6. Mulliken atomic charges
The Mulliken atomic charges of the estimated compounds (1–3) were calculated the DFT using B3LYP 6-311G (d,p) at a basis set, the data were tabulated in Table 5 . It showed that the C5 is the most positive and O6 have the most negative charge for L1H. In case of L2H it is observed that the most nucleophilic centers are N4, N5 and N17 which are the most electrophilic susceptibility positions. On the other hand, it is obvious that the nucleophilic susceptibility of the L2H is recognized on C16 sites. However, N4, N5 and N16 are the most negative charges of L3H, while its respective positively charged atoms are C5 and S19. The positively charged centers are the most susceptible sites for nucleophilic attacks i.e., electron donation. However, the most negatively charged centers are the most susceptible sites for electrophilic one [ 32 ].
Table 5.
The Mulliken atomic charges of L1H, L2H, and L3H.
| L1H | L2H | L3H | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | C | -0.11122 | 1 | C | -0.01515 | 1 | C | -0.2013 |
| 2 | C | 0.106601 | 2 | C | 0.003903 | 2 | C | 0.280357 |
| 3 | C | 0.134528 | 3 | C | 0.130135 | 3 | C | 0.068197 |
| 4 | N | -0.16705 | 4 | N | -0.26368 | 4 | N | -0.35022 |
| 5 | C | 0.511645 | 5 | N | -0.33433 | 5 | N | -0.3283 |
| 6 | O | -0.39734 | 6 | C | 0.205425 | 6 | C | 0.106955 |
| 7 | C | -0.09115 | 7 | C | -0.02753 | 7 | C | 0.039048 |
| 8 | N | -0.4852 | 8 | C | 0.005259 | 8 | C | 0.026534 |
| 9 | C | 0.051185 | 9 | C | 0.051085 | 9 | C | 0.060093 |
| 10 | C | -0.20289 | 10 | C | 0.067703 | 10 | C | -0.03639 |
| 11 | C | -0.09894 | 11 | C | -0.03372 | 11 | C | 0.026019 |
| 12 | C | -0.09495 | 12 | C | 0.028768 | 12 | C | -0.02273 |
| 13 | C | -0.1082 | 13 | C | -0.07759 | 13 | C | 0.096392 |
| 14 | C | -0.16576 | 14 | C | 0.106634 | 14 | C | 0.137059 |
| 15 | C | -0.12612 | 15 | C | 0.123458 | 15 | C | 0.177593 |
| 16 | C | -0.16461 | 16 | C | 0.192665 | 16 | N | -0.65011 |
| 17 | C | -0.08972 | 17 | N | -0.38104 | 17 | C | 0.285513 |
| 18 | C | -0.17746 | 18 | C | 0.119451 | 18 | C | -0.48668 |
| 19 | C | -0.00069 | 19 | C | 0.056393 | 19 | S | 0.552361 |
| 20 | N | -0.33001 | 20 | C | -0.01049 | 20 | C | 0.167863 |
| 21 | C | -0.03167 | 21 | C | 0.034302 | 21 | C | 0.035451 |
| 22 | C | -0.08019 | 22 | C | -0.05817 | 22 | C | -0.23807 |
| 23 | N | -0.56129 | 23 | C | 0.008089 | 23 | C | 0.13635 |
| 24 | C | 0.005574 | 24 | Cl | 0.118015 | |||
| 25 | C | 0.000038 | ||||||
| 26 | C | -0.01433 | ||||||
| 27 | C | 0.077149 | ||||||
3.5. Molecular docking
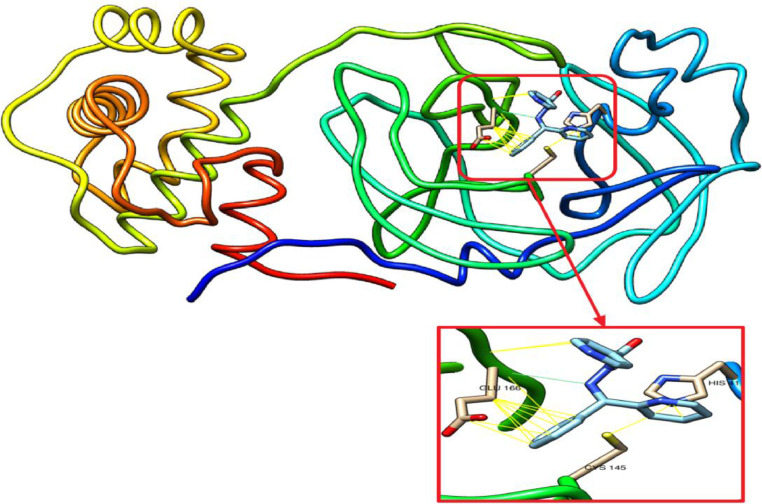
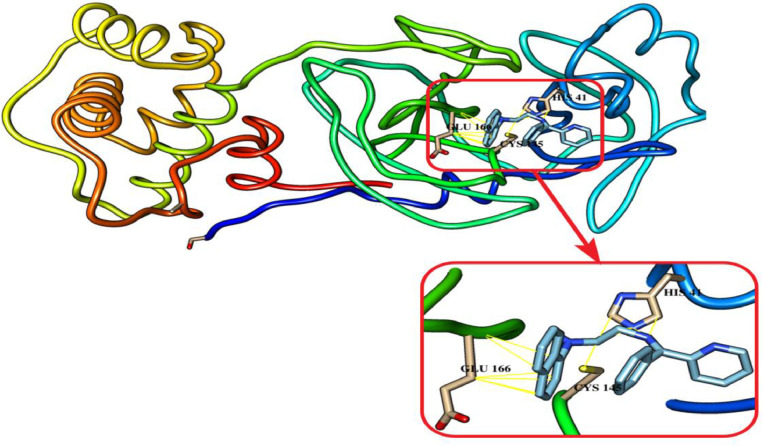
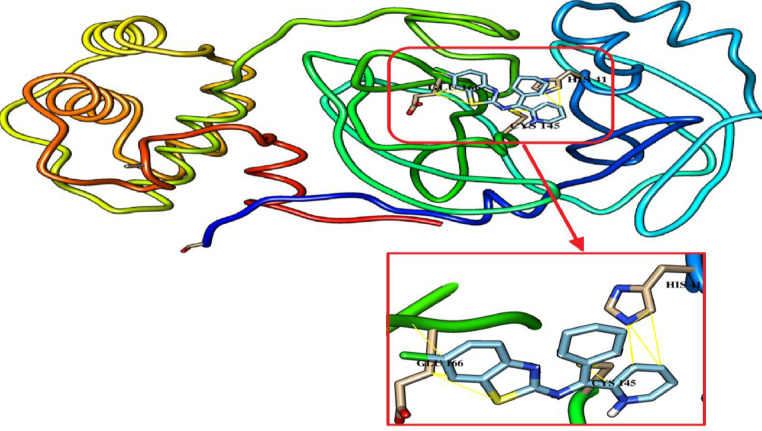
The synthetic novel Schiff bases have shown a significant rule against CLpro, the important enzyme for proliferation of SARS-COV-2 virus. The inhibitory effect was investigated based on their interaction to the catalytic residues of His41 and Cys145, the essential residues of Glu166 and Ser1 for maintaining CLpro on the correct conformation [33]. The synthetic compounds have been docked with 3CLpro. They form hydrogen bonds (blue stripes) and Van der Waal's interactions (yellow stripes) as depicted in Fig. 7, Fig. 8, Fig. 9 . L2H and L3H have shown the best binding affinity of -7.6 for both. Of them, L3H had a better RMSD as presented it Table 6 . the synthetic Schiff bases L1H OPT has shown a tendency to form hydrogen bonds with the active residue of Glu166. Whereas, they are all interact with others catalytic residues of of His41 and Cys145 of CLpro. Our results suggest these compounds as a potent CLpro inhibitors, particularly L3H and thus could be used for the treatment of COVID-19 after a suitable in vitro and in vivo validation as well as clinical trials.
Fig. 7.
L1H docked with 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2. Hydrocarbon skeleton of L1H is cyan, nitrogen atoms are blue, oxygens red. Below, different side of view of magnified images of its contact sites to HIS41, CYS145 and GLU166.
Fig. 8.
L2H docked with 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2. Hydrocarbon skeleton of L2H is cyan, nitrogen atoms are blue, oxygens red. Below, different side of view of magnified images of its contact sites to HIS41, CYS145 and GLU166.
Fig. 9.
L3H docked with 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2. Hydrocarbon skeleton of L3H is cyan, nitrogen atoms are blue, oxygens red. Below, different side of view of magnified images of its contact sites to HIS41, CYS145 and GLU166.
Table 6.
The binding interactions of L1H, L2H, and L3H to 3-chymotrypsin-like protease of SARS-CoV-2.
| Pharmaceutical name | Score (kcal/mol) | RMSD | Hydrogen bond (distance) | Van der Waal's (distance) |
|---|---|---|---|---|
| L1H | -6.6 | 27.120 – 29.360 | Glu166 (2.275 Å) | His41 (3.664 Å/ 3.777 Å). Cys145 (3.470 Å/ 3.490 Å). Glu166 (14 side contacts, distance range: 2.275 Å to 3.826Å). |
| L2H | -7.6 | 28.639 – 31.280 | - | His41 (5 side contacts, distance range 3.464 Å to 3.785 Å). Cys145 (3.707 Å) Glu166 (6 side contacts, distance range 3.440 Å to 3.780 Å). |
| L3H | -7.6 | 1.524 - 6.174 | - | His41 (4 side contacts, distance range 3.624 Å to 3.826 Å). Cys145 (3.779 Å) Glu166 (4 side contacts, distance range 3.390 Å to 3.955 Å). |
4. Conclusion
New Schiff bases, N'-(phenyl(pyridin-2-yl)methylene)isoni-cotinohydrazide (L1H), N-(2-((phenyl(pyridin-2-yl)methylene)amino)ethyl)naphthalen-1-amine (L2H), and N-(6-chlorobenzo[d]thiazol-2-yl)-1-phenyl-1-(pyridin-2-yl)methanimine (L3H) were synthesized by condensation of 2-benzoylpyridine with three different amines and characterized by different physicochemical studies. The antimicrobial activities of all complexes were tested at three concentrations 50, 100 and 200 μg/mL, against five bacterial types. L3H is more active for the tested bacteria compared with other Schiff bases (L1H, and L2H) and has highest effective against the tested bacteria S. aureus, M. luteus, S. pyogenes, B. subtilis with MIC values of 6.25, 25, 25, and 25μg/mL, respectively, whereas a less active against E. coli, with MIC value 100μg/mL. The L3H compound is more active than the other Schiff bases because it has a (Cl and S) atoms in the structure, these atoms possibly via enhanced membrane transport into the cell or some other mode of action. The compounds have also been docked with 3CLpro. They have shown good interaction with the catalytic sites of 3CLpro, thus could be consider as a potent inhibitors and therapeutic agents for COVID-19 after suitable in vitro and in vivo validation as well as clinical trials. The molecular docking results were illustrated in terms of the DFT calculations. The results of the DFT showed that L3H is the most lying HOMO and therefore it may be the best to serve as an electron donor. In addition, N4, N5 and N16 are the most electrophilic centers of L3H and this may be due to the presence of the lone pair of electrons on these atoms. Finally, performing further in vitro and small animal models is very promising in vivo studies establishing solid experimental evidence of its activity as COVID-19 inhibitors.
CRediT authorship contribution statement
Ahmed S.M. Al-Janabi: Supervision, Conceptualization, Methodology, Investigation, Writing - review & editing. Amin O. Elzupir: Writing - review & editing, Data curation, Formal analysis. Tarek A. Yousef: Methodology, Investigation, Software, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2020.129454.
Appendix. Supplementary materials
References
- 1.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D., Zhang P., Bao C., Zhang Y., Zhu N. Emerging understanding of etiology and epidemiology of the novel coronavirus (COVID-19) infection in Wuhan, China. Preprints. 2020 doi: 10.20944/preprints202002.0283.v1. [DOI] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus I, Research T. A novel coronavirus from patients with pneumonia in China, 2019. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng F. Cancer Bioinformatics. Springer; Cham, Switzerland: 2019. In silico oncology drug repositioning and polypharmacology; pp. 243–261. [DOI] [PubMed] [Google Scholar]
- 5.Liu C., Ma Y., Zhao J., Nussinov R., Zhang Y.-C., Cheng F., Zhang Z.-K. Computational network biology: data, model, and applications. Phys. Rep. 2019;846:1–66. [Google Scholar]
- 6.Theerawatanasirikul S., Kuo C.J., Phetcharat N., Lekcharoensuk P. In silico and in vitro analysis of small molecules and natural compounds targeting the 3CL protease of feline infectious peritonitis virus. Antivir. Res. 2020;174 doi: 10.1016/j.antiviral.2019.104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagar M., Chaieb K., Parveen S., Ahmed H., Alnoman R. N-alkyl 2-pyridone versus O-alkyl 2-pyridol: ultrasonic synthesis, DFT, docking studies and their antimicrobial evaluation. J. Mol. Struct. 2020;1199 [Google Scholar]
- 8.Alnoman R.B., Parveen S., Hagar M., Ahmed H.A., Knight J.G. A new chiral boron–dipyrromethene (BODIPY)–based fluorescent probe: molecular docking, DFT, antibacterial and antioxidant approaches. J. Biomol. Struct. Dyn. 2019;19:1–14. doi: 10.1080/07391102.2019.1701555. [DOI] [PubMed] [Google Scholar]
- 9.Alnoman R.B., Hagar M., Parveen S., Ahmed H.A., Knight J.G. Computational and molecular docking approaches of a new axially chiral BODIPY fluorescent dye. J. Photochem. Photobiol. A: Chem. 2020;395 [Google Scholar]
- 10.Kumar K.S., Ganguly S., Veerasamy R., De Clercq E. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4(3)H-ones. Eur. J. Med. Chem. 2010;45(11):5474–5479. doi: 10.1016/j.ejmech.2010.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang D., Lin M., Wei L., Xie L., Zhu G., Cruz C.S.D., Sharma L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P., Fan H., Lan T., Yang X.L., Shi W.F., Zhang W., Zhu Y., Zhang Y.W., Xie Q.M., Mani S., Zheng X.S., X.S. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556(2020):255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019‐new coronavirus epidemic: evidence for virus evolution. J. Med. Vir. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer A.W. Single-disk antibiotic-sensitivity testing staphylococci. Arch. Internal Med. 1959;104(2):208–216. doi: 10.1001/archinte.1959.00270080034004. [DOI] [PubMed] [Google Scholar]
- 15.P.R. Murray, E.J. Baron, M.A. Pfaller, F.C. Tenover, R.H. Yolken, G.L. Wood, J.A. Washington, Manual of clinical microbiology, Washington. DC. (1995).
- 16.Gaussian 09 . gaussian. Inc.; Wallingford, CT, USA: 2009. Revision a. 02. [Google Scholar]
- 17.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 19.Trott O., Olson A.J. Auto dock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi J., Batra N., Malhotr R. Ligational behavior of Schiff bases towards transition metal ion and metalation effect on their antibacterial activity. Spectrochimica Acta A. 2012;97:397–405. doi: 10.1016/j.saa.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Ali O.A.M., El-Medani S.M., Ahmed D.A., Nassar D.A. Synthesis, characterization, fluorescence and catalytic activity of some new complexes of unsymmetrical Schiff base of 2-pyridinecarboxaldehyde with 2,6-diaminopyridine. Spectrochimica Acta Part A. 2015;144:99–106. doi: 10.1016/j.saa.2015.02.078. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein R.M., Webster F.X. 6th ed. John Wiley & Sons; New York, NY, USA: 1997. Spectrometric Identification of Organic Compounds. [Google Scholar]
- 23.Tarek A.F., Fayzah A.S., Jubair B., Omima A.W. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixicacidcarbohydrazide, synthesis, antitubercular activity and pharmacophoricmodelbuilding. Eur. J. Med. Chem. 2010;45:4578–4586. doi: 10.1016/j.ejmech.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Küçükgüzel G., Rollas S., Küçükgüzel M. Kiraz I. Synthesis and antimycobacterial activity of some coupling products from 4-aminobenzoic acid hydrazones. Eur. J. Med. Chem. 1999;34(12):1093–1100. [Google Scholar]
- 25.Sridhar S.K., Saravanan M., Ramesh A. Synthesis and antibacterial screening of hydrazones, Schiff and MANNICH bases of isatin derivatives. Eur. J. Med. Chem. 2001;36(7-8):615–625. doi: 10.1016/s0223-5234(01)01255-7. (2001) [DOI] [PubMed] [Google Scholar]
- 26.Prabhu N.V., Sharp K.A. Heat capacity in proteins. Annu. Rev. Phys. Chem. 2005;56:521–548. doi: 10.1146/annurev.physchem.56.092503.141202. [DOI] [PubMed] [Google Scholar]
- 27.Sato 3R., Vohra S., Yamamoto S., Suzuki K., Pavel K., Shulga S., Blume Y., Kurita N. Specific interactions between tauprotein and curcumin derivatives: molecular docking and abinitio molecular orbital simulations. J. Mol. Graph. Model. 2020;98 doi: 10.1016/j.jmgm.2020.107611. [DOI] [PubMed] [Google Scholar]
- 28.Majumdar D., Singh D.K., Pandey D.K., Parai D., Bankura K., Mishra D. DFT investigations of linear Zn3-typecomplexwithcompartmentalN/O-donor Schiff base: synthesis, characterizations, crystal structure, fluorescence and molecular docking. J. Mol. Struct. 2020;1209 [Google Scholar]
- 29.Almutairi M.S., Leenaraj D., Ghabbour H.A., Joe I.H., Attia M.I. Spectroscopic identification, structural features, hirshfeld surface analysis and molecular docking studies on stiripentol: an orphan antiepileptic drug. J. Mol. Struct. 2019;1180:110–118. [Google Scholar]
- 30.Yousef T.A., Abu El-Reash G.M., El Morshedy R.M. Quantum chemical calculations, experimental investigations and DNA studies on (E)-2-((3-hydroxynaphthalen-2-yl) methylene)-N-(pyridin-2-yl) hydrazinecarbothio- amide and its Mn (II), Ni (II), Cu (II), Zn (II) and Cd (II) complexes. Polyhedron. 2012;45:71–85. [Google Scholar]
- 31.Yousef T.A., Alduaij O.K., Abu El-Reash G.M., El Morshedy R.M. Semiempirical studies, spectral analysis, in vitro antibacterial and DNA degradation studies of heterocyclic thiosemicarbazone ligand and its metal complexes. J. Mol. Liq. 2016;222:762–776. [Google Scholar]
- 32.Yousef T.A. Structural, optical, morphology characterization and DFT studies of nano sized Cu (II) complexes containing schiff base using green synthesis. J. Mol. Struct. 2020;1215 [Google Scholar]
- 33.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.