Abstract
This study aimed to assess the efficacy of Saccharomyces boulardii, a yeast probiotic, in the management of acute diarrhoeal disorders in the paediatric population in outpatient settings. It was a multicentre retrospective analysis of medical records of children who were treated for acute diarrhoea by routine treatment (oral rehydration solution and zinc) with or without S. boulardii. Overall, 160 children presenting with acute diarrhoea at seven different outpatient paediatric settings were included in the study. Children were divided into two categories based on their treatment with S. boulardii (SB group) or without S. boulardii (Non-SB group). Baseline demographic, anthropometric and clinical variables were compared between the two groups. The median duration of diarrhoea post-treatment was significantly shorter in the S. boulardii group (3 days) than in the non-SB group (4 days). A significant reduction in the frequency of stools was observed post-treatment in the S. boulardii group (1.7 versus 2.5 in the non-SB group). There was a significant weight gain in the S. boulardii group post-treatment (300 g) in comparison with the non-SB group (mean loss of 400 g). This study established the positive role of S. boulardii in the management of acute diarrhoeal diseases in children. Moreover, the S. boulardii probiotic was seen to be effective in diarrhoeal diseases in children with dehydration.
Keywords: Acute diarrhoea, Children, Probiotic, Real-world evidence, Retrospective, Saccharomyces boulardii
Introduction
According to the Global Burden of Disease study [1], there were 134.94 million (95% uncertainty interval (UI) 115.53 million–159.12 million) episodes of diarrhoea with 66 157 (95% UI 56 001–78 160) deaths among children under 5 years of age in 2016 in India. Overall incidence per child was estimated at 1.20 episodes (95% UI 1.03–1.42) and the incidence of deaths due to diarrhoea was 59.0 (95% UI 49.9–69.7) per 100 000 under-5 children. According to the Global Burden of Disease study, diarrhoea was the fifth leading cause of mortality among children under 5 years in 2016 worldwide and rotavirus was the leading aetiology for the diarrhoeal mortality.
Diarrhoea is defined by the WHO as the passage of three or more loose or watery stools per day [2]. The aetiological factors may be bacterial, viral, protozoal and other causes such as certain medications [3]. Treatment of diarrhoea involves the maintenance of hydration by oral rehydration or intravenous fluids [4]; oral rehydration solution is intended to decrease the mortality and morbidity due to diarrhoea, as it balances the water and electrolytes [5]. Besides maintaining hydration, it is equally important to substantially shorten the duration/frequency of diarrhoea, such as by using probiotics. Probiotics are living non-pathogenic microorganisms that, when administered in specific amounts, confer health benefits, as defined by the joint committee of WHO and Food and Agriculture Organization (Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, 01 till 04 October 2001, Cordoba, Argentina). A study by Dinleyici et al. [6] established evidence from the western world in which Saccharomyces boulardii significantly reduced the duration of diarrhoea by approximately 24 hours. Compared with probiotics (bacterial in nature), S. boulardii yeast cells are resistant to antibiotics due to their fungal nature and no DNA exchange has been observed, for example of resistance genes, to bacteria [7].
Numerous clinical studies have discussed the role of S. boulardii for the management of diarrhoea associated with acute and chronic infections, antibiotics or Clostridium difficile [[8], [9], [10], [11]]. In a research study conducted by Kurugöl and Koturoğlu in 2005, medians of average stool frequency were found to be significantly lower in the S. boulardii group compared with the placebo group after the second day of therapy [8]. Also, the probiotic effectively reduces the risk of antibiotic-associated diarrhoea and has been useful in acute rotaviral diarrhoea [8,9]. This is also ascertained by a systematic review and meta-analysis of 31 randomized controlled trials (RCTs) conducted by McFarland [11].
There is established evidence from the western world that the S. boulardi probiotic reduces the duration of diarrhoea, so studies based on real-world data would generate real-world evidence in the Indian population and help in designing treatment strategies and relevant longitudinal studies. Hence, we conducted the study using real-world data to assess the efficacy of the S. boulardii probiotic in the management of acute diarrhoea in Indian children.
Material and methods
The study was a retrospective, follow-up study based on data extracted from medical records of children, who visited various outpatient settings for acute diarrhoea.
Period of study
A total of 510 electronic medical records of children who had visited outpatient clinics at seven different paediatric centres located across five different states in India between January 2019 to November 2019 were reviewed for the following inclusion/exclusion criteria. Inclusion criteria were: children from 0 to 18 years) of either gender who received treatment for acute diarrhoea in outpatient settings during routine clinical practice, were treated with oral rehydration solution and zinc with or without S. boulardii CNCM I-745 (Econorm sachet; Biocodex, Gentilly, France; 250 mg twice daily), and who had revisited within 5 days of the index visit for follow up. Exclusion criteria were chronic or persistent diarrhoea (≥2 weeks) or severe diarrhoea, i.e. diarrhoea managed with intravenous fluids or requiring hospital admission.
Sampling technique and sample size
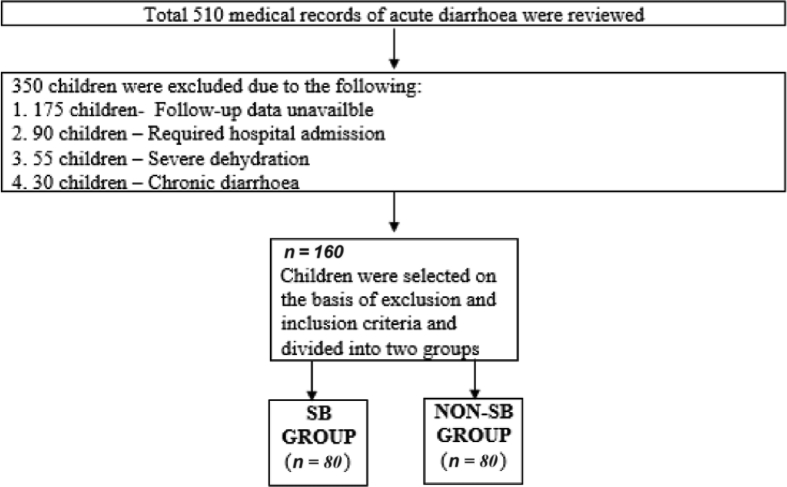
In this multicentre retrospective analysis of medical records at seven different outpatient paediatric settings across five different states (Gujarat, Delhi, Bengaluru, Haryana and Bihar) in India, 160 children were treated for acute diarrhoea by routine treatment (oral rehydration solution and zinc) with (SB group) or without (Non-SB group) the yeast probiotic S. boulardii. It was a convenient sampling based on the availability of medical records with essential variables for analysis as records of all patients were taken up for analysis (Fig. 1).
Fig. 1.
Flow diagram of data collection.
Statistical analysis and outcome variables
Data analysis was carried out using Microsoft Office Excel (version 365) and R software (3.6.2). Continuous variables are presented as means and categorical variables were presented in the form of proportions.
Descriptive statistics for both the groups including baseline demographic variables (age, gender), anthropometric (weight), clinical (duration and frequency of diarrhoea) and aetiological factors were included. Final outcome post-treatment was analysed in terms of the mean duration of diarrhoea, mean frequency of stools per day and mean change in body weight in both groups. Means and medians were compared by t-statistics and Mann–Whitney U test, respectively, and categorical variables were compared using χ2 statistics. A p value was considered significant at <0.05. Effect of significant, independent variables on the final outcome was assessed by using binary logistic regression. Mean duration of diarrhoea post-treatment was taken as a dependent variable, whereas age, current body weight, frequency of stools at the time of presentation and the use of S. boulardii were taken as independent variables.
The propensity score was calculated to obtain an unbiased estimate of S. boulardii probiotic effect adjusted for the impact of given confounding factors. For this, the covariates capable of explaining differences in the likelihood of receiving S. boulardii probiotic were identified. These variables included age, current weight, frequency of stools at the time of presentation and dehydration status and were used as covariates in the estimation of the propensity scores obtained through logistic regression. One-to-one matching was done based on the propensity score, which resulted in 37 well-matched pairs. Given this balance in the propensity score and covariates between groups, an independent samples t test was conducted on the resolution of diarrhoea in both groups.
Ethical issues
Confidentiality of subjects has been maintained as only anonymized and de-identified (at facility level) variables were used for the data analysis.
Results
Descriptive statistics of patient population
The baseline assessment and comparison of demographic and anthropometric characteristics of the patients in the two groups is shown in Table 1. The mean ages were 35.2 months and 59.5 months in the SB and non-SB group (p < 0.05), respectively. Age-wise distribution was significantly different in both groups (p < 0.05). The SB group had a higher proportion (32.5% versus 10%) of children under 1 year of age than the non-SB group (p < 0.05). There were more boys than girls in both groups though gender distribution was not found to be significantly different.
Table 1.
Baseline demographic, anthropometric and clinical characteristics of the children
| Characteristics | SB group | Non-SB group | p value |
|---|---|---|---|
| Age (in months) | |||
| Mean (SD) | 35.2 (32.8) | 59.5 (50.0) | |
| Median (IQR) | 24 (11–48) | 48 (24–91.5) | <0.05 |
| Age-wise distribution of study population, n (%) | |||
| 0–1 years | 26 (32.5) | 8 (10.0) | <0.05 |
| 1–2 years | 17 (21.2) | 14 (17.5) | |
| 2–3 years | 13 (16.2) | 12 (15.0) | |
| 3–4 years | 6 (7.5) | 8 (10.0) | |
| 4–5 years | 5 (6.3) | 4 (5.0) | |
| 5–11 years | 13 (16.3) | 30 (37.5) | |
| >11 years | 0 (0.0) | 4 (5.0) | |
| Gender distribution, n (%) | |||
| Male | 45 (56.3) | 43 (53.8) | 0.8 |
| Female | 35 (43.7) | 37 (46.2) | |
| Birthweight and current weight | |||
| Birthweight (kg) | |||
| Mean (SD) | 3.0 (0.5) | 2.9 (0.4) | 0.3 |
| Median (IQR) | 3 (2.6–3.3) | 2.9 (2.6–3.1) | |
| Current weight (kg) | |||
| Mean (SD) | 12.9 (7.3) | 16.7 (8.5) | <0.05 |
| Median (IQR) | 10.8 (8–15) | 13.5 (10–22.5) | |
| Frequency of stools (number of stools/day) | |||
| Mean (SD) | 9.1 (2.2) | 7.5 (2.3) | <0.05 |
| Median (IQR) | 9 (8–11) | 7 (6–10) | |
| Dehydration status, n (%) | |||
| No dehydration | 22 (27.5) | 58 (72.5) | |
| Some dehydration | 58 (72.5) | 22 (27.5) | <0.05 |
| Severe dehydration | 0 (0.0) | 0 (0.0) | |
| Distribution according to presumptive diagnosis, n (%) | |||
| Acute diarrhoea | 55 (68.7) | 58 (72.5) | 0.4 |
| Acute dysentery | 14 (17.5) | 11 (13.8) | |
| Antibiotic-associated diarrhoea | 4 (5.0) | 6 (7.5) | |
| Watery diarrhoea (cholera) | 0 (0.0) | 1 (1.3) | |
| Rotaviral diarrhoea | 7 (8.8) | 2 (2.5) | |
| Total | 80 (100.0) | 80 (100.0) | |
Abbreviations: IQR, interquartile range; SB, Saccharomyces boulardii; SD, standard deviation.
There was no significant difference in mean birthweight of both groups, but the current weight was significantly (p < 0.05) less in the SB group (12.9 kg versus 16.7 kg) than the non-SB group.
Clinical presentation of diarrhoea cases
As shown in Table 1, the mean frequency of stools (episodes/day) before the treatment was reported to be 9.1 in the SB group versus 7.5 in the non-SB group, which was statistically significant (p < 0.05). The SB group had a significantly higher proportion of children presenting with dehydration in comparison with the non-SB group (p < 0.05). Distribution of aetiological factors (clinical and diagnostic) was similar in both groups (p=0.4). Acute diarrhoea was the commonest presentation followed by acute dysentery in both groups. There was a single case of culture-positive cholera (in the non-SB group). Rotaviral diarrhoea was diagnosed by the detection of rotavirus antigen in stool specimens, as per record. There were nine cases of rotavirus (seven in the SB group and two in the non-SB group) in the study population.
Post-treatment outcome
In the univariate analysis, the mean duration of diarrhoea post-treatment in the SB group was 3 days in comparison with 4.4 days in the non-SB group (p < 0.05). The post-treatment duration of diarrhoea and stool frequency were statistically significantly shorter in the SB group than in the non-SB group. The post-treatment weight gain was also statistically significantly higher in the SB than in the non-SB group (Table 2).
Table 2.
Comparative efficacy of Saccharomyces boulardii in the management of acute diarrhoea
| Outcome variables | SB group | Non-SB group | p value |
|---|---|---|---|
| Mean duration of diarrhoea post-treatment (days) | |||
| Mean (SD) | 3.0 (1.0) | 4.4 (1.1) | |
| Median (IQR) | 3 (2–4) | 4 (4–5) | <0.05 |
| Change in mean body weight (kg) | |||
| Mean (SD) | 0.3 (0.4) | –0.4 (1.8) | |
| Median (IQR) | 0.3 (0.02–0.4) | 0 (–0.2 to 0.1) | <0.05 |
| Mean frequency of stools post-treatment as per reported on the follow-up visit (loose stools/day) | |||
| Mean (SD) | 1.7 (0.6) | 2.5 (1.5) | |
| Median (IQR) | 2 (1–2) | 2 (2–2.3) | <0.05 |
| Dehydration status, n (%) | 80 (50) | 80 (50) | |
| No dehydration, n, mean duration of diarrhoea post-treatment (SD) | 22, 2.6 (0.9) | 58, 4.3 (0.2) | <0.05 |
| Some dehydration, n, mean duration of diarrhoea post-treatment (SD) | 58, 3.2 (0.9) | 22, 4.7 (0.7) | |
| Total, n, mean (SD) | 80, 3.0 (1.1) | 80, 4.4 (1.1) | |
Abbreviations: IQR, interquartile range; SB, Saccharomyces boulardii; SD, standard deviation.
Utilization patterns of SB probiotic: most frequently prescribed to dehydrated children
Our analysis showed that children with dehydration had significantly higher odds (6.9, p < 0.05) of receiving S. boulardii in comparison with children with no dehydration. Saccharomyces boulardii was used in 72.5% of dehydrated children compared with 27.5% of children without dehydration. The S. boulardii probiotic was associated with a reduced mean duration of diarrhoea post-treatment in children with or without dehydration. In the dehydration category, mean duration of diarrhoea post-treatment among children prescribed S. boulardii was 3.2 days compared with 4.7 days without S. boulardii (p < 0.05). Though in children with no dehydration the use of S. boulardii was comparatively less frequent, it still had a significant effect on outcome in terms of the reduced mean duration of diarrhoea post-treatment (2.6 versus 4.3 days) (Table 2).
Resolution of diarrhoea post-treatment
Resolution of diarrhoea is defined as the stool frequency ≤3 episodes/day with the normal consistency of stools. As shown in Table 3, the majority of the patients (71.2%) reported complete resolution within 2 days post-treatment in the SB group in comparison with the non-SB group (18.7%). This difference was found to be statistically significant (p < 0.05). All the cases were resolved in the SB group, but 25% of the cases in the Non-SB group were unresolved.
Table 3.
Comparative duration of resolution of diarrhoea in SB and non-SB group
| Follow up (days) | SB group versus non-SB group, n (%) |
|---|---|
| Day 1 | 29 (36.2) versus 4 (5.0) |
| Day 2 | 28 (35.0) versus 11 (13.7) |
| Day 3 | 16 (20.0) versus 22 (27.5) |
| Day 4 | 7 (8.8) versus 23 (28.8) |
| Total | 80 (100.0) versus 60 (75.0) |
Abbreviations: SB, Saccharomyces boulardii.
Factors associated with post-treatment outcome in diarrhoea
Multivariate linear regression was conducted to assess important parameters associated with post-treatment outcome. All variables that were significantly different in both groups (in univariate analysis) were included as independent variables in the regression. These variables included age (months), current weight (kg), frequency of stools (as reported by parents), and S. boulardii in the treatment regimen. Dehydration status was not included in the multivariate analysis because it was significantly associated with the higher probability of getting S. boulardii treatment. Mean duration of diarrhoea post-treatment was taken as an outcome or dependent variable.
Presence of S. boulardii in the treatment was associated with a significant reduction in the duration of diarrhoea post-treatment and was the only significant factor (Table 4).
Table 4.
Multivariate linear regression of the parameters with the duration of diarrhoea post-treatment
| Variables | p value |
|---|---|
| Age | 0.2 |
| Current body weight | 0.3 |
| Frequency of stools at the time of presentation | 0.1 |
| Treatment (SB versus non-SB group) | <0.05 |
Abbreviation: SB, Saccharomyces boulardii.
Propensity score suggested that those receiving the S. boulardii probiotic showed significantly shorter duration of diarrhoea post-treatment with an average of 2.7 days relative to the control group (Table 5).
Table 5.
Covariate balance before and after matching covariates
| Outcome | SB group |
Non-SB group |
p value |
|---|---|---|---|
| Mean (±SD) | Mean (±SD) | ||
| Pre-matching | |||
| Duration of diarrhoea post-treatment (days) | 3 (1) | 4.4 (1.1) | <0.05 |
| Post-matching | |||
| Duration of diarrhoea post-treatment (days) | 2.7 (0.9) | 4.4 (1.1) | <0.05 |
Abbreviations: SB, Saccharomyces boulardii; SD, standard deviation.
Discussion
The human intestinal microbiota can play a key role in modulating health and disease over a lifetime. Saccharomyces boulardii has a number of distinct mechanisms of action such as luminal action, trophic action and mucosal anti-inflammatory signalling effects. Saccharomyces boulardii has antimicrobial properties, and secretes 54 kDa, 63 kDa and 120 kDa proteins that exhibit intestinal microflora protection against pathogenic bacteria, either by clotting the toxin or by reducing the cAMP level [12].
Role of S. boulardii in management of acute diarrhoea
Our real-world evidence analysis showed that the mean duration and frequency of diarrhoea was significantly reduced in the SB group compared with the non-SB group. This was similar to the findings in the RCT study conducted by Das et al., wherein the median duration (hours) of diarrhoea was significantly less in the S. boulardii group (60 versus 89; 95% CI –41.2 to 16.8) compared with the control group [10]. The same is observed in the RCT carried out by Dash et al., in which the duration of diarrhoea was comparatively less (26.31 versus 47.81 hours) in the group given S. boulardii than the control group [13]. Another study, by Riaz et al., purported that the duration of diarrhoea was significantly less in the group given S. boulardii by 11.9 (52.1 versus 64.0) hours when compared with the control group [14]. A meta-analysis of seven RCTs (944 respondents) concluded that those children treated with S. boulardii, a decrease in the duration of acute diarrhoea was seen by approximately 1 day compared with placebo [15].
Billoo [16] reported a significant role of S. boulardii in the prevention of diarrhoeal episodes in the following months post-treatment. Similar findings were seen in the RCTs conducted in Turkey [16], Argentina and Belgium [17]. Htwe et al. [18] found that the use of S. boulardii therapy for 5 days considerably decreases the mean duration of acute diarrhoea and stool frequency and normalizes stool consistency. The mean duration of diarrhoea was comparatively less than in the control group (3.08 versus 4.68 days). According to a systematic review [15] that included 11 randomized controlled trials (1306 children, 651 in the S. boulardii and 655 control groups), S. boulardii was able to reduce the duration of diarrhoea by approximately 24 hours (–0.99 days; 95% CI –1.40 to –0.58).
Saccharomyces boulardii probiotic is not only effective in children, but has also shown good efficacy in adults. A prospective RCT (May 2012 to January 2013 in Boston, Massachusetts) on 53 adults performed by Kabbani et al. observed that when the S. boulardii probiotic was used along with amoxicillin-clavulanate, there were fewer changes in the gut microbiota and antibiotic-associated diarrhoea was prevented [19]. Another meta-analysis by Szajewska and Kołodziej in 2015, had conducted a study on 21 RCTs (4780 participants, 2441 in the S. boulardii group and 2339 in the control group). It included both children and adults, in which the duration of diarrhoea was shortened, compared with the placebo or no treatment group [20].
In our study, there was a significantly mild increase in mean body weight in the SB group when compared with the non-SB group. According to Le Luyer et al. [21], when children were given the lactose-free formula mixed with S. boulardii, there was an average daily weight gain (74.2 ± 26.4 g versus 23.7 ± 6.7 g; p < 0.05) in the group given S. boulardii compared with the non-S. boulardii group.
The current study is one among the few multicentric real-world evidence studies performed in Indian settings and consolidates the role of probiotics in diarrhoeal management. This study also highlights the common practices associated with use of probiotics in the management of acute diarrhoea. As S. boulardii was found to be significantly effective in reducing frequency and duration of diarrhoea even in mild cases (no dehydration), it will be useful to inform and educate health-care providers regarding the usefulness of S. boulardii in decreasing the morbidity associated with diarrhoea. Saccharomyces boulardii was found to be effective in diarrhoea irrespective of associated aetiology.
Inherent to any real-world retrospective study, there are a few limitations. It was not possible to ascertain time intervals in hours (recovery time), which causes slight overestimation or underestimation of time intervals. Impact on other important manifestations such as vomiting and fever also could not be assessed. The study included only those cases with at least one follow up, which might have excluded potential cases either recovered early or switched over to another doctor or setting (inpatient). Also, it was not possible to adjust for all confounding factors that might be associated with the prescription of probiotics such as cost, severity of disease and other clinical parameters like randomization was not carried out in the study. We were not able to ensure the standard procedures for data recording at the various facilities because it was a retrospective study.
Conclusion
Diarrhoea is not only associated with high morbidity and mortality in children. Probiotics such as S. boulardii can help in early recovery in such patients. Health-care providers need to be educated regarding the benefit of S. boulardii probiotics in the management of acute diarrhoea in addition to standard treatment agents.
Conflicts of interest
The authors have stated that there are no conflicts of interest.
Status of ethical clearance
This study was approved by the Good Society for Ethical Research.
Funding
None.
References
- 1.GBD 2016 Diarrhoeal Disease Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Diarrheal disease https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease Available at:
- 3.World Health Organization (n.d WHO | dysentery. https://www.who.int/topics/dysentery/en/ Available at:
- 4.World Health Organization 2005. https://apps.who.int/iris/bitstream/handle/10665/43209/9241593180.pdf?sequence=1 Available at:
- 5.Farthing M., Salam M.A., Lindberg G., Dite Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol. 2013;47:12–20. doi: 10.1097/MCG.0b013e31826df662. [DOI] [PubMed] [Google Scholar]
- 6.Dinleyici E., Eren M., Ozen M., Yargic Z., Vandenplas Y. Effectiveness and safety of Saccharomyces boulardii for acute infectious diarrhea. Exp Opin Biol Ther. 2012;12:395–410. doi: 10.1517/14712598.2012.664129. [DOI] [PubMed] [Google Scholar]
- 7.Moré M., Swidsinski A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis: a review. Clin Exp Gastroenterol. 2015;8:237–255. doi: 10.2147/CEG.S85574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurugöl Z., Koturoğlu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediat. 2005;94:44–47. doi: 10.1111/j.1651-2227.2005.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 9.Buck M.L. Saccharomyces boulardii as a probiotic for children. Pediat Pharmacother. 2009;15(7) [Google Scholar]
- 10.Das S., Gupta P.K., Das R.R. Efficacy and safety of Saccharomyces boulardii in acute rotaviral diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediat. 2016;9:464–470. doi: 10.1093/tropej/fmw032. [DOI] [PubMed] [Google Scholar]
- 11.McFarland L.V. Systematic review and metanalysis of Saccharomyces boulardii in adult patients World. J Gastroenterol. 2010;14(16):2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatri I., Tomar R., Ganesan K., Prasad G.S., Subramanian S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci Rep. 2017;7:371. doi: 10.1038/s41598-017-00414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash D., Dash M., Mohanty M., Acharya N. Efficacy of probiotic Saccharomyces boulardii as an adjuvant therapy in acute childhood diarrhoea. J Nepal Paediat Soc. 2017;36:250–255. [Google Scholar]
- 14.Riaz M., Alam S., Malik A., Ali S. Efficacy and safety of Saccharomyces boulardii in acute childhood diarrhea: a double blind randomised controlled trial. Ind J Pediat. 2011;79:478–482. doi: 10.1007/s12098-011-0573-z. [DOI] [PubMed] [Google Scholar]
- 15.Szajewska H., Skórka A. Saccharomyces boulardii for treating acute gastroenteritis in children: updated meta-analysis of randomized controlled trials. Alim Pharmacol Ther. 2009;30:960–961. doi: 10.1111/j.1365-2036.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- 16.Billoo A. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J Gastroenterol. 2006;12:4557. doi: 10.3748/wjg.v12.i28.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarruel G., Rubio D., Lopez F., Cintioni J., Gurevech R., Romero G. Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study. Acta Paediat. 2007;96:538–541. doi: 10.1111/j.1651-2227.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Htwe K., Yee K., Vandenplas Y., Tin M. Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized controlled study. Am J Trop Med Hyg. 2008;78:214–216. [PubMed] [Google Scholar]
- 19.Kabbani T., Pallav K., Dowd S., Villafuerte-Galvez J., Vanga R., Castillo N. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbe. 2016;8:17–32. doi: 10.1080/19490976.2016.1267890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szajewska H., Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801. doi: 10.1111/apt.13344. [DOI] [PubMed] [Google Scholar]
- 21.Le Luyer B., Makhoul G., Duhamel J. Étude multicentrique, contrôlée en double insu d’une formule adaptée enrichie en Saccharomyces boulardii dans le traitement des diarrhées aiguës du nourrisson. Archiv Pédiat. 2010;17:459–465. doi: 10.1016/j.arcped.2010.02.004. [DOI] [PubMed] [Google Scholar]