Significance
Oxidative stress, which accompanies sterile inflammation, induces release of extracellular vesicles (stressEVs), which activate Toll-like receptor 4 (TLR4), resulting in a pattern of gene expression distinct from response triggered by bacterial lipopolysaccharide. The synergy between 15-lipoxygenase and secreted phospholipase A2, both of which are induced by stress, is necessary for the formation of oxidized lysophospholipids, TLR4 endogenous agonists. Moreover, TLR4 was necessary for sPLA2 promoted K/BxN serum-induced arthritis. As the formation of TLR4 agonists is enzyme driven, it provides an opportunity to inhibit these enzymes to limit sterile inflammation.
Keywords: oxidative stress, extracellular vesicles, Toll-like receptor 4, 15-lipoxygenase, phospholipase A2
Abstract
Damage-associated endogenous molecules induce innate immune response, thus making sterile inflammation medically relevant. Stress-derived extracellular vesicles (stressEVs) released during oxidative stress conditions were previously found to activate Toll-like receptor 4 (TLR4), resulting in expression of a different pattern of immune response proteins in comparison to lipopolysaccharide (LPS), underlying the differences between pathogen-induced and sterile inflammation. Here we report that synergistic activities of 15-lipoxygenase (15-LO) and secreted phospholipase A2 (sPLA2) are needed for the formation of TLR4 agonists, which were identified as lysophospholipids (lysoPLs) with oxidized unsaturated acyl chain. Hydroxy, hydroperoxy, and keto products of 2-arachidonoyl-lysoPI oxidation by 15-LO were identified by mass spectrometry (MS), and they activated the same gene pattern as stressEVs. Extracellular PLA2 activity was detected in the synovial fluid from rheumatoid arthritis and gout patients. Furthermore, injection of sPLA2 promoted K/BxN serum-induced arthritis in mice, whereby ankle swelling was partially TLR4 dependent. Results confirm the role of oxidized lysoPL of stressEVs in sterile inflammation that promotes chronic diseases. Both 15-LO and sPLA2 enzymes are induced during inflammation, which opens the opportunity for therapy without compromising innate immunity against pathogens.
The innate immune system protects the organism against pathogens and endogenous damage; however, dysregulated inflammation may lead to pathology. In infection, tissue damage caused by acute inflammation is usually limited, but sterile inflammation may lead to chronic inflammation and tissue damage (1). Toll-like receptors (TLRs) and inflammasomes are important for recognizing pathogen-associated molecular patterns (PAMPs). In addition, they can also be activated by endogenous factors, generally known as damage-associated molecular patterns (DAMPs) that are released as a consequence of trauma and tissue injury in a broad range of sterile inflammatory diseases (2) such as: ischemia/reperfusion injury (IRI) (3), autoimmune diseases (4), and aging-related pathologies (5). Sterile inflammation, which is evoked by tissue damage, is accompanied by oxidative stress, whereby oxidative damage of biologically important molecules, such as phospholipids (PLs), DNA, proteins, and other cellular components, can occur and may convert them to DAMPs, which act as stress-induced signaling molecules (6–8). According to recent reports, the latter can be released from cells into the bloodstream by extracellular vesicles (9). Additionally, several enzymes, among them lipoxygenases and phospholipases, are induced by stress and contribute to inflammation (10, 11), participating in the formation of DAMPs.
Extracellular vesicles (microvesicles and exosomes; EVs) are released by cells under normal and stress conditions and have been detected in all body fluids (12). They can carry proinflammatory molecules (13) and as such contribute to disease progression. We have reported previously that the TLR4/MD-2 complex is activated by the EVs released after oxidative stress (stressEVs). As inactive EVs were converted into active EVs by partial oxidation through the Fenton reaction, we concluded that oxidative stress and oxidation of phospholipids are important for TLR4 agonist formation (14). Unsaturated fatty acid oxidizing enzymes like lipoxygenases (LOs) are induced by stress and contribute to inflammation (15). They are one of two types of enzymes capable of direct peroxidation of PLs and actually contribute to the regulation of the inflammatory response as their oxidized products can exert proinflammatory activity distinct from the action triggered by bacterial products (16). Also several phospholipases A2 (especially secreted PLA2 [sPLA2]) are elevated during inflammation, correlating with severity of the condition (17). sPLA2 enzymatic activity was detected in several examined synovial fluids, including rheumatoid, osteoarthritic, psoriatic, and gouty fluids (18). Duchez et al. (19) observed increased ankle thickness and inflammation in K/BxN serum-induced arthritis in mice with 12-LO (a mouse ortholog of human 15-LO) and human sPLA2-IIA expression in comparison to mice lacking one or both enzymes. In addition, several other studies have shown that oxidized PLs modulate TLR4 activity (14, 20–22) and that they can be involved in sterile inflammation-promoted diseases including atherosclerosis and rheumatoid arthritis (RA) (23–26). Published data imply that both enzymes could contribute to TLR4 agonist formation in chronic diseases.
RNA microarray analysis revealed differences between LPS and stress-derived EV (stressEVs) signaling (14). In this new study several differently expressed genes involved in immune response were selected and their expression was compared after activation of TLR4/MD-2 by LPS, stressEVs, and two putative mouse TLR4/MD-2 agonists (lipid IVa and paclitaxel). StressEVs and paclitaxel activated proinflammatory Ccl24 and Il23 cytokine gene expression and antiinflammatory genes, Tnfaip6 and Socs2. No expression or only minor expression of these genes was detected after LPS or lipid IVa stimulation. Additionally, no induction of tolerance in macrophages by stressEVs was observed. The results underlie the differences in classical (pathogen-induced) and sterile inflammation. Eicosanoid-lysolipids, namely lysophospholipids (lysoPLs) with oxidized arachidonoyl acyl chain (e.g., HpETE-LPC, HETE-LPC) were first determined by Liu et al. (27, 28) in mice and human tissues and their role as possible signaling molecules was purposed, but neither their receptor nor signaling pathway had been identified. Herein we found that 15-LO oxidized 2-arachidonoyl-lysoPLs are more potent agonists for TLR4/MD-2 than oxidized PLs with two acyl chains. Decreased 15-LO expression in cells resulted in release of stressEVs with reduced potency of TLR4 activation confirming the role of 15-LO as the physiological producer of biologically active oxidized PL agonists. Additionally, we show that the formation of lysoPLs depends on the activity of sPLA2s, which are activated during inflammation. Incubation of different sPLA2s with 15-LO oxidized synthetic EVs (synEVs) resulted in higher TLR4 activity. To summarize, we identified and confirmed that eicosanoid-lysolipids can act as signaling molecules that can be delivered to other cells as part of EVs and we identified TLR4/MD-2 as their receptor. Moreover, synovial fluids from patients with RA and gout exerted sPLA2 activity and injection of sPLA2-IIA to mice with K/BxN-induced arthritis promoted the ankle swelling and was partially TLR4 dependent, confirming that both enzymes are needed for the formation of TLR4 agonists and contribute to the disease progression. As the formation of TLR4 agonists is enzyme driven, it opens an option of applying specific inhibitors that could limit the sterile inflammation.
Materials and Methods
More details are provided in SI Appendix.
Isolation and Preparation of EVs.
StressEVs were collected after a 50-min to 1-h stimulation (when cells start to detach) of HEK293 cells with 10 or 12 μM calcium ionophore A23187 (Sigma) in Hanksʾ balanced salt solution (HBSS) buffer (Invitrogen) containing 2.5 mM CaCl2. Supernatants were centrifuged at 800 × g to remove the cells and by two time ultracentrifugation at 100,000 × g (rotor types 50TI or 42.1; Beckman Coulter) stressEVs were isolated. StressEVs were resuspended in phosphate buffered saline (PBS) and their size was analyzed by dynamic light scattering (DLS; Malvern). Their concentration was determined by the bicinchonic acid (BCA) assay (Sigma).
SynEVs composed of only PLs were prepared in two ways: First, natural PLs and lysoPLs were dissolved and mixed in organic solvent in ratios determined in EVs by Weerheim et al. (29) (SI Appendix, Table S2). Second, 30% of 1,2-diarachidonoyl-sn-glycero-3-PE (AAPE) and 70% of 1-palmitoyl-2-oleoyl-glycero-3-PC (POPC) (% weight) were dissolved and mixed in organic solvent. Organic solvent was evaporated. PLs were hydrated in PBS at 65 °C for 2 h. The hydrated lipid solution went through two freeze-thaw-vortex cycles in liquid nitrogen. The synEVs were prepared by extrusion through 100-nm polycarbonate membranes (Avanti Polar Lipids). The size of synEVs was confirmed by DLS.
Lipoxygenase 15-LO Oxidation and PLA2 Hydrolysis.
Lipoxygenase 15-LO was immobilized to magnetic beads according to manufacturers’ instructions. A total of 0.2 mg of 15-LO was bound to 100 μL of beads. According to spectra at 280 nm, approximately 30% of the added 15-LO bound to the beads. SynEVs, single PLs, and lysoPLs from natural sources or synthetic 20:4 lysoPI (all dissolved in organic solvent, evaporated, resuspended and hydrated in PBS) were incubated with 10 μg of 15-LO in 50 mM borate buffer, pH 9.0, at room temperature for 10 min. The 15-LO was removed with a magnet and oxidized compounds were tested for their TLR4 activity or further hydrolyzed.
SynEVs or oxidized synEVs (composed of 30% AAPE and 70% POPC) (160 μg/mL) were incubated with porcine sPLA2-IB, recombinant human group IIA (sPLA2-IIA), or recombinant human group X sPLA2 (sPLA2-X) (both were prepared as described in refs. 30 and 31) (all 30 μg/mL) or with 5× concentrated synovial fluid for 2 h at 37 °C in the presence of 2 mM CaCl2. The reaction was stopped by the addition of the pan-sPLA2 inhibitor varespladib (50 μM).
Gene Set Enrichment Analysis.
Data that we deposited to Gene Expression Omnibus (accession number: GSE45234) (14) were obtained from bone marrow-derived macrophages (BMDM) stimulation with LPS or stressEVs. To determine which sets of genes are statistically significantly different in expression between stressEVs- and LPS-stimulated cells we analyzed the microarray data using gene set enrichment analysis (GSEA) (32). We used Gene Ontology for gene set annotation. We displayed stressEVs correlated gene sets with high significance only (false discovery rate [FDR] < 0.001) using the Enrichment Map visualization tool (33). We manually identified clusters of gene sets so that there are no shared genes between sets in different clusters. Moreover, for each cluster we merged all of the gene sets into a gene list and sorted it according to the default GSEA ranking metric signal to noise. We display the expression values for the top 15 genes according to that metric.
Determination of sPLA2 Activity in Synovial Fluid.
Synovial fluids from patients with RA and gout were collected during routine clinical procedures, transferred into 15-mL tubes, and immediately centrifuged for 10 min at 1,300 × g, at room temperature. Supernatants were frozen at −80 °C until analysis. Approval for the use of patient synovial fluid aspirates was obtained after informed consent which was approved by the Slovene National Medical Ethics Committee (approval no. 0120-623/2017/6).
Samples were ultracentrifuged at 100,000 × g for 1 h to remove EVs at 3× concentration. sPLA2 enzymatic activity was assayed using a sensitive fluorometric assay (34, 35) with sonicated vesicles composed of 1-palmitoyl-2-pyrenedecanoyl-sn-glycero-3-phosphoglycerol (Invitrogen) on a Safire 2 microplate reader (Tecan). The specific amount of recombinant sPLA2 was used as standard.
To reduce viscosity, samples were incubated with 10 units/mL of hyaluronidase (Sigma) for 1 h at 37 °C. Samples were then concentrated at 3-kDa cutoff (Amicon Ultra; Millipore) five times and added to the 15-LO oxidized synEVs.
Arthritis Model.
C57BL/6 OlaHsd and B6.129-TLR4tm1Aki (TLR4KO) mice were used for K/BxN serum transfer-induced arthritis model. Eight-week-old female mice were used for the experiments. Animals were housed in individually ventilated cages under specific pathogen-free conditions. Mice were housed in a 12-h light/dark cycle and given standard mouse chow and water ad libitum. All animal experiments were performed according to the directives of the EU 2010/63 and approved by the Administration of the Republic of Slovenia for Food Safety, Veterinary and Plant Protection of the Ministry ofAgriculture, Forestry and Foods, Republic of Slovenia (approval no. U34401-14/2019/8).
For K/BxN serum-induced arthritis (36), we injected mice with 150 μL of K/BxN serum intraperitoneally (i.p.). On day 2, an additional 90 μL of K/BxN serum was i.p. administered. To observe additional inflammatory effects of sPLA2 on arthritis severity and development, 10 μL of recombinant human sPLA2-IIA (9.5 μg) was injected into the left knee joint using a 30-G needle (Beckmen). Then mice were monitored for 14 d for arthritis development. Thickness of knee joints was measured daily using calipers under isoflurane anesthesia. In wild-type (WT) and TLR4 knockout (KO) mice (both n = 5) both ankles were measured in WT+sPLA2 (n = 5) and TLR4+sPLA2 (n = 4) mice. Only left ankles were measured.
Statistical Analysis.
GraphPad Prism 5 was used for preparing the graphs and performing statistical analysis. In vitro data from two or three independent experiments are shown. Statistical analysis (SEM and Student’s t test) was done from pooled data from independent experiments. When values between WT and KO cells were compared, one-tailed tests were used. When variance between independent experiments was high, a logarithmic (natural logarithm - LN) transformation was applied for the data used in statistical tests. In vivo data showing delta ankle thickness are presented as mean ± SEM. For this experiment, two-way ANOVA was used for statistical analysis.
Results
LPS and StressEVs Differently Activate Immune Response Genes.
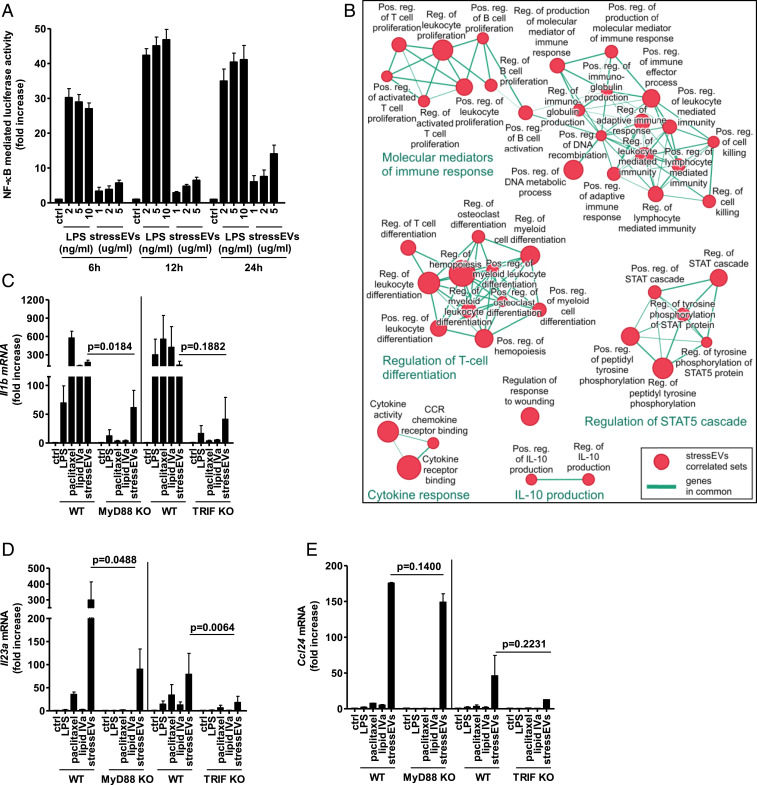
Our previous analysis determined that LPS and stressEVs stimulate gene expression differently in BMDMs (14), which led us to propose that distinct signaling pathways are induced by the two TLR4 agonists. To further elucidate the differences, stressEVs were isolated after oxidative stress and analyzed for their size distribution by DLS. The presence of exosomes and microvesicles was determined (SI Appendix, Fig. S1A) and confirmed in fractions (fr.) collected after density gradient ultracentrifugation by Tsg101 and CD81 markers (mainly in fr. 6 and 7) (SI Appendix, Fig. S1B). Annexin V was detected in most fractions (fr. 3 to 8) and calnexin was detected mainly in fr. 8, showing that different populations of EVs are present. The activation of NF-κB was tested and it was found that LPS and stressEVs differed in the strength and the time course of gene expression. LPS was an inducer of NF-κB (Fig. 1A) with the maximum response reached after 12 h in comparison to stressEVs where increase in NF-κB activity was observed even after 24 h. On the other hand, stressEVs but not LPS were able to activate several other transcription factor (TF) and response elements (such as AP-1, CRE, SRE, and in a minor way also NF-κB) in a TLR4-independent manner as well (SI Appendix, Fig. S2 A–C), implying that different molecules present in stressEVs can mediate signaling, thus showing the heterogeneity of stressEVs.
Fig. 1.
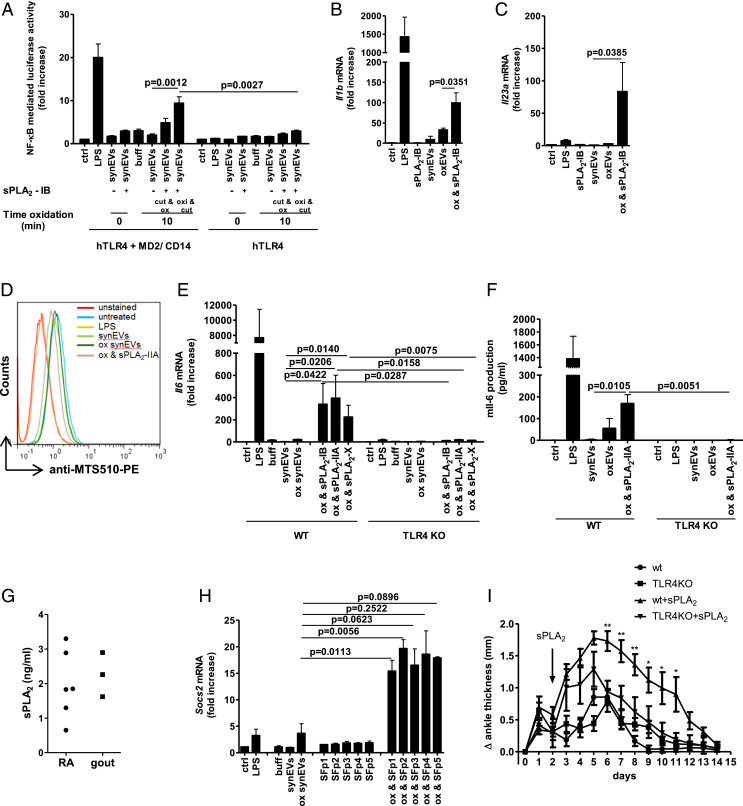
StressEVs and paclitaxel stimulate TLR4/MD-2 differently from LPS and lipid IVa. StressEVs were isolated from HEK293 cells stimulated with A23187. (A) HEK293T cells were transfected with plasmids for hTLR4, hMD-2/CD14, firefly luciferase under NF-κB promotor and Renilla luciferase for normalization. HEK293T cells were stimulated with stressEVs (1, 2, or 5 μg/mL) or LPS (2, 5, or 10 ng/mL) for 6 h, 12 h, or 24 h. A dual luciferase test was performed. Negative controls are transfected but unstimulated cells. (B) The Enrichment Map displays the enriched gene sets in stressEV-stimulated cells as analyzed by GSEA. Red dots represent increased expression, and the dot size is proportional to the enrichment significance. Related gene sets (with some genes in common) were manually grouped into clusters and assigned a label. We display only the sets with high significance of differential expression (FDR < 0.001). (C–E) WT or MyD88 KO macrophages and WT or TRIF KO BMDMs were stimulated for 6 h with paclitaxel (50 μM), lipid IVa (100 ng/mL), stressEVs (10 μg/mL), or with LPS (2 ng/mL). Il1b, Il23a, and Ccl24 mRNA levels were determined using qPCR. (A) Three independent experiments (indep. exps.) (n = 9); (C and D) three indep. exps. (n = 3); t test (one-tailed; paired); (E) two indep. exps. (n = 2); t test (one-tailed; paired).
Several genes that were up-regulated in the microarray analysis by both LPS and stressEVs (such as Il6 and Il1b) and several genes that showed significant differences, when induced by stressEVs in comparison to LPS (ref. 14 and SI Appendix, Fig. S3), were selected. Il23, Ccl24, Tnfaip6, and Socs2 are involved in the immune response and by using GSEA analysis they were evaluated as being a part of clusters of gene sets assigned as the molecular mediator of immune response, regulation of the response to wounding, cytokine response, and others (Fig. 1B).
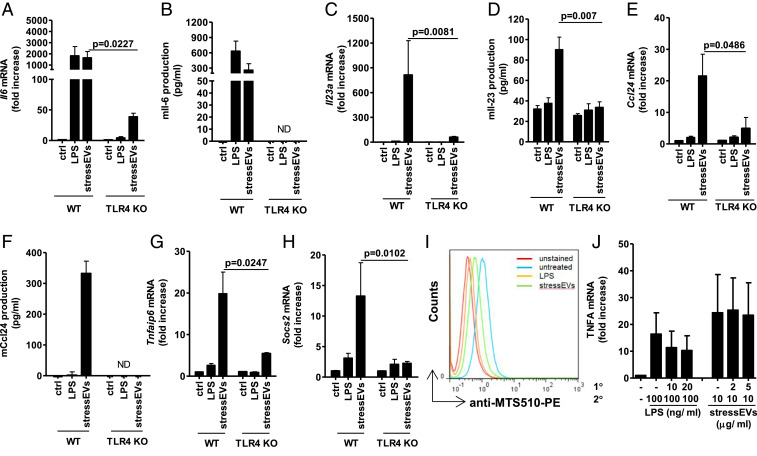
Along with LPS and stressEVs, lipid IVa, and paclitaxel, both putative mouse TLR4 agonists (37, 38) were tested for their effects on gene expression in macrophages. The expressions of Il1b and Il6 (Fig. 1C and SI Appendix, Fig. S4A) were augmented by all agonists, whereas Il23, Ccl24 (Fig. 1 D and E), Tnfaip6, and Socs2 (SI Appendix, Fig. S4 B and C) were more strongly induced by stressEVs and paclitaxel stimulation than by LPS or lipid IVa. However, all of the observed agonist-induced changes were dependent on both MyD88 and TRIF pathways (Fig. 1 C–E and SI Appendix, Fig. S4 A–C), showing the differences in signaling downstream of these two adapter proteins. Additionally, for stressEVs we showed that activation of these genes and cytokine expression were indeed to a large degree TLR4 dependent also at the cytokine level (Fig. 2 A–H). The addition of stressEVs to macrophages prevented recognition of TLR4/MD-2 monomer by MTS510 Ab (Fig. 2I), implying clustering of the receptor. The results show that structurally different agonists targeting the same receptor may trigger different responses.
Fig. 2.
StressEVs activate immune response genes through TLR4/MD-2, but do not induce cell tolerance. (A, C, E, G, and H) WT or TLR4 KO BMDMs were stimulated for 6 h with stressEVs (10 μg/mL) or LPS (2 ng/mL). Il6, Il23a, Ccl24, Tnfaip6, and Socs2 mRNA levels were determined using qPCR. (B, D, and F) WT or TLR4 KO BMDMs were stimulated for 16 h with LPS (2 ng/mL) or stressEVs (5 μg/mL in F or 10 μg/mL). Il-6 and Ccl24 concentrations in supernatants or Il-23 production in cell lysate were measured by ELISA. ND, not detected. (I) RAW 264.7 cells were stimulated with stressEVs (10 μg/mL) or LPS (1 μg/mL) as a positive control for 4 h. TLR4/MD-2 dimerization using anti-MTS510-PE Ab was determined by flow cytometry. (J) THP-1 cells were treated for 8 h with low concentrations of LPS (10 or 20 ng/mL) or stressEVs (1 or 2 μg/mL), followed by additional LPS (100 ng/mL) or stressEV (10 μg/mL) challenge for 18 h. TNFα levels were determined using qPCR. (A, C, and E) Four indep. exps. (n = 5); t test (one-tailed; paired); (B and D) two indep. exps. (n = 4); t test (one-tailed; unequal variance); (F) two indep. exps. (n = 3); (G) three indep. exps. (n = 3); t test (one-tailed; paired); (H) three indep. exps. (n = 4); t test (one-tailed; paired); (J) three indep. exps. (n = 3). For I, an additional indep. exp. is shown in SI Appendix, Additional data.
LPS stimulation of monocytes/macrophages makes them temporarily refractory to the subsequent LPS challenge, a phenomenon called endotoxin tolerance, an important protective mechanism (39). Interestingly the stimulation of THP-1 macrophages by the stressEVs did not induce the tolerance to the subsequent stressEV (Fig. 2J) or LPS treatment (SI Appendix, Fig. S4D), pointing to an additional difference that might contribute to the persistent inflammation in chronic diseases.
The 15-Lipoxygenase-Oxidized lysoPLs Are TLR4/MD-2 Ligands.
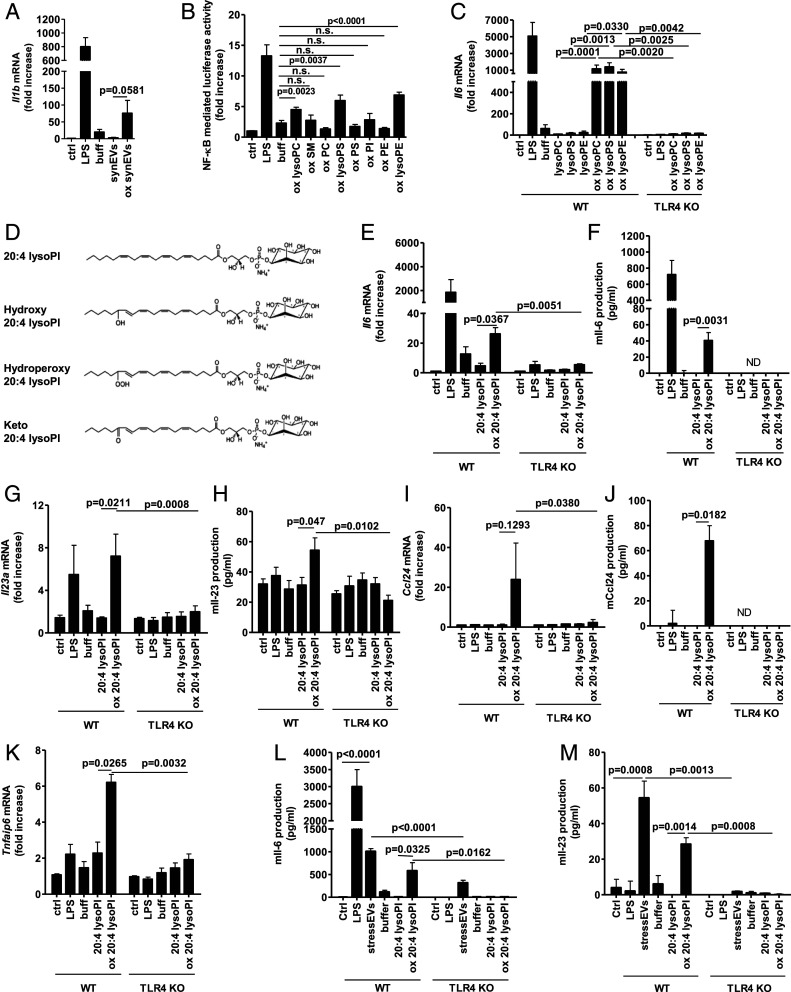
Lipoxygenase 15-LO is involved in several chronic diseases from diabetes to atherosclerosis (40). Its role in the formation of TLR4/MD-2 ligands has already been suggested (14). PLs share some structural motifs with LPS, therefore we prepared synEVs from a mixture of natural PLs that are the constituents of EVs (29). SynEVs were oxidized by 15-LO, immobilized to magnetic beads so that the enzyme could be easily removed, and their activity was tested. 15-LO-oxidized but not untreated synEVs triggered Il1b and Il6 gene expression (Fig. 3A and SI Appendix, Fig. S5A). To determine the identity of TLR4/MD-2 activating molecular species, each PL and lysoPL component was oxidized separately. Interestingly, only 15-LO-oxidized lysoPLs (lysoPC, lysoPS, and lysoPE) but not oxidized PLs activated NF-κB (Fig. 3B) and Il6 and Il1b gene expression in a TLR4-dependent manner (Fig. 3SI Appendix, Fig. S5B). The PLs and lysoPLs used were extracted from natural sources and comprise hydrophilic head groups with diverse acyl chains, which makes it difficult to determine the structural determinants of an endogenous TLR4 agonist. To confirm that lysoPLs are indeed TLR4/MD-2 ligands we used the synthetic 2-arachidonoyl-lysophosphatidylinositol (20:4 lysoPI), a substrate for 15-LO oxidation. The oxidized products of 20:4 lysoPI were analyzed by mass spectrometry (MS) (SI Appendix, Fig. S5C), which identified eicosanoid-lysoPL products, namely with hydroxyl (HETE-LPI product), hydroperoxyl (HpETE-LPI product), or keto group (Fig. 3D). This oxidized 20:4 lysoPI activated cells where gene and cytokine expression was mainly TLR4-dependent, as shown by a low response in TLR4 KO macrophages (Fig. 3 E–K). The MyD88 pathway was involved as well (SI Appendix, Fig. S5 D and E) and the same genes were activated as by stressEVs (Fig. 2 A–H). As low cytokine release was observed in BMDMs after stimulation especially with oxidized 20:4 lysoPI, dendritic cells, as another type of relevant immune cell in chronic diseases, were stimulated as well. BMDCs responded with increased Il-6 expression to both stressEVs and even better to oxidized 20:4 lysoPI (Fig. 3L) in comparison to BMDMs. Furthermore, Il-23 was detected in supernatants (Fig. 3M) in a TLR4-dependent manner.
Fig. 3.
The 15-LO-oxidized lysoPLs are TLR4-activating ligands. SynEVs composed of PLs and lysoPLs were prepared. (A, C, E, G, I, and K) WT or TLR4 KO BMDMs were stimulated for 6 h with synEVs or oxidized synEVs (10 μg/mL) in A, singlePLs or oxidized single PLs (10 μg/mL) in C, 20:4 lysoPI or oxidized 20:4 lysoPI (5 μg/mL) in E, G, I, and K, or LPS (2 ng/mL) as a positive control. Il1b, Il6, Il23a, Ccl24, and Tnfaip6 mRNA levels were determined using qPCR. (B) HEK293T cells expressing hTLR4 and hMD-2/CD14 as well as firefly luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with oxidized single PLs (5 μg/mL) or LPS (10 ng/mL) as a positive control for 24 h. Negative controls are transfected but unstimulated cells. A dual luciferase test for NF-κB activity was performed. (D) The 15-LO oxidation products of 20:4 lysoPI that were determined by MS. (F, H, and J) WT or TLR4 KO BMDMs were stimulated for 16 h with LPS (2 ng/mL), 20:4 lysoPI, or oxidized 20:4 lysoPI (10 μg/mL). Il-6 and Ccl24 concentrations in supernatants or Il-23 production in cell lysate were measured by ELISA. ND, not detected. (L and M) WT or TLR4 KO BMDCs were stimulated for 16 or 8 h to detect Il-6 and Il-23, respectively, with LPS (2 ng/mL), stressEVs (10 μg/mL), 20:4 lysoPI, or oxidized 20:4 lysoPI (10 μg/mL). Il-6 and Il-23 concentrations in supernatants were measured by ELISA. (A, C, and K) Three indep. exps. (n = 3); t test (two-tailed; paired or one-tailed; paired for KO); (B) three indep. exps. (n = 6 or 9); t test (two-tailed; unequal var.); n.s., not significant; (E and G) three indep. exps. (n = 4); t test (two-tailed; paired or one-tailed; paired for KO); (I) two indep. exps. (n = 2); t test (two-tailed; paired or one-tailed; paired for KO); (F) two indep. exps. (n = 5); t test (two-tailed; unequal var.); (H) two indep. exps. (n = 4); t test (two-tailed; unequal var. or one-tailed; unequal var. for KO); (J) two indep. exps. (n = 3); t test (two-tailed; unequal var.); and (L and M) two indep. exps. (n = 6); t test (two-tailed; unequal var. or one-tailed; unequal var. for KO).
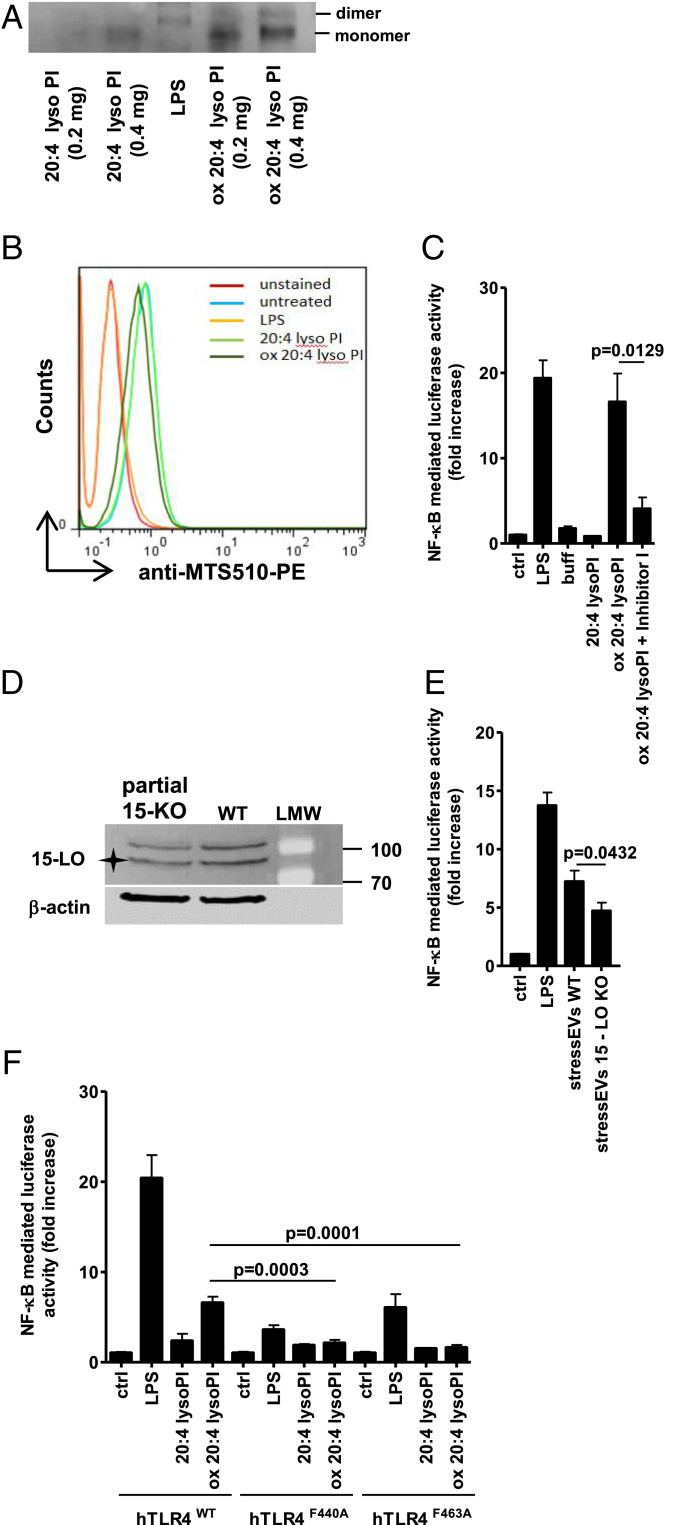
Oxidized 20:4 lysoPI-induced TLR4 dimerization was observed by native polyacrylamide gel electrophoresis (PAGE) though less prominent than with LPS (Fig. 4A). Also a minor shift in binding of MTS510 Ab to TLR4/MD-2 monomer was observed (Fig. 4B). Importantly, oxidized 20:4 lysoPI activated NF-κB TFs in a TLR4-dependent manner, but not other response elements, which were activated by stressEVs (SI Appendix, Fig. S6 A and B), further confirming oxidized lysoPLs as the TLR4-activating ligands.
Fig. 4.
Mechanism of oxidized PL formation and TLR4 signaling. (A) Indicated amounts of 20:4 lysoPI or oxidized 20:4 lysoPI were incubated with TLR4/MD-2 recombinant protein (0.5 μg) and then samples were loaded to 10% native PAGE. The dimerization of TLR4/MD-2 was analyzed by Western blot. (B) RAW 264.7 cells were stimulated with 20:4 lysoPI, oxidized 20:4 lysoPI (40 μg/mL), or LPS (1 μg/mL) as a positive control for 6 h. TLR4/MD-2 dimerization using anti-MTS510-PE Ab was determined by flow cytometry. (C and E) HEK293T cells expressing hTLR4, hMD-2/CD14, as well as firefly luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with 20:4 lysoPI (5 μg/mL), oxidized 20:4 lysoPI (5 μg/mL) in the presence or absence of Inhibitor I (1 mM) in C, stressEVs WT (5 μg/mL), stressEVs 15-LO KO (5 μg/mL) in E or LPS (10 ng/mL) as a positive control for 24 h. Dual luciferase tests for NF-κB activity were performed. (D) Western blot detection of 15-LO and β-actin (loading control) in WT and partial 15-LO KO HEK293 cells. Asterisk indicates specific band. (F) HEK293T cells expressing hMD-2/CD14 and hTLR4WT, hTLR4F440A, or hTLR4F463A as well as luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with 20:4 lysoPI (5 μg/mL), oxidized 20:4 lysoPI (5 μg/mL), or LPS (10 ng/mL) as a positive control for 24 h. Dual luciferase test for NF-κB activity was performed. (C) Two indep. exps. (n = 6); t test (two-tailed; unequal var.); (E) three indep. exps. (n = 6 or 12); t test (two-tailed; unequal var.); (F) three indep. exps. (n = 8); t test (two-tailed; unequal var.). For B an additional indep. exp. is shown in SI Appendix, Additional data.
To further confirm that 15-LO-mediated lysoPI oxidation is important for the agonistic activity Inhibitor I, a specific inhibitor of 15-LO, was used. Inhibitor I, when added during 15-LO oxidation, significantly reduced TLR4 activation by oxidized 20:4 lysoPI (Fig. 4C). Additionally, to confirm the role of 15-LO in the formation of TLR4-active ligands at the cellular level, we tried to prepare the 15-LO KO HEK293 cells by using CRISPR/Cas9 technology. We were able to decrease but not fully abolish 15-LO expression (Fig. 4D and SI Appendix, Fig. S7), which was sufficient to observe reduced activity of isolated stressEVs in comparison to stressEVs isolated from WT cells (Fig. 4E).
LPS mediates dimerization of the TLR4/MD-2 complex by interacting with the secondary hydrophobic binding site on the TLR4 ectodomain involving Phe residues at positions 440 and 463 in hTLR4, and mutants at those sites lost the ability for activation by LPS (41). TLR4F440A and TLR4F463A mutants expressed in HEK293T cells were stimulated with LPS or 15-LO-oxidized 20:4 lysoPI. Decreased NF-κB activity was detected by both ligands, implying that LPS and oxidized lysoPI share the same mechanism of interaction with TLR4 (Fig. 4F).
Lipoxygenase 15-LO and PLA2 Activities Are Necessary for Endogenous Agonist Formation.
LysoPLs are not present as abundantly as PLs, so there might be a mechanism to increase their formation. Several human PLA2 variants exist and, in particular, secreted PLA2 proteins (sPLA2s) are elevated during inflammation (10). PLA2s hydrolyze PLs at the sn-2 position, releasing free fatty acids and lysoPLs. To test whether PLA2 activity may be important for providing lysoPLs as the substrate for oxidation by 15-LO, we used AAPE. As PLA2s exhibit increased activity when the substrate is presented in aggregates rather than in the monomeric form (42) we prepared synEVs with a diameter of 100 nm (as determined by DLS; SI Appendix, Fig. S1C) containing 30% AAPE and 70% POPC. The synEVs were oxidized with 15-LO and then hydrolyzed by porcine pancreatic sPLA2-IB or first hydrolyzed and then oxidized. Oxidation followed by the hydrolysis of synEVs resulted in higher TLR4/MD-2 activity (Fig. 5A) than the reverse order and Il1b gene expression (Fig. 5B). Both enzymes were also needed for the increased Il23 and Il6 gene expressions by synEVs (Fig. 5C and SI Appendix, Fig. S8A). Along with the porcine pancreatic sPLA2-IB, recombinant human sPLA2-IIA and sPLA2-X were tested. None of the sPLA2s alone activated TLR4/MD-2 receptor (SI Appendix, Fig. S8B), but similarly both human sPLA2s promoted formation of TLR4 agonists together with 15-LO synergy, which bound to TLR4/MD-2 receptor (Fig. 5D), resulting in cytokine expression and Il-6 release (Fig. 5 E and F and SI Appendix, Fig. S8C), confirming the role of both enzymes in ligand formation.
Fig. 5.
Concerted 15-LO and sPLA2 activity is needed for formation of TLR4-activating ligands in synEVs. SynEVs composed of 30% AAPE and 70% POPC were prepared. (A) HEK293T cells expressing hTLR4 and hMD-2/CD14 or hTLR4 alone as well as firefly luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with synEVs (10 μg/mL), hydrolyzed and oxidized synEVs, oxidized and hydrolyzed synEVs (10 μg/mL), or LPS (10 ng/mL) as a positive control for 24 h. Negative controls are transfected but unstimulated cells. A dual luciferase test for NF-κB activity was performed. (B, C, E, and H) WT macrophages (B, C, and H) and WT or TLR4 KO BMDMs (E) were stimulated for 6 h with indicated sPLA2 (1.9 μg/mL) or synovial fluid (SF) (14,7 μL), synEVs (10 μg/mL), oxidized synEVs (10 μg/mL), indicated oxidized and hydrolyzed synEVs (10 μg/mL) or LPS (2 ng/mL) as a positive control. Il1b, Il6, Il23a, and Socs2 mRNA levels were determined using qPCR. (D) RAW 264.7 cells were stimulated with synEVs, oxidized synEVs, oxidized and hydrolyzed sPLA2-IIA synEVs (40 μg/mL), or LPS (1 μg/mL) as a positive control for 6 h. TLR4/MD-2 dimerization using anti-MTS510-PE Ab was determined by flow cytometry. (F) WT or TLR4 KO BMDMs were stimulated for 16 h with synEVs (10 μg/mL), oxidized synEVs (10 μg/mL), oxidized and hydrolyzed sPLA2-IIA synEVs (10 μg/mL), or LPS (2 ng/mL) as a positive control. Il-6 in supernatants was measured by ELISA. (G) PLA2 activity was measured in the SF (ultracentrifuged and concentrated 3×) from RA and gout patients. (G) Mice (n = 4 to 5 per group) were injected with 150 and 90 μL K/BxN serum on days 0 and 2. On day 2 recombinant human sPLA2-II (9.5 μg) was injected. The development of arthritis was followed for 14 d. (A) Three indep. exps. (n = 6 or 9); t test (two-tailed; unequal var.); (B) three indep. exps. (n = 3); t test (two-tailed; paired); (C and E) three indep. exps. (n = 4); t test (two-tailed; paired or one-tailed; paired for KO). For D, additional an indep. exp. is shown in SI Appendix, Additional data. (F) Two indep. exps. (n = 6); t test (two-tailed; unequal var. or one-tailed; unequal var. for KO); (H) two indep. exps. (n = 2); t test (two-tailed; paired); (I) two-way ANOVA between WT+sPLA2 and TLR4KO+sPLA2 (n = 4 or 5); *P < 0.05, **P < 0.01.
Recently it was shown that arachidonic acid inhibits TLR4 receptor signaling (43). PLA2 hydrolysis of oxAAPE could also result in the release of the 15-LO-oxidized arachidonic acid signaling molecule (15-hydroperoxyeicosatetraenoic acid; 15-HpETE), however 15-HpETE did not activate TLR4/MD-2 receptor (SI Appendix, Fig. S9) and activation of TLR4 KO BMDMs with oxidized and hydrolyzed synEVs was minimal (Fig. 5 E and F and SI Appendix, Fig. S8C), excluding 15-HpETE as the major contributor to TLR4-dependent gene expression.
PLA2 Promotes TLR4-Mediated Arthritis.
Synovial fluid from RA patients exerts sPLA2 activity and the presence of several sPLA2 isoforms has been reported (44) that might contribute to ligand formation (17). Synovial fluid samples from RA and gout patients were collected and indeed sPLA2 activity was detected (Fig. 5G). The synovial fluid supernatants were ultracentrifuged at 100,000 × g to remove the EVs, and added to 15-LO-oxidized synEVs. No increase in proinflammatory cytokine mRNAs was detected (SI Appendix, Fig.S10 A and B), but an increase in antiinflammatory Socs2 and Tnfaip6 mRNA was measured (Fig. 5H and SI Appendix, Fig. S10C).
Several eicosanoid-lysolipids (oxidized 2-arachidonoyl-lysoPLs), among them also 15-HETE-lysoPC, have been identified in murine hepatic tissue and human myocardium (27). Especially the amount of 15-HETE-lysoPC was increased in lung fibroblasts when they were treated with calcium ionophore (27). We tried to detect 15-HETE-lysoPLs in synovial fluid by mass spectroscopy. Although 2-arachidonoyl-lysoPLs were detected, we were not able to detect oxidized 15-HETE-lysoPL products. We only detected compounds that had very similar retention times as the 15-LO-oxidized lysoPL standards, so we assume that they are very likely very similar compounds.
To further confirm the pathophysiological relevance of sPLA2, K/BxN serum was injected i.p. into WT and TLR4KO mice. No difference between ankle thickness of WT and TLR4KO mice was observed when only serum was injected (Fig. 5I). As C57BL/6 mice express no sPLA2-IIA (44), we injected human recombinant sPLA2-IIA into the left knee on day 2. Injection of sPLA2-IIA promoted swelling of joints. The ankle thickness was higher in WT than in TLR4KO mice (Fig. 5I), pointing out the possible role of TLR4 signaling during sPLA2-promoted arthritis.
Discussion
Sterile inflammation occurs under diverse pathological conditions, such as ischemia/reperfusion or trauma and it also underlies the pathologies of diseases with chronic inflammatory conditions, including atherosclerosis, autoimmune diseases, aging-related pathologies, and even cancer (45, 46). The innate immune signaling pathways (TLRs and inflammasomes are the most extensively studied) sense infection but also contribute to the sterile inflammation. Differences in the outcome of both conditions exist, but the mechanisms have not been fully determined yet.
In this study we showed that stressEVs, released during oxidative stress and representing DAMPs, are a diverse mixture of EVs according to size and composition. Different distribution of several markers after gradient ultracentrifugation indicates that EVs are of different origin and composition. The presence of annexin V points to similar mechanism of EV release as shown by Gong et al. (47), whereby activation of necroptosis-involved mixed lineage kinase-like (MLKL) protein resulted in rapid influx of Ca2+ followed by exposure of PS and annexin V at the cell surface. As a result, shedding of vesicles from the damaged cell surface was observed, which serves to preserve the plasma membrane integrity and support cell survival.
Despite their heterogenicity, we were able to elucidate their TLR4-activating potential among several other signaling properties. StressEVs but not LPS activated TLR4-dependent expression of Ccl24 and Il-23, important in inflammatory autoimmune diseases such as RA, psoriasis, or in ischemia/reperfusion injury (48, 49), that has already been targeted by therapeutic agents (50). On the other hand, several antiinflammatory proteins were expressed during stressEV activation such as Socs2, or Tsg6 (Tnfaip6 gene), which participate in conversion of macrophages from pro- to an antiinflammatory type (51). These differences between LPS-mediated and sterile inflammation result from the activation of different signaling molecules and transcription factors downstream of MyD88 and TRIF. We observed minor Il-23 mRNA expression after LPS expression, but no Il-23 cytokine was detected (Figs. 2D, 3H and 3M), although some publications reported that LPS can stimulate Il-23 production in dendritic cells (52) and also in macrophages at high concentration (1 μg/mL) (53).
Interestingly, no induction of tolerance was observed after stressEV stimulation, which makes them different from other DAMPs (54, 55) and may be a result of activation/interplay between signaling pathways that stressEVs can induce in a TLR4-dependent and -independent manner. In RA, the stimulation of TLR4 was also shown to be potentiated by cell type-specific intolerance in synovial fibroblasts (56) further contributing to the disease. Interestingly, paclitaxel, an anticancer drug and an agonist of mouse TLR4/MD-2 (57), induced the same response pattern as stressEVs, implying it might be possible to design molecules that activate different immune responses from LPS although they target the same receptor.
OxPLs have been reported to exert pro- and antiinflammatory activities, mostly identified by indirect mechanisms of oxPLs action (58). OxPLs also induce differentiation of macrophages to Mox type, which is distinct from M1 and M2 types (59). Various receptors of structurally distinct oxPLs compounds have been identified such as TLR2, which is not activated by short chain oxPLs but rather by oxPLs that contain reducible functional groups on nonfragmented sn-2 acyl chains (60) or caspase-11, which is activated by short chain 1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-PC (POVPC) or 1-palmitoyl-2-glutaryl-sn-glycero-3-PC (PGPC) in dendritic cells (61). A contradictory role on TLR4 as a receptor of oxPLs was partially elucidated by Oskolkova et al. (22). They showed that oxPLs like 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-PC (PEIPC) and POVPC inhibit LPS stimulation at low concentrations, but stimulate cells at high concentrations, though this effect could also be TLR4 independent. We showed before that PLs oxidized with the Fenton reaction for 30 min but not for 24 h activated TLR4 (14). Interestingly, although there are reports on detection and signaling of oxPLs and oxidized arachidonic acid (eicosanoids like prostaglandins, thromboxanes), which is released from PLs after PLA2 activity, lysoPL products of PLs hydrolysis received almost no attention. Herein we focused on oxidized lysoPLs where we identified the structural features of these TLR4 agonists. We showed that lysoPLs oxidized with 15-LO, independently of the type of hydrophilic head (PE, PI, PS, or PC), activated TLR4/MD-2 with higher potency than PLs comprising two acyl chains. Importantly, the oxidized lysoPI induced the same gene expression profile as stressEVs. Mass spectrometry identified keto, hydroxyl (HETE), and hydroperoxyl (HpETE) groups, but whether all species can act as TLR4 agonists still remains open. Il-6 cytokine expression from oxidized 20:4 lysoPI-stimulated cells was low (Fig. 3F) in comparison to LPS, which indicates it is a weak TLR4 agonist, although the local concentration of ox lysoPLs may be high. Some publications indicate that dendritic cells might respond better to oxidized PLs than macrophages (61), so BMDCs were stimulated with either stressEV or oxidized 20:4 lysoPI. DCs released higher amounts of Il-6 as well as Il-23 to supernatant in response to both agonists (Fig. 3 L and M) in a TLR4-dependent manner, not only confirming oxidized 20:4 lysoPI as a TLR4 agonist, but also showing that DCs may contribute more to innate immune response against oxidative stress. Additionally, in contrast to lysoPI added in a suspension, when synEVs were oxidized and hydrolyzed, more PL bound to TLR4/MD-2 receptor (Fig. 5D) with increased expression of Il-6 (Fig. 5F) although the cells were stimulated with lower concentration (3 μg/mL of ox lysoPE vs. 10 μg/mL of ox lysoPI). The results imply that EVs preserve unspecific binding of PLs to serum proteins and promote their recognition.
Structurally ox lysoPLs are not ideally suited as agonists of TLR4/MD-2. Most likely several molecules bind into the MD-2 hydrophobic pocket, as it was shown for myristic acid molecules in the MD-2 crystal structure (62) and for neoseptin (63). Lower stability of TLR4/MD-2 dimers is expected, probably resulting in lower activation of signaling pathways. Weaker stimulation and slower kinetics are favorable characteristics of chronic diseases and might also be one of the reasons that tolerance was not observed.
Lipoxygenase 12-LO was detected in EVs isolated from mice platelets (19). Although we were not able to detect 15-LO inside stressEVs from human cells we propose that 15-LO activity is important for stressEV TLR4 agonist activity as stressEV isolated from human cells with impaired 15-LO expression had significantly lower activity. The 15-LO can oxidize both arachidonic acyl chains in AAPE. PLA2 can thus release free oxidized arachidonic acid (15-HpETE) as well. However, 15-HpETE did not activate HEK293 cells expressing TLR4/MD-2 receptor.
According to the phospholipid composition in EVs, the fraction of lysoPLs is low (29), so it is reasonable to expect there is a mechanism that could increase the formation of lysoPLs during inflammation. PC/lysoPC ratio in the synovial fluid and plasma of RA patients decreases, which might be due to the increased PLA2 activity (64) and microvesicles are the preferred substrate for sPLA2s (65). We confirmed the role of 15-LO and secreted PLA2 enzymes in the formation of TLR4 agonists. Results imply that more agonists are formed when PLs are first oxidized and then hydrolyzed, probably because the oxidized sn-2 fatty acid moiety protrudes into the aqueous phase resulting in the formation of “whiskers” (66) thus increasing availability to sPLA2. This result is in agreement with the observation that sPLA2-IIA has enhanced ability to hydrolyze oxidized low-density lipoprotein (LDL) in comparison with unmodified LDL (10).
sPLA2s act on various membranes in the extracellular environment, including intact and apoptotic cells, lipoproteins, and microvesicles, but different isoforms exhibit different abilities to bind to and hydrolyze target membranes (31). Human and mouse sPLA2-IIA enzymes are inactive toward intact cells, due to their poor binding to the PC-rich plasma membrane (31), but they act on membranes containing negatively charged PLs, whereas groups V and X sPLA2s bind with high affinity to PC-rich membranes and efficiently hydrolyze lipoproteins and plasma membranes of intact cells (31, 67, 68). Intriguingly, while the antibacterial role of sPLA2-IIA has been well documented (69), it has been revealed only recently that its endogenous substrates may be mitochondria released from activated platelets, whereby their hydrolysis leads to exacerbation of inflammation (70). sPLA2 activity was detected in synovial fluid from patients with RA, gout, psoriasis (18), and especially two isoforms are elevated (proinflammatory sPLA2-IIA and anti-inflammatory sPLA2-V) in synovial fluid from RA patients (44). Transgenic expression of sPLA2-X resulted in higher induction of cytokines in response to LPS (71), showing its role in potentiating the immune response although not detected in synovial fluid. In this study we did not determine the identity of the sPLA2 isoforms in synovial fluid of RA and gout patients, but given that all three sPLA2s (sPLA2-IB, sPLA2-IIA, and sPLA2-X) tested could equally contribute to the ligand formation from synEVs, we suggest that in principle any sPLA2 present in synovial fluid may contribute to the action and that sPLA2s in synovial fluid are probably not the only source for TLR4 agonist formation in sterile inflammation.
Using mass spectrometry, we detected 2-arachidonoyl-lysoPLs in the synovial fluid, but not oxidized 15-HETE-lysoPL species. According to the oxidized standards we used (SI Appendix, Tab. S1), molecules with very similar structure (likely different position of double bond) were detected. Given that different lysoPLs are the source and that oxidized molecules are very unstable probably reduces the probability of detecting specific molecular species especially in the extracellular fluid. In support of our data Liu et al. (27, 28) detected eicosanoid-lysolipids formed by either COX-2 or 12-LO and iPLA2γ activity in murine hepatic tissue, murine and human myocardium, as well as in cells exposed to calcium ionophore. As increased concentrations of 12-HETE-LPC were detected in serum of old male mice they proposed them as specific biomarkers for various age-associated diseases (28).
Increased expression of human sPLA2-IIA in mice results in dermatopathies, similar to psoriasis, but with no significant inflammatory-cell influx (17) showing the necessity of an additional mechanism. Concerted activity of 12-LO and sPLA2-IIA has been shown in the uptake of microparticles by neutrophils (19). The authors also observed increased ankle thickness and inflammation in K/BxN serum-induced arthritis in mice with both enzymes in comparison to mice lacking one or both enzymes in agreement with our observations where injection of sPLA2-IIA into mice with K/BxN serum-induced arthritis promoted the disease. We additionally showed that sPLA2-IIA contribution to ankle thickness was partially TLR4 dependent, so formation of TLR4 agonist might be a relevant process. To conclude, we revealed the mechanism of endogenous ligand formation through activity of 15-LO and sPLA2, which contributes to sterile inflammation in chronic conditions like RA with differences to classical inflammation on the cytokine level, thereby giving the option to design specific inhibitors that will limit sterile inflammation but will not globally affect systemic innate immunity.
Supplementary Material
Acknowledgments
This study was financially supported by the H2020 Marie Sklodowska-Curie Innovative Training Networks project TOLLerant (grant no. 642157) and the Slovenian Research Agency (research core no. P4-0176 to R.J. and project no. J3-9257 to M.M.-K). We thank Anja Perčič and Peter Pečan for technical support.
Footnotes
This article is a PNAS Direct Submission.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005111117/-/DCSupplemental.
Data Availability.
The data that support the graphs within this paper were deposited to public respository Figshare (72) (DOI: 10.6084/m9.figshare.10008044); more detailed protocols and materials are available from the corresponding author.
References
- 1.Rock K. L., Latz E., Ontiveros F., Kono H., The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L., Wang L., Chen S., Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 14, 2592–2603 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni M., Gemma S., Rossetti C., The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016, 6978936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Yin H., Zhao M., Lu Q., TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 47, 136–147 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Feldman N., Rotter-Maskowitz A., Okun E., DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 24, 29–39 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Chakravarti B., Chakravarti D. N., Oxidative modification of proteins: Age-related changes. Gerontology 53, 128–139 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Cooke M. S., Evans M. D., Dizdaroglu M., Lunec J., Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 17, 1195–1214 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Poli G., Leonarduzzi G., Biasi F., Chiarpotto E., Oxidative stress and cell signalling. Curr. Med. Chem. 11, 1163–1182 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Yarana C., St Clair D. K., Chemotherapy-induced tissue injury: An insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants (Basel) 6, 75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambeau G., Gelb M. H., Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77, 495–520 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Uderhardt S., Krönke G., 12/15-lipoxygenase during the regulation of inflammation, immunity, and self-tolerance. J. Mol. Med. (Berl.) 90, 1247–1256 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Keller S., Ridinger J., Rupp A. K., Janssen J. W., Altevogt P., Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 9, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y. et al., Inflammasome-derived exosomes activate NF-κB signaling in macrophages. J. Proteome Res. 16, 170–178 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Manček-Keber M. et al., Toll-like receptor 4 senses oxidative stress mediated by the oxidation of phospholipids in extracellular vesicles. Sci. Signal. 8, ra60 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Singh N. K., Rao G. N., Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 73, 28–45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochkov V. N. et al., Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 12, 1009–1059 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grass D. S. et al., Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Invest. 97, 2233–2241 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruzanski W. et al., Enzymatic activity and immunoreactivity of extracellular phospholipase A2 in inflammatory synovial fluids. Inflammation 16, 451–457 (1992). [DOI] [PubMed] [Google Scholar]
- 19.Duchez A. C., et al. , Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. U.S.A. 112, E3564–E3573 (2015).Correction in: Proc. Natl. Acad. Sci. U.S.A. 112, E6825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erridge C., Kennedy S., Spickett C. M., Webb D. J., Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: Roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283, 24748–24759 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitinger N., Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr. Opin. Lipidol. 14, 421–430 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Oskolkova O. V. et al., Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J. Immunol. 185, 7706–7712 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Stocker R., Keaney J. F. Jr., Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84, 1381–1478 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Lankin V. Z. et al., Oxidative stress in atherosclerosis and diabetes. Bull. Exp. Biol. Med. 140, 41–43 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Tak P. P., Zvaifler N. J., Green D. R., Firestein G. S., Rheumatoid arthritis and p53: How oxidative stress might alter the course of inflammatory diseases. Immunol. Today 21, 78–82 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Fairburn K. et al., Oxidative stress and its control: A pathogenetic role in inflammatory joint disease. Biochem. Soc. Trans. 21, 371–375 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Moon S. H., Jenkins C. M., Sims H. F., Gross R. W., Cyclooxygenase-2 mediated oxidation of 2-arachidonoyl-lysophospholipids identifies unknown lipid signaling pathways. Cell Chem. Biol. 23, 1217–1227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X. et al., 12-LOX catalyzes the oxidation of 2-arachidonoyl-lysolipids in platelets generating eicosanoid-lysolipids that are attenuated by iPLA2γ knockout. J. Biol. Chem. 295, 5307–5320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weerheim A. M., Kolb A. M., Sturk A., Nieuwland R., Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal. Biochem. 302, 191–198 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Petan T., Krizaj I., Gelb M. H., Pungercar J., Ammodytoxins, potent presynaptic neurotoxins, are also highly efficient phospholipase A2 enzymes. Biochemistry 44, 12535–12545 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Singer A. G. et al., Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277, 48535–48549 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Subramanian A. et al., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merico D., Isserlin R., Stueker O., Emili A., Bader G. D., Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS One 5, e13984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacic L., Novinec M., Petan T., Baici A., Krizaj I., Calmodulin is a nonessential activator of secretory phospholipase A(2). Biochemistry 48, 11319–11328 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Radvanyi F., Jordan L., Russo-Marie F., Bon C., A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal. Biochem. 177, 103–109 (1989). [DOI] [PubMed] [Google Scholar]
- 36.Monach P. A., Mathis D., Benoist C., The K/BxN arthritis model. Curr. Protoc. Immunol. 15, 15.22 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki K., Nogawa H., Nishijima M., Identification of mouse MD-2 residues important for forming the cell surface TLR4-MD-2 complex recognized by anti-TLR4-MD-2 antibodies, and for conferring LPS and taxol responsiveness on mouse TLR4 by alanine-scanning mutagenesis. J. Immunol. 170, 413–420 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Muroi M., Tanamoto K., Structural regions of MD-2 that determine the agonist-antagonist activity of lipid IVa. J. Biol. Chem. 281, 5484–5491 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Biswas S. K., Lopez-Collazo E., Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Dobrian A. D. et al., Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 50, 115–131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resman N. et al., Essential roles of hydrophobic residues in both MD-2 and toll-like receptor 4 in activation by endotoxin. J. Biol. Chem. 284, 15052–15060 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelb M. H., Jain M. K., Hanel A. M., Berg O. G., Interfacial enzymology of glycerolipid hydrolases: Lessons from secreted phospholipases A2. Annu. Rev. Biochem. 64, 653–688 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y. et al., Arachidonic acid inhibits inflammatory responses by binding to myeloid differentiation factor-2 (MD2) and preventing MD2/toll-like receptor 4 signaling activation. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165683 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Boilard E. et al., A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol. Med. 2, 172–187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw A. C., Goldstein D. R., Montgomery R. R., Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 13, 875–887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y., Gardner S. E., Clarke M. C., Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 31, 2781–2786 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Gong Y. N. et al., ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croxford A. L., Mair F., Becher B., IL-23: One cytokine in control of autoimmunity. Eur. J. Immunol. 42, 2263–2273 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Hu X. et al., IL-23 promotes myocardial I/R injury by increasing the inflammatory responses and oxidative stress reactions. Cellular Physiol. Biochem. 38, 2163–2172 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Teng M. W. et al., IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21, 719–729 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Mittal M. et al., TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. U.S.A. 113, E8151–E8158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang J., Voorhees T. J., Liu Y., Zhao Y., Chang C. H., Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A. Proc. Natl. Acad. Sci. U.S.A. 107, 8340–8345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W. et al., AP-1 activated by toll-like receptors regulates expression of IL-23 p19. J. Biol. Chem. 284, 24006–24016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aneja R. K. et al., Preconditioning with high mobility group box 1 (HMGB1) induces lipopolysaccharide (LPS) tolerance. J. Leukoc. Biol. 84, 1326–1334 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Austermann J., et al. , Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions. Cell Rep. 9, 2112–2123 (2014).Correction in: Cell Rep. 11, 849 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Klein K. et al., The epigenetic architecture at gene promoters determines cell type-specific LPS tolerance. J. Autoimmun. 83, 122–133 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Kawasaki K., Gomi K., Nishijima M., Cutting edge: Gln22 of mouse MD-2 is essential for species-specific lipopolysaccharide mimetic action of taxol. J. Immunol. 166, 11–14 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Briot A. et al., Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J. Exp. Med. 212, 2147–2163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serbulea V. et al., Macrophages sensing oxidized DAMPs reprogram their metabolism to support redox homeostasis and inflammation through a TLR2-Syk-ceramide dependent mechanism. Mol. Metab. 7, 23–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadl A. et al., Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 51, 1903–1909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanoni I., Tan Y., Di Gioia M., Springstead J. R., Kagan J. C., By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47, 697–709.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohto U., Fukase K., Miyake K., Satow Y., Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science 316, 1632–1634 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Wang Y. et al., TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc. Natl. Acad. Sci. U.S.A. 113, E884–E893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs B., Schiller J., Wagner U., Häntzschel H., Arnold K., The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: Investigations by 31P NMR and MALDI-TOF MS. Clin. Biochem. 38, 925–933 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Fourcade O. et al., Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 80, 919–927 (1995). [DOI] [PubMed] [Google Scholar]
- 66.Greenberg M. E. et al., The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 283, 2385–2396 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Bezzine S. et al., On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277, 48523–48534 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Jarc E. et al., Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 247–265 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Nevalainen T. J., Graham G. G., Scott K. F., Antibacterial actions of secreted phospholipases A2. Review. Biochim. Biophys. Acta 1781, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Boudreau L. H. et al., Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shridas P. et al., Group X secretory phospholipase A2 enhances TLR4 signaling in macrophages. J. Immunol. 187, 482–489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ha V. T., et al. , Synergy between 15-lipoxygenase and secreted PLA2 promotes inflammation by formation of TLR4 agonists from extracellular vesicles. Figshare respository. https://figshare.com. DOI: 10.6084/m9.figshare.10008044. Deposited 21 October 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the graphs within this paper were deposited to public respository Figshare (72) (DOI: 10.6084/m9.figshare.10008044); more detailed protocols and materials are available from the corresponding author.