Significance
The specialized assembly requirements of Rubisco hamper its bioengineering in plants, especially in regard to transforming in “red” Rubiscos from algae with better CO2-fixing properties that could enhance crop photosynthesis and growth. We show this assembly incompatibility does not extend to the “red” type Rubisco from Rhodobacter sphaeroides. Despite evolving from a different phylogenetic lineage to plant Rubisco, the assembly requirements of RsRubisco are readily met in chloroplasts as well as Escherichia coli. Coexpressing its cognate Rubisco activase enhanced RsRubisco activity and improved plant photosynthesis and growth twofold. RsRubisco provides a protein scaffold for red Rubisco bioengineering in E. coli and plants—requiring future optimisation of chloroplast RsRubisco expression and catalytic repair.
Keywords: chloroplast transformation, Rubisco activase, carbon fixation, photosynthesis
Abstract
Plant photosynthesis and growth are often limited by the activity of the CO2-fixing enzyme Rubisco. The broad kinetic diversity of Rubisco in nature is accompanied by differences in the composition and compatibility of the ancillary proteins needed for its folding, assembly, and metabolic regulation. Variations in the protein folding needs of catalytically efficient red algae Rubisco prevent their production in plants. Here, we show this impediment does not extend to Rubisco from Rhodobacter sphaeroides (RsRubisco)—a red-type Rubisco able to assemble in plant chloroplasts. In transplastomic tobRsLS lines expressing a codon optimized Rs-rbcLS operon, the messenger RNA (mRNA) abundance was ∼25% of rbcL transcript and RsRubisco ∼40% the Rubisco content in WT tobacco. To mitigate the low activation status of RsRubisco in tobRsLS (∼23% sites active under ambient CO2), the metabolic repair protein RsRca (Rs-activase) was introduced via nuclear transformation. RsRca production in the tobRsLS::X progeny matched endogenous tobacco Rca levels (∼1 µmol protomer·m2) and enhanced RsRubisco activation to 75% under elevated CO2 (1%, vol/vol) growth. Accordingly, the rate of photosynthesis and growth in the tobRsLS::X lines were improved >twofold relative to tobRsLS. Other tobacco lines producing RsRubisco containing alternate diatom and red algae S-subunits were nonviable as CO2-fixation rates (kcatc) were reduced >95% and CO2/O2 specificity impaired 30–50%. We show differences in hybrid and WT RsRubisco biogenesis in tobacco correlated with assembly in Escherichia coli advocating use of this bacterium to preevaluate the kinetic and chloroplast compatibility of engineered RsRubisco, an isoform amenable to directed evolution.
Increasing global crop productivity is of paramount importance to address the rising demands posed by our escalating global population, arable land limitations, and dwindling yield gains afforded by plant breeding. Innovation to produce a step change in yield improvement is essential (1). Improving photosynthetic efficiency offers one frontier strategy with a core objective being to enhance the CO2-fixing properties of the enzyme Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco), the point of carbon entry into the biosphere (2, 3). Plant Rubisco is located in the chloroplast and is considered inefficient as it is slow (∼2–5 s−1 turnover rate), necessitating its production in large amounts (20–50% leaf soluble protein in C3 plants; ref. 4). Rubisco can also fix O2 instead of CO2 leading to 2-phosphoglycolate production whose recycling by photorespiration consumes energy and releases fixed CO2 (5). Rubisco content and catalysis in plants therefore strongly influences photosynthesis, growth, and resource use efficiency, making the enzyme a prized target for improvement (6, 7).
Surveys of plant Rubisco natural catalytic diversity have identified evidence of increased carboxylation potential (8–10). Cross-scale modeling however suggests the extent of this kinetic variation may be insufficient for appreciable crop productivity gains (11). Broader kinetic diversity has been discovered in Rubisco from marine phototrophs (12), some with matching potential to support crop photosynthesis (13), others with capacity to improve yield by >30% (14, 15). A structural rationale to explain this kinetic diversity remains unforthcoming as the crystal structures of plant and nongreen algae are highly superimposable. Both comprise Form I Rubisco L8S8 complexes comprising eight ∼50-kDa large (L)-subunits and eight ∼15- to 17-kDa small (S)-subunits (16) with structurally conserved catalytic sites housed within each L2 dimer pair (17). Form I Rubisco divides into distinct “green”-type (Form IA, IB) and “red”-type (Form IC, ID) L-subunit lineages (18) with the plant Form IB Rubisco rbcL gene located in the chloroplast genome (plastome) and multiple RbcS copies in the nucleus while nongreen algae Form ID Rubisco is coded by a rbcL-rbcS operon in the plastome (19).
Differences in the folding and assembly requirements of Form IB and ID Rubisco prevent expression of kinetically superior red-algae Rubisco in leaf chloroplasts (20, 21). Indeed, the biogenesis requirements of Form IB plant Rubisco are extensive, requiring at least seven auxiliary chaperone and chaperonin components (22) with differing L-subunit specificities that can require highly harmonious structural compatibility (e.g., Rubisco accumulation factor 1, Raf1; ref. 23) or little recognition specificity (e.g., Bundle-Sheath-Deficient II, BSD2; ref. 24). Differing assembly requirements for distinct Rubisco isoforms have posed significant challenges to expressing heterologous plant and cyanobacteria “green”-type Rubiscos in leaf chloroplasts (6, 10).
Efforts to improve the carboxylation properties of Form I Rubisco have been achieved by directed evolution using Rubisco-dependent E. coli screens (25). Among these is the “red”-type Form IC Rubisco from the proteobacterium Rhodobacter sphaeroides (26) whose simpler biogenesis requirements allow for its abundant production in Escherichia coli (27). Study of R. sphaeroides Rubisco is aided by the availability of crystal structures of both RsRubisco (27) and its metabolic repair Rubisco activase (Rca) protein (RsRca) encoded by the cbbX gene (28). Comparable to plant Rca, RsRca forms a hexameric, AAA+-like ring structure to facilitate the removal of inhibitory sugars bound within the RsRubisco catalytic sites using the power of ATP hydrolysis. Unlike plant Rca however, RsRca activity is allosterically controlled by RuBP availability with inhibitor release invoked by a pulling motion on the RsL-subunit C terminus (29). As this mechanism is not shared by plant Rca, RsRca cannot activate plant Rubisco and plant Rca is unable to activate RsRubisco (30).
Here, we demonstrate that unlike nongreen algae Form ID Rubisco, the biogenesis requirements of the phylogenetically related Form IC RsRubisco are readily met in tobacco chloroplasts. We show metabolic repair of RsRubisco requires RsRca coexpression to elevate its functionality, leaf photosynthesis, and plant growth. Comparison of hybrid RsRubisco production in E. coli and tobacco advocate E. coli as a suitable proxy for screening the biogenesis potential and kinetics of mutant RsRubisco prior to transplanting into tobacco chloroplasts.
Results
Generating the Transplastomic tobRsLS Lines.
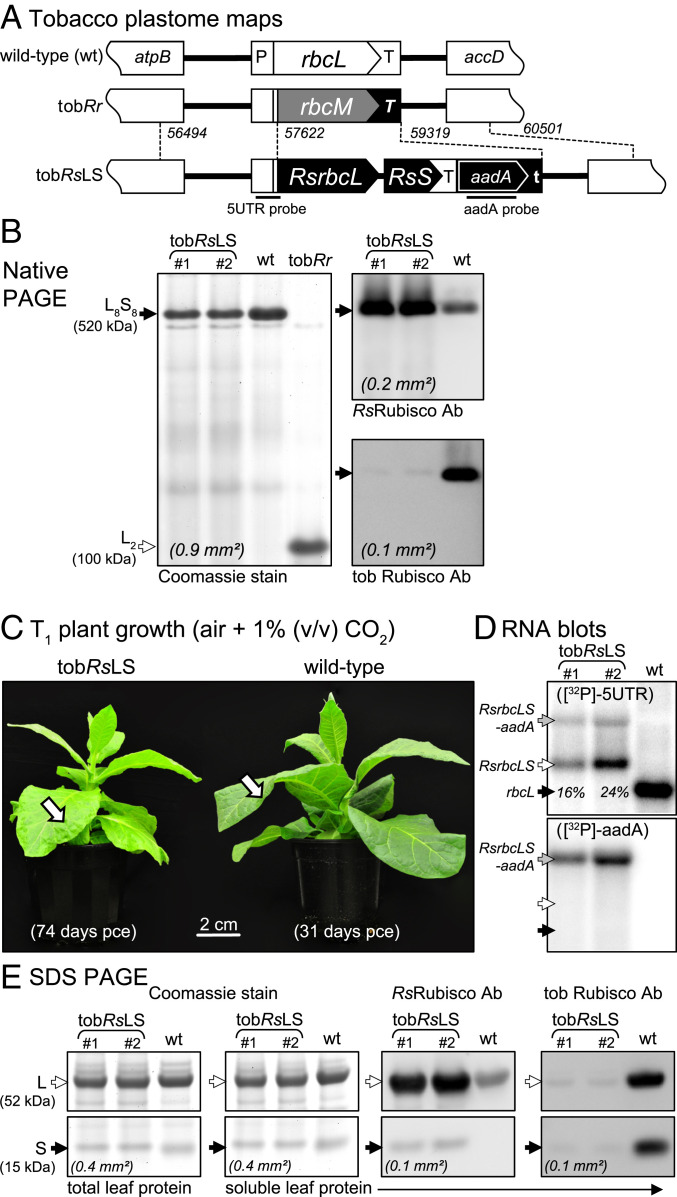
The plastome transforming plasmid pLEVRsLS contains a synthetic Rs-rbcL-rbcS operon whose codon use was modified to match the tobacco rbcL gene (GenBank KM464722; ref. 26) (Fig. 1A). Moreover, the Rs-rbcL gene maintains the first 42 nucleotides of the native tobacco rbcL 5′-translational control region (31), which replaces the RsL-subunit native MDTKTTEIKGKERY N terminus with the MSPQTETKASVGF sequence of the tobacco L-subunit. In pLEVRsLS, the aadA selectable marker gene was inserted downstream of the Rs-rbcL-rbcS genes near the end of the tobacco rbcL 3′ untranslated region at a position shown to facilitate sufficient transcription read-through of a polycistronic-aadA messenger RNA (mRNA) for spectinomycin selection (32). The pLEVRsLS plasmid was transformed into the plastome of the Rhodospirillum rubrum L2 Rubisco-producing tobacco master-line tobRr (Fig. 1A and ref. 32) and eight spectinomycin-resistant tobRsLS lines screened by native-PAGE. Each tobRsLS line produced ∼520-kDa L8S8 complexes and confirmed to be RsRubisco on replicate immunoblots probed with antibodies to RsRubisco or tobacco Rubisco (Fig. 1B). Two tobRsLS lines (#1, #2) were maintained and soil grown to fertile maturity in air containing 1% (vol/vol) CO2.
Fig. 1.
RsRubisco expression in tobacco chloroplasts. (A) Transformation of the tobRr plastome with plasmid pLEVRsLS produced tobRsLS lines where the rbcM gene coding R. rubrum L2 Rubisco (located in place of the native rbcL in WT tobacco) was replaced with the RsRubisco operon (RsrbcL-RsS) and aadA selectable marker gene. Dashed lines and numbering relative to tobacco plastome (GenBank accession no. Z00044) indicate plastome sequence in pLEVRsLS used to facilitate homologous recombination. The tobacco rbcL promoter/5′UTR (P) and first 42 nucleotides of native rbcL sequence (the 5UTR probe) are conserved in each tobacco genotype. The aadA probe DNA region is shown. accD, plastome genes; T, tobacco rbcL 3′UTR; T, psbA 3′UTR; t, rps16 3′UTR; atpB. (B) Coomassie stain and immunoblot native-PAGE analyses confirming the production of RsL8S8 Rubisco complexes in tobRsLS. The varying area of soluble leaf protein analyzed is indicated in italics. (C) Phenotype of a T1 tobRsLS plant (line #1) and WT tobacco grown at 25 °C in air containing 1% (vol/vol) CO2. Arrows indicate leaves sampled for RNA and protein analyses in B, D, and E. (D) Blots of 3 µg of total leaf RNA hybridized with the 5UTR-probe showing the WT rbcL mRNA levels are four- to fivefold higher than the Rs-rbcLS and Rs-rbcLS-aadA polycistronic mRNAs (the latter detected by the aadA probe) produced in tobRsLS lines #1 and #2. (E) SDS/PAGE–immunoblot analysis of the total and soluble leaf protein fractions showing the RsRubisco produced in the tobRsLS lines is fully soluble and does not contain tobacco S-subunits.
RsRubisco Production and Function in Tobacco Chloroplasts.
The tobRsLS lines grew slower than WT tobacco under elevated CO2 (Fig. 1C), primarily due to a 60% reduction in RsRubisco content (relative to WT tobacco) where only ∼40% of the catalytic sites were functionally active (Table 1). RNA blot analyses showed tobRsLS leaves produced two polycistronic transcripts, a Rs-rbcL-rbcS mRNA and ∼fivefold less abundant Rs-rbcL-rbcS-aadA mRNA. Despite the operons being regulated by the endogenous tobacco rbcL promoter and terminator regulatory sequences (Fig. 1A), the combined abundance of both RsRubisco transcripts was <25% the native rbcL mRNA content in WT tobacco (Fig. 1D).
Table 1.
Rubisco content, activation, and catalysis at 25 °C
| Rubisco parameter | Source tissue | ||
| Tobacco* | tobRsLS* | E. coli-RsLS† | |
| kcatc | 3.2 ± 0.2 | 3.9 ± 0.2 | 3.7 ± 0.2 |
| Kc | 11.3 ± 0.8 | 58.4 ± 2.1 | 59.7 ± 0.1 |
| Ko | 283 ± 15 | n.d | 1,742 ± 280 |
| Kc21%O2 | 21.4 ± 0.9 | 71.4 ± 2.3 | 68.8‡ |
| Sc/o | 81.9 ± 1.6 | 59.7 ± 1.3 | 58.4 ± 1.2 |
| kcato§ | 1.0 | n.d. | 1.4 |
| kcatc/Kc21%O2 | 148 | 57 | 54 |
| µmol sites·m2 ¶ | 22.1 ± 3.5 | 9.3 ± 2.3 | n.a. |
| % sites active¶ | 69 ± 5 | 40 ± 3 | n.a. |
n.a., not applicable; n.d., not determined.
Data ± SD of three or more biological samples.
Data from ref. 26.
Kc21%O2, the apparent Km for CO2 (Kc) at atmospheric [O2] (assumed 252 µM at 25 °C) calculated as Kc(1+[O2]/Ko).
Calculated from the equation Sc/o = (kcatc/Kc)/(kcato/Ko).
Measured in young leaves (n = 6 ± SD) of 15-cm-high plants growing in air +1% (vol/vol) CO2.
Comparative SDS/PAGE analysis of the total and soluble tobRsLS leaf protein indicated the RsRubisco subunits were fully soluble with no tobacco S-subunits, only RsS-subunits, detected by immunoblotting (Fig. 1E). This was confirmed through kinetic analysis, which found the carboxylation rate (kcatc), the Michaelis constant for CO2 under ambient O2 (Kc21%O2), and the Sc/o of the RsRubisco produced in tobRsLS matched that of RsRubisco produced in E. coli (26) (Table 1).
RsRca Enhances tobRsLS Photosynthesis and Growth.
To increase the activation status of RsRubisco in tobRsLS the plasmid pBinTP-cbbX was transformed by Agrobacterium into the nucleus of tobRsLS#1 (Fig. 2A). Thirty kanamycin-resistant F0 tobRsLS::X lines were obtained from which two heterozygous, single allelic insertion tobRsLS::X F1 lines (#7, #8) producing high levels of RsRca were identified (SI Appendix, Fig. S1). In air supplemented with 1% (vol/vol) CO2, the tobRsLS-X7 and tobRsLS-X8 lines both grew faster than tobRsLS, albeit slower than WT controls (Fig. 2 B and C). To test the high-CO2 requirement of the tobRsLS and tobRsLS::X lines, a representative juvenile plant of each genotype at ∼3 cm in height was taken from the growth experiment and transferred to ambient CO2 where their development ceased with necrosis and death following after 1–2 mo (SI Appendix, Fig. S2).
Fig. 2.
Expressing RsRca in tobRsLS improved growth. (A) Genetic detail of the nucleus transforming plasmid pBinTP-cbbX. LB/RB, Left/Right T-DNA borders; nptII, gene coding kanamycin resistance; PCaMV, cauliflower mosaic virus 35S promoter; PMS/TMS, mannopine synthase promoter/terminator; Pnos/Tnos, nopaline synthase promoter/terminator; TP, tobacco S-subunit transit peptide. (B) Phenotype of the plants at the indicated ages postcotyledon emergence (pce) grown at 25 °C in air with 1% (vol/vol) CO2 under ∼400 ± 100 µmol photons.m−2·s−1. (C) Comparative growth measured as a function of plant height of WT tobacco (squares), tobRsLS#1 (circles), and RsRca-expressing tobRsLS::X lines (triangles; line #7 white, #8 black), n = 5 ± SD for each genotype. Age of plants analyzed by leaf gas exchange in Fig. 3 is shaded gray. Comparison of the Rubisco (D), tobacco Rubisco activase (NtRca) (E), and RsRca contents (F) in the youngest nearly fully expanded leaves (14 ± 1 cm in diameter) from five (±SD) plants at 30 ± 3 cm in height of each genotype. See SI Appendix, Fig. S3 for example quantification of NtRca and RsRca. Letters show the ranking of the means following a one-way ANOVA and post hoc Tukey test (different letters indicate statistical differences at the 5% level, P < 0.05).
Comparison of the Rubisco and tobacco Rca (NtRca) content in comparable upper canopy leaves showed the tobRsLS and tobRsLS::X plants produced the same levels of RsRubisco (∼40% the amount produced in wild type, Fig. 2D) and NtRca levels remained unchanged in both transplastomic genotypes despite producing a noncognate Rubisco (Fig. 2E). RsRca production in the tobRsLS::X leaves matched that of the endogenous NtRca content (Fig. 2F). Leaf gas exchange measurements on the same leaves showed faster photosynthetic rates (A) under varying intercellular CO2 concentrations (Ci) in tobRsLS::X leaves relative to tobRsLS (Fig. 3A), consistent with their differing growth rates (Fig. 2 B and C). The faster A-Ci response in wild type showed A was Rubisco activity (Ac) limited up to ∼400 µbar CO2 before becoming electron transport rate limited (AJ). In both tobRsLS::X and tobRsLS, photosynthesis remained Ac limited over the Ci range tested, albeit at much lower rates than expected (Fig. 3A, dashed gray line). The discrepancy was attributed to the attenuated RsRubisco activation state in tobRsLS (∼23% sites activated) and tobRsLS::X (∼45% sites activated) in the plants left overnight in the laboratory under ambient CO2 prior to measuring leaf gas exchange. When adjusted for the level of functional RsRubisco, the simulated A-Ci responses in tobRsLS::X and tobRsLS matched the rates measured by gas exchange (Fig. 3A, solid lines). Notably, the in vivo RsRubisco activation states in tobRsLS and tobRsLS::X leaves sampled within their elevated CO2 growth chambers were higher, at ∼55% and 75% respectively, the latter matching the activation status of tobacco Rubisco in plants grown under ambient CO2 (Fig. 3B). Unlike tobRsLS and tobRsLS::X however, the Rubisco activation status in wild type grown under elevated CO2 was reduced. As seen previously, lowering the activation status of Rubisco occurs under elevated CO2 when photosynthesis is limited by electron-transport product availability (33). Taken together, the faster photosynthetic and growth rates by tobRsLS::X correlate with an increase in the amount of active RsRubisco due to metabolic repair (activation) by RsRca.
Fig. 3.
RsRca and high CO2 is needed to activate RsRubisco and stimulate leaf photosynthesis. (A) Leaf gas exchange measurements of CO2-assimilation rates (A) at 25 °C under varying intercellular CO2 pressures (Ci) made at 1,200 µmol photons·m−2·s−1 illumination (plants analyzed indicated in Fig. 2C). Despite sharing comparable levels of RsRubisco (8.2 ± 0.7 µmol active site·m−2; see Fig. 2D), the photosynthetic rates in the tobRsLS::X lines (open symbols) were ∼twofold higher than the tobRsLS lines but lower than that modeled (see SI Appendix, Supplementary Information Text) using the RsRubisco kinetics in Table 1. (B) The effect of growth CO2 and RsRca expression on the in vivo activation status of RsRubisco in plants acclimated overnight to air (ambient 0.04% (vol/vol) CO2, open bars) or in the high CO2 (1% [vol/vol]) growth chamber (filled bars). The activation status of R. sphaeroides Rubisco was stimulated by high CO2 growth, and RsRca coexpression while in wild type the activation status of tobacco Rubisco declined with increasing CO2. n = 3 and 5 (±SD) for air and high CO2 samples, respectively. Solid lines in A represent the modeled A-Ci response accounting for the low activation status of RsRubisco (as indicated) in the tobRsLS and tobRsLS::X leaves during gas exchange. Different letters in B indicate statistical differences (P < 0.05) following a one-way ANOVA and post hoc Tukey test.
Using E. coli as a Proxy for RsRubisco Production in Chloroplasts.
Kinetic mutants of bacterial Form I Rubisco selected by directed evolution using E. coli screens typically involve changes in solubility (i.e., L8S8 biogenesis potential; refs. 25, 26, and 34). Differences in the assembly compatibility of WT and mutant cyanobacteria Form IA and IB Rubiscos in E. coli do not correlate with their biogenesis potential in tobacco chloroplasts (25), which is problematic as generating plastome transformants is a lengthy and costly process. By contrast the high levels of RsRubisco produced in tobRsLS and tobRsLS::X correlate with its more permissive assembly in E. coli (26, 27). RsRubisco biogenesis in E. coli is, however, impaired when comprising a hybrid complex containing Form ID S-subunits from nongreen algae (27). We used this property to test how well the biogenesis of RsRubisco mutants in E. coli can serve as a proxy for screening their expression in leaf chloroplasts by expressing a range of RsRubisco mutants in both expression hosts.
E. coli and transplastomic tobacco producing hybrid RsRubisco incorporating the Form ID algae S-subunits from P. tricornutum (Pt, diatom), G. monilis (Gs, filamentous red algae) or Galdieria sulphuraira (Gs, unicellular red algae) were produced as shown in Fig. 4A. Structural comparisons show that while the algae S-subunits share only ∼50% sequence identity with the RsS-subunit, they all code a truncated βA-βB loop and extended C-terminal βE-βF loop relative to plant Form IB Rubisco SI Appendix, Fig. S4 A–C. Nevertheless, the L8S8 quaternary complex of all Form I Rubisco are highly superimposable SI Appendix, Fig. S4 D and E. Accordingly, each hybrid L8S8 RsRubisco was produced in E. coli and in the leaves of the tobRsLGmS, tobRsLGsS, and tobRsLPtS lines (Fig. 4B). Each hybrid RsRubisco isoform migrated at differing rates through native-PAGE, their relative mobilities mirrored between the E. coli and leaf protein samples. Moreover, the intensity of the Coomassie-stained L8S8 bands matched the Rubisco contents quantified by CABP binding (Fig. 4B). The differing levels of hybrid RsRubisco produced in E. coli (relative to cell soluble protein content) were equivalent to the amounts produced in tobacco leaves (Fig. 4B). These findings suggest E. coli may be a suitable proxy for preevaluating mutant RsRubisco expression in chloroplasts.
Fig. 4.
Comparing WT and hybrid RsRubisco production in E. coli and tobacco chloroplasts. (A) RsRubisco operons comprising an Rs-rbcL gene and differing rbcS genes coding either G. sulphuraria (GsS), G. monilis (GmS), P. tricornutum (PtS), or tobacco (NtS) S-subunits (see SI Appendix, Fig. S4 for sequence and structure information) were cloned into pLEV4 and transformed into the tobRr plastome (Fig. 1A) or cloned into pET28a(+) and expressed in E. coli BL21(DE3). (B) Differences in the assembly of the WT and hybrid RsRubisco isoforms in E. coli (n = 3 ± SD) and tobacco chloroplasts (n = 3 or 4 ± SD) was analyzed by native-PAGE and quantified by [14C]-CABP binding (Rubisco amount loaded per lane shown in brackets). The relative levels of each RsRubisco produced in E. coli (filled bars) matched that made in tobacco chloroplasts (open bars). Hybrid RsRubisco comprising NtS-subunits were not detected (nd). The measured CO2-fixation rates (kcatc) and CO2/O2 specificity (SC/O) for each Rubisco is shown in italics. Further analysis of the transformed samples is shown in SI Appendix, Fig. S5.
The Dependence of S-Subunit Compatibility for RsRubisco Catalysis.
Despite producing appreciable levels of hybrid RsRubisco, the tobRsLGmS, tobRsLGsS, and tobRsLPtS lines produced pale green leaves, could not be grown in soil under elevated CO2, and required continued maintenance in tissue culture (SI Appendix, Fig. S5A). SDS/PAGE analysis of the total and soluble leaf protein found the RsL-subunit and algae S-subunits produced were entirely soluble, with the production of NtRca still maintained despite producing noncognate hybrid RsRubiscos SI Appendix, Fig. S5B. The impaired growth stemmed from >95% reductions in the kcatc of each hybrid RsRubisco (<0.1 s−1, Fig. 4B). While these low rates precluded determination of Kc and Ko, measures of Sc/o made using extended [1-3H]-RuBP consumption assays showed the specificities of hybrid enzymes comprising GmS, GsS, or PtS subunits were reduced 36%, 46%, and 51%, respectively (Fig. 4B). Evidently the sequence diversity between the algae and the RsS-subunits (SI Appendix, Fig. S4 A and B) structurally disrupt the catalytic sites located >20 Å within the RsL8 core, albeit without detectable detriment to tight CABP binding.
Discussion
There have been significant advances in solving the extensive, sometimes highly specialized, ancillary chaperone needs for Form IB L-subunit folding and assembly into L8–BSD2 complexes receptive to S-subunit binding (22, 35). These discoveries have helped advance crop Rubisco bioengineering by increasing our appreciation of the need for heterologous L-subunit sequence compliance with the chloroplast stroma chaperone machinery (6, 24). Not meeting the compatibility requirements has impaired, or prevented, almost all efforts to engineer foreign Rubisco in tobacco chloroplasts. By contrast, we demonstrate the assembly requirements of the Form IC RsRubisco are readily met in chloroplasts, accumulating to ∼40% the Rubisco abundance in WT tobacco. By comparison, using the same genetic approach to introduce tobacco or potato rbcL-rbcS operons into the plastome of a tobRr genotype where S-subunit synthesis had been RNA interference (RNAi)-silenced saw the level of tobacco or potato Rubisco production was, at best, also ∼40–50% the Rubisco abundance in WT tobacco (36).
Our discovery that the assembly requirements of RsRubisco are readily met in chloroplasts suggest this Form IC isoform may provide a promising protein chassis to progress the long-awaited capability to bioengineer “red”-lineage Rubisco in chloroplasts (21, 37). Our preliminary work on this found the kinetics of hybrid RsRubisco incorporating differing algae S-subunits was impaired with kcatc reduced >95% and Sc/o lowered up to 50%. The work also showed differing structural compatibilities between the RsL-subunit and algae S-subunits perturbed L8S8 holoenzyme biogenesis by comparable extents in both E. coli and chloroplasts (Fig. 4). These findings indicate the RsRubisco biogenesis requirements are suitably met by the protein folding and assembly machineries in both expression hosts without the need to coexpress cognate R. sphaeroides chaperone(s). Nevertheless, complementation with RsRca was needed in tobacco to boost RsRubisco function, reinforcing the pervasive influence this metabolic repair chaperone has on carbon assimilation and growth in phototrophs and underscoring the mechanistic incompatibility between RsRca and plant Rca (30, 35).
RsRubisco, an Enzyme Scaffold for Red-Rubisco Bioengineering in Chloroplasts.
One objective to improve plant photosynthesis has been the replacement of crop Rubisco with a Form ID isoform from a red-algae such as G. monilis whose 20% higher carboxylation efficiency and twofold improved Sc/o are predicted to improve yield by up to 30% (37). Success has not been forthcoming as the assembly requirements of Form ID Rubisco are not met in chloroplasts (20, 21). Although structural variations between the red and green Form I Rubisco lineages seem subtle (16), they clearly have a dramatic effect on catalysis and pinpointing the sequence changes which impart kinetic variability has proven problematic (17). The chloroplast and E. coli assembly limitations of Form ID Rubisco however do not extend to RsRubisco, suggesting it may possibly serve as a viable protein scaffold for bioengineering the “red”-Rubisco lineage. As shown here, while the RsRubisco kcatc is 20% higher than tobacco, its CE (kcatc/Kc21%O2) is reduced threefold and Sc/o decreased by 25% (Table 1). Accordingly, the growth of the tobRsLS and tobRsLS::X lines could not be supported under ambient CO2 (SI Appendix, Fig. S2) due to unsustainable CO2-assimilation rates of less than 1 µmol·m2·s−1 (Fig. 3A). Efforts to further enhance the CE and Sc/o by directed evolution (26) are underway, in addition to integrating prospective catalysis-enhancing structural elements from Form ID Rubisco into RsRubisco by phylogenetic grafting. The experimental pipeline includes kinetic characterization and evaluating mutant RsRubisco expression initially in E. coli before transforming the enzymes simulated to improve photosynthesis into RsRca-expressing tobacco.
The Requirement for a Compatible Activase in Rubisco Bioengineering.
Our understanding of the phylogenetic diversity and mechanistic function of the Rubisco activase superfamily has advanced dramatically over the last decade (30, 35, 38). Underpinning these endeavors is the desire to modify Rca function in crops to augment Rubisco activity and, hence, photosynthesis, under fluctuating light and elevated temperature (1, 7). Until now it has been assumed the excess Rca inherently produced in tobacco (39) provides ample regulatory potential to the low levels of recombinant plant Rubisco produced, as shown by ref. 40. This was not the case in the tobRsLS lines that necessitated RsRca coexpression to metabolically repair RsRubisco catalytic competency (Fig. 3B) leading to >twofold improvements in tobRsLS::X photosynthesis (Fig. 3A) and growth (Fig. 2 B and C). This queries whether deficiencies in Rubisco activation status have contributed to the higher CO2-requiring, poorer photosynthesis and growth of tobacco-expressing cyanobacteria Rubisco (41, 42), especially with the recent discovery that Rca in cyanobacteria serves both a catalytic and structural role in activating and packaging Form IB Rubisco in carboxysomes (38). Notably Rubisco activation by the plant-like cyanobacteria Rca involves interactions between the L-subunit N terminus and the central pore loops of hexameric Rca. To what extent appending the tobacco L-subunit N terminus onto the RsL-subunit in the tobRsLS and tobRsLS::X lines might enable limited activation of the RsRubisco by NtRca remains to be experimentally tested.
Strategies for Increasing RsRubisco Production in Chloroplasts.
Compared with cyanobacteria L8S8 expression in tobacco (41, 42), RsRubisco production is >threefold higher and matches the Rubisco content in tobacco-expressing different Flaveria rbcL genes (23) and rbcL-rbcS plastome transformed RNAi-RbcS tobacco lines producing tobacco or potato Rubisco (36). Notably, the Rs-rbcL-rbcS mRNA abundance in tobRsLS was >75% lower than rbcL levels in wild type (Fig. 1D). Other transplastomic studies also replacing the tobacco rbcL gene with comparable Arabidopsis rbcL-raf1 operon (23) or synthetic plant rbcL-rbcS polycistrons (36) have similarly shown reductions in transgene mRNA abundance and Rubisco expression. This suggests elevating RsRubisco production may be achieved by increasing transcript abundance and stability. This might be achieved by equipping the rbcS gene (and aadA gene) with independent regulatory (promoter/terminator) elements or insertion into the plastome inverted repeat regions to duplicate copy number. Alternatively, incorporating an intercistronic expression element (IEE) upstream of rbcS to facilitate rbcLS transcript processing into stable monocistronic mRNAs (43–45) may potentially increase RsL- and RsS-subunit translation and RsRubisco production.
Importance of Subunit Complementarity for Assembly and Function.
Although RsRubisco and the Form ID red algal enzymes share a common L-subunit lineage and strong S-subunit structural relatedness (SI Appendix, Fig. S4), they have evolved in contrasting cellular contexts (19). Nevertheless, sufficient structural complementarity exists between the RsL8 core and algae Form ID S-subunits to allow hybrid RsRubisco assembly in both E. coli and tobacco chloroplasts (Fig. 4 A and B). As shown previously, RsRubisco assembly depends on the RsS-subunit to mediate RsL8 core formation, L8S8 stability, and conformational change within the active sites to initiate catalytic competency (27). The impaired kinetics of each hybrid RsRubisco (Fig. 4B) is congruent with the known influence the S-subunit has on the catalysis of all Form I Rubisco isoforms (17, 46, 47). As indicated above, targeted mutagenic changes to the RsL- and RsS-subunits based on structure-function phylogenetic grafting may provide solutions beneficial to catalysis. Such efforts however require more extensive Form ID Rubisco sequence and kinetic investigation as first, their carboxylation properties diverge significantly from Form IB Rubisco (8, 12, 13, 16, 48) and second, the subunits of both lineages show sufficient structural differences that prevent the production of hybrid RsRubisco comprising tobacco S-subunits in both E. coli (Fig. 4 A and B) and chloroplasts (Fig. 1E). The absence of detectable tobacco S-subunits in tobRsLS validates prior findings that unassembled S-subunits are rapidly degraded by proteolysis in the chloroplast stroma (32, 49).
Conclusion
A major hindrance to bioengineering Rubisco in chloroplasts is uncertainty in the compatibility of recombinant Rubisco with the chloroplast protein folding machinery. Likewise, incongruence between Form IB Rubisco expression in E. coli and chloroplasts limits the throughput of in planta Rubisco bioengineering. Whether the capabilities for expressing plant Rubisco in E. coli can be employed to predetermine expression in chloroplasts remains unclear (22). Nevertheless, our findings that the biogenesis requirements of RsRubisco are readily met in both E. coli and chloroplasts, the cognate RsRca metabolic repair chaperone expresses well and functions in tobacco, that RsRubisco can be evolved by directed evolution (27) and provides a compatible structural scaffold to Form ID enzymes all bode well for advances being made to improving crop photosynthesis via RsRubisco bioengineering.
Materials and Methods
Tobacco Chloroplast and Nuclear Transformation.
The pLEVRsLS plasmid was derived from the plastome transforming vector pLEV4 (23) containing a Rs-rbcLS operon (GenBank accession no. KM464722) whose codon used matches the tobacco rbcL gene. The Rs-rbcS gene in pLEVRsLS was replaced with synthetic Gs-rbcS, Gm-rbcS, and Pt-rbcS genes (GenBank accessions MT833926, MT833925, and MT833927, respectively) to produce pLEVRsLGsS, pLEVRsLGmS, and pLEVRsLPtS, respectively. The plasmids were transformed into tobRr leaves (32) by biolistic bombardment and the transformed lines identified by native-PAGE (50). Only the tobRsLS lines could be grown to maturity in soil and required elevated (1% [vol/vol]) CO2. A synthetic TP-cbbX gene was transformed into tobRsLS#1 using Agrobacterium tumefaciens LB4404 and tobRsLS::X lines identified by immunoblot analysis SI Appendix, Fig. S1A and F1 heterozygous lines 7 and 8 identified by segregation analyses SI Appendix, Fig. S1B.
E. coli Expression of WT and Hybrid RsRubisco.
The rbcL-rbcS operon from each pLEV construct were cloned as NcoI/SalI fragments into pET28a(+) to produce plasmids pETRsLS, pETRsLGsS, pETRsLGmS, and pETRsLPtS. The tobacco S-subunit producing plasmid pETRsLNtS contained a codon modified tobacco (Nt) rbcS gene (GenBank accession no. MT596796). Expression of hybrid and WT RsRubisco in BL21(DE3) E. coli was undertaken in triplicate in 25 mL of Lysogeny broth and 30 µg·mL−1 kanamycin cultures grown at 37 °C to an OD600 of 0.8 before inducing with 0.5 mM IPTG, grown at 28 °C for 7 h before centrifuging (4 °C, 8,000 × g, 5 min), N2 freezing the cells and storing at −80 °C.
Cell Protein Analyses.
The protein in tobacco leaf samples and lysed E. coli cells were extracted, quantified, and analyzed by native- and SDS/PAGE as described (10, 25, 50). The Rubisco content was quantified by [14C]-CABP binding, its activation status was determined using the NADH-coupled spectrophotometric assay (51), and the kinetics was determined as described (25, 40, 50).
Preparation and Hybridization with 32P-Labeled DNA Probes.
Total leaf RNA was extracted, separated on denaturing formaldehyde agarose gels, transferred onto nylon membrane, hybridized with 32P-labeled DNA probes, and signal densitometry was analyzed as previously described (23).
Supplementary Material
Acknowledgments
This research was supported by the Australian Government through the Australian Research Council Centre of Excellence for Translational Photosynthesis Grant CE140100015.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011641117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Bailey-Serres J., Parker J. E., Ainsworth E. A., Oldroyd G. E. D., Schroeder J. I., Genetic strategies for improving crop yields. Nature 575, 109–118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans J. R., Lawson T., From green to gold: Agricultural revolution for food security. J. Exp. Bot. 71, 2211–2215 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Simkin A. J., López-Calcagno P. E., Raines C. A., Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70, 1119–1140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans J. R., Clarke V. C., The nitrogen cost of photosynthesis. J. Exp. Bot. 70, 7–15 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Bauwe H., Photorespiration, (eLS, 2019), pp. 1–9. [Google Scholar]
- 6.Sharwood R. E., Engineering chloroplasts to improve Rubisco catalysis: Prospects for translating improvements into food and fiber crops. New Phytol. 213, 494–510 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Carmo-Silva E., Scales J. C., Madgwick P. J., Parry M. A. J., Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 38, 1817–1832 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Iñiguez C. et al., Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 101, 897–918 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Orr D. J. et al., Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiol. 172, 707–717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharwood R. E., Ghannoum O., Kapralov M. V., Gunn L. H., Whitney S. M., Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nat. Plants 2, 16186 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Wu A., Hammer G. L., Doherty A., von Caemmerer S., Farquhar G. D., Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5, 380–388 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Young J. N. et al., Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. J. Exp. Bot. 67, 3445–3456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heureux A. M. C. et al., The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae. J. Exp. Bot. 68, 3959–3969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long S. P., Zhu X. G., Naidu S. L., Ort D. R., Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29, 315–330 (2006). [DOI] [PubMed] [Google Scholar]
- 15.John Andrews T., Whitney S. M., Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Arch. Biochem. Biophys. 414, 159–169 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Valegård K. et al., Structural and functional analyses of Rubisco from arctic diatom species reveal unusual posttranslational modifications. J. Biol. Chem. 293, 13033–13043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Lun M., van der Spoel D., Andersson I., Subunit interface dynamics in hexadecameric rubisco. J. Mol. Biol. 411, 1083–1098 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Badger M. R., Bek E. J., Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 59, 1525–1541 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Allen J. F., de Paula W. B., Puthiyaveetil S., Nield J., A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci. 16, 645–655 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Lin M. T., Hanson M. R., Red algal Rubisco fails to accumulate in transplastomic tobacco expressing Griffithsia monilis RbcL and RbcS genes. Plant Direct 2, e00045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitney S. M., Baldet P., Hudson G. S., Andrews T. J., Form I Rubiscos from non-green algae are expressed abundantly but not assembled in tobacco chloroplasts. Plant J. 26, 535–547 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Aigner H. et al., Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 358, 1272–1278 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Whitney S. M., Birch R., Kelso C., Beck J. L., Kapralov M. V., Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by coexpressing its ancillary RAF1 chaperone. Proc. Natl. Acad. Sci. U.S.A. 112, 3564–3569 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlan B. et al., BSD2 is a Rubisco-specific assembly chaperone, forms intermediary hetero-oligomeric complexes, and is nonlimiting to growth in tobacco. Plant Cell Environ. 42, 1287–1301 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Wilson R. H., Martin-Avila E., Conlan C., Whitney S. M., An improved Escherichia coli screen for Rubisco identifies a protein-protein interface that can enhance CO2-fixation kinetics. J. Biol. Chem. 293, 18–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Whitney S. M., Directed evolution of an improved Rubisco; In vitro analyses to decipher fact from fiction. Int. J. Mol. Sci. 20, 5019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi J., Mueller-Cajar O., Tsai Y. C., Hartl F. U., Hayer-Hartl M., Role of small subunit in mediating assembly of red-type form I Rubisco. J. Biol. Chem. 290, 1066–1074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller-Cajar O. et al., Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase. Nature 479, 194–199 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Bhat J. Y. et al., Mechanism of enzyme repair by the AAA+ chaperone Rubisco activase. Mol. Cell 67, 744–756.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Mueller-Cajar O., The diverse AAA+ machines that repair inhibited Rubisco active sites. Front. Mol. Biosci. 4, 31 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda H., Maliga P., Sequences downstream of the translation initiation codon are important determinants of translation efficiency in chloroplasts. Plant Physiol. 125, 430–436 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitney S. M., Sharwood R. E., Construction of a tobacco master line to improve Rubisco engineering in chloroplasts. J. Exp. Bot. 59, 1909–1921 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Whitney S. M., von Caemmerer S., Hudson G. S., Andrews T. J., Directed mutation of the Rubisco large subunit of tobacco influences photorespiration and growth. Plant Physiol. 121, 579–588 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson R. H., Whitney S. M., “Improving CO2 fixation by enhancing Rubisco performance” in Directed Enzyme Evolution: Advances and Applications, Alcalde M., Ed. (Springer International Publishing, Cham, 2017), pp. 101–126. [Google Scholar]
- 35.Bracher A., Whitney S. M., Hartl F. U., Hayer-Hartl M., Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 68, 29–60 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Martin-Avila E. et al., Modifying plant photosynthesis and growth via simultaneous chloroplast transformation of Rubisco large and small subunits. Plant Cell 32, 2898–2916 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X. G., Portis A. R., Long S. P., Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ. 27, 155–165 (2004). [Google Scholar]
- 38.Flecken M, et al. , Dual role of a Rubisco activase in metabolic repair and carboxysome organization. BioRxiv:2020.2005.2016.099382 (16 May 2020).
- 39.Yamori W., von Caemmerer S., Effect of Rubisco activase deficiency on the temperature response of CO2 assimilation rate and Rubisco activation state: Insights from transgenic tobacco with reduced amounts of Rubisco activase. Plant Physiol. 151, 2073–2082 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharwood R. E., von Caemmerer S., Maliga P., Whitney S. M., The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiol. 146, 83–96 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long B. M. et al., Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nat. Commun. 9, 3570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Occhialini A., Lin M. T., Andralojc P. J., Hanson M. R., Parry M. A. J., Transgenic tobacco plants with improved cyanobacterial Rubisco expression but no extra assembly factors grow at near wild-type rates if provided with elevated CO2. Plant J. 85, 148–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock R., Genetic engineering of the chloroplast: Novel tools and new applications. Curr. Opin. Biotechnol. 26, 7–13 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Lu Y., Rijzaani H., Karcher D., Ruf S., Bock R., Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proc. Natl. Acad. Sci. U.S.A. 110, E623–E632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou F., Karcher D., Bock R., Identification of a plastid intercistronic expression element (IEE) facilitating the expression of stable translatable monocistronic mRNAs from operons. Plant J. 52, 961–972 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa C., Hatanaka T., Misoo S., Miyake C., Fukayama H., Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiol. 156, 1603–1611 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spreitzer R. J., Peddi S. R., Satagopan S., Phylogenetic engineering at an interface between large and small subunits imparts land-plant kinetic properties to algal Rubisco. Proc. Natl. Acad. Sci. U.S.A. 102, 17225–17230 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson D. T., Breaking the rules of Rubisco catalysis. J. Exp. Bot. 67, 3180–3182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt G. W., Mishkind M. L., Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc. Natl. Acad. Sci. U.S.A. 80, 2632–2636 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitney S. M., Sharwood R. E., Plastid transformation for Rubisco engineering and protocols for assessing expression. Methods Mol. Biol. 1132, 245–262 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Sharwood R. E., Sonawane B. V., Ghannoum O., Whitney S. M., Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. J. Exp. Bot. 67, 3137–3148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.