Significance
Ionotropic glutamate receptors (iGluRs) are a diverse family of tetrameric ligand-gated ion channels. Recent structural information has provided valuable insight into the conformational dynamics and assembly properties of iGluRs, most importantly on heteromeric iGluRs, which are prevalent in the nervous system. However, little is known about how channel gating is controlled by the 4 subunits within the tetramers, different ligand occupancies, and intersubunit cooperativity. In this study, we probe how subunit-selective ligands affect activation and desensitization of heteromeric iGluRs and we provide mechanistic insight into ligand dissociation. Our findings have direct pharmacologic implications, as we show that subunit-selective antagonists can block desensitization of heteromeric iGluRs, thereby causing potentiation instead of inhibition.
Keywords: ligand-gated ion channel, medicinal chemistry, non-NMDA receptor, receptor modulation
Abstract
Ionotropic glutamate receptors (iGluRs) are key molecules for synaptic signaling in the central nervous system, which makes them promising drug targets. Intensive efforts are being devoted to the development of subunit-selective ligands, which should enable more precise pharmacologic interventions while limiting the effects on overall neuronal circuit function. However, many AMPA and kainate receptor complexes in vivo are heteromers composed of different subunits. Despite their importance, little is known about how subunit-selective ligands affect the gating of heteromeric iGluRs, namely their activation and desensitization properties. Using fast ligand application experiments, we studied the effects of competitive antagonists that block glutamate from binding at part of the four subunits. We found that UBP-310, a kainate receptor antagonist with high selectivity for GluK1 subunits, reduces the desensitization of GluK1/GluK2 heteromers and fully abolishes the desensitization of GluK1/GluK5 heteromers. This effect is mirrored by subunit-selective agonists and heteromeric receptors that contain binding-impaired subunits, as we show for both kainate and GluA2 AMPA receptors. These findings are consistent with a model in which incomplete agonist occupancy at the four receptor subunits can provide activation without inducing desensitization. However, we did not detect significant steady-state currents during UBP-310 dissociation from GluK1 homotetramers, indicating that antagonist dissociation proceeds in a nonuniform and cooperativity-driven manner, which disfavors nondesensitizing occupancy states. Besides providing mechanistic insights, these results have direct implications for the use of subunit-selective antagonists in neuroscience research and envisioned therapeutic interventions.
Glutamate is an important neurotransmitter in the mammalian brain. Its excitatory action is mediated by ionotropic glutamate receptors (iGluRs), a family of tetrameric ligand-gated ion channels comprising 18 different genes (1, 2). The different iGluR subtypes play key roles in synaptic transmission, modulation, and plasticity (3–5); thus, controlling iGluR function may be useful for treating a wide range of diseases, such as pain, migraine, epilepsy, mood disorders, and addiction, as well as for managing ischemic stroke, glioblastoma, and neurodegeneration.
The discovery of agonists and antagonists that discriminate between the AMPA (GluA), kainate (GluK), and NMDA (GluN) receptor subfamilies (1, 6) was a key step in dissecting their physiological functions. However, the clinical utility of iGluR agonists and antagonists has remained limited, because broad activation or inhibition of iGluRs is associated with severe side effects (e.g., refs. 7–9). To overcome these side effects, current medicinal research is focusing on allosteric ligands with modulatory effects and subunit-specific ligands that show selective binding at only one or a few iGluR subtypes (10–12). For instance, subunit-selective kainate receptor antagonists have been proposed for treating cerebral ischemia (LY377770) (13), anxiety (LY382884) (14), and epilepsy (UBP-310) (15, 16) and have been in clinical trials for treating pain (LY5454694) (17). In the case of AMPA receptors, which have more closely related ligand-binding domains (LBDs), some moderately selective agonists have been reported, but subunit-selective antagonists are rare (18).
Subunit-specific ligands are thought to allow for more precise intervention, as their action remains confined to a small set of receptor subtypes and to the brain regions where these subtypes are expressed. However, many or even most neuronal iGluRs are heteromeric receptors (3, 4, 19–23), which increases the versatility of iGluR signaling (24–26) and allows for fine-tuned regulation. This motivated us to investigate how heteromeric kainate and AMPA receptors are impacted by subunit-specific ligands.
One hallmark of AMPA and kainate receptor gating is their fast (millisecond) desensitization, which is induced by prolonged glutamate binding. Desensitization is tightly regulated by, for example, RNA splicing (27) and auxiliary subunits (28–31). Depending on the glutamate dynamics in the synaptic cleft, desensitization exerts important physiological functions. It can terminate the current flow after glutamate release and can shape the responses to low residual concentrations of glutamate (e.g., refs. 32, 33). Recovery from desensitization is slow, taking tens of milliseconds in the case of AMPA receptors but seconds for kainate receptors (34). Desensitization and recovery may thus control the responses to subsequent release events and may affect postsynaptic short-term plasticity and frequency dependence (29). Suppression of AMPA receptor desensitization causes severe developmental defects (35).
Recent full-length iGluR structures provide insight into the conformational changes occurring on ligand binding (36–39), but how the ligand occupancy at the four LBDs controls receptor activation and desensitization remains unclear. Structure-function studies have revealed that glutamate binding leads to closure of the clamshell-like LBDs, which appears to be a requirement for efficient activation (40). Single-channel recordings on desensitization-blocked receptors have demonstrated that at least two subunits must be occupied by agonists for substantial activation to occur (31, 41). Desensitization is thought to originate from rearrangements of the interface between two adjacent LBDs, as stabilization of this interface can slow or abolish desensitization (42–45).
Based on the observation that even low glutamate concentrations elicit efficient desensitization in the absence of significant activation (31, 46, 47), it was further concluded that a single occupied subunit may be sufficient to cause receptor desensitization, which became part of some widely used models to describe iGluR gating (26, 47). However, the interpretation of experiments with low ligand concentrations is complicated by slow binding kinetics and the fact that mid- and high-occupancy states are still sampled occasionally (48).
More recent work on a special class of heteromeric kainate receptors—those incorporating both low- and high-affinity subunits (e.g., GluK2 and GluK5, respectively; formerly known as GluR6 and KA2)—has shown that these receptors can evade desensitization with two occupied subunits while showing substantial activation (48–50). In these experiments, subunit-selective agonists (48, 51), the incorporation of mutated subunits with lowered affinity (49, 50), and tethered photoswitchable ligands (48) were used to ensure less than full receptor occupancy. However, whether this behavior also applies to other types of kainate receptors and the postsynaptically far more abundant AMPA receptors remains unclear. It also raises the important question of how subunit-selective antagonists would affect the gating of heteromeric iGluRs, many of which are prevalent in the nervous system.
Here we show that UBP-310, a GluK1-selective antagonist, reduces the desensitization of GluK1/GluK2 heteromers (formerly known as GluR5/GluR6) and fully abolishes the desensitization of GluK1/GluK5 heteromers (formerly GluR5/KA2). We find that similar effects are elicited by subunit-selective agonists and subunit-specific manipulations at heteromeric AMPA receptors. Antagonist dissociation experiments provide additional mechanistic insight into the behavior of homomeric receptors. Overall, we find that partial ligand occupancy causes reduced desensitization, which may have direct implications for the use of subunit-selective ligands in neuroscience research and therapeutic applications.
Results
UBP-310 Antagonizes GluK1 Homomers but Reduces Desensitization of GluK1/GluK2 Heteromers.
To test the influence of competitive subtype-specific antagonists on kainate receptor desensitization, we chose UBP-310. This willardiine derivative shows varying binding affinities at different GluK subunits, with the highest affinity reported for GluK1 (52–54).
We assessed the effect of UBP-310 on different kainate receptor combinations by performing fast glutamate perfusion experiments on outside-out patches from human embryonic kidney (HEK) cells (Fig. 1A). Fast application of 3 mM glutamate to GluK1 homotetramers yielded characteristic inward currents (Fig. 1B and SI Appendix, Fig. S1). Fast activation and channel opening (rise time <0.4 ms; SI Appendix, Fig. S1A) was followed by rapid and almost complete desensitization (mean τdesen = 0.8 ± 0.1 ms; mean Isteady-state/peak = 1.0 ± 0.4%, n = 7 patches; Fig. 1 B, D, and E). Recovery from glutamate-induced desensitization occurred on the seconds timescale (τrecov = 2.0 s; SI Appendix, Fig. S1B), in line with previous reports (28).
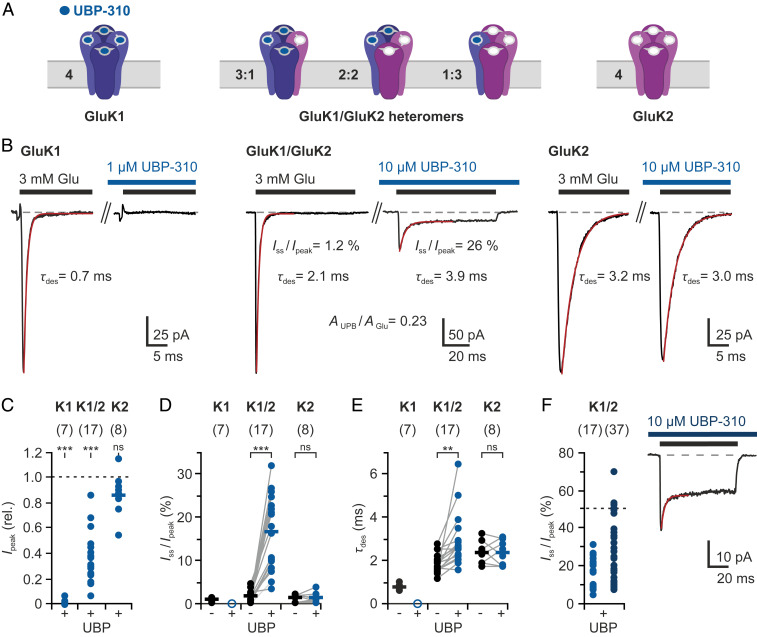
Fig. 1.
Effects of UBP-310, a GluK1-selective antagonist, on GluK1/GluK2 heteromers. (A) Cartoon representation of GluK1/GluK2 receptors with variable subunit composition. UBP-310 (blue circles) prevents glutamate from binding at GluK1 subunits (blue). (B) Typical current responses from GluK1 homomers (Left), GluK1/GluK2 heteromers (Middle), and GluK2 homomers (Right) to 3 mM Glu in the absence and presence of UBP-310 (concentrations as indicated). (C) Peak current reduction by UBP-310. (D) Relative steady-state currents in the absence and presence of UBP-310. (E) Effects on desensitization time constants (τdes). (F) Additional data obtained in the continued presence of UBP-310 (dark blue). Measurements were performed by fast ligand application to outside-out patches from transfected HEK cells. The bars indicate mean values of normally distributed data from (n) different patches. Statistical testing was performed using paired t tests: **P ≤ 0.01; ***P ≤ 0.001; ns, not significant. More details are provided in SI Appendix, Figs. S1 and S2.
When we added 1 μM UBP-310 to the glutamate and wash solutions during individual recordings, the glutamate-induced currents were fully suppressed (Fig. 1 B and C), confirming that UBP-310 acts as high-affinity antagonist at GluK1 (52, 53). In contrast, the addition of UBP-310 had no significant effect on the responses of GluK2 homotetramers, even when applied at a concentration of 10 μM (Fig. 1 B and C). However, at 100 μM UBP-310 the responses of GluK2 homotetramers to 3 mM glutamate were partially antagonized (SI Appendix, Fig. S1E), which is in line with previous reports (55).
To test the influence of UBP-310 on heteromeric receptors, we coexpressed GluK1 and GluK2 subunits, which are expected to form receptor complexes with varying stoichiometries of GluK1 and GluK2 subunits (20, 24). Indeed, currents from cotransfected cells showed desensitization kinetics intermediate between fast GluK1 and slow GluK2 desensitization (Fig. 1 B and E and SI Appendix, Fig. S2). The addition of 10 μM UBP-310 caused a current reduction in all patches ranging from 14% to 93% (n = 17 patches; Fig. 1 B and C), which indicates that different amounts of GluK1 subunits were present. Desensitization slowed in the presence of UBP-310 (Fig. 1E), mainly because the remaining currents were now dominated by GluK2-containing receptors.
Most importantly, the addition of antagonist had a marked effect on the amount of desensitization. The relative steady-state current, Iss/peak, increased from mean 1.9 ± 1.2% in the absence of UBP-310 to up to 32% in the presence of UBP-310 (mean Iss/peak = 16.8 ± 8.3%, n = 17 patches; Fig. 1 B and D). Double-pulse experiments show that these steady-state currents do not desensitize further, which indicates that they originate from stable receptor subpopulations that do not desensitize (SI Appendix, Fig. S2B).
The highest steady-state currents were observed for patches in which UBP-310 binding caused the strongest current reduction, that is, in patches from cells that apparently expressed higher levels of GluK1 than GluK2 (SI Appendix, Fig. S2C). Analyzing additional patches in the continued presence of UBP-310 showed that the steady-state currents can rise to >50% (mean Iss/peak = 29.7 ± 16.9%, n = 37 patches; Fig. 1F and SI Appendix, Fig. S2D). Overall, these data indicate that some receptor combinations with mixed agonist/antagonist occupancies give nondesensitizing currents (SI Appendix, Note 1 and Fig. S8).
UBP-310 Fully Suppresses Desensitization of GluK1/GluK5 Heteromers.
Endogenous kainate receptors often incorporate the high-affinity subunits GluK4 or GluK5 in addition to the low-affinity subunits, GluK1 to GluK3. The GluK5 subunit is widely expressed in the central nervous system (3, 19) and the incorporation of high-affinity subunits has profound consequences on receptor trafficking and gating (26, 56, 57). It has been shown that coexpression of GluK2 and GluK5 subunits yields functional heteromers with defined 2:2 stoichiometry next to GluK2 homotetramers (58). GluK5 homotetramers remain nonfunctional and do not traffic to the membrane (56–59).
Here we coexpressed GluK1 and GluK5 subunits, which resulted in small but completely desensitizing currents with fast activation and desensitization kinetics (mean Iss/peak = 2.1 ± 0.4%, mean τdesen = 0.7 ± 0.1 ms, n = 9 patches; Fig. 2 and SI Appendix, Fig. S3), similar to GluK1 homomers. In the subsequent experiments, we lowered the UBP-310 concentration to 1 μM, since UBP-310 at 10 μM also blocked GluK5 subunits (SI Appendix, Fig. S3B) (55).
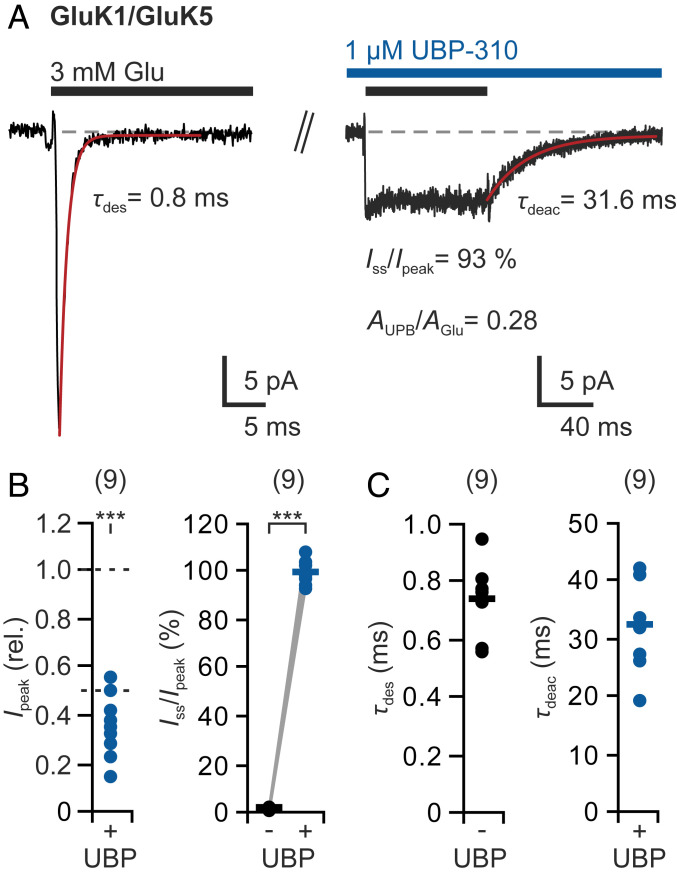
Fig. 2.
The antagonist UBP-310 abolishes desensitization of GluK1/GluK5 heteromers. (A) Typical glutamate responses after coexpression of GluK1 and GluK5 subunits before (Left) and after (Right) the addition of 1 μM UBP-310. (B) UBP-310 reduced the current (Ipeak ≤56%) and completely blocked desensitization (Iss/Ipeak = ∼99%). (C) Desensitization time constants (τdes) in the absence of UBP-310 and deactivation time constants (τdeac) in the presence of UBP-310. Data are from n = 9 patches; bars indicate mean values. Statistical testing was performed with paired t tests: ***P ≤ 0.001. See also SI Appendix, Fig. S3.
The addition of 1 μM UBP-310 led to a strong but variable current reduction to 14% to 56% (n = 9 patches; Fig. 2 A and B), which indicates variable amounts of heteromeric GluK1/GluK5 receptors next to GluK1 homomers. Most striking was the effect on desensitization. In the presence of 1 μM UBP-310, all currents were completely nondesensitizing, that is, desensitization became fully blocked (mean Iss/peak = 99.2 ± 4.3%, n = 9 patches; Fig. 2B), which was also confirmed in double-pulse experiments (SI Appendix, Fig. S3E). As a result, the use of a GluK1-specific antagonist caused a strong overall potentiation of the currents originating from GluK1/GluK5 heteromers. After glutamate removal, the currents slowly deactivated (mean τdeac = 32.6 ± 7.3 ms, n = 9 patches; Fig. 2C), which matches the glutamate deactivation kinetics that have been reported for GluK5 subunits (26).
Overall, these data suggest that GluK1/GluK5 heteromers yield half-maximal but nondesensitizing currents when glutamate is prevented from binding at the GluK1 subunits. This behavior resembles the situation in GluK2/GluK5 heteromers, in which agonist binding at either the two GluK2 subunits or the two GluK5 subunits produces half-maximal activation but no desensitization (48) (SI Appendix, Note 1).
Desensitization during Antagonist Dissociation from GluK1 and GluK1/GluK5 Receptors.
Our experiments show that desensitization is reduced when antagonists occupy only a part of the subunits that are being present in heteromeric receptors. This suggests that desensitization may also be reduced during antagonist dissociation while receptors are progressing through different occupancy states (Fig. 3A).
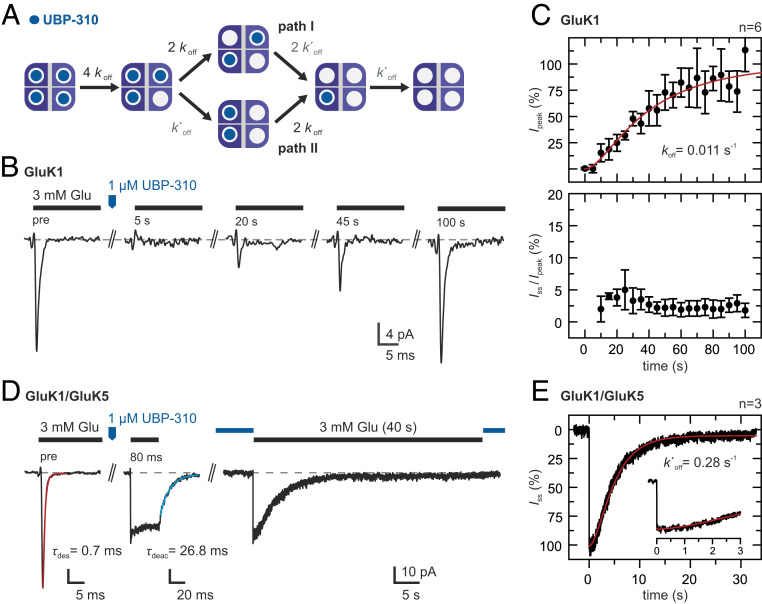
Fig. 3.
Glutamate-induced currents during UBP-310 dissociation. (A) Kinetic scheme showing the different occupancy states that can become populated during antagonist dissociation. Two antagonists may be bound at different LBD dimers (path I) or at the same LBD dimer (path II). More details are provided in SI Appendix, Note 2. (B) Glutamate responses during UBP-310 dissociation from GluK1 homotetramers. Test pulses of 3 mM glutamate were applied before and at 5-s intervals after applying a pulse of 1 μM UBP-310. (C) Peak currents (Top) and steady-state currents (Bottom) normalized to the peak currents before UBP-310 application (mean ± SD, n = 6 patches). Fitting the kinetic scheme in A with k′off = 0.28 s−1 (see below) yields koff = 0.011 s−1 (red line). (D, Left) Glutamate responses of GluK1/GluK5 heteromers. UBP-310 binding at the GluK1 subunits blocks glutamate-induced desensitization. (D, Right) After UBP-310 dissociation, the GluK1 subunits become available for glutamate binding, and the steady-state current slowly desensitizes. (E) The disappearance of the steady-state current (average current of three patches) shows a clear lag phase. Fitting of a three-state kinetic scheme yields a k′off = 0.28 s−1 (red line). Details of the model are provided in SI Appendix, Note 2, and details on the fits are provided in SI Appendix, Fig. S4.
We first monitored the recurrence of glutamate-induced currents from GluK1 homotetramers after a saturating pulse of UBP-310 had been applied (Fig. 3B). The peak current recovered slowly on the tens of seconds timescale with a pronounced lag phase (t1/2 = ∼35 s, n = 6 patches; Fig. 3C), as expected for receptors that are transitioning through states that yield no or little activation. Similar kinetics were also reported for UBP-310 dissociation from desensitization-blocked GluK1 receptors (52). However, a key observation is that we did not observe any significant steady-state currents during recovery from UBP-310 block (Iss/peak ≤5%, n = 6 patches; Fig. 3C). Apparently, occupancy states that produce nondesensitizing currents are not rate-limiting and do thus not accumulate during dissociation. No significant changes in current rise time and desensitization kinetics were observed (SI Appendix, Fig. S4A).
For comparison, we measured glutamate-induced currents during UBP-310 dissociation from GluK1/GluK5 heteromers (Fig. 3D). Binding of UBP-310 at the GluK1 subunits resulted in nondesensitizing glutamate currents, as shown previously (Fig. 2). On dissociation, also the GluK1 subunits became available for glutamate binding, and the current desensitized (Fig. 3D). The current ceased within seconds, showing a minor lag phase (t1/2 = ∼4.3 s, n = 3 patches; Fig. 3E). Thus, the loss of nondesensitizing configurations in GluK1/GluK5 heteromers during UBP-310 dissociation is considerably faster than the peak current recovery of GluK1 homotetramers.
We analyzed our findings with a simplified model (Fig. 3A and SI Appendix, Note 2 and Fig. S9). Since iGluR tetramers assemble with pseudo-twofold symmetry (36; but see ref. 60 for a recent exception) we assumed that UBP-310 dissociation starts from a symmetric configuration. Different configurations can be distinguished during antagonist dissociation, however. Most obviously, two antagonists (agonists) can be bound at different LBD dimers (path I) or at the same LBD dimer (path II). The situation can be summarized in a branched kinetic scheme (Fig. 3A). The implications of a more detailed version of this model are described in SI Appendix, Note 2.
If dissociation proceeded equally fast from all four subunits (koff = k′off), then all possible occupancy states would become significantly populated during UBP-310 dissociation from GluK1 homotetramers (SI Appendix, Fig. S10A). For statistical reasons, path I would be slightly favored over path II (2:1). This model would reproduce the observed lag phase if we assume that receptors with one agonist bound do not yield current and that receptors with two, three, and four agonists bound contribute increasingly more current (SI Appendix, Note 1 and Fig. S8B). Least-squares fitting yields koff = k′off = 0.023 s−1 (SI Appendix, Fig. S4B); however, this model also predicts significant steady-state currents (SI Appendix, Note 2), in contrast to our experimental observations.
To explain this discrepancy, we explored alternative models. For instance, if cooperativity is at play, then dissociation of the second antagonist, k′off, may be faster than dissociation of the first antagonist from this dimer, koff. In this case, path II would become increasingly favored over path I (SI Appendix, Fig. S10B). In addition, receptors with one antagonist (three agonists) bound would then accumulate to a lesser extent.
Evidence for the second scenario is provided by our data indicating that UBP-310 dissociation from GluK1 subunits is much faster in the context of GluK1/GluK5 heteromers than in GluK1 homotetramers (Fig. 3). If we assume that GluK1/GluK5 heteromers incorporate two GluK1 subunits, as is the case in GluK2/GluK5 heteromers (48, 58) (SI Appendix, Note 1), then a fit of the corresponding model gives k′off = 0.28 s−1 (Fig. 3E and SI Appendix, Fig. S4C). The observed lag phase suggests that receptors with one subunit blocked by UBP-310 (three agonists bound) produce partly nondesensitizing currents (SI Appendix, Note 2 and Figs. S4C and S10C).
Once we incorporate k′off = 0.28 s−1 to describe UBP-310 dissociation from GluK1 homotetramers (Fig. 3C), we obtain koff = 0.011 s−1, a ∼25-fold difference in dissociation rate constants (k′off/koff). This readily explains why nondesensitizing or partially desensitizing configurations, namely path I and receptors with three bound agonists, do not significantly accumulate during antagonist dissociation (SI Appendix, Fig. S10D), in agreement with our experimental observations (Fig. 3 B and C).
The Effect of Subunit-Selective Antagonists Is Mirrored by Subunit-Selective Agonists.
We found that desensitization is reduced or fully abolished when a part of the subunits is occupied by UBP-310. This inhibiting effect on desensitization could be specific to UBP-310 or could be due to reduced agonist occupancy. To test the latter hypothesis, we investigated responses from heteromeric receptors using subunit-selective agonists as well as binding-impaired subunits.
First, we used the agonist 5-iodowillardiine (5-IW), which is known to activate GluK1 and GluK5 but not GluK2 subunits (12, 24, 51). When we applied 100 μM 5-IW after coexpressing GluK1 and GluK2 subunits, we obtained a similar outcome as was seen in the presence of UBP-310: a part of the 5-IW current desensitized rapidly, but substantial steady-state currents remained (mean Iss/peak = 15.6 ± 6.3%, n = 9 patches; Fig. 4A and SI Appendix, Fig. S5). This is in contrast to the full desensitization induced by 5-IW binding at all four subunits, as observed for GluK1 homomers (SI Appendix, Fig. S5A) and GluK1/GluK5 heteromers (mean Iss/peak = 2.0 ± 1.3%, n = 6 patches; Fig. 4A and SI Appendix, Fig. S5B).
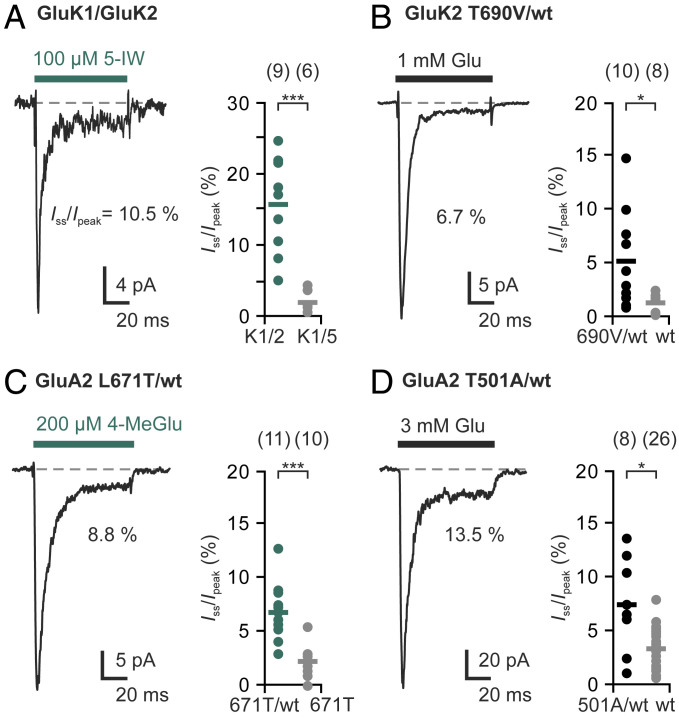
Fig. 4.
Subunit-specific agonists and binding-impaired subunits reduce desensitization of heteromeric kainate and AMPA receptors. (A) Application of the GluK1-specific agonist 5-IW causes significant steady-state currents of GluK1/GluK2 heteromers (green) but not of GluK1/GluK5 heteromers (gray). (B) Incorporation of binding-impaired GluK2(T690V) subunits significantly increases glutamate-induced steady-state currents compared with homomeric GluK2(wt) receptors (gray). (C) GluA2(L671T) is activated by 4-MeGlu, resulting in small steady-state currents that are typical for GluA2 (gray). The 4-MeGlu-induced steady-state currents are significantly higher in heteromers with GluA2(wt) (green). (D) Incorporation of binding-impaired GluA2(T501A) subunits into GluA2 receptors significantly increases glutamate-induced steady-state currents. The bars indicate mean values, and n indicates the number of different patches. Statistical testing was performed with Welch's t tests: *P ≤ 0.05, ***P ≤ 0.001. More details on the experiment and the controls are provided in SI Appendix, Figs. S5–S7.
The range of steady-state currents observed on 5-IW binding to GluK1/GluK2 heteromers (5% to 25%; Fig. 4A) likely reflects heterogeneous receptor compositions, as has been described in a similar experiment on GluK1(Q)/GluK2(R) heteromers (24). In contrast, 5-IW binding to the GluK5 subunits in GluK2/GluK5 heteromers, which assemble in defined stoichiometries (58, 61), results in completely nondesensitizing currents (48). Our findings are also supported by observations reported for ATPA, another GluK1- and GluK5-selective agonist (20).
Similarly, when we coexpressed GluK2(wt) and a binding-impaired GluK2 subunit, GluK2(T690V) (59), we again observed incomplete desensitization with steady-state currents up to 15% (mean Iss/peak = 5.2 ± 4.3%, n = 10 patches; Fig. 4B), in contrast to the pronounced desensitization of GluK2(wt) homotetramers (mean Iss/peak = 1.3 ± 0.8%, n = 8 patches; Fig. 1). Taken together, these experiments confirm that partial agonist occupancies can result in incomplete desensitization of various kainate receptor types.
Partial Agonist Occupancy Also Reduces Desensitization of Heteromeric AMPA Receptors.
We next tested whether subunit-specific manipulations would also affect AMPA receptor desensitization. For this, we artificially modified the GluA2 ligand selectivity by substituting an amino acid side chain in the binding pocket (leucine 671 to threonine; L671T). The L671T substitution is known to increase kainate binding and efficacy at GluA2 receptors (62), and it has been suggested that leucine 671 obstructs binding of the kainate receptor agonist (2S,4R)-4-methylglutamic acid (4-MeGlu; SYM 2081) (63, 64). Indeed, we found that GluA2(L671T) homomers were rapidly activated and desensitized by 200 μM 4-MeGlu, a concentration that does not elicit currents at GluA2(wt) homotetramers (SI Appendix, Fig. S6).
Strikingly, when we applied 4-MeGlu after coexpressing GluA2(L671T) and GluA2(wt) subunits, we observed steady-state currents of up to 13% (mean Iss/peak = 6.9 ± 2.5%, n = 11 patches), significantly higher than those seen on application of glutamate (mean Iss/peak = 3.6 ± 1.7%, n = 6; SI Appendix, Fig. S6B) or application of 4-MeGlu at GluA2(L671T) homotetramers (mean Iss/peak = 2.2 ± 1.4%, n = 10 patches; Fig. 4C). The latter experiment confirms that the reduced desensitization does not result from a low ligand efficacy of 4-MeGlu.
Similarly, coexpression of GluA2(wt) and the low-affinity variant GluA2(T501A) (SI Appendix, Fig. S7) (42) resulted in significantly higher steady-state currents on glutamate application (mean Iss/peak = 7.5 ± 4.1%, n = 8 patches) compared with GluA2(wt) homotetramers (mean Iss/peak = 3.4 ± 1.7%, n = 26 patches; Fig. 4D). Thus, we conclude that AMPA receptors behave similarly to kainate receptors, with partial agonist occupancy resulting in partial desensitization.
Discussion
Heteromeric iGluRs are widely expressed in the central nervous system, but little is known about how subunit-specific antagonists and agonists affect their activation and desensitization properties. In the first part of our study, we relied on UBP-310, a competitive antagonist with reported selectivity for several kainate receptor subunits (52, 53) that is being increasingly used in neuroscience research (e.g., refs. 55, 65). Our fast-ligand application experiments confirm high selectivity for GluK1 receptors over GluK2 receptors and, to a lesser degree, over GluK5-containing receptors (Figs. 1 and 2 and SI Appendix, Figs. S1–S3), which is in line with ligand-binding assays and work on desensitization blocked receptors (52–54). However, in our hands, this specificity was not high enough to clearly differentiate between GluK2 and GluK5 subunits (SI Appendix, Fig. S3B; but see ref. 55).
Most importantly, our observations show that by acting as a subunit-specific antagonist, UBP-310 reduces the glutamate-induced desensitization of heteromeric GluK1/GluK2 receptors (Fig. 1) and fully abolishes the desensitization of GluK1/GluK5 heteromers (Fig. 2). We further show that desensitization is similarly reduced by subunit-selective agonists or the incorporation of binding-impaired subunits, as demonstrated by 5-IW binding to GluK1/GluK2 heteromers and GluK2/GluK2(T690V) pseudoheteromers, respectively (Fig. 4 A and B). This suggests that the inhibitory effects on desensitization result from incomplete agonist occupancy. Thus, we expect that reduced desensitization will be generally observed when purely competitive antagonists block agonist binding at only a part of the four subunits.
We next asked whether AMPA receptor desensitization would be similarly affected. AMPA receptors are the dominant postsynaptic receptor species at excitatory synapses and are frequently heteromers (4, 21–23). Since only few subunit-selective AMPA receptor agonists are currently available (1, 18), we engineered an AMPA receptor subunit, GluA2(L671T), that allows for 4-MeGlu binding and also used a binding-impaired subunit, GluA2(T501A), to form pseudoheteromeric receptors with GluA2(wt) subunits (Fig. 4 C and D). We found that, as in kainate receptors, partial agonist occupancies caused partial desensitization. Thus, one occupied subunit is not sufficient to efficiently trigger full receptor desensitization, in contrast to the predictions of models typically used to describe iGluR gating (26, 47) (SI Appendix, Note 1).
The extent of desensitization block depends on receptor composition and thus on the relative expression levels and assembly preferences of the respective subunits. Steady-state currents from GluK1/GluK2-coexpressing cells and GluK2 or GluA2 pseudoheteromers were variable and mostly rather small, although in some patches steady-state currents reached > 50% (Fig. 1F and SI Appendix, Note 1). A different behavior is seen for kainate receptor complexes that incorporate the high-affinity subunit GluK5. Previous work with tethered photoswitchable ligands and selective agonists has provided detailed insight into the activation and desensitization of GluK2/GluK5 heteromers, which assemble with defined 2:2 stoichiometry (58) and where liganding of either the two GluK2 or the two GluK5 subunits provides half-maximal activation but no desensitization (48). Here we obtained similar results for GluK1/GluK5 heteromers using UBP-310 to block glutamate from binding at GluK1 subunits (Figs. 2 and 3 D and E). This indicates that GluK1/GluK5 heteromers also have a strong preference for assembling into nondesensitizing configurations, possibly with 2:2 stoichiometry (SI Appendix, Note 1). Studies with isolated amino-terminal domains (ATDs) suggest a strong preference for heterodimeric GluK1/GluK5 assemblies, similar to those of GluK2/GluK5 (61, 66).
In another set of experiments, we addressed whether nondesensitizing states are also populated during antagonist dissociation (Fig. 3). We found that UBP-310 dissociates on slower timescales than typical quinoxalinedione antagonists (31, 41), making it well suited for these experiments. In the case of GluK1/GluK5 heteromers, the UBP-310 block was lost within seconds, as demonstrated by the concomitant loss of steady-state current (Fig. 3 D and E). The kinetics shows a lag phase, indicating that receptors with three bound agonists also contribute to this nondesensitizing current (SI Appendix, Note 2). In contrast, UBP-310 dissociation from GluK1 homotetramers was slower, occurring on the tens of seconds timescale, during which we did not detect any significant steady-state currents (Fig. 3 B and C). These observations can be readily explained if we assume that antagonist dissociation is not equally fast at all four receptor subunits. These differences could be due to binding cooperativity and/or positional differences within the tetramers (SI Appendix, Note 2). Our data also show heterogeneity in the receptor configurations with two bound agonists: some configurations produce nondesensitizing currents, while other configurations with two bound agonists produce either no or fully desensitizing responses. This situation can be illustrated by a branched kinetic scheme (Fig. 3A).
More generally, our experiments show that ligand occupancy is an important determinant of AMPA and kainate receptor desensitization, in addition to ligand efficacy (67, 68) and structural rearrangements of the LBD dimer interface (42–44, 69). The prevailing view that one agonist-occupied subunit is sufficient to induce efficient desensitization of the tetrameric receptors (26, 47) had already been challenged by work on kainate receptors incorporating the high-affinity subunits GluK4 and GluK5 (48–50) and here is also disproven for other types of kainate receptors, as well as GluA2 AMPA receptor pseudoheteromers. Further experimental work is needed to address the role of binding cooperativity and interdimer coupling (34), the behavior of different types of AMPA receptor heteromers, and the contribution of auxiliary subunits. Besides the mechanistic implications of our findings, we show that partial receptor occupancies can have significant practical consequences, for instance, when subunit-selective antagonists are used to treat heteromeric receptor populations.
The desensitization block that can arise from using subunit-selective antagonists may be small and variable, but the physiological consequences could be large. Heteromeric AMPA receptors play an important role in the nervous system (4, 21–23), and recent work suggests that their assembly occurs in a biased, nonrandom fashion (22, 25, 70). In this case, the formation of nondesensitizing configurations may be favored, similar to GluK5-containing kainate receptor heteromers in which preferential assembly (58, 61, 66) yields nondesensitizing configurations while high-affinity glutamate binding is preserved (Fig. 2). The impact on synaptic function may be profound if iGluRs maintain nondesensitizing currents in the presence of falling or low basal glutamate concentrations. The use of subunit-specific antagonists may prolong synaptic activations or cause unwanted tonic activations. Moreover, since blocking of desensitization circumvents the need for slow recovery, postsynaptic short-term depression also may be affected.
In summary, we show that competitive antagonists with high subunit-selectivity can cause unintended effects at heteromeric iGluRs. Depending on the receptor composition, they reduce desensitization. This is important to keep in mind when subunit-selective antagonists are used in neurophysiological experiments, for example, when UBP-310 or its derivative ACET is used to dissect metabotropic kainate receptor signaling (55, 65, 71). Furthermore, subunit-selective ligands may cause new adverse effects in therapeutic applications. These findings should motivate more detailed investigations analyzing the effects of subunit-selective ligands and modulators on iGluR function.
Materials and Methods
Currents were measured by fast piezo-driven glutamate application to outside-out patches from HEK cells transiently expressing the indicated kainate and AMPA receptor heteromers. Voltage-clamp measurements were performed at −70 mV using the indicated ligands. Details of the constructs, measurements, and data analysis are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank P. H. Seeburg (MPI Heidelberg), Y. Stern-Bach (Hebrew University), K. M. Partin (Colorado State University), L. Chen (Stanford University), and E.Y.I. for iGluR expression plasmids and Hendrik Margis, Laura Hönig, and Jeannette Gebel for mutagenesis and/or initial whole-cell characterization of receptor variants. We also thank Nadine Hube for excellent technical assistance and Adela Dudić for discussion. This project was funded by a grant from the NRW-Rückkehrprogramm (to A.R.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007471117/-/DCSupplemental.
Data Availability.
All study data are included in the main text and SI Appendix.
References
- 1.Traynelis S. F. et al., Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiner A., Levitz J., Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 98, 1080–1098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contractor A., Mulle C., Swanson G. T., Kainate receptors coming of age: Milestones of two decades of research. Trends Neurosci. 34, 154–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley J. M., Wilkinson K. A., Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17, 337–350 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Paoletti P., Bellone C., Zhou Q., NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Watkins J. C., Krogsgaard-Larsen P., Honoré T., Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol. Sci. 11, 25–33 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Walters M. R. et al., The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: A double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc. Dis. 20, 304–309 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Petty M. A., Weintraub P. M., Maynard K. I., ACEA 1021: Flip or flop? CNS Drug Rev. 10, 337–348 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Mancilla B. et al.; BGG492 Study Group , Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 34, 103–113 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Jane D. E., Lodge D., Collingridge G. L., Kainate receptors: Pharmacology, function and therapeutic potential. Neuropharmacology 56, 90–113 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Larsen A. M., Bunch L., Medicinal chemistry of competitive kainate receptor antagonists. ACS Chem. Neurosci. 2, 60–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alt A. et al., Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 46, 793–806 (2004). [DOI] [PubMed] [Google Scholar]
- 13.O’Neill M. J. et al., LY377770, a novel iGlu5 kainate receptor antagonist with neuroprotective effects in global and focal cerebral ischaemia. Neuropharmacology 39, 1575–1588 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Alt A. et al., Anxiolytic-like effects through a GLUK5 kainate receptor mechanism. Neuropharmacology 52, 1482–1487 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Smolders I. et al., Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat. Neurosci. 5, 796–804 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Crépel V., Mulle C., Physiopathology of kainate receptors in epilepsy. Curr. Opin. Pharmacol. 20, 83–88 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Chappell A. S., Iyengar S., Lobo E. D., Prucka W. R., Results from clinical trials of a selective ionotropic glutamate receptor 5 (iGluR5) antagonist, LY5454694 tosylate, in 2 chronic pain conditions. Pain 155, 1140–1149 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Fleming J. J., England P. M., Developing a complete pharmacology for AMPA receptors: A perspective on subtype-selective ligands. Bioorg. Med. Chem. 18, 1381–1387 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Herb A. et al., The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 8, 775–785 (1992). [DOI] [PubMed] [Google Scholar]
- 20.Paternain A. V., Herrera M. T., Nieto M. A., Lerma J., GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 20, 196–205 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu W. et al., Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Chen S., Swensen A. C., Qian W. J., Gouaux E., Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364, 355–362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenthold R. J., Petralia R. S., Blahos J I. I., Niedzielski A. S., Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui C., Mayer M. L., Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J. Neurosci. 19, 8281–8291 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herguedas B. et al., Architecture of the heteromeric GluA1/2 AMPA receptor in complex with the auxiliary subunit TARP γ8. Science 364, eaav9011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barberis A., Sachidhanandam S., Mulle C., GluR6/KA2 kainate receptors mediate slow-deactivating currents. J. Neurosci. 28, 6402–6406 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosbacher J. et al., A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266, 1059–1062 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Straub C., Zhang W., Howe J. R., Neto2 modulation of kainate receptors with different subunit compositions. J. Neurosci. 31, 8078–8082 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constals A. et al., Glutamate-induced AMPA receptor desensitization increases their mobility and modulates short-term plasticity through unbinding from Stargazin. Neuron 85, 787–803 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Eibl C., Plested A. J., AMPA receptors: Mechanisms of auxiliary protein action. Curr. Opin. Physiol. 2, 84–91 (2018). [Google Scholar]
- 31.Coombs I. D., MacLean D. M., Jayaraman V., Farrant M., Cull-Candy S. G., Dual effects of TARP γ-2 on glutamate efficacy can account for AMPA receptor autoinactivation. Cell Rep. 20, 1123–1135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otis T., Zhang S., Trussell L. O., Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. J. Neurosci. 16, 7496–7504 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H. W., Balmer T. S., Romero G. E., Trussell L. O., Slow AMPAR synaptic transmission is determined by stargazin and glutamate transporters. Neuron 96, 73–80.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowie D., Lange G. D., Functional stoichiometry of glutamate receptor desensitization. J. Neurosci. 22, 3392–3403 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christie L. A. et al., AMPA receptor desensitization mutation results in severe developmental phenotypes and early postnatal lethality. Proc. Natl. Acad. Sci. U.S.A. 107, 9412–9417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobolevsky A. I., Structure and gating of tetrameric glutamate receptors. J. Physiol. 593, 29–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyerson J. R. et al., Structural basis of kainate subtype glutamate receptor desensitization. Nature 537, 567–571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S. et al., Activation and desensitization mechanism of AMPA receptor-TARP complex by cryo-EM. Cell 170, 1234–1246.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twomey E. C., Yelshanskaya M. V., Grassucci R. A., Frank J., Sobolevsky A. I., Structural bases of desensitization in AMPA receptor-auxiliary subunit complexes. Neuron 94, 569–580.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin R., Banke T. G., Mayer M. L., Traynelis S. F., Gouaux E., Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 6, 803–810 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Rosenmund C., Stern-Bach Y., Stevens C. F., The tetrameric structure of a glutamate receptor channel. Science 280, 1596–1599 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Stern-Bach Y., Russo S., Neuman M., Rosenmund C., A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron 21, 907–918 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Armstrong N., Jasti J., Beich-Frandsen M., Gouaux E., Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 127, 85–97 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Weston M. C., Schuck P., Ghosal A., Rosenmund C., Mayer M. L., Conformational restriction blocks glutamate receptor desensitization. Nat. Struct. Mol. Biol. 13, 1120–1127 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Dawe G. B. et al., Defining the structural relationship between kainate-receptor deactivation and desensitization. Nat. Struct. Mol. Biol. 20, 1054–1061 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heckmann M., Bufler J., Franke C., Dudel J., Kinetics of homomeric GluR6 glutamate receptor channels. Biophys. J. 71, 1743–1750 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert A., Howe J. R., How AMPA receptor desensitization depends on receptor occupancy. J. Neurosci. 23, 847–858 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiner A., Isacoff E. Y., Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat. Chem. Biol. 10, 273–280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher J. L., Mott D. D., Distinct functional roles of subunits within the heteromeric kainate receptor. J. Neurosci. 31, 17113–17122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher M. T., Fisher J. L., Contributions of different kainate receptor subunits to the properties of recombinant homomeric and heteromeric receptors. Neuroscience 278, 70–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson G. T., Green T., Heinemann S. F., Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Mol. Pharmacol. 53, 942–949 (1998). [PubMed] [Google Scholar]
- 52.Mayer M. L., Ghosal A., Dolman N. P., Jane D. E., Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists. J. Neurosci. 26, 2852–2861 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolman N. P. et al., Synthesis and pharmacological characterization of N3-substituted willardiine derivatives: Role of the substituent at the 5-position of the uracil ring in the development of highly potent and selective GLUK5 kainate receptor antagonists. J. Med. Chem. 50, 1558–1570 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Atlason P. T. et al., Mapping the ligand binding sites of kainate receptors: Molecular determinants of subunit-selective binding of the antagonist [3H]UBP310. Mol. Pharmacol. 78, 1036–1045 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinheiro P. S. et al., Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex 23, 323–331 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Ren Z. et al., Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J. Neurosci. 23, 6608–6616 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasu-Nishimura Y. et al., Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J. Neurosci. 26, 7014–7021 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiner A., Arant R. J., Isacoff E. Y., Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Rep. 1, 234–240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scholefield C. L., Atlason P. T., Jane D. E., Molnár E., Assembly and trafficking of homomeric and heteromeric kainate receptors with impaired ligand binding sites. Neurochem. Res. 44, 585–599 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumari J., Vinnakota R., Kumar J., Structural and functional insights into GluK3-kainate receptor desensitization and recovery. Sci. Rep. 9, 10254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar J., Schuck P., Mayer M. L., Structure and assembly mechanism for heteromeric kainate receptors. Neuron 71, 319–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong N., Mayer M., Gouaux E., Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes. Proc. Natl. Acad. Sci. U.S.A. 100, 5736–5741 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayer M. L., Crystal structures of the GluR5 and GluR6 ligand binding cores: Molecular mechanisms underlying kainate receptor selectivity. Neuron 45, 539–552 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Donevan S. D., Beg A., Gunther J. M., Twyman R. E., The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J. Pharmacol. Exp. Ther. 285, 539–545 (1998). [PubMed] [Google Scholar]
- 65.Petrovic M. M. et al., Metabotropic action of postsynaptic kainate receptors triggers hippocampal long-term potentiation. Nat. Neurosci. 20, 529–539 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Zhao H. et al., Preferential assembly of heteromeric kainate and AMPA receptor amino terminal domains. eLife 6, e32056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holm M. M., Lunn M. L., Traynelis S. F., Kastrup J. S., Egebjerg J., Structural determinants of agonist-specific kinetics at the ionotropic glutamate receptor 2. Proc. Natl. Acad. Sci. U.S.A. 102, 12053–12058 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robert A., Armstrong N., Gouaux J. E., Howe J. R., AMPA receptor binding cleft mutations that alter affinity, efficacy, and recovery from desensitization. J. Neurosci. 25, 3752–3762 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litwin D. B., Carrillo E., Shaikh S. A., Berka V., Jayaraman V., The structural arrangement at intersubunit interfaces in homomeric kainate receptors. Sci. Rep. 9, 6969 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mansour M., Nagarajan N., Nehring R. B., Clements J. D., Rosenmund C., Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron 32, 841–853 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Ryazantseva M. et al., Kainate receptors regulate development of glutamatergic synaptic circuitry in the rodent amygdala. eLife 9, e52798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.