Significance
The advancement of breeding behavior is a well-documented response to changing climate conditions, as timing reproduction with resource availability is critical for success in many species. However, the relationship between cues that prompt reproduction and resource availability can become decoupled, reducing success. Tree swallows have advanced reproduction in response to warming springs, but now chicks are exposed to twice the risk of inclement weather conditions, resulting in high rates of chick mortality. Mass mortality events appear to be driven by decreasing insect availability at low daytime temperatures. Our findings highlight the complex effects of climate change on animal life cycles and demonstrate the urgency of understanding how animals balance information from the environment when making crucial life history decisions.
Keywords: migration, life history, climate change, climate variability
Abstract
In response to a warming planet with earlier springs, migratory animals are adjusting the timing of essential life stages. Although these adjustments may be essential for keeping pace with resource phenology, they may prove insufficient, as evidenced by population declines in many species. However, even when species can match the tempo of climate change, other consequences may emerge when exposed to novel conditions earlier in the year. Here, using three long-term datasets on bird reproduction, daily insect availability, and weather, we investigated the complex mechanisms affecting reproductive success in an aerial insectivore, the tree swallow (Tachycineta bicolor). By examining breeding records over nearly half a century, we discovered that tree swallows have continuously advanced their egg laying by ∼3 d per decade. However, earlier-hatching offspring are now exposed to inclement weather events twice as often as they were in the 1970s. Our long-term daily insect biomass dataset shows no long-term trends over 25 y but precipitous drops in flying insect numbers on days with low ambient temperatures. Insect availability has a considerable impact on chick survival: Even a single inclement weather event can reduce offspring survival by >50%. Our results highlight the multifaceted threats that climate change poses on migrating species. The decoupling between cold snap occurrence and generally warming spring temperatures can affect reproductive success and threaten long-term persistence of populations. Understanding the exact mechanisms that endanger aerial insectivores is especially timely because this guild is experiencing the steepest and most widespread declines across North America and Europe.
Climate change is one of the most pervasive threats of the modern era, with far-reaching effects on the phenology and fitness of individuals to entire ecosystems around the globe. Organisms have responded by shifting the timing of key seasonal events, such as spring migration or reproduction, to earlier in the year (1, 2). Long-distance migrants are at risk for mistiming their breeding seasons because they often rely on cues separated from environmental conditions by thousands of kilometers. However, this likely extends to even shorter-distance migrants as well, as timing adjustments may be constrained by a suite of factors, including inherited circannual programs, time to accumulate fat stores, migration distance, stopover quality, and conditions encountered along the way (3–8). Consequently, there is growing concern that an inability to adjust reproductive timing in pace with advancing breeding ground conditions can cause decreased breeding success and juvenile survival in migrant birds (9–12).
Optimizing the timing of migration and subsequent breeding is further complicated by the fact that phenological responses to climate change are often dissimilar between trophic levels (13, 14), and such differential rates of advancement have the potential to create “mismatches,” or disruptions of historical relationships between predators and the availability of their prey (9, 13–17). For species such as insectivores whose developing offspring rely on brief resource pulses from often ephemeral prey, phenological mismatches are likely to cause declines in reproductive success (10, 18). Moreover, even for species that may lack specific constraints for reproductive adjustment, climate change may produce conditions in which it is difficult to optimize the timing of reproduction. For example, the relationship between mean temperature and temperature variability for a given day of year can change (19, 20), resulting in reduced reliability of mean temperature as a cue for timing life history decisions. For the majority of temperate species whose reproductive success depends on temperature-driven resource availability, increasingly variable conditions can create a particularly challenging environment for phenological adjustment.
Whereas previously documented mismatches driven by climate change have resulted from poor or misleading information due to geographic separation (21), changes in the rates of environmental change along a migratory route (3), or quality of information available over time at different breeding habitats (22), returning migrants may also experience a mismatch between two different aspects of climate at the same site and season. Changing temperatures may well be the most salient cue driving phenological advancement, but organisms must also moderate their responses to temperature as a consequence of other fluctuating aspects of the environment, including precipitation (23), light (24), nutrients (25), and prey availability (26). For example, by responding to one aspect of climate that is historically associated with earlier and more successful reproduction (27), migratory animals may become more vulnerable to a different aspect of climate, such as periodic inclement weather events, which tend to be less predictable on short time scales.
Aerial insectivores, a diverse group of migratory bird species that includes flycatchers, swifts, swallows, and nightjars, have significantly declined by ∼15 to 40% in recent decades across North America (28) and Europe (29). Multiple mechanisms related to the drivers of overall insect abundance [e.g., due to agricultural intensification (29), pesticide use (30), overall insect decline] or insect availability [e.g., due to phenological mismatch (31), temperature (32), precipitation (33), or wind (34)] are thought to contribute to these declines, but a single guild-wide cause has yet to be identified. Because all aerial insectivores forage in flight and rely upon actively flying insects (28), they are especially sensitive to environmental factors, like temperature, which influence insect flight behavior (32). Thus, the reproductive success of aerial insectivores may be uniquely susceptible to short-term variations in prey activity to longer-term phenological shifts in overall prey abundance.
This study uses three standardized, long-term datasets spanning more than 25 y on bird reproduction, daily insect availability, and weather conditions (Methods) to investigate the extent to which phenological mismatches affect the reproductive performance of a declining aerial insectivore, the tree swallow Tachycineta bicolor (35). We predicted that the annual timing of tree swallow reproduction would be driven by year-to-year differences in temperatures, with earlier reproduction occurring in years with earlier warmth. To test our predictions, we analyzed annual trends in lay and hatch records from two sites separated by <35 km, spanning 43 y from 1972 to 2015. Our dataset included a total of 11,236 chicks recorded in records from 2,041 nests. We compared fledging rates with local weather conditions when chicks were nestlings. To better understand the mechanism between fledging rates and weather, we explored how daytime temperatures affect insect biomass using a daily dataset of aerial insects collected from 1989 to 2014. We also determined whether the occurrence of cold snaps during the nestling period has changed over time and how cold snap events influence overall fledgling production. Finally, we examined our results in light of overall reproductive metrics to understand how short-term weather events could affect long-term population trends.
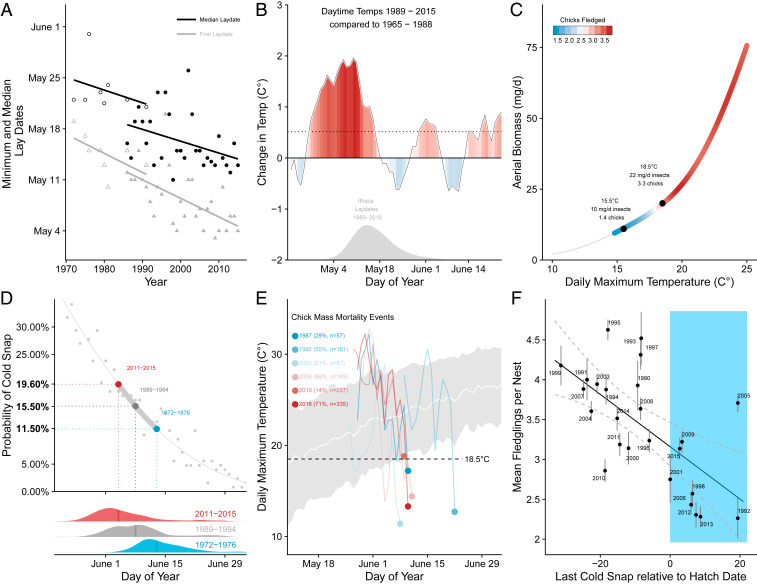
Across their entire North American range (36), tree swallows have on average advanced their reproduction by 9 d between 1950 and 1990, and we found that this trend has accelerated in New York with an advancement of lay dates by approximately 13 d from 1972 to 2015 (linear regression, F1, 36 = 28.1, P < 0.001, adjusted R2 = 0.42) (Fig. 1A). From 1989 to 2015, overall mean annual temperatures in Ithaca, New York increased by half a degree (0.51 °C) compared to the preceding 25 y (1963 to 1988). However, during May to early June when tree swallows are laying eggs, temperatures increased by nearly fourfold, by 1.9 °C, over the same interval (Fig. 1B). Tree swallows laid earlier when it was warmer in early spring, also resulting in a concerted advancement in hatch dates (Spearman’s ρ = −0.64, linear regression, F1, 28 = 13.9, P < 0.001, adjusted R2 = 0.35) (Fig. 1A). Selection for earlier reproduction is expected, as early clutches tend to produce more eggs and fledglings than do later ones (27, 37). However, selection for early breeding is likely driven by a complex suite of factors that translate to increased offspring production and survival (37), including overall insect abundance during egg formation (38), access to aquatic food sources that are rich in omega-3 highly unsaturated fatty acids (e.g., refs. 39 and 40, and the availability of higher-quality mates and nest sites (27).
Fig. 1.
Patterns in the changes of temperatures and lay dates in tree swallows. (A) Trends in tree swallow lay dates. Over the last 43 y, both earliest and median lay dates have advanced by nearly 2 wk at both study sites (solid symbols, Ithaca, NY; hollow symbols, Newark Valley, NY). (B) Daytime maximum temperatures from 1989 to 2015, compared with those from the previous 25 y (1965 to 1988), have averaged an annual average increase of only 0.51 °C (horizontal dotted line). However, seasonal temperatures immediately preceding egg laying (gray density plot) have been nearly 2 °C warmer, likely driving tree swallows to initiate earlier clutches. (C) Aerial insect biomass is positively correlated with daytime maximum temperature, and below a certain threshold (18.5 °C), tree swallows successfully fledge fewer chicks. A 3 °C decrease in daily maximum temperature from 18.5 to 15.5 °C results in a 50% decrease in the available aerial biomass to feed young. (D) The overall distribution of tree swallow hatch dates (with solid vertical lines at the medians) have shifted earlier from 1972 to 1976 (blue), to 1989 to1994 (gray), and 2011 to 2015 (red). Because random cold snap occurrence has not changed at the same rate as spring warming, the probability of nestlings experiencing a cold snap has almost doubled from once every 10 y to once every 5 y (change in probability from 11.5 to 19.6%) (filled gray line and points). (E) The effects of cold snap events are often dramatic, as even 1- to 2-d events of temperatures less than 18.5 °C can result in mass die-off events for young chicks. For example, 71% of all chicks died in a single day event on June 9, 2016. The white line is the average daily maximum temperature and the gray shading is ±1 SD. The colored points are the date of a peak mortality event. The colored trajectories to the left of each point are the daily maximum temperatures preceding the event and all are at or below 18.5 °C. (F) There is a significant relationship between the mean number of fledglings produced per nest and the difference in dates between the median annual hatch and that year’s last cold snap. Years in the blue region were on average exposed to cold-snap events. Positive numbers on the x axis correspond to the cold snap occurring after the median hatch date, whereas negative numbers are before median hatch (i.e., no cold snap experienced that year). Error bars correspond with ±SE.
Despite the advantages of breeding earlier, early reproduction also comes with an associated cost: Increased exposure to inclement weather events that decrease chick survival. When tree swallow chicks are exposed to low daytime temperatures more frequently, on average fewer individuals survive to fledging (Fig. 1C). The consequences of exposure to low temperatures are dramatic, and when temperatures descend through a 3 °C range (15.5 to 18.5 °C) the average number of tree swallows fledglings produced per nest decreases rapidly from 3.3 to 1.4 chicks. This appears to be a consequence of aerial insect availability, which is positively correlated with daytime temperature (linear regression, F1, 1,621 = 879, P < 0.001, adjusted R2 = 0.36) and drops below an apparent threshold in our population where chicks can be successfully fledged at 18.5 °C (32), due to greatly reduced insect activity (Fig. 1C). This relationship between food availability and reproductive success is a key factor shaping aerial insectivore reproductive strategies: Reduced aerial insect activity is brought on almost immediately as daytime temperatures fall, and these cold snaps greatly reduce food availability for parents provisioning nestlings. Thus, if it were possible to do so, tree swallows deciding when to lay eggs would benefit by being able to trade off the benefits of early breeding with the risks of unpredictable inclement weather and low food availability during the most intensive chick-rearing period, which occurs 15 to 20 d after clutches are completed.
In contrast to advancing tree swallow egg laying and hatch dates, seasonal patterns of 1-, 2-, and 3-d cold snaps below 18.5 °C show no clear trends and have not changed significantly in the past 125 y (SI Appendix). However, adults apparently have not been able to time reproduction in relation to when the last annual cold snap occurs (linear regression, F1, 24 = 0.67, P = 0.42, adjusted R2 = 0.01). Thus, by laying progressively earlier, tree swallows are advancing their reproduction into riskier times of year when daytime temperatures are less likely to provide sufficient insect activity for adults to meet the demands of rapidly developing offspring (Fig. 1D). Consequently, tree swallow nestlings hatched since 2011 have nearly twice the risk of experiencing a cold snap compared to those hatched in the 1970s (increase from 11.5 to 19.6%) (Fig. 1D) and the mean proportion of complete nest failures has increased as well (from 15.8 to 33.2% from 1989 to 2015) (SI Appendix). Thus, exposure to low daytime temperatures associated with reduced food availability often translates to punctuated mass mortality events in which the majority of nests completely fail (Fig. 1E). For example, on and around June 9, 2016, 71% of all nests completely failed as temperatures fell to a daytime maximum of 14.3 °C.
Finally, we discovered that breeding earlier when cold snap risks are greater has widespread impacts on population-wide reproductive success. The annual mean number of fledglings produced per nest decreased as the risk of exposure to cold snaps increased (weighted linear regression: F1, 23 = 19.7, P < 0.01, adjusted R2 = 0.44). The costs of reproduction mistimed to cold snaps are substantial (Fig. 1F): Nests that hatch after the last annual cold snap fledge an additional chick compared to nests that hatch before this date (Mann–Whitney–Wilcoxon: W = 122, P < 0.001, mean chicks fledged after: 3.78 ± 0.50 chicks vs. before: 2.67 ± 0.43 chicks).
Parental decisions about when to reproduce can have cascading effects on the developmental environment that offspring experience. Periods of low temperatures present a three-pronged challenge for young altricial nestlings: With reduced food densities, parents must make prolonged foraging bouts away from the nest and therefore provide chicks with less warmth while nestlings simultaneously experience increased energy demands from thermoregulation (41–43) coupled with reduced energy inputs, often resulting in fatal consequences for chicks (32, 42). Here we show that reduced food availability due to low ambient temperatures may result in population-wide consequences in annual breeding success and is due to a mismatch between the cues that time egg laying and local environmental conditions that determine the success of hatchling development.
Population growth rates for short-lived species are typically thought to be driven by fecundity and juvenile survival (44, 45), as adults generally experience few breeding seasons in their short lives (46). Among aerial insectivores, swallow species have been undergoing some of the most rapid declines observed over the last 45 y (35), and tree swallows are declining at multiple sites across North America (47, 48). Recent studies have shown that, while adult survival rates appear relatively stable (49, 50), fledgling survival and juvenile and adult return rates largely determine population viability due to their high elasticity and sensitivity (33). Poor local weather conditions have been shown to negatively affect tree swallow fledgling survival (32, 33), and some declining populations likely depend on recruitment via immigration to maintain viability (51).
In resident bird populations, phenological mismatches may not necessarily negatively affect mean lifetime reproductive success or population trajectories. The effects of variation in reproductive output on such populations are buffered by density-dependent variation in juvenile survival rates: Years of high reproductive output lead to lower overwinter juvenile survival rates than do those following summers with low reproductive output (52, 53). But in migratory species, especially those that, like swallows, are nonterritorial, the effects of density are not nearly as age-dependent, and density-dependence is more diffuse and weaker than factors such as spring weather, with its density-independent effect (48). Thus, in swallow populations there is likely little of the age-structured buffering seen in some resident populations, and reproductive failures are much more likely to have direct demographic consequences.
Recent studies have drawn considerable public attention to the potential widespread decline of insect populations (54, 55), although the generality of these observed patterns remains unclear (50–52). For example, we find no evidence of decreased aerial insect availability at our study site in terms of either total biomass (linear model, F1, 22 = 1.79, P = 0.19, R2 = 0.03) or total insects captured during the tree swallow breeding season (linear model, F1, 22 = 0.33, P = 0.57, R2 = 0.00) (SI Appendix). Perhaps more importantly, the mechanism we have observed does not depend on concerted trends in insect abundance across North America or Europe to drive or reinforce aerial insectivore declines. Rather, because of the strong relationship between insect flight activity and temperature, shifting breeding earlier necessarily incurs greater risk of low temperatures and reproductive failure.
We do not yet fully understand which cues migrating birds such as the tree swallow use when timing breeding, but some combination of temperatures in spring (36) and insect food availability (27, 56) seem likely to be particularly relevant sources of information. Given the persistent drive to breed as early as possible, the picture that emerges is that these swallows are laying eggs on the basis of environmental conditions just a few days before laying (57) and that they have no way to anticipate the random occurrence of a cold snap 2 to 3 wk after egg laying. To gain the benefits of earlier laying, they must take on the increased risk of unpredictable cold snaps and the decreased chances of successful reproduction that such events entail. Thus, swallows may not be able to respond optimally to this change because opposing selection for early versus late breeding fluctuates from year to year. These findings highlight the complexity of climate change effects on migratory species and demonstrate the urgency of understanding how animals balance information from historical cues that may become decoupled from current conditions by climate change.
Methods
Tree Swallow Life History Data.
We studied two tree swallow populations near Ithaca, New York, separated by less than 35 km, from 1972 to 2015. Laying date was known for both populations through direct observation and hatching dates were recorded for the Ithaca population in the years 1989 to 2015. We estimated the hatching date for the 1972 to 1989 dataset by calculating the average time of incubation added to the number of eggs in each clutch. We excluded data from individual nests in our calculations that were known second broods or that had documented experimental manipulation during incubation (58). We also excluded years from the analysis where there were fewer than 15 total observed nests. These filtering criteria left us with a total of 2,041 nest records with 11,236 chicks spanning 43 y. From this dataset, we calculated the first observed and median lay and hatch date for each year from 1972 to 2015 and tested for temporal trends using generalized linear models. We modeled our data using linear models and calculated confidence intervals using a bias-corrected and accelerated bootstrap by resampling the original dataset 1,000 times using the R package “arm” (59). All models were inspected for evidence of temporal autocorrelation using the function “acf” in base R and, for models based on annual averages, we used an inverse variance weighting procedure to account for differences in precision of the estimates. We evaluated the fit of linear models by examining the residual versus fitted values and qq-plot for departures from normality, and we visually checked for equal variance using a spread-location plot. We also explored the potential of influential outliers in the data using a scale-leverage plot. All of our analyses are available as a single annotated rMarkdown file with model diagnostics (60).
The work with live animals was approved under protocol 2001-0051 of Cornell University’s Institutional Animal Care and Use Committee.
Weather Data.
We downloaded weather data from seven weather stations within 50 km of Ithaca from 1893 to 2019 using the Climate Data Online Tool (CDO), an online service provided by the National Climatic Data Center (https://www.ncdc.noaa.gov/cdo-web/). We averaged the daily minimum (typically nighttime) and maximum (typically daytime) temperatures from each of the weather stations to generate a dataset with these two average values for each day. We then divided the minimum and maximum daily temperatures into deciles and calculated 3-d rolling averages of each decile to test for temperature trends over two time periods, since 1893 and since 1970, using linear models as listed previously. In addition, we compared daily average daytime temperatures since the start of the Rothamsted insect sampler (below) at the Ithaca field site (1989 to 2015) to data from the previous 25 y (1963 to 1988) to determine the relative temperature trends occurring before, during, and immediately after reproduction.
Insect Activity Data.
We used a 12-m Rothamsted aerial insect vacuum sampler at the Cornell Experimental Ponds near Ithaca, New York (42.504371°N, −76.465949°W) to collect insect samples from April 1st to the end of the swallow breeding season spanning 24 y from 1989 to 2013. The insect sampling schedule encompasses the entire tree swallow breeding season and often precedes the earliest swallow egg dates by over 3 wk. The sampler was operated from 1 h after sunrise to 1 h before sunset and samples were collected once per day. Within each daily sample, individual insects were classified into 1 of 12 insect orders and then grouped into size categories resulting in a total of 1,097,035 total insect samples. The number of insects per size category was then converted into a measure of biomass by using a series of allometric length to mass equations for each of the insect orders (see table S1 in ref. 40). We then merged the insect data with the daily weather data to determine the effects of temperature on measured aerial insect biomass using a generalized additive model with “mgcv” in R.
Previous research has suggested that the activity and overall numbers of aerial insects decreases rapidly over a narrow temperature range between 15.5 °C and 18.5 °C (32). We labeled the occurrence of 1, 2, and 3 consecutive days with daily maximum temperatures below 18.5 °C as “cold snaps” in the dataset. From these data we calculated the average daily probability of a cold snap occurring and at what day the last cold snap occurred each year. We then tested for temporal trends in cold snap occurrence from 1893 to 2015 using both linear and generalized additive models. To compare the number of chicks fledged when they did, or did not, experience a cold snap, we used a nonparametric Mann–Whitney–Wilcoxon test.
Supplementary Material
Acknowledgments
J.R.S. thanks Jesko Partecke for thoughtful discussions on the ideas presented in the report, and we thank the dozens of undergraduate field crew who collected data and analyzed insect samples at the Ithaca field site, making this project possible. The work reported here was supported by NSF IBN-0131437, DEB-0717021, and DEB-1242573 (to D.W.W.) and NSF IOS-1457251 (to M.N.V.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009864117/-/DCSupplemental.
Data Availability.
All data needed to evaluate the conclusions presented in this report are in SI Appendix, which is provided as an rMarkdown file. Original data used in the analysis have been deposited at the Knowledge Network for Biocomplexity with the following identifier: doi:10.5063/F1SF2TJX (61).
References
- 1.Pecl G. T. et al., Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Møller A. P., Fiedler W., Berthold P., Effects of Climate Change on Birds, (Oxford University Press, Oxford, 2010). [Google Scholar]
- 3.Both C., Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Curr. Biol. 20, 243–248 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Both C. et al., Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. London Ser. B 277, 1259–1266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmaljohann H., Both C., The limits of modifying migration speed to adjust to climate change. Nat. Clim. Chang. 7, 573–576 (2017). [Google Scholar]
- 6.Lindström Å., Alerstam T., Hedenström A., Faster fuelling is the key to faster migration. Nat. Clim. Chang. 9, 288–289 (2019). [Google Scholar]
- 7.Zurell D., Graham C. H., Gallien L., Thuiller W., Zimmermann N. E., Long-distance migratory birds threatened by multiple independent risks from global change. Nat. Clim. Chang. 8, 992–996 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Åkesson S. et al., Timing avian long-distance migration: From internal clock mechanisms to global flights. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Both C. et al., Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. Biol. Sci. 277, 1259–1266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Both C., Bouwhuis S., Lessells C., Visser M. E., Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (2006). [DOI] [PubMed] [Google Scholar]
- 11.van Gils J. A. et al., Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science 352, 819–821 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Radchuk V. et al., Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 10, 3109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thackeray S. J. et al., Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Renner S. S., Zohner C. M., Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165–182 (2018). [Google Scholar]
- 15.Visser M. E., Both C., Shifts in phenology due to global climate change: The need for a yardstick. Proc. Biol. Sci. 272, 2561–2569 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayor S. J. et al., Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Sci. Rep. 7, 1902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser M. E., Gienapp P., Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 3, 879–885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M. E., Holleman L. J., Gienapp P., Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Ma Q., Huang J. G., Hänninen H., Berninger F., Divergent trends in the risk of spring frost damage to trees in Europe with recent warming. Glob. Change Biol. 25, 351–360 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Martinuzzi S. et al., Future frequencies of extreme weather events in the National Wildlife Refuges of the conterminous US. Biol. Conserv. 201, 327–335 (2016). [Google Scholar]
- 21.Both C., Visser M. E., Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Hollander F. A., Titeux N., Holveck M.-J., Dyck H. V., Timing of breeding in an ecologically trapped bird. Am. Nat. 189, 515–525 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. M., Lajeunesse M. J., Rohr J. R., A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 8, 224–228 (2018). [Google Scholar]
- 24.Poloczanska E. S. et al., Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3, 62 (2016). [Google Scholar]
- 25.Greaver T. et al., Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 6, 836–843 (2016). [Google Scholar]
- 26.Guiden P. W., Bartel S. L., Byer N. W., Shipley A. A., Orrock J. L., Predator–prey interactions in the Anthropocene: Reconciling multiple aspects of novelty. Trends Ecol. Evol. 34, 616–627 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Winkler D. W., Allen P. E., The seasonal decline in tree swallow clutch size: Physiological constraint or strategic adjustment? Ecology 77, 922–932 (1996). [Google Scholar]
- 28.Nebel S., Mills A., McCracken J., Taylor P., Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 5, 1 (2010). [Google Scholar]
- 29.Bowler D. E., Heldbjerg H., Fox A. D., de Jong M., Böhning‐Gaese K., Long‐term declines of European insectivorous bird populations and potential causes. Conserv. Biol. 33, 1120–1130 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Hallmann C. A., Foppen R. P., van Turnhout C. A., de Kroon H., Jongejans E., Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Saino N. et al., Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. Biol. Sci. 278, 835–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler D. W., Luo M. K., Rakhimberdiev E., Temperature effects on food supply and chick mortality in tree swallows (Tachycineta bicolor). Oecologia 173, 129–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox A. R., Robertson R. J., Lendvai Á. Z., Everitt K., Bonier F., Rainy springs linked to poor nestling growth in a declining avian aerial insectivore (Tachycineta bicolor). Proc. Biol. Sci. 286, 20190018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irons R. D. et al., Wind and rain are the primary climate factors driving changing phenology of an aerial insectivore. Proc. Biol. Sci. 284, 20170412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg K. V. et al., Decline of the North American avifauna. Science 366, 120–124 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Dunn P. O., Winkler D. W., Climate change has affected the breeding date of tree swallows throughout North America. Proc. R. Soc. Lond. B Biol. Sci. 266, 2487–2490 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler D. W. et al., Full lifetime perspectives on the costs and benefits of lay date variation in tree swallows. Ecology 101, e03109 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Dunn P. O., Winkler D. W., Whittingham L. A., Hannon S. J., Robertson R. J., A test of the mismatch hypothesis: How is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92, 450–461 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Twining C. W. et al., Omega-3 long-chain polyunsaturated fatty acids support aerial insectivore performance more than food quantity. Proc. Natl Acad. Sci. U.S.A. 113, 10920–10925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twining C. W., Shipley J. R., Winkler D. W., Aquatic insects rich in omega‐3 fatty acids drive breeding success in a widespread bird. Ecol. Lett. 21, 1812–1820 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Ardia D. R., Pérez J. H., Clotfelter E. D., Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc.Biol. Sci. 277, 1881–1888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shipley J. R., “Environmental effects on developing birds: Short-term adjustments with life-long impacts,” PhD dissertation, Cornell University, Ithaca, NY (2018).
- 43.McCarty J. P., “Effects of short-term changes in environmental conditions on the foraging ecology and reproductive success of tree swallows, Tachycineta bicolor,” PhD dissertation, Cornell University, Ithaca, NY (1995).
- 44.Lack D., The Natural Regulation of Animal Numbers, (Clarendon Press, Oxford, 1954). [Google Scholar]
- 45.Sæther B.-E. et al., Demographic routes to variability and regulation in bird populations. Nat. Commun. 7, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sæther B.-E., Bakke Ø., Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81, 642–653 (2000). [Google Scholar]
- 47.Cox A. R., Robertson R. J., Fedy B. C., Rendell W. B., Bonier F., Demographic drivers of local population decline in tree swallows (Tachycineta bicolor) in Ontario, Canada. The Condor 120, 842–851 (2018). [Google Scholar]
- 48.Cox A. R., Robertson R. J., Rendell W. B., Bonier F., Population decline in tree swallows (Tachycineta bicolor) linked to climate change and inclement weather on the breeding ground. Oecologia 192, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Weegman M. D., Arnold T. W., Dawson R. D., Winkler D. W., Clark R. G., Integrated population models reveal local weather conditions are the key drivers of population dynamics in an aerial insectivore. Oecologia 185, 119–130 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Clark R. G. et al., Geographic variation and environmental correlates of apparent survival rates in adult tree swallows Tachycineta bicolor. J. Avian Biol. 49, jav-012514 (2018). [Google Scholar]
- 51.Taylor L. U., Woodworth B. K., Sandercock B. K., Wheelwright N. T., Demographic drivers of collapse in an island population of tree swallows. The Condor 120, 828–841 (2018). [Google Scholar]
- 52.Reed T. E., Jenouvrier S., Visser M. E., Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Reed T. E., Grøtan V., Jenouvrier S., Sæther B.-E., Visser M. E., Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Hallmann C. A. et al., More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12, e0185809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lister B. C., Garcia A., Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. U.S.A. 115, E10397–E10406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowlin M. S., Winkler D. W., Natural variation in flight performance is related to timing of breeding in tree swallows (Tachycineta bicolor) in New York. Auk 121, 345–353 (2004). [Google Scholar]
- 57.Ardia D. R., Wasson M. F., Winkler D. W., Individual quality and food availability determine yolk and egg mass and egg composition in tree swallows Tachycineta bicolor. J. Avian Biol. 37, 252–259 (2006). [Google Scholar]
- 58.Orzechowski S. C., Shipley J. R., Pegan T. M., Winkler D. W., Negligible effects of blood sampling on reproductive performance and return rates of Tree Swallows. J. Field Ornithol. 90, 21–38 (2019). [Google Scholar]
- 59.Gelman A., Su Y.-S., Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package version 1.11-2. https://CRAN.R-project.org/package=arm. Accessed 14 September 2020.
- 60.Xie Y., Allaire J., Grolemund G., R Markdown: The Definitive Guide, (Chapman and Hall/CRC, 2018). [Google Scholar]
- 61.Shipley Jeremy Ryan and Winkler David. 2020. Ithaca, New York Tree Swallow (Tachycineta bicolor) study site reproduction data (1972-2015). Knowledge Network for Biocomplexity. doi:10.5063/F1SF2TJX.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions presented in this report are in SI Appendix, which is provided as an rMarkdown file. Original data used in the analysis have been deposited at the Knowledge Network for Biocomplexity with the following identifier: doi:10.5063/F1SF2TJX (61).