Significance
Endometriosis is a highly prevalent proinflammatory disease without reliable diagnostic biomarkers and cannot be cured by nowadays’ medical treatments. Herein, we identified that extracellular vesicle (EV)-associated VEGF-C, secreted by proinflammatory cytokine-stimulated endometriotic stromal cells, is a critical modulator for endometriosis progression by promoting lymphangiogenesis. Invaded lymphatic vessels may serve as a canal for the infiltration of immune cells, which further enhances the inflammatory status in the endometriotic microenvironment and produces more EV-VEGF-C. The elevated circulating EV-VEGF-C is a sensitive and reliable biomarker for detecting endometriosis. These data advance our understanding of the pathophysiology of endometriosis and suggest VEGF-C can be a noninvasive diagnostic biomarker and a potential therapeutic target for endometriosis.
Keywords: lymphangiogenesis, VEGF-C, COUP-TFII, EV, biomarker
Abstract
Endometriosis is a highly prevalent gynecological disease with severe negative impacts on life quality and financial burden. Unfortunately, there is no cure for this disease, which highlights the need for further investigation about the pathophysiology of this disease to provide clues for developing novel therapeutic regimens. Herein, we identified that vascular endothelial growth factor (VEGF)-C, a potent lymphangiogenic factor, is up-regulated in endometriotic cells and contributes to increased lymphangiogenesis. Bioinformatic analysis and molecular biological characterization revealed that VEGF-C is negatively regulated by an orphan nuclear receptor, chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII). Further studies demonstrated that proinflammatory cytokines, via suppression of COUP-TFII level, induce VEGF-C overexpression. More importantly, we show that functional VEGF-C is transported by extracellular vesicles (EVs) to enhance the lymphangiogenic ability of lymphatic endothelial cells. Autotransplanted mouse model of endometriosis showed lenvatinib treatment abrogated the increased lymphatic vessels development in the endometriotic lesion, enlarged retroperitoneal lymph nodes, and immune cells infiltration, indicating that blocking VEGF-C signaling can reduce local chronic inflammation and concomitantly endometriosis development. Evaluation of EV-transmitted VEGF-C from patients’ sera demonstrates it is a reliable noninvasive way for clinical diagnosis. Taken together, we identify the vicious cycle of inflammation, COUP-TFII, VEGF-C, and lymphangiogenesis in the endometriotic microenvironment, which opens up new horizons in understanding the pathophysiology of endometriosis. VEGF-C not only can serve as a diagnostic biomarker but also a molecular target for developing therapeutic regimens.
Endometriosis is a chronic inflammatory gynecological disease defined as the presence of endometriotic lesions outside of the uterine cavity. Clinical symptoms include dysmenorrhea, dyspareunia, and even infertility, which severely reduce the life quality of affected women and their families. In 1927, Sampson proposed that the retrograded menstrual flow is the main force for tissues to reach the implanted sites, which remains as the most accepted hypothesis so far (1). However, more than 90% women of reproductive age have retrograded menstruation while only 8–15% of them develop endometriosis (2, 3), suggesting the involvement of some other critical factors in the pathogenesis of endometriosis. So far, a number of studies have reported that endometriotic tissues have the capability to survive in the unfavorable conditions, including having better adhesion ability, higher proliferation rate, and more resistant to apoptosis (4–6). Nevertheless, since endometriosis is a multifactorial and complicated disease, the etiology remains largely unclear.
The theory of lymphatic system dissemination has been proposed for almost 100 years (7). Recent data also reported the involvement of lymphatic system in disease occurrence in distant/rare sites among patients with endometriosis (8). A few studies suggest that the lymphatic system may play some roles in the pathogenesis of endometriosis (9–11), especially in the highly recurrent cases after medical treatment and/or surgical removal (12). However, how the lymphatic system is involved in endometriosis and how lymphangiogenic factors are regulated during the progression of endometriosis are still undefined. Lymphangiogenesis is mainly promoted by the effect of vascular endothelial growth factor (VEGF)-C, which is a well-known growth factor responsible for both angiogenesis and, in particular, lymphangiogenesis. Although it has been reported that level of VEGF-C is higher in endometriotic lesions (13, 14), the pathological function and the regulatory mechanism remain uncharacterized. VEGF-C activity is mediated by binding to receptors including VEGF receptor-2 and -3 (VEGFR-2 and VEGFR-3). Aberrant angiogenic and lymphangiogenic potential in eutopic endometrium of women with endometriosis is suggested to favor the survival of endometrial cell in ectopic sites. However, the hypothesis that lymphangiogenesis may promote the development of endometriosis remains to be tested.
Endometriosis is a disease with prolonged inflammatory response. The presence of ectopic tissues in peritoneal cavity, without clear mechanism, reaches a state of balance with a high level of proinflammatory cytokines and immune cells. Our previous studies have shown these highly expressed proinflammatory cytokines play crucial roles in the pathogenesis of endometriosis (15, 16). It is found that proinflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α suppress chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII, also known as NR2F2) (15). As a transcription repressor, COUP-TFII binds to the motif containing the AGGTCA half site on promoter sequence to regulate clusters of genes functionally involved in vital cellular processes including organogenesis, angiogenesis, metabolism, cell fate determination, and cancer progression (17). Down-regulation of COUP-TFII resulted in up-regulation of cyclooxygenase-2 (COX-2), an inducible enzyme responsible for prostaglandins E2 (PGE2) production (15), angiogenin (18), a potent angiogenic factor, and aromatase (19), the key enzyme regulating steroidogenesis, providing evidence to correlate the proinflammatory cytokines, gene regulation, and pathogenesis of endometriosis. Nevertheless, whether cytokines-COUP-TFII axis contributes to lymphangiogenesis in endometriotic lesions remains unattested.
Here, we hypothesize that the expression of VEGF-C may promote the infiltration of lymphatic vessels into endometriotic lesions. The newly developed lymphatic system not only serves as a channel to drain the wastes out of endometriotic cells but also provides a network for immune cells to reach the endometriotic lesions. Thus, the infiltration of lymphatic system enhances the inflammatory microenvironment, which forms a vicious cycle that favors the development of endometriosis. The purpose of this study is to characterize the unrevealed mechanism of lymphangiogenesis in endometriotic lesion and employ this finding to develop a potential diagnostic or therapeutic strategy for endometriosis.
Results
Elevation of VEGF-C in Endometriosis Is Mediated by Down-Regulation of COUP-TFII.
To investigate the clinical relevance of VEGF-C in endometriosis, we first analyzed two publicly available microarray datasets, GSE23339 (20) and GSE7305 (21). Results showed that VEGF-C mRNA was up-regulated in endometriotic tissues compared to disease-free endometria (Fig. 1A). We then checked the expression of VEGF-C using clinical samples and found that levels of VEGF-C protein were increased in ectopic lesion and peritoneal fluid of patients with endometriosis (Fig. 1 B–D). More importantly, data showed VEGF-C protein was increased along with the severity of disease (Fig. 1D).
Fig. 1.
COUP-TFII mediated VEGF-C expression in endometriotic stromal cells. (A) Expression of VEGF-C in two public datasets (GSE23339 and GSE7305). Original data were downloaded from GEO and analyzed by in-house bioinformatic tools. Statistical analysis was performed using two-tailed unpaired Student’s t test. (B) Representative immunohistochemistry images show VEGF-C expression in normal endometrium (Nor) and endometriotic lesion (Endo). Note that fibroblasts near by the endometriotic cells and infiltrated macrophages (big, round cells indicated by arrows) also stained positive for VEGF-C besides endometriotic stromal cells. (C) Levels of VEGF-C in peritoneal fluid of women without (Nor, n = 6) and with (Endo, n = 42) endometriosis. (D) Representative Western blot (Upper) and quantitative results (Lower) of the VEGF-C protein in peritoneal fluids from groups of the patients without/with endometriosis: normal (n = 6), stage I (n = 5), stage II (n = 9), stage III (n = 18), stage IV (n = 10). (E) Quantitative results of COUP-TFII (Left) and VEGF-C (Right) mRNA level in human endometrial stromal cells transfected with control siRNA (siNC) or COUP-TFII siRNA (siCII#1, siCII#2) for 24 h (n = 4). (F) Representative images (Upper) and quantitative results (Lower) of VEGF-C protein in control and COUP-TFII knockdown cells (n = 5). CL, total cell lysate; CM, conditioned media. (G) Representative images of COUP-TFII, Flag, and VEGF-C protein (Left) and quantitative results of VEGF-C protein (Right) in control (Vector) and COUP-TFII overexpression (COUP-TFII) ectopic stromal cells (n = 4). Asterisks indicate P < 0.05.
To investigate the underlying mechanism responsible for VEGF-C up-regulation in endometriosis, we reanalyzed our own microarray data derived from COUP-TFII-knockdown human endometrial stromal cells as reported previously (18). Gene set enrichment analysis (GSEA) revealed that genes involved in vasculature development are enriched in COUP-TFII knockdown eutopic endometrial stromal cells (SI Appendix, Fig. S1A), and VEGF-C is one of the up-regulated angiogenic genes under COUP-TFII knockdown (SI Appendix, Fig. S1B). Consistent with our result, analysis of Gene Expression Omnibus (GEO) datasets also revealed inverse correlation of COUP-TFII and VEGF-C (SI Appendix, Fig. S1C). To further verify that VEGF-C expression is negatively correlated with COUP-TFII levels, we knocked down COUP-TFII in endometrial stromal cells (with higher COUP-TFII levels) (SI Appendix, Fig. S1D) by two sets of small interfering RNA (siRNA) (siCII#1 and siCII#2). Coherent to the analytic data, the results showed both VEGF-C mRNA and secreted VEGF-C proteins were significantly increased when COUP-TFII was knocked down (Fig. 1 E and F). In contrast, overexpression of COUP-TFII in ectopic stromal cells (with low COUP-TFII levels) reduced VEGF-C secretion (Fig. 1G).
Expression of VEGF-C Is Induced by Proinflammatory Cytokine-Mediated COUP-TFII Down-Regulation.
Our previous data demonstrated that COUP-TFII was down-regulated by proinflammatory cytokines (15); we thus aimed to investigate whether treatment with proinflammatory cytokines will cause VEGF-C up-regulation. Treatment of normal endometrial stromal cells with IL-1β and TNF-α caused a decrease in COUP-TFII protein (Fig. 2A) and concomitantly an increase in VEGF-C mRNA and protein (Fig. 2 B and C). In contrast, forced expression of COUP-TFII abolished IL-1β– and TNF-α–induced VEGF-C up-regulation (Fig. 2D). Promoter activity assay demonstrated that VEGF-C was up-regulated by IL-1β and TNF-α at the transcription level (SI Appendix, Fig. S2A). Bioinformatic analysis predicted a potential COUP-TFII binding site in the upstream of VEGF-C promoter (Fig. 2E). Chromatin immunoprecipitation (ChIP)-qPCR assay showed COUP-TFII indeed binds to the VEGF-C promoter region, and the binding is decreased under cytokines treatment (Fig. 2E). Similarly, there was a reduced binding of COUP-TFII on VEGF-C promoter in ectopic endometriotic stromal cell as compared to that in the eutopic counterpart (SI Appendix, Fig. S2B).
Fig. 2.
Proinflammatory cytokines suppress COUP-TFII to induce VEGF-C expression. (A) Representative Western blot (Left) and quantification result (Right) showed down-regulation of COUP-TFII protein in endometrial stromal cells treated with IL-1β (1 ng/mL) and TNF-α (10 ng/mL) for 24 h (n = 8 biological repeats). (B) Up-regulation of VEGF-C mRNA in endometrial stromal cells treated with IL-1β (1 ng/mL) and TNF-α (10 ng/mL) for 24 h (n = 6 biological repeats). (C) Representative Western blot (Upper) and quantification result (Lower) show of VEGF-C protein in cultured media of endometrial stromal cells treated with IL-1β (1 ng/mL) and TNF-α (10 ng/mL) for 24 h (n = 6 biological repeats). (D) Representative Western blot (Left) and quantitative results (Right) show overexpression of COUP-TFII (indicated by Flag) inhibits IL-1β and TNF-α–induced increases of VEGF-C (n = 7). (E) The schematic drawing (Left) shows the predicted COUP-TFII–binding sites (-0.6K) in VEGF-C promoter. Two pairs of arrows indicate PCR primers. Right shows quantified ChIP-qPCR result (n = 4 biological repeats). *P < 0.05 compared to respective controls for all images; #P < 0.05 compared to vector control in D.
Knockdown of COUP-TFII in Endometrial Cells Stimulates Tube Formation of Lymphatic Endothelial Cells.
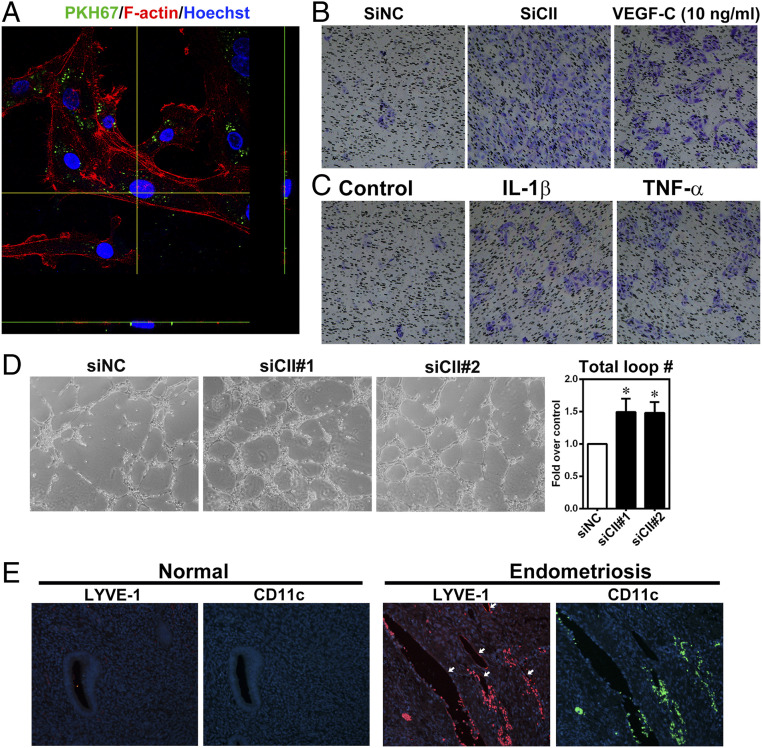
Treatment of lymphatic endothelial cells (LEC) with conditioned media collected from COUP-TFII knockdown endometrial stromal cells enhanced tube formation (SI Appendix, Fig. S3A). To confirm this effect is mediated by VEGF-C signaling, we first treated LEC with different doses of recombinant VEGF-C and evaluated cell proliferation and migration. Results show that VEGF-C dose dependently induced LEC proliferation and migration (SI Appendix, Fig. S3 B and C). Next, lenvatinib, a selective VEGFR2/3 receptor inhibitor, was preincubated with the LEC for 30 min prior to the addition of conditioned media. Results showed that pretreatment with lenvatinib abolished COUP-TFII knockdown-induced tube formation (Fig. 3A).
Fig. 3.
Knockdown of COUP-TFII stimulates lymphatic endothelial cell tube formation via VEGF-C–VEGFR signaling. (A) Representative images and quantitative result show total loop number of lymphatic endothelial cells treated with conditioned media collected from COUP-TFII knockdown primary cultured endometrial stromal cells with or without pretreatment of Lenvatinib (Lenv, 20 nM, n = 10). *P < 0.05. (B) Results of NTA of conditioned media collected from eutopic stromal cells and ectopic endometriotic stromal cells (n = 3). (C) Quantitative results show nanoparticle profile in conditioned media collected from paired eutopic endometrial stromal cells and ectopic endometriotic stromal cells (n = 3). (D) Representative transmission electron microscopic images show EV-associated immunogold-stained VEGF-C (black dots) from conditioned media collected from paired eutopic (Eu) and ectopic (Ec) endometrial stromal cells. (E) Representative Western blot (Left) and quantitative result (Right) show VEGF-C protein in EV fraction but not EV-free conditioned media (n = 3). Conditioned media collected from control siRNA or si-COUP-TFII (siCII#1 and siCII) transfected endometrial stromal cells. (F) Representative Western blot (Upper) and quantitative result (Lower) show VEGF-C protein in EV fraction (n = 3) of endometrial stromal cells transfected with empty vector (indicated as −) or COUP-TFII expressing plasmids (indicated as +). (G) Representative Western blot (Upper) and quantitative result (Lower) show VEGF-C protein in EV fraction (n = 3) of endometrial stromal cells treated with vehicle (Ctl), IL-1β (IL), or TNF-α (TNF) for 24 h. CD63 and TSG101 are EV markers. Asterisks indicate P < 0.05.
Endometriotic Stromal Cells-Secreted VEGF-C Is Carried by Extracellular Vesicles.
It is always a concern how endometriotic cell-produced lymphoattractant reaches the existing lymphatic endothelial cells, which are physically away from the endometriotic cells. We hypothesized that extracellular vesicles (EVs) may serve as cargos that carry and deliver VEGF-C molecules to the remote lymphatic vessels. To test this hypothesis, we first used a nanoparticle tracking assay to analyze the production and distribution of EVs. As seen in Fig. 3B, the total amount of EVs was much greater in ectopic stromal cells as compared to the eutopic counterparts. Further analysis revealed that amounts of nanoparticles were significantly greater in size: 95–165 nm (exosome) and size 175–245 nm (microvesicles) (Fig. 3C and SI Appendix, Fig. S4 A and B). Transmission electron microscope examination showed VEGF-C is carried by EVs, and more VEGF-C molecules are associated with EVs isolated from ectopic endometriotic stromal cells (Fig. 3D). Further analyses revealed that VEGF-C proteins were detected in EVs but not in EV-depleted conditioned media, and knockdown of COUP-TFII significantly increased EV-associated VEGF-C (Fig. 3E). In contrast, forced expression of COUP-TFII in ectopic stromal cells reduced EV-associated VEGF-C (Fig. 3F). Treatment with IL-1β and TNF-α significantly increased EV-associated VEGF-C production by endometrial stromal cells (Fig. 3G). Interestingly, the number and size of EVs were not affected by COUP-TFII knockdown or cytokines treatment (SI Appendix, Fig. S4 C and D), suggesting other factors are responsible for the increased EV number seen in endometriotic stromal cells.
EV-Associated VEGF-C Stimulates Lymphatic Vessel Development and Immune Cell Infiltration.
Endometriotic stromal cells were prelabeled with PKH67 fluorescent dye to label EVs (SI Appendix, Fig. S5), which were then used to treat LEC. Confocal images show EVs secreted by ectopic endometriotic stromal cells can be uptaken by lymphatic endothelial cells (Fig. 4A). Results from transwell migration assay and tube formation assay demonstrated that EVs isolated from COUP-TF knockdown or cytokine-treated cells significantly induced LEC migration (Fig. 4 B and C) and tube formation (Fig. 4D), respectively. Finally, we evaluated the clinical impact of lymphangiogenesis in endometriosis. Immunofluorescence staining showed LYVE-1–positive signals were evident in ectopic endometriotic lesion while the signal was barely detected in eutopic endometrium (Fig. 4E and SI Appendix, Fig. S6). More importantly, we observed CD11c+ immune cells within and around the lumen of lymphatic vessels in ectopic endometriotic lesion (Fig. 4E) but not in the eutopic endometrial tissues (SI Appendix, Fig. S6).
Fig. 4.
Increased lymphangiogenesis and immune cell infiltration by EV-associated VEGF-C. (A) The representative confocal microscopic image shows two-dimensional view (x and left y axes) of EVs uptaken by lymphatic endothelial cells. F-actin was stained with phalloidin (red) and nuclei were stained with Hoechst 33258 (blue) for visualization. (B and C) Representative images of transwell migration assays using EVs collected from COUP-TFII knockdown (B) or IL-1β or TNF-α–treated (C) endometrial stromal cells. VEGF-C was used as positive control. (D) Representative images and quantitative results show increased total loop number of lymphatic endothelial cells treated with EV fraction collected from COUP-TFII knockdown primary cultured endometrial stromal cells (n = 5). *P < 0.05. (E) Representative immunofluorescence staining images show the expression and distribution of LYVE-1 and CD11c (a marker expressed in certain groups of immune cells) in human normal endometrium (n = 32) and endometriotic tissues (n = 10). White arrows indicate the LYVE-1–positive lymphatic endothelial cells.
Increased Lymphatic Vessel Infiltration in Endometriotic Lesion Is Mediated by VEGF-C.
We then tested our hypothesis using mouse model of endometriosis (SI Appendix, Fig. S7A). We first observed that induction of endometriosis-like lesions in mice caused severe adhesion in the peritoneum (SI Appendix, Fig. S7B) and enlargement of retroperitoneal lymph nodes (Fig. 5A), a phenomenon like what was observed in women with endometriosis by using computed tomography scanning (Fig. 5B). Immunofluorescence staining using Lyve-1 and CD11c+ antibodies revealed that immune cells are detected around the Lyve-1–positive lymphatic vessels (Fig. 5C). Treatment with lenvatinib reduced lesion sizes as compared to mice receiving DMSO (vehicle) injection (Fig. 5D) without causing the weight loss or organ failure (SI Appendix, Fig. S7 C–F). Immunohistochemical staining also showed that less lymphatic vessels (stained by Lyve-1 antibody) in lenvatinib-treated group (Fig. 5E). In contrast, numbers of infiltrated blood vessels (stained by CD31 antibody) were not affected by treatment with lenvatinib (SI Appendix, Fig. S8), indicating the specificity of lenvatinib in blocking lymphangiogenesis. Lastly, we examined lymph nodes in the peritoneal cavity and found that treatment with lenvatinib significantly reduced the size of lymph nodes (Fig. 5F).
Fig. 5.
VEGF-C enhances lymphatic system development in endometriotic tissues. (A) Representative pictures (Left) and quantified result (Right) showed lymph nodes recovered from peritoneal cavity of mice with (n = 6 mice) or without (control, n = 4 mice) surgery-induced endometriosis. Two to three lymph nodes were obtained from each mouse. (B) Transversal view (Left) of endometrioma (indicated by a circle) and both transversal (Middle) and coronal (Right) views of lymph nodes (indicated by red arrows) in patients with endometriosis were taken by computed tomography scanning. (C) Representative images show immunofluorescence staining of endometrium and endometriotic-like lesion from mice (n = 7 mice per group) using anti-Lyve-1 (red) and anti-CD11c (green) antibodies. (D) Gross view (Left) and quantified result (Right) of endometriotic lesions (n = 7 mice, four lesions per mouse) collected from mice treated with vehicle or Lenvatinib for 1 mo. (E) Representative IHC image (Left) and quantified result (Right) showed Lyve-1–positive signals in endometriotic lesions treated with vehicle or Lenvatinib. (F) Gross view (Left) and quantified results (Right) of sizes of sentinel lymph nodes (n = 7 mice, two lymph nodes per mouse) collected from mice treated with vehicle or Lenvatinib. Asterisks indicate P < 0.05.
We then examined effects of lenvatinib-inhibited lymphatic system development on immune cells infiltration. Consistent with the notion that reduced lymphatic vessels results in less inflammation, infiltrated immune cells including F4/80- and CD11c-positive macrophages, granzyme B-positive NK cells/cytotoxic T lymphocytes, and IL-17–positive Th-17 cells were abundant in endometriotic lesions (Fig. 6 A and B). Treatment with lenvatinib markedly reduced numbers of infiltrated immune cells (Fig. 6 A and B). Taken together, these data suggest that VEGF-C and lymphatic system play an important role in immune cells infiltration and endometriotic lesion progression (Fig. 6C).
Fig. 6.
Blocking VEGF-C signaling reduces immune cells infiltration to endometriotic tissues. (A and B) Representative immunofluorescence staining images (A) and quantitative results (B) showed infiltrated immune cells in endometriotic lesion collected from mice (n = 7 mice per group) treated with vehicle or lenvatinib. Granzyme B as a marker for natural killer cells and T cells. CD11c as a marker for some lineages of granulocytes, monocytes/macrophages, and lymphocytes. F4/80 as an immune marker for mice macrophages. IL-17 as an immune marker for T helper 17 (Th17) cell. Nuclei were stained with Hoechst33258. *P < 0.05. (C) Schematic drawing of working model (see text for details).
EV-Associated VEGF-C in Patient’s Serum Is a Potential Diagnostic Biomarker.
To investigate whether VEGF-C can be used as a diagnostic biomarker, we isolated EVs from an independent cohort of patients’ sera and quantified levels of VEGF-C (Fig. 7A and SI Appendix, Fig. S9A). The size and number of EVs from fractions 14–19 of eluents in a small set of samples were analyzed by nanoparticle tracking analysis (NTA) (SI Appendix, Fig. S9B). Results from Western blotting and transmission electron microscopic analyses showed that EV-associated VEGF-C were significantly more in patients with endometriosis than that in normal ones (Fig. 7 B–D). The area under receiver-operating characteristic (ROC) curve is 0.8175 (95% CI, 0.6973–0.9376), with the optimal sensitivity of 81.3%, and specificity of 71.4% compared to normal group by using 912.4 pg/mL as the cutoff value (Fig. 7E).
Fig. 7.
Extracellular vesicles-transmitted VEGF-C from patients’ sera is a potential diagnostic marker for endometriosis. (A) Schematic drawing of the procedure analyzing EV-transmitted VEGF-C from patients’ sera. (B) Representative Western blot image shows VEGF-C of pooled fractions of isolated EVs from patients’ sera by SEC. VEGF-C was higher in patients with endometriosis than normal ones. CD9, EV’s marker. (C) Representative TEM images of EV-associated immunogold-stained VEGF-C (black dots) isolated from patients’ sera. (D) Quantified ELISA result of VEGF-C in pooled fractions of exosomes isolated from patients’ sera (normal, n = 21; endometriosis, n = 48). (E) ROC curve for distinguishing endometriosis patients from normal ones (AUC = 0.8175; P < 0.0001).
Discussion
Despite being identified for more than a century, the etiology of endometriosis, unfortunately, remains largely unknown. The lack of understanding of the underlying mechanisms responsible for the pathological processes of endometriosis hampers the development of efficacious treatment regimens for this disease. Herein, we unravel the molecular mechanism of lymphangiogenesis and its function in the pathogenesis of endometriosis (Fig. 6C). Proinflammatory cytokines, such as IL-1β and TNF-α, suppress COUP-TFII expression in endometrial stromal cells, which causes VEGF-C secretion. The secreted VEGF-C is mainly transmitted by nanoscale extracellular vesicles. Carried by these EV nanoparticles ensures VEGF-C a more stable and effective means of transportation to reach the sentinel lymph nodes, where VEGF-C binds to VEGFR2/R3 on lymphatic endothelial cells to induce lymphangiogenesis toward endometriotic lesions. The formation of lymphatic vessels provides further routes for immune cells to infiltrate into endometriotic tissue and secrete more proinflammatory cytokines. Thus, the feedforward loop involving proinflammatory cytokines, COUP-TFII, VEGF-C, and immune cells establishes a vicious cycle of inflammation and lymphangiogenesis to promote the progression of endometriosis.
Endometriosis is known as a chronic proinflammatory disease. Although many studies have partially reported the involvement of immune cells, proinflammatory cytokines, and intrinsic/extrinsic factors in causing inflammation in the endometriotic microenvironment (see refs. 22 and 23 for review), there are still many unknown mechanisms that need to be explored. For example, how immune cells are recruited to the endometriotic tissues and what the impact of such immune cell infiltration is have not been fully elucidated. Herein, we provide evidence to demonstrate that immune cells infiltration may be due to loss of COUP-TFII–mediated VEGF-C expression. Loss of COUP-TFII leads to overproduction of VEGF-C in ectopic endometriotic stromal cells, which plays an important role in recruiting immune cells to the endometriotic lesion. COUP-TFII is a versatile transcription repressor, which has been shown to regulate the expression of aromatase (24), COX-2 (15), and angiogenin (18) in endometriotic stromal cells. In this study, we add another important player, VEGF-C, to the COUP-TFII–regulated gene list. These findings not only concur with previous reports that VEGF-C is up-regulated in women with endometriosis (13, 14) but also provide the underlying mechanism to explain why VEGF-C is overexpressed in endometriotic cells. More intriguingly, we demonstrate that the secreted VEGF-C is carried by EVs. As EVs are nanoscale cargos that efficiently transport mediators from one location to the other, even to the remote sites, EV-associated VEGF-C is likely to transmit from endometriotic stromal cells to sentinel lymph nodes to induce the migration of lymphatic endothelial cells toward endometriotic lesions. Indeed, our in vitro and in vivo data demonstrate that EV-associated VEGF-C is capable of inducing tube formation and lymphangiogenesis, respectively. Taken together, our current findings uncover another important pathophysiological process of endometriosis regulated by COUP-TFII.
The function of lymphatic system in endometriotic lesion has been discussed some 90 y ago. In 1927, Sampson proposed the possibility that lymphatic vessel may serve as an alternative route for disseminating endometriotic lesions into peritoneal cavity, but he was not able to prove it (7). Since then, not much progress was made regarding the regulation or function of lymphangiogenesis in endometriosis. Recently, it has been reported that endometrial cells are detected in uterine lymphatic vessels and obturator lymph nodes in women with endometriosis (10, 11, 25) and in induced endometriosis model of baboon (26). However, whether these lymphatic vessel-disseminated cells cause endometriosis metastasis has not been proven. We suggest higher activity of lymphangiogenesis found in endometriotic lesions is not for dissemination of endometriotic tissues but for other fundamental functions such as infiltration of immune cells to enhance the local inflammation. Indeed, computed tomography image shows women with endometriosis had large reactive peritoneal lymph nodes, a phenomenon that indicates local inflammation and an increase number of immune cells in lymph node (25). We also demonstrated that retroperitoneal lymph nodes are enlarged in mice with induced endometriosis and treatment with lenvatinib ameliorated this phenomenon. These data suggest that the lymphatic system is an important reservoir of immune cells in response to local inflammation such as aberrant growth of endometriotic lesions.
Besides inducing lymphangiogenesis, VEGF-C has also been found to promote macrophage and lymphocyte migration in cancer microenvironment (27–29). Thus, increased secretion of VEGF-C from endometriotic cells promotes lymphatic vessel formation, which might provide a speedy highway for immune cells to migrate. Since the concentration of VEGF-C is high in the lesion, this creates a chemoattractant gradient favoring the migration of immune cells toward the lesion. As a result, the number of immune cells is increased in endometriotic tissues. Indeed, we observed a marked increase in macrophages, regulatory T cells, and Th-17 lymphocytes accumulating near lymphatic vessels in the endometriotic lesion, and these infiltrated immune cells population was significantly reduced by treatment with lenvatinib, a selective VEGFR2/R3 inhibitor, to disrupt VEGF-C signaling. Our findings are supported by previous studies in human melanoma and in mouse model of lymphedema that immune cells can infiltrate to cancer region and injured site, respectively, through lymphatic vessels (30, 31).
Previous studies have shown that immune cells including neutrophils (32), macrophages (33–35), regulatory T cells (36, 37), Th-17 cells (38), and even NK cells (39) all contribute to disease pathogenesis of endometriosis either by increased secretion of proinflammatory cytokines or reduced phagocytic ability. Since we demonstrated the importance of VEGF-C in regulating lymphangiogenesis and immune cells infiltration, specifically targeting VEGF-C signaling may exert beneficial effect in ameliorating endometriosis progression. Indeed, treatment with lenvatinib, a selective blocker for VEGF-R2/R3, successfully prevents the growth of endometriotic lesions. Since the lymph nodes are also reduced by lenvatinib treatment concomitantly with marked reduction in infiltrating immune cells in endometriotic lesions, we reason the regression of endometriotic lesion is likely mediated by reduced inflammation. Lenvatinib is a promising drug for treating cancers such as thyroid cancer and metastatic renal cell carcinoma, and has already underwent clinical trials (40, 41). In our in vivo murine model, we showed lenvatinib can reduce lesions size accompanied by decreased lymphangiogenesis and immune cells infiltration and, more importantly, without damaging other organs or causing weight loss in mice.
Owing to its biological characteristics, EVs have been emphasized having extraordinary potential for future applications particularly in clinical diagnosis and therapy (42). Emerging evidence shows high correlation between certain molecules (proteins, DNA, or RNA) transported by these nanoscale membranous vesicles in patients’ blood and disease progression (43), suggesting patients can possibly be diagnosed in a noninvasive and faster way for certain diseases. So far, laparoscopy is the only definite way to diagnose endometriosis, which is relatively risky and time-consuming. In our study, we proved patients with endometriosis have higher VEGF-C, and VEGF-C is transmitted by EVs in blood circulation throughout the whole body. This finding not only further demonstrates the importance of VEGF-C involved in the pathogenesis of endometriosis, but also provides a way for diagnosing endometriosis without receiving surgery. However, it must bear in mind that EVs and/or VEGF-C can be secreted by many types of cells in the body, and we did not directly measure EV–VEGF-C secreted by endometriotic cells in vivo, which is the limitation of current study.
Taken together, our data not only demonstrate the pathological role of VEGF-C signaling in endometriosis but also show serum VEGF-C can be used as a diagnostic biomarker for endometriosis. Furthermore, results from in vivo study also suggest the feasibility of using lenvatinib or other VEGFR inhibitors to treat endometriosis.
Materials and Methods
Ethical Approval and Clinical Samples from Patients.
The experimental procedure was approved by the Institutional Review Board of National Cheng Kung University Medical Center and informed consent was obtained from individual patients. Thirty-five endometriosis-free (defined as normal) women and 111 women with endometriosis were recruited to this study. Severity of endometriosis was classified based on the revised American Society of Reproductive Medicine classifications (1997) and were histologically confirmed by pathologists. Patients information was listed in Dataset S1.
Primary Stromal Cells, Cell Culture Condition, and Treatments.
The procedure for purification of stromal cells from uterine endometrium and ectopic endometriotic tissues was described previously (44). Stromal cells were routinely stained with vimentin and cytokeratin to check the purity (SI Appendix, Methods and Fig. S9A). In vitro decidualization assay was also performed to verify the cell originality (SI Appendix, Fig. S9B).
Microarray Data Mining and GSEA.
The procedure for microarray analysis of endometrial stromal cells from patients with or without COUP-TFII knockdown was described previously (18). Herein, we reanalyzed the raw data and employed GSEA by cross-referencing genes with significant difference in COUP-TFII–knockdown endometrial stromal cells with biological processes from the Gene Ontology database (Molecular Signatures Database, version 6.2) (45).
ChIP Coupled with qPCR.
The procedure for ChIP-qPCR was performed as previously described (44). More detailed information is described in SI Appendix, Methods. Primers are listed in Dataset S2.
Constructs, siRNA, and Transfection.
The procedures of COUP-TFII overexpression and siRNA knockdown were described previously (15). Reagents and siRNA information are listed in Dataset S3.
Tube Formation Assay, Transwell Migration Assay, and Cell Proliferation Assay.
Human Dermal LECs were cultured in Endothelial Cell Growth Medium MV2 (ECGM-MV2; PromoCell, C-22022) with SupplementMix, at 37 °C and subjected for different assays (SI Appendix, Methods).
Extracellular Vesicles Extraction from Cultured Conditioned Medium and Nanoparticle Tracing Analysis.
Extracellular vesicles, including exosomes and microvesicles, were isolated from conditioned media by using ExoQuick-TC Exosome Precipitation Solution (System Biosciences, catalog no. EXOTC10A-1) according to the manufacturer’s protocol. However, serum-free conditioned media from control or treated endometrial stromal cells were spun under low centrifugation rate to remove debris (500 × g, 10 min), followed by high centrifugation rate (16,000 × g, 30 min) to collect extracellular vesicles. The supernatant was diluted by phosphate buffered saline (PBS) and then sent to the Center for Micro/Nano Science and Technology at National Chung Kung University for nanoparticle tracking analysis.
Tracking Extracellular Vesicles Uptake.
Extracellular vesicles labeled with PKH67 Fluorescent Cell Linker Kit (Sigma-Aldrich, PKH67GL) were used for membrane tracking analysis according to the manufacturer’s protocol.
Murine Model of Endometriosis.
Autotransplantation model of endometriosis was performed by sewing the eutopic tissues onto the peritoneal wall as previously described (44). More detailed information is described in SI Appendix, Methods.
Immunohistochemistry Staining and Immunofluorescence Staining.
Paraffin-embedded endometrial tissues and endometriotic lesions from human or mice were stained by standard protocols as previously described (46). Detailed procedures are described in SI Appendix, Methods.
Extracellular Vesicles Isolation from Serum by Size Exclusion Chromatography.
Patient’s serum was first centrifuged at 300 × g for 10 min, and followed by 1,200 × g for another 20 min. One hundred microliters of supernatant of centrifuged patient’s serum was used for miniPURE-EVs: size exclusion chromatography (SEC) (HansaBioMed Life Sciences, Lonza, HBM-mPEV-10), and the fractions were collected. We used PBS as mobile phase and counted three drops (around 100 μL) as one fraction. A total of 20 fractions were collected.
Enzyme‐Linked Immunosorbent Assay.
Levels of EV-associated VEGF-C in patients’ sera were assessed using Human VEGF-C Quantikine enzyme‐linked immunosorbent assay (ELISA) Kit (R&D Systems, DVEC00) according to the manufacturer’s instructions.
Transmission Electron Microscopic Examination of Immunogold-Stained VEGF-C.
The EV fractions from cell culture or patients’ sera were purified and subjected to transmission electron microscopic examination as described in SI Appendix, Methods.
Statistical Analysis.
Differences between two groups were analyzed by Student’s t test. One-way analysis of variance (ANOVA) and two-way ANOVA were used to compare multiple groups and followed by posttest analysis in GraphPad Prism 5.0 (GraphPad Software). Serum VEGF-C was analyzed using ROC to determine the area under the curve, sensitivity, and specificity. Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Yen‐Yu Lai, Yi‐Chen Tang, Mei-Feng Huang, Yi-Shan Ya, Yi-Jou Chung, and Ting-Ting Chan for technical assistance. We also thank the Bioinformatics Center at National Cheng Kung University for providing excellent assistance in bioinformatic analyses. This work was supported by Ministry of Science and Technology, Taiwan, Research Grants 106‐2320‐B‐006‐072‐MY3, 105-2320-B-006-036-MY3 (to S.-J.T.), and 108-2314-B-00 -059-MY3 (to M.-H.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.K.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920037117/-/DCSupplemental.
Data Availability.
Microarray data are available at the National Center for Biotechnology Information GEO, accession no. GSE107469. All other study data are included in the article and supporting information.
References
- 1.Sampson J. A., Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–425 (1927). [Google Scholar]
- 2.Cramer D. W., Missmer S. A., The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 955, 11–22 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Halme J., Hammond M. G., Hulka J. F., Raj S. G., Talbert L. M., Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64, 151–154 (1984). [PubMed] [Google Scholar]
- 4.Hsiao K. Y., Lin S. C., Wu M. H., Tsai S. J., Pathological functions of hypoxia in endometriosis. Front. Biosci. (Elite Ed.) 7, 309–321 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Wu M. H., Hsiao K. Y., Tsai S. J., Hypoxia: The force of endometriosis. J. Obstet. Gynaecol. Res. 45, 532–541 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Wu M. H., Lu C. W., Chuang P. C., Tsai S. J., Prostaglandin E2: The master of endometriosis? Exp. Biol. Med. (Maywood) 235, 668–677 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Sampson J. A., Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 3, 93–110.43 (1927). [PMC free article] [PubMed] [Google Scholar]
- 8.Fargas Fabregas F., Cusido Guimferrer M., Tresserra Casas F., Baulies Caballero S., Fabregas Xaurado R., Malignant transformation of abdominal wall endometriosis with lymph node metastasis: Case report and review of literature. Gynecol. Oncol. Case Rep. 8, 10–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insabato L., Pettinato G., Endometriosis of the bowel with lymph node involvement. A report of three cases and review of the literature. Pathol. Res. Pract. 192, 957–961 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Mechsner S. et al., Immunohistochemical evaluation of endometriotic lesions and disseminated endometriosis-like cells in incidental lymph nodes of patients with endometriosis. Fertil. Steril. 94, 457–463 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Tempfer C. B. et al., Lymphatic spread of endometriosis to pelvic sentinel lymph nodes: A prospective clinical study. Fertil. Steril. 96, 692–696 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Jerman L. F., Hey-Cunningham A. J., The role of the lymphatic system in endometriosis: A comprehensive review of the literature. Biol. Reprod. 92, 64 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Takehara M. et al., Vascular endothelial growth factor A and C gene expression in endometriosis. Hum. Pathol. 35, 1369–1375 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Xu H. et al., Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis 16, 541–551 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Lin S. C. et al., Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 99, E427–E437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M. H. et al., Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J. Clin. Endocrinol. Metab. 90, 286–295 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Lin F. J., Qin J., Tang K., Tsai S. Y., Tsai M. J., Coup d’Etat: An orphan takes control. Endocr. Rev. 32, 404–421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J. L. et al., Suppression of COUP-TFII upregulates angiogenin and promotes angiogenesis in endometriosis. Hum. Reprod. 333, 1517–1527 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Zeitoun K., Takayama K., Michael M. D., Bulun S. E., Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol. Endocrinol. 13, 239–253 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Hawkins S. M. et al., Functional microRNA involved in endometriosis. Mol. Endocrinol. 25, 821–832 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hever A. et al., Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc. Natl. Acad. Sci. U.S.A. 104, 12451–12456 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W. N., Wu M. H., Tsai S. J., Critical factors involved in chronic inflammation in the pathophysiology of endometriosis. Adaptive Medicine 10, 1–9 (2018). [Google Scholar]
- 23.Izumi G. et al., Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 44, 191–198 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Attar E. et al., Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 94, 623–631 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berbic M. et al., A novel pilot study of endometrial stromal cells and immune cell populations in sentinel uterine-draining lymph nodes during the menstrual cycle and in endometriosis. Reprod. Sci. 20, 1339–1348 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Hey-Cunningham A. J. et al., Endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. Reprod. Sci. 18, 747–754 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y. et al., Paracrine signaling by VEGF-C promotes non-small cell lung cancer cell metastasis via recruitment of tumor-associated macrophages. Exp. Cell Res. 364, 208–216 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Iwami D., Brinkman C. C., Bromberg J. S., Vascular endothelial growth factor c/vascular endothelial growth factor receptor 3 signaling regulates chemokine gradients and lymphocyte migration from tissues to lymphatics. Transplantation 99, 668–677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skobe M. et al., Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 159, 893–903 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordry N. et al., Lymphatic vessel density is associated with CD8(+) T cell infiltration and immunosuppressive factors in human melanoma. OncoImmunology 7, e1462878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gousopoulos E., Proulx S. T., Scholl J., Uecker M., Detmar M., Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 186, 2193–2203 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Takamura M. et al., Neutrophil depletion reduces endometriotic lesion formation in mice. Am. J. Reprod. Immunol. 76, 193–198 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Chuang P. C. et al., Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am. J. Pathol. 176, 850–860 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu M. H., Chuang P. C., Lin Y. J., Tsai S. J., Suppression of annexin A2 by prostaglandin E(2) impairs phagocytic ability of peritoneal macrophages in women with endometriosis. Hum. Reprod. 28, 1045–1053 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Wu M. H. et al., Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis. Am. J. Pathol. 167, 1061–1069 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M. Q. et al., CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 5, e1436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei C. et al., 1-Methyl-tryptophan attenuates regulatory T cells differentiation due to the inhibition of estrogen-IDO1-MRC2 axis in endometriosis. Cell Death Dis. 7, e2489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirata T. et al., Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology 149, 1260–1267 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Kang Y. J. et al., An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 29, 2176–2189 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Iniguez-Ariza N. M., Ryder M. M., Hilger C. R., Bible K. C., Salvage lenvatinib therapy in metastatic anaplastic thyroid cancer. Thyroid 27, 923–927 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Motzer R. J. et al., Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 16, 1473–1482 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Yanez-Mo M. et al., Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meningher T. et al., Schistosomal MicroRNAs isolated from extracellular vesicles in sera of infected patients: A new tool for diagnosis and follow-up of human schistosomiasis. J. Infect. Dis. 215, 378–386 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Lin S. C. et al., Targeting anthrax toxin receptor 2 ameliorates endometriosis progression. Theranostics 9, 620–632 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian A. et al., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin S. C. et al., Targeting hypoxia-mediated YAP1 nuclear translocation ameliorates pathogenesis of endometriosis without compromising maternal fertility. J. Pathol. 242, 476–487 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microarray data are available at the National Center for Biotechnology Information GEO, accession no. GSE107469. All other study data are included in the article and supporting information.