Significance
Networks of no-take marine reserves support local fisheries by ensuring a consistent supply of juvenile fish. We measured larval dispersal patterns for a highly exploited coral grouper and quantified temporal fluctuations in the recruitment contribution from a network of no-take marine reserves on the Great Barrier Reef. Although recruitment contributions from individual reserves are extremely variable, the reserve network generates a connectivity portfolio effect that successfully dampens the volatility of larval supply to nearby coral reefs. Our findings demonstrate that effective reserve networks can yield previously unrecognized stabilizing benefits that ensure a consistent replenishment of exploited fish stocks.
Keywords: marine reserve, larval dispersal, connectivity, portfolio effects, marine spatial planning
Abstract
Well-managed and enforced no-take marine reserves generate important larval subsidies to neighboring habitats and thereby contribute to the long-term sustainability of fisheries. However, larval dispersal patterns are variable, which leads to temporal fluctuations in the contribution of a single reserve to the replenishment of local populations. Identifying management strategies that mitigate the uncertainty in larval supply will help ensure the stability of recruitment dynamics and minimize the volatility in fishery catches. Here, we use genetic parentage analysis to show extreme variability in both the dispersal patterns and recruitment contribution of four individual marine reserves across six discrete recruitment cohorts for coral grouper (Plectropomus maculatus) on the Great Barrier Reef. Together, however, the asynchronous contributions from multiple reserves create temporal stability in recruitment via a connectivity portfolio effect. This dampening effect reduces the variability in larval supply from individual reserves by a factor of 1.8, which effectively halves the uncertainty in the recruitment contribution of individual reserves. Thus, not only does the network of four marine reserves generate valuable larval subsidies to neighboring habitats, the aggregate effect of individual reserves mitigates temporal fluctuations in dispersal patterns and the replenishment of local populations. Our results indicate that small networks of marine reserves yield previously unrecognized stabilizing benefits that ensure a consistent larval supply to replenish exploited fish stocks.
Marine reserves are a comprehensive tool to mitigate the overexploitation of marine resources and to enhance the recovery of marine ecosystems following disturbances (1–3). They are being implemented globally to preserve biodiversity (4, 5), improve livelihoods in coastal communities (6), and indirectly benefit local fisheries by protecting spawning stocks and replenishing exploited populations beyond reserve boundaries (7). In principle, the greater biomass of exploited species in reserves (8, 9), combined with greater per-capita reproductive outputs (10, 11), generates positive ecological and socioeconomic value to fisheries by contributing to the replenishment of local populations and enhancing population persistence via the supply of larval offspring (12–15). However, larval contributions from individual reserves are likely to be highly variable (16, 17), both because local population abundances vary and because complex oceanographic processes and larval behaviors produce spatial and temporal variability in connectivity patterns (16–19). Such volatility in larval supply can lead to temporal fluctuations in recruitment (20, 21) and uncertainty concerning the value of marine reserves to either biodiversity conservation or fisheries management (20, 22–24). Clearly, the long-term ecological and economic benefits of no-take marine reserves depend on significant and consistent larval supply among reserves, and from reserves to neighboring habitats (7, 22–24). This has yet to be established.
Decades of ecological theory on risk spreading in spatially structured populations shows how variability in the contribution of separate subpopulations can deliver net benefits for metapopulation growth and persistence (25–28). In general, more subpopulations and greater population connectivity reduce the probability of local extinctions via a “rescue effect” and dampen local fluctuations in population replenishment (29–32) and fishery catches (33, 34). If correct, effective networks of no-take marine reserves could mitigate against the volatility of larval supply provided the network can dampen the spatially and temporally variable contributions of individual reserves (20, 21). In the context of optimal reserve design, variability in the aggregate performance of a reserve network hinges on covariation among its individual components (34, 35) so that overall stability in larval supply can theoretically be achieved despite volatility in the performance of individual reserves.
Such variance dampening has more recently been referred to as a “portfolio effect,” and negative spatial covariation in population sizes (a “subpopulation portfolio effect”) has been observed in a diversity of biological systems, including the population dynamics of fishes (36–38). Modern portfolio theory emerged from financial economics and is increasingly applied in resource management settings to optimize the design of reserve networks and mitigate against disturbance events (39–42). In marine ecology, individual populations can be thought of as different financial stocks and their larval supply are the returns they generate. Creating a marine reserve will reliably increase the abundance in a protected population and generate larger larval supply (43), like purchasing more of a particular stock. Just as a diverse portfolio of uncorrelated financial assets minimizes an investor’s exposure to stock market volatility (44), replication of reserves in an interconnected network could mitigate against fluctuations in larval supply and minimize the risk of recruitment failure. However, to date there has been no empirical estimation of the portfolio effect for any existing network of reserves. This knowledge gap is significant given the large and rapidly increasing global investment in interconnected networks of marine reserves, the success of which hinges on the assumption that networks have emergent benefits that are greater than the sum of the constituent reserves.
For any established marine reserve network, a portfolio effect for a protected species can be measured by observing temporal fluctuations in the recruitment contribution of multiple reserves, over multiple recruitment cohorts. Here, we evaluate variability in the aggregate performance of no-take marine reserves in the Great Barrier Reef Marine Park (GBRMP), using a unique dataset of six discrete recruitment cohorts of juvenile coral grouper (Plectropomus maculatus, Serranidae), spanning 6 y. We use genetic parentage analysis on a sample of adult fish from four no-take marine reserves and juvenile fish that recruited to nearby coral reefs to reveal temporal trends in larval connectivity patterns. These results allow us to measure fluctuations in the performance of individual reserves, and reveal the existence of a “connectivity portfolio effect,” a reduction in the volatility of larval recruitment that results from asynchronous variation in larval connectivity patterns.
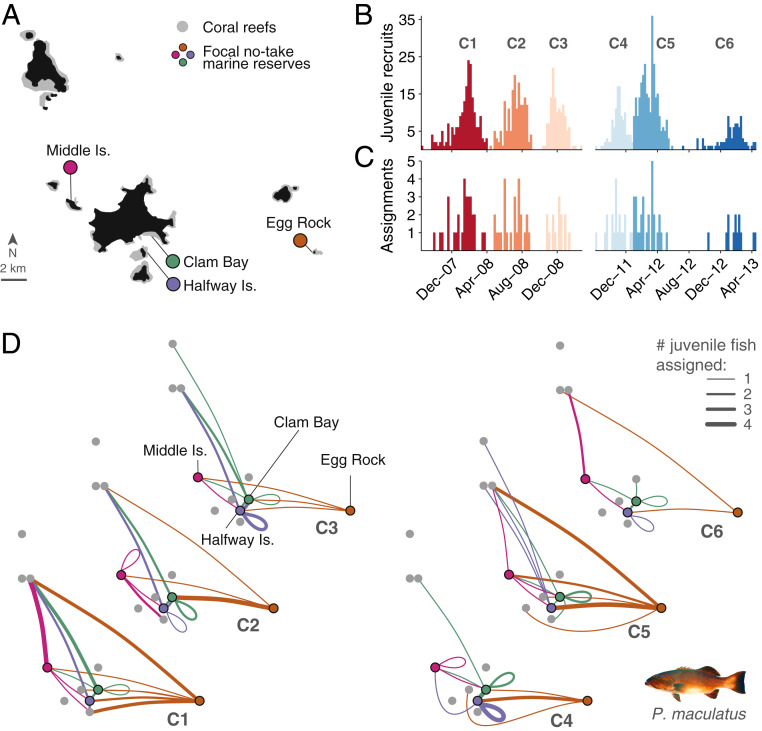
In the GBRMP, coral grouper (Plectropomus spp.) are highly targeted by commercial and recreational fishers and have responded positively to protection inside no-take marine reserves (1). In the Keppel Islands, the biomass of coral grouper is two to three times higher on no-take reserve reefs than on neighboring fished reefs (45); hence we expect their contribution to local larval replenishment to be high relative to fished reefs (11). We collected tissue samples of adult coral grouper from four reserves in the Keppel Islands (Fig. 1A) during the peak reproductive season in the austral summers of 2007–2008 and 2011–2012 (Methods). Our sample of 877 adults represents 19.2 ± 3.0% SE and 22.5 ± 4.7% SE of the reproductively mature population of coral grouper in reserves during the sampling periods (SI Appendix, Table S1). We also collected 981 juveniles from reserve and fished reefs throughout the island group. Six discrete recruitment cohorts were identified based on interruptions between periods of unimodal distributions of spawning times of juvenile fish (Fig. 1B and SI Appendix), providing information on recruitment and dispersal patterns at an unusually high temporal resolution.
Fig. 1.
Realized larval dispersal patterns of coral grouper from a network of no-take marine reserves. (A) The Keppel Islands in the southern Great Barrier Reef, where adult coral grouper (Plectropomus maculatus) were sampled in four no-take marine reserves (Middle Island, Halfway Island, Clam Bay, and Egg Rock). Juveniles were sampled from all known suitable coral reef habitat throughout the island group. Focal reefs are highlighted by colored dots, consistent across panels. (B) We used daily otolith increments and length–age relationships (SI Appendix, Fig. S1) to identify six distinct spawning periods and settlement cohorts (C1–C6) between September 2007 and April 2013 among the 981 sampled juvenile fish. (C) Parentage analysis identified 125 parent–offspring pairs spanning all six cohorts. (D) All assignments were to parents sampled from four no-take marine reserves. For each dispersal network, colored circles (network nodes) represent reserves and gray circles correspond to other reefs in the Keppel Islands. Lines (network edges) represent juvenile fish that successfully dispersed from reserves to neighboring reefs, where line thickness indicates the number of assigned juveniles and line color indicates their origin.
Results
Based on the unique genetic profiles of adult and juvenile coral trout collected in the Keppel Islands, we identified 125 juvenile fish as the progeny of adults sampled within the four reserves (Methods). Assigned juvenile fish were distributed among six recruitment cohorts that represent an exhaustive sample of all juvenile fish that settled in the island group (Fig. 1C and SI Appendix, Table S2). In each cohort, we identified juvenile fish that dispersed from no-take marine reserves to both fished areas (85 juveniles) and other reserves (40 juveniles). However, dispersal patterns varied substantially among recruitment cohorts, showing no consistent trend or single underlying structure among dispersal networks (Fig. 1D, average Pearson correlation: 0.43 ±0.03 SE). The distance, direction, and strength of larval connections from each of the four reserves (SI Appendix, Figs. S2 and S3) were inconsistent among successive cohorts, indicating highly variable connectivity patterns among reserve reefs and neighboring reefs.
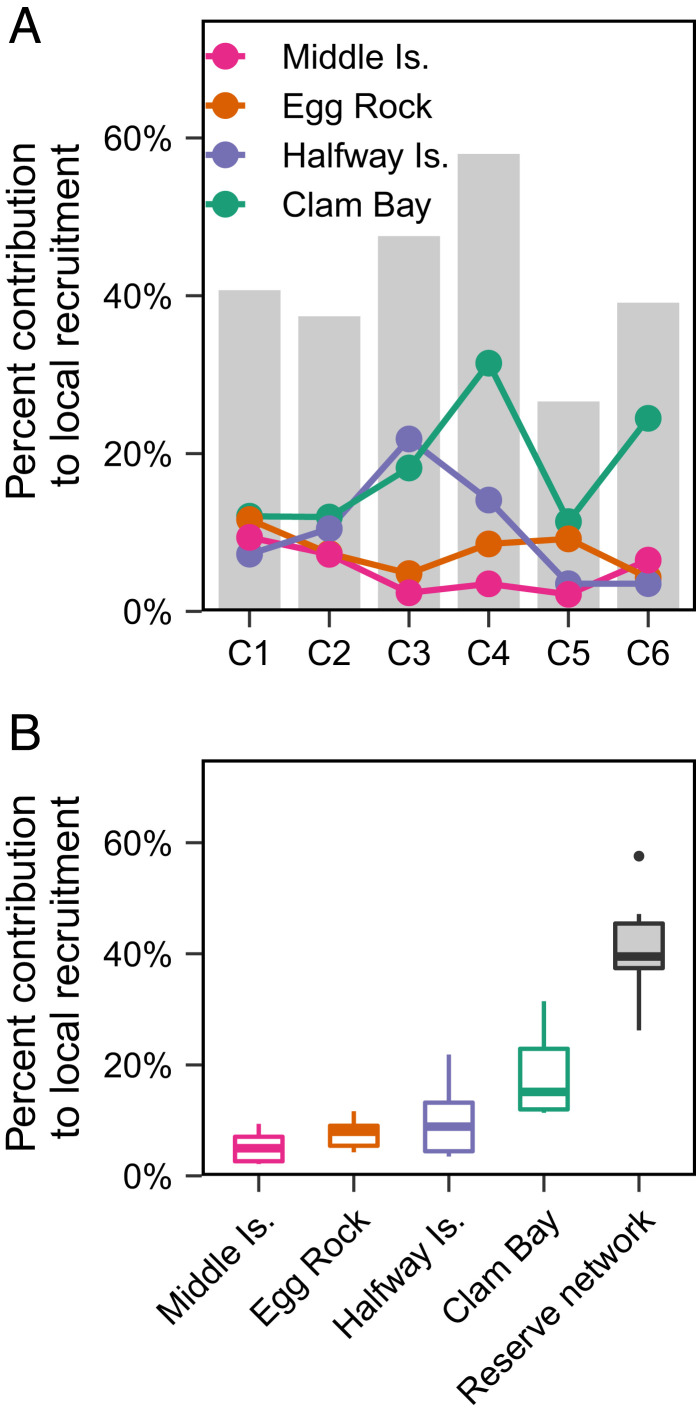
Adjusting for unsampled adults, we estimate the performance of each reserve as their proportional contribution to the overall recruitment in the island group (Methods). The results show that the aggregate network of four no-take marine reserves consistently generates between 26 and 58% of all local recruitment for any given cohort (Fig. 2A). On average, the aggregate performance of the reserve network, which represents only 14% of coral reef habitat, is responsible for 41 ± 11% SD of all recruitment in the island group. Our findings reinforce the importance of reserves as a source of juvenile fish for local population replenishment. However, they also reveal substantial fluctuations and uncertainty in the performance of individual reserves. The median contribution of a single reserve to local recruitment varied between 5 and 15% with a coefficient of variation (CV) of 0.71 (Fig. 2B), meaning that the degree of variation between reserves and between cohorts is extremely high. Incidentally, the time series also reveals asynchronous fluctuations in the local recruitment contribution of the four reserves across the six cohorts [synchrony index: 0.25, where 0 is maximally asynchronous and 1 is maximally synchronous (46); Fig. 2A]. So, while the performance of a single reserve varies with each cohort, it varies independently of other reserves in the network. This weak covariation in the performance of individual reserves dampens the temporal variance in the recruitment contribution of the aggregate network of marine reserves (CV = 0.26).
Fig. 2.
Variable and asynchronous performance of no-take marine reserves. (A) Our time series of larval dispersal patterns indicate temporal fluctuations in the contribution of no-take marine reserves to local recruitment in the Keppel Islands (colored lines). However, when combined (gray bars), the four reserves generate between 26 and 58% of all local recruitment for any given cohort (C1–C6). (B). Boxplots of the relative performance of each reserve indicate they contribute unevenly to local recruitment. The median contribution of individual reserves ranges from 5 to 15% and reveals an extremely high degree of variance in their performance through time. Collectively, the median contribution of the aggregate reserve network is higher and less variable.
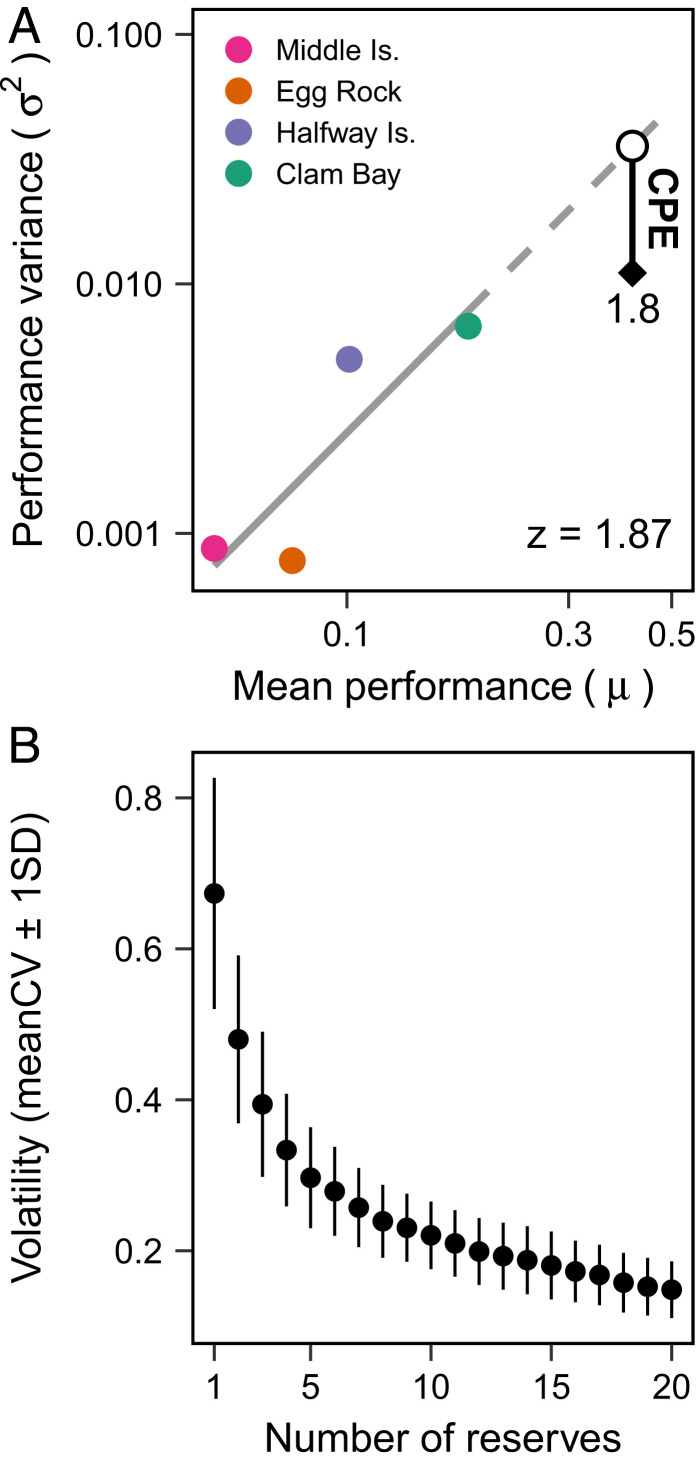
The high variance in the performance of individual reserves and the negative covariance within the aggregate network generates a substantial portfolio effect that reduces the overall volatility in the larval contributions of the reserve network. If we measure the mean and variance in the local recruitment contribution of reserves across all six cohorts, we see that reserves with greater average contribution to local recruitment also have greater variance among discrete cohorts (Fig. 3A and Methods). Such mean–variance scaling relationships are common in ecological systems and typically follow a power law with an exponent z lower than 2. A portfolio effect indicates the temporal variance of the aggregate components is less than predicted based on its average performance over time. Here, the observed variance in the aggregate network of reserves in the Keppel Islands is 1.8 (95% CI, 1.2–2.4) times less than predicted based on the mean-variance relationship of individual reserves (Fig. 3A). This indicates that the combined performance of the four reserves exhibits a substantial portfolio effect to minimize volatility in the recruitment contribution of individual reserves.
Fig. 3.
The connectivity portfolio effect (CPE) reduces volatility in reserve performance. (A) We calculate the CPE from the temporal mean (μ) and variance (σ2) of each reserve’s contribution to local recruitment of coral grouper on log–log axes and extrapolate the mean–variance relationships (z) to the aggregate mean contribution of the reserve network across the six discrete cohorts in the Keppel Islands. The difference between the predicted (circle) and observed variability (diamond) represents the strength of the connectivity portfolio effect. (B) Based on the measured performance of reserves in the Keppel Islands for each cohort (Fig. 2B), we estimate the coefficient of variation (CV) in the aggregate recruitment contribution of reserves in a network. We use a bootstrap resampling procedure to estimate the mean and SD of CV, which reflects the volatility in reserve performance (Methods).
We can extend these findings by using the observed variation in the performance of marine reserves in the Keppel Islands to estimate the shape and strength of the connectivity portfolio effect with increasing number of marine reserves. From the recruitment contribution of reserves in the Keppel Islands, we estimate that the volatility in the local recruitment contribution of a single marine reserve would be on average 0.66 ± 0.16 SD (Fig. 3B). This indicates that the supply of juvenile fish from a single reserve can fluctuate widely between recruitment cohorts. By bootstrap resampling from the set of reserves, we predict that volatility will fall rapidly with every additional reserve in the network (Fig. 3B), so that it is halved with only four reserves (0.34 ± 0.08 SD). Therefore, by dampening the volatility of their aggregate contribution to recruitment, networks of marine reserves increase temporal stability in the replenishment of local populations.
Discussion
Consistent with previous findings (12, 13), our results reveal no-take marine reserves generate considerable larval subsidies to neighboring habitats and are responsible for generating a disproportionally large proportion of local recruitment. However, our unique temporal dataset also reveals high spatial and temporal variability in connectivity patterns with extreme fluctuations in the recruitment contribution of individual reserves through time. Furthermore, scale dependency in the performance of marine reserves indicates that large mean contributions to local recruitment are also associated with larger fluctuations in their performance. While individual no-take marine reserves clearly enhance long-term recruitment in the island group, the benefits of a single reserve are spatially and temporally unpredictable.
Despite the volatility in larval dispersal patterns, asynchrony in the larval supply from reserves promotes the temporal stability of local recruitment patterns in the Keppel Islands. The presence of a portfolio effect from the aggregate performance of the network of reserves effectively dampens temporal fluctuations in larval supply to yield previously unrecognized stabilizing benefits that ensure a consistent source of local recruitment. In doing so, networks of no-take marine reserves minimize the risk of recruitment failure to local fisheries and promote positive ecological and socioeconomic values beyond the simple increase in fish biomass and larval subsidies (6, 7, 22–24).
Portfolio effects are common to a variety of ecological systems where demographic and environmental processes fluctuate asynchronously or are negatively correlated over time (34). The connectivity portfolio effect is analogous to other ecological portfolio effects in that it is driven by a highly stochastic process: larval dispersal. The successful dispersal and recruitment of marine larvae depend on both behavioral and physical processes (16, 17, 20, 21), which creates uncertainty in connectivity patterns between coral reef habitats. We therefore expect to see evidence of the connectivity portfolio effect in all marine populations regulated by larval exchange.
Our results are based on one of the most intensive and extensive genetic parentage assignment datasets available (47), but positive parentage assignments still only represent a subsample of the recruitment occurring at each location. Although our sampling of juvenile fish was well distributed among reefs, the presence of uncorrelated sampling noise would augment the strength of the connectivity portfolio effect; however, it does not create it. We would strongly expect connectivity patterns to contain the negatively correlated structure that drives portfolio effects. Physical drivers of oceanographic flows contain large stochastic components (16–19), and advective current structures will naturally create negative connectivity correlations in a reef matrix.
Since the supply of larval offspring is linked to the size and structure of populations (10, 11), we also anticipate the connectivity portfolio effect works in conjunction with subpopulation portfolio effects previously described in marine fishes (34). When fluctuations in population size lead to fluctuations in larval supply, these are likely to accentuate the spatial and temporal variance of recruitment patterns. Networks of no-take marine reserves, which accumulate larger biomass of exploited species (8, 9) and generate substantial larval subsidies, may therefore effectively mitigate local fluctuations in spawning stock biomass, larval supply, and rates of population replenishment.
Our findings demonstrate that effective reserve networks take advantage of a connectivity portfolio effect that mitigates temporal volatility in larval supply to ensure the stability of recruitment dynamics, with potential long-term sustainability benefits for exploited fish stocks. Replication of no-take marine reserves within networks provides an essential hedge against uncertainty in the dynamic processes that sustain fisheries stocks (48, 49) and may moderate the effects of large-scale climatic disturbances (1–3) that are projected to escalate as global warming progresses (39, 40, 42).
Methods
Sample Collections and Cohort Identification.
This study focuses on the bar-cheek coral grouper (Plectropomus maculatus, Serranidae). Like most species of groupers, it is heavily targeted by commercial, recreational, and subsistence fishers throughout the Indo-Pacific region (50). We sampled adult and juvenile coral grouper from fringing coral reefs in the Keppel Islands between September 2007 and April 2013. We sampled adult fish intensively from reefs in four focal no-take marine reserves, and juvenile fish on all protected and fished reefs in the island group, with effort distributed proportionally to the area of each reef (Fig. 1A). We measured the size of each fish and aged juvenile fish from sagittal otolith to determine the age–length relationship for juvenile P. maculatus in the Keppel Islands (Age = Total Length × 1.159 – 4.283, R2 = 0.81) and estimate the date of spawn (SI Appendix, Fig. S1). We defined six discrete recruitment cohorts in the data, which we believe correspond to six different adult spawning events (SI Appendix, Table S2). A spawning event was defined as a unimodal pulse of reproduction, which resulted in the observed dispersal and recruitment patterns.
Underwater Visual Census Surveys and Population Size Estimates.
We conducted underwater visual census (UVC) surveys of P. maculatus populations to quantify their density, biomass, and length–frequency distributions on all focal reserve and fished reefs. These UVC surveys were part of a broader long-term reef biodiversity monitoring program that was initiated in the Keppel Island group in 2002 (see ref. 45 for a detailed methodology). For the present study, we conducted standard UVC surveys along 50 × 6-m belt transects on reef slope, crest, and flat habitat-strata for nine monitoring sites on focal reserve reefs (Middle Island, Clam Bay, Halfway Island, and Egg Rock) prior to each round of sample collection. To quantify reef habitat areas, we used a combination of high-resolution satellite imagery and stratified habitat surveys (reef slope, crest, flat) to map reef habitats areas within each focal reserve. All spatial analyses were conducted using ArcGIS (ESRI). We estimated total population size for each focal reserve, and the proportion of adults sampled, by scaling up length-specific P. maculatus density estimates to the total area within each reef habitat strata (SI Appendix, Table S1) (12, 51).
Parentage Analysis.
We first extracted genomic DNA from ∼2 mm2 of fin or muscle tissue and screened each individual at 23 microsatellite loci (52). We identified parent–offspring pairs in two periods, with each period composed of three successive cohorts. Period 1 included all sampled juvenile fish that recruited to reefs in the Keppel Islands between September 2007 and March 2009 (n = 559) and all sampled adult fish that were mature during the same period (n = 686), including large adults (>500 mm) captured between September 2011 and April 2013. Period 2 included juveniles that recruited between September 2011 and April 2013 (n = 454) and adults that were mature during that period (n = 891), including individuals captured between September 2007 and March 2009. For each period, we used a maximum-likelihood approach implemented in the software program FAMOZ (53, 54) to reveal parent–offspring relationships in our samples.
Reserve Performance.
In the context of this study, the performance of a single no-take marine reserve is measured by its relative contribution to local recruitment across all sampled reefs in the island group in each cohort. Since we sampled only a fraction of all reproductively mature adults in each reserve (SI Appendix, Table S2), the observed number of assigned juveniles (Fig. 1D) represents only a fraction of a reserve’s contribution to local recruitment. In order to compare the performance of each reserves across different cohort, we estimated the number of juveniles we would have assigned to each reserve had all adults been sampled in the populations. Since we can assign parentage to fathers, mothers, or both, the relationship between the number assignments and the proportion of parents sampled is nonlinear (12). The expected recruitment contribution (R) accounts for the number of assigned juveniles given the proportion of adults sampled from reserve i so that:
where n represents the number of assigned juveniles and P is the proportion of sampled adults in the focal reef or reefs. We assume that all adult P. maculatus within each reserve boundary have an equal probability of contributing to local recruitment and that our sample of juveniles represents a random sample of recruitment in the study area for each cohort. We then estimate the percent contribution to local recruitment contribution of reserves by standardizing for the number juveniles sampled in each cohort.
Measuring the Mean–Variance Corrected Portfolio Effect.
We correct our estimates of the portfolio effect by accounting for the natural scale dependence of population processes. In financial systems, the variance in returns scales linearly with the mean return (since every stock yields the same dividend). In ecological systems, by contrast, larger populations exhibit lower variability than we would expect from proportional scaling. Such mean–variance scaling is common across ecological systems and predicts that the temporal variance of individual components (σ2) increases with the mean value (μ) according to a power-law relationship with exponent (38, 55). Using the mean–variance exponent fit to the sampled reserves , we predict the average recruitment contribution and variance of a single reserve with a mean output equal to the sum of the mean outputs of the four individual reserves. We compare the predicted CV of this hypothetical single reserve to the observed CV of the contributions made by the portfolio of four reserves , to calculate the strength of the connectivity portfolio effect. We analyzed the sensitivity of our results to the definition of cohorts and found that the results were almost identical.
A bootstrap resampling protocol estimates the volatility in the recruitment contribution of an arbitrary number of reserves. First, we create a set of reserves by randomly resampling (with replacement) from the observed recruitment contribution timeseries of the reserves in the Keppel Islands . We repeat this procedure 100 times for each value of to estimate the mean and SD of the CV. This method assumes that recruitment timeseries from additional reserves would have similar correlation structure to those observed in the Keppel Islands.
Supplementary Material
Acknowledgments
We acknowledge this research took place on the traditional land and sea country of the Woppaburra people and recognize their continuing connection to land, water, and community in the Keppel Islands. We thank our field assistants for support with sample collection, and Lisa Boström Einarsson, Darren Cameron, Graeme Cummings, and Rachel Pears for their thoughtful comments on an earlier version of this manuscript. This work was funded by an Australian Research Council (ARC) Discovery Early Career Research Award to H.B.H. (DE160101141); an ARC Discovery Grant to G.P.J., M.B., and M.L.B. (DP190103056); and an ARC Future Fellowship to M.B. (FT170100274). Additional support was provided by the ARC Centre of Excellence for Coral Reef Studies (Grant CE140100020) and King Abdullah University of Science and Technology baseline research funds.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920580117/-/DCSupplemental.
Data and Code Availability.
All study data are included in the article and SI Appendix. All R scripts for the calculation of the CPE are available in SI Appendix.
References
- 1.Emslie M. J. et al., Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park. Curr. Biol. 25, 983–992 (2015). [DOI] [PubMed] [Google Scholar]
- 2.MacNeil M. A. et al., Recovery potential of the world’s coral reef fishes. Nature 520, 341–344 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Roberts C. M. et al., Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. U.S.A. 114, 6167–6175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubchenco J., Grorud-Colvert K., OCEAN. Making waves: The science and politics of ocean protection. Science 350, 382–383 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Worm B., Marine conservation: How to heal an ocean. Nature 543, 630–631 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Ban N. C. et al., Well-being outcomes of marine protected areas. Nat. Sustain. 2, 524–532 (2019). [Google Scholar]
- 7.Watson J. E. M., Dudley N., Segan D. B., Hockings M., The performance and potential of protected areas. Nature 515, 67–73 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Edgar G. J. et al., Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Gill D. A. et al., Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543, 665–669 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Barneche D. R., Robertson D. R., White C. R., Marshall D. J., Fish reproductive-energy output increases disproportionately with body size. Science 360, 642–645 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Marshall D. J., Gaines S., Warner R., Barneche D. R., Bode M., Underestimating the benefits of marine protected areas for the replenishment of fished populations. Front. Ecol. Environ. 73, 1651–413 (2019). [Google Scholar]
- 12.Harrison H. B. et al., Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023–1028 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Le Port A. et al., Temperate marine protected area provides recruitment subsidies to local fisheries. Proc. Royal Soc. B 284, 20171300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Aloia C. C., Bogdanowicz S. M., Majoris J. E., Harrison R. G., Buston P. M., Self-recruitment in a Caribbean reef fish: A method for approximating dispersal kernels accounting for seascape. Mol. Ecol. 22, 2563–2572 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Almany G. R. et al., Larval fish dispersal in a coral-reef seascape. Nat. Ecol. Evol. 1, 148 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Cowen R. K., Sponaugle S., Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 1, 443–466 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Bode M. et al., Successful validation of a larval dispersal model using genetic parentage data. PLoS Biol. 17, e3000380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James M. K., Armsworth P. R., Mason L. B., Bode L., The structure of reef fish metapopulations: Modelling larval dispersal and retention patterns. Proc. Biol. Sci. 269, 2079–2086 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowen R. K., Paris C. B., Srinivasan A., Scaling of connectivity in marine populations. Science 311, 522–527 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Sale P. F. et al., Critical science gaps impede use of no-take fishery reserves. Trends Ecol. Evol. 20, 74–80 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Almany G. R. et al., Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28, 339–351 (2009). [Google Scholar]
- 22.Gell F., Roberts C., Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 18, 448–455 (2003). [Google Scholar]
- 23.Hastings A., Botsford L. W., Comparing designs of marine reserves for fisheries and for biodiversity. Ecol. Appl. 13, S65–S70 (2003). [Google Scholar]
- 24.Gaines S. D., White C., Carr M. H., Palumbi S. R., Designing marine reserve networks for both conservation and fisheries management. Proc. Natl. Acad. Sci. U.S.A. 107, 18286–18293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levins R., Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Am. 15, 237–240 (1969). [Google Scholar]
- 26.Chesson P. L., Warner R. R., Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 117, 923–943 (1981). [Google Scholar]
- 27.Chesson P., Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000). [Google Scholar]
- 28.Hanski I., Metapopulation dynamics. Nature 396, 41–49 (1998). [Google Scholar]
- 29.Bascompte J., Possingham H., Roughgarden J., Patchy populations in stochastic environments: Critical number of patches for persistence. Am. Nat. 159, 128–137 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Kritzer J. P., Sale P. F., Metapopulation ecology in the sea: From Levins’ model to marine ecology and fisheries science. Fish Fish. 5, 131–140 (2004). [Google Scholar]
- 31.Vergara P. M., Saravia-Zepeda A., Castro-Reyes N., Simonetti J. A., Is metapopulation patch occupancy in nature well predicted by the Levins model? Popul. Ecol. 58, 335–343 (2016). [Google Scholar]
- 32.Van Schmidt N. D., Beissinger S. R., The rescue effect and inference from isolation-extinction relationships. Ecol. Lett. 23, 598–606 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Hastings A., Botsford L. W., Equivalence in yield from marine reserves and traditional fisheries management. Science 284, 1537–1538 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Schindler D. E., Armstrong J. B., Reed T. E., The portfolio concept in ecology and evolution. Front. Ecol. Environ. 13, 257–263 (2015). [Google Scholar]
- 35.White J., Botsford L., Hastings A., Largier J., Population persistence in marine reserve networks: Incorporating spatial heterogeneities in larval dispersal. Mar. Ecol. Prog. Ser. 398, 49–67 (2010). [Google Scholar]
- 36.Schindler D. E. et al., Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Thibaut L. M., Connolly S. R., Sweatman H. P. A., Diversity and stability of herbivorous fishes on coral reefs. Ecology 93, 891–901 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Anderson S. C., Cooper A. B., Dulvy N. K., Ecological prophets: Quantifying metapopulation portfolio effects. Methods Ecol. Evol. 4, 971–981 (2013). [Google Scholar]
- 39.Ando A. W., Mallory M. L., Optimal portfolio design to reduce climate-related conservation uncertainty in the Prairie Pothole Region. Proc. Natl. Acad. Sci. U.S.A. 109, 6484–6489 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ando A. W. et al., When portfolio theory can help environmental investment planning to reduce climate risk to future environmental outcomes—and when it cannot. Conserv. Lett. 11, e12596 (2018). [Google Scholar]
- 41.Beyer H. L. et al., Risk‐sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 11, e12587 (2018). [Google Scholar]
- 42.Runting R. K. et al., Reducing risk in reserve selection using Modern Portfolio Theory: Coastal planning under sea-level rise. J. Appl. Ecol. 55, 2193–2203 (2018). [Google Scholar]
- 43.Pelc R. A., Warner R. R., Gaines S. D., Paris C. B., Detecting larval export from marine reserves. Proc. Natl. Acad. Sci. U.S.A. 107, 18266–18271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markowitz H., Portfolio selection. J. Finance 7, 77–91 (1952). [Google Scholar]
- 45.Williamson D. H., Ceccarelli D. M., Evans R. D., Jones G. P., Russ G. R., Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol. Evol. 4, 337–354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thibaut L. M., Connolly S. R., Understanding diversity-stability relationships: Towards a unified model of portfolio effects. Ecol. Lett. 16, 140–150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bode M., Williamson D. H., Harrison H. B., Outram N., Jones G. P., Estimating dispersal kernels using genetic parentage data. Methods Ecol. Evol. 9, 490–501 (2018). [Google Scholar]
- 48.McCook L. et al., Management under uncertainty: Guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 28, 353–366 (2009). [Google Scholar]
- 49.Pauly D. et al., Towards sustainability in world fisheries. Nature 418, 689–695 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Sadovy de Mitcheson Y. et al., Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 14, 119–136 (2013). [Google Scholar]
- 51.Williamson D. H. et al., Large-scale, multidirectional larval connectivity among coral reef fish populations in the Great Barrier Reef Marine Park. Mol. Ecol. 25, 6039–6054 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Harrison H. B. et al., Validation of microsatellite multiplexes for parentage analysis and species discrimination in two hybridizing species of coral reef fish (Plectropomus spp., Serranidae). Ecol. Evol. 4, 2046–2057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerber S., Chabrier P., Kremer A., FAMOZ: A software for parentage analysis using dominant, codominant and uniparentally inherited markers. Mol. Ecol. Notes 3, 479–481 (2003). [Google Scholar]
- 54.Marshall T. C., Slate J., Kruuk L. E., Pemberton J. M., Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Tilman D., Biodiversity: Population versus ecosystem stability. Ecology 77, 350–363 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix. All R scripts for the calculation of the CPE are available in SI Appendix.