Summary
Background
Muscle biopsy is the gold standard for diagnosis of mitochondrial disorders because of the lack of sensitive biomarkers in serum. Fibroblast growth factor 21 (FGF-21) is a growth factor with regulatory roles in lipid metabolism and the starvation response, and concentrations are raised in skeletal muscle and serum in mice with mitochondrial respiratory chain deficiencies. We investigated in a retrospective diagnostic study whether FGF-21 could be a biomarker for human mitochondrial disorders.
Methods
We assessed samples from adults and children with mitochondrial disorders or non-mitochondrial neurological disorders (disease controls) from seven study centres in Europe and the USA, and recruited healthy volunteers (healthy controls), matched for age where possible, from the same centres. We used ELISA to measure FGF-21 concentrations in serum or plasma samples (abnormal values were defined as >200 pg/mL). We compared these concentrations with values for lactate, pyruvate, lactate-to-pyruvate ratio, and creatine kinase in serum or plasma and calculated sensitivity, specificity, and positive and negative predictive values for all biomarkers.
Findings
We analysed serum or plasma from 67 patients (41 adults and 26 children) with mitochondrial disorders, 34 disease controls (22 adults and 12 children), and 74 healthy controls. Mean FGF-21 concentrations in serum were 820 (SD 1151) pg/mL in adult and 1983 (1550) pg/mL in child patients with respiratory chain deficiencies and 76 (58) pg/mL in healthy controls. FGF-21 concentrations were high in patients with mitochondrial disorders affecting skeletal muscle but not in disease controls, including those with dystrophies. In patients with abnormal FGF-21 concentrations in serum, the odds ratio of having a muscle-manifesting mitochondrial disease was 132·0 (95% CI 38·7–450·3). For the identification of muscle-manifesting mitochondrial disease, the sensitivity was 92·3% (95% CI 81·5–97·9%) and specificity was 91·7% (84·8–96·1%). The positive and negative predictive values for FGF-21 were 84·2% (95% CI 72·1–92·5%) and 96·1 (90·4–98·9%). The accuracy of FGF-21 to correctly identify muscle-manifesting respiratory chain disorders was better than that for all conventional biomarkers. The area under the receiver-operating-characteristic curve for FGF-21 was 0·95; by comparison, the values for other biomarkers were 0·83 lactate (p=0·037, 0·83 for pyruvate (p=0·015), 0·72 for the lactate-to-pyruvate ratio (p=0·0002), and 0·77 for creatine kinase (p=0·013).
Interpretation
Measurement of FGF-21 concentrations in serum identified primary muscle-manifesting respiratory chain deficiencies in adults and children and might be feasible as a first-line diagnostic test for these disorders to reduce the need for muscle biopsy.
Funding
Sigrid Jusélius Foundation, Jane and Aatos Erkko Foundation, Molecular Medicine Institute of Finland, University of Helsinki, Helsinki University Central Hospital, Academy of Finland, Novo Nordisk, Arvo and Lea Ylppö Foundation.
Introduction
Mitochondrial diseases constitute a group of metabolic disorders that affect people of all ages. The estimated prevalence in England and Australia is around one per 5000 of the general population.1–3 The clinical features vary from one to multiple affected organs, and the disorders can occur with or without lactic acidosis, in neonates, children, and adults.4–6
Serum biomarkers for mitochondrial disorders include lactate, pyruvate, aminoacids, creatine kinase, and possibly serum creatine.7,8 The ratio of lactate to pyruvate is sometimes raised, especially in children and in adults with encephalomyopathies, but is frequently normal in patients with progressive myopathies. Creatine kinase concentrations in serum are occasionally increased in people with mitochondrial diseases, mostly in the disorders that cause muscle damage. Nevertheless, histological, biochemical, and mitochondrial DNA analyses of muscle biopsy samples remain the gold standard diagnostic methods.7 Biopsy is invasive, prone to complications, and expensive; surgical biopsy in children requires general anaesthesia and, together with the operation and the analyses, costs can be thousands of euros per biopsy. Furthermore, biochemical analysis of the mitochondrial respiratory chain (comprising the ATP-producing enzyme complexes I–IV) is technically challenging and results vary between centres.9 The activities of complexes IV (cytochrome-c oxidase [COX]) and II (succinate dehydrogenase) can be analysed by histochemistry, but those of the other complexes cannot. Additional approaches for the diagnosis of mitochondrial respiratory chain deficiencies would, therefore, be useful.
We have reported increased expression of fibroblast growth factor 21 (FGF-21), a circulating hormone-like cytokine and an established regulator of lipid metabolism and starvation response,10–12 in the muscles of mice with mitochondrial myopathies.13 The increased expression correlated with the number of COX-negative fibres, which is associated with disease severity.13 Furthermore, the mice had raised concentrations of FGF-21 in serum and showed signs of a chronic starvation response, including mobilisation of lipids from adipose tissue and the liver, which is similar to that seen in mice overexpressing FGF-21.10 In this retrospective diagnostic study, we measured FGF-21 concentrations in serum of patients with metabolic and muscle disorders to assess whether it is a feasible biomarker for human respiratory chain deficiencies.
Methods
Participants
Patients who had been diagnosed by the researchers as having a mitochondrial disorder were enrolled from one of seven medical centres involved in mitochondrial disease diagnosis in Finland, Netherlands, Sweden, Norway, Estonia, and the USA, in 2002–11. Eligible patients had a mitochondrial disease diagnosed by DNA analysis. The control group consisted of retrospectively identified adult and child patients with non-mitochondrial neurological disorders, with an emphasis on muscle disorders (disease controls), and prospectively recruited healthy adult and child volunteers or children who had undergone investigation for disorders of non-mitochondrial origin (healthy controls) in the Helsinki, Nijmegen and Gothenburg centres. We matched all controls and patients for age whenever possible (webappendix p 1).
As patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) have exceptionally low body-mass index (BMI), we tested the effect of body composition on FGF-21 concentrations. We assessed serum samples provided by KHP from eight obese adults (BMI 35–49 kg/m2) and from five volunteer patients with anorexia nervosa (BMI 15–17 kg/m2) from the clinic of AR, none of whom was in the study. As only FGF-21 concentrations in serum were measured in these individuals, they were not included in the control group.
The study was approved by the institutional ethics review boards of all centres (coordinator Helsinki University Central Hospital, #43/13/03/04/2008 and 74/13/03/00/09) and the participants gave written informed consent.
Procedures
We analysed serum or plasma samples that had previously been collected from patients and disease controls or that were specifically collected for this study in 2009–11, and those collected from controls at enrolment to assess metabolic phenotype and to measure mitochondrial biomarkers. Samples were stored at −80°C until analysis. We measured FGF-21 concentration in duplicate samples by ELISA (BioVendor, Brno, Czech Republic), according to the manufacturer’s instructions. The values from every assay were compared with reference FGF-21 concentrations, and results were extrapolated from a linear standard curve. Independent replication of measurements was done for all samples and the average of four values was reported.
To investigate whether FGF-21 concentrations would be stable over time, we tested samples from four randomly selected patients contacted before clinic visits (one with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes [MELAS], one with Alpers’ encephalopathy, and two with mitochondrial myopathy) taken 1·0–1·5 years after the initial serum samples were collected.
All analyses were done by specifically trained scientists (JME and HT) who were unaware of group allocations. Values were matched to diagnoses after interpretation of the results.
Needle biopsy of muscle was done in two patients with MELAS and three with mitochondrial recessive ataxia syndrome (MIRAS) who volunteered to undergo a second biopsy for the study, and in six controls. Samples were snap-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR was done on complementary DNA generated from 1 μg total RNA with a DyNAmo Flash SYBR Green qPCR Kit (Thermo Fisher Scientific, Waltham, MA, USA) on an CFX96 system (Bio-Rad Hercules, CA, USA). Primers 5′-ACCTGGAGATCAGGGAGGAT-3′ and 5′-AGTGGAGCGATCCATACAGG-3′ were used to analyse FGF-21, and 5′-CCTGGCACCCAGCACAAT-3′ and 5′-GGGCCGGACTCGTCATAC-3′ were used to analyse β actin as a control gene. We calculated the proportion of all fibres in the muscle biopsy that were COX negative.
See Online for webappendix
Liver has been reported to be a major source of FGF-21.14,15 Therefore, we retrospectively collected results of liver function tests for all patients with available data and assessed whether FGF-21 values correlated with liver dysfunction.
Statistical analysis
We used the Mann-Whitney U test to compare FGF-21 concentrations between patients and controls. Spearman’s rank correlation coefficient was applied to investigate the associations between FGF-21 and other biomarkers, BMI, and the proportion of COX-negative muscle fibres. We did multivariate regression analyses to test whether any biomarker was independently associated with FGF-21 concentrations. Data are expressed as mean (SD).
Biomarker concentrations were classified as being either normal or abnormal; for FGF-21 the cutoff was based on the concentrations in 95% of controls, and for other markers cutoffs were based on the reference ranges of the study centres. The following cutoff values were used: FGF-21 200 pg/mL; lactate 2·4 mmol/L; pyruvate 70 μmol/L; lactate-to-pyruvate ratio 30; and creatine kinase 270 U/L in children younger than 2 years, 220 U/L in children aged 3–15 years, 270 U/L in boys aged 16–17 years, 150 U/L in girls aged 16–17 years, 400 U/L in men aged 18–49 years, 282 U/L for men aged 50 years or older, and 210 U/L for women older than 18 years (for liver cutoff values see webappendix p 5). For each cutoff we used logistic regression analysis to calculate odds ratios (ORs) for having a mitochondrial disease compared with controls.
We assessed the sensitivity, specificity, and positive and negative predictive values ofeach biomarker to distinguish correctly mitochondrial diseases from non-mitochondrial disease states. We also compared areas under receiver-operating-characteristic curves (AUC) for biomarkers to assess the diagnostic usefulness of FGF-21 against each of the other biomarkers in turn and to compare the diagnostic usefulness of all biomarkers in the same model, with FGF-21 as the reference.16 The data for sensitivity (true-positive rate) and 100% specificity (false-positive rate) were plotted against each other. For a more comprehensive comparison of the tests, we analysed AUC with the biomarkers on a continuous scale.
We did all statistical analyses with Stata (version 11.0) and PRISM 5. Two-sided p values were used with a significance level of 0·05.
Role of the funding source
The sponsors of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We assessed samples from 67 patients (41 adults and 26 children) with confirmed mitochondrial disorder. 63 patients had diagnoses confirmed by DNA analysis; four children were included who had evidence of severe respiratory chain deficiency (residual acivity of ≥1 respiratory chain complexes <25% of mean control values) on biochemical analysis or typical histological findings for muscle dystrophies and inclusion body myositis. Patients’ characteristics are summarised in table 1 and table 2. None of the patients was receiving a ketogenic diet at the time of sampling. To the disease control group we recruited 34 patients (22 adults—19 with a genetic diagnosis and three with typical clinical or histological muscle findings— and 12 children). To the healthy control group we recruited 74 individuals: 49 adult and seven child volunteers, and 18 children who had undergone investigation for non-mitochondrial disorders (type 1 diabetes mellitus [n=5], nephropathy [n=4], early puberty [n=3], delayed growth [n=4], suspected endocrinopathy [n=1], and narcolepsy [n=1]). We tested 164 serum and 11 plasma samples (table 1, table 2, and webappendix p 1).
Table 1:
Clinical symptoms and signs, morphological and biochemical findings, and serum FGF-21 concentrations in adults with mitochondrial disorders and adult disease controls, listed by participants’ numbers
| Sex | Age at onset (years) | Age at serum sampling (years) | Age at death (years) | BMI (kg/m2) | Main clinical features | Lactate (mmol/L) | Pyruvate (μmol/L) | Lactate: pyruvate | CK (U/L) | Liver dys-function* | Muscle histology | Biochemical analysis | DNA diagnosis | FGF-21 (pg/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle-manifesting mitochondrial disorders | |||||||||||||||
| 1 | F | 42 | 55 | Alive | 18.3 | Cardiomyopathy, diabetes mellitus, hearing impairment | 2.4 | 97† | 25 | 140 | No | 30% COX-neg | CI+CIV 60% | mtDNA, tRNA-Leu, 3243A→G, 82% muscle | 1762† |
| 2 | F | 31 | 47 | Alive | 24.0 | Diabetes mellitus, hearing impairment, GI, myalgia | 2.3 | ND | ND | 67 | ND | ND | ND | mtDNA, tRNA-Leu, 3243A→G | 641† |
| 3 | F | NA | 79 | Alive | 18.3 | Deafness, myalgia, cataract | 1.6 | ND | ND | 130 | ND | 5% COX-neg | ND | mtDNA, tRNA-Leu, 3243A→G | 624† |
| 4 | M | 40 | 46 | Alive | 20.4 | Diabetes mellitus, hearing impairment, epilepsy, myopathy | 5.7† | ND | ND | 408† | ND | 4% COX-neg | ND | mtDNA, tRNA-Leu, 3243A→G, 31% blood | 580† |
| 5 | F | 31 | 47 | Alive | 17.1 | Short stature, PEO, myopathy, diabetes mellitus, GI | 1.3 | ND | ND | 45 | ND | ND | ND | mtDNA, tRNA-Leu, 3243A→G | 572† |
| 6 | M | 48 | 53 | Alive | 25.1 | Cardiomyopathy, myopathy, hearing impairment, diabetes mellitus, RR | 1.6 | 72† | 22 | 143 | No | ND | ND | mtDNA, tRNA-Leu, 3243A→G, 17% blood | 562† |
| 7 | F | NA | 67 | Alive | 18.9 | Diabetes mellitus, hearing impairment | 2.5† | 97† | 26 | 62 | No | ND | ND | mtDNA, tRNA-Leu, 3243A→G, in son | 395† |
| 8 | M | 35 | 40 | Alive | 21.2 | Hearing impairment, epilepsy, ataxia, strokes, cardiomyopathy, myopathy, neuropathy | 5.6† | ND | ND | 2546† | ND | 20% COX-neg | ND | mtDNA, tRNA-Leu, 3243A→G | 289† |
| 9 | M | 30 | 52 | Alive | 21.0 | Diabetes mellitus, hearing impairment, ataxia, ptosis | 3.9† | 136† | 29 | 65 | No | 7% COX-neg | ND | mtDNA, tRNA-Leu, 3243A→G, 77% urine | 289† |
| 10 | M | 38 | 39 | Alive | 22.6 | Mildly affected exercise-related myalgia, mild hearing impairment | 1.0 | ND | ND | 129 | ND | ND | ND | mtDNA, tRNA-Leu, 3243A→G | 219† |
| 11 | M | 37 | 39 | Alive | 21.0 | Headaches | 1.1 | ND | ND | 120 | No | ∼2% COX-neg | ND | mtDNA, tRNA-Leu, 3243A→G 90% in urine | 88 |
| 12 | M | 13 | 43 | Alive | NA | Exercise-induced muscle pain, aercise intolerance | 1.6 | ND | ND | 331 | ND | Myopathic, COX-intensity variable | Partial CI | mtDNA, tRNA mutation | 2125† |
| 13 | F | 50 | 65 | Alive | NA | Ptosis and restricted eye movements | 0.5 | ND | ND | 106 | No | 3% COX-neg | ND | mtDNA, tRNA mutation | 1283† |
| 14 | F | 34 | 50 | Alive | 21.9 | PEO, myopathy | 1.5 | 50 | 30 | 471† | No | 11% COX-neg | Partial CI+CIV | TWINKLE‡- dominant, 13dupAA | 659† |
| 15 | M | 30 | 49 | Alive | 28.8 | PEO, myopathy, fatty liver | 1.5 | 78† | 19 | 715† | Yes | 8% COX-neg | Partial CI+CIV | TWINKLE‡- dominant, 13dupAA | 586† |
| 16 | M | 25 | 51 | Alive | 23.0 | PEO, myopathy | 1.2 | 54 | 22 | 1300† | No | 12% COX-neg | Partial CI+CIV | TWINKLE‡- dominant, 13dupAA | 570† |
| 17 | M | 30 | 60 | Alive | NA | PEO, proximal muscle weakness in lower extremities sensory polyneuropathy | 4.2† | ND | ND | 234 | No | 5% COX-neg | ND | POLG-dominant, P.Y955C | 347† |
| 18 | M | 35 | 56 | Alive | 34.9 | PEO, myopathy | 1.2 | ND | ND | 147 | Yes | 5% COX-neg | ND | TWINKLE‡- dominant, 13dupAA | 338† |
| 19 | M | 25 | 38 | Alive | 26.7 | PEO, myopathy | 1.2 | ND | ND | 196 | No | 5% COX-neg | Partial CI+IV | TWINKLE‡- dominant, 13dupAA | 237† |
| 21 | F | 15 | 18 | 18 | 10.1 | Severe MNGIE | 4.8† | 290† | 17 | ND | ND | ND | TP 1% | TYMP homozygous, c.112G→T | 3738† |
| 22 | M | 18 | 21 | 21 | 13.3 | Severe MNGIE | 6.1† | 210† | 29 | ND | ND | Normal at age 18 years | TP 4% | TYMP homozygous, c.854T→C | 3771† |
| 23 | M | 22 | 24 | 24 | NA | Severe MNGIE | 5.0† | ND | ND | <20 | ND | ND | Elevated P-thymidine | TYMP homozygous, c.457G→A | 3750† |
| 24 | M | 31 | 32 | Alive | 13.9 | Severe MNGIE | 1.3 | 6 | ND | ND | ND | RRFs, COX-neg | TP 0% | TYMP homozygous, c.893G→A | 2323† |
| 25 | M | na | 22 | 24 | NA | Moderate MNGIE | ND | ND | ND | ND | ND | ND | TP 9.5% | TYMP homozygous, c.1327-1346del | 317† |
| 26 | M | 22 | 29 | Alive | 13.5 | Moderate MNGIE | 4.1† | 40 | 103† | 46 | ND | ND | TP 0% | TYMP homozygous, c.433G→A | 283† |
| 27 | F | 15 | 47 | 49 | 16.3 | Moderate MNGIE, diabetes mellitus | ND | ND | ND | ND | ND | RRF | TP 2% | TYMP IVS5+1G→A c715G→A | 281† |
| 28 | M | NA | 16 | Alive | NA | Moderate MNGIE | ND | ND | ND | ND | ND | ND | TP 4% | TYMP c.1319insG, c.1311G→A | 266† |
| 29 | M | 7 | 11 | Alive | 13.3 | Moderate MNGIE | ND | ND | ND | ND | ND | ND | TP 3% | TYMP c.433G>A, IVS4-1G→A | 65 |
| Mitochondrial disease with nervous system involvement | |||||||||||||||
| 30 | F | 16 | 24 | 24 | NA | MIRAS, epilepsy, terminal status epilepticus | ND | ND | ND | 234† | ND | Normal at age 19 | ND | POLG homozygous, p.W748S | 4374† |
| 31 | M | >40 | 51 | Alive | 31.3 | MIRAS | 1.7 | 69 | 25 | 89 | No | ND | ND | POLG homozygous, p.W748S+E1143G | 537† |
| 32 | F | 21 | 31 | Alive | NA | MIRAS | 1.8 | ND | ND | 115 | ND | ND | ND | POLG homozygous, p.A467T | 357† |
| 33 | M | 27 | 43 | Alive | 33.4 | MIRAS | 2.1 | 98† | 21 | 96 | No | 4.5% COX-neg | ND | POLG homozygous, p.W748S+E1143G | 291† |
| 34 | M | 32 | 51 | Alive | 25.6 | MIRAS, hearing impairment | 2.0 | 119† | 17 | 188 | No | ND | ND | POLG homozygous, p.W748S+E1143G | 246† |
| 35 | F | 20 | 22 | Alive | 22.5 | MIRAS, epilepsy, migraine, liver transplant | 1.2 | 121† | 10 | 38 | No | ND | ND | POLG homozygous, p.W748S+E1143G | 173 |
| 36 | M | 20 | 38 | Alive | 32.0 | MIRAS | 3.0† | 130† | 23 | 172 | No | 0.4% COX-neg | ND | POLG homozygous, p.W748S+E1143G | 149 |
| 37 | M | 13 | 30 | Alive | 38.7 | MIRAS | 1.9 | 94† | 20 | 263 | No | ND | ND | POLG homozygous, p.W748S+E1143G | 131 |
| 38 | M | 44 | 52 | Alive | 25.0 | MIRAS | 0.9 | 47† | 19 | 103 | No | 5.4% COX-neg | ND | POLG homozygous, p.W748S+E1143G | 116 |
| 39 | M | 38 | 49 | Alive | 21.7 | MIRAS | 1.6 | 82† | 20 | 318 | No | 3.7% COX-neg | ND | POLG homozygous, p.W748S+E1143G | 54 |
| 40 | F | 19 | 25 | Alive | 28.0 | MIRAS | 1.3 | ND | ND | 102 | No | ND | ND | POLG homozygous, p.W748S+E1143G | 50 |
| 41 | M | 28 | 41 | Alive | 28.6 | MIRAS | 2.2 | 104† | 21 | 571† | No | 0.5% COX-neg | ND | POLG homozygous, p.W748S+E1143G | 25 |
| Other disorders affecting muscle | |||||||||||||||
| 42 | F | NA | 57 | Alive | NA | Myotonic dystrophy type 2 | ND | ND | ND | 1095† | No | ND | ND | Expansion in ZNF9§ | 701† |
| 43 | M | 2 | 40 | Alive | NA | Spinal muscular atrophy type 2 | ND | ND | ND | 36 | No | ND | ND | Homozygotic deletion in SMN1 | 436† |
| 44 | F | 50 | 71 | Alive | NA | Inclusion body myositis | ND | ND | ND | 555† | No | Myositis, <1% COX-neg | ND | Unknown | 392† |
| 45 | F | 67 | 76 | Alive | NA | Inclusion body myositis | ND | ND | ND | 310† | No | Myositis, 8% COX-neg | ND | Unknown | 253† |
| 46 | F | NA | 77 | Alive | NA | Oculopharyngeal muscular dystrophy | ND | ND | ND | 38 | Yes | <1% COX-neg | ND | PABPN1 (GCG)6 expanded to (GCG)9 | 251† |
| 47 | M | 60 | 65 | Alive | NA | Motoneuron disease | ND | ND | ND | 1760† | No | Denervation myopathy, 2% COX-neg | ND | Unknown | 190 |
| 48 | F | 40 | 54 | Alive | NA | Myotonic dystrophy type 1 | ND | ND | ND | 330† | No | ND | ND | 1500 CTG repeats in DMPK | 162 |
| 49 | F | 2 | 40 | Alive | NA | Spinal muscular atrophy type 3 | ND | ND | ND | 137 | No | ND | ND | Unknown | 135 |
| 50 | F | 44 | 51 | Alive | NA | Myotonic dystrophy type 2 | 0.6 | ND | ND | 7794† | No | Dystrophy | ND | CCTG ecpansion in ZNF9§ | 131 |
| 51 | M | 6 | 39 | Alive | NA | Becker’s muscle dystrophy | ND | ND | ND | 3725† | No | ND | ND | ND | 114 |
| 52 | F | 50 | 68 | Alive | NA | Cardiomyopathy, muscle weakness of lower limbs | 1.2 | ND | ND | 42 | Yes | <1% COX-neg | ND | LMNA mutation | 98 |
| 53 | F | 30 | 54 | Alive | NA | Muscle weakness | ND | ND | ND | 937† | ND | Dystrophy | ND | CAPN3 compound heterozygous, p.F454S; p.Y745X | 81 |
| 54 | M | 83 | 85 | Alive | NA | Inclusion body myositis | 1.3 | ND | ND | 650† | Yes | Myositis 8% COX-neg | ND | Unknown | 68 |
| 55 | F | 40 | 55 | Alive | NA | Myotonic dystrophy type 2 | ND | ND | ND | 310† | No | Dystrophy | ND | CCTG expansion in ZNF9§ | 56 |
| 56 | M | NA | 65 | Alive | NA | Inclusion body myositis | ND | ND | ND | 321 | Yes | Myositis <1% COX-neg | ND | Unknown | 46 |
| 57 | F | NA | 48 | Alive | NA | Myotonic dystrophy type 2 | 0.8 | ND | ND | 175 | No | ND | ND | CCTG expansion in ZNF9§ | 42 |
| 58 | F | 58 | 71 | Alive | NA | Inclusion body myositis | 0.4 | ND | ND | 283† | No | Myositis 3% COX-neg | ND | Unknown | 40 |
| 59 | F | NA | 57 | Alive | NA | Welander’s muscular dystrophy | ND | ND | ND | 254† | No | ND | ND | Founder haplotype of Welander’s disease | 24 |
| 60 | F | 37 | 48 | Alive | NA | Myotonic dystrophy type 1 | 0.5 | ND | ND | 155 | No | ND | ND | 300 CTG repeats in DMPK | 24 |
| 61 | F | 15 | 23 | Alive | NA | Late-onset Pompe’s disease | 0.6 | ND | ND | 1807† | Yes | Gtycogen increase | ND | α-l,4-glucosidase mutations | 18 |
| 62 | F | 13 | 41 | Alive | NA | Myotonic dystrophy type 1 | ND | ND | ND | 740† | No | Dystrophy | ND | 1300 CTG repeats in DMPK | 17 |
| 63 | M | 49 | 58 | Alive | NA | Inclusion body myositis | 0.4 | ND | ND | 2114† | No | Myositis <1% COX-neg | ND | Unknown | 17 |
BMI=body-mass index. CK=creatine kinase. F=female. COX-neg=cytochrome-c-oxidase-negative muscle fibres. CI=respiratory chain complex IV deficiency. CIV=respiratory chain complex IV deficiency. mtDNA=mitochondrial DNA. tRNA=transfer RNA. ND=not determined. GI=gastrointestinal. NA=not available. M=male. RR=increased blood pressure. PEO=progressive external ophthalmoplegia. MNGIE=mitochondrial neurogastrointestinal encephalomyopathy. TP=thymidine phosphorylase. RRF=ragged red fibre. MIRAS=mitochondrial recessive ataxia syndrome.
For liver function test results see webappendix p2.
Abnormal biomarker values.
Also known as C10orf2.
Also known as CNBP.
Table 2:
Clinical symptoms and signs, morphological and biochemical findings, and serum FGF-21 concentrations in children with mitochondrial disorders, child disease controls, and children with LCHADs, listed by participants’ numbers
| Sex | Age at onset (years) | Age at serum sampling (years) | Age at death (years) | Main clinical features | Lactate (mmol/L) | Pyruvate (μmol/L) | Lactate: pyruvate | CK (U/L) | Liver dys-function* | Muscle histology | Biochemical analysis | DNA diagnosis | FGF-21 (Pg/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle-manifesting mitochondrial disorders | ||||||||||||||
| 64 | M | 0.3 | l.3† | 1.4 | Alpers-Huttenlocher syndrome | 7.2‡ | 156‡ | 46‡ | 163 | Yes | RRF, COX-neg | CI, CIV | POLG heterozygous | 4358‡ |
| 65 | M | 0.2 | 0.3† | Alive | Reversible COX deficiency | 7.0‡ | 125‡ | 56‡ | 662‡ | No | RRF, COX-neg | CIV | mtDNA, tRNA-Glu | 4248‡ |
| 66 | F | 7.0 | 10.0† | Alive | Encephalomyopatty, cardiomyopathy | 15.0‡ | 319‡ | 47‡ | 2288‡ | No | RRF, few COX - neg | CI, CIV | mtDNA, tRNA-Leu | 4232‡ |
| 67 | M | 0.1 | 0.1† | Alive | Reversible COX deficiency | 7.5‡ | 118‡ | 64‡ | 193 | No | RRF, COX-neg | CIV | mtDNA, tRNA-Glu | 4161‡ |
| 68 | M | 0.8 | NA | 2 | Psychomotor retardation, epilepsy | 20.0‡ | ND | ND | ND | ND | ND | ND | POLG p.A467T/G848S | 4126‡ |
| 69 | F | 16.0 | NA | 16 | Alpers-Huttenlocher syndrome | 2.8‡ | ND | ND | ND | ND | Critical illness myopathy | ATP 34 nmol/h | POLG c.13399G→A | 3901‡ |
| 70 | M | At birth | 0.2 | Alive | LCHAD crisis, delayed growth decreased liver function | ND | ND | ND | 294‡ | Yes | ND | ND | HADHA c.1528G→C homozygous | 3314‡ |
| 71 | M | At birth | NA | Alive | Severe myopathy | 6.3‡ | ND | ND | ND | ND | Fat droplets | CIV (23%) | Unknown | 3001‡ |
| 72 | M | 7.0 | 13.5† | Alive | Retinitis pigmentosa, short stature | 3.4‡ | 137‡ | 25 | 325‡ | No | RRF, COX-neg | CIV | mtDNA, tRNA-Arg | 2134‡ |
| 73 | F | At birth | NA | Alive | Psychomotor retardation, vision impairment | 4.7‡ | ND | ND | ND | ND | COX-neg general | CI (4%), CIV (19%) | Unknown | 1866‡ |
| 74 | M | 3.0 | 13.8† | Alive | MERRF | 6.4‡ | 84‡ | 76‡ | 229‡ | ND | RRF, COX-neg | CI, CIV | mtDNA, tRNA-Lys | 1612‡ |
| 75 | F | 0.2 | 0.3† | Alive | Reversible COX-deficiency | 3.9‡ | 73‡ | 53‡ | ND | No | RRF, COX-neg | CIV | mtDNA, tRNA-Glu | 1465‡ |
| 76 | M | 0.8 | NA | Alive | Leigh syndrome | 4.3‡ | ND | ND | ND | ND | ND | ND | NDUFS7 | 1335‡ |
| 77 | F | 6.0 | 9.9† | Alive | MELAS | 7.0‡ | 122‡ | 57‡ | 205 | no | RRF, COX-pos | CI | mtDNA, tRNA-Leu | 1090‡ |
| 78 | F | 2.0 | 3.5 | Alive | Alpers’ hepato-encephalopathy, refractoiy epilepsy, psychomotor retardation | ND | 37 | 22 | ND | No | No findings | ND | POLG p.W748S/T914P | 1062‡ |
| 79 | F | 14.0 | 17† | Alive | Mitochondrial myopathy | 5.7‡ | 118‡ | 48‡ | 247‡ | No | RR COX-neg | CI, CIV | mtDNA, tRNA-Phe | 728‡ |
| 80 | F | At birth | NA | Alive | Mitochondrial myopathy, failure to thrive LVH | 5.8‡ | ND | ND | ND | ND | Type I predominance | CI(30%), CIII (13%), CIV (62%) | Unknown | 638‡ |
| 81 | M | 0.8 | NA | Alive | Leigh syndrome | 3.2‡ | ND | ND | ND | ND | Basophilic | CI 70% | mtDNA, 3697G→A | 576‡ |
| 82 | F | At birth | NA | Alive | Psychomotor retardation | 10.5‡ | ND | ND | ND | ND | COX-neg general | CI (16%), CIV (16%) | Unknown | 442‡ |
| 83 | F | At birth | 220 | Alive | Leigh syndrome | 2.6‡ | 38 | 68‡ | ND | ND | No findings | CI | mtDNA, 10191T→C70% | 439‡ |
| 84 | M | At birth | NA | Alive | Psychomotor retardation, PEO | 2.1‡ | ND | ND | ND | ND | RRF, COX-neg | ND | mtDNA deletion | 436‡ |
| 85 | M | 0.5 | 3.3 | Alive | Leigh syndrome | 4.4‡ | 161‡ | 27 | 620‡ | no | COX-neg general | CIV | SURF1 | 335‡ |
| 86 | F | 14.0 | NA | Alive | Severe myopathy | 2.3 | ND | ND | ND | ND | Type I low | CI (7%) | LHON mtDNA, 3460G→A | 116 |
| No severe respiratory chain deficiency or non-mitochondrial disease | ||||||||||||||
| 87 | M | At birth | 2.8 | 3.0 | Progeria, cardiomyopathy, Raynaud’s syndrome, growth retardation | 1.8 | 63 | 29 | 70 | ND | Normal | Normal | LMNC mutation p.L306R | 556‡ |
| 88 | F | At birth | 14.0 | Alive | Smith-Lemly-Opitz syndrome | ND | ND | ND | ND | ND | ND | ND | KCNMA1§ | 89 |
| 89 | F | At birth | NA | Alive | Leigh | 3.3‡ | ND | ND | ND | ND | ND | CII (73%) | Unknown | 76 |
| 90 | F | 3.0 | 11.0 | Alive | Failure to thrive, cardiomyopathy | 3.4‡ | ND | ND | ND | ND | ND | ND | SLC22A5¶ | 65 |
| 91 | M | 0.5 | NA | Alive | Psychomotor retardation | 1.8 | ND | ND | ND | ND | ND | ATP 14 nmol/h | Unknown | 58 |
| 92 | M | 2.0 | 6.0 | Alive | Duchenne muscular dystrophy | ND | ND | ND | 16000‡ | ND | Dystrophy | ND | Deletion in exons 45–50 of DMD | 34 |
| 93 | M | At birth | NA | Alive | Dysmorphia | 0.8 | ND | ND | ND | ND | ND | ATP 22 nmol/h | Unknown | 32 |
| 94 | F | 1.1 | NA | Alive | Psychomotor retardation, epilepsy | 2.0 | ND | ND | ND | ND | ND | ATP 25 nmol/h | Unknown | 22 |
| 95 | M | 0.5 | 16.0 | Alive | Feeding difficulties | 2.7‡ | ND | ND | ND | ND | ND | ND | Gyrate atrophy II | 17 |
| 96 | M | 1.4 | NA | Alive | Failure to thrive | 2.0 | ND | ND | ND | ND | ND | ND | Unknown | 5 |
| 97 | F | 2.0 | NA | Alive | Slight mental retardation | 1.9 | ND | ND | ND | ND | ND | CI (91%) | Unknown | 1 |
| 98 | F | At birth | 10.0 | Alive | Myopathy | ND | ND | ND | ND | ND | ND | ND | Fatty acid oxidation | 0 |
| LCHAD in diet | ||||||||||||||
| 99 | F | At birth | 9.2 | Alive | LCHAD in diet | 1.0 | ND | ND | 437‡ | Yes | ND | ND | HADHA c.1528 G→C homozygous | 185 |
| 100 | F | At birth | 9.0 | Alive | LCHAD in diet, cardiomyopathy | 2.7‡ | ND | ND | 43740‡ | Yes | ND | ND | HADHA c.1528 G→C homozygous | 83 |
| 101 | F | At birth | 0.7 | Alive | LCHAD in diet, delayed growth, cardiomyopathy, mild retinopathy | ND | ND | ND | 390‡ | ND | ND | ND | HADHA c.1528 G→C homozygous | 76 |
CK=creatine kinase. M=male. RRF=ragged red fibre. COX-neg=cytochrome-c-oxidase-negative muscle fibres. CI=respiratory chain complex I deficiency. CIV=respiratory chain complex IV deficiency. mtDNA=mitochondrial DNA. tRNA=transfer RNA. F=female. NA=not available. ND=not determined. ATP=measurement of adenosine triphosphate and phosphocreatine production from pryruvate (reference range 42.1-81.2 nmol/h). MERRF=myoclonic epilepsy with ragged red fibres. MELAS=mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. LVH=left ventricular hypertrophy.
CIII=respiratory chain complex III deficiency. PEO=progressive external ophthalmoplegia. CII=respiratory chain complex II deficiency. LCHAD=long-chain 3 hydroxyacyl CoA dehydrogenase deficiency.
For liver function test results see webappendix p2.
Measured in plasma samples.
Abnormal biomarker values.
Previously SLO.
Also known as OCTN2.
FGF-21 antigen in serum or plasma samples was stable at room temperature over 2 days, at 4°C over 2 days, and after repeated freeze–thaw cycles. Long-term storage and handling of serum and plasma samples also did not affect the stability of FGF-21, as concentrations were consistent in samples stored at −80°C for up to 9 years and in fresh samples.
The mean concentrations of FGF-21 in serum were 70 pg/mL (range 15–309 pg/mL) in healthy adults, 114 pg/mL (42–244 pg/mL) in healthy children, and 77 pg/mL (47–329 pg/mL) in children with symptoms of non-mitochondrial origin, giving a mean FGF-21 concentration of 76 pg/mL in the healthy control group. FGF-21 concentrations in serum did not differ by age, or sex, and the period of fasting before sampling did not seem to lead to differences, although these features were not tested statistically. The FGF-21 concentrations in plasma and in serum were similar in range in controls and similarly raised in patients.
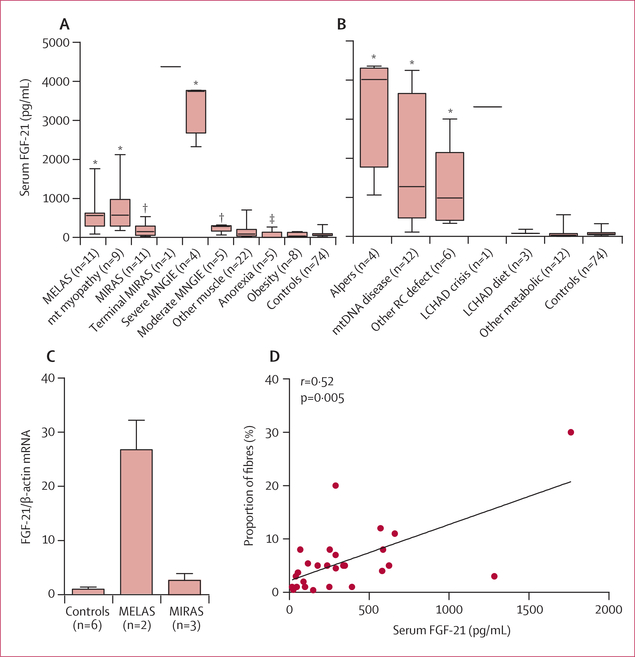
Mean FGF-21 concentrations in serum were on average ten times higher in adult patients with all types of respiratory chain deficiencies affecting muscles than in healthy controls (820 [SD 1151] pg/mL vs 76 [58] pg/mL; p<0.0001; table 1, figure 1), but were up to 57 times higher in one patient with MIRAS whose serum sample was taken during terminal status epilepticus (4374 pg/mL; table 1). FGF-21 concentrations in serum seemed to be associated with increased severity of symptoms. For example, oftwo siblings with MELAS, one with subclinical cardiomyopathy had moderate increases in FGF-21 concentration compared with the other, who had severe myopathy and cardiomyopathy (562 pg/mL vs 1762 pg/mL). Except for the patient with terminal status epilepticus, patients with MIRAS, which mainly affects the nervous system, generally had lower FGF-21 concentrations in serum than patients who had disease manifesting in skeletal muscle. Moderate MNGIE was associated with lower FGF-21 concentrations in serum than was severe disease (table 1). The adult disease controls mainly had low FGF-21 concentrations in serum (table 1). Of patients with inclusion body myositis, who frequently also have COX-negative muscle fibres, four had normal and two had slightly increased FGF-21 concentrations (table 1).
Figure 1: FGF-21 concentrations in serum in patients with mitochondrial disorders and healthy controls.
Data for patients with mitochondrial disorders are separated shown by specific disease groups. (A) Adult patients. (B) Paediatric patients. (C) FGF-21 messenger RNA expression in the skeletal muscle of six controls, two patients with MELAS, and three patients with MIRAS measured by quantitative PCR relative to β-actin. (D) Correlation between FGF-21 concentrations in serum and proportion of COX-negative fibres (n=28). Mann-Whitney U test was used to compare FGF-21 concentrations between subgroups of patients and between patients and controls, and data in A, B, and C are mean (SD) and range, and p values are for comparisons with controls. MELAS=mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. mt=mitochondrial. MIRAS=mitochondrial recessive ataxia syndrome. MNGIE=mitochondrial neurogastrointestinal encephalomyopathy. Alpers=Alpers’ encephalopathy. RC=respiratory chain. LCHAD=long-chain 3 hydroxyacyl CoA dehydrogenase deficiency. COX= cytochrome-c oxidase. *p<0·001. †p<0·01. ‡p<0·05.
Children with respiratory chain deficiencies affecting muscles (eg, deficiencies in complexes I, III, or IV, Alpers’ hepatoencephalopathy, or infantile COX deficiency) had very high FGF-21 concentrations in serum and plasma, with the average increase being 26 times the control value (mean 1983 [SD 1550] pg/mL vs 76 [58] pg/mL, p<0·0001); the greatest increase was 57 times the mean control value in a patient with Alpers-Huttenlocher syndrome (4358 pg/ml; table 2, figure 1). Patients with long-chain 3 hydroxyacyl CoA dehydrogenase deficiency had FGF-21 concentrations in the lower range of normal, although one untreated patient with this disorder who was in crisis had very high concentrations (3314 pg/mL). Most children with other non-mitochondrial muscle disorders had normal FGF-21 concentrations (table 2).
Six patients had hepatopathy (two of 23 adults and four of 13 children with mitochondrial disease in whom liver function was assessed). Among these patients, the adults had moderately increased FGF-21 concentrations in serum. By contrast, among the adults whose liver function was assessed, those with the highest FGF-21 concentrations in serum showed no signs of liver involvement. Three children with long-chain 3 hydroxyacyl CoA dehydrogenase deficiency had liver dysfunction, but only the child in crisis had a high FGF-21 concentration. A patient with Alpers’ hepatoencephalopathy had the highest FGF-21 concentration in serum of child patients and also had liver dysfunction, but most of the child patients with high FGF-21 values who were tested had no liver involvement. The difference in mean concentration between patients with and without liver dysfunction was not significant (893 [SD 1486] pg/mL vs 659 [1062] pg/mL, p=0·94; webappendix p 2). Several patients with high creatine kinase concentrations also had raised values for alanine aminotransferase and aspartate aminotransferase, which is indicative of muscle degeneration and not liver disease if other liver tests are normal. Thus, the raised concentrations of FGF-21 in serum were not explained by liver damage.
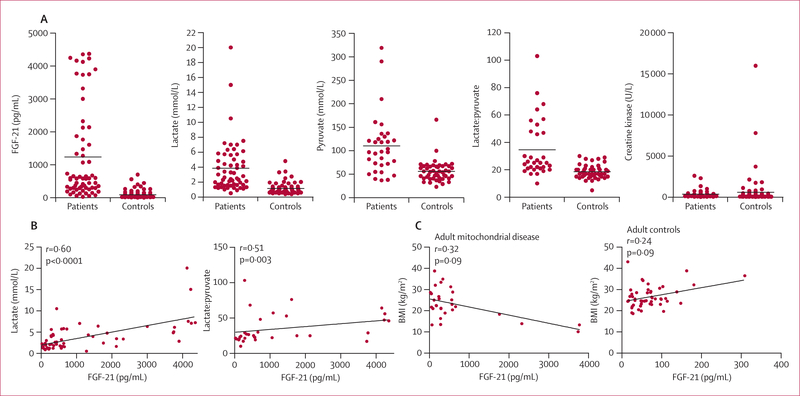
In the assessment of effects of body composition, four of the five patients with anorexia nervosa (BMI 15–17 kg/m2) had very low FGF-21 concentrations (0, 1, 3, and 7 pg/mL) and one had a slightly raised concentration (267 pg/mL). In general, FGF-21 concentrations in serum correlated negatively with BMI in patients with respiratory chain deficiencies, whereas in healthy controls the correlation was generally positive (figure 2). In the eight very obese patients (BMI 35–49 kg/m2), FGF-21 concentrations in serum were within the normal range (9–147 pg/mL, figure 1).
Figure 2: Comparison of different biomarkers in serum.
(A) Biomarker valúes in patients with mitochondrial disease and in a combined group of disease controls and healthy controls. Horizontal line indicates the mean value in each group. (B) Correlation between serum FGF-21 concentration and standard serum biomarkers in patients with mitochondrial disease (n=63 for lactate and n=32 for lactate-to-pyruvate ratio). (C) Correlation between FGF-21 concentrations in serum and BMI in patients with mitochondrial disorders (n=29) and controls (n=49). BMI=body-mass index.
FGF-21 messenger RNA was almost undetectable in the six healthy controls from whom muscle biopsy samples were tested. In the two MELAS retested patients, who had high FGF-21 concentrations in serum, muscle expression levels were 27 times higher than in the controls (figure 1). The three MIRAS patients who were retested, who had low FGF-21 concentrations in serum, had only slightly increased muscle expression levels for FGF-21.
In the 28 patients with COX-negative fibres, the proportion of affected fibres correlated significantly with FGF-21 concentrations in serum (figure 1), whereas no correlation was found between other biomarkers and COX-negative fibres.
In patients with respiratory chain deficiencies, the mean concentrations of biomarkers in serum or plasma were higher than those in healthy and disease controls: 13·5 times higher for FGF-21, 3·3 for lactate, 2·0 for pyruvate, 1·9 for the lactate-to-pyruvate ratio, and 1·7 for creatine kinase. Lactate concentration and the ratio of lactate to pyruvate correlated significantly with FGF-21 concentration in serum in patients (figure 2) but not in disease controls. Creatine kinase and pyruvate showed no correlation in any group. This result was confirmed for lactate in a multiple regression analysis with all biomarkers included simultaneously in the same model (p<0·0001), but not for lactate-to-pyruvate ratio. FGF-21 concentrations remained the same or increased with disease progression in the four patients for whom follow-up serum samples were tested (table 3).
Table 3:
Results from repeat measurement of FGF-21 in serum, listed by patients’ numbers
| Diagnosis | FGF-21 concentration in serum (pg/mL) |
Disease progression between measurements | ||
|---|---|---|---|---|
| 2009 or 2010 | 2011 | |||
| 4 | MELAS | 580 | 1048 | Active disease progression |
| 15 | Mitochondrial myopathy | 586 | 915 | Moderate disease progression |
| 16 | Mitochondrial myopathy | 570 | 514 | Stable disease |
| 78 | Alpers’ hepatoencephalopathy | 1062* | 2250 | Progressive disease, remitting status epilepticus |
Analysis done in 2010.
FGF-21 concentrations in serum were useful to differentiate patients with muscle-manifesting mitochondrial diseases from healthy and disease controls. If FGF-21 concentrations were abnormal, the odds ratio for having a muscle-manifesting respiratory chain deficiency in adults and children combined was 132·0 (95% CI 38·7–450·3). The risk was also significant but was lower for lactate (21·8, 7·3–64·9) and pyruvate (19·4, 5·5–68·6), but not for creatine kinase (2·2, 0·9–5·2; p=0·078). Among patients who had mitochondrial diseases with muscle involvement, measurement of FGF-21 concentrations in serum would have missed only 3% of the correct diagnoses, whereas lactate and pyruvate would have missed 38%, lactate-to-pyruvate ratio 54%, and creatine kinase 61%.
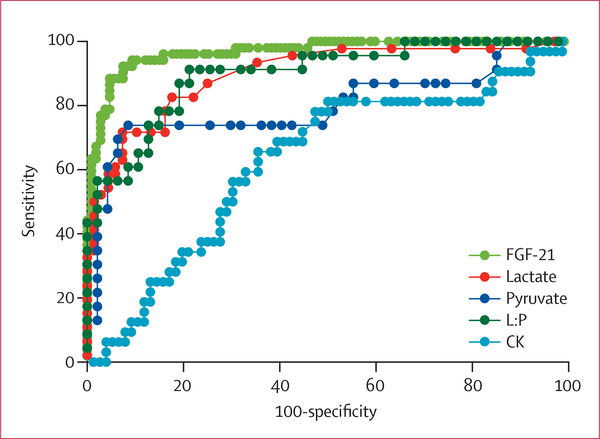
The distributions of normal and abnormal values for all biomarkers are shown in table 4. FGF-21 concentrations showed higher sensitivity than and similar specificity to lactate, pyruvate, and lactate-to-pyruvate ratio, and showed higher sensitivity and specificity than creatine kinase (table 5). The AUC showed that sensitivity and specificity were significantly better for FGF-21 than for lactate (p<0·0001), pyruvate (p=0·003), lactate-to-pyruvate ratio (p<0·0001), and creatine kinase (p<0·0001), separately and when FGF-21 concentrations in serum were analysed against all the other biomarkers simultaneously (AUC: FGF-21 0·95; lactate 0·83, p=0·037; pyruvate 0·83, p=0·015; lactate-to-pyruvate ratio 0·72, p=0·0002; and creatine kinase 0·77, p=0·013). These values suggest a 95% chance that FGF-21 concentrations in serum will correctly distinguish patients with a muscle-manifesting mitochondrial disease from those with non-mitochondrial disease, compared with 83% for lactate and pyruvate, 72% for lactate-to-pyruvate ratio, and 77% for creatine kinase. The AUC for continuous biomarker values (figure 3) indicated similar results to those for dichotomised variables.
Table 4:
Distribution of abnormal values in different patient groups, by biomarker*
| FGF-21 (pg/mL) |
Lactate (mmol/L) |
Pyruvate (μmol/L) |
Lactate:pyruvate† | Creatine kinase (U/L)‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <200 | >200 | <2.4 | >2.4 | <70 | >70 | <30 | >30 | Normal | Raised | |
| Adult muscle-manifesting mitochondrial disorders (MELAS, PEO, and MNGIE; n=29) | 3 | 26 | 15 | 10 | 4 | 7 | 10 | 1 | 15 | 7 |
| Adult mitochondrial disorders with nervous system involvement (MIRAS; n=12) | 7 | 5 | 10 | 1 | 2 | 7 | 9 | 0 | 11 | 1 |
| Child muscle-manifesting mitochondrial disorders (n=23) | 1 | 22 | 2 | 19 | 2 | 10 | 3 | 9 | 3 | 7 |
| Non-mitochondrial neurological disorders (adults [n=22]/children [n=15]) | 17/11 | 5/1 | 8/6 | 0/3 | 0/1 | 0/0 | 0/1 | 0/0 | 5/1 | 17/1 |
| Controls | 71 | 3 | 50 | 2 | 40 | 6 | 46 | 0 | 50 | 2 |
MELAS=mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. PEO=progressive external ophthalmoplegia. MNGIE=mitochondrial neurogastrointestinal encephalomyopathy. MIRAS=mitochondrial recessive ataxia syndrome.
Numbers vary across biomarkers because all data were not available for all patients.
Calculated as 1000×(lactate)/(pyruvate).
Reference values for age-groups are described on webappendix p 5.
Table 5:
Sensitivity, specificity, and positive and negative predictive values for FGF-21 and conventional serum biomarkers for mitochondrial diseases*
| Sensitivity | Specificity | Positive predictlvevalue | Negative predictlvevalue | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGF-21 | Lac | Pyru | L:P† | CK | FGF-21 | Lac | Pyru | L:P† | CK | FGF-21 | Lac | Pyru | L:P† | CK | FGF-21 | Lac | Pyru | L:P† | CK | |
| Adult muscle-manifesting mitochondrial disorders (MELAS, PEO, and MNGIE; n=29) | 89.7 (72.7–97.8) | 40.0 (21.161–3) | 63.6 (30.8–891) | 9.0 (0.2–41.3) | 31.8 (13.9–549) | 91.7 (84.8–96.1) | 92.7 (83.9–97.6) | 87.2 (74.2–95.2) | 100.0 (92.5–100.0) | 73.7 (62.3–83.1) | 74.3 (56.7–87.5) | 66.7 (38.4–88.2) | 53.9 (25.1–80.8) | 100.0 (2.5–100.0) | 25.9 (11.1–46.3) | 97.1 (91.6–99.4) | 81.0 (70.6–89.0) | 91.1 (78.8–97.5) | 82.5 (70.1–91.3) | 78.9 (67.6–87.7) |
| Adult mito-chondrial disorders with nervous system involvement (MIRAS; n=12) | 41.7 (15.2–72.3) | 9.1 (0.2–41.3) | 77.8 (40.0–97.2) | 0(0–7.6) | 8.3 (0.2–38.5) | 91.7 (84.8–96.1) | 92.8 (83.9–97.6) | 87.2 (74.3–95.2) | 100.0 (66.4–100.0) | 73.7 (62.3–83.1) | 35.7 (12.8–64.9) | 16.7 (0.4–64.1) | 53.9 (25.1–80.8) | NA‡ | 4.8 (0.1–23.8) | 93.4 (86.9–97.3) | 86.5 (76.6–93.3) | 95.4 (84.2–99.4) | 83.9 (717–92.4) | 83.6 (72.5–91.5) |
| Child muscle-manifesting mitochondrial disorders (n=23) | 95.7 (78.1–99.9) | 90.5 (69.6–98.8) | 83.3 (51.6–97.9) | 75.0 (42.8–94.5) | 70.0 (34.8–93.3) | 91.7 (84.8–96.1) | 92.8 (83.9–97.6) | 83.3 (51.6–97.9) | 100.0 (92.5–100.0) | 73.7 (62.3–83.1) | 71.0 (52.0–85.8) | 79.2 (57.9–92.9) | 62.5 (35.4–84.8) | 100.0 (66.4–100.0) | 25.9 (11.1–46.3) | 99.0 (94.6–100.0) | 87.0 (89.5–99.6) | 95.4 (84.2–99.4) | 94.0 (83.5–98.8) | 94.9 (85.9–98.9) |
| Adult and child muscle-manifesting mitochondrial disorders (n=52) | 92.3 (81.5–97.9) | 63.0 (47.6–76.8) | 73.9 (51.6–89.8) | 43.5 (23.2–65.5) | 43.8 (26.4–62.3) | 91.7 (84.8–96.1) | 92.8 (83.9–97.6) | 87.2 (74.3–95.2) | 100.0 (92.5–100.0) | 73.7 (62.3–83.1) | 84.2 (72.1–92.5) | 85.3 (68.9–95.1) | 73.9 (51.6–89.8) | 100.0 (69.2–100.0) | 41.2 (24.7–59.3) | 96.1 (90.4–94.9) | 79.0 (68.5–87.3) | 87.2 (74.3–95.2) | 78.3 (65.8–87.9) | 75.7 (64.3–84.9) |
| All mito-chondrial disorders (n=64) | 82.8 (71.3–91.1) | 52.6 (39.0–66.0) | 75.0 (56.6–88.5) | 31.3 (16.1–50.0) | 34.1 (20.5–49.9) | 91.7 (84.8–96.1) | 92.8 (83.9–97.6) | 87.2 (74.3–95.2) | 100.0 (92.5–100.0) | 73.7 (62.3–83.1) | 85.5 (74.2–93.1) | 85.7 (69.7–95.2) | 80.8 (61.4–92.3) | 100.0 (69.2–100.0) | 42.9 (26.3–60.7) | 90.0 (82.8–94.9) | 70.3 (59.8–79.5) | 83.7 (70.3–92.6) | 68.1 (55.8–78.8) | 65.9 (54.8–75.8) |
Values are percentages (95% CI). Lac=lactate. Pyru=pyruvate. L:P=lactate-to-pyruvate ratio. CK=creatine kinase. MELAS=mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. PEO=progressive external ophthalmoplegia. MNGIE=mitochondrial neurogastrointestinal encephalomyopathy. MIRAS=mitochondrial recessive ataxia syndrome.
Each mitochondrial disease group was compared with all disease controls and healthy controls.
Calculated as 1000×(lactate/pyruvate).
L:P was normal in all MIRAS patients and controls and, therefore, the positive predictive value could not be calculated.
Figure3: Receiver-operating-characteristic curves for different biomarkers (continuous values) of muscle-manifesting respiratory chain deficiencies in adults and children.
Areas under the curves are: 0·97 (95% CI 0·94–0·99) for FGF-21 in serum; 0·90 (0·84–0·96) for lactate; 0·80 (0·70–0·93) for pyruvate; 0·90 (0·82–0·98) for L:P; and 0·63 (0·51–0·74) for CK. L:P=ratio of lactate to pyruvate. CK=creatine kinase.
For the detection of MIRAS, pyruvate showed the highest sensitivity (table 5) and odds ratio (23·9, 95% CI 4·0–143·2), followed by FGF-21 (7·9, 2·1–29·9), lactate (1·3, 0·1–12·1), and creatine kinase (0·3, 0·03–2·1). However, when the biomarkers were compared pairwise against pyruvate, the AUC for FGF-21 was similar (p=0·15), whereas the AUC for lactate and creatine kinase were worse (p=0·0019 and p=0·0014, respectively).
Discussion
FGF-21 in serum seems to be a sensitive and specific quantitative biomarker for muscle pathology in a wide range of mitochondrial disorders in adults and children, including deficiencies in one or more of the respiratory chain enzyme complexes in the skeletal muscle. Lactate and lactate-to-pyruvate ratio were specific biomarkers for muscle-manifesting respiratory chain deficiencies but pyruvate was sensitive only for MIRAS. Pyruvate has not, however, been previously reported to be a good biomarker for MIRAS, and large-scale analyses are needed to confirm this finding.
Our multicentre approach enabled the testing of serum samples from patients with diagnoses confirmed by DNA analysis. The control range of FGF-21 in serum was also in accordance with ranges in previous publications (webappendix p 6).14,17–21 AUC values suggested that FGF-21 concentrations in serum could be used to detect most adult mitochondrial myopathies, which typically showed normal values for lactate and ratio of lactate to pyruvate. Concentrations were highest in severe infantile disorders, such as Alpers’ hepatoencephalopathy or severe infantile COX deficiency, in which lactate concentrations and lactate-to-pyruvate ratios were normal. However, if lactate concentrations in children were even slightly raised, FGF-21 concentrations in serum increased by more than ten times. Our approach overestimated the specificity and sensitivity of lactate and took even marginal increases to be pathological (eg, 2·5 mmol/L compared with the cutoff of 2·4 mmol/L). In practice, small rises in lactate concentrations are rarely deemed diagnostic because they fluctuate and results are prone to artifacts (eg, from stasis or a struggling child). FGF-21 concentrations seemed to remain raised, albeit in four patients.
FGF-21 concentrations in serum seemed to increase with increasing clinical severity of the mitochondrial disease and muscle pathology. A correlation was seen with the proportion of COX-negative fibres, and the retesting of samples from four patients suggested that concentrations of FGF-21 increased as disease progressed. A role of FGF-21 in disease monitoring, however, needs further study. In patients with long-chain 3 hydroxyacyl CoA dehydrogenase deficiency, measurement of FGF-21 concentrations in serum might be useful in the assessment of treatment response after crisis. The concentration was very high in one patient in crisis, but levels were low in patients in remission and being treated by dietary modification.22
Respiratory chain deficiencies of single or multiple complexes resulted in high concentrations of FGF-21 in serum, including complex I deficiency, which is the most common form.23 Reliable diagnosis ofcomplex I deficiency is challenging for routine laboratories because this complex cannot be assessed by histology, it is prone to inactivation during handling ofsamples, and measurement of its activity is technically challenging.9 Thus, the use of a low-cost test would be useful (an ELISA done in a diagnòstic laboratory typically costs €100–200, compared with thousands of euros for surgical muscle biopsy, when salaries, surgery and analysis costs are summed).
FGF-21 regulates lipid metabolism and starvation response in mice10–12 and is produced by the liver, adipose tissue, and muscle.10,13,24,25 After 12 h of fasting, secretion increases by 28 times in mouse liver,11 although in human beings expression is notably altered only after 7 days of fasting.26 Respiratory chain defects induce FGF-21 expression in mouse skeletal muscle, which leads to raised concentrations in serum.13 We show here that the response is similar in human beings. We cannot exclude the contribution of liver or adipose tissue to abnormal serum concentrations ofFGF-21, but found no correlation with liver dysfunction in tested participants.
Previous studies have reported positive17,27,28 or no26,29,30 correlation between FGF-21 concentrations in serum and BMI in healthy populations. A study in healthy twins strongly suggested that increased liver fat, which frequently co-occurs with obesity, is a crucial factor in raising FGF-21 concentrations rather than overall adiposity.30 Widespread metabolic alterations, especially cachexia or leanness,31 are frequently associated with mitochondrial disorders, yet we found significant negative correlations between raised FGF-21 concentrations in serum and BMI, which is consistent with lipid-recruiting function of FGF-21. Whether the tissue-of-origin dictates the lipid metabolic effects or FGF-21 response is dependent on the amount of liver fat needs further study.
Reports of increased concentrations in serum of patients with non-alcoholic fatty liver disease support the view that liver fat and raised FGF-21 concentrations in serum are related.14,20,28 By contrast with populations in those studies, however, our patients had confirmed diagnoses of mitochondrial disorders. Nevertheless, concomitant disorders, such as non-alcoholic fatty liver disease, cannot be genetically diagnosed and represent a heterogeneous group of diseases in which mitochondrial contribution cannot be excluded. The increased FGF-21 concentrations in serum in adults with non-alcoholic fatty liver disease overlap with the lower range of concentrations in our adult patients with myopathy (webappendix p 6), but the symptoms of non-alcoholic fatty liver disease do not overlap with muscle-manifesting mitochondrial disease.
Confounding factors might affect the use of FGF-21 concentrations in serum as a diagnostic marker. However, we found no correlation between serum concentrations and age, liver dysfunction, non-mitochondrial muscle disease, chronic or acute fasting, or obesity. Additionally, the serum and plasma samples were collected from patients who originated from seven different centres in Europe and the USA, which suggests no link to specific European populations.
Only one of 22 children with mitochondrial disease had normal FGF-21 concentrations in serum. She carried a mutation typically causing Leber’s hereditary optic neuropathy. The lactate concentration and muscle histology were normal in this child, but complex I activity in muscle was low. Other patients with isolated complex I deficiency had high FGF-21 concentrations in serum, and the reason for this one patient’s low concentration is unclear. FGF-21 expression was induced in a wide selection of respiratory chain deficiencies, but because most cases directly or indirectly involved mitochondrial DNA, we cannot exclude the possibility that increased FGF-21 concentrations in serum are typical for mitochondrial DNA involvement. Among patients with non-mitochondrial disorders, we found moderate increases in the concentrations of FGF-21 in serum in a cachexic child with progeria, and further samples need to be analysed to establish whether abnormal concentrations are a feature of this rare disease group. Most patients with inclusion body myositis, with or without COX-negative muscle fibres, had normal FGF-21 concentrations in serum, which suggests that induction is triggered by primary respiratory chain deficiency. An inherited form of inclusion body myositis has been associated with defective autophagy,32,33 which is a crucial component of the starvation response. Further studies are required to clarify whether autophagy induction is linked to FGF-21 secretion.
Although the multicentre approach enabled us to collect a representative number of serum samples of various disease groups of interest, the retrospective nature of the study meant that sampling protocols were not predetermined and patients’ fasting and the timing of sample collection were not standardised. Additionally, whether FGF-21 secretion is induced in other diseases not included in this study remains to be tested.
Our results for the sensitivity of FGF-21 to identify mitochondrial disorders with muscle involvement compared with those for lactate, pyruvate, lactate-to-pyruvate ratio, and creatine kinase suggest that measurement of FGF-21 in serum by ELISA would be a useful first-line test in patients with suspected respiratory chain deficiencies to prioritise patients for muscle biopsy (panel). Analysis of FGF-21 concentrations in serum might make the diagnosis ofrespiratory chain deficiencies possible in non-specialist hospital laboratories and reduce the need for invasive biopsy procedures in children and adults.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the term “FGF21” for any published articles. In 2005 this cytokine was associated with lowered lipid and glucose concentrations when overexpressed in mice, and in 2007 was deemed a major regulator of starvation response.10–12 Of 161 articles on FGF-21 identified, none described studies of human patients with mitochondrial disorders, or with FGF-21 as a neurological disease biomarker. We limited our discussion to studies in which FGF-21 was measured in human samples for any disorder and those of approaches for diagnosis of mitochondrial disorders.
Interpretation
We found in a mouse model of late-onset mitochondrial myopathy that FGF-21 was induced in skeletal muscle when respiratory chain deficiency manifested.13 Our results in human beings are consistent with those in mice: combined or isolated mitochondrial respiratory chain deficiencies in muscle were associated with raised concentrations of FGF-21 in serum. Histological and biochemical analysis of skeletal muscle biopsy samples is the gold standard for diagnosis of mitochondrial disorders in adults and children but is invasive and expensive, and the conventional serum biomarkers lactate, pyruvate, ratio of lactate to pyruvate, and creatine kinase have poor sensitivity, specificity, or both. We found that FGF-21 had good sensitivity and specificity for detection of primary respiratory chain deficiencies with muscle manifestation in adults and children. Concentrations of FGF-21 in serum correlated with the number of respiratory chain deficient muscle fibres and was highest in the most disabling disorders. We suggest that measurement of FGF-21 concentrations in serum be considered as a first-line test for mitochondrial disorders to prioritise patients for muscle biopsy.
Acknowledgments
We thank Sigrid Juselius Foundation, Jane and Aatos Erkko Foundation, Molecular Medicine Institute of Finland, University of Helsinki and Helsinki University Central Hospital, Academy of Finland, Novo Nordisk, and Arvo and Lea Ylppö Foundation for funding support, Anu Harju and Markus Innilä for technical help, and the patients and their families for participating in the study.
Footnotes
Conflicts of interest
AS and HT have submitted an international patent application for serum diagnostics of mitochondrial disease. KHP has been paid to give lectures for Eli Lilly, Abbott, and Novo Nordisk. JS is the Chief Executive Officer of Khondrion, a spin-off company of the Radboud University Nijmegen Medical Centre. The other authors declare that they have no conflicts of interest.
Contributor Information
Anu Suomalainen, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland; Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland; Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland.
Jenni M Elo, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland.
Kirsi H Pietiläinen, Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland; Obesity Research Unit, Department of Medicine, Division of Internal Medicine, University of Helsinki, Helsinki, Finland.
Anna H Hakonen, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland.
Ksenia Sevastianova, Department of Medicine, University of Helsinki, Helsinki, Finland.
Mari Korpela, Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland.
Pirjo Isohanni, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland; Department of Paediatrics and Adolescents, Helsinki University Central Hospital, Helsinki, Finland.
Sanna K Marjavaara, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland.
Tiina Tyni, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland; Department of Paediatrics and Adolescents, Helsinki University Central Hospital, Helsinki, Finland.
Sari Kiuru-Enari, Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland.
Helena Pihko, Department of Paediatrics and Adolescents, Helsinki University Central Hospital, Helsinki, Finland.
Niklas Darin, Sahlgrenska Academy of the University of Gothenburg, Gothenburg, Sweden.
Katrin Õunap, Tartu University Hospital, Tartu, Estonia.
Leo A J Kluijtmans, Radboud University, Nijmegen Medical Centre, Nijmegen, Netherlands s.
Anders Paetau, Department of Pathology, Helsinki University Central Hospital, Helsinki, Finland.
Jana Buzkova, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland.
Laurence A Bindoff, University of Bergen and Haukeland University Hospital, Departments of Clinical Medicine and Neurology, Bergen, Norway.
Johanna Annunen-Rasila, Oulu University Hospital, Department of Neurology and Clinical Research Centre, Oulu, Finland.
Johanna Uusimaa, Oulu University Hospital, Department of Neurology and Clinical Research Centre, Oulu, Finland.
Aila Rissanen, Obesity Research Unit, Department of Medicine, Division of Internal Medicine, University of Helsinki, Helsinki, Finland.
Hannele Yki-Järvinen, Department of Medicine, University of Helsinki, Helsinki, Finland.
Michio Hirano, Columbia University, Presbyterian Medical Center, New York, NY, USAs.
Mar Tulinius, Sahlgrenska Academy of the University of Gothenburg, Gothenburg, Sweden.
Jan Smeitink, Radboud University, Nijmegen Medical Centre, Nijmegen, Netherlands.
Henna Tyynismaa, Research Programmes Unit, Molecular Neurology, University of Helsinki, Helsinki, Finland.
References
- 1.Elliott H R, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet 2008; 83: 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer AM, McFarland R, Blakely EL, et al. Prevalence of mitochondrial DNA disease in adults. Ann Neurol 2008; 63: 35–39. [DOI] [PubMed] [Google Scholar]
- 3.Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 2003; 126: 1905–12. [DOI] [PubMed] [Google Scholar]
- 4.Di Donato S Multisystem manifestations of mitochondrial disorders. J Neurol 2009; 256: 693–710. [DOI] [PubMed] [Google Scholar]
- 5.Smeitink JA, Zeviani M, Turnbull DM, Jacobs HT. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab 2006; 3: 9–13. [DOI] [PubMed] [Google Scholar]
- 6.Finsterer J, Harbo HF, Baets J, et al. EFNS guidelines on the molecular diagnosis of mitochondrial disorders. Eur J Neurol 2009; 16: 1255–64. [DOI] [PubMed] [Google Scholar]
- 7.Suomalainen A Biomarkers for mitochondrial respiratory chain disorders. J Inherit Metab Dis 2011; 34: 277–82. [DOI] [PubMed] [Google Scholar]
- 8.Shaham O, Slate NG, Goldberger O, et al. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci USA 2010; 107: 1571–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medja F, Allouche S, Frachon P, et al. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 2009;9: 331–39. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415–25. [DOI] [PubMed] [Google Scholar]
- 12.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426–37. [DOI] [PubMed] [Google Scholar]
- 13.Tyynismaa H, Carroll CJ, Raimundo N, et al. Mitochondrial myopathy induces a starvation-like response. Hum Mol Genet 2010. 19: 3948–58. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010; 53: 934–40. [DOI] [PubMed] [Google Scholar]
- 15.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009; 71: 369–75. [DOI] [PubMed] [Google Scholar]
- 16.Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med 1981; 94: 557–92. [PubMed] [Google Scholar]
- 17.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–53. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Bao Y, Xy A, et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab 2009; 94: 2151–56. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas-Ramos D, Almeda-Valdes P, Gomez-Perez FJ, et al. Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur J Endocrinol 2010; 163: 469–77. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz Y, Eren F, Yonal O, et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest 2010; 40: 887–92. [DOI] [PubMed] [Google Scholar]
- 21.Lin Z, Wu Z, Yin X, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profiles. PLoS One 2010; 5: e15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiekerkoetter U, Bastin J, Gillingham M, Morris A, Wijburg F, Wilcken B. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis 2010; 33: 555–61. [DOI] [PubMed] [Google Scholar]
- 23.Janssen RJ, Nijtmans LG, van den Heuvel LP, Smeitink JA. Mitochondrial complex I: structure, function and pathology. J Inherit Metab Dis 2006; 29: 499–515. [DOI] [PubMed] [Google Scholar]
- 24.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett 2008; 582: 3805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muise ES, Azzolina B, Kuo DW, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor Y and altered metabolic states. Mol Pharmacol 2008; 74: 403–12. [DOI] [PubMed] [Google Scholar]
- 26.Galman C, Lundasen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–74. [DOI] [PubMed] [Google Scholar]
- 27.Dostalova I, Kavalkova P, Haluzikova D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008; 93: 3627–32. [DOI] [PubMed] [Google Scholar]
- 28.Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domingo P, Gallego-Escuredo JM, Domingo JC, et al. Serum FGF21 levels are elevated in association with lipodystrophy, insulin resistance and biomarkers of liver injury in HIV-1-infected patients. AIDS 2010; 24: 2629–37. [DOI] [PubMed] [Google Scholar]
- 30.Tyynismaa H, Raivio T, Hakkarainen A, et al. Liver fat but not other adiposity measures influence circulating FGF21 levels in healthy young adult twins. J Clin Endocrinol Metab 2011; 96: E351–55. [DOI] [PubMed] [Google Scholar]
- 31.Hakonen AH, Heiskanen S, Juvonen V, et al. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet 2005; 77: 430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ju JS, Fuentealba RA, Miller SE, et al. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Celi Biol 2009; 187: 875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 2004;36: 377–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.