Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an autosomal recessive disorder caused by mutations in the TYMP gene, which encodes thymidine phosphorylase (TP). TP dysfunction results in systemic thymidine (dThd) and deoxyuridine (dUrd) overload, which selectively impair mitochondrial DNA replication. Allogeneic hematopoietic transplantation has been used to treat MNGIE patients; however, this approach has serious adverse effects, including the toxicity of myeloablative conditioning, graft rejection and graft-versus-host disease. With the aim of testing the feasibility of gene therapy for MNGIE, we transduced TP-deficient B-lymphoblastoid cells from two MNGIE patients, with lentiviral vectors carrying a functional copy of the human TYMP DNA coding sequence. This restored TP activity in the cells, which reduced the excretion of dThd and dUrd and their concentrations when added in excess. Additionally, lentiviral-mediated hematopoietic gene therapy was used in partially myeloablated double TYMP/Upp1 knockout mice. In spite of the relatively low levels of molecular chimerism achieved, high levels of TP activity were observed in the peripheral blood of the transplanted mice, with a concomitant reduction of nucleoside concentrations. Our results suggest that hematopoietic gene therapy could be an alternative treatment for this devastating disorder in the future.
Keywords: MNGIE, lentiviral vector, thymidine phosphorylase, TYMP, mitochondria
INTRODUCTION
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a multisystemic disease caused by autosomal recessive mutations in the TYMP gene encoding thymidine phosphorylase (TP).1 The main clinical features of MNGIE are progressive external ophthalmoplegia, severe gastrointestinal dysmotility, cachexia, peripheral neuropathy, diffuse leukoencephalopathy on brain magnetic resonance imaging and evidence of mitochondrial dysfunction (histological, biochemical and genetic abnormalities of mitochondria).2 Patients usually die from complications of their gastrointestinal problems and their critical nutritional status, with an average life expectancy of 37 years.2,3 MNGIE is a rare disease, with fewer than 200 patients known to be affected worldwide, but the true incidence of the disease is unknown and calculations based on known cases may lead to underestimations because MNGIE patients are often misdiagnosed.4
TP catalyzes the phosphorolysis of thymidine (dThd) or deoxyuridine (dUrd) nucleosides to the corresponding bases, thymine or uracil, and deoxyribose-1-phosphate.5 In MNGIE patients, mutations in TYMP markedly reduce TP activity, resulting in a systemic accumulation of dThd and dUrd. Plasma and tissue dThd and dUrd, which are undetectable (<0.05μM) in normal individuals, reach micromolar concentrations in MNGIE patients.6–8 This is relevant from a pathogenic point of view as in vitro and in vivo studies have demonstrated that an excess of dThd induces an increase of thymidine triphosphate (dTTP), which interferes with the correct replication of mitochondrial DNA, causing mitochondrial dysfunction.9,10
Therapeutic approaches have so far focused on reducing the systemic overload of these nucleosides.7,11–14 In recent years, several MNGIE patients have been treated with allogeneic hematopoietic stem cell transplantation (HSCT).4,12,15,16 After successful HSCT, donorderived nucleated blood cells and platelets provided enough TP activity to reduce the dThd and dUrd concentrations to undetectable or nearly undetectable levels, and subsequent slowing of disease progression or mild clinical improvement.4,12,15,16 However, this approach has several limitations, which include the high morbidity and mortality of allogeneic HSCT, and the difficulty of obtaining suitable donors for a high proportion of patients. In MNGIE patients, HSCT implies additional difficulties. MNGIE patients are generally in poor medical condition, with limited capacity to tolerate transplant-related complications. The gastrointestinal function is disturbed in most cases, with potential impairment of absorption, so that parenteral application of drugs is needed. In addition, drugs with possible mitochondrial toxicity must be avoided.4
As occurs with many monogenic disorders that can benefit from an allogeneic HSCT, MNGIE is probably a very good candidate for hematopoietic stem cell gene therapy (HSCGT). Robust and stable TP expression in hematopoietic cells should clear the systemic accumulation of dThd and dUrd, as observed in MNGIE patients undergoing an allogeneic HSCT.12 Here, we show that gene transfer using lentiviral vectors results in stable TP activity in cell lines from MNGIE patients and in a double knockout (Tymp−/−; Upp1−/−) murine model of the disease,10 reverting dThd and dUrd overload to normal levels. Our results suggest that gene therapy is a promising therapeutic approach for MNGIE.
RESULTS
Transduction of TP-deficient B-lymphoblastoid cell lines (B-LCLs)
We generated B-LCLs through Epstein–Barr virus (EBV) infection of lymphocytes from two MNGIE patients (P1 and P2) and two normal controls (C1 and C2). These B-LCLs were transduced with a lentiviral vector containing the TYMP DNA coding sequence (cDNA) (p305-TP) or the same vector without the TYMP cDNA (p305-sham) (Figure 1a). Both vectors contained human phosphoglycerate kinase (hPGK) promoter and also included the sequence encoding the enhanced green fluorescent protein (EGFP) as a marker gene (see Materials and Methods).
Figure 1.
Transduction efficiency in B-LCLs. (a) Schematic representation of the lentiviral vectors. p305-TP, p305-sham and p-sham vectors and their elements. RRE, rev-responsive element; cPPT, central polypurine tract; hPGK, human phosphoglycerate kinase promoter; TYMP, coding sequence of the TYMP gene; IRES, internal ribosome entry site; eGFP, enhanced green fluorescent protein; WPRE, woodchuck hepatitis post-transcriptional regulatory element; LTR, long terminal repeat. Both circular vectors are schematically represented as linear forms. (b) Flow cytometry analysis of B-LCLs obtained from two controls (C1, C2) and two MNGIE patients (P1, P2) transduced at 15 MOI and cultured for at least 10 days before analysis (pre-sorted) and 10 days after sorting of EGFP+ cells (post-sorted). The x axis shows green fluorescence intensity, y axis shows cell counts. Open histograms: non-transduced cell lines used as negative controls. Filled histograms: transduced cell lines.
Flow cytometry analyses revealed variable levels of transduction in B-LCLs derived from MNGIE patients and controls (Figure 1b). Cell sorting was performed to enrich cell lines in EGFP+ cells. TP- and sham-transduced cells from P2 did not survive after sorting, which prompted us to use unsorted transduced B-LCLs from patients and EGFP+-sorted cell population from control B-LCLs for further experiments. Transduction efficiencies were studied by measuring the copy number of TYMP and EGFP sequences in transduced cells, using quantitative real-time PCR (qRT-PCR) (Table 1). For p305-TP-transduced cell lines, post-sorted C1, C2 and pre-sorted P1 showed values between 1.1 and 1.7 average TYMP copies per cell, and only 0.12 copies per cell for pre-sorted P2. Similar results were obtained for the copy number of the EGFP gene, consistent with the fact that the p305-TP vector carries one TYMP copy per EGFP copy. Higher averages of EGFP copy numbers per cell were observed, indicating higher efficiencies of transduction in sham-transduced cells relative to TP-transduced B-LCLs. These differences were not observed when transduction was assessed by flow cytometry (Figure 1b), probably because of a low level of EGFP expression of the p305-sham vector when compared with that of p305-TP. This notion was supported by the observation of lower EGFP mRNA levels per EGFP DNA copy in sham-transduced cells as compared with TP-transduced cells (Table 1).
Table 1.
Transduction efficiencies and EGFP expression in B-LCLs
| Cell line | Vector | EGFP+ cells (%) | EGFP DNA copy number | TYMP DNA copy number | EGFP mRNA | |||
|---|---|---|---|---|---|---|---|---|
| per cell | per transduced cell | per cell | per transduced cell | referred to PPIA mRNA | referred to EGFP DNA copy number | |||
| C1 | p305-TP | 96 | 1.43 | 1.49 | 1.46 | 1.52 | 2.37 | 1.66 |
| p305-sh | 97 | 2.86 | 2.95 | UND | — | 0.83 | 0.29 | |
| C2 | p305-TP | 98 | 1.43 | 1.46 | 1.74 | 1.78 | 1.21 | 0.85 |
| p305-sh | 33 | 3.15 | 9.55 | UND | — | 0.32 | 0.10 | |
| P1 | p305-TP | 31 | 0.89 | 2.87 | 1.10 | 3.55 | 1.00 | 1.12 |
| p305-sh | 31 | 2.23 | 7.19 | UND | — | 0.71 | 0.32 | |
| P2 | p305-TP | 6 | 0.10 | 1.67 | 0.12 | 2.00 | 0.08 | 0.80 |
| p305-sh | 3 | 0.17 | 5.67 | UND | — | 0.06 | 0.38 | |
Abbreviations: B-LCLs, B-lymphoblastoid cell lines; EGFP, enhanced green fluorescent protein; sh, sham; TP, thymidine phosphorylase; UND, undetectable.
Percentages of EGFP+ cells were determined by flow cytometry. EGFP and TYMP DNA copies were assessed by quantitative real-time PCR (qRT-PCR), referred to the single-copy gene RNase P, and expressed as average copy number per cell, or per transduced cell. EGFP mRNA levels were determined by qRT-PCR, referred to endogenous levels of PPIA mRNA (cyclophilin A) and shown as fold increase referred to the result obtained for the P1 transduced with p305-TP. In addition (last column), this value was referred to the EGFP DNA copy number.
TYMP mRNA and TP protein levels were increased in TP-transduced cell lines
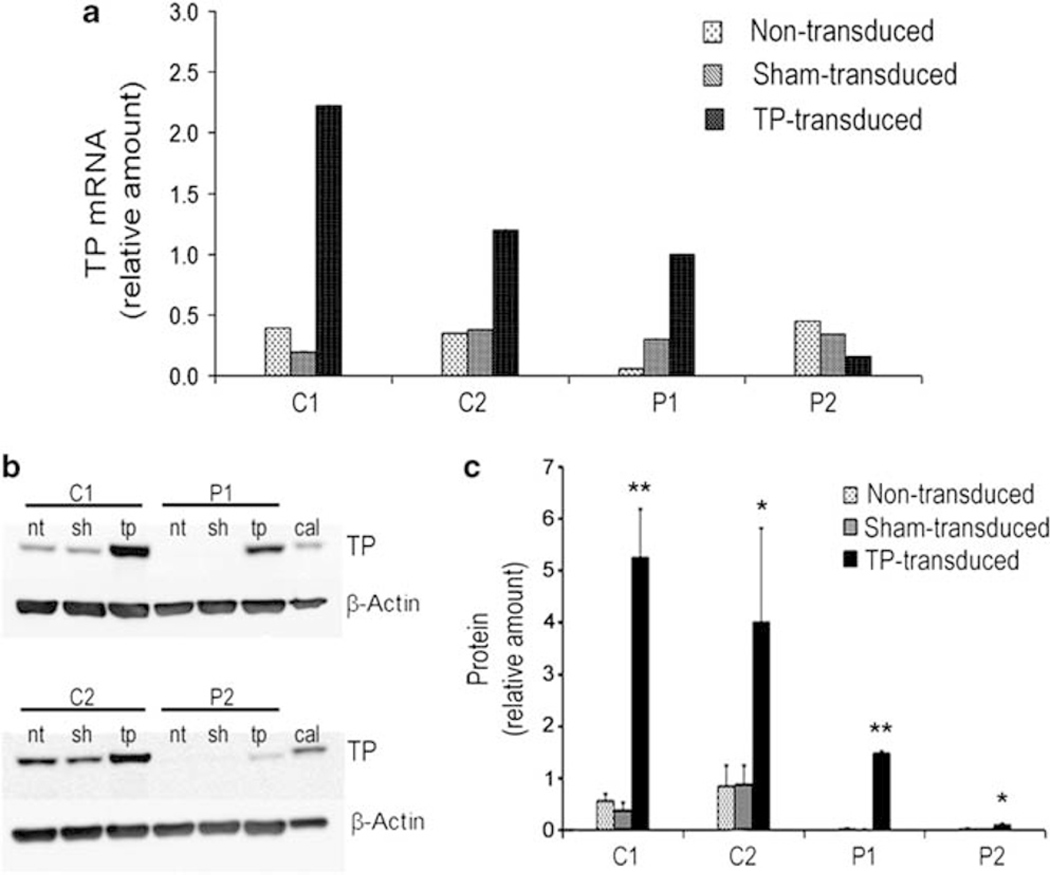
To determine whether the integrated p305-TP vector can efficiently drive TYMP expression in B-CLs, levels of TYMP mRNA and TP protein in cell homogenates were quantified. Analyses were performed at least 2 weeks after transduction to avoid possible interference from non-integrated vector expression. TYMP mRNA levels were measured by qRT-PCR and normalized to cyclophilin A (PPIA) mRNA, which was homogenously expressed (data not shown). Untransduced and p305-sham-transduced B-LCLs showed similar levels of TYMP mRNA in both control and patients’ cell lines, except for non-transduced B-LCLs from P1, which had lower amounts of TYMP mRNA (Figure 2a). Transduction with p305-TP increased three- to sixfold the levels of TYMP mRNA in controls and P1. Surprisingly, B-LCLs of P2 transduced with p305-TP showed reduced TYMP mRNA levels relative to untransduced and sham-transduced cells. Levels of TP protein were similar in non-transduced or sham-transduced B-LCLs from controls, and were undetectable or barely detectable in patients’ cells (Figures 2b and c). p305-TP transduction significantly increased TP protein levels, which paralleled increases observed in TYMP mRNA in controls and in P1. In P2, p305-TP induced low, but clearly measurable levels of TP protein, which were undetectable in their non-transduced and sham-transduced lines.
Figure 2.
Expression of TP. (a) TYMP mRNA levels in B-LCLs. Values are normalized to the endogenous PPIA gene mRNA (cyclophilin A) levels and shown as fold increase with respect to the result obtained for the P1 transduced with p305-TP. Data are expressed as mean of two independent determinations on the same cDNA. (b) Representative western blot showing the amounts of TP protein in B-LCL extracts. nt, non-transduced cell lines; sh and tp, cell lines transduced with p305-sham and p305-TP, respectively. (c) Quantitative levels of TP protein obtained by densitometry; all values are normalized to β-actin and shown as fold increase referred to the result obtained for a calibrator protein extract (cal). Results are expressed as the mean (±s.d.) of three independent experiments (three different protein extracts). Asterisks indicate statistically significant differences between TP transduced as compared with both non-transduced and sham transduced (*P<0.05; **P<0.001; one-way analysis of variance with Bonferroni correction for multiple comparisons).
p305-TP restored TP activity in TP-deficient cells
Figure 3 shows the results for TP activity in cell lines from patients and controls. Enzyme activity was undetectable or barely detectable in B-LCLs from MNGIE patients (below 5nmol of thymine formed h−1 per mg protein), and between 550 and 1100 nmol h−1 per mg protein in B-LCLs from controls. TP activity was strongly increased in B-LCLs transduced with p305-TP. A strong correlation was observed between TP protein levels and TP activity, and the linear distribution of the data in this representation suggests that both transgenic and endogenous TP proteins have similar enzymatic Vmax, as our enzyme assay is performed at substrate saturation. TP activity gained after TP transduction in B-LCLs (TP activity in TP-transduced cells TP activity in sham-transduced cells) was directly proportional to the average transgenic TYMP DNA copy number per cell (r=0.978; P=0.022, Pearson correlation). We also assessed the effects of TP transduction on HEK293T cells. Although non-transduced and sham-transduced HEK293T cells had undetectable TP activity, after transduction with p305-TP the activity reached 6345nmol h−1 per mg protein.
Figure 3.
TP activity. (a) TP activity in B-LCLs from controls and MNGIE patients. Results are expressed as mean (±s.d.) of four different protein extracts. Asterisks indicate statistically significant differences between TP transduced and either non-transduced or sham transduced (**P<0.001; one-way analysis of variance with Bonferroni correction for multiple comparisons). (b) Linear correlation (Spearman) between relative TP protein amount (data from Figure 2c) and TP activity (data from a).
Both endogenous and transgenic TP activity prevented or limited dThd and dUrd accumulation in the culture medium (Figure 4). Extracellular concentrations of dThd and dUrd increased with cultured TP-deficient cells over time, whereas control and p305-TPtransduced cells (patients and controls) showed a very low excretion or catabolic reduction of these nucleosides in the culture medium (Figures 4a–c). The addition of 10 and 20μM of exogenous dThd and dUrd to the medium (mimicking extracellular concentrations observed in MNGIE patients17) showed that TP-deficient cells are unable to catabolize an excess of nucleosides, but this catabolic ability was restored after transduction with p305-TP (Figures 4d–f). In the case of B-LCLs from patient 2, which achieved only moderate TP activity after p305-TP transduction (Figure 3), the catabolic reduction was less pronounced, but it was still noticeable compared with the non-transduced or sham-transduced counterparts.
Figure 4.
Metabolism of dThd and dUrd. B-LCLs were seeded in medium with dialyzed fetal bovine serum and maintained for 4 days in culture without any medium exchange; culture medium was then recovered to measure dThd and dUrd excretion (shown as nucleoside concentration in the medium) in non-transduced (a), p305-sham-transduced (b) and p305-TP-transduced (c) cultures. dThd and dUrd catabolism was studied for non-transduced (d), p305-sham-transduced (e) and p305-TP-transduced (f) B-LCLs in a similar experiment in which 10μm dThd and 20μm dUrd were added to the medium at day 0. Results are expressed as mean of two independent experiments, which are represented as vertical bars (not s.d.).
p305-TP-mediated TYMP expression did not induce cytotoxicity
As p305-TP transduction in C1, C2 and P1 B-LCLs resulted in high TP activities, well above those observed in non-transduced B-LCLs from controls, we investigated whether this increase could result in dTTP depletion. Thus, we determined deoxyribonucleoside triphosphate amounts in cellular extracts, including the HEK293T cell line. Although we did not observe dTTP depletion in B-LCLs from patients or controls overexpressing TYMP after p305-TP transduction, TP-transduced HEK293T cells had a significant reduction ~40% of the dTTP percentage (Figure 5a). To determine whether this biochemical imbalance was associated with alterations in the cell cycle status, we analyzed cell cycle by flow cytometry of Hoechst-stained non-transduced, sham-transduced and TP-transduced cells (Figure 5b). No significant changes in the percentage of proliferating cells (S+M phases) after TP transduction or sham transduction were detected, either in B-LCLs or in HEK293T cells. However, slightly higher percentages of proliferating cells were observed in EGFP+ subpopulations (cells effectively transduced) than in EGFP+ subpopulations, although this difference was not statistically significant. This trend was observed in both TP-transduced and sham-transduced cells, indicating that this effect was TP independent.
Figure 5.
Deoxyribonucleoside triphosphates (dNTPs) in cell homogenates and cytotoxicity. (a) Percentages of dTTP in cell homogenates. Cultured cells were lysed and dNTPs extracted from the homogenates. dATP, dGTP, dCTP and dTTP concentrations were determined. The results are expressed as the percentage of dTTP on the total dNTP pool (dTTP×100/(dATP+dGTP+dCTP+dTTP)). The mean (±s.d.) of three independent experiments is depicted. Asterisk indicates statistically significant differences between TP transduced and either non-transduced and sham transduced (*P<0.01; one-way analysis of variance with Bonferroni correction for multiple comparisons). (b) Percentages of proliferating cells (S+M phases) as assessed by flow cytometry of Hoechst 33342-stained cells. Bars represent the percentages of proliferating cells in non-transduced cultures (open bars), and those observed in the EGFP+ (gray bars) and EGFP (black bars) cell populations. Numbers under the cell line label indicate the percentage of EGFP+ cells out of the total cells in the transduced cell line. Owing to the reduced number of EGFP events in sham-transduced 293T and TP-transduced C2, no reliable results for this fraction could be obtained in these cell lines (asterisks).
Stability of transgenic TYMP
Figure 6 shows the stability of TP activity provided by p305-TP vector in B-LCLs from P1. At early passages after transduction, cells increased TP activity to 1490nmol thymine h−1 per mg protein, compared with undetectable TP in sham and untransduced B-LCLs (Figures 3a and 6a). Although cell counts were not performed, microscopic observation of the culture and stability of the medium color indicated that growth rate suddenly decreased between 4 and 16 weeks, and TP activity almost disappeared. Growth rate and TP activity recovered after week 16, reaching stable values of around 5000nmol thymine h−1 per mg protein at week 28 and over 17 subsequent weeks (Figure 6a). In parallel, the percentages of EGFP+ cells initially declined and, after week 16, increased (Figure 6b). In this experiment, sham-transduced cells did not survive beyond week 20, probably because true immortalization by EBV was not achieved.
Figure 6.
Stability of transgenic TP expression. Monitoring of transgenic TP activity and the percentage of EGFP+ cells in B-LCLs from P1 (a, b), and HEK293T cells (c, d) after transduction. Cells were maintained in culture and samples were collected for TP activity assay and flow cytometry analysis. TP activity is expressed as 103 nmol of thymine formed h−1 per mg protein. When present, error bars indicate duplicate results obtained from the same homogenate (not s.d.). Note different scales for different series in plots a and c. TP activities close or below 5nmol thymine h−1 per mg protein are detectable but barely quantifiable. Note that sham-transduced B-LCL from P1 stopped proliferating and did not survive beyond week 20.
We additionally used the immortalized HEK293T cell line to study long-term transgene expression in p305-TP-transduced cells. As shown in Figure 6c, non-transduced and sham-transduced HEK293T cells had barely detectable TP activity (around or below 5nmol thymine h−1 per mg protein), whereas p305-TP-transduced HEK293T cells achieved activities of 5000nmol h−1 per mg protein or higher, which were maintained over at least 15 weeks in culture. The percentage of EGFP+ cells was also stable (Figure 6d), around 70%, suggesting that there was not any selective advantage for p305-TP-transduced cells due to differential survival or differential rates of growth in culture.
p305-TP provided high levels of TP activity and reduced nucleoside overload in vivo
To test the efficacy of our lentiviral vector in vivo, we used a double Tymp/Upp1 knockout murine model.10 As the in vitro study had shown that EGFP was poorly expressed by the p305-sham vector (Figure 1b and Table 1), we decided to use a modified version of the sham vector in the animal model (p-sham), identical to p305-sham but lacking the internal ribosomal entry site (IRES) (Figure 1a). Previous work in our laboratory showed that this vector resulted in higher EGFP expression levels than p305-sham. Immunoselected hematopoietic lineage negative (Lin−) cells from double KO mice were transduced with our p305-TP or p-sham vector. Flow cytometric analysis revealed p305-TP transduction efficiencies of 26–28% in two different extractions of donor cells, and 16–18% for the corresponding p-sham transductions. Transduced cells were infused into partially myeloablated syngenic double KO mice. Four weeks after transplantation, the level of gene marking in the peripheral blood (PB) of TP-transplanted mice (n=8) ranged between 2.0 and 10.5%, with a mean copy number per transduced cell ranging from 0.5 to 1.5. As shown in Figure 7a, high TP activities (median: 11.3nmol thymine h−1 per mg protein, range: 7.6–13.3) were achieved in PB cells of treated mice, as compared with undetectable or negligible values in untreated and sham-treated double KO. Interestingly, wild-type (wt) mice had detectable but very low TP activity in the blood cell fraction (median: 0.04; range: undetectable to 0.10), in contrast to other tissues that show higher TP activities.10 Circulating dThd and dUrd concentrations in untreated and sham-treated double KO mice were between two- and fivefold higher than those observed in the wt mice. After treatment with the p305-TP vector, double KO mice reduced their plasma dThd and dUrd concentrations to levels in the range of wt mice (Figures 7b and c). In fact, plasma dThd levels in TP-treated double KO mice were lower than those of wt mice (median 3.3, range 2.0–4.2μM for wt, versus 1.6 and 0.7–2.9 for TP-treated double KO).
Figure 7.
Effect of p305-TP in vivo. (a, b and c): Effect of HSCGT in a Tymp/Upp1 double KO mice, 1 month after transplantation. Box plots represent the median (horizontal line), inter-quartile range (box) and minimum and maximum (whiskers), except outliers, which are depicted as dots. WT, untreated wild-type mice; KO, untreated Tymp−/−/Upp1−/− mice; SHAM, p-sham-treated Tymp−/−/Upp1−/−mice; TP, p305-TP-treated Tymp−/−/Upp1−/−mice. (a) TP activity in the PB cell fraction. (b) plasma dThd, and (c) plasma dUrd. Open triangles indicate significant differences with respect to wt mice (P<0.01) and open stars indicate significant differences with respect to KO and sham-treated mice (P<0.01). Note the discontinuity of the y axis scale of a. (d, e and f): Long-term monitoring in vivo obtained from peripheral mice’s blood (weeks 4 to 26) or from intracardiac puncture blood (dashed lines, week 29). Values for each animal are depicted in individual lines with individual symbols. Some symbols are slightly displaced at the last time points to facilitate identification. (d) TP activity in p305-TP treated mice. The gray area at the bottom indicates the range of TP values observed in n=15 untreated wt mice (undetectable for untreated and p-sham-treated Tymp−/−/Upp1−/−mice). (e) Percentage of EGFP+ cells in p305-TP-treated mice. (f) Percentage of EGFP+ cells in p-sham-treated mice.
In order to assess the stability of TP expression, TP activity was monitored over 29 weeks after transplantation in blood samples of the mice. Figure 7d shows that, after a moderate decline of TP activity between the fourth and eighth weeks, all eight TP-treated mice maintained high TP activities up to 29 weeks after the transplantation, more than 20-fold higher than those of wt animals. At week 25, gene-marking levels based on EGFP fluorescence ranged between 1.0 and 7.5%, (Figure 7e) indicating that low levels of molecular chimerism are able to provide high TP activity in vivo. At this point, TP activity did not significantly correlate with molecular chimerism levels (percentage of EGFP+ cells) in PB, but it did with bone marrow chimerism (P=0.038; Supplementary Figure 1).
The sham group (Figure 7f) showed higher percentages of transduction in vivo, and three out of eight mice showed a progressive increase of the %EGFP+ cells between weeks 8 and 26. These increases and those observed in the TP-treated group were not associated with apparent hematopoietic malignant transformation in these mice. Absolute and differential blood cell counts performed in all the animals 29 weeks after treatment were within the normal range for C57BL/6J mice (Peters LL. Aging study: Blood hematology in 31 inbred strains of mice. MPD: Peters4. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, ME, USA. http://phenome.jax.org, December 2010) and did not reveal different distributions of lineages between TP-treated (lymphocytes 84.4±3.1%; granulocytes 12.5±1.9%; monocytes 3.2±1.6%; N=8), sham-treated (lymphocytes 82.6±6.6%; granulocytes 14.6±6.2%; monocytes 2.8±0.7%; N=10), KO (lymphocytes 85.3±1.4%; granulocytes 12.1±1.4%; monocytes 2.7±0.5%; N=8) and wt animals (lymphocytes 83.1±3.1%; granulocytes 14.0±2.2%; monocytes 2.9±1.1%; N=9). In addition, increased TP activity did not affect the ability of the hematopoietic stem cells to differentiate into multiple lineages. Flow cytometric analysis of two TP-treated mice’s PB 33 weeks after treatment revealed that all lineages analyzed (CD11b+, Gr-1+, CD19+ and CD4/8a+) contributed to the EGFP+ cell subpopulation (data not shown).
DISCUSSION
In this work, we show that TP can be restored in human TP-deficient cells and that HSCGT using lentiviral vectors can ameliorate the metabolic abnormalities in a murine model of MNGIE. All therapeutic strategies have been aimed at normalizing the accumulation of the toxic metabolites dThd and dUrd in patients.7,13,14 The observation that platelet transfusions transiently reduced the levels of dThd and dUrd in MNGIE patients,18 suggested that HSCT from healthy donors could provide a permanent source of TP at levels, producing a significant clinical benefit. Patients undergoing a successful allogeneic HSCT show permanent restoration of TP activity with correction of biochemical abnormalities and progressive improvements in clinical parameters.4,12,15,16 However, allogeneic HSCT is not an ideal therapy because of toxicity of the conditioning regimen and the risk of severe complications, such as graft failure and graft-versus-host disease.19
We hypothesized that HSCGT using autologous cells could overcome some of these limitations, and represents a reasonable therapeutic alternative for MNGIE patients. To this aim, we have used lentiviral-mediated gene transfer to demonstrate the feasibility of restoring TP activity in cellular models, and in vivo in a murine model of the disease. HSCGT has already been proven successful in a number of human disorders,20–22 but these successes were partially overshadowed by the observation of severe adverse effects (insertional oncogenesis) in some of the patients.23,24 In order to minimize such risks, novel integrative vectors, which show increased safety, have been developed and some of them have already been used in clinical trials.25 Two main strategies are currently under investigation to minimize potential risks and increase the biosafety profile of vectors. One is the use of lentiviral instead of γ-retroviral vectors, as they were shown to be less prone to integrate near oncogenes and near their origin of transcription.26,27 A second strategy is the use of vectors with self-inactivating (SIN) configuration, which greatly reduces the transactivating potential of the viral long terminal repeat sequences.26
We used EBV transformation to obtain B-LCLs as cellular models for our study. We transduced B-LCLs from two MNGIE patients and two controls, and HEK293T cells with our TP vector, and observed significant transduction efficiencies and good expression levels of functional TP. Only in one case (P2), we observed low levels of transduction, due to unknown reasons. However, this low efficiency was sufficient to provide a TP activity of a third of the normal value for B-LCLs from healthy controls. Overall, the TP activities obtained were directly proportional to the transduction efficiency. Higher copy numbers were detected in the p305-sham-transduced lines as compared with those TP transduced. Rather than a more efficient transduction by the p305-sham vector, this difference could be explained if we take in account that EGFP was expressed at lower levels in the p305-sham vector, as compared with the p305-TP vector. Therefore, the titrations to obtain batches of p305-sham vectors and the multiplicity of infection (MOI) used for the p305-sham transductions were most likely underestimated. To avoid this problem, we used a modified version of the sham vector lacking the IRES for the animal studies.
We used B-LCLs from two patients. P1 was a compound heterozygote for a mutation involving the splicing of intron 2 (c.215–1G>C) and a missense mutation in the exon 7 (c.886A>C),18 and P2 was homozygote for an in-frame 18-bp duplication in the exon 8 (c.994_1011dup).28 In both cases, negligible or undetectable levels of TP protein were observed in cell homogenates from non-transduced and sham-transduced lines, even though they showed similar TYMP mRNA levels as controls (except for non-transduced B-LCLs from P1, which had lower mRNA levels). The presence of mRNAs and absence of both TP protein and enzyme activity suggest that either mutant mRNAs are not translated or that aberrant TP protein is rapidly degraded. Transduction with TP resulted in significant increases in TYMP mRNA levels in C1, C2 and P1, whereas lower transcript levels were observed in P2. The reason for this unexpected reduction is unknown, but it could be partly explained by a decrease in the transcription of the endogenous TYMP gene in response to the transcription of the transgene. This putative repression would be masked in C1, C2 and P1 by the higher levels of transgenic TYMP mRNA, which is indistinguishable from the endogenous mRNA. In fact, reduced TYMP mRNA observed after p305-TP transduction in P2 was accompanied by the expression of TP protein and TP activity, both undetectable in the corresponding sham-transduced or non-transduced B-LCL from P2.
The ratios between TP protein amounts and TP activities in B-LCL homogenates were similar for endogenous and transgenic TP, indicating that both forms of TP have similar Vmax. Transgenic TYMP induced higher levels of protein than those observed in non-transduced control cells, probably due to a higher potency of the hPGK promoter as compared with the endogenous human TYMP promoter. This prompted us to test whether this excess of TP activity could lead to intracellular dTTP depletion, which would be a potentially deleterious effect. Significant dTTP depletion was observed only in the HEK293T cells, probably because this cell line, with a virtually absolute lack of TP activity, achieved the highest enzyme activity among all TP-transduced cell lines. On the other hand, flow cytometry analysis of Hoechst-stained cells did not reveal detectable cytotoxicity or abnormal proliferation associated to this partial dTTP depletion. Interestingly, for almost all cell lines, EGFP+ cell subpopulations showed slightly higher percentages of proliferating cells than EGFP+ subpopulations for both TP-transduced and sham-transduced cells, which could be reflecting the fact that cells or clones with higher proliferative potential were preferentially transduced. At the same time, TP-deficient lines recovered the capacity to catabolize an excess of exogenous dThd and dUrd after TP transduction, which is an important goal from the therapeutic point of view.
Transgenic TP activity was stable in HEK293T cells over at least 15 weeks. Our long-term TP expression studies in the B-LCL model were partially affected by the special features of EBV transformation of B-lymphocytes. Several reports have demonstrated that true immortalization only occurs in some B-LCL clones that survive after long periods of proliferation in pre-immortal phases.29 Our experiments to assess lentiviral transduction and TP expression were performed in B-LCLs with less than 100 culture passages; therefore, it is unlikely that cell lines achieved true immortalization. TP-transduced B-LCLs from P1 survived after a growth crisis and reached stable levels of TP activity for at least 17 weeks. In fact, the results depicted in Figures 6a and b indicate a clonal selection of p305-TP-transduced cells, probably related with the EBV-induced immortalization process discussed above,29 although we cannot rule out the possibility that this proliferative advantage was induced by the increased TP activity or by a vector insertion enhancing cell proliferation. Alternatively, a biased transduction of rapidly proliferative cells that eventually predominate in the cultures could also account for this observation. Although the molecular reasons for this clonal selection should be further investigated, our results show that, at late passages, TP activity was high and stable in this transduced B-LCL and HEK293T cells. Therefore, we can conclude that our p305-TP vector was able to provide long-term expression of active TP to two different TP-deficient cell lines.
We used double Tymp/Upp1 KO mice to evaluate our therapeutic tool in vivo because this model recapitulates the biochemical imbalances and some other features observed in MNGIE patients.10 However, several differences in the pyrimidine deoxynucleoside metabolism between human and mice have to be taken into account. First, both TP and uridine phosphorylase contribute to the catabolism of dThd and dUrd in mice, whereas this role is played entirely (or almost entirely) by TP in humans.30 Second, white blood cells and platelets are among the richest tissues in TP activity in humans,8 whereas in mice, blood cells have the lowest TP activity among all tissues analyzed.10 Third, normal dThd and dUrd plasma levels are higher in mice (1–4μM) than in humans (below 0.05μM). Therefore, HSCGT may constitute a more rational approach in MNGIE patients than in the murine model because restoration in humans would take place in a more relevant target cell type.
Our preliminary in vivo results demonstrate that p305-TP is fully functional as a potential therapeutic vector. We provided allotopic restoration of TP activity to the mice and reached an average TP activity around 11nmol thymine h−1 per mg protein in blood cellular fraction, 1 month after transplantation, and high activities (between 2–30nmol h−1 per mg protein) were maintained at least 29 weeks after transplantation. TP activity of blood cells significantly correlated with %EGFP+ bone marrow cells, but did not with %EGFP+ blood cells. The reasons for this discrepancy are unknown, but could be related with factors masking the correlation in PB but not in bone marrow. For example, high white cell and platelet counts should be associated to higher TP activity, and our TP activity test does not control this variable because most of the protein homogenate comes from erythrocytes, which presumably contain very low TP, and would mask the protein of TP-expressing cells. We measured TP in total blood cells instead of buffy coat because of the limited volumes of blood available. Although reference values for circulating TP activity in humans have been reported only for buffy coat,7,17 we have measured activities in PB cells of healthy controls, which ranged between 4–8nmol h−1 per mg protein (Torres-Torronteras and Martí, unpublished results), indicating that our vector has potential to restore circulating TP activity to normal levels in humans. This activity was sufficient to maintain plasma dThd and dUrd concentrations within the normal range. dThd (but not dUrd) concentrations were moderately over-reduced, but it is unlikely that this difference would produce detrimental effects, given the broad range of dThd levels in the wt mice, which partially overlaps that observed for the TP-treated double KO. This point will have to be analyzed further in the future. As the main objective of a potential treatment for MNGIE is to reduce the systemic overload of dThd and dUrd, these results strongly support further studies of this therapy in this animal model. This will allow us to address several questions raised by our results. For example, pronounced increases of blood TP activity and/or %EGFP+ cells were observed in some mice 26–29 weeks after the treatment, although these increases were not associated to apparent hematopoietic malignant transformation. This and other important issues will have to be clarified with more specific tests in an extended follow-up.
Unlike most hereditary diseases, TP deficiency constitutes a unique situation for several reasons. The molecular defect can be corrected, theoretically, in any tissue or cell type, and the enzyme does not need to be secreted because its substrates are small diffusible molecules. Moreover, the correction of a limited number of cells providing enough TP activity can be sufficient to clear the systemic overload of nucleosides. We used bone marrow cells as target and sublethal total body irradiation as a conditioning regimen before transplantation. As transduced cells expressing TP are not expected to have a selective advantage, as opposed to what occurs in patients with adenosine deaminase-deficient severe combined immunodeficiency,20 we think that some amount of myeloablation is required to facilitate engraftment of the gene-corrected hematopoietic cells. In this regard, regimes based on chemotherapeutic agents as those successfully used in human clinical trials can make the therapy safer. Our results suggest that the level of correction required to obtain a therapeutic benefit is probably very low, and that, for therapeutic purposes, transgene expression does not seem to require a strict regulation, at least in the murine model tested.
In summary, we have generated a lentiviral vector capable of introducing a fully functional version of the TYMP gene in vitro and in vivo. Transduction of human TP-deficient cells with this vector provided stable expression of functional TP that catabolizes an excess of dThd and dUrd present in the extracellular medium. Overexpression of TYMP did not produce cytotoxicity in our conditions, and the allotopic targeting of the gene in a Tymp/Upp1 double KO model restored the correct balanced dThd and dUrd concentrations. Our results strongly suggest that treatment of MNGIE with HSCGT using lentiviral vectors is feasible, and support carrying out long-term studies in the animal model to ensure full correction of the phenotype and the safety of the procedure.
MATERIALS AND METHODS
This study was approved by our institutional review board and animal care and use committee.
Cell culture conditions and lymphocyte transformation
Peripheral blood mononuclear cells were obtained from two patients previously diagnosed with MNGIE by clinical, biochemical and genetic criteria, after informed consent. P1 was compound heterozygote for a missense mutation (c.886A>C), and a mutation in the splice site of intron 2 (c.215–1G>C).18 P2 harbored a homozygous 18-bp duplication in exon 8 (c.994_1011dup).28 Peripheral blood mononuclear cells were obtained from both patients and two healthy controls through Ficoll gradient centrifugation, and cultured in 3.2ml of complete medium (RPMI-1640, Invitrogen, Carlsbad, CA, USA; 15% dialyzed heat-inactivated fetal bovine serum, Invitrogen; 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; 2mML-glutamine; 1Ul−1 penicillin; 1Ul−1 streptomycin; 50μgml−1 gentamicin), supplemented with 1.8ml of EBV-containing supernatant from cultured B95–8 marmoset cells. In all, 1μgml−1 of anti-CD3 OKT3 (eBioscience, San Diego, CA, USA) was added to avoid T-cell response. Cells were maintained in culture for 15–21 days and subcultured in complete medium until cell clumps were visible. Flow cytometry analysis revealed that in all EBV-transformed lines, more than 90% of the cells were CD45+ and CD19+ (data not shown). HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium containing 4.5g l−1 of glucose (Invitrogen), supplemented with 10% heat-inactivated fetal bovine serum, 2mML-glutamine, 1Ul−1 penicillin and 1Ul−1 streptomycin.
Animal model
A Tymp1/Upp1 double KO murine model in C57BL/6J genetic background previously characterized 10 was used for in vivo tests. Two separated reproductive groups were segregated: homozygous Tymp−/−/Upp1−/− (KO group) and homozygous Tymp+/+/Upp1+/+ (wt group). Both inbred groups had isogenic background, except for the Tymp/Upp1 genotype.
Vector construction
The IRAT p970B1069D6 clone from IRAT MGC Human-verified full-length cDNA library, containing the human TYMP full cDNA (NCBI accession BC052211) was purchased from RZPD (Berlin, Germany). The coding fragment was amplified by PCR, excluding the polyA tract (forward primer:5’-GA ACCCTGAACCCTACGGTC-3’ and reverse: 5’-CGCGGCAAAGGAGCTTTATT-3’). A high fidelity polymerase (Platinum Taq DNA polymerase, Invitrogen) was used to reduce the rate of amplification errors. PCR was carried out as follows: one denaturation step at 94°C 2min; 25 cycles at 94°C 30s, 55°C 30s, 68°C 100s; and one final extension step at 68°C 7min. The amplified cDNA was cloned into pCR4-TOPO using TOPO TA Cloning kit for sequencing (Invitrogen), and sequenced with BigDye and ABI-3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). We flanked the TYMP cDNA with the appropriate restriction targets for further directional cloning into the lentiviral vector (EcoRVat 5’ and XbaI at 3’) by subcloning into pBluescript SK plasmid. A clone with the insert in the correct orientation was selected and digested with EcoRV (blunt ends) and XbaI (New England Biolabs, Ipswich, MA, USA) to obtain the TYMP cDNA fragment ready to be cloned into the vector. The self-inactivated lentiviral vector pCCL.sin.PPT.hPGK.deltaNGFR. IRESemvcv.wt.eGFP.Wpre (p305) was kindly provided by Dr L Naldini (San Raffaele Telethon Institute for Gene Therapy, Milano, Italy). We removed the deltaNGFR fragment by SmaI (blunt ends) and XbaI digestion, and introduced our TYMP cDNA fragment to obtain our active vector (p305-TP). We obtained a sham vector (p305-sham) by simply removing the deltaNGFR fragment through BamHI digestion and re-ligation. The p305-sham vector was used for the in vitro study in cultured cells. A different sham vector, identical to p305-sham but lacking the IRES (p-sham) was kindly provided by L Naldini, and used for the animal studies. Previous work in our laboratory showed that this vector resulted in higher EGFP expression levels than p305-sham. Figure 1a shows the representation of p305-TP (containing the lentiviral backbone, the human phosphoglycerate kinase, hPGK, promoter to drive the bicistronic expression of TYMP and EGFP), the p305-sham and the p-sham. No mutations were detected in the TP-coding region, and correct orientation of integration was confirmed by direct sequencing, as compared with the wt sequence number NM_001953.3 in the NCBI (data not shown).
Vector production, titration and transduction
Lentiviral vectors were produced by transient co-transfection of HEK293T cells with p305-TP (or p305-sham, or p-sham) and the packaging plasmids pRSV REV, pMDLg RRE and pMD VSVG at 37°C and 5% CO2. The supernatant medium containing transductant particles was recovered 48, 56 and 72 h after transfection, filtered through a 0.45-μm membrane and enriched by at least 100-fold through ultracentrifugation at 50000g, for 2h at 4 °C. Titration was carried out by treating HEK293T cells with serial dilutions of the enriched supernatants. After 4 days in culture, HEK293T cells were trypsinized and EGFP fluorescence was analyzed by flow cytometry to estimate transduction rates, and viral titers were calculated.31
B-LCL transductions were performed at 15 MOI in 14 μg cm2 RetroNectin (Takara, Otsu, Japan)-coated 24 multi-well plates in the presence of 4 μg ml−1 protamine sulfate. HEK293T cells were transduced at 10 MOI without Retro-Nectin. After addition of vectors, the plates were centrifuged at 300g, 32°C for 90min, and transductions were allowed to proceed for an additional period of 48h at 37°C, 5% CO2. Then, cells were washed once with phosphate-buffered saline (PBS) and fresh medium was added to the cultures.
For transduction of murine hematopoietic progenitors, 5×105 Lin− cells per well, obtained as detailed below, were seeded in 24-well plates at 5×105cells per ml in Iscove’s modified Dulbecco’s medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 2mML-glutamine, 1Ul−1 penicillin, 1Ul−1 streptomycin and recombinant murine growth factors (rmSCF 10ngμl−1, rmTPO 10ngμl−1, rmIL3 10ngμl−1 and rmFlt3 10ngμl−1) (ImmunoTools, Friesoythe, Germany). Lentiviruses were added at 100 MOI and cells were centrifuged at 300g, 32°C for 90min. Transductions were allowed to proceed overnight at 37°C, 5% CO2. Then, cells were washed with PBS and re-suspended (2.5×106cells per ml) in PBS immediately before the infusion to the recipient mice.
Flow cytometry analysis
EGFP fluorescence was analyzed by flow cytometry to assess the percentage of transduced cells. Cultured cells, or buffy coat and bone marrow cells from mice, were re-suspended in fresh medium and fluorescence was measured through a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The fluorescence cutoff was chosen at that value that rendered a 1% or less positive events in a negative control for EGFP or the fluorescent antibody indicated in the specific experiment. For cell cycle analyses, cultured cells were stained with Hoechst 33342, then EGFP- and Hoechst-derived fluorescence was simultaneously measured using a MoFlo Legacy cell sorter device (Beckman Coulter, Brea, CA, USA). Data was analyzed using the FCS Express version 3 software (De Novo Software, Los Angeles, CA, USA).
PB cell differential counts were performed using a BC-2800 Auto Hematology analyzer (Mindray, Mahwah, NJ, USA).
Assessment of DNA copy number and mRNA levels by qRT-PCR
qRT-PCR determinations were performed using pre-designed or custom-designed TaqMan assays in the ABI PRISM 7500 sequence detection system (Applied Biosystems). For copy number quantification of specific DNA sequences, total DNA was obtained by phenol–chloroform extraction from 107 cells. Transgenic TYMP cDNA copy number (integrated in the genome from p305-TP transduction) was quantified using the pre-designed TaqMan MGB gene expression assay Hs00157317_m1 (Applied Biosystems), which amplifies a 95-nucleotide amplicon covering the exon 4–5 junction. Transgenic TYMP cDNA is detected by this assay because it does not contain intronic sequences. EGFP gene copy number (integrated in the genome from p305-TP, p305-sham or p-sham transduction) was quantified using custom-designed specific primers and TaqMan MGB probe, as previously described.31 Both TYMP cDNA and EGFP copy numbers were normalized to the copy number of human RNase P gene (TaqMan RNase P detection reagents kit) in B-LCLs, and to the copy number of murine Ang1 gene (pre-designed TaqMan MGB gene expression assay, Id assay Mm00833184_s1) for murine samples. As both RNase P and Ang1 are single copy genes (two copies per diploid cell), we could express the results as copy number per cell. In all experiments, the quantification was based on a standard curve prepared with different dilutions of DNA from a control transduced with p305-TP (which contains the same number of copies of TYMP and EGFP) or with a plasmid containing the RNAse P or Ang1 fragments amplified in the assay. The ratios between TYMP, EGFP and RNAse P or Ang1 were calculated and a correction factor obtained from different amplification efficiencies was applied.
For mRNA quantification, total RNA was extracted from 5×106 cells using Trizol reagent (Invitrogen). The amount and integrity of the RNA obtained was assessed by capillary electrophoresis using the RNA 6000 Nano Chip kit (Agilent Technologies, Santa Clara, CA, USA). The levels of RNA degradation were always negligible (data not shown). In all, 2μg of RNA was treated with DNase I (Invitrogen). Reverse transcription was performed on DNA-free RNA using the High-capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resulting cDNA was used for qRT-PCR assays using the probes indicated above for TYMP and EGFP. Results of TYMP and EGFP mRNA levels were referred to PPIA mRNA (pre-designed TaqMan MGB gene expression assay, Id assay Hs99999904_m1). Amplifications were performed as follows: one step at 501C 2min; one step at 951C 10min; 40 cycles at 951C 15s, 601C 1min. For each experiment, all reactions were run in triplicates.
Western blot analysis
Protein extracts were obtained as described below for TP activity determination. In all, 3.5μg of protein extracts were electrophoresed in a 10% SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with an anti-TP mouse monoclonal antibody (Calbiochem, Darmstadt, Germany) and an anti-β-actin mouse monoclonal antibody (Sigma-Aldrich, St Louis, MO, USA), and then with peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Bands were visualized by treating the membranes with ECL chemiluminescent kit (GE Healthcare, Buckinghamshire, England), and quantified using Quantity One software (Bio-Rad, Hercules, CA, USA). All the TP-to-β-actin ratios were referred to the corresponding ratio obtained from a control extract, used as a calibrator that was run in all gels.
Deoxyribonucleoside triphosphate determination
Deoxyribonucleoside triphosphate determinations were performed using a polymerase assay based on the incorporation of 3H-dATP or 3H-dTTP in synthetic oligonucleotides,32 in total cell homogenates obtained as previously described.33 Briefly, cells were lysed by mechanical disruption through a 27-gauge1-inch needle in 2ml of isolation buffer (220mM mannitol, 70mM sucrose, 5mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.2, 1mM ethylene glycol tetraacetic acid, 0.5% bovine serum albumin), and proteins were precipitated through addition of 60% methanol (final concentration). Supernatants containing total cell deoxyribonucleoside triphosphates were dried under speed vacuum and stored at −80°C. Dry pellets were re-suspended in 400μl of water and 10μl were added to each polymerase reaction.32
Transplantation of mice with transduced bone marrow Lin−
Double KO donors (8- to 12-week-old) were killed by CO2 inhalation, and bone marrow was obtained by crushing femorae, tibiae and iliac crests with mortar and pestle. Lin− bone marrow cells were immunoselected by lineage depletion (Lineage Cell Depletion Kit for Mouse, Miltenyi Biotech, Bergisch Gladbach, Germany). After enrichment, cells were transduced with p305-TP or p-sham vectors, as detailed above. Between 2×105 and 5×105 transduced cells were injected in the tail vein of 8- to 12-week-old double KO recipient mice, after sublethal myeloablation using total body irradiation (6Gy, in two doses of 3Gy at a dose rate of 2.24Gy min−1 given at a 2-h interval). Mice were housed in filter-isolated ventilated cages for 15 days after transplantation to reduce the risk of infection, and then housed in conventional cages. PB was collected at different times from the saphenous vein for different determinations. At week 29, p305-TP- and p-sham-treated mice were killed by CO2 inhalation, and blood was collected immediately by intracardiac puncture, as well as bone marrow, as detailed above.
Nucleoside levels and TP activity determination
dThd and dUrd were measured in cell culture media and mice plasma by a binary gradient-elution ultra performance liquid chromatography (UPLC) method. Cultured cells were centrifuged at 300g for 5min, and the supernatant was recovered and deproteinized through ultrafiltration (Microcon YM-10, Millipore, Billerica, MA, USA) at 14000g, 30min, 25°C. The cellular pellet was PBS washed and kept at −80°C for TP assay. In all, 50μl of mouse anticoagulated (EDTA) PB was collected from the saphenous vein and diluted in 400ml PBS, centrifuged at 3000g for 5min at 4°C and the supernatant (diluted plasma) was deproteinized through addition of 0.5M (final concentration) perchloric acid. Blood cell fraction was kept at −80°C for TP assay. In all, 5ml of medium or diluted plasma, both deproteinized, were injected into an Acquity UPLC apparatus (Waters, Milford, MA, USA) and eluted at 0.5ml min−1 with a saline buffer eluent A (20mM ammonium acetate, pH 5.6) and an organic eluent B (methanol gradient grade), according to the following gradient: 0 to 1.1min, 100% eluent A; 1.1 to 5 min, 100 to 86.4% eluent A; 5 to 6.1 min, 0% eluent A; 6.1 to 7.2min, 100% eluent A. The column used was an Acquity UPLC BEH C18 column 1002.1mm, 130Å pore size, 1.7mm particle size (Waters). Optical absorbance of the eluate was monitored at 267nm, and definitive identification of the dThd and dUrd peaks was based upon retention time and by treatment of a second aliquot of the sample with a large excess of purified Escherichia coli TP (Sigma-Aldrich) to eliminate the dThd and dUrd. The quantitation of the nucleosides was based on peak areas using external aqueous standards.
TP activity was determined in cultured cells and PB cell fraction. Cultured cells (5106) or PB cell fraction obtained as detailed above were homogenized in 200ml of lysis buffer (50mM Tris-HCl, pH 7.2; 10ml l−1 Triton X-100; 2mM phenylmethylsulfonyl fluoride; 0.2mll−1 2-mercaptoethanol) by passing 12 times through a 27-gauge×1-inch needle. TP activity was determined as detailed elsewhere.8 Briefly, 74μl of homogenate containing 10mg (cultured cells) or 2mg (blood cell fraction) of total protein was incubated at 37°C for 1h in the presence of 10mM dThd in the reaction buffer,8 and the thymine formed was measured through the UPLC method described above for nucleoside determination. The protein content was determined through the Bradford method.34
Statistical analysis
Statistical analysis was performed with the SPSS 15.0 software. Tests used are indicated in the Results section and/or the figure legends. For statistical purposes, undetectable values were considered as zero.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Luigi Naldini for kindly providing the lentiviral vectors p305 and p-sham, and Michael Terry for his English Language assistance. This work was supported by the Spanish Instituto de Salud Carlos III [PI 06/0735, PS09/01591, Miguel Servet grants to JB and RM] and the United Mitochondrial Disease Foundation [UMDF 04/042].
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999; 283: 689–692. [DOI] [PubMed] [Google Scholar]
- 2.Hirano M, Nishigaki Y, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes. Neurologist 2004; 10: 8–17. [DOI] [PubMed] [Google Scholar]
- 3.Lara MC, Valentino ML, Torres-Torronteras J, Hirano M, Martí R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): biochemical features and therapeutic approaches. Biosci Rep 2007; 27: 151–163. [DOI] [PubMed] [Google Scholar]
- 4.Halter J, Schuüpbach WM, Casali C, Elhasid R, Fay K, Hammans S et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant 2011; 46: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desgranges C, Razaka G, Rabaud M, Bricaud H. Catabolism of thymidine in human blood platelets: purification and properties of thymidine phosphorylase. Biochim Biophys Acta 1981; 654: 211–218. [DOI] [PubMed] [Google Scholar]
- 6.Marti R, Nishigaki Y, Hirano M. Elevated plasma deoxyuridine in patients with thymidine phosphorylase deficiency. Biochem Biophys Res Commun 2003; 303: 14–18. [DOI] [PubMed] [Google Scholar]
- 7.Spinazzola A, Marti R, Nishino I, Andreu AL, Naini A, Tadesse S et al. Altered thymidine metabolism due to defects of thymidine phosphorylase. J Biol Chem 2002; 277: 4128–4133. [DOI] [PubMed] [Google Scholar]
- 8.Valentino ML, Martí R, Tadesse S, López LC, Manes JL, Lyzak J et al. Thymidine and deoxyuridine accumulate in tissues of patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). FEBS Lett 2007; 581: 3410–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro P, Pontarin G, Crocco L, Fabris S, Reichard P, Bianchi V et al. Mitochondrial deoxynucleotide pools in quiescent fibroblasts: a possible model for mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). J Biol Chem 2005; 280: 24472–24480. [DOI] [PubMed] [Google Scholar]
- 10.Lopez LC, Akman HO, García-Cazorla A, Dorado B, Martí R, Nishino I et al. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet 2009; 18: 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vocht C, Ranquin A, Willaert R, Van Ginderachter JA, Vanhaecke T, Rogiers V et al. Assessment of stability, toxicity and immunogenicity of new polymeric nanoreactors for use in enzyme replacement therapy of MNGIE. J Control Release 2009; 137: 246–254. [DOI] [PubMed] [Google Scholar]
- 12.Hirano M, Martí R, Casali C, Tadesse S, Uldrick T, Fine B et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology 2006; 67: 1458–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran NF, Bain MD, Muqit MM, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIE. Neurology 2008; 71: 686–688. [DOI] [PubMed] [Google Scholar]
- 14.Yavuz H, Ozel A, Christensen M, Christensen E, Schwartz M, Elmaci M et al. Treatment of mitochondrial neurogastrointestinal encephalomyopathy with dialysis. Arch Neurol 2007; 64: 435–438. [DOI] [PubMed] [Google Scholar]
- 15.Hirano M, Casali C, Tadesse S, Stanzani M, Savage DG. Sustained biochemical and clinical improvements two years post-allogeneic stem cell transplantation in a patient with MNGIE. Neurology 2008; 70 (Suppl 1): A406–A407. [Google Scholar]
- 16.Schupbach M, Benoist JF, Casali C, Elhasid R, Fay K, Hahn D et al. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) for Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE). Neurology 2009; 73: 332–332. [Google Scholar]
- 17.Marti R, Spinazzola A, Tadesse S, Nishino I, Nishigaki Y, Hirano M. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem 2004; 50: 120–124. [DOI] [PubMed] [Google Scholar]
- 18.Lara MC, Weiss B, Illa I, Madoz P, Massuet L, Andreu AL et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology 2006; 67: 1461–1463. [DOI] [PubMed] [Google Scholar]
- 19.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826. [DOI] [PubMed] [Google Scholar]
- 20.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413. [DOI] [PubMed] [Google Scholar]
- 21.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823. [DOI] [PubMed] [Google Scholar]
- 22.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409. [DOI] [PubMed] [Google Scholar]
- 23.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419. [DOI] [PubMed] [Google Scholar]
- 24.Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, kinner A et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 2010; 16:198–204. [DOI] [PubMed] [Google Scholar]
- 25.Matrai J, Chuah MK, Vandendriessche T. Recent advances in lentiviral vector development and applications. Mol Ther 2010; 18: 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 2009; 119: 964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003; 300: 1749–1751. [DOI] [PubMed] [Google Scholar]
- 28.Gamez J, Lara MC, Mearin F, Oliveras-Ley C, Raguer N, Olive M et al. A novel thymidine phosphorylase mutation in a Spanish MNGIE patient. J Neurol Sci 2005; 228: 35–39. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M, Tahara H, Ide T, Furuichi Y. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res 2004; 64: 3361–3364. [DOI] [PubMed] [Google Scholar]
- 30.el Kouni MH, el Kouni MM, Naguib FN. Differences in activities and substrate specificity of human and murine pyrimidine nucleoside phosphorylases: implications for chemotherapy with 5-fluoropyrimidines. Cancer Res 1993; 53: 3687–3693. [PubMed] [Google Scholar]
- 31.Meza NW, Puyet A, Pérez-Benavente S, Quintana-Bustamante O, Diez A, Bueren JA et al. Functional analysis of gammaretroviral vector transduction by quantitative PCR. J Gene Med 2006; 8: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 32.Ferraro P et al. Quantitation of cellular deoxynucleoside triphosphates. Nucleic Acids Res 2010; 38: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pontarin G, Gallinaro L, Ferraro P, Reichard P, Bianchi V. Origins of mitochondrial thymidine triphosphate: dynamic relations to cytosolic pools. Proc Natl Acad Sci USA 2003; 100: 12159–12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.