Abstract
Our assignment was to review the development of the face-processing network, an assignment that carries the presupposition that a face-specific developmental program exists. We hope to cast some doubt on this assumption and instead argue that the development of face processing is guided by the same ubiquitous rules that guide the development of cortex in general.
Keywords: faces, domains, development, inferotemporal cortex
1. INTRODUCTION
The prevalent assumption of a face-specific program likely arises from our extensive experience with faces and their importance for social communication. The behavioral significance of faces in combination with their visual complexity has undoubtedly fostered a view that faces must be neurally special. Much research has been focused on resolving how neurons could develop tuning for such a specific class of stimuli. Disparate views on the development of face perception generally differ along one dimension: innateness. ; i.e., what is built into the system and what requires extrinsic experience. One point of view is that the brain is innately organized into anatomically distinct regions, each processing different biologically important image categories, or image categories that resemble biologically important things (McKone et al. 2012, Robbins & McKone 2007, Wilmer et al. 2010). The alternative point of view is that extrinsic experience is required for the development of specialized circuits for processing features in one’s environment (Gauthier 2000, Gauthier & Bukach 2007, Tarr & Cheng 2003, Tarr & Gauthier 2000). Our goal is to try to reconcile discrepant views on the neural development of face-processing regions by taking a bottom-up, reductionistic approach to see whether seemingly complex phenomena can be accounted for by ubiquitous, experimentally established mechanisms.
Even early in life, faces have social importance, and infants will preferentially look at the faces of their parents morethan at strangers (Bushnell et al. 1989). The importance grows as developing primates learn that faces convey a wealth of information about their social environment. Identity, thoughts, emotions, and intentions can all be inferred from faces—all in a single glance. Despite this fast processing, primates spend an extraordinary amount of time attending to faces in social contexts. What’s more, we are so primed to see faces that we perceive them even in things like toast or water stains that share only slight similarity in structure (face pareidolia). The ethological importance of faces and the prioritization of facial processing in our visual behavior have been interpreted by many to indicate that the primate brain must have specialized circuitry for face recognition (Grill-Spector et al. 2004, McKone et al. 2012, Yovel & Kanwisher 2004).
The ventral part of the primate temporal lobe [termed the inferotemporal cortex (IT)] is important for the recognition of faces and, more generally, objects, because lesions in IT result in object recognition deficits, or agnosias. The agnosias resulting from such lesions can be surprisingly specific, affecting particular object categories, such as faces, text, or tools, while sparing others (Farah 1990, 1992; Moscovitch et al. 1997) (Figure 1a). Konorski was probably the first to suggest that the specificity of agnosias indicates that there must be ‘gnostic fields’ (Konorski 1967 p 106), or parts of the brain dedicated to processing specific perceptual categories, and indeed functional MRI (fMRI) studies much later provided evidence for the functional specialization of IT. Both humans (Clark et al. 1996, Kanwisher et al. 1997, Puce et al. 1996) and monkeys (Bell et al. 2009, Pinsk et al. 2005, Tsao et al. 2003) have fMRI domains that are more responsive to faces than to other object categories (Figure 1b). There are also domains selective for places (Aguirre et al. 1998b, Epstein et al. 1999, Kornblith et al. 2013, Nasr et al. 2011), buildings (Aguirre et al. 1998a), tools (Beauchamp et al. 2003, Chao et al. 2002, Downing et al. 2006), bodies (Bell et al. 2009, Downing et al. 2001, Pinsk et al. 2005, Tsao et al. 2003), and, in literate humans, text (Cohen et al. 2000). These domains are found in stereotyped locations in both species.
Figure 1.
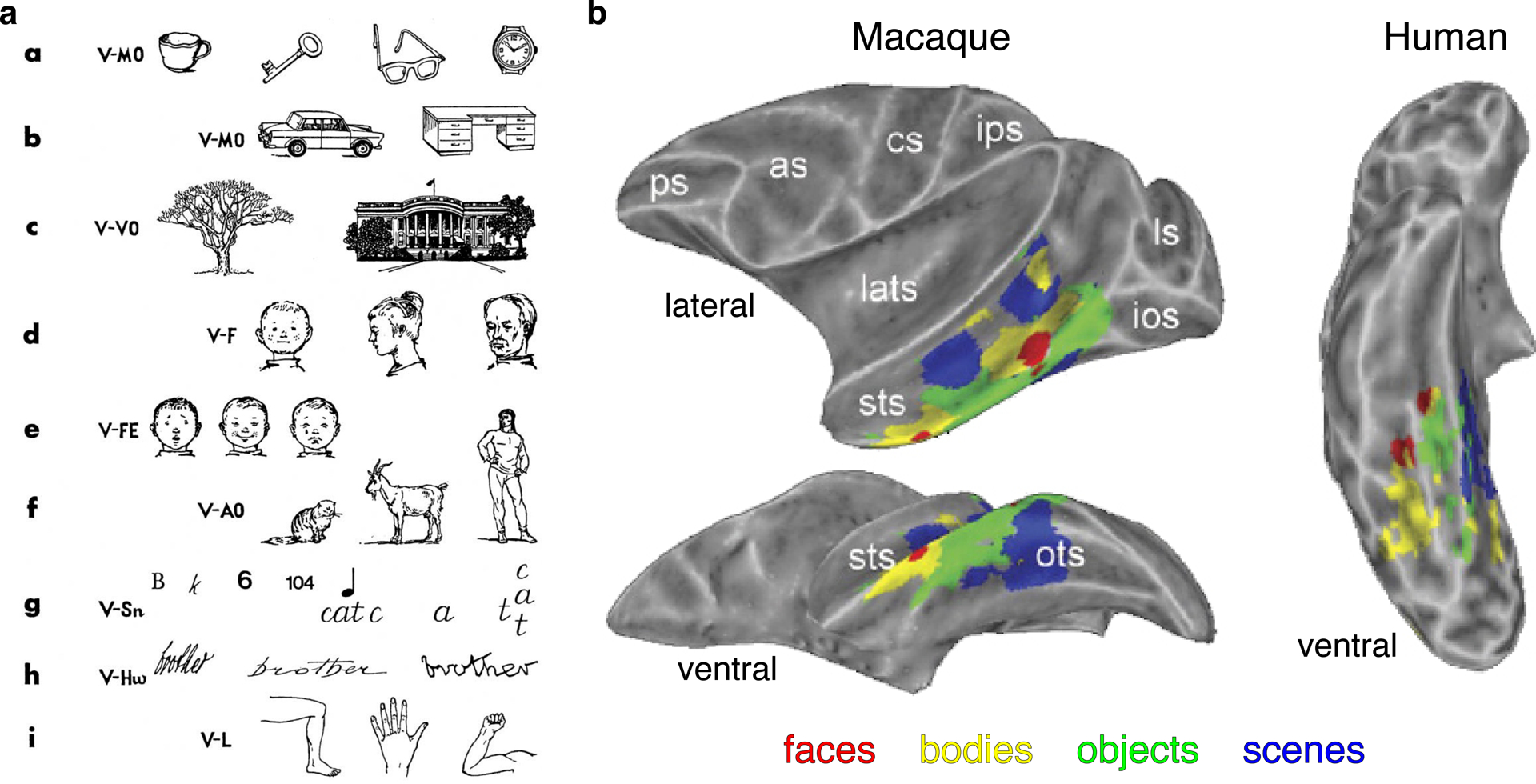
Behavioral and neural correlates of object processing. (a) Different kinds of visual agnosias according to Konorski (1967; reproduced with permission). (b) Face, body, object, and scene domains in inferior temporal cortex of macaques (left) and humans (right). Image adapted with permission from Bell et al. (2009). Abbreviations: as, arcuate sulcus; cs, central sulcus; ios, inferior occipital sulcus; ips, intraparietal sulcus; lats, lateral sulcus; ls, lunate sulcus; ps, principal sulcus; sts, superior temporal sulcus.
Konorski predicted not only the existence of domains dedicated to processing particular categories but also the existence of individual neurons that would be selective for such high-level percepts as faces, facial expressions, or places. He proposed that such gnostic neurons were a logical extension of the hierarchy proposed by Hubel & Wiesel (1962, 1965) for the processing of visual information from geniculate to primary visual cortex and thence to extrastriate visual areas. In this hierarchy, center/surround receptive fields in the geniculate combine to form simple cells in visual cortex, which are then combined to generate complex cells and then hypercomplex cells. Hubel & Wiesel proposed that such a progression could be accomplished by iterative rules, performing similar calculations at each successive stage, reresulting in sequentially more complex properties. Face areas in IT are situated at late stages of this hierarchy which comprises a few dozen visual areas in total. In IT, neurons can be selective for quite complex stimuli, such as bodies, places, or faces (Bruce et al. 1981, Desimone et al. 1984, Kobatake & Tanaka 1994, Perrett et al. 1987). Because we see IT as one (or several) stage(s) in this hierarchy, we want to back away not only from the idea that the circuitry of face domains is qualitatively different from those of other kinds of domains in IT, but also from the idea that the mechanisms involved in the development of domains in IT are qualitatively different from the mechanisms involved in the development of early cortical visual areas. Indeed, we hope to convince the reader that these mechanisms are universal to all of cortex.
2. VISUAL HIERARCHY
Mammalian brain evolution is characterized by increasing cortical territory interspersed between primary sensory areas (Krubitzer 2007). The stereotyped location of sensory areas relative to one another can be found in all mammals (Figure 2a), suggesting that the mechanisms guiding the general topological organization of the cortical surface must be evolutionarily old. The organization of cortex is determined by a combination of intrinsic genetic factors and activity-dependent processes. The size of cortex is determined by genes that control cell death and cell cycle in the ventricular zone (Kornack & Rakic 1998). Primary (thalamo-recipient) sensory areas are specified early in development by molecular markers and receive thalamic inputs mapped, by means of molecular gradients, according to the physical layout of the peripheral sense organ (Flanagan 2006). Primary sensory areas project to adjacent cortex in a mirror-image fashion that preserves the topography of the sensory space (Cragg 1969, Kaas et al. 1979, Zeki 1969) (Figure 2b). Secondary areas in turn project in mirror-image fashion to the next adjacent cortex (Van Essen et al. 2001) (Figure 2c). At each successive stage, individual neurons receive input from groups of neurons in the previous layer that overlap in their sensory representations and vary to some extent in their tuning, resulting in progressively larger and more complex representations of sensory space at each successive stage. In the visual cortical hierarchy, the primary visual area (V1) comprises a map of the contralateral visual field in each hemisphere; the secondary visual area (V2) receives input from V1 and comprises a second distinct cortical map of contralateral visual space that is mirror symmetric to V1’s map and that has slightly larger receptive fields with more complex tuning; and so on. Within a species, individuals have the same number of maps laid out in a stereotypical fashion across the cortical surface. Thus, a stereotypical layout of cortical areas could unfold in a given species, requiring only a molecular predetermination of the primary, thalamo-recipient, zones. Though it is unknown whether the number of mirror maps is predetermined, or whether mirroring occurs until available cortical territory is occupied, a huge fraction of cortex is organized into continuous mirror maps (Kaas 1997) that appear to be established by or shortly after birth (Arcaro & Livingstone 2017a). The regularity of these sequential mirror maps throughout the visual system, like the progressively more complex neuronal tuning, supports Konorski’s contention that higher visual areas, despite their adaptive and disparate selectivities, are formed by iterative rules acting similarly at each level of the hierarchy, anchored to the primary sensory map, as opposed to area-specific mechanisms.
Figure 2.
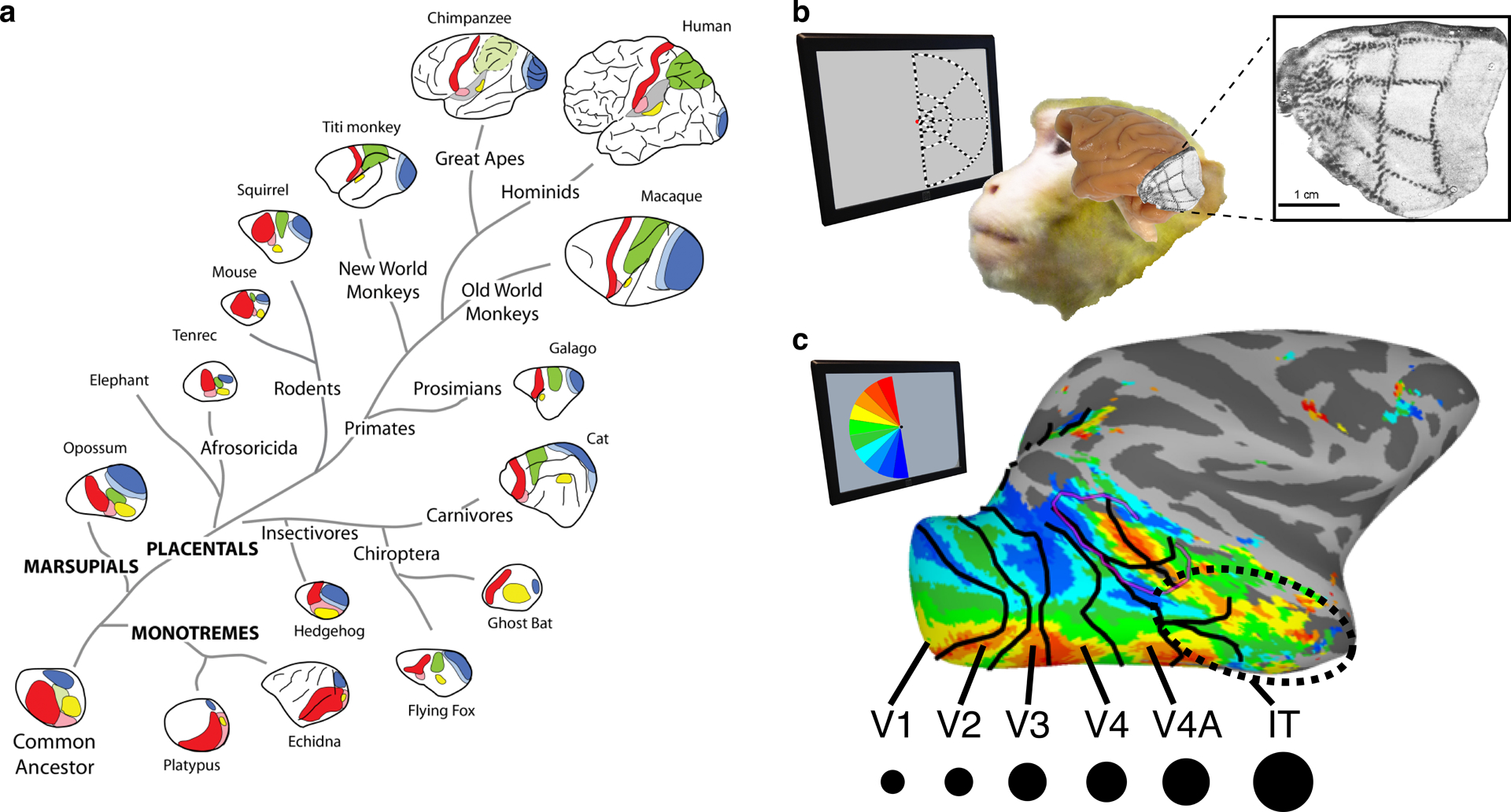
Topological organization of sensory maps. (a) Primary sensory areas lie adjacent to each other in lower mammals but spread apart over evolution; areas between comprise increasing numbers of mirror-image maps (reproduced with permission from Krubitzer 2007). (b) Representation of contralateral visual space in V1 (Tootell et al. 1988; adapted with permission). (c) Mirror-image maps cover almost the entire macaque visual system. Moving up the visual hierarchy from V1 to IT, receptive field size (black dots) increases. Abbreviations: IT, inferotemporal cortex
IT cortex sits at later stages within the visual hierarchy, comprising neurons with large receptive fields and complex tuning that reflect the aggregation of inputs at several stages (Figure 2c). Though the precise number of areas within IT is unknown, three subdivisions along the caudo-rostral axis have been proposed based on anatomy (posterior, central, and anterior IT). Yet finer areal distinctions may exist as several retinotopic representations within the two posterior-most subdivisions have been identified (Arcaro & Livingstone 2017b, Janssens et al. 2014, Kolster et al. 2014).
3. UBIQUITOUS RULES GUIDE VISUAL DEVELOPMENT
3.1. Complexity from Simple Rules
The rules driving the organization within IT are apparent at the earliest stages of the visual hierarchy. It is well accepted that the exquisite organization of V1 arises according to developmental mechanisms ubiquitous across cortex and across species: (a) Axons from the thalamus spread out across V1 according to gradients of trophic molecules, creating a topographic map of visual space (O’Leary et al. 1999, Tessier-Lavigne & Goodman 1996). (b) Activity-dependent mechanisms cause neurons to retain and strengthen synaptic inputs that fire in a similar manner to each cell’s most active inputs, and to lose inputs that differ in firing pattern (Katz & Shatz 1996). Such mechanisms thus encourage the clustering of inputs with similar activity patterns and segregation of inputs with differing activity patterns, yet preserve the overarching retinotopic map. This short-range affinity/long-range repulsion is a special case of the reaction/diffusion mechanism proposed by Alan Turing (1952), whose simple rules can generate stable, regular, striped patterns (Figure 3a) in simple chemical reactions (Castets et al. 1990, Oyuyang & Swinney 1991), symmetry-breaking in development (Soriano et al. 2009), and myriad striped and spotted patterns observed in nature (Meinhardt & Gierer 2000, Painter et al. 1999). Activity-dependent sorting causes refinement of the V1 retinotopic map (driven by retinal proximity) (Cang et al. 2005, Penn et al. 1998, Shatz & Stryker 1988) but also produces clustered segregation of inputs from the two eyes (Hubel et al. 1975) (Figure 3b,c) and segregation by retinal cell type (Kremkow et al. 2016). The regularity of the organization of V1 (Hubel & Wiesel 1974) and the profound effects on this organization arising from experimental manipulations (Hubel & Wiesel 1970, Hubel et al. 1977, Sengpiel et al. 1999, Tanaka et al. 2006) together indicate that this complex arrangement of multiple features develops by activity-dependent self-organizing mechanisms without the need for prescribed functions.
Figure 3.
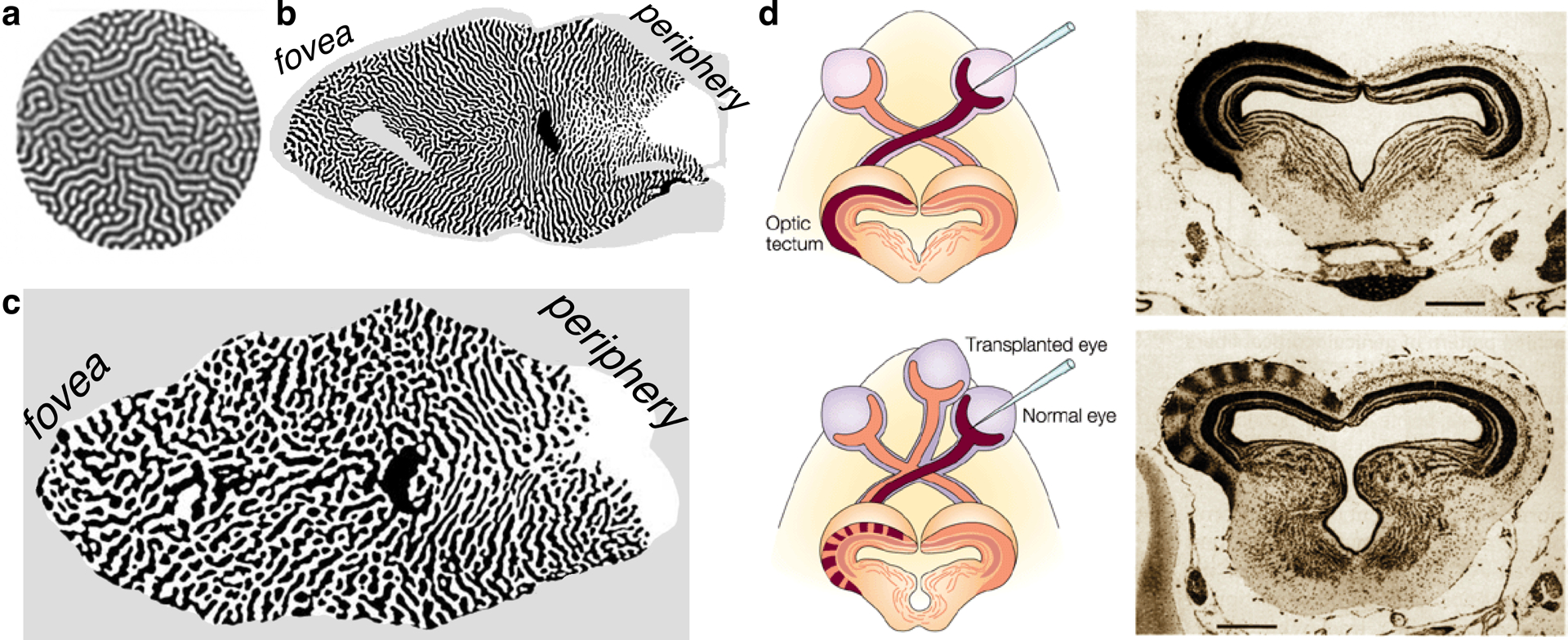
Self-organizing patterns. (a) Spontaneous stable Turing patterns in a chlorine dioxide–iodine–malonic acid reaction (Peña et al. 2003; reproduced with permission). (b) Ocular dominance stripes in macaque V1 run perpendicular to the V1/V2 border (Sincich & Horton, 2002; reproduced with permission) (c) Ocular dominance stripes in human V1 run perpendicular to the V1/V2 border (Adams et al. 2007; modified with permission). (d) Self-organizing rules lead to ocular dominance columns in a three-eyed frog, even though the frog tectum is normally innervated entirely by only the contralateral eye; scale bars 400 micrometers (Constantine-Paton & Law 1978; reproduced with permission).
3.2. Function Follows Form
What drives the development of visual domains? The input layers of V1 reiterate a high-resolution map of the surface of the retina, or visual field, but they are also organized into an almost crystalline array along several other parameters: ocular dominance, color, orientation, and sign of contrast (Figure 4). These domains are present at birth (Blasdel et al. 1995, Wiesel & Hubel 1974) and are organized in a similar, regular pattern in Old World monkeys (Hubel et al. 1975), great apes (Tigges & Tigges 1979), and humans (Adams et al. 2007). The apparent utility of extracting information about contrast, orientation, and eye of origin for visual perception suggests that this intrinsic organization reflects an evolutionary advantage to organizing these features in a regular and precise way. Yet visual perception is not dependent on these architectural features. Some New World monkeys lack ocular dominance columns yet have stereoscopic vision comparable to that of Old World monkeys (Livingstone et al. 1995). Further, columnar organization can be induced in animal species that normally lack such neural architecture (Constantine-Paton & Law 1978, Livingstone 1996). Frogs, which do not normally have ocular dominance columns because their optic projections are entirely crossed, develop clear ocular dominance columns if an extra eye is transplanted onto a tadpole (Constantine-Paton & Law 1978) (Figure 3d). Therefore, complex domain architecture such as the columnar architecture of V1 likely does not emerge to serve a particular function but rather arises as a consequence of more general wiring rules. When present, ocular dominance columns tend to run perpendicular to the V1/V2 border, suggesting that in those species eye of origin is the strongest activity-dependent discrimination. Orientation stripes, in turn, run perpendicular to ocular dominance stripes, but, in species without ocular dominance, orientation stripes instead run perpendicular to the V1/V2 border (Blasdel & Campbell 2001). V2 stripes that constitute color, form, or motion processing domains run perpendicular to V1/V2 and V2/V3 borders (Livingstone & Hubel 1984, Tootell et al. 1983). The fungibility of features in the organization of stripes at map borders further indicates that universal activity-dependent segregation rules, rather than innate programs specific to each feature, govern the complex pattern of features in V1.
Figure 4.
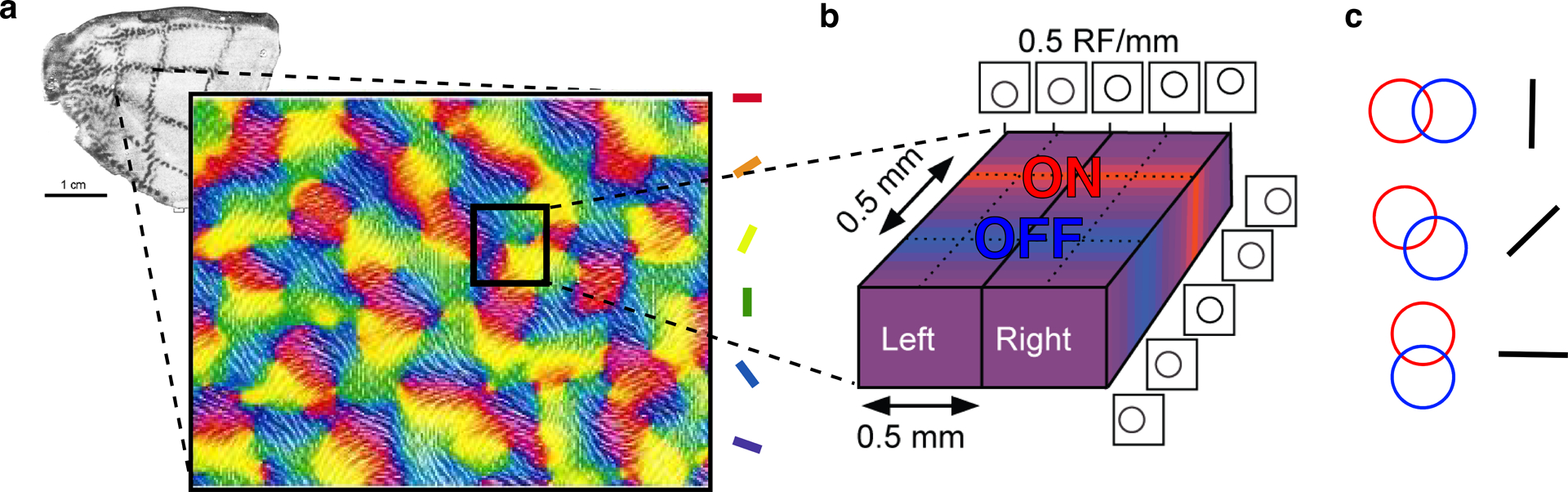
Self-organization of macaque V1 by activity-dependent clustering of inputs with similar response properties. (a) Map of orientation selectivity in macaque V1. (left adapted with permission from Tootell et al. (1988); large orientation map reproduced with permission from (Blasdel 1992). (b) The input layer of V1 is segregated into domains for left and right eyes as well as ON and OFF and, more globally, comprises a map of visual space (Kremkow et al. 2016; reproduced with permission). (c) Different locations of ON and OFF inputs lead to orientation selectivity in layers above and below L4 (Kremkow et al. 2016; reproduced with permission).
The same activity-dependent mechanisms that drive formation of the crystalline pattern of stripes of ocular dominance and orientation selectivity in V1 prenatally continue to act postnatally. Many studies have shown that activity-dependent synaptic plasticity not only sets up the initial organization of V1 prenatally but remains such a powerful influence postnatally that if the animal’s visual experience is abnormal, the wiring can be dramatically altered and V1 can become responsive to only the kinds of things the animal experienced. If a young animal sees through only its left eye, V1 becomes unresponsive to the right eye (Figure 5a). If the animal never sees horizontal contours, it loses the ability to process them (Figure 5b). This postnatal plasticity holds true for other primary sensory cortices in many species (Figure 5c), which implies that the cortex is a statistical learning machine that adapts to process whatever it is presented within its environment.
Figure 5.
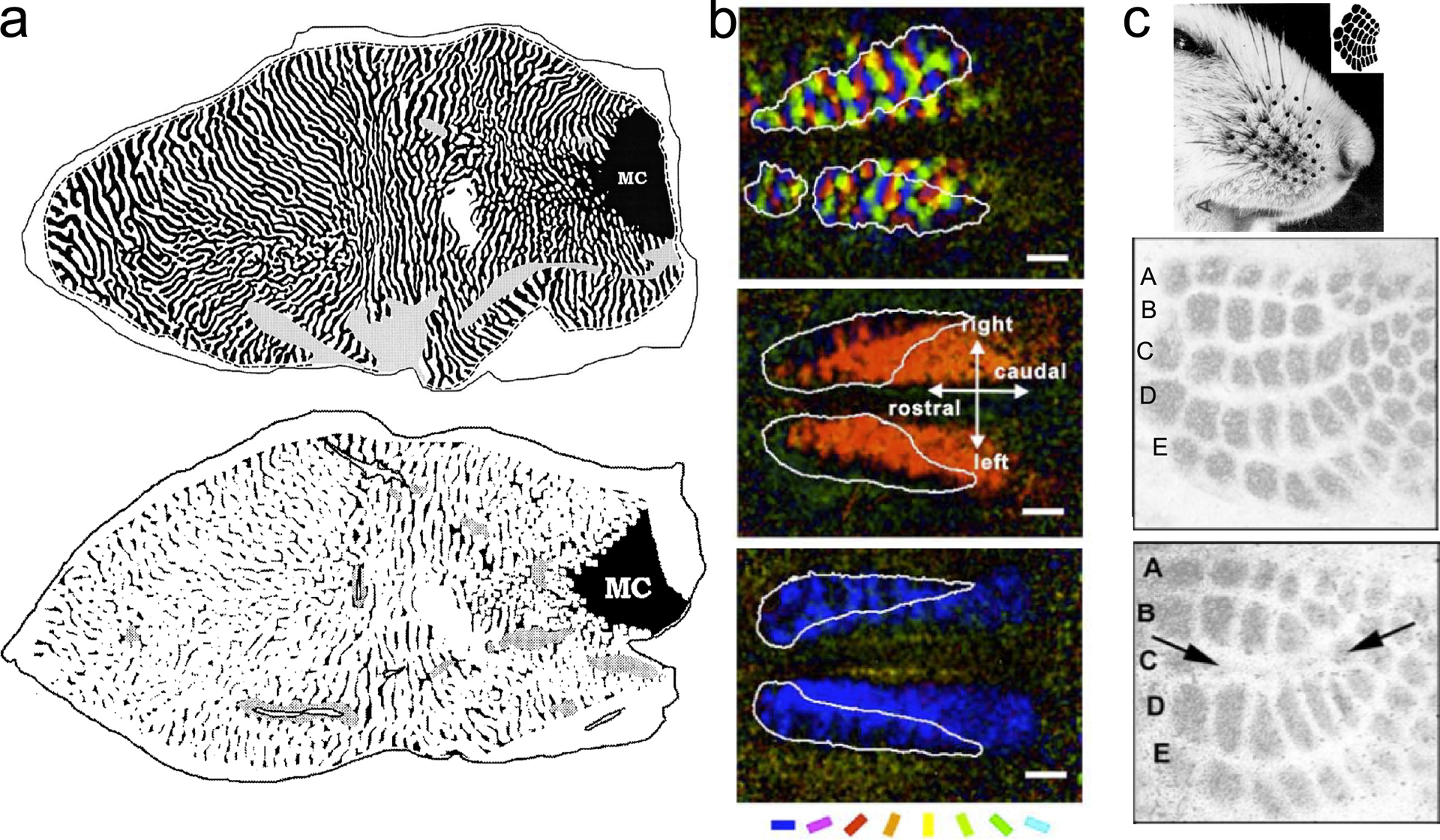
Synaptic plasticity is so strong during early postnatal development that the cortex comes to respond to stimuli it experiences early in development and to not respond to stimuli it does not experience. (a, top) Normal pattern of contralateral (black) and ipsilateral (white) inputs to macaque V1 after late monocular deprivation and (a, bottom) loss of contralateral inputs after early contralateral monocular deprivation. The cortex responds almost entirely to the seeing eye. The monocular crescent (MC) is the far peripheral part of the visual field with input from only the contralateral eye, the view of the ipsilateral eye being blocked by the nose (Horton & Hocking 1997; reproduced with permission). (b) Effects of wearing orientation-selective lenses on kitten orientation tuning. (Top) Normal map of orientation selectivity after lens rearing. (Middle) Map of effects of early exposure to only right-oblique orientations. (Bottom) Map of cortex after early exposure to only horizontal orientations. Cortex responds only to stimuli the animal experienced; scale bars 2mm (Tanaka et al. 2006; adapted with permission). (c, top) Effects of whisker removal on primary somatosensory cortex in mouse (Van der Loos & Woolsey 1973; reproduced with permission). (Middle) Normal pattern five rows of barrels (A-E), each barrel corresponding to an individual whisker. (Bottom) Cortex missing middle row (C) of barrels (arrows) after removal of middle whisker row (Datwani et al. 2002; modified with permission).
Successive stages in the visual hierarchy adhere to these common organizing principles. The pioneering work of Semir Zeki (1973, 1974a, b) revealed that different areas of extrastriate cortex were specialized for different visual modalities, such as motion, binocular disparity, or color. It therefore became a commonly held idea that each map, or area, must be specialized to process different kinds of visual information, such as color or motion. Horace Barlow (1986) proposed that the reason we have so many visual areas is so that maps of sensory space can be reconfigured into feature maps. However, an alternative interpretation is that each map in the hierarchy is self-organized into subregions with similar response properties, so that nearby (and therefore interconnected) neurons all convey information about the same modality, such as color, orientation, or motion. Thus, each map carries different kinds of information covering the entire visual field, rather than each map containing only one kind of modality, thereby allowing incrementally farther feature linking at each stage along the hierarchy (Zeki & Shipp 1989; Levitt et al. 1994; Livingstone and Hubel 1984b, 1987). This second explanation has the appeal that it makes domains in extrastriate areas qualitatively similar to the much smaller domains in V1, but would require a systematic increase in the scale of clustering from early to higher visual cortex. Such a gradation in scale can be seen in the patterns of ocular dominance, orientation, color, and disparity domains across extrastriate areas V2, V3, and V4 (Figure 6). Thus, the regular, repeating mirror organization of higher visual areas and the regular, striped, interdigitating organizations of different modalities running perpendicular to areal borders are all consistent, with, we suggest, higher areas being formed by the same kinds of self-organizing activity-based clustering rules as V1.
Figure 6.
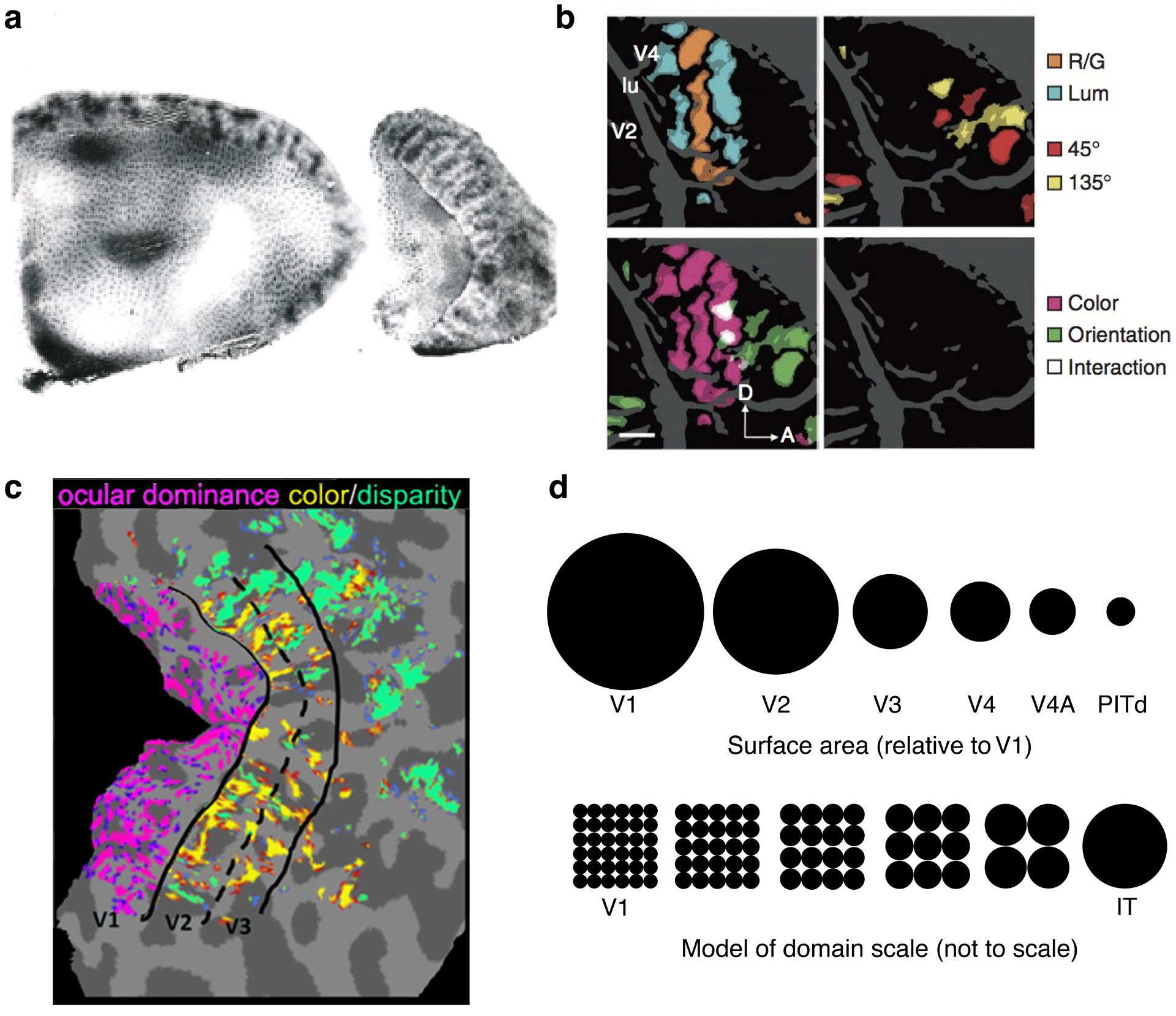
Increasing scale of domains moving from V1 to V2, V3 and anterior areas. (a) V2 stripes run perpendicular to the V1/V2 border in a (left) macaque, (right) squirrel monkey (Livingstone & Hubel 1984; reproduced with permission). Note that the V2 stripes are coarser than the V1 blob pattern. (b) Color, luminance, and orientation domains are similarly interdigitated in macaque V4 (Tanigawa et al. 2010; modified with permission). (c) Composite of ocular dominance, color, and disparity domains in human V1–V3 (Nasr et al. 2016; modified with permission). (d, top) Surface area relative to macaque V1 for the central 10° of eight retinotopic areas (Arcaro & Livingstone 2017b; reproduced with permission). Surface area systematically decreases moving up the visual hierarchy. (Bottom) Domain scale moving from V1 to IT. Abbreviations: A, anterior; D, dorsal; IT, inferotemporal cortex; lu, lunate sulcus; LUM, luminance; PITd, peripheral inferotemporal cortex ddorsal; RG, red vs green.
3.3. The Importance of Being Retinotopic
Retinotopic mapping is the fundamental organizing principle of visual cortex. While the domain architecture of V1 is modifiable by early visual experience, such experimental manipulations do not change the large-scale map of contralateral visual space. Postnatal experience may refine V1’s retinotopy, but only genetic manipulations or changes to the thalamic innervation yield large deviations from a species’ stereotypical map (Cang et al. 2005, 2008; Fukuchi-Shimogori & Grove 2001; Katz & Shatz 1996; Magrou et al. 2018; Rakic et al. 1991). The modular organization of early visual cortex adheres to areal borders (Figure 6), illustrating the intrinsic link between the domain architecture and the retinotopy of early visual cortex. This link extends to IT cortex. IT domains as identified by fMRI are distributed along the anterior–posterior axis, and clusters of adjacent face, body, object, color, and scene domains together form stripe-like patterns, with each domain spanning bands of iso-eccentricity (Arcaro & Livingstone 2017b, Janssens et al. 2014, Lafer-Sousa & Conway 2013, Srihasam et al. 2012) (Figure 7). However, in contrast to V1 through V4, these IT domains are not restricted to individual retinotopic maps. For example, individual scene-selective domains span at least two retinotopic maps in both humans and monkeys (Arcaro & Livingstone 2017b, Arcaro et al. 2009). Similarly, individual face-selective domains are found in central visual-field representations but are not localized to individual maps (Arcaro et al. 2017, Janssens et al. 2014). This may reflect a finer-scale domain architecture not resolvable at current fMRI resolutions, where multiple domains with similar tuning properties are mirrored across parallel sections of retinotopic maps and thus, at the scale of fMRI, appear as a single domain spanning multiple retinotopic areas. Alternatively, a single domain in IT may actually span multiple retinotopic maps, reflecting an inversion of dominance between clustering and hierarchy. That is, moving up the cortical hierarchy, areal size decreases (Figure 6d), modular scale increases (Figure 6d), and receptive field size increases (Figure 2c) as each successive cortical area pools inputs from spatially adjacent receptive fields of the antecedent cortical area. The integration of information across successively larger swaths of the visual field may, at the hierarchical level of IT, result in an inversion of the large-scale structure where domain scale exceeds the extent of individual retinotopic maps. As such, domains would not be restricted to individual areas but would still be anchored to the underlying retinotopic architecture that emerges early in development from an interplay between intrinsic and extrinsic self-organizing rules.
Figure 7.
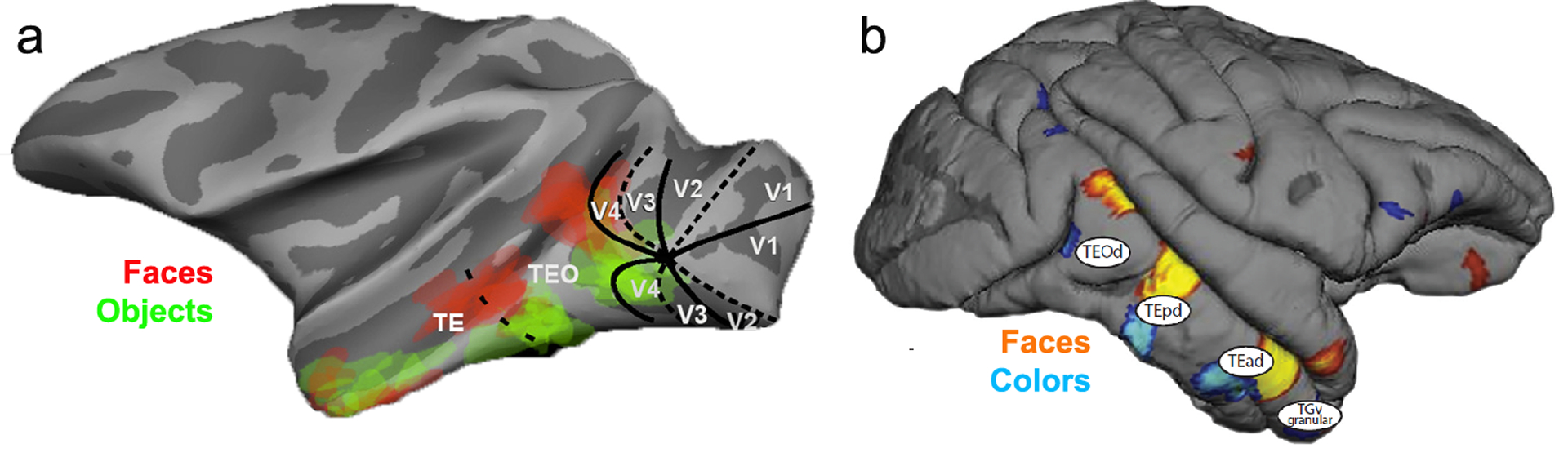
Domains in IT are distributed along the anterior–posterior extent of the STS in three stripe-like clusters, but they are huge compared to stripes in V1 and V2. (a) fMRI activation of face vs object domains in macaque IT (Srihasam et al. 2012; reproduced with permission); (b) fMRI activation of face vs color domains in macaque IT (Lafer-Sousa & Conway 2013; reproduced with permission). Abbreviations: IT, inferotemporal cortex; STS, superior temporal sulcus
4. HOW IT GETS ITS SPOTS
Could the same kinds of simple, spread-out-but-cluster (short-range facilitation/long-range inhibition) rules that produce the ocular dominance, sign-of-contrast, color, and orientation domains in V1 produce the much larger modality and category domains in IT? Extrapolating from how early visual areas develop, we should not be surprised if even more complex response selectivities and stripe-like clusters of cells with similar response properties could arise from activity-dependent sorting rules acting on hierarchically organized maps. While experimental evidence for such self-organization in IT has yet to be demonstrated, the distribution of face, body, object, color, and scene domains along the anterior–posterior axis of IT and their juxtaposition to one another (Figure 7) are certainly consistent with a rule-based segregation acting across a visual hierarchy. Further, computational models that adhere to the same principles enforcing short-range facilitation and long-range repulsion spatially segregate faces, words, and houses across processing units after sufficient training (i.e., experience) (Plaut & Behrmann 2011, Wang & Cottrell 2017), demonstrating that these organizing principles are computationally sufficient to produce the domain architecture of IT.
Opinions on how IT domains develop range from convictions that domains are innately programmed to develop specialized circuitry designed to process biologically important categories (e.g., McKone et al. 2012) to the equally strongly held conviction that IT domains represent levels of learned expertise (e.g., Gauthier 2000). Any hypothesis for how IT domains form needs to account for the stereotyped locations of domains from one individual to the next for both natural and unnatural categories. The stereotyped location of IT domains could be taken as evidence that these regions are innately prespecified either from yet-to-be-discovered genetic markers or from the interplay of intrinsic factors that somehow culminate in the clustering of a few ethologically relevant visual categories without the need for extrinsic experience. In either case, the nativist position would be that, through evolutionary pressures, these brain regions are predestined to process these particular categories. However, the fact that domains for unnatural categories, such as buildings, tools, or text, are also found in stereotyped locations poses a challenge for domains being genetically hardwired. A modified account for the innate prespecification of domains, the ‘recycling theory’ (Dehaene & Cohen 2007), posits that experience acts on domains that were innately programmed to recognize some evolutionarily important category, such as tree branches, to be co-opted to a novel task, such as recognizing text. Another hypothesis, based on the caudo-rostral maturational gradient of visual cortex (Quartz & Sejnowski 1997), proposes that timing during development determines where domains end up, and attributes the stereotyped location of different domains to the typical sequence of different life experiences. A third hypothesis, the expertise model, explains the stereotyped localizations by different degrees of categorical discrimination required for different kinds of things (Gauthier & Palmeri 2002). Malach and colleagues (Levy et al. 2001, Malach et al. 2002) suggested that retinotopic maps are fundamental, and looking behavior determines where along these maps different categories become localized. Tootell and colleagues (Tootell et al. 2012, Yue et al. 2014) proposed that degree of curvature versus rectilinearity was involved in domain localization. While there are still more variants of these hypotheses, they all plausibly explain why domains might wind up in anatomically consistent locations by appealing to some combination of innateness, experience, and age of learning in the development of IT.
To try to disentangle these factors , in particular, expertise, age of learning, and innateness, our laboratory trained monkeys at different ages to discriminate symbols in order to receive different reward amounts. Juvenile monkeys learned 25 symbols over many months of daily training. After training, these monkeys showed fMRI domains that were selectively responsive to the trained symbolscompared to untrained similar shapes (Figure 8). Given that monkeys are not typically literate and do not normally have symbol domains, this development means that experience is sufficient to produce IT domains (Srihasam et al. 2012). We also asked whether degree of expertise or timing during development could affect the location of these training-induced domains by training juvenile monkeys on different sets of symbols at different ages during prepubertal development (Srihasam et al. 2014). We chose symbol sets that were visually distinct, and trained some monkeys on one symbol set first and then trained them on a second symbol set many months later Other monkeys received the reverse order of training. All the monkeys developed domains that were selectively responsive to the trained symbols, but surprisingly, the locations of these novel domains did not depend on training order or degree of expertise; rather, each symbol set domain mapped to a different stereotyped location in IT (Figure 8). The only reasonable explanation for this result is that the stereotyped localizations depended on the visual features comprising each set. That in turn implies that there must have been some kind of shape-based proto-organization that dictated where training-induced domains would develop.
Figure 8.
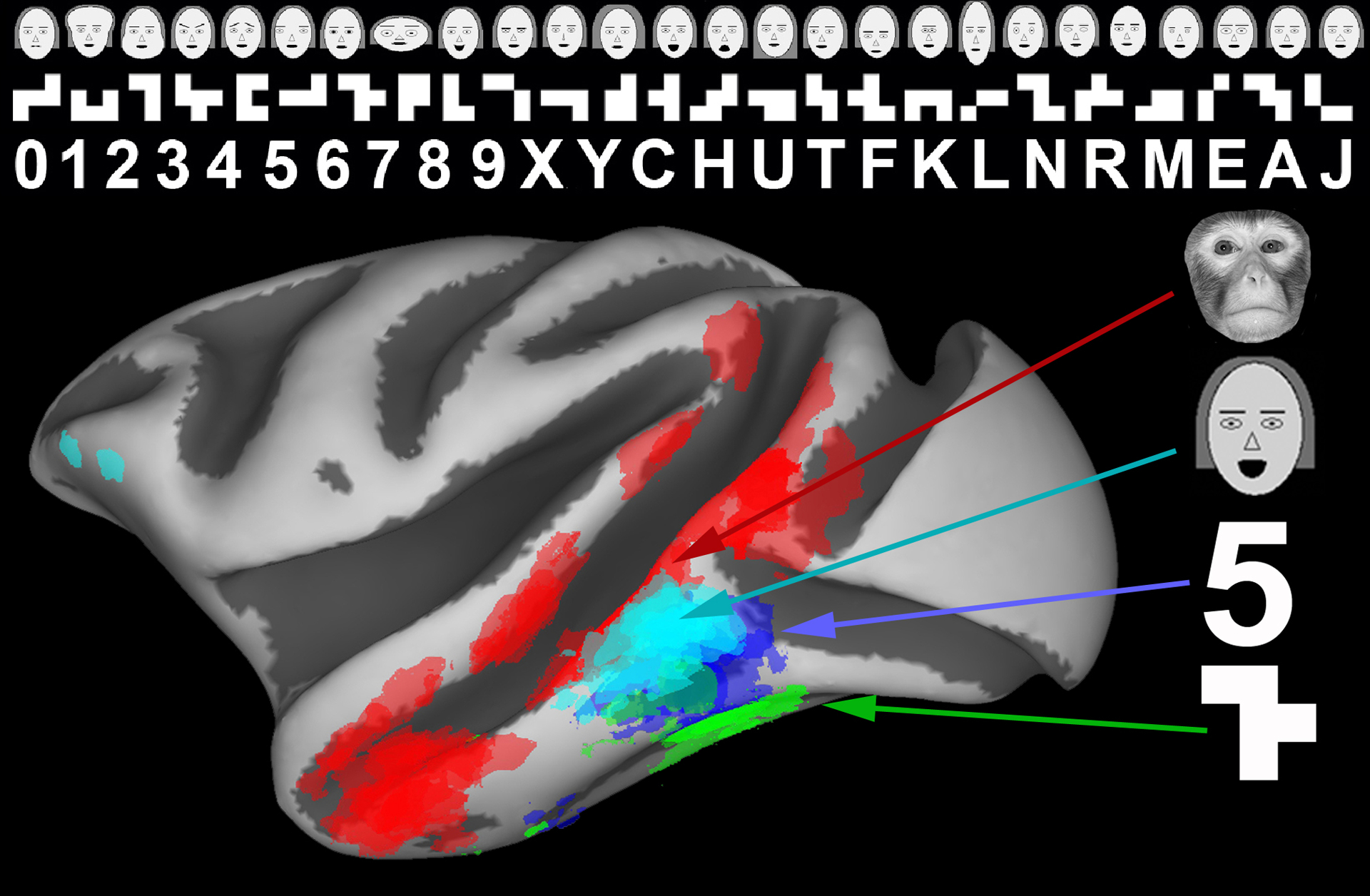
Domains that develop as a consequence of intensive early-life training form in stereotyped locations. The locations of these artificial domains did not depend on degree of expertise or age of learning. The stereotyped locations indicate the existence of some kind of shape-based proto-architecture on which experience exerts plasticity (Srihasam et al. 2014; reproduced with permission).
Any model of IT development also needs to account for why domains covary with a broad shape-based architecture. Tootell and colleagues (Srihasam et al. 2014, Tootell et al. 2012, Yue et al. 2014) found that face domains are selectively responsive not only to faces but also to other round objects and that conversely scene domains are selectively responsive to rectilinear images. Neurons in face domains will respond to objects that are round like faces such as clocks or balls (Tsao et al. 2006). Likewise, other domains will respond to images that have shape features similar to those of the preferred category (Andrews et al. 2015, Rice et al. 2014), and the layout of domains covaries with midlevel shape features (Long et al. 2018). The recycling model would explain such co-occurrences by positing that category selectivity is the fundamental organizing principle for IT and that novel, or unnatural, domains arise in particular locations because of their similarity in shape to that of natural object categories (Dehaene & Cohen 2007).
Malach and colleagues’ (Hasson et al. 2002, Levy et al. 2001) proposal that retinotopy might be the fundamental organizing principle over category in IT is based on the observation that the stereotyped locations of face, text, and scene domains in humans invariably correlates with eccentricity biases for central-left, central-right, and peripheral visual fields, respectively. A covariance between eccentricity and domains is also seen in monkeys (Janssens et al. 2014, Lafer-Sousa & Conway 2013, Rajimehr et al. 2014, Srihasam et al. 2014), and this covariance can emerge in neural network models trained to discriminate between image categories (Plaut & Behrmann 2011, Wang & Cottrell 2017). The correlation between category, curvature selectivity, and eccentricity in IT in adult humans, monkeys (Figure 9), and computer simulations of brain dynamics does not help us resolve which is the primary principle. Exponents of category being the fundamental organization argue that the eccentricity biases observed for different category domains arise from preferred viewing behavior for different categories, in that we look at faces but use peripheral vision to process scenes. However, curvature selectivity and eccentricity are also strongly correlated in early visual areas (Srihasam et al. 2014), which are not category selective. Furthermore, the correlation between curvature and eccentricity can be explained by the ubiquitous receptive-field tuning property of end-stopping, which would result in sharper curvature tuning at central eccentricities (Hubel & Wiesel 1965, Ponce et al. 2017, Srihasam et al. 2014). This interrelationship between eccentricity and shape selectivity suggests a topographic mapping not only for retinotopy but also for shape selectivity.
Figure 9.
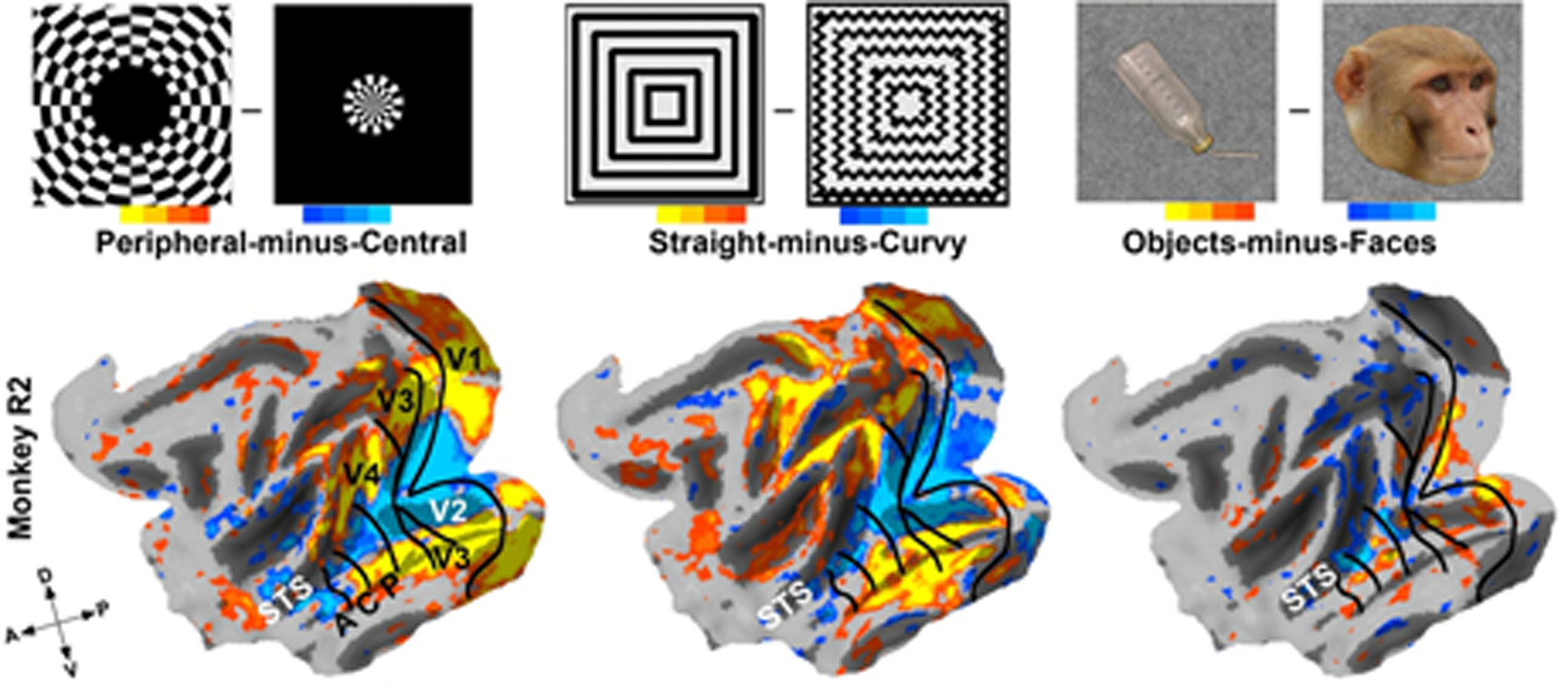
Face-selective regions map to central parts of the visual field and respond more to curvy than to straight stimuli. But the correlation between eccentricity and curvature extends to early visual areas too, indicating that eccentricity-based shape selectivity may be a more fundamental organizing principle than category selectivity (Srihasam et al. 2014; reproduced with permission). Abbreviations: A, anterior; C, central; D, dorsal; P, posterior; V, ventral; STS, superior temporal sulcus.
Tracking the emergence of curvature, eccentricity, and category selectivity may help resolve the mechanisms guiding IT domain formation. One would think we could resolve this question by resolving the developmental time line and finding out which comes first—retinotopy or category selectivity—but this is surprisingly not that simple. Electroencephalogram and functional near-infrared spectroscopy studies of human infants as young as 3 months have shown differential brain activity between faces and general object categories as well as between face orientations (upright versus inverted) (de Haan et al. 2002, Halit et al. 2003, Johnson et al. 2005, Otsuka et al. 2007). Electrophysiological recordings in infant monkeys as young as 39 days found cells in IT that responded to faces (Rodman et al. 1991). While these studies show some specificity of brain activity in infants, the specificity of face selectivity (versus other image categories and versus low-level shape selectivity) and the relation to the domain architecture of IT were not rigorously addressed.
Recently, two studies, one of human infants (Deen et al. 2017) (Figure 10a,b) and the other of macaque infants (Livingstone et al. 2017) (Figure 10c,d), reported similar results on the early emergence of face domains, but the studies gave different interpretations of the results. Both studies found early (4–6 months in humans and 1 month in monkeys) selectivity for face movies versus scene movies in some of the brain regions that, in adults, would show the same selectivity, but both studies found no selectivity for faces versus objects until later in development. Deen and colleagues found face versus scene selectivity within lateral occipital cortex (OFA) and superior temporal sulcus (STS), but not fusiform cortex (FFA), from a group-average analysis of human infants (Figure 10a). They interpreted their findings in human infants to mean that face patches are present at birth but are not as selective early in development as they are in adults; that is, face patches are present but immature. Our laboratory interpreted our failure to find face versus object selectivity until later in development as evidence that the early differential responsiveness to monkey movies versus scene movies represented differences in activity across the visual field from noncategorical shape selectivity or retinotopic motion energy rather than category selectivity. We also found an early differential response between pixelated face movies versus scene movies, further indicating that noncategorical image statistics were the driving force for these early movie data. The face movies used in the infant macaque study contained monkeys grooming each other, so most of the motion in these clips was spatially focal and centered, whereas the nature movies generally panned entire scenes. Similarly, face and object movies in the human infant study consisted of human children and toys in front of a black background, whereas scene movies comprised backgrounds that spanned the full extent of the stimulus display. It seems to us that a ‘face’ domain, no matter how immature, ought to respond better to faces than to objects. In adults, face domains respond more strongly to face images than to a wide range of stimuli (Mur et al. 2012). Given the difficulty of collecting large amounts of data from infants, face activity has been compared with only a few image categories (e.g., objects, scrambled images, scenes). It remains to be seen to what extent the selectivity profile of face domains in infants reflects that found in adults, though changes in the anatomical structure and selectivity have been observed into adolescence (Golarai et al. 2010b, 2015) and even early adulthood (Germine et al. 2011). Together, these data suggest that IT face domains are undeveloped at birth and demonstrate an important role for postnatal development.
Figure 10.
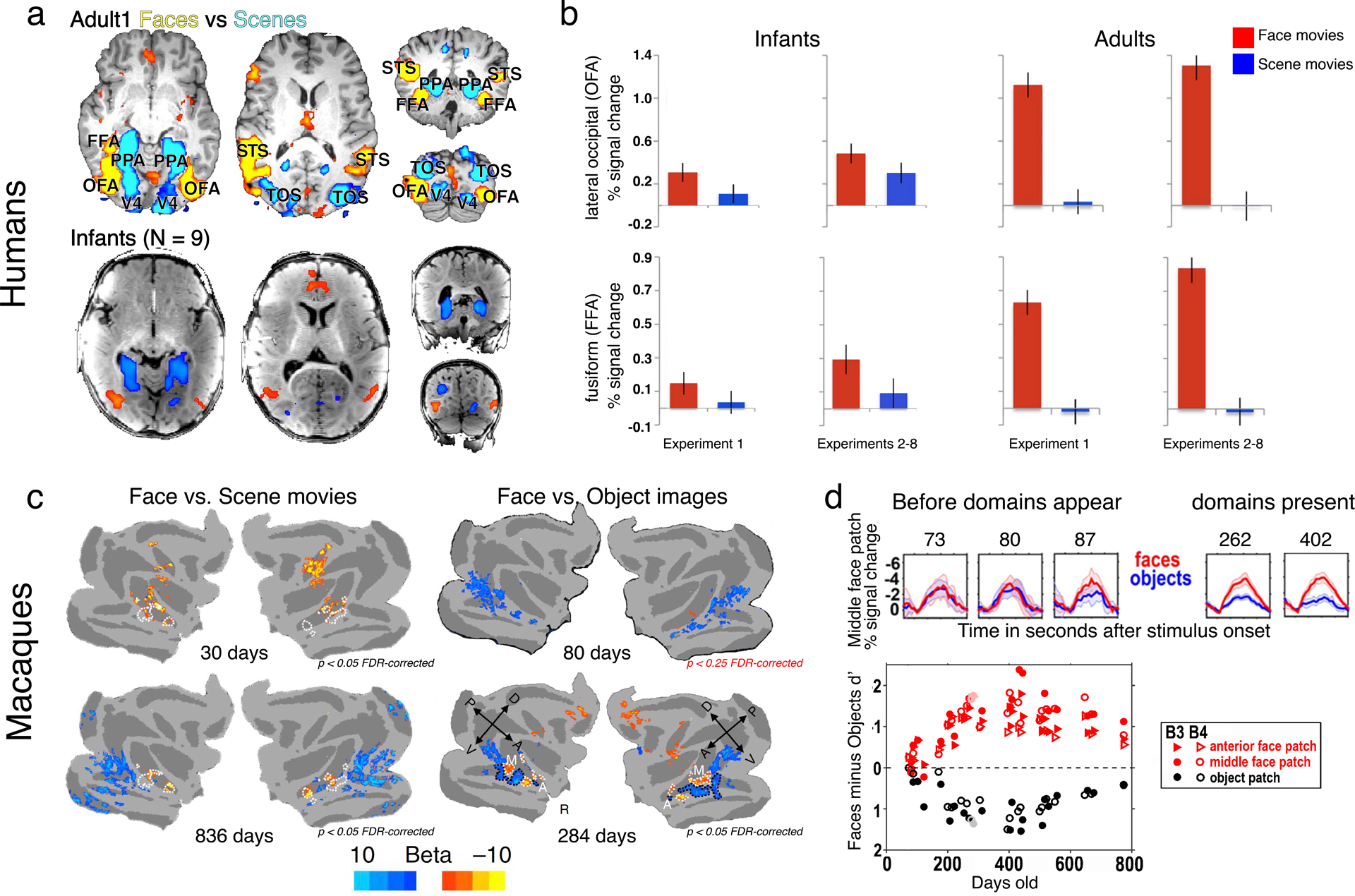
Face activity in human and monkey infants. (a) Group-average fMRI activity maps for human (top) adults and (bottom) infants (Deen et al. 2017; modified with permission). (b) ROI analysis of data from panel a for OFA and FFA in infants and adults]. Infant data have been rescaled to match adult data. (c) fMRI activity maps for face movies versus scene movies and face images versus object static images in (top) infant macaques and (bottom) juvenile macaques. Selective responsiveness to faces and objects appears around 3 months of development (Livingstone et al. 2017; reproduced with permission). (d, top) Developmental timecourse of face and object d-primes in face and object domains in macaque IT. (Bottom) Regions of IT that will become face and object selective are nonselectively responsive to both categories before domains appear. Abbreviations: A, anterior; D, dorsal; FDR, false discovery rate; FFA, fusiform cortex; fMRI, functional MRI; IT, inferotemporal cortex; M, xI dont see an M in this figurex; OFA, lateral occipital; P, posterior; PPA, parahippocampal place area; R, rostral; ROI, region of interest; TOS, transverse occipital sulcus; V, ventral.
These early imaging studies provide some evidence for a caudo-rostral maturation of face domains. In human IT, only the occipital face area (OFA) showed greater responses in group-average maps to faces versus scenes at 3 months (Deen et al. 2017) (Figure 10a). A region-of-interest (ROI) analysis (Figure 10b) found weak selectivity for faces near FFA and nothing in anterior temporal cortex. While caution should be exercised in interpreting null or weak effects, especially in studies of young children, greater activity for scenes versus faces was observed in parahippocampal place area (PPA). PPA is medial to FFA and thus farther from the head coils, indicating that signal quality was not a precluding factor in detecting face-selective effects in FFA at this young age. In monkeys, face domains PL (posterior lateral), ML (middle lateral), MF (middle fundal), AL (anterior lateral), and AF (anterior fundal) were selective for faces as compared with inanimate objects by 3 months. There were no clear timing differences in the development of these domains, though face selectivity was not consistently mapped in the anterior-most IT face domain, AM (anterior medial), nor in the frontal face domain, even at 2 years of age (Livingstone et al. 2017). Together, these data are consistent with the idea of a caudo-rostral maturation of visual cortex and the notion that the primate face domain system continues to mature into adolescence.
In contrast to the lack of clear face domains in newborns, robust retinotopic organization throughout the visual system is present at birth. Within the first 2 weeks, adult-like patterns of anatomical connections are present between V1 and V2 and between V2 and V3, V4, and MT in monkeys (Baldwin et al. 2012, Barone et al. 1996, Coogan & Van Essen 1996), though the functional development of these pathways does not reach adult-like maturity until 3 months (Distler et al. 1996). Using functional connectivity analyses on fMRI data, we found that the entire macaque visual system, from V1 through anterior IT, exhibits a clear retinotopic organization by 10 days after birth (the earliest timepoint tested; Figure 11), and the scaling of receptive fields from V1 through IT was present by 110 days (the earliest timepoint tested with spatial frequency stimuli) (Arcaro & Livingstone 2017a). This organization was comparable to that typically found in adult monkeys. That is, a series of hierarchical retinotopic maps in newborn monkeys span from subcortical structures to V1 and up through anterior IT. This organization was present well before face domains were identified, and regions that later exhibited face selectivity already had a prominent bias for the central visual field, similar to adults. The persistence of retinotopic organization in congenitally blind humans (Bock et al. 2015, Butt et al. 2013, Striem-Amit et al. 2015) and in monkeys deprived of form vision from birth (Arcaro et al. 2018) is consistent with the idea that this organization is present at birth and further indicates that retinotopy is the primary organizing principle that guides subsequent functional development. Because eccentricity carries with it a low-level organization for scale, spatial frequency, and curvature, at birth there would already exist a proto-architecture constraining postnatal experience-dependent modifications.
Figure 11.
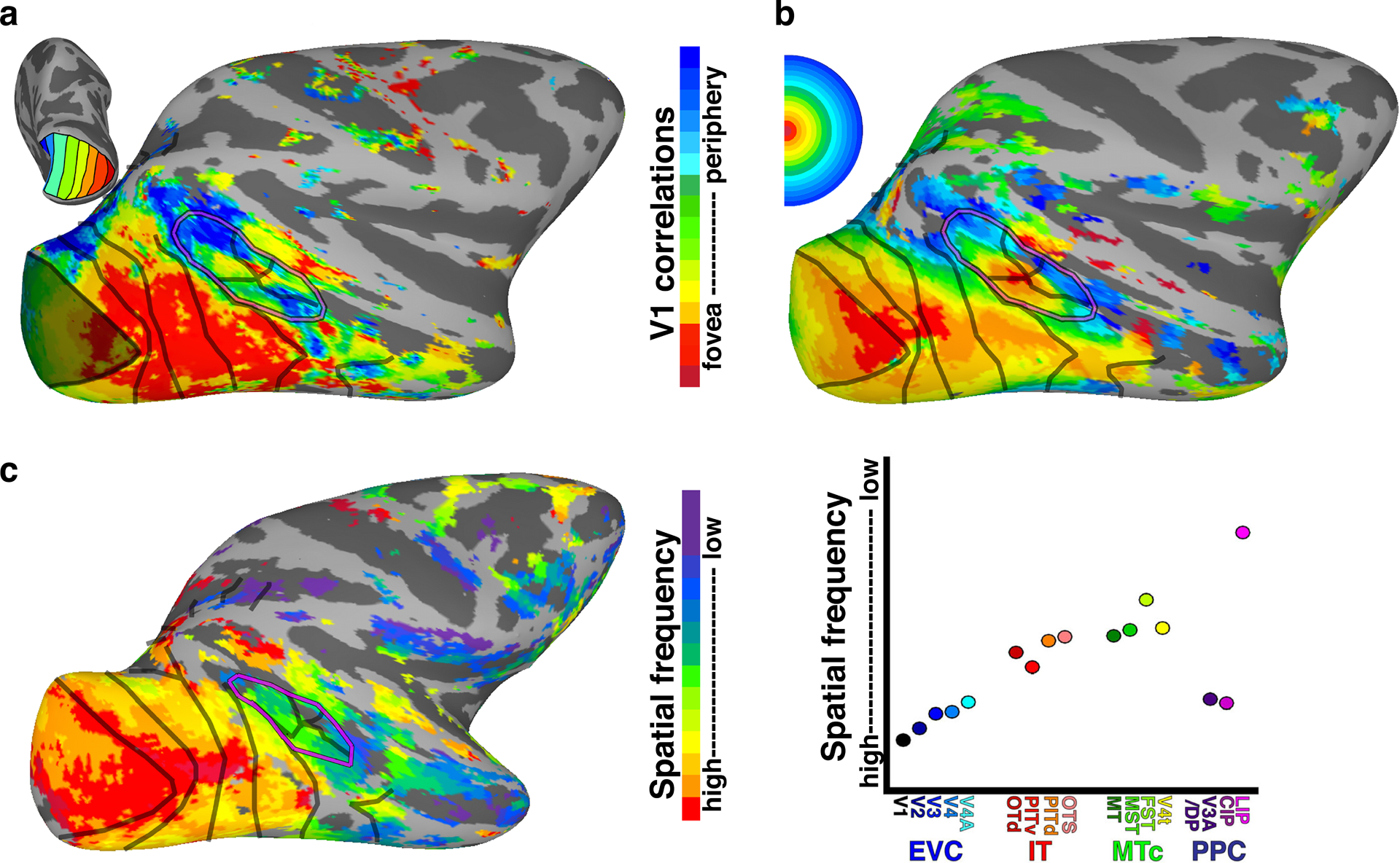
Retinotopic organization is present at birth (Arcaro & Livingstone 2017a; reproduced with permission). (a) Retinotopic organization mapped in newborn monkey using functional connectivity with a V1 eccentricity-map template. (b) Eccentricity organization mapped in the same monkey at 2 years of age using conventional expanding ring stimuli. (c) Spatial frequency mapped in a newborn monkey. Mean spatial frequency shifts from fine to coarse both with eccentricity and up the visual hierarchy. Abbreviations: DP, xdorsal prelunatex; CIP, caudal intraparietal; EVC, early visual cortex; FST, xFST is just a name, not an abbreviationx; IT, inferotemporal complex; LIP, just a name; MST, just a name; MT, it’s a name; MTc, MT complex; OTd, xjust a namex; OTS, occipitotemporal sulcus; PITd, PIT dorsal; PITv, xPITventralx; PPC, posterior parietal cortex.
The development of symbol domains in symbol-trained juvenile monkeys means that experience is sufficient to induce IT domain formation, but is experience necessary for face domain formation? This would be extremely difficult to answer for humans since it would require either an early intervention that would be deemed too invasive or the discovery of someone who was naturally raised without seeing faces but had otherwise normal visual experience and acuity. No such cases exist to our knowledge. So to address this question, we raised three monkeys by hand without letting them see any kind of face (outside of images shown during experiments) for the first year of life. None of the face-deprived monkeys developed face domains, but they did have normal domains for hands, bodies, and scenes (Arcaro et al 2017). Therefore, they were missing domains only for the single object category they had not seen, indicating that experience is necessary for IT domain formation. Control monkeys look disproportionately at faces in free-viewing situations. In contrast, the face-deprived monkeys did not look disproportionately at faces during free viewing but looked more at hands (Figure 12). These results are consistent with the idea that domain formation is driven by extensive early experience looking at what is important in the environment. Centrally biased parts of IT that preferentially respond to faces in control monkeys did not respond to faces in face-deprived monkeys, and instead became dominated by hands over all other categories (e.g., objects), even though the hand responsiveness profile did not shift away from the usual hand locations, which are normally located slightly farther into the fundus of the STS than face domains. Importantly, the hand responsiveness profile was not expected to shift, given that we did not manipulate their hand experience in any way, and both deprived and control monkeys likely experienced their hands in similar parts of their visual field in daily life, such as when eating, manipulating objects, and navigating the environment. These data provide causal evidence that postnatal experience is necessary for the development of face domains.
Figure 12.

Free-viewing looking behavior in control and face-deprived monkeys. Control monkeys look at faces in various images but face-deprived monkeys do not, indicating that face-looking behavior is unlikely to be innate (Arcaro et al. 2017; reproduced with permission).
The type of face experience and amount of exposure required for face-domain development remain to be resolved. For example, neurons in adult face domains are particularly sensitive to the eyes (Issa & DiCarlo 2012). This eye preference might emerge early in life from experience with conspecifics (Simion & Di Giorgio 2015), as newborns attend equally to internal and external features of faces (Turati et al. 2006). Several interesting questions follow from these findings. Would a monkey raised seeing only the outline of a head and the mouth develop face domains? Would neurons be more sensitive to other facial features such as the mouth (versus eyes)?
The early retinotopic organization of the visual system, the subsequent development of IT domains, and the importance of early visual experience for IT domain formation impose constraints on possible theories of face development. We have discussed two distinct hypotheses for the development of IT domains: (a) IT is organized innately into anatomically distinct regions that process different biologically important image categories, or image categories that resemble biologically important things. Such a domain-specific hypothesis must be mitigated by the fact that face domains do not develop for the first several months after birth and remain immature through adolescence, and a domain-specific hypothesis also needs to include the constraint that maintenance of the postulated innate face selectivity requires face experience. (b) IT contains a domain-general architecture at birth that is molded by experience. This domain-general organization comprises a series of retinotopic maps that carry with them low-level shape selectivity. Eccentricity-biased looking behavior and/or low-level shape selectivity determine what parts of this prewired architecture become specialized for different categories of objects. Thus, we propose, IT is not a tabula rasa at birth but has a well-defined retinotopic architecture to which experience-driven modifications adhere.
5. JUST LOOK FOR THE BARE NECESSITIES
We considered subheadings, but felt it would arbitrarily break up the continuity of this section (with the exception of the subcortical paragraph, but journal conventions want 2 or more subheadings) Can a model of an intrinsic domain-general architecture emerging as an activity-dependent self-organizing system that is then molded by early postnatal extrinsic experience explain the observations that have been previously interpreted as evidence that face processing must arise by some face-specific innate mechanism? There are three main lines of evidence invariably given in support of the innate face domain hypothesis:(a) the stereotyped location of face domains in humans and monkeys; (b) the optimal processing of faces when they are intact and upright; and (c) the very early preferential face looking in human and monkey infants. We have already discussed how the stereotyped location of face (and other category) domains can be explained by activity-dependent rules acting on a protomap that carries with it an eccentricity-based shape selectivity: This proto-architecture acts as a scaffolding on which environmentally driven looking behavior exerts activity-dependent modifications. We do not know which—looking behavior or shape tuning—is more important in localizing domains in relation to a retinotopic map. A recent study of adult humans who played Pokémon extensively as children found that activity patterns to this highly experienced stimulus set reflected retinal eccentricity (Gomez et al. 2018a), suggesting that looking behavior plays a major role in localization. On the other hand, our finding of stereotyped localization of trained-symbol domains in monkeys implicates a shape-map effect. Given the intrinsic link between shape selectivity and retinotopy, it is probable that both factors are crucial.
Yet a suitable neural architecture alone is not enough for our neural networks to develop face domains; a specific training set is required. From the very first day of birth, our visual experience is heavily biased toward seeing faces (Figure 13). When babies are not aimlessly staring at the ceilings and backgrounds of their environments, most of their visual experience over the first several months consists of faces of parents and caretakers (Jayaraman et al. 2015). Such extensive experience with a small number of faces would enable the visual system to build up a prototypical face across a wide range of viewing angles and conditions. Over the course of the first few years, the variability of face experience increases as babies experience more individuals, which could serve to strengthen invariant representations and categorical learning of faces.
Figure 13.
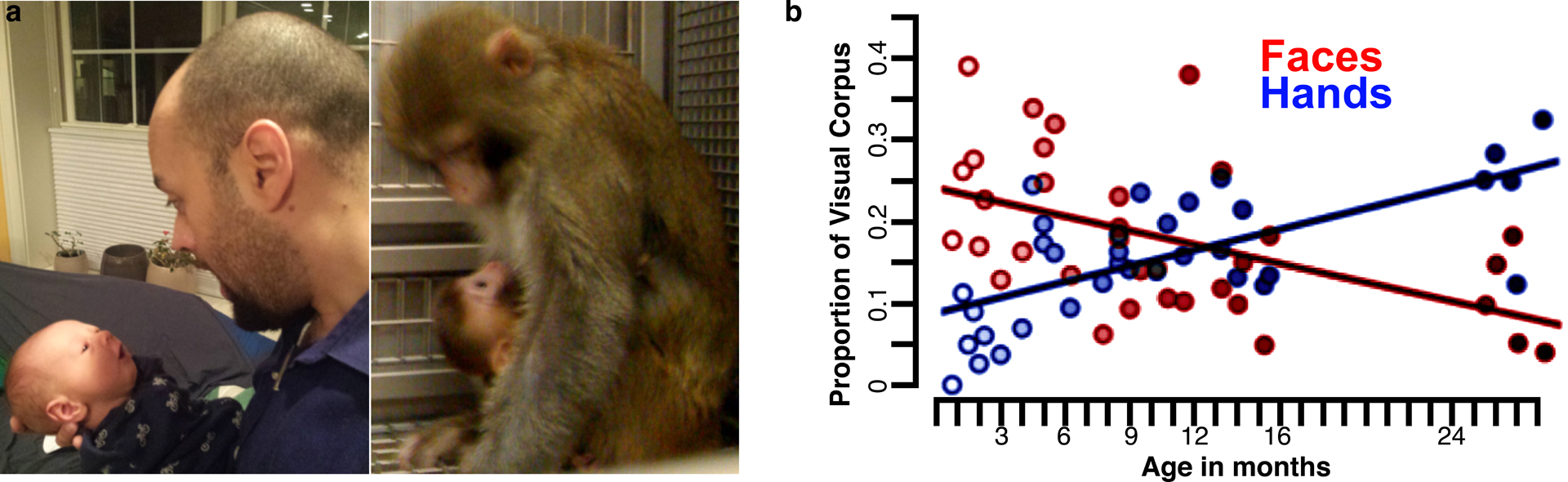
Early visual experience. (a) Primate infants look at faces early in development. Photos provided by (left) J. Gamble and (right) M.S. Livingstone. (b) Early visual experience of faces (red) and hands (blue) by human infants (Fausey et al. 2016; reproduced with permission). While early visual experience comprises primarily a limited number of faces (i.e., caregivers), overall visual human experience shifts to include hands as babies become more mobile and interact with the environment. The prominence of hands in early experience of human infants parallels our finding that face-deprived monkeys preferentially look at hands in the absence of visually experiencing faces. It remains to be resolved whether a comparable shift of visual experience from faces to hands is present in normally reared monkeys.
The second line of evidence considered to support the innate model is that faces are processed differently from other kinds of objects. In particular, it is claimed that faces are processed holistically, as intact upright wholes. Faces are recognized better when viewed upright (Yin 1969) and face features are recognized better when they are viewed as part of an intact face (Tanaka & Farah 1993, Young et al. 1987). These observations have been interpreted to indicate that face domains must be innate neural structures specialized to process faces in a specific orientation. However, the link between prioritized processing of upright faces and the innateness of IT domains is tenuous: Our alternative interpretation of prioritized processing is that faces are not a special category of objects except insofar as they are a homogeneous stimulus set, compared with other objects (Dailey & Cottrell 1999) with which we have extensive early experience, and the prioritized nature of upright, intact face processing arises because faces are recognized better when viewed in the same configurations in which they have been extensively experienced starting at birth (e.g., Figure 13). In support of this, other categories for which we have IT domains, such as text (Cohen et al. 2000), and even categories for which we lack specialized domains, such as cars (Aguirre et al. 1999), are also recognized better when viewed as they are typically experienced. Thus, specialized domain architecture may support, but is not necessary for, the behavioral prioritization of processing faces or other stimuli in intact, upright configurations.
The third line of evidence given to support the hypothesis that face domains are innate is how early face prioritization appears in development (McKone et al. 2012). Within minutes after birth human infants will turn their heads to follow a schematic face more than a scrambled face (Goren et al. 1975, Johnson et al. 1991). Such studies of newborns have engendered the idea that babies are born predisposed to gaze at and to bond with their mother, and therefore there must be some kind of innate face template. By 3 months of age, infants already exhibit several behavioral signatures of adult face processing, such as sensitivity to configurational properties (Turati et al. 2010) and recognition across views (Pascalis et al. 1998). While such early behavior could be taken as evidence for an intrinsic bias in face processing, experience has a significant effect on behavior very early in development. Three-month-old infants show perceptual narrowing, such as a decreased ability to recognize other-race faces (Kelly et al. 2007, Sangrioli & de Schonen 2004), which is not present at birth (Kelly et al. 2005) and is anticorrelated with the amount of ethnic diversity in the infant’s community (Bar-Haim et al. 2006). Further, infants look preferentially at faces of the same gender as the primary caregiver, for both male and female caregivers (Quinn et al. 2002). Thus, even before face-selective domains develop in IT, behavioral signatures of adult face processing are present and reflect the effects of experience.
Early face-viewing bias is stipulated by an innate model of face processing but is also consistent with a model positing that experience drives the development of face domains. Even within hours of birth and with relatively little visual experience, a mother’s face is recognized and preferred over a stranger’s face (Bushnell 2001, Bushnell et al. 1989, Pascalis et al. 1995). However, this preferential looking is correlated with the amount of visual exposure in newborns (Bushnell et al. 1989) (Figure 14a). Further, brain activity even within the first day of life can adapt to structure in the environment from minimal experience (1 hour) (Teinonen et al. 2009). These studies illustrate that our brains are statistical learning machines, even at birth. Thus, studies of newborns establish the presence of early looking biases, but these biases do not necessitate an innate face template.
Figure 14.
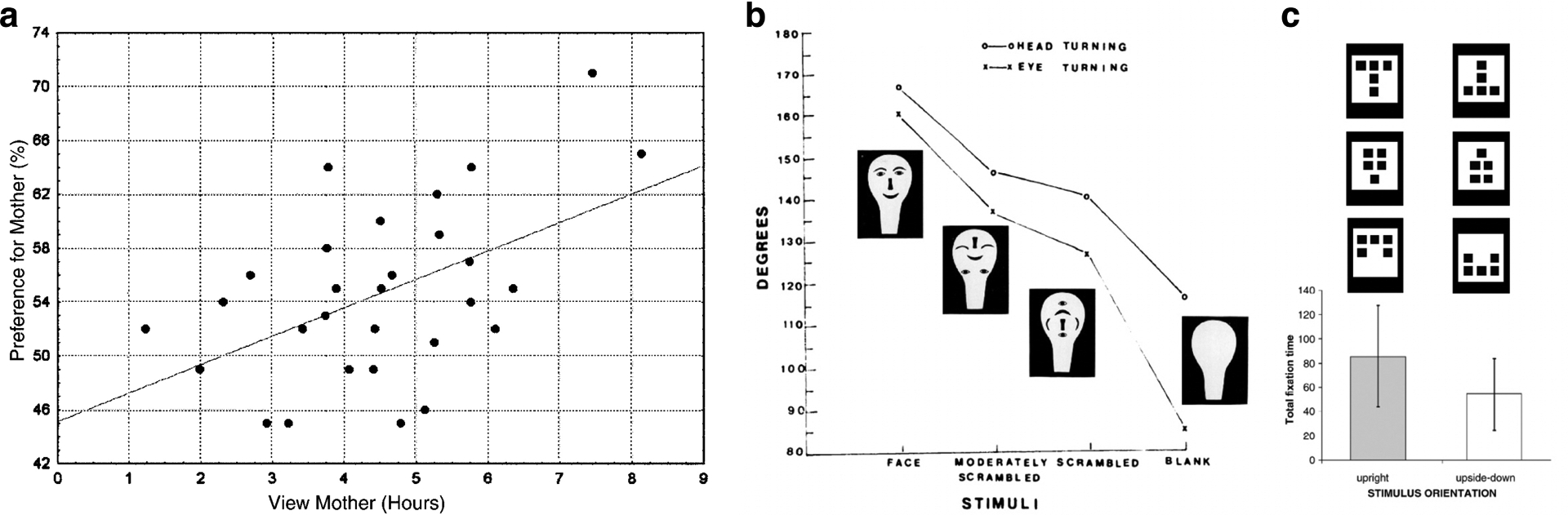
Face-looking behavior in human infants. (a) Newborn human infants will preferentially turn their heads toward their mothers versus strangers with as little as 6 hours of visual experience of the mother (Bushnell et al. 1989; reproduced with permission). (b) Newborn human infants turn their heads farther to follow face-like images compared to scrambled images. Image (Goren et al. 1975; reproduced with permission). (c) Newborn human infants look longer at images with more things in the upper part than in the lower part (Simion et al. 2002b; reproduced with permission).
If looking behavior acting on a retinotopic proto-architecture drives domain development, what is driving looking behavior? While preferential face looking in newborns has been demonstrated many times, some studies have shown that what may drive this preferential looking behavior are nonspecific structural and geometric properties, such as the relative density of small dark things in the upper half of a light disk compared to the lower half (Cassia et al. 2002, 2004; Simion et al. 2002a; Turati et al. 2002). Given the poor visual acuity of newborns at birth, such early preferences realistically could not amount to much more than a preference for a top-heavy image. A contrast polarity preference for dark in the eye region may also be present early in life (Farroni et al. 2005). However, the degree to which this finding reflects eye-specific features versus a low-level contrast saliency has been challenged (Simion & Di Giorgio 2015). Moreover, this contrast polarity template does not match many nonhuman primate faces and the degree and often sign of contrast vary across ethnicities, both of which challenge any face-specific evolutionary explanation. Further, in naturalistic settings, a model of low-level image saliency captures looking behavior of 3-month old infants better than does the location of faces, whereas looking behavior at 6 months reflects actual face location (Frank et al. 2009). Thus, preferential looking toward faces and face-like images in newborns may reflect intrinsic biases in the processing of structural features that are common but not exclusive to faces.
What neural structures could facilitate early preferred looking at top-heavy salient features? Given that preferential face looking is apparent well before IT domains develop (i.e., selective responses to faces versus inanimate objects) (Arcaro et al. 2017, Deen et al. 2017, Livingstone et al. 2017), it is implausible to us that early looking behavior is driven by a face-specific cortical template at birth. A subcortical template specific to faces has been proposed to explain early face-looking behavior (Morton & Johnson 1991), though direct evidence for such an early template is lacking; for example, neither our study of infant macaques (Livingstone et al. 2017) nor recent human infant imaging (Deen et al. 2017) found subcortical face-selective regions. However, it seems plausible that subcortical structures guide early preferential looking. Infants look preferentially at whatever is visually salient. Infants will look at checker patterns they can distinguish in preference to spatial frequencies too high for them to resolve (Banks & Ginsburg 1985). Such general processes may be driven by the superior colliculus (Sprague et al. 1973), an evolutionarily old part of the brain that drives eye movements to salient stimuli. The colliculus has a map of the visual field in the superficial layers, and stimulation of the deeper layers produces eye movements toward the corresponding part of the visual field. It is visually responsive in neonatal monkeys (Figure 15a) (unpublished data from M.S. Livingstone and J.L. Vincent), and in adult monkeys there are stronger responses to visual stimuli presented in the upper field compared to the lower field (Hafed & Chen 2016) (Figure 15b). Something as simple as an upper-visual-field bias in the colliculus could account for early face-looking behavior in primate infants (Atkinson et al. 1992). The evolutionary driving force may be that in ground-dwelling animals’ visual input from above is often critical for survival (Wallace et al. 2013). The upper visual field is overrepresented in mice, whose eyes point upward (Figure 15c), as do the eyes of many animals. In animals with forward pointing eyes, an enhancement in the neural machinery for attending to the upper visual field could serve a similar purpose. Thus, an evolutionarily old bias for detecting things in the upper visual field, in combination with the saliency of small, round, dark things, may underlie the seemingly social behavior of human infants orienting toward faces or eyes.
Figure 15.
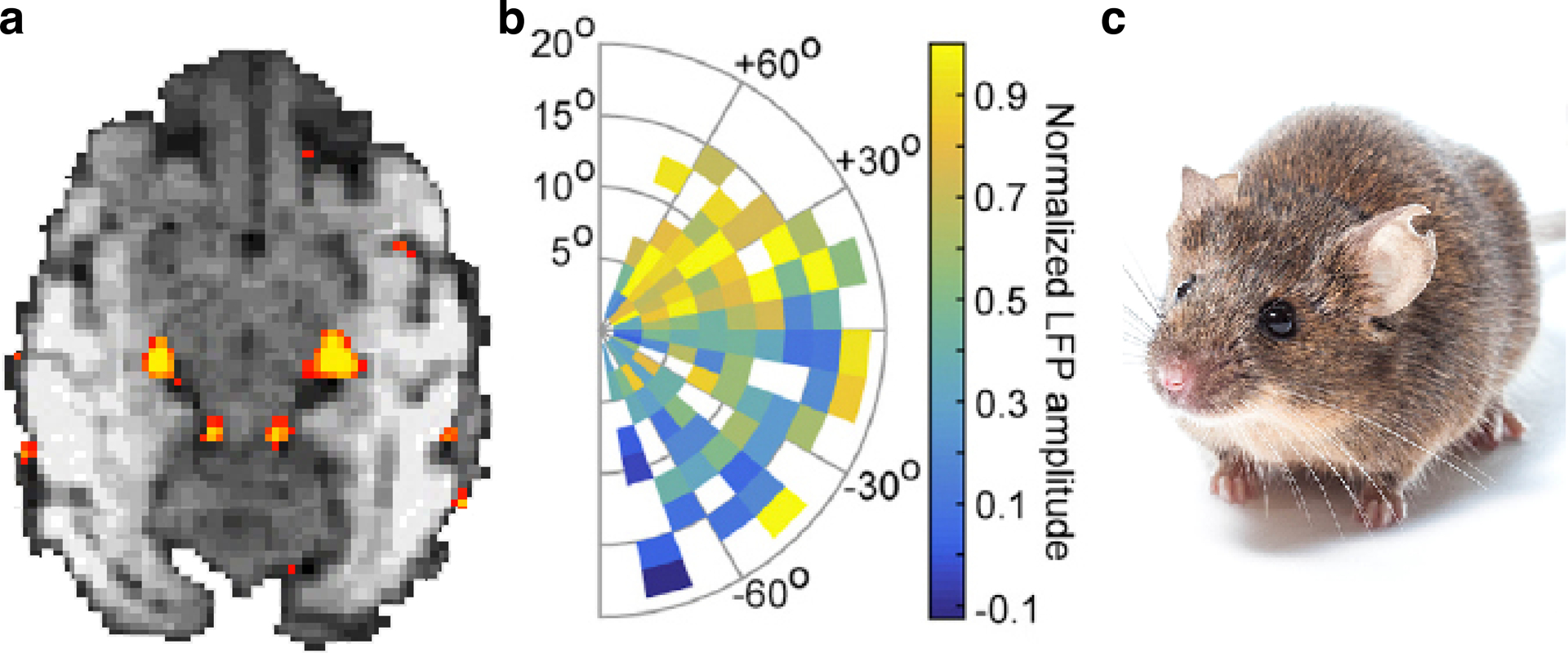
The superior colliculus and upper-visual-field bias. (a) Visually evoked activity in a 7-day-old macaque. The lateral geniculate is visually responsive (large bilateral activations) as is the superior colliculus (smaller bilateral activations) (M.S. Livingstone & J.L. Vincent, unpublished data). (b) In the superior colliculi of adult macaques, stimuli in the upper visual field evoke larger responses than do stimuli presented in the lower visual field (Hafed & Chen 2016; reproduced with permission). (c) Many animals have eyes that tilt upward, favoring detection of things that appear from above. Photo provided by A. Sugden. Abbreviation: LFP, local field potential.
6. CHILDHOOD MATURATION
At what age are face domains adult-like? In contrast to IT domains in adults, at 4 to 6 months in humans (Deen et al. 2017) and 1 month in monkeys (Livingstone et al. 2017), IT domains are not selectively responsive to faces as compared to inanimate objects. At approximately 3 months, face domains in monkeys are selective for faces compared with objects, scrambled faces, and scenes. The magnitude of selectivity and areal extent of face domains at 3 months were comparable to those in older monkeys. Given that only a limited number of categories were probed and modulations such as adaptation were not tested, it remains unclear whether these IT face domains are truly adult-like at this age. Further, face-selective responses were not consistently identified in the AM nor in the frontal face domains during the first two years. These regions are commonly face selective in adults (Tsao et al. 2008), suggesting that development of the macaque face domain system continues into adolescence.
Development of face domains in humans also appears to continue into adolescence. Several human fMRI studies have reported an increase in the size and/or selectivity of face domain FFA with age (Aylward et al. 2005; Cantlon et al. 2011; Golarai et al. 2007, 2010a; Scherf et al. 2007, 2011). Changes in selectivity and microstructure of face domains between children and adults are correlated with performance on face recognition tasks (Golarai et al. 2007, Gomez et al. 2018b), suggesting that experience continues to shape face domains throughout adolescence. Maturation of face domains includes refinement of the underlying retinotopic organization. A recent fMRI study found that the visual field coverage of fusiform face domain (pFus/ FFA) in the right hemisphere and the text domain in the left hemisphere had a larger foveal bias in adults (22–28 years) as compared to children (5–12 years) (Gomez et al. 2018b). The changes in visual field coverage were correlated with fixation patterns during a free-viewing task. These findings suggest that the foveal bias, which is present at birth (Arcaro & Livingstone 2017a), increases in adults to reflect changes in the viewing patterns of faces and text. Thus, behaviorally relevant changes in the functional and anatomical architecture of face domains continue well beyond the initial detection of face selectivity and are dependent on experience.
7. PLASTICITY
There is a final set of results that have been interpreted as supporting the innateness of category-specific IT domains, namely, demonstrations of cross-modal correlations in category selectivity in IT. For example, congenitally blind adults show a mediolateral distinction in the ventral visual pathway between animate and inanimate auditorily presented objects (Mahon et al. 2009). Congenitally blind adults presented with sounds characteristic of faces, bodies, objects, and scenes show a similar category organization as is typically found in sighted controls for visually presented stimuli (van den Hurk et al. 2017). Lastly, the visual word form area (VWFA) is activated by Braille reading in both blind and Braille-literate sighted subjects and by finger spelling in deaf subjects (Buchel et al. 1998, Reich et al. 2011, Siuda-Krzywicka et al. 2016, Waters et al. 2007). We would like to suggest again that there may be an alternative, map-based explanation for these observations. Given that humans have been reading and writing for at most 5,000 years, and literacy has been common for only the last few hundred years, there is no way humans have evolved an area in the brain specialized specifically for reading. These cross-modal plasticity results have been interpreted as demonstrating that these areas must have some innate predisposition to represent certain categories irrespective of modality; i.e. the face domain has innate connectivity to some social network (Powell et al 2018) or the VWFA has innate preferential connectivity with grammatical/speech/language areas (Mahon & Caramazza 2011).
We suggest again that there may be an alternative, map-based explanation for these observations. We propose that the observed cross-modal plasticity results could reflect map-based connectivity biases that support the interlinking of general processes across modalities, rather than connectivities that support specific functions. Much of the primate brain consists of maps of the world, in various modalities (Figure 16a). These maps share developmental gradients (O’Leary et al. 1999, Tessier-Lavigne & Goodman 1996; Flanagan 2006) that lead to a similar topographic organization of sensory space, including inversion of space and expansions of regions with high resolution -- acuity in the visual system, touch discrimination in somatosensation, and fineness of motor control. With cross-modal topography-preserving links point-specific connectivity naturally follows. This reduces the daunting challenge of interlinking seemingly discrete points within a high-dimensional space to a more tractable transformation of low-dimensional spaces (Figure 16b). Indeed, it is well established that the spatially specific interconnectivity between individual sensory maps (e.g., V1 and V2) arises from topography-preserving self-organizing processes , not point-to-point pre-specification (Figure 16c, left). Similarly, seemingly specific functions come to be linked across modalities based simply on the co-alignment of broad topographic gradients. For example, reading, irrespective of modality, requires high-resolution discrimination - recognizing learned patterns of many small elements. Evolutionary pressures may have promoted the interlinking of general computations (e.g., fine discrimination in one modality to fine discrimination in another) thereby allowing a biological system to remain flexible and adaptive to the environment rather than a large-scale rewiring to support a limited number of specific functions (e.g., reading or face recognition). In section 4 we described how a retinotopic map carries with it a map of sensitivity to scale and curvature and how such non-specific tuning may underlie the development of differential selectivity to more complex shapes. It should be no surprise that complexity from simple principles emerges naturally in other modalities as well and that such complex tuning systematically maps onto the underlying topography (Aflalo & Graziano 2006). In this framework what is interlinked is general features across maps, not specific functions. Indeed, cross-modal plasticity adheres to the intrinsic topographic organization of visual cortex; nonvisual category representations are spatially differentiated within IT in blind subjects along the medial-lateral axis (van den Hurk et al. 2017), which differentiates eccentricity representations and low-level shape tuning of the topographic architecture. We therefore propose that these results on cross-modal plasticity indicate commonalities in general computational requirements rather than commonalities in what the computations are used for.
Figure 16.
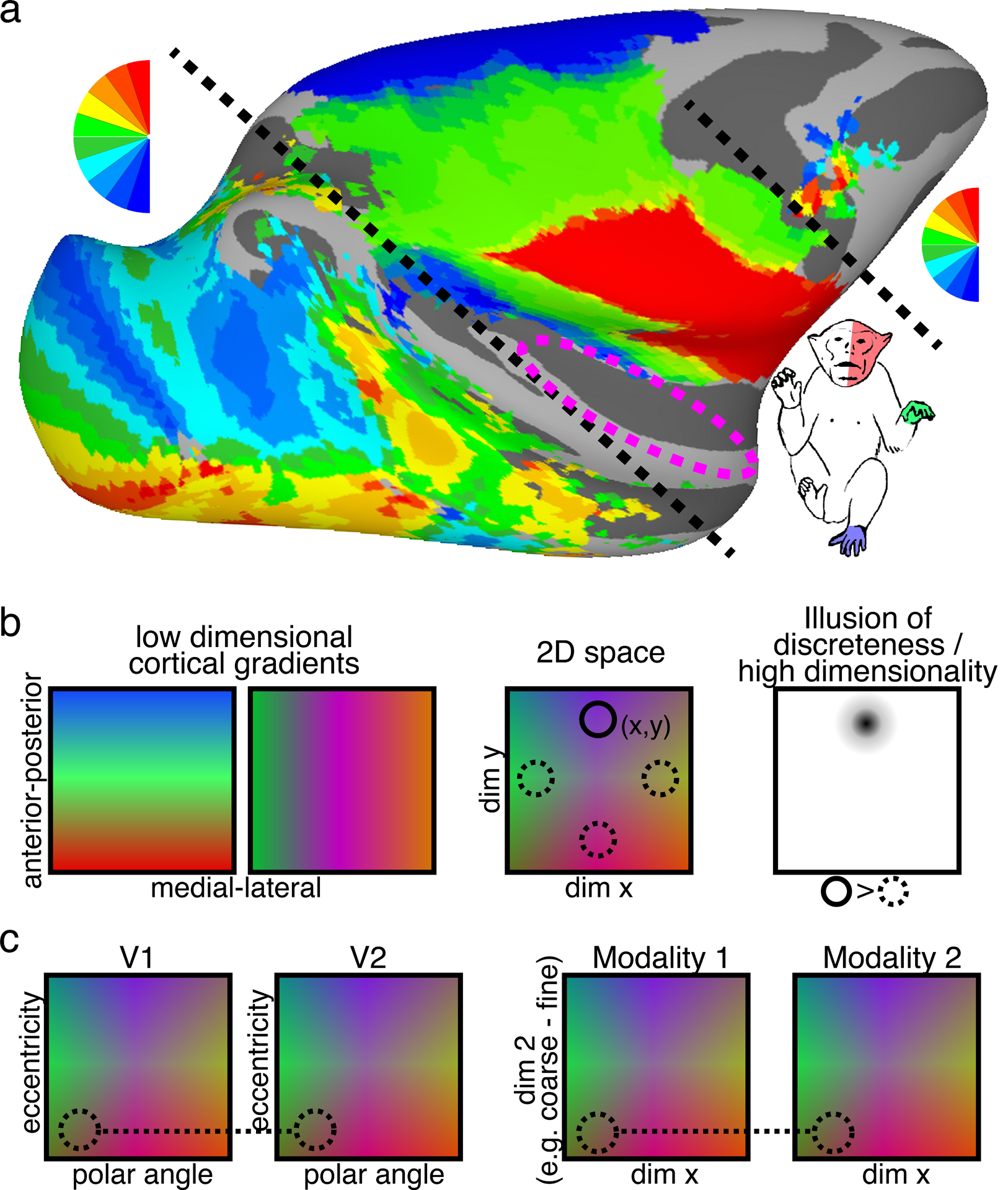
Maps, maps, maps. (a) Most of the primate cortical surface comprises topographic gradients of sensory space. Group average retinotopic (polar angle, n=6) and somatotopic (whole body, n=8) maps are differentiated by black dashed lines. Magenta dashed circle illustrates the cortical region comprising tonotopic maps. Arcaro and Livingstone unpublished data. (b, left) Topographic gradients systematically vary across the cortical surface. (Middle) The intersection of these gradients leads to 2-dimensional topographic maps along the cortical surface. The (x,y) coordinates of these gradients leads to unique features. (Right) Contrasting features within this topographic space can give the illusion of discreteness and high dimensionality (e.g., contrasting faces and objects with fMRI) while the ground truth is a continuum within a low-dimensional space. (c, left) Point-to-point connectivity between areas is not pre-determined, but arises from ubiquitous activity-dependent wiring rules. (c, right) Likewise, point specific links (direct or indirect) between modalities that each comprise topographic representations of space may emerge naturally from alignment of low-dimensional maps based on similarity of general computation requirements such as coarse-to-fine resolution and/or configural processing.
8. CONCLUSION
In conclusion, we advocate caution and parsimony in interpreting features of the brain as being innately optimized to process abstract, cognitive, or social behaviors, extrapolating from suggestions that cortical columns serve no particular purpose but are instead an epiphenomenon of a self-organizing mapping strategy (Horton & Adams 2005, Law & Constantine-Paton 1981, Livingstone et al. 1995). We are not questioning the utility of studying face domains. Even as an epiphenomenon, face domains hold tremendous value for understanding the neural correlates of face processing and broader computations within IT. However, as scientists wanting to understand the primate brain, we often ask what a brain region is good for, assuming that a brain region is for something—that it has a purpose. Instead, we favor asking how universal rules could lead to circuits that, along with environmental reinforcements, could come to underlie complex, adaptive behaviors.
Acknowledgments:
This work was supported by NIH National Eye Institute grants EY16187, EY25670, P30EY012196. We thank A. Schapiro for helpful comments on the paper.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adams DL, Sincich LC, Horton JC. 2007. Complete pattern of ocular dominance columns in human primary visual cortex. J. Neurosci 27:10391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflalo TN & Graziano MSA. 2006. Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array. J. Neurosci 26:6288–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Singh R, D’Esposito M. 1999. Stimulus inversion and the responses of face and object-sensitive cortical areas. Neuroreport 10:189–94 [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. 1998a. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron 21:373–83 [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. 1998b. Neural components of topographical representation. PNAS 95:839–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Watson DM, Rice GE, Hartley T. 2015. Low-level properties of natural images predict topographic patterns of neural response in the ventral visual pathway. J. Vis 15(7):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro M, Schade PF, Livingstone MS. 2018. Preserved cortical organization in the absence of early visual input. J. Vis 18(10):27 [Google Scholar]
- Arcaro MJ, Livingstone MS. 2017a. A hierarchical, retinotopic proto-organization of the primate visual system at birth. eLife 6:e26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Livingstone MS. 2017b. Retinotopic organization of scene areas in macaque inferior temporal cortex. J. Neurosci 37:7373–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, McMains SA, Singer BD, Kastner S. 2009. Retinotopic organization of human ventral visual cortex. J. Neurosci 29:10638–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Schade PF, Vincent JL, Ponce CR, Livingstone MS. 2017. Seeing faces is necessary for face-domain formation. Nat. Neurosci 20:1404–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Hood B, Wattam-Bell J, Braddick O. 1992. Changes in infants’ ability to switch visual attention in the first three months of life. Perception 21:643–53 [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, et al. 2005. Brain activation during face perception: evidence of a developmental change. J. Cogn. Neurosci 17:308–19 [DOI] [PubMed] [Google Scholar]
- Baldwin MK, Kaskan PM, Zhang B, Chino YM, Kaas JH. 2012. Cortical and subcortical connections of V1 and V2 in early postnatal macaque monkeys. J. Comp. Neurol 520:544–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MS, Ginsburg AP. 1985. Infant visual preferences: a review and new theoretical treatment. Adv. Child Dev. Behav 19:207–46 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. 2006. Nature and nurture in own-race face processing. Psychol. Sci 17:159–63 [DOI] [PubMed] [Google Scholar]
- Barlow HB. 1986. Why have multiple cortical areas? Vis. Res 26:81–90 [DOI] [PubMed] [Google Scholar]
- Barone P, Dehay C, Berland M, Kennedy H. 1996. Role of directed growth and target selection in the formation of cortical pathways: prenatal development of the projection of area V2 to area V4 in the monkey. J. Comp. Neurol 374:1–20 [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. 2003. FMRI responses to video and point-light displays of moving humans and manipulable objects. J. Cogn. Neurosci 15:991–1001 [DOI] [PubMed] [Google Scholar]
- Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RB, Ungerleider LG. 2009. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J. Neurophysiol 101:688–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G, Campbell D. 2001. Functional retinotopy of monkey visual cortex. J. Neurosci 21:8286–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G, Obermayer K, Kiorpes L. 1995. Organization of ocular dominance and orientation columns in the striate cortex of neonatal macaque monkeys. Vis. Neurosci 12:589–603 [DOI] [PubMed] [Google Scholar]
- Blasdel GG. 1992. Orientation selectivity, preference, and continuity in monkey striate cortex. J. Neurosci 12:3139–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock AS, Binda P, Benson NC, Bridge H, Watkins KE, Fine I. 2015. Resting-state retinotopic organization in the absence of retinal input and visual experience. J. Neurosci 35:12366–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross CG. 1981. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol 46:369–84 [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K. 1998. A multimodal language region in the ventral visual pathway. Nature 394:274–77 [DOI] [PubMed] [Google Scholar]
- Bushnell IWR. 2001. Mother’s face recognition in newborn infants: learning and memory. Infant Child Dev. 10:67–74 [Google Scholar]
- Bushnell IWR, Sai F, Mullin JT. 1989. Neonatal recognition of the mother’s face. Br. J. Dev. Psychol 7:3–5 [Google Scholar]
- Butt OH, Benson NC, Datta R, Aguirre GK. 2013. The fine-scale functional correlation of striate cortex in sighted and blind people. J. Neurosci 33:16209–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. 2008. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron 57:511–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. 2005. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron 48:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. 2011. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex 21:191–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassia VM, Simion F, Milani I, Umilta C. 2002. Dominance of global visual properties at birth. J. Exp. Psychol. Gen 131:398–411 [PubMed] [Google Scholar]
- Cassia VM, Turati C, Simion F. 2004. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychol. Sci 15:379–83 [DOI] [PubMed] [Google Scholar]
- Castets V, Dulos E, Boissonade J, De Kepper P. 1990. Experimental evidence of a sustained standing Turing-type nonequilibrium chemical pattern. Phys. Rev. Lett 64:2953–56 [DOI] [PubMed] [Google Scholar]
- Chao LL, Weisberg J, Martin A. 2002. Experience-dependent modulation of category-related cortical activity. Cereb. Cortex 12:545–51 [DOI] [PubMed] [Google Scholar]
- Clark VP, Keil K, Maisog JM, Courtney S, Ungerleider LG, Haxby JV. 1996. Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography. Neuroimage 4:1–15 [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, et al. 2000. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123(Part 2):291–307 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Law MI. 1978. Eye-specific termination bands in tecta of three-eyed frogs. Science 202:639–41 [DOI] [PubMed] [Google Scholar]
- Coogan TA, Van Essen DC. 1996. Development of connections within and between areas V1 and V2 of macaque monkeys. J. Comp. Neurol 372:327–42 [DOI] [PubMed] [Google Scholar]
- Cragg BG. 1969. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vis. Res 9:733–47 [DOI] [PubMed] [Google Scholar]
- Dailey MN, Cottrell GW. 1999. Organization of face and object recognition in modular neural network models. Neural Netw. 12:1053–74 [DOI] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. 2002. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J. Neurosci 22:9171–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. 2002. Specialization of neural mechanisms underlying face recognition in human infants. J. Cogn. Neurosci 14:199–209 [DOI] [PubMed] [Google Scholar]
- Deen B, Richardson H, Dilks DD, Takahashi A, Keil B, et al. 2017. Organization of high-level visual cortex in human infants. Nat. Commun 8:13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. 2007. Cultural recycling of cortical maps. Neuron 56:384–98 [DOI] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. 1984. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci 4:2051–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG. 1996. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb. Cortex 6:184–95 [DOI] [PubMed] [Google Scholar]
- Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. 2006. Domain specificity in visual cortex. Cereb. Cortex 16:1453–61 [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. 2001. A cortical area selective for visual processing of the human body. Science 293:2470–73 [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. 1999. The parahippocampal place area: recognition, navigation, or encoding? Neuron 23:115–25 [DOI] [PubMed] [Google Scholar]
- Farah MJ. 1990. Visual Agnosia: Disorders of Object Recognition and What They Tell Us About Normal Vision. Cambridge, MA: MIT Press [Google Scholar]
- Farah MJ. 1992. Is an object an object an object? Cognitive and neuropsychological investigations of domain specificity in visual object recognition. Curr. Dir. Psychol. Sci 1:164–69 [Google Scholar]
- Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G. 2005. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. PNAS 102:17245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausey CM, Jayaraman S, Smith LB. 2016. From faces to hands: changing visual input in the first two years. Cognition 152:101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG. 2006. Neural map specification by gradients. Curr. Opin. Neurobiol 16:59–66 [DOI] [PubMed] [Google Scholar]
- Frank MC, Vul E, Johnson SP. 2009. Development of infants’ attention to faces during the first year. Cognition 110:160–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. 2001. Neocortex patterning by the secreted signaling molecule FGF8. Science 294:1071–74 [DOI] [PubMed] [Google Scholar]
- Gauthier I. 2000. What constrains the organization of the ventral temporal cortex? Trends Cogn. Sci 4:1–2 [DOI] [PubMed] [Google Scholar]
- Gauthier I, Bukach C. 2007. Should we reject the expertise hypothesis? Cognition 103:322–30 [DOI] [PubMed] [Google Scholar]
- Gauthier I, Palmeri TJ. 2002. Visual neurons: categorization-based selectivity. Curr. Biol 12:R282–84 [DOI] [PubMed] [Google Scholar]
- Germine LT, Duchaine B, Nakayama K. 2011. Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition 118:201–10 [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, et al. 2007. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci 10:512–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Hong S, Haas BW, Galaburda AM, Mills DL, et al. 2010a. The fusiform face area is enlarged in Williams syndrome. J. Neurosci 30:6700–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Grill-Spector K. 2015. Experience shapes the development of neural substrates of face processing in human ventral temporal cortex. Cereb. Cortex 27:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Yoon JM, Grill-Spector K. 2010b. Differential development of the ventral visual cortex extends through adolescence. Front. Hum. Neurosci 3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J, Barnett M, Grill-Spector K. 2018a. Novel childhood experience suggests eccentricity drives organization of human visual cortex. bioRxiv 415729. 10.1101/415729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J, Natu V, Jeska B, Barnett M, Grill-Spector K. 2018b. Development differentially sculpts receptive fields across early and high-level human visual cortex. Nat. Commun 9:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. 1975. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 56:544–49 [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. 2004. The fusiform face area subserves face perception, not generic within-category identification. Nat. Neurosci 7:555–62 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen CY. 2016. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr. Biol 26:1647–58 [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. 2003. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage 19:1180–93 [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. 2002. Eccentricity bias as an organizing principle for human high-order object areas. Neuron 34:479–90 [DOI] [PubMed] [Google Scholar]
- Horton JC, Adams DL. 2005. The cortical column: a structure without a function. Philos. Trans. R. Soc. B 360:837–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. 1997. Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. J. Neurosci 17:3684–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. 2002. Divided by Cytochrome Oxidase: A Map of the Projections from V1 to V2 in Macaques. Science. 295:1734–1737. [DOI] [PubMed] [Google Scholar]
- Hubel DH, LeVay S, Wiesel TN. 1975. Mode of termination of retinotectal fibers in macaque monkey: an autoradiographic study. Brain Res. 96:25–40 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol 160:106–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1965. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J. Neurophysiol 28:229–89 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol 206:419–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1974. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J. Comp. Neurol 158:267–93 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. 1977. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. B 278:377–409 [DOI] [PubMed] [Google Scholar]
- Issa EB, DiCarlo JJ. 2012. Precedence of the eye region in neural processing of faces. J. Neurosci 32:16666–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens T, Zhu Q, Popivanov ID, Vanduffel W. 2014. Probabilistic and single-subject retinotopic maps reveal the topographic organization of face patches in the macaque cortex. J. Neurosci 34:10156–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Fausey CM, Smith LB. 2015. The faces in infant-perspective scenes change over the first year of life. PLOS ONE 10:e0123780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. 1991. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40:1–19 [DOI] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, et al. 2005. The emergence of the social brain network: evidence from typical and atypical development. Dev. Psychopathol 17:599–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. 1997. Topographic maps are fundamental to sensory processing. Brain Res. Bull 44:107–12 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin C-S, Merzenich MM. 1979. Multiple representations of the body within the primary somatosensory cortex of primates. Science 204:521–23 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci 17:4302–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. 1996. Synaptic activity and the construction of cortical circuits. Science 274:1133–38 [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Ge L, Quinn PC, Slater AM, et al. 2007. Cross-race preferences for same-race faces extend beyond the African versus Caucasian contrast in 3-month-old infants. Infancy 11:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, et al. 2005. Three-month-olds, but not newborns, prefer own-race faces. Dev. Sci 8:F31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake E, Tanaka K. 1994. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J. Neurophysiol 71:856–67 [DOI] [PubMed] [Google Scholar]
- Kolster H, Janssens T, Orban GA, Vanduffel W. 2014. The retinotopic organization of macaque occipitotemporal cortex anterior to V4 and caudoventral to the middle temporal (MT) cluster. J. Neurosci 34:10168–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. 1967. Integrative Activity of the Brain: An Interdisciplinary Approach. Chicago: Univ. Chicago Press [Google Scholar]
- Kornack DR, Rakic P. 1998. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. PNAS 95:1242–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith S, Cheng X, Ohayon S, Tsao DY. 2013. A network for scene processing in the macaque temporal lobe. Neuron 79:766–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremkow J, Jin J, Wang Y, Alonso JM. 2016. Principles underlying sensory map topography in primary visual cortex. Nature 533:52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L. 2007. The magnificent compromise: cortical field evolution in mammals. Neuron 56:201–8 [DOI] [PubMed] [Google Scholar]
- Lafer-Sousa R, Conway BR. 2013. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat. Neurosci 16:1870–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MI, Constantine-Paton M. 1981. Anatomy and physiology of experimentally produced striped tecta. J. Neurosci 1:741–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Yoshioka T, Lund JS 1994. Intrinsic cortical connections in macaque visual area V2: Evidence for integration between different functional streams. J Comp Neurol 342: 551–570. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. 2001. Center-periphery organization of human object areas. Nat. Neurosci 4:533–39 [DOI] [PubMed] [Google Scholar]
- Livingstone MS. 1996. Ocular dominance columns in New World monkeys. J. Neurosci 16:2086–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. 1984. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci 4:309–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. 1984b. Specificity of intrinsic connections in primate primary visual cortex. J. Neurosci 4:2830–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. 1987. Connections between layer 4B of Area 17 and the thick cytochrome-oxidase stripes of Area 18 in the squirrel monkey. J. Neurosci 7:3371–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Nori S, Freeman DC, Hubel DH. 1995. Stereopsis and binocularity in the squirrel monkey. Vis. Res 35:345–54 [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Vincent JL, Arcaro MJ, Srihasam K, Schade PF, Savage T. 2017. Development of the macaque face-patch system. Nat. Commun 8:14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B, Yu C-P, Konkle T. 2018. Mid-level visual features underlie the high-level categorical organization of the ventral stream. PNAS 115:E9015–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrou L, Barone P, Markov NT, Killackey HP, Giroud P, et al. 2018. How areal specification shapes the local and interareal circuits in a macaque model of congenital blindness. Cereb. Cortex 28:3017–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. 2009. Category-specific organization in the human brain does not require visual experience. Neuron 63:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. 2011. What drives the organization of object knowledge in the brain? Trends Cogn. Sci 15:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. 2002. The topography of high-order human object areas. Trends Cogn. Sci 6:176–84 [DOI] [PubMed] [Google Scholar]
- McKone E, Crookes K, Jeffery L, Dilks DD. 2012. A critical review of the development of face recognition: Experience is less important than previously believed. Cogn. Neuropsychol 29:174–212 [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. 2000. Pattern formation by local self-activation and lateral inhibition. Bioessays 22:753–60 [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. 1991. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev 98:164–81 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, Behrmann M. 1997. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but normal face recognition. J. Cogn. Neurosci 9:555–604 [DOI] [PubMed] [Google Scholar]
- Mur M, Ruff DA, Bodurka J, De Weerd P, Bandettini PA, Kriegeskorte N. 2012. Categorical, yet graded—single-image activation profiles of human category-selective cortical regions. J. Neurosci 32:8649–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, et al. 2011. Scene-selective cortical regions in human and nonhuman primates. J. Neurosci 31:13771–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S, Polimeni JR, Tootell RB. 2016. Interdigitated color- and disparity-selective columns within human visual cortical areas V2 and V3. J. Neurosci 36:1841–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Yates PA, McLaughlin T. 1999. Molecular development of sensory maps: representing sights and smells in the brain. Cell 96:255–69 [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Watanabe S, Kakigi R. 2007. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. Neuroimage 34:399–406 [DOI] [PubMed] [Google Scholar]
- Oyuyang Q, Swinney HL. 1991. Transition from a uniform state to hexagonal and striped Turing patterns. Nature 352:610–12 [Google Scholar]
- Painter KJ, Maini PK, Othmer HG. 1999. Stripe formation in juvenile Pomacanthus explained by a generalized Turing mechanism with chemotaxis. PNAS 96:5549–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. 1998. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. J. Exp. Psychol. Learn. Mem. Cogn 24:249–60 [DOI] [PubMed] [Google Scholar]
- Pascalis O, De Schonen S, Morton J, Deruelle C. 1995. Mother’s face recognition by neonates: a replication and an extension. Infant Behav. Dev 18:79–85 [Google Scholar]
- Peña B, Pérez-García C, Sanz-Anchelergues A, Míguez DG, Muñuzuri AP. 2003. Transverse instabilities in chemical Turing patterns of stripes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys 68:056206. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. 1998. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279:2108–12 [DOI] [PubMed] [Google Scholar]
- Perrett DI, Mistlin AJ, Chitty AJ. 1987. Visual neurones responsive to faces. Trends Neurosci. 10:358–64 [Google Scholar]
- Pinsk MA, Desimone K, Moore T, Gross CG, Kastner S. 2005. Representations of faces and body parts in macaque temporal cortex: a functional MRI study. PNAS 102:6996–7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut DC, Behrmann M. 2011. Complementary neural representations for faces and words: a computational exploration. Cogn. Neuropsychol 28:251–75 [DOI] [PubMed] [Google Scholar]
- Ponce CR, Hartmann TS, Livingstone MS. 2017. End-stopping predicts curvature tuning along the ventral stream. J. Neurosci 37:648–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LJ, Kosakowski HL, Saxe R. 2018. Social origins of cortical face areas. Trends in Cognitive Sciences. 22:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. 1996. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J. Neurosci 16:5205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. 1997. The neural basis of cognitive development: a constructivist manifesto. Behav. Brain Sci 20:537–56 [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalils O. 2002. Representation of the gender of human faces by infants: a preference for female. Perception 31:1109–21 [DOI] [PubMed] [Google Scholar]
- Rajimehr R, Bilenko NY, Vanduffel W, Tootell RB. 2014. Retinotopy versus face selectivity in macaque visual cortex. J. Cogn. Neurosci 26:2691–700 [DOI] [PubMed] [Google Scholar]
- Rakic P, Suñer I, Williams RW. 1991. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. PNAS 88:2083–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich L, Szwed M, Cohen L, Amedi A. 2011. A ventral visual stream reading center independent of visual experience. Curr. Biol 21:363–68 [DOI] [PubMed] [Google Scholar]
- Rice GE, Watson DM, Hartley T, Andrews TJ. 2014. Low-level image properties of visual objects predict patterns of neural response across category-selective regions of the ventral visual pathway. J. Neurosci 34:8837–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R, McKone E. 2007. No face-like processing for objects-of-expertise in three behavioural tasks. Cognition 103:34–79 [DOI] [PubMed] [Google Scholar]
- Rodman HR, Skelly JP, Gross CG. 1991. Stimulus selectivity and state dependence of activity in inferior temporal cortex of infant monkeys. PNAS 88:7572–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangrioli S, de Schonen S. 2004. Recognition of own-race and other-race faces by three-month-old infants. J. Child Psychol. Psychiatry 45:1219–27 [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. 2007. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci 10:F15–30 [DOI] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Avidan G, Behrmann M. 2011. “What” precedes “which”: developmental neural tuning in face- and place-related cortex. Cereb. Cortex 21:1963–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Stawinski P, Bonhoeffer T. 1999. Influence of experience on orientation maps in cat visual cortex. Nat. Neurosci 2:727–32 [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. 1988. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242:87–89 [DOI] [PubMed] [Google Scholar]
- Simion F, Di Giorgio E. 2015. Face perception and processing in early infancy: inborn predispositions and developmental changes. Front. Psychol 9:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion F, Farroni T, Cassia VM, Turati C, Barba BD. 2002a. Newborns’ local processing in schematic facelike configurations. Br. J. Dev. Psychol 20:465–78 [Google Scholar]
- Simion F, Valenza E, Cassia VM, Turati C, Umilta C. 2002b. Newborns’ preference for up-down asymmetrical configurations. Dev. Sci 5:427–34 [Google Scholar]
- Siuda-Krzywicka K, Bola L, Paplinska M, Sumera E, Jednorog K, et al. 2016. Massive cortical reorganization in sighted Braille readers. eLife 5:e10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J, Rudiger S, Pullarkat P, Ott A. 2009. Mechanogenetic coupling of Hydra symmetry breaking and driven Turing instability model. Biophys. J 96:1649–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Berlucchi G, Rizzolatti G. 1973. The role of the superior colliculus and pretectum in vision and visually-guided behavior In Handbook of Sensory Physiology, ed. Jung R, pp. x–x. Berlin: Springer-Verlag [Google Scholar]
- Srihasam K, Mandeville JB, Morocz IA, Sullivan KJ, Livingstone MS. 2012. Behavioral and anatomical consequences of early versus late symbol training in macaques. Neuron 73:608–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihasam K, Vincent JL, Livingstone MS. 2014. Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat. Neurosci 17:1776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem-Amit E, Ovadia-Caro S, Caramazza A, Margulies DS, Villringer A, Amedi A. 2015. Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain 138:1679–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. 1993. Parts and wholes in face recognition. Q. J. Exp. Psychol. A 46:225–45 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ribot J, Imamura K, Tani T. 2006. Orientation-restricted continuous visual exposure induces marked reorganization of orientation maps in early life. Neuroimage 30:462–77 [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Lu HD, Roe AW. 2010. Functional organization for color and orientation in macaque V4. Nat. Neurosci 13:1542–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr MJ, Cheng YD. 2003. Learning to see faces and objects. Trends Cogn. Sci 7:23–30 [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. 2000. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat. Neurosci 3:764–69 [DOI] [PubMed] [Google Scholar]
- Teinonen T, Fellman V, Naatanen R, Alku P, Huotilainen M. 2009. Statistical language learning in neonates revealed by event-related brain potentials. BMC Neurosci 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. 1996. The molecular biology of axon guidance. Science 274:1123–33 [DOI] [PubMed] [Google Scholar]
- Tigges J, Tigges M. 1979. Ocular dominance columns in the striate cortex of chimpanzee (Pan troglodytes). Brain Res. 166:386–90 [DOI] [PubMed] [Google Scholar]
- Tootell RB, Nasr S, Yue X. 2012. Selectivity for different shapes drives ‘face selective’ and ‘scene selective’ areas in human visual cortex. SFN Abstr. 2012:624.04 [Google Scholar]
- Tootell RB, Silverman MS, De Valois RL, Jacobs GH. 1983. Functional organization of the second cortical visual area in primates. Science 220:737–39 [DOI] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. 1988. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J. Neurosci 8:1531–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. 2003. Faces and objects in macaque cerebral cortex. Nat. Neurosci 6:989–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. 2006. A cortical region consisting entirely of face-selective cells. Science 311:670–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. 2008. Comparing face patch systems in macaques and humans. PNAS 105:19514–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turati C, Di Giorgio E, Bardi L, Simion F. 2010. Holistic face processing in newborns, 3-month-old infants, and adults: evidence from the composite face effect. Child Dev. 81:1894–905 [DOI] [PubMed] [Google Scholar]
- Turati C, Macchi Cassia V, Simion F, Leo I. 2006. Newborns’ face recognition: role of inner and outer facial features. Child Dev. 77:297–311 [DOI] [PubMed] [Google Scholar]
- Turati C, Simion F, Milani I, Umilta C. 2002. Newborns’ preference for faces: What is crucial? Dev. Psychol 38:875–82 [PubMed] [Google Scholar]
- Turing A. 1952. The chemical basis of morphogenesis. Philos. Trans. R. Soc. B 237:37–52 [Google Scholar]
- van den Hurk J, Van Baelen M, Op de Beeck HP. 2017. Development of visual category selectivity in ventral visual cortex does not require visual experience. PNAS 114:E4501–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. 1973. Somatosensory cortex: structural alterations following early injury to sense organs. Science 179:395–98 [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Lewis JW, Drury HA, Hadjikhani N, Tootell RB, et al. 2001. Mapping visual cortex in monkeys and humans using surface-based atlases. Vis. Res 41:1359–78 [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JN. 2013. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498:65–69 [DOI] [PubMed] [Google Scholar]
- Wang P, Cottrell GW. 2017. Central and peripheral vision for scene recognition: a neurocomputational modeling exploration. J. Vis 17(4):9. [DOI] [PubMed] [Google Scholar]
- Waters D, Campbell R, Capek CM, Woll B, David AS, et al. 2007. Fingerspelling, signed language, text and picture processing in deaf native signers: the role of the mid-fusiform gyrus. Neuroimage 35:1287–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. 1974. Ordered arrangement of orientation columns in monkeys lacking visual experience. J. Comp. Neurol 158:307–18 [DOI] [PubMed] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Williams M, et al. 2010. Human face recognition ability is specific and highly heritable. PNAS 107:5238–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R. 1969. Looking at upside-down faces. J. Exp. Psychol 81:141–45 [Google Scholar]
- Young AW, Hellawell D, Hay DC. 1987. Configurational information in face perception. Perception 16:747–59 [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. 2004. Face perception: domain specific, not process specific. Neuron 44:889–98 [DOI] [PubMed] [Google Scholar]
- Yue X, Pourladian IS, Tootell RB, Ungerleider LG. 2014. Curvature-processing network in macaque visual cortex. PNAS 111:E3467–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. 1969. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 14:271–91 [DOI] [PubMed] [Google Scholar]
- Zeki SM. 1973. Colour coding in rhesus monkey prestriate cortex. Brain Res. 53:422–27 [DOI] [PubMed] [Google Scholar]
- Zeki SM. 1974a. Cells responding to changing image size and disparity in the cortex of the rhesus monkey. J. Physiol 242:827–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. 1974b. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J. Physiol 236:549–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki & Shipp 1989. Modular Connections between Areas V2 and V4 of Macaque Visual Cortex. Euro J of Neurosci 1: 494–506 [DOI] [PubMed] [Google Scholar]


