Abstract
In addition to a nutritive role, human milk also guides the development of a protective intestinal microbiota in the infant. Human milk possesses an overabundance of complex oligosaccharides that are indigestible by the infant yet are consumed by microbial populations in the developing intestine. These oligosaccharides are believed to facilitate enrichment of a healthy infant gastrointestinal microbiota, often associated with bifidobacteria. Advances in glycomics have enabled precise determination of milk glycan structures as well as identification of the specific glycans consumed by various gut microbes. Furthermore, genomic analysis of bifidobacteria from infants has revealed specific genetic loci related to milk oligosaccharide import and processing, suggesting coevolution between the human host, milk glycans, and the microbes they enrich. This review discusses the current understanding of how human milk oligosaccharides interact with the infant microbiota and examines the opportunities for translating this knowledge to improve the functionality of infant formulas.
Keywords: milk oligosaccharides, bifidobacteria, prebiotics
INTRODUCTION
The process of bacterial colonization of the intestine begins naturally in a stepwise manner with three phases: delivery, breastfeeding, and weaning (Penders et al. 2006, Sherman et al. 2009). By the age of 18 months, the colonic bacterial microbiota is considered complete (Harmsen et al. 2000, Palmer et al. 2007, Rubaltelli et al. 1998). The gut microbiota of breastfed infants is modulated by human milk, the predominant diet of newborn infants. Milk is a truly unique food that has been shaped by mammalian evolution to provide both nutrition and protection to the developing infant, all at an energetic cost to the mother. As a result of this distinctive evolutionary tension, milk is unlikely to have retained superfluous contents that do not benefit the infant, as their presence comes at a cost to the mother. The benefits of breastfeeding in terms of infant development and protection have been well documented (Wu & Chen 2009). Various factors present in milk are known to modulate the developing microbiota within the infant gastrointestinal tract (GIT), including immunoglobulins, lactoferrin, lysozyme, bioactive lipids, leukocytes, and various milk glycans (glycolipids, glycoproteins, and free oligosaccahrides) among others (Newburg 2005). Although some of these bioactive components are known for their functionality in reducing pathogens in the infant GIT, others are believed to encourage specific bacterial populations, such as bifidobacteria—a genus first identified more than 100 years ago in the feces of breastfed infants (Moro 1900, Tissier 1900). Such a prebiotic function of human milk was originally described by Gyorgy and coworkers (1954), who first identified N-acetyl-glucosamine (GlcNAc)-containing oligosaccharides, generally termed human milk oligosaccharides (HMOs), as the bifidus factor responsible for enrichment of bifidobacteria (Gauche et al. 1954, German et al. 2008).
HMOs are believed to have many roles in a developing infant in addition to putative prebiotic functions. HMOs may possess antiadhesive effects that reduce the binding of pathogenic bacteria to colonocytes (Lane et al. 2010). HMOs have modulating effects on immunologic processes at the level of gut-associated lymphoid tissue (Guarner 2009) and may also decrease intestinal permeability in preterm infants in a dose-related manner in the first postnatal month (Taylor et al. 2009). Others have suggested that HMOs are an important source of N-acetyl-neuraminic acid (NeuAc; sialic acid), an essential monosaccharide during the period of neonate brain development and myelination (Wang et al. 2001).
The newborn infant gastrointestinal tract is initially colonized by aerobic and facultatively anaerobic bacteria, often species of enterobacteria, enterococci, and staphylococci (Adlerberth & Wold 2009). As these initial bacteria consume oxygen present in the intestine, anaerobic genera such as bifidobacteria, clostridia, and bacteroides are enriched. A number of studies have demonstrated that bifidobacteria are overrepresented in the gastrointestinal microbiota of breastfed infants by comparison with adults (Favier et al. 2003, Harmsen et al. 2000,Mariat et al. 2009, Penders et al. 2006). More recently, some studies have illustrated the sporadic and individualized nature of microbial colonization of infants (Koenig et al. 2010, Palmer et al. 2007). These recent approaches have also challenged the often-reported observation of bifidobacterial dominance of the breastfed infant GIT. Unfortunately, technical biases in these studies derived from sequencing V2 16S rDNA region amplicons may have led to an underestimation of the actinobacterial clade—as has been observed in studies comparing different 16S variable region amplicons (Turnbaugh et al. 2006) or by comparing to complementary metagenomic approaches (Koenig et al. 2010). Regardless, these new approaches hold significant promise in more comprehensively characterizing the influence of breast milk on the developing infant gut microbiota and its inherent metabolic capacity.
In a recent review of a number of studies undertaken in the past 20 years, Alderberth & Wold (2009) reported only minor differences in the levels of bifidobacteria present in formula-fed infant feces compared with breastfed infant feces, contrasting a commonly held perception of a low bifidobacterial presence in formula-fed infants. Certain microbial clades, such as clostridia, bacteroides, and Enterobacteriaceae, are more often observed in formula-fed than breastfed infants, resulting in a common description of formula-fed infants as having a more adult-like gastrointestinal microbiota (Adlerberth & Wold 2009). Moreover, a recent study noted that the specific bifidobacterial species diversity present in formula-fed infants is more adult-like (Haarman & Knol 2005).
The World Health Organization has clearly identified breastfeeding as providing the optimum nutrition and protection for developing infants (WHO 2009). In spite of this advice, there remains a great need for optimal infant formulas as a substitute for breastfeeding in cases where the latter is simply not possible. Recent advances in our understanding of the complex functions of human milk create a conceptual path for the design of more functional formulas. Such formulas would not only provide the complex nutritional needs for infants but also facilitate the microbial successions witnessed in breastfed infant GIT.
A variety of options are available for specific modification of microbial colonization in infants fed formula. Besides adding live bacteria, such as bifidobacteria and lactobacilli as probiotic additives, prebiotic oligosaccharides can be added as substrates that arrive undigested to the colon (Boehm & Moro 2008) and stimulate the growth and/or metabolic activity of beneficial bacterial species such as bifidobacteria (Manning & Gibson 2004). Interestingly, the structure of HMOs, compromising more than 200 different molecular structures (Bode 2006, Coppa et al. 2004, Ninonuevo et al. 2006), differs significantly from plant-derived fructooligosaccharides (FOS) or enzymatically synthesized galactooligosaccharides (GOS) (Fanaro et al. 2005a). To date, the variable oligosaccharide content of human milk cannot be successfully reproduced on a large scale for inclusion in infant formulas. (German et al. 2008, Guarner 2009, Manning & Gibson 2004, Sarney et al. 2000, Taylor et al. 2009, Wang et al. 2001). This manuscript focuses on the latest discoveries in the field of milk oligosaccharides as well their potential use in infant formula. In addition, structure and effects of bovine milk oligosaccharides (BMOs) and plant-derived oligosaccharides are briefly compared with those of HMOs.
HUMAN MILK OLIGOSACCHARIDES
HMOs are the third most abundant component of human milk (Kunz et al. 2000). Among all of the components, such as proteins, lactose, and nucleotides, the HMO is the only component that has been demonstrated to play a significant role in the stimulation of the growth of specific bacteria (Coppa et al. 2006). HMO-like structures are also found as components of glycolipids and glycoproteins (Newburg 1999). There are approximately 200 known compositions incorporating ≥3 carbohydrate monomers via 13 possible glycosidic linkages (Kunz et al. 2000, Ninonuevo et al. 2006). The molecular structure of these oligosaccharides is highly variable; additionally, the composition and concentration change significantly during lactation (described below) (Boehm & Moro 2008). After ingestion, HMOs pass mainly unabsorbed through the small intestine into the colon, where they are fermented to short-chain fatty acids (SCFA) and lactic acids, creating an acidic environment (Ogawa et al. 1992).
HMO Composition and Structure
The composition of milk oligosaccharides, as well as other milk components, differs among mammalian species and also during the course of lactation. Oligosaccharides in human milk are characterized by an enormous structural diversity (Chaturvedi et al. 2001). HMOs are formed by the attachment of a single glucose (Glc) molecule at the reducing end to galactose (Gal; bound to the Glc) to form a lactose core (Bode 2006). A linear chain is formed via β1–3 linkage attached to the core structure of GlcNAc, whereas a branched chain results when two GlcNAcs are added on both the β1–3 and β1–6 positions (Wu et al. 2010). After addition of the GlcNAc, another Gal is added either at β1–4 or β1–3. The resulting GlcNAc and Gal disaccharide may repeat multiple times. There are at least 12 different types of glycosidic bonds described in HMO (Newburg et al. 2005, Kobata 2003).). The smallest oligosaccharides are generated either when fucose (Fuc) is added to lactose, thus generating the trisaccharide fucosyllactose (2′FL; Fucα1–2, Galβ1–4Glc, and 3′FL; Gal β1–4[Fucα1–3]Glc), or when NeuAc is added to lactose, generating the sialyllactoses (3′SL; NeuAcα1–3Gal β1–4Glc and 6′SL; NeuAcα1–6Galβ1–4Glc) (Espinosa et al. 2007). However, these small oligosaccharides are generally less abundant than the larger, more complicated structures.
The synthesis of these oligosaccharides within the lactating mammary gland is catalyzed by a number of specific glycosyltransferases, including galactotransferases, N-acetylglucosaminyltransferases, fucosyltransferases, and sialyltransferases, whose expression is required for the synthesis of various glycoconjugates that are normally found in both lactating and nonlactating mammary tissue (Kelder et al. 2001). Because the human intestine does not express the luminal enzymes to cleave the α-glycosidic linkages of Fuc and sialic acid, as well as β-glycosidic linkages in the core HMO molecule, these acids are resistant to enzymatic cleavages in the intestine (Engfer et al. 2000, Gnoth et al. 2000). As a result, HMOs can be detected in the feces of breastfed infants (Coppa et al. 2001). HMOs are also absorbed in vivo (Engfer et al. 2000, Gnoth et al. 2001, Kunz et al. 2000, Rudloff et al. 2006) through the intestinal wall in small amounts, possibly by receptor-mediated endocytosis (~1% of intake) and can be detected in urine (Coppa et al. 1990, Coppa et al. 2001). In this process, HMOs are taken unmodified up via trans- and para-cellular pathways (Gnoth et al. 2001).
HMOs are especially rich in the type 1 oligosaccharides. Lacto-N-biose (LNB; Galβ1–3GlcNAc) is a building unit of the three type 1 HMOs, such as lacto-N-tetraose (LNT; Galβ1–3GlcNAcβ1–3Galβ1–4Glc), lacto-N-fucopentaose I (LNFP; Fucα1–2Galβ1–3GlcNAcβ1–3Galβ1–4Glc), and lacto-N-difucohexaose I (Fucα1–2Galβ1–3[Fucα1–4]GlcNAcβ1–3Galβ1–4Glc)]. More detail on the structure and function of selected HMOs is available in a recent review by Bode (2006).
Although researchers began describing analytical methods for the isolation and characterizing of HMO over 40 years ago (Kobata & Ginsburg 1969), only recently has the technology advanced to precisely present and differentiate those complex glycans. Recently, Ninonuevo & Lebrilla (2009) discussed in detail the current methods for analysis of oligosaccharides in human milk. In recent years, more published data on the structure and function of HMOs allowed defining of the Lewis and Secretor blood groups corresponding to the specific HMOs produced by lactating mothers (Kunz & Rudloff 2008).
Lewis Blood Group and Secretor Status
Studies have shown that milk from different mothers may be qualitatively and quantitatively different with regards to its oligosaccharide content (Newburg 2000). A close relationship exists between HMO profiles, the structures of milk oligosaccharides, and the Lewis Blood Group and Secretor status (Kobata 1992, Thurl et al. 1997). The main criteria in the predicted variability of phenotypes seems to depend on the expression and activity of specific fucosyltransferases in the lactating mammary gland (Kunz & Rudloff 2008). Fucosyltransferase and fucosidase activities vary in milk specimens, both from different donors and from the same donors at different stages of lactation (Wiederschain & Newburg 1996). Additionally, these differences might also apply to other glycoconjugates (e.g., glycoproteins that are constructed via the same glycosyltransferases that synthesize HMOs). In human milk from individuals with blood type Le(a − b +) (~70% of the population), HMO with α1-2, α1-3, and α1-4-linked fucosyl residues occur (Kobata 1992). The second group, Le(a + b −) (20%) lacks compounds with α1-2-linked fucosyl compounds. Finally, in the remaining 10% of the population (Le(a − b −)), oligosaccharides with α1-4-linked fucose residues are missing. This system could potentially play a significant role in synchronizing select bacterial microbiota in infants with the mother’s blood group. Population-based studies support this concept, and a high level of α1-2-linked Fuc relative to total HMOs has been shown to correlate with a lowered incidence of infant diarrhea (Morrow et al. 2005).
Variations in HMO Production
HMOs are solely produced in the lactating mammary gland and vary over the course of lactation (Chaturvedi et al. 2001). HMOs achieve a maximum concentration in the colostrum (above 20 g L−1) with 5–14 g L−1 in mature milk (Kunz et al. 2000, Kunz et al. 1996). One limitation in the attempts to characterize HMO is a lack of availability in sufficient quantities and purity for in vitro and clinical studies. The majority of clinical studies using breast milk have to be interpreted carefully because assumed biological effects of HMO have also been credited to glycoproteins, glycolipids, and other milk constituents (Espinosa et al. 2007), and numerous other glycoconjugates might share the structural features with HMOs.
Given that various functions are associated with the diverse HMO structures, the details of variations in composition and differences of oligosaccharides among humans in remote populations need to be defined. Current analytical methods to characterize oligosaccharides in human milk include high-performance liquid chromatography (HPLC) (Chaturvedi et al. 1997, Leo et al. 2010), high pH anion exchange chromatography (HPAEC) (Thurl et al. 1996), capillary electrophoresis (CE) (Bao & Newburg 2008, Shen et al. 2000), and mass spectrometry (MS) (Albrecht et al. 2010, LoCascio et al. 2007, Marcobal et al. 2010, Niñonuevo & Lebrilla 2009). A method for precise quantification of consumption of individual HMOs named matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry (MALDI-FTICR MS) allows detection of individual neutral oligosaccharides, which represent the majority of total HMOs (Ninonuevo et al. 2006).
Chaturvedi and coworkers have demonstrated that the ratio of fucosylated α1–2-linked oligosaccharide concentrations to oligosaccharides devoid of α1–2 linked Fuc changed during the first year of lactation from 5:1 to 1:1 (Chaturvedi et al. 2001). Furthermore, the concentrations of individual oligosaccharides varied substantially, both between the mothers and over the course of lactation. Those results suggest that the protective activities of HMOs might vary among the individuals and during the lactation. Interestingly, the attachment of Fuc was shown to be based on the Secretor status and Lewis blood group of the individual mother (Thurl et al. 1997). Another study analyzed the level of major neutral oligosaccharides for three consecutive days in human milk colostrums (Asakuma et al. 2007). Concentrations of 2′FL and lactodifucotetraose on day 1 were found to be substantially higher than those on day 2 and 3, whereas LNT concentration increased from day 1 to day 3.
Using HPLC-Chip/time-of-flight (TOF)-MS technology, Ninonuevo et al. (2008) reported significant variations in the oligosaccharide contents primarily with the minor HMO components, whereas there was a tendency to produce a single component in very large quantities among lactating mothers. In that study, the most abundant components were identified to be lacto-N-neotetraose (LNnT), LNT, and LNFP. These authors noted that LNnT, LNT, and LNFP were also preferentially consumed by Bifidobacterium longum subsp. infantis (B. infantis), illustrating a unique correspondence between these most abundant oligosaccharides and the bacteria they enrich. Interestingly, adult-type bifidobacteria Bifidobacterium adolescentis and Bifidobacterium animalis do not degrade LNT (Xiao et al. 2010). It is generally accepted that the mother’s diet, physiology, and feeding behavior may have an impact on the daily HMO production.
HMOs AS A DEFENSE MECHANISM
HMOs play a critical role in the infant’s defense system, the development of a specific intestinal microbiota, and the inflammatory processes (Zopf & Roth 1996). Numerous local and systemic effects of HMOs have been described previously, including protective functions of HMOs against enteropathogens (Newburg et al. 2004a). Also, antipathogenic effects of fucosylated oligosaccharides, specifically those that contain the Fucα1–2 structural motif were elucidated (Newburg et al. 2004a, Newburg et al. 2004b, Ruiz-Palacios et al. 2003). For example, Ruiz-Palacios and coworkers have reported that infants fed with human milk having low concentrations of 2′FL may be more susceptible to diarrhea than babies fed the breast milk containing high concentrations of 2′FL (Ruiz-Palacios et al. 2003).
Prevention of Pathogen Adhesion
Other researchers reported that HMOs serve as soluble ligand analogs and block pathogen adhesion (Newburg et al. 2005). The chemical structures of HMO are homologous to the carbohydrate units of glycoconjugates, especially of glycolipids, on cell surfaces of mammalian epithelial cells. For example, binding of Escherichia coli, Streptococcus pneumonia, Campylobacter jejuni, Helicobacter pylori, and Vibrio cholerae was inhibited by the glycoconjugates present in HMOs (e.g., 2′fucosyllactosamine) (Bode 2009, Leach et al. 2005, Morrow et al. 2004, Newburg et al. 2005, Ruiz-Palacios et al. 2003). It is also possible that HMOs can have glycome-modifying effects through changing of the expression of intestinal epithelial cell surface glycans. Angeloni et al. (2005) demonstrated that Caco-2 cells change their surface glycan profile after the exposure to 3′SL, a constituent of HMOs. In that study, the expression of α2–3- and α2–6-linked sialic acid residues in Caco-2 cells was significantly downregulated. Thus, this particular HMO appears to modify the glycan content of the epithelial cell surface and the receptor sites for some pathogens. The same researchers further confirmed that the adhesion of enteropathogenic E. coli (EPEC) was reduced upon treatment with 3′SL. Table 1 lists the HMOs that inhibit specific pathogens in vitro, ex vivo, or in vivo.
Table 1.
Pathogen inhibition by select HMOs in in vivo, ex vivo, and in vitro studies
| Pathogen | HMO tested | Reference |
|---|---|---|
| Norwalk virus | Fucosylated oligosaccharides | (Ruvoën-clouet et al. 2006) |
| Campylobacter jejuni | (Morrow et al. 2004, Ruiz-Palacios et al. 2003) | |
| Vibrio cholera | (Ruiz-Palacios et al. 2003) | |
| Escherichia coli (heat-stable enterotoxin) | (Newburg et al. 1990, Newburg et al. 2004b) | |
| Streptococcus pneumoniae | Sialyllactose | (Leach et al. 2005) |
| Cholera toxin | (Idota et al. 1995) | |
| E. coli | (Virkola et al. 1993) | |
| Pseudomonas aeruginosa | (Devaraj et al. 1994) | |
| Aspergillus fumigates conidia | (Bouchara et al. 1997) | |
| Influenza virus | (Gambaryan et al. 1997, Matrosovich et al. 1993) | |
| Polymavirus | (Stehle et al. 1994) | |
| Helicobacter pylori | (Mysore et al. 1999) | |
| HIV-1 | Oligosaccharides | (Hong et al. 2009 |
| Streptococcus pneumoniae | (Andersson et al. 1986, Idänpään-Heikkilä et al. 1997) | |
| Enteropathogenic E. coli (EPEC) | (Cravioto et al. 1991) | |
| Haemophilius influenzae | (Idänpään-Heikkilä et al. 1997) |
Role of Oligosaccharides in the Development of the Immune System
Previous research has demonstrated that HMOs directly affect the immune system (Eiwegger et al. 2004, Newburg 2009, Velupillai & Harn 1994). For example, HMOs have been shown to interact with selectins (Schumacher et al. 2006), integrins (Bode et al. 2004a), and toll-like receptors (Vos et al. 2007), as well as to affect leukocyte-endothelial cell and leukocyte-platelet interactions (Bode et al. 2004a, Bode et al. 2004b, Lasky 1995, McEver 1994, Schwertmann et al. 1996). Many sialylated and fucosylated HMOs may block the latter interactions by having significant effects on the progression of inflammatory responses (Kunz et al. 1999). A recent study has also shown that HMOs can inhibit transfer of HIV-1 virus to CD4+ lymphocytes (Hong et al. 2009). Furthermore, HMOs induce intracellular processes, including differentiation and apoptosis of intestinal epithelial cells. (Kuntz et al. 2009, Kuntz et al. 2008). Neutral HMO structures, such as LNFP III and LNnT, affect murine IL-10 production (Velupillai and Harn 1994), suggesting that HMOs might be involved in the production of antiinflammatory mediators that suppress proinflammatory Th1 response in mice (Terrazas et al. 2001). In another study, HMOs affected Th1/Th2 skewing via production of cytokines as well as maturation and activation of human cord blood–derived T cells (Eiwegger et al. 2004). More recently, Eiwegger et al. (2010) demonstrated a novel, direct immunomodulatory effect of acidic fraction of HMO when compared with the same fraction from cow’s milk. In this study, acidic HMOs stimulated production of IFN-γ and IL-10, directing the neonatal Th2-type T-cell phenotype toward a Th-0-type profile in cord blood–derived mononuclear cells. This effect also impacted Th-2-type immune response of allergen-specific T cells from peanut allergic individuals. Both results strongly suggest antiallergic properties of certain acidic HMOs.
HMOs AS GROWTH FACTORS FOR BIFIDOBACTERIA
A bifidobacterial presence in the feces of breastfed infants was described by Moro in 1900, who reported that human milk contains a growth factor for these bacteria (Moro 1900). Fifty years later, György identified the bifidus factor to be GlcNAc (previously named gynolactose) (Gyorgy 1953, Hoover et al. 1953) using growth of Bifidobacterium bifidum subsp. Pennsylvanicum. In vitro studies have demonstrated that GlcNAc-containing oligosaccharides are indeed able to enhance the growth of this bifidobacteria, while other sugars showed less growth-promoting activity (Petschow & Talbott 1991). The bifidogenic effect in infants is often associated with a reduction of stool pH and changes in SCFA pattern (Rinne et al. 2005). The ability of selected bifidobacteria to consume prebiotic oligosaccharides from human milk is likely an essential trait enabling this genera to be one of the most abundant colonizers of the breastfed infant gut (LoCascio et al. 2009, LoCascio et al. 2007).
Ward et al. (2006) first demonstrated vigorous growth of B. infantis on HMOs as a sole carbon, whereas Lactobacillus gasseri, a common inhabitant of the adult intestine, grew poorly. Further analysis revealed bifidobacterial species-specific differences in HMO growth, with B. infantis reaching a cell density threefold higher than B. longum, Bifidobacterium breve, B. bifidum, and B. adolescentis (Ward et al. 2007). Further work by LoCascio and colleagues (LoCascio et al. 2009, LoCascio et al. 2007) demonstrated preferential consumption by B. infantis of the smaller HMO species (degree of polymerization <7). These small HMO species represent the bulk of the HMOs present in pooled samples and are consistently presented over lactation (Ninonuevo et al. 2008). Tellingly, only B. infantis and B. breve could grow on the individual monosaccharide constituents of HMOs (Glc, Gal, GlcNAc, Fuc, NeuAc), suggesting another mechanism for these species to garner energy from these substrates within the intestine (Ward et al. 2007). Although growth of B. bifidum on HMO was less vigorous than B. infantis, direct consumption of HMO was observed (Ward et al. 2007). Interestingly, Fuc, GlcNAc, and NeuAc were not consumed by B. bifidum and remained in the media suggesting that this species is capable of deconstructing HMOs outside the cell to gain access to Glc and Gal constituents as growth substrates (Ward et al. 2007).
The recent genome sequence of B. infantis has enabled a more comprehensive analysis of the HMO growth phenotype by this species. Notably, Sela et al. (2008) described a 43 Kb gene HMO cluster containing the four glycosyl hydrolase activities needed to cleave HMO into its constituent monosaccharides (sialidase, fucosidase, galactosidase, and hexosaminidase) as well as an array of oligosaccharide transport-related genes (Figure 1). Proteomic analysis revealed that genes in this locus are induced upon growth on HMOs, suggesting that B. infantis imports the HMO, whereby the internalized oligosaccharides are catabolized by glycosidases prior to entry of the monosaccharides into the fructose-6-phosphate phosphoketolase central metabolic pathway (Sela et al. 2008). The transport proteins within the main HMO cluster contained six Family 1 extracellular solute binding proteins (SBP) predicted to bind oligosaccharides and to be a part of ABC transporters facilitating import and metabolism. A phylogenetic analysis of these six SBPs indicated a specific evolutionary divergence from other bifidobacterial SBP Family 1 proteins (Sela et al. 2008), suggesting a unique relationship to HMO metabolism. Table 2 lists several bifidobacterial strains and corresponding genes related to HMO metabolism.
Figure 1.
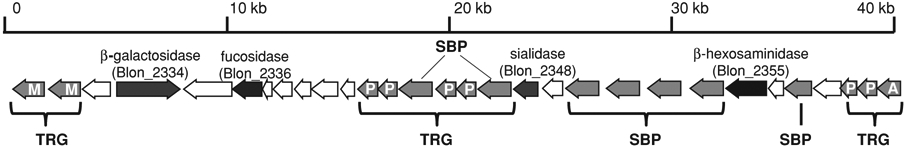
43 Kb gene cluster in B. infantis ATCC15697 containing glycosyl hydrolases and transport-related genes (TRG) required for importing and metabolizing HMOs. SBP: solute binding protein, M: major facilitator superfamily, P: ABC transporter permease component, and A: ABC transporter ATPase subunit. Adapted from Sela et al. (2008).
Table 2.
Presence of α-fucosidase, α-sialidase, and lacto-N-biose phosphorylase genes in sequenced bifidobacterial genomes
| Sequenced bifidobacterial genomes | Fucosidases | Sialidases | Lacto-N-biose phosphorylases |
|---|---|---|---|
| Bifidobacterium adolescentis ATCC 15703; L2–32 | – | – | – |
| Bifidobacterium animalis lactis AD011; HN019 | – | – | – |
| Bifidobacterium animalis subsp. lactis BI-04; DSM 10140 | – | – | – |
| Bifidobacterium bifidum NCIMB 41171 | 1 | 2 | 4 |
| Bifidobacterium breve DSM 20213 | – | 1 | 2 |
| Bifidobacterium catenulatum DSM 16992 | – | – | – |
| Bifidobacterium dentium ATCC 27678 | 1 | – | – |
| Bifidobacterium dentium Bd1 | – | – | – |
| Bifidobacterium gallicum DSM 20093 | – | – | – |
| Bifidobacterium longum DJO10A | – | – | 2 |
| Bifidobacterium longum NCC2705 | 1 | ||
| Bifidobacterium longum subsp. infantis ATCC 15697 | 4 | 2 | 1 |
| Bifidobacterium longum subsp. infantis ATCC 55813a | – | – | 1 |
| Bifidobacterium longum subsp. infantis CCUG 52486a | – | – | 1 |
| Bifidobacterium pseudocatenulatum DSM 20438 | – | – | – |
LoCascio et al. (2010) indicated these strains to be B. longum subsp. longum.
LoCascio et al. (2009) confirmed that the ability to consume HMOs is conserved in the entire B. infantis lineage, whereas other bifidobacteria isolated from infants reveal more strain-specific phenotypic variation. Recently, the same authors used comparative genomic hybridization to demonstrate a unique conservation of the HMO locus across the B. infantis subspecies. This work also revealed a mutant B. infantis strain (JCM1260) for which specific transporter genes within the main HMO cluster are absent (LoCascio et al. 2010). This mutant strain did not grow vigorously on HMOs unlike the other wild-type B. infantis strains (LoCascio et al. 2009), providing the first genetic evidence specifically linking the main HMO locus to the HMO growth phenotype. For a more detailed discussion of the phylogenomic aspects of HMO consumption in bifidobacteria, readers are referred to a recent review by Sela & Mills (2010).
Nishimoto & Kitaoka (2007a) identified the novel degradation pathway in bifidobacteria specific for LNB, an HMO building block. Wada et al. (2008) further described the pathway in bifidobacteria involving both LNB and galacto-N-biose (GNB; Galβ1–3GalNAc), a core structure of the mucin sugar that is present in the human intestine and milk (Lloyd et al. 1996). The latter pathway involves proteins and enzymes that are required for the uptake and degradation of disaccharides such as the GNB/LNB transporter (Suzuki et al. 2008, Wada et al. 2007), galacto-N-biose/lacto-N-biose I phosphorylase (GLNBP; LnpA) (Kitaoka et al. 2005, Nishimoto & Kitaoka 2007b), UDP-glucose-hexose 1-phosphate uridyltransferase (GalT), and UDP-galactose epimerase (GalE). Other researchers confirmed that several bifidobacteria strains, including B. longum subsp. longum, B. infantis, B. breve, and B. bifidum were able to grow on LNB (Groschwitz et al. 2009), whereas none of the strains for B. adolescentis, B. catenulatum, B. dentium, B. angulatum, B. animalis subsp. lactis, and B. thermophilum showed any growth. The presence of the LnpA gene coincided with the LNB utilization in that study. Furthermore, previous studies have shown that some bifidobacterial strains have a unique pathway for the degradation of HMO, specifically with a type 1 chain (β-linked LNB) involving lacto-N-biosidase (LnbB). It has been suggested that the presence of the LnbB and GNB/LNB pathways in some bifidobacterial strains could provide a nutritional advantage for these organisms, thereby increasing their populations within the ecosystem of the breastfed newborns (Wada et al. 2008). Among mammalian milk oligosaccharides, those of Homo sapiens are especially rich in the type 1 LNB structure (Asakuma et al. 2007) and LnbB activity was found in the strains of B. longum and B. bifidum but not in B. animalis and B. pseudolongum (Wada et al. 2008). Indeed, LoCascio et al. (2007) and Ward et al. (2006) suggested that the ability to assimilate type 1 HMO is limited to certain species of bifidobacteria, e.g., B. bifidum and B. infantis.
Using 2′FL as a substrate, Katayama et al. (2008) examined bifidobacterial strains for the occurrence of fucosidase. Those researchers reported that several bifidobacteria strains, including B. bifidum JCM1254 and B. longum JCM1217, produce 1,2-α-L-fucosidase; this enzyme cleaves α-L-fucosyl residue bound to Gal through the α-1–2 linkages found at the nonreducing termini of HMO (Podolsky 1985, Song et al. 2002). Beside 2′-FL, this enzyme readily hydrolyzed lacto-N-fucopentaose I; however, it showed a very limited activity for α-(1–3) -linked L-fucosyl residues of 3-fucosyllactose and lacto-N-fucopentaose V, and had no action on the α-1–4 linkage and α-1–6 linkage.
The various catabolic strategies for HMO observed among different infant-borne bifidobacteria suggest different evolutionary adaptations to the gain a growth advantage from the same complex substrate (Sela & Mills 2010). B. infantis appears to internalize small HMO species (LoCascio et al. 2009, LoCascio et al. 2007), whereas B. bifidum exports enzymes to selectively remove LNB from the HMO structure and processes LNB intracellularly (Katayama et al. 2004). B. breve and B. longum subsp. longum are able to consume free LNnT from the HMO pool (LoCascio et al. 2007, Ward et al. 2007). However, B. breve is also able to grow on the monosaccharide constituents of HMO (Ward et al. 2007). Given that these species are often isolated from the same infant feces, it is tempting to speculate that the various mechanisms may be linked to niche partitioning among bifidobacteria within the developing infant gastrointestinal tract.
ALTERNATIVE SOURCES FOR HMO-LIKE PREBIOTICS IN INFANT FORMULA: ANIMAL MILKS
Breastfed infants are better protected against several types of infections than formula-fed infants (Newburg 1997). Several researchers suggested supplementing infant formula with oligosaccharides similar to those found in human milk (McVeagh & Miller 1997, Motil 2000). Given that human milk is obviously not amenable to large-scale production, there is an urgent demand for alternative, yet functionally comparable, oligosaccharide sources from which to obtain sufficient amounts to perform clinical studies and examine the potential for use in infant nutrition. It was shown previously that the bioactivity of oligosaccharides from bovine and human milk is similar (Gopal & Gill 2000), and therefore BMOs could be used in milk products as bioactive components in human nutrition.
The oligosaccharides in milk of domestic animals, including bovine, differ in structure and have less complex structures with fewer isomers compared with HMOs, whereas the similarities include β-glycosidic linkage of Gal and N-acetylhexosamine to lactose (Urashima et al. 2001). The linkages to Fuc are rare, whereas linkages of Gal or N-acetylglucosamine are dominant. The human intestine lacks enzymes able to hydrolyze all β-glycosidic linkages except the one in lactose. Thus, β-glycosidically bound Gal is the structural element that protects these molecules from digestion during passage through the small intestine (Boehm and Stahl 2007). Sialic acid is a major structural element in the BMOs (Kunz & Rudloff 1993, Saito et al. 1984); however, in contrast to HMO, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are present (Bode 2006). It is important because differences in the chemical structure of sialic acid in human versus cow’s milk are likely to influence bioavailability. It was previously reported that sialylated oligosaccharides also have a role in the initial stage of inflammation and may be effective against the influenza virus and ulcers caused by Helicobacter pylori (Parente et al. 2003). Unique differences between BMOs and HMOs were reported previously in terms of the size, type, and relative amounts (Tao et al. 2008). Bovine colostrums contain sialyl N-acetyllactosamine (Gal(β1–4)GlcNAc) as well as 3′SL, whereas human milk contains only 3′SL (Martin-Sosa et al. 2003). Mature bovine milk also contains galactosyllactoses at concentrations of 40 to 60 mg L−1 (Davis et al. 1983). In a study by Tao et al. (2009), sialylated BMOs made up to 70% of colostrum and 50% of mature milk with the majority of the sialic acid Neu5Ac. Gopal & Gill (2000) reported 10 sialylated and eight neutral oligosaccharides in bovine milk and colostrums. Sialyllacto-N-tetraose c (LSTc), 6′SL, and disialyllacto-N-tetraose were the most representative constituents among sialyl-oligosaccharides (Coppa et al. 1999). Martin-Sosa et al. (2003) have shown that 3′SL was the most representative species in the bovine colostrums, although 6′SL remained at constantly high values during the lactation. The dominant neuramin lactose from human milk has the Neu5Ac acid linked to Gal via an α2-6 bond, whereas the dominant bovine neuramin lactose is linked α2–3.
It is possible that BMOs could have similar functions to HMOs in terms of pathgoen deflection. The free trisaccharide, Galα1–3Galβ1–4Glc, which is found in the bovine colostrum (Urashima et al. 1991), is thought to be an inhibitor of the binding of pathogenic organisms (e.g., Clostridium difficile) to the intestinal mucosa of newborn calves. Bovine colostrum also contains a potential prebiotic isoglobotriose (Galα1–3Galβ1–4Glc), which has not been described in human milk.
Tao et al. (2008) integrated the nanoflow liquid chromatography (nanoLC) with MS in a HPLC-Chip/TOF-MS instrument to profile HMO and BMO. In that study, 40 BMOs were identified, most of which were sialylated; fucosylation was not observed in any of the samples. The same researchers demonstrated that anionic oligosaccharides are minor components in HMOs (<20%) but represent about 70% of the total oligosaccharides in bovine colostrums. Also, sialylated BMOs decreased dramatically during the first 24 hours of lactation, whereas neutral oligosaccharides increased (Nakamura et al. 2003).
Among the other milks from domesticated mammals, goat milk is especially rich in complex lactose-derived oligosaccharides. Interestingly, goat milk oligosaccharides (GMOs) have been shown to contain higher levels of oligosaccharides than bovine milks and are also reported to contain fucosylated species (Nakamura & Urashima 2004). Lara-Villoslada (Lara-Villoslada et al. 2006) recently demonstrated that GMOs reduced the inflammation and body weight loss in rats exposed to dextran sodium sulfate, a common model of colitis and inflammatory bowel disease. Other effects of GMOs included less severe colonic lesions, more favorable intestinal microbiota, and increased intestinal function. Thus, goat milk is another potential source of GMOs for human nutrition applications, including infant formulas (Martinez-Ferez et al. 2006).
Future Opportunities with Animal Milks
The milk contents of each mammalian species are precisely customized to meet the specific needs of the cognate newborns. Although recent data indicate significant differences among milk from domestic animals (Martinez-Ferez et al. 2006), milk and colostrum of domestic animals uniformly contain large amounts of sialyl oligosaccharides as well as many kinds of neutral oligosaccharides (Nakamura & Urashima 2004). Clearly, of the highest priority for infant formula is the search for the structural elements of HMO that are considered crucial to their biological effect and would serve as scientific basis for the selection of oligosaccharides from sources other than human. BMOs are particularly attractive candidates because the large size of the existing bovine dairy industry positions them as a readily available source for significant amounts of oligosaccharides with biological functions close to HMO. Recently, Barile et al. (2009) determined the composition of a variety of neutral and sialylated oligosaccharides in whey permeate using a MALDI-FTICR technique. Seven of the 15 oligosaccharides identified in that study had the same composition as some HMO structures and contained NeuAc. Those results suggest that whey permeate, a common waste stream in cheesemaking, could be a source of oligosaccharides with compositions similar to those present in human milk.
It has been previously demonstrated that sialylated oligosaccharides are important in brain development and increased immunity in infants (Boehm & Stahl 2007, Montserrat & Alicia 2001, Wang & Brand-Miller 2003). Bovine mature milk, which is used currently to produce infant formulas, has a relatively low sialic acid content (Carlson 1985, Neeser et al. 1991, Sánchez-Díaz et al. 1997, Wang et al. 2001). In humans, the sialyloligosaccharides range from 1 g L−1 in colostrums to 90–450 mg L−1 in mature milk (Martin-Sosa et al. 2003, Martín-Sosa et al. 2004, whereas in bovine-based infant formulas the content of sialyloligosaccharides is as low as 15–35 mg L−1 (Martin-Sosa et al. 2003, Wang et al. 2001). The majority of sialic acid in infant formulas is bound to protein (70%), followed by free oligosaccharides, with only 1% in the free form (Wang et al. 2001). Thus, infants fed bovine-based formulas receive significantly less sialic acid compared with breastfed infants. Several researchers attempted to concentrate and isolate the milk sialyloligosaccharides naturally present in whey (described in Barile et al. 2009). However, the exact number and type of monosaccharide residues forming sialyloligosaccharides in whey currently is not well known.
As mentioned above, both fucosylation and sialylation play an important role in the prevention of pathogens binding to the intestinal epithelia and promotion of the growth of beneficial bacteria. However, although HMO are highly fucosylated (Ninonuevo et al. 2006), the BMOs examined to date are not (Tao et al. 2009). The lack of fucosylation in BMOs is interesting given that the recent analysis of the bovine genome clearly indicates that the genetic capacity for creation of fucosylated oligosaccharides is present (Elsik et al. 2009). Certainly, more research on the molecular basis for the observed lack of fucosylation in bovine milk is warranted.
CURRENT COMMERCIAL OLIGOSACCHARIDES USED IN INFANT FORMULA
Among the rather large array of currently available and emerging prebiotics (Crittenden & Playne 2009), relatively few have been examined for use in infant formulas. Stemming from the common observation of bifidobacteria in the feces of breastfed infants, attempts have been made to reproduce this bifidogenic aspect in formulas by adding commercial prebiotics, in particular FOS and GOS, which are known to be broadly bifidogenic (Crittenden & Playne 2009).
Although HMOs are complex glycans composed of five different monosaccharides, FOS and GOS are much simpler structures. FOS are linear fructose polymers, whereas the basic structure of GOS incorporates lactose at the reducing end that is typically elongated with up to six Gal residues, which can contain different branching ([Gal(β1–3/4/6)]1–6Gal(β1–4)Glc). FOS can be commercially produced through the reverse reaction of fructanases and sucrases or via enzymatic hydrolysis of inulin (Espinosa et al. 2007). FOS produced by the first method lacks a reducing end and contains one Glc residue and two or more fructose moieties [short chain (sc) FOS; degree of polymerization (DP) 2–6)] (Fanaro et al. 2005b), whereas hydrolysis of inulin produces free anomeric carbons and contains one fructose [long chain (lc) FOS; DP 7–60)] (Roberfroid 2005). Commercial GOS preparations are mostly produced by enzymatic treatment of lactose with β-galactosidases from different sources, such as fungi, yeast, or bacteria, which results in a mixture of oligomers with various chain lengths (Park & Oh 2010).
Previous work suggested that the upper limit for the DP for GOS is eight (Macfarlane et al. 2008). However, recent work by Barboza et al. (2009) clearly demonstrated that there were oligosaccharides with a DP of up to fifteen. The same researchers reported in vitro growth behavior of different bifidobacterial strains of disaccharide- and monosaccharide-free fractions of GOS (pGOS). MALDI-FTICR MS analysis demonstrated that although all the strains tested were able to grow on the pGOS substrate, there were strain- and DP-specific bifidobacterial preferences for pGOS utilization. In general, the infant borne–isolates (B. infantis and B. breve) were able to consume the GOS species with DP ranging from three to eight more efficiently, while B. adolescentis and B. longum subsp. longum exhibited more differential consumption of select DP. Previously, GOS consumption with specific DP preferences had been determined only for B. adolescentis DSM 20083 (Van Laere et al. 2000). The selective consumption of certain GOS structures by different bifidobacterial species hints at the intriguing possibility of targeting GOS prebiotics to enrich select bifidobacterial species.
Falony et al. (2009) investigated FOS and inulin degradation by a wide range of Bifidobacterium species, focusing in particular on the presence of a preferential FOS breakdown mechanism. That study revealed the existence of a limited number of phenotypically distinct clusters among the tested bifdiobacterial strains, however none of the species was able to degrade inulin or FOS completely. Noteworthy, common infant isolates B. bifidum and B. breve did not degrade inulin and FOS.
Perhaps the most studied prebiotic additive to infant formula is a GOS and FOS mixture, added at a 9:1 ratio (GOS:FOS) (Fanaro et al. 2005a). This particular ratio of prebiotics has been shown to increase bifidobacteria in infant feces (Boehm et al. 2002, Haarman & Knol 2005, Knol et al. 2005) and lower the incidence ofpathogens (Knol et al. 2005). Other studies showed positive outcomes in terms of stool consistency and intestinal transit time with GOS/FOS (Mihatsch et al. 2006). Kapiki and colleagues showed that formula supplemented with FOS resulted in increased bifidobacteria and reduction in E. coli and enterococci (Kapiki et al. 2007). A recent study by Nakamura et al. (2009) demonstrated that fecal samples from infants fed formula supplemented with polydextrose, GOS, and lactulose (8 g L−1) contained significantly less bifidobacteria (20.7%) than fecal samples from infants fed breast milk (83.5%). Interestingly, the same study also confirmed that the prebiotic blend may have a greater impact on infant fecal bacterial populations in younger than in older infants.
Future Opportunities with Commercial Prebiotics
Although the studies employing commercial prebiotic additions to infant formula look promising, at least in terms of enriching bifidobacteria, several questions remain. Growth on HMOs is restricted to select bifidobacteria, primarily B. infantis and B. bifidum, species that possess the requisite genetic capacity (in particular fucosidase and sialidase functions) to deconstruct the HMO polymer. In contrast, FOS and GOS are more broadly utilized across the genus and thus may more nonspecifically enrich from this clade. Assuming HMOs evolved in concert with both the human host and the cognate infant-borne bifidobacteria (as postulated in Sela & Mills 2010), is a nonspecific enrichment of any bifidobacterial species, perhaps as the result of a GOS or FOS treatment, inherently of value to an infant? In other words, is any bifidobacteria resulting from a bifidogenic prebiotic a good outcome? As described above, the functions of HMOs are multifold, and it is unlikely that FOS and GOS possess similar developmental, immunological, or antiadherence functions. A recent study by Shoaf et al. (2006) demonstrated the ability of GOS to reduce enteropathogenic E. coli adherence to tissue culture cells. However, these authors noted the high level of GOS required to witness a significant reduction in E. coli adherence, and they speculated that this antiadherence activity might be enhanced by fucosylation or sialyation of the GOS.
Thus, one clear opportunity is to decorate existing prebiotics to obtain more HMO-like structures and functions. The technology for chemoenzymatic construction of complex carbohydrates has advanced tremendously enabling both decoration of existing structures and wholesale construction of HMO-like structures (Muthana et al. 2009). Following such a path, the generation of specifically designed, individual HMO structures would greatly enhance our ability to link biological function to specific glycan structural motifs. Perhaps more importantly, this would also set the conceptual stage for the creation of tailored synbiotic partners—very specifically designed and constructed HMOs paired with specific cognate bifidobacteria—to achieve a regulatable colonization of either the infant, or adult, GIT.
Table 3.
Specific monosaccharide linkages in HMO, BMO, and commercial oligosaccharides. Adapted from Kuntz et al. (2009)
| Glycans | Monosaccharide linkages |
|---|---|
| HMO | |
| Lacto-N-tetraose/hexaose/octaose/decaose | β1–3, β1–4, β1–6 |
| Fucosyllactose | α1–2, α1–3, α1–4, β1–3, β1–4 |
| Sialyllactose | α2–3, α2–6, β1–4 |
| Sialyl-lacto-N-tetraose | α2–3, α2–6, β1–3, β1–4 |
| BMO | |
| Acetyllactosamine | β1–4 |
| Galactosyllactose | β1–3, β1–4, β1–6 |
| Acetylneuraminyllactose (Neu5Ac) | α2–3, α2–6, α2–8, β1–4 |
| Glycolylneuraminyllactose (Neu5Gc) | α2–3, α2–6, β1–4 |
| Galactooligosaccharides (GOS) | α1–2, α1–4, α1–6 |
| Fructooligosaccharides (FOS) | β1–2 |
ACKNOWLEDGMENTS
Authors thank David Sela and Daniel Garrido for their assistance in preparing this manuscript. This publication was made possible in part by grant support from the University of California Discovery Grant Program, the California Dairy Research Foundation, Dairy Management Inc., the Gates Foundation, USDA NRI-CSREES Award 2008-35200-18776, NIEHS Superfund P42 ES02710, the Charge study P01 ES11269, and by NIH-NICID awards 5R01HD059127 and 1R01HD061923.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adlerberth I, Wold AE. 2009. Establishment of the gut microbiota in Western infants. Acta Paediatr. 98:229–38 [DOI] [PubMed] [Google Scholar]
- Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. 2010. CE-LIF-MS n profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis. 31(7):1264–73 [DOI] [PubMed] [Google Scholar]
- Andersson B, Porras O, Hanson LA, Lagergård T, Svanborg-Edén C. 1986. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J. Infect. Dis 153(2):232–37 [DOI] [PubMed] [Google Scholar]
- Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, et al. 2005. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 15:31–41 [DOI] [PubMed] [Google Scholar]
- Asakuma S, Urashima T, Akahori M, Obayashi H, Nakamura T, et al. 2007. Variation of major neutral oligosaccharides levels in human colostrum. Eur. J. Clin. Nutr 62:488–94 [DOI] [PubMed] [Google Scholar]
- Bao Y, Newburg DS. 2008. Capillary electrophoresis of acidic oligosaccharides from human milk. Electrophoresis 29:2508–15 [DOI] [PubMed] [Google Scholar]
- Barboza M, Sela DA, Pirim C, LoCascio RG, Freeman SL, et al. 2009. Glycoprofiling bifidobacterial consumption of galacto-oligosaccharides by mass spectrometry reveals strain specific, preferential consumption of glycans. Appl. Environ. Microbiol 75(23):7319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile D, Tao N, Lebrilla CB, Coisson JD, Arlorio M, German JB. 2009. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int. Dairy J 19:524–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L 2006. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J. Nutr 136:2127–30 [DOI] [PubMed] [Google Scholar]
- Bode L 2009. Human milk oligosaccharides: prebiotics and beyond. Nutr. Rev 67:S183–S91 [DOI] [PubMed] [Google Scholar]
- Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. 2004a. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost 92(6):1402–10 [DOI] [PubMed] [Google Scholar]
- Bode L, Rudloff S, Kunz C, Strobel S, Klein N. 2004b. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil {beta} 2 integrin expression. J. Leukoc Biol 76:820–26 [DOI] [PubMed] [Google Scholar]
- Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, et al. 2002. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed 86:F178–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm G, Moro G. 2008. Structural and functional aspects of prebiotics used in infant nutrition. J. Nutr 138:1818S–28 [DOI] [PubMed] [Google Scholar]
- Boehm G, Stahl B. 2007. Oligosaccharides from milk. J. Nutr 137:847S–49 [DOI] [PubMed] [Google Scholar]
- Bouchara J, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky J, et al. 1997. Sialic acid–dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect. Immun 65:2717–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S 1985. N-acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am. J. Clin. Nutr 41:720–26 [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, et al. 2001. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 11:365–72 [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Ruiz-Palacios GM, Pickering LK, Newburg DS. 1997. Milk oligosaccharide profiles by reversed-phase HPLC of their perbenzoylated derivatives. Anal. Biochem 251(1):89–97 [DOI] [PubMed] [Google Scholar]
- Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. 2004. The first prebiotics in humans: human milk oligosaccharides. J. Clin. Gastroenterol 38(6 Suppl):S80–83 [DOI] [PubMed] [Google Scholar]
- Coppa GV, Gabrielli O, Giorgi P, Catassi C, Montanari MP, et al. 1990. Preliminary study of breastfeeding and bacterial adhesion to uroepithelial cells. Lancet 335:569–71 [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. 2001. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Adv. Exp. Med. Biol 501:307–14 [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. 1999. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. Suppl 88(430):89–94 [DOI] [PubMed] [Google Scholar]
- Coppa GV, Zampini L, Galeazzi T, Gabrielli O. 2006. Prebiotics in human milk: a review. Dig. Liver Dis 38:S291–S94 [DOI] [PubMed] [Google Scholar]
- Cravioto A, Tello A, Villafán H, Ruiz J, del Vedovo S, Neeser JR. 1991. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J. Infect. Dis 163(6):1247–55 [DOI] [PubMed] [Google Scholar]
- Crittenden R, Playne MJ. 2009. Prebiotics In Handbook of Probiotics and Prebiotics, ed. Lee YK, Salminen S, pp. 535–81. Hoboken, NJ:John Wiley Sons [Google Scholar]
- Davis DT, Holt C, Christie WW. 1983. The Composition of Milk In Biochemistry of Lactation, ed. Mepham TB, pp. 71–117. Amsterdam-New York: Elsevier [Google Scholar]
- Devaraj N, Sheykhnazari M, Warren WS, Bhavanandan VP. 1994. Differential binding of Pseudomonas aeruginosa to normal and cystic fibrosis tracheobronchial mucins. Glycobiology 4:307–16 [DOI] [PubMed] [Google Scholar]
- Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, et al. 2010. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol 21(8):1179–88 [DOI] [PubMed] [Google Scholar]
- Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, et al. 2004. Human milk–derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res 56(4):536–40 [DOI] [PubMed] [Google Scholar]
- Elsik CG, Tellam RL, Worley KC, Gibbs RA, Muzny DM, et al. 2009. The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science 324:522–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. 2000. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr 71:1589–96 [DOI] [PubMed] [Google Scholar]
- Espinosa RM, Taméz M, Prieto P. 2007. Efforts to emulate human milk oligosaccharides. Br. J. Nutr 98(Suppl 1):S74–79 [DOI] [PubMed] [Google Scholar]
- Falony G, Lazidou K, Verschaeren A, Weckx S, Maes D, De Vuyst L. 2009. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by bifidobacterium species reveals four different phenotypes. Appl. Environ. Microbiol 75:454–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, et al. 2005a. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr. Suppl 94(449):22–26 [DOI] [PubMed] [Google Scholar]
- Fanaro S, Jelinek J, Stahl B, Boehm G, Kock R, Vigi V. 2005b. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: effect on intestinal flora, stool characteristics, and pH. J. Pediatr. Gastroenterol. Nutr 41:186–90 [DOI] [PubMed] [Google Scholar]
- Favier CF, de Vos WM, Akkermans ADL. 2003. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe 9:219–29 [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Tuzikov AB, Piskarev VE, Yamnikova SS, Lvov DK, et al. 1997. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine). Virology 232:345–50 [DOI] [PubMed] [Google Scholar]
- Gauhe A, György P, Hoover JRE, Kuhn R, Rose CS, et al. 1954. Bifidus factor. IV. Preparations obtained from human milk. Arch. Biochem. Biophys 48:214–24 [DOI] [PubMed] [Google Scholar]
- German JB, Freeman SL, Lebrilla CB, Mills DA. 2008. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr. Workshop Ser. Pediatr. Program 62:205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. 2000. Human milk oligosaccharides are minimally digested in vitro. J. Nutr 130:3014–20 [DOI] [PubMed] [Google Scholar]
- Gnoth MJ, Rudloff S, Kunz C, Kinne RKH. 2001. Investigations of the in vitro transport of human milk oligosaccharides by a caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J. Biol. Chem 276:34363–70 [DOI] [PubMed] [Google Scholar]
- Gopal PK, Gill HS. 2000. Oligosaccharides and glycoconjugates in bovine milk and colostrum. Br. J. Nutr 84(Suppl. 1):S69–74 [DOI] [PubMed] [Google Scholar]
- Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, et al. 2009. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci 106:22381–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F 2009. Prebiotics, probiotics and helminths: the “natural” solution? Dig. Dis 27:412–17 [DOI] [PubMed] [Google Scholar]
- Gyorgy P 1953Ahitherto unrecognized biochemical difference between human milk and cow’s milk. Pediatrics 11(2):98–108 [PubMed] [Google Scholar]
- Gyorgy P, Norris RF, Rose CS. 1954. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch. Biochem. Biophys 48:193–201 [DOI] [PubMed] [Google Scholar]
- Haarman M, Knol J. 2005. Quantitative real-time PCR assays to identify and quantify fecal bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol 71:2318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen HJM, Wildeboer-Veloo ACM, Raangs GC, Wagendorp AA, Klijn N, et al. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr 30:61–67 [DOI] [PubMed] [Google Scholar]
- Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. 2009. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN). Br. J. Nutr 101(4):482–86 [DOI] [PubMed] [Google Scholar]
- Hoover JR, Braun GA, Gyorgy P. 1953. Neuraminic acid in mucopolysaccharides of human milk. Arch. Biochem. Biophys 47(1):216–17 [DOI] [PubMed] [Google Scholar]
- Idänpään-Heikkilä I, Simon PM, Zopf D, Vullo T, Cahill P, et al. 1997. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J. Infect. Dis 176(3):704–12 [DOI] [PubMed] [Google Scholar]
- Idota T, Kawakami H, Murakami Y, Sugawara M. 1995. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci. Biotechnol. Biochem 59(3):417–19 [DOI] [PubMed] [Google Scholar]
- Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. 2007. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum. Dev 83:335–39 [DOI] [PubMed] [Google Scholar]
- Katayama T, Sakuma A, Kimura T, Makimura Y, Hiratake J, et al. 2004. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-{alpha}-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol 186:4885–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Wada J, Fujita K, Kiyohara M, Ashida H, Yamamoto K. 2008. Functions of novel glycosidases isolated from bifidobacteria. J. Appl. Glycosci 55:101–9 [Google Scholar]
- Kelder B, Erney R, Kopchick J, Cummings R, Prieto P. 2001. Glycoconjugates in human and transgenic animal milk. Adv. Exp. Med. Biol 501:269–78 [DOI] [PubMed] [Google Scholar]
- Kitaoka M, Tian J, Nishimoto M. 2005. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol 71:3158–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, et al. 2005. Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr. Suppl. 94(449):31–33 [DOI] [PubMed] [Google Scholar]
- Kobata A 1992. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 209:483–501 [DOI] [PubMed] [Google Scholar]
- Kobata A 2003. Possible application of milk oligosaccharides for drug development. Chang Gung Med J. 26(9):621–36 [PubMed] [Google Scholar]
- Kobata A, Ginsburg V. 1969. Oligosaccharides of human milk. J. Biol. Chem 244:5496–502 [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, et al. 2010. Microbes and Health Sackler Colloquium: Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz S, Kunz C, Rudloff S. 2009. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br. J. Nutr 101:1306–15 [DOI] [PubMed] [Google Scholar]
- Kuntz S, Rudloff S, Kunz C. 2008. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br. J. Nutr 99:462–71 [DOI] [PubMed] [Google Scholar]
- Kunz C, Rodriguez-Palmero M, Koletzko B, Jensen R. 1999. Nutritional and biochemical properties of human milk. Part I: general aspects, proteins, and carbohydrates. Clin. Perinatol 26(2):307–33 [PubMed] [Google Scholar]
- Kunz C, Rudloff S. 1993. Biological functions of oligosaccharides in human milk. Acta Paediatr. 82(11):903–12 [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S. 2008. Potential anti-inflammatory and anti-infectious effects of human milk oligosaccharides. Adv. Exp. Med. Biol 606:455–66 [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr 20:699–722 [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Hintelmann A, Pohlentz G, Egge H. 1996. High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides. J. Chromatogr. B Biomed. Appl 685(2):211–21 [DOI] [PubMed] [Google Scholar]
- Lane JA, Mehra RK, Carrington SD, Hickey RM. 2010. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int. J. Food Microbiol 142:1–13 [DOI] [PubMed] [Google Scholar]
- Lara-Villoslada F, Debras E, Nieto A, Concha A, Gálvez J, et al. 2006. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr 25:477–88 [DOI] [PubMed] [Google Scholar]
- Lasky LA. 1995. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem 64:113–40 [DOI] [PubMed] [Google Scholar]
- Leach JL, Garber SA, Marcon AA, Prieto PA. 2005. In vitro and in vivo effects of soluble, monovalent globotriose on bacterial attachment and colonization. Antimicrob. Agents Chemother 49:3842–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo F, Asakuma S, Fukuda K, Senda A, Urashima T.2010. Determination of sialyl and neutral oligosaccharide levels in transition and mature milks of Samoan women, using anthranilic derivatization followed by reverse phase high performance liquid chromatography. Biosci. Biotechnol. Biochem 74(2):298–303 [DOI] [PubMed] [Google Scholar]
- Lloyd KO, Burchell J, Kudryashov V, Yin BWT, Taylor-Papadimitriou J. 1996. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. J. Biol. Chem 271:33325–34 [DOI] [PubMed] [Google Scholar]
- LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. 2010. Comparative genomic hybridization of Bifidobacterium longum strains reveals broad conservation of milk utilization genes in subsp. infantis. Appl. Environ. Microbiol 76:7373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio RG, Ninonuevo M, Kronewitter S, Freeman SL, German JB, et al. 2009. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol 2333–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, et al. 2007. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem 55:8914–19 [DOI] [PubMed] [Google Scholar]
- Macfarlane GT, Steed H, Macfarlane S. 2008. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol 104:305–44 [DOI] [PubMed] [Google Scholar]
- Manning TS, Gibson GR. 2004. Prebiotics. Best Pract. Res. Clin. Gastroenterol 18:287–98 [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, et al. 2010. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem 58:5334–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, et al. 2009. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Sosa S, Martín M-J, García-Pardo L-A, Hueso P. 2003. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J. Dairy Sci 86:52–59 [DOI] [PubMed] [Google Scholar]
- Martín-Sosa S, Martín M-J, García-Pardo LA, Hueso P. 2004. Distribution of sialic acids in the milk of Spanish mothers of full term infants during lactation. J. Pediatr. Gastroenterol. Nutr 39:499–503 [DOI] [PubMed] [Google Scholar]
- Martinez-Ferez A, Rudloff S, Guadix A, Henkel CA, Pohlentz G, et al. 2006. Goats’ milk as a natural source oflactose-derived oligosaccharides: isolation by membrane technology. Int. Dairy J 16:173–81 [Google Scholar]
- Matrosovich MN, Gambaryan AS, Tuzikov AB, Byramova NE, Mochalova LV, et al. 1993. Probing of the receptor-binding sites of the H1 and H3 influenza A and influenza B virus hemagglutinins by synthetic and natural sialosides. Virology 196:111–21 [DOI] [PubMed] [Google Scholar]
- McEver RP. 1994. Role of selectins in leukocyte adhesion to platelets and endothelium. Ann. N. Y. Acad. Sci 714:185–89 [DOI] [PubMed] [Google Scholar]
- McVeagh P, Miller JB. 1997. Human milk oligosaccharides: only the breast. J. Paediatr. Child Health 33(4):281–86 [DOI] [PubMed] [Google Scholar]
- Mihatsch WA, Hoegel J, Pohlandt F. 2006. Prebiotic oligosaccharides reduce stool viscosity and accelerate gastrointestinal transport in preterm infants. Acta Paediatrica 95:843–48 [DOI] [PubMed] [Google Scholar]
- Montserrat R-U, Alicia S-O. 2001. Oligosaccharides: application in infant food. Early Hum. Dev 65:S43–52 [DOI] [PubMed] [Google Scholar]
- Moro E 1900. Morphologische und bakterogische untersuchungen uber die Dambakterien des Sauglings: die bacterium flora des normalen frauenmilch stuhls. Jahb Kinderheilkd 61:686–734 [Google Scholar]
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Lourdes Guerrero M, et al. 2004. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr 145:297–303 [DOI] [PubMed] [Google Scholar]
- Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. 2005. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr 135:1304–7 [DOI] [PubMed] [Google Scholar]
- Motil KJ. 2000. Infant feeding: a critical look at infant formulas. Curr. Opin. Pediatr 12:469–76 [DOI] [PubMed] [Google Scholar]
- Muthana S, Cao H, Chen X. 2009. Recent progress in chemical and chemoenzymatic synthesis of carbohydrates. Curr. Opin. Chem. Biol 13:573–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore JV, Wigginton T, Simon PM, Zopf D, Heman-Ackah LM, Dubois A. 1999. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology 117(6):1316–25 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Gaskins HR, Collier CT, Nava GM, Rai D, et al. 2009. Molecular ecological analysis of fecal bacterial populations from term infants fed formula supplemented with selected blends of prebiotics. Appl. Environ. Microbiol 75:1121–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kawase H, Kimura K, Watanabe Y, Ohtani M, et al. 2003. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J. Dairy Sci 86:1315–20 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Urashima T. 2004. The milk oligosaccharides of domestic farm animals. Trends Glycosci. Glycotechnol 16:135–42 [Google Scholar]
- Neeser J-R, Golliard M, Del Vedovo S. 1991. Quantitative determination of complex carbohydrates in bovine milk and in milk-based infant formulas. J. Dairy Sci 74:2860–71 [DOI] [PubMed] [Google Scholar]
- Newburg DS. 1997. Do the binding properties of oligosaccharides in milk protect human infants from gastrointestinal bacteria? J. Nutr 127:980S–84 [DOI] [PubMed] [Google Scholar]
- Newburg DS. 1999. Human milk glycoconjugates that inhibit pathogens. Curr. Med. Chem 6(2):117–27 [PubMed] [Google Scholar]
- Newburg DS. 2000. Are all human milks created equal? Variation in human milk oligosaccharides. J. Pediatr. Gastroenterol. Nutr 30(2):131–33 [DOI] [PubMed] [Google Scholar]
- Newburg DS. 2005. Innate immunity and human milk. J. Nutr 135:1308–12 [DOI] [PubMed] [Google Scholar]
- Newburg DS. 2009. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci 87:26–34 [DOI] [PubMed] [Google Scholar]
- Newburg DS, Pickering LK, McCluer RH, Cleary TG. 1990. Fucosylated oligosaccharides of human milk protect suckling mice from heat-stabile enterotoxin of Escherichia coli. J. Infect. Dis 162(5):1075–80 [DOI] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, et al. 2004a. Innate protection conferred by fucosylated oligosaccharides ofhuman milk against diarrhea in breastfed infants. Glycobiology 14:253–63 [DOI] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Guerrero ML, et al. 2004b. Human milk alphal,2-linked fucosylated oligosaccharides decrease risk of diarrhea due to stable toxin of E. coli in breastfed infants. Adv. Exp. Med. Biol 554:457–61 [DOI] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Morrow AL. 2005. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr 25:37–58 [DOI] [PubMed] [Google Scholar]
- Niñonuevo MR, Lebrilla CB. 2009. Mass spectrometric methods for analysis of oligosaccharides in human milk. Nutr. Rev 67:S216–S26 [DOI] [PubMed] [Google Scholar]
- Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, et al. 2006. A strategy for annotating the human milk glycome. J. Agric. Food Chem 54:7471–80 [DOI] [PubMed] [Google Scholar]
- Ninonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, et al. 2008. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem 56:618–26 [DOI] [PubMed] [Google Scholar]
- Nishimoto M, Kitaoka M. 2007a. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol 73:6444–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto M, Kitaoka M. 2007b. Identification of the putative proton donor residue of lacto-N-biose phosphorylase (EC 2.4.1.211). Biosci. Biotechnol. Biochem 71:1587–91 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Ben RA, Pons S, de Paulo MI, Bustos Fernández L. 1992. Volatile fatty acids, lactic acid, and pH in the stools of breast-fed and bottle-fed infants. J. Pediatr. Gastroenterol. Nutr 15:248–52 [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente F, Cucino C, Anderloni A, Grandinetti G, Porro GB. 2003. Treatment of Helicobacter pylori infection using a novel antiadhesion compound (3′ sialyllactose sodium salt). A double blind, placebo-controlled clinical study. Helicobacter 8:252–56 [DOI] [PubMed] [Google Scholar]
- Park A-R, Oh D-K. 2010. Galacto-oligosaccharide production using microbial β-galactosidase: current state and perspectives. Appl. Microbiol. Biotechnol 85:1279–86 [DOI] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, et al. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–21 [DOI] [PubMed] [Google Scholar]
- Petschow BW, Talbott RD. 1991. Response of bifidobacterium species to growth promoters in human and cow milk. Pediatr. Res 29(2):208–13 [DOI] [PubMed] [Google Scholar]
- Podolsky DK. 1985. Oligosaccharide structures of human colonic mucin. J. Biol. Chem 260:8262–71 [PubMed] [Google Scholar]
- Rinne MM, Gueimonde M, Kalliomäki M, Hoppu U, Salminen SJ, Isolauri E. 2005. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol. Med. Microbiol 43:59–65 [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. 2005. Introducing inulin-type fructans. Br. J. Nutr 93(Suppl. 1):S13–25 [DOI] [PubMed] [Google Scholar]
- Rubaltelli FF, Biadaioli R, Pecile P, Nicoletti P. 1998. Intestinal flora in breast- and bottle-fed infants. J. Perinat. Med 26(3):186–91 [DOI] [PubMed] [Google Scholar]
- Rudloff S, Obermeier S, Borsch C, Pohlentz G, Hartmann R, et al. 2006. Incorporation of orally applied 13C-galactose into milk lactose and oligosaccharides. Glycobiology 16:477–87 [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem 278:14112–20 [DOI] [PubMed] [Google Scholar]
- Ruvoën-Clouet N, Mas E, Marionneau S, Guillon P, Lombardo D, Le Pendu J. 2006. Bile-salt-stimulated lipase and mucins from milk of “secretor” mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem. J 393:627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Itoh T, Adachi S. 1984. Presence of two neutral disaccharides containing N-acetylhexosamine in bovine colostrum as free forms. Bioch. Biophys. Acta (BBA): Gen. Subj 801:147–50 [DOI] [PubMed] [Google Scholar]
- Sánchez-Díaz A, Ruano M-J, Lorente F, Hueso P. 1997. A critical analysis of total sialic acid and sialoglycoconjugate contents of bovine milk-based infant formulas. J. Pediatr. Gastroenterol. Nutr 24:405–10 [DOI] [PubMed] [Google Scholar]
- Sarney DB, Hale C, Frankel G, Vulfson EN. 2000. A novel approach to the recovery of biologically active oligosaccharides from milk using a combination of enzymatic treatment and nanofiltration. Biotechnol. Bioeng 69:461–67 [DOI] [PubMed] [Google Scholar]
- Schumacher G, Bendas G, Stahl B, Beermann C. 2006. Human milk oligosaccharides affect P-selectin binding capacities: in vitro investigation. Nutrition 22:620–27 [DOI] [PubMed] [Google Scholar]
- Schwertmann A, Rudloff S, Kunz C. 1996. Potential ligands for cell adhesion molecules in human milk. Ann. Nutr. Metab 40(5):252–62 [DOI] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, et al. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 105:18964–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, Mills DA. 2010. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 18:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Warren CD, Newburg DS. 2000. High-performance capillary electrophoresis of sialylated oligosaccharides of human milk. Anal. Biochem 279:37–45 [DOI] [PubMed] [Google Scholar]
- Sherman PM, Cabana M, Gibson GR, Koletzko BV, Neu J, et al. 2009. Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27–28, 2008. J. Pediatr 155:S61–70 [DOI] [PubMed] [Google Scholar]
- Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun 74:6920–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-F, Weng M-Q, Wu S-M, Xia Q-C. 2002Analysis of neutral saccharides in human milk derivatized with 2-aminoacridone by capillary electrophoresis with laser-induced fluorescence detection. Anal. Biochem 304:126–29 [DOI] [PubMed] [Google Scholar]
- Stehle T, Yan Y, Benjamin TL, Harrison SC. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369(6476):160–63 [DOI] [PubMed] [Google Scholar]
- Suzuki R, Wada J, Katayama T, Fushinobu S, Wakagi T, et al. 2008. Structural and thermodynamic analyses of solute-binding protein from Bifidobacterium longum specific for core 1 disaccharide and lacto-N-biose I. J. Biol. Chem 283:13165–73 [DOI] [PubMed] [Google Scholar]
- Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. 2008. Bovine milk glycome. J. Dairy Sci 91:3768–78 [DOI] [PubMed] [Google Scholar]
- Tao N, DePeters EJ, German JB, Grimm R, Lebrilla CB. 2009. Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J. Dairy Sci 92:2991–3001 [DOI] [PubMed] [Google Scholar]
- Taylor SN, Basile LA, Ebeling M, Wagner CL. 2009. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed. Med 4:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA Jr. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization in helminth infections. J. Immunol 167:5294–303 [DOI] [PubMed] [Google Scholar]
- Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. 1997. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J 14:795–99 [DOI] [PubMed] [Google Scholar]
- Thurl S, Müller-Werner B, Sawatzki G. 1996. Quantification of individual oligosaccharide compounds from human milk using high-pH anion-exchange chromatography. Anal. Biochem 235:202–6 [DOI] [PubMed] [Google Scholar]
- Tissier H 1900. Recherches sur la Flore Intestinale des Nourrissons. Paris: Univ. Paris [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–31 [DOI] [PubMed] [Google Scholar]
- Urashima T, Saito T, Nakamura T, Messer M. 2001. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J 18:357–71 [DOI] [PubMed] [Google Scholar]
- Urashima T, Saito T, Ohmisya K, Shimazaki K. 1991. Structural determination of three neutral oligosaccharides in bovine (Holstein-Friesian) colostrum, including the novel trisaccharide; GalNAc alpha 1–3Gal beta 1–4Glc. Biochim. Biophys. Acta 1073(1):225–29 [DOI] [PubMed] [Google Scholar]
- Van Laere KMJ, Abee T, Schols HA, Beldman G, Voragen AGJ. 2000. Characterization of a novel beta-galactosidase from Bifidobacterium adolescentis DSM 20083 active towards transgalactooligosaccharides. Appl. Environ. Microbiol 66:1379–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velupillai P, Harn DA. 1994. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc. Natl. Acad. Sci. USA 91(1):18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkola R, Parkkinen J, Hacker J, Korhonen TK. 1993. Sialyloligosaccharide chains of laminin as an extracellular matrix target for S fimbriae of Escherichia coli. Infect. Immun. 61:4480–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. 2007. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev. Immunol 27(2):97–140 [DOI] [PubMed] [Google Scholar]
- Wada J, Ando T, Kiyohara M, Ashida H, Kitaoka M, et al. 2008. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl. Environ. Microbiol 74:3996–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J, Suzuki R, Fushinobu S, Kitaoka M, Wakagi T, et al. 2007. Purification, crystallization and preliminary X-ray analysis of the galacto-N-biose-/lacto-N-biose I-binding protein (GL-BP) of the ABC transporter from Bifidobacterium longum JCM1217. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun 63(Pt 9):751–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Brand-Miller J. 2003. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr 57:1351–69 [DOI] [PubMed] [Google Scholar]
- Wang B, Brand-Miller J, McVeagh P, Petocz P. 2001. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr 74:510–15 [DOI] [PubMed] [Google Scholar]
- Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. 2006. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol 72:4497–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Niñonuevo M, Mills DA, Lebrilla CB, German JB. 2007. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res 51:1398–405 [DOI] [PubMed] [Google Scholar]
- WHO. 2009. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. pp. 1–111 [PubMed] [Google Scholar]
- Wiederschain GY, Newburg DS. 1996. Compartmentalization of fucosyltransferase and alpha-L-fucosidase in human milk. Biochem. Mol. Med 58:211–20 [DOI] [PubMed] [Google Scholar]
- Wu S, Tao N, German JB, Grimm R, Lebrilla CB. 2010. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 9:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Chen PH. 2009. Health consequences of nutrition in childhood and early infancy. Pediatr. Neonatol 50:135–42 [DOI] [PubMed] [Google Scholar]
- Xiao J-Z, Takahashi S, Nishimoto M, Odamaki T, Yaeshima T, et al. 2010. Distribution of in vitro fermentation ability of lacto-N-biose I, a major building block of human milk oligosaccharides, in bifidobacterial strains. Appl. Environ. Microbiol 76:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D, Roth S. 1996. Oligosaccharide anti-infective agents. Lancet 347(9007):1017–21 [DOI] [PubMed] [Google Scholar]


