Abstract
BACKGROUND:
Bipolar disorder shows significant variability in clinical presentation. Here we adopt a personalized approach to quantify the brain structural and functional similarity of each individual patient to other patients and to healthy individuals.
METHODS:
Brain morphometric and resting-state functional connectivity measures from two independent samples of patients with bipolar disorder and healthy individuals (total number of participants=215) were modeled as single vectors to generated individualized morphometric and connectivity profiles. These profiles were then used to compute a person-based similarity indices which quantified the similarity in neuroimaging profiles amongst patients and between patients and health individuals.
RESULTS:
The morphometric and connectivity profiles of patients showed within-diagnosis similarity which was comparable to that observed in healthy individuals. They also showed minimal deviance from those of healthy individuals; the correlation between the profiles of patients and healthy individuals was high (range: 0.71–0.94, p<10−5). The degree of similarity between imaging profiles was associated with IQ (for cortical thickness) and age (functional integration) rather than clinical variables. Patients who were prescribed lithium, compared to those who were not, showed greater similarity to healthy individuals in terms of network integration (t = 2.2, p = 0.03).
LIMITATIONS:
We focused on patients with Bipolar disorder, type I only.
CONCLUSIONS:
High inter-individual similarity in neuroimaging profiles was observed amongst patients with bipolar disorder and between patients and healthy individuals. We infer that brain alterations associated with bipolar disorder may be nested within the normal biological diversity consistent with the high prevalence of mood symptoms in the general population.
Keywords: Inter-individual correlation, Bipolar disorder, Magnetic resonance imaging, normative modeling, Resting-state
1. Introduction
Bipolar disorder is a severe mental illness, characterized by mood episodes and variable inter-episode remission (American Psychiatric Association, 2013), and a major cause of disease burden worldwide (Benjamini and Yekutieli, 2001). Biological models of bipolar disorder emphasize differences between patients and healthy individuals in brain morphology and functional organization. The most consistent findings involve volumetric reductions in the amygdala, hippocampus, and thalamus (Arnone et al., 2009; Hallahan et al., 2011; Hibar et al., 2016), cortical thinning in prefrontal and occipital regions (Hibar et al., 2018), and dysfunctional engagement of subcortical and prefrontal regions during tasks of affective processing and cognitive control (Chen et al., 2011; Janiri et al., 2019). Further, bipolar disorder has been associated with resting-state dysconnectivity primarily of the default mode, sensorimotor, and central executive networks (Birur et al., 2017; Doucet et al., 2017; Meda et al., 2014; Vargas et al., 2013). However, the neuroimaging literature in bipolar disorder shows marked inter-study variability and attempts to differentiate patients from healthy individuals on the basis of neuroimaging measures have generally shown low accuracy and reproducibility (Frangou et al., 2017; Nunes et al., 2018; Rocha-Rego et al., 2014).
Heterogeneity is commonly invoked to explain inter-study inconsistencies based on findings of variability in the genetic architecture (Charney et al., 2017; Song et al., 2017), cognitive profiles (Bora, 2017; Jensen et al., 2016; Karantonis et al., 2020; Lima et al., 2019; Martino et al., 2014), and the clinical presentation of bipolar disorder (Karanti et al., 2019; Wallace et al., 2016). However, “apparent heterogeneity” (i.e., heterogeneity attributable to methodological factors rather being a true attribute of a population) in any feature could arise as a result of a shift in the overall distribution of one group relative to the other, or from differential sampling from distributions with similar variance. Wolfers and colleagues (2018) partially addressed this issue with respect to brain structure in patients with bipolar disorder. They determined the extent to which each discrete brain regional measure in each patient deviated from the normative mean. Most of the regions where individual-level deviation was noted were regions where case-control differences were also observed using traditional group-level analyses, indicating significant inter-patient similarity.
A key limitation of most previous research is that it models the brain as a collection of independent regions or functional networks. As both morphometry and functional connectivity show marked covariance (Alexander-Bloch et al., 2013; Zhang et al., 2011), a crucial, but as yet unanswered question, pertains to the degree to which the neuroimaging profiles of individual patients differ from other patients or from healthy individuals.
Here we use a novel metric, the person-based similarity index (PBSI) (Doucet et al., 2019b), which quantifies the in-group similarity between the brain structural and connectivity profile of an individual to that of the other members of a group. We have previously demonstrated that the PBSI is biologically and functionally meaningful as it is reproducible, heritable and associated with cognitive ability (Doucet et al., 2019b). Using this index, we examined the person-specific in-group similarity in regional cortical thickness and subcortical volumes and resting state connectivity in bipolar disorder (n = 44) and healthy individuals (n = 52). Data acquired data on an independent sample (patients=78; healthy individuals=41) were used to test reproducibility. We hypothesized that significant disorder-related heterogeneity in brain morphometry and connectivity, if present, would be associated with lower within-group inter-individual similarity. Conversely, high levels of within-group similarity in amongst patients would argue against significant heterogeneity at least in terms of neuroanatomical and connectivity profiles. Accordingly, high levels of similarity between patients and healthy individuals would indicate that disease-related changes in brain morphometry and connectivity are likely to be subtle and nested within the normal variation. To complement these analyses and to allow comparison with prior studies we also tested whether bipolar disorder is associated with increased variance in the morphometry of discrete regions or the connectivity of discrete functional networks.
2. Materials and methods
2.1. Samples
We analyzed data from two samples, the discovery sample assessed at the Icahn School of Medicine at Mount Sinai (ISMMS), USA and the replication sample assessed at the Olin Neuropsychiatric Research Center, Yale University, USA. The ISMMS sample comprised 44 patients with bipolar disorder, Type I, and 52 healthy individuals (Table 1, Supplementary Table S1) and the Yale sample comprised 78 patients with bipolar disorder, Type I, and 41 healthy individuals (Table 1, Supplementary Table S2). Details of the recruitment and assessment are provided in the Supplementary Material. Participants in both the ISMMS and the Yale sample were screened to exclude IQ less than 70; presence of a systemic medical illnesses or central nervous system disorders; history of significant head trauma; substance use disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; (American Psychiatric Association, 2013)) and contra-indications for magnetic resonance imaging (MRI). In both samples, the diagnostic status of all participants was assessed using the Structured Clinical Interview for DSM-5 (First et al., 2015), IQ was evaluated using Wechsler Abbreviated Scale of Intelligence (Wechsler, 2011) and psychopathology was rated with the 24-item Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993). In BPRS, items are coded from 1 (absent) to 7 (extremely severe). BPRS subscale scores were also calculated as follows: positive symptoms subscale=sum of scores for hallucination, unusual thought content, and bizarre behavior; negative symptoms subscale=sum of scores for blunted affect, emotional withdrawal, and motor retardation; depression/anxiety subscale=sum of scores for anxiety, depression, suicidality, and guilt; mania/disorganization subscale=sum of scores for motor hyperactivity, elevated mood, excitement, distractibility, and grandiosity. In patients, clinical information was confirmed through their medical records, medication type and dose were recorded on the day of scanning and the daily antipsychotic dose was converted to chlorpromazine equivalents (CPZE) (Gardner et al., 2010).
Table 1.
Site Sample Characteristics For Each Diagnostic Group.
| Discovery Sample | Replication Sample | |||
|---|---|---|---|---|
| Healthy Individuals N = 52 |
Patients with Bipolar Disorder N = 44 |
Healthy Individuals N = 41 |
Patients with Bipolar Disorder N = 78 |
|
| Male Sex (n,%) | 28 (53.8) | 29 (65.9) | 13 (31.7) | 26 (33.3) |
| Age (mean, years) | 29.8 (8.3) | 27.6 (8.3) | 33.2 (11.8) | 34.2 (12.6) |
| IQ (mean) | 115.2 (16.6) | 103.0 (17.9) | 104.1 (19.7) | 106.0 (17.1) |
| BPRS Total Score (mean) | 24.2 (0.4) | 46.3 (19.8) | 25.5 (3.9) | 32.6 (8.7) |
| Psychotic Symptoms (n,%) | – | 44 (100) | – | 21 (26.9) |
| Unmedicated (n,%) | – | 4 (9.3) | – | 15 (19.2) |
| Lithium (n,%) | – | 18 (41.9) | – | 11 (14.1) |
| Antipsychotics (n,%) | – | 34 (79.1) | – | 24 (30.8) |
| Daily Antipsychotic Dose (CPZE) | – | 275.9 (339.3) | – | 92.2 (224.5) |
Continuous variables are shown as mean (standard deviation); BPRS: Brief Psychiatric Rating Scale; CPZE: Chlorpromazine equivalents.
2.2. Neuroimaging
The neuroimaging data at both sites were acquired using Siemens 3T scanners (Erlangen, Germany) and were processed separately using identical protocols. Details of the acquisition sequences, data preprocessing and image analyses are presented in the Supplementary Material. Cortical reconstruction, based on the Desikan atlas (Desikan et al., 2006), and volumetric segmentation of structural datasets were implemented in the FreeSurfer image analysis suite (version 5.3.0; http://surfer.nmr.mgh.harvard.edu/). In each participant, 64 cortical thickness and 18 subcortical volume measures were extracted from the structural dataset (detailed in Supplementary Table S3). Analyses of the resting-state functional magnetic resonance imaging (rsfMRI) data were implemented using the Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the Data Processing and Analysis for Brain Imaging Toolbox (Yan et al., 2016) (details in the Supplementary Material). We did not include surface area as a previous large-scale analysis did not identify disorder-related abnormalities in this measure (Hibar et al., 2018).
We used previously established templates Doucet et al., 2019a Doucet et al., 2018 to extract connectivity measures from the 5 most reproducible resting-state brain networks and their constituent modules (Doucet et al., 2018): default mode (4 modules), central executive (2 modules), salience, sensorimotor (2 modules) and visual (4 modules) (Supplementary Figure S1). Functional connectivity within and between modules was calculated using Pearson’s correlation coefficients which were then Fisher z-transformed to yield 13 within- and 78 between-module functional connectivity measures per participant (Supplementary Table S3). The within-module connectivity represents the cohesiveness of each module and the between-module functional connectivity represents the integration of modules within the functional connectome. In generating the PBSI we considered cortical thickness, subcortical volume, functional cohesiveness and functional integration separately because they have partially distinct genetic, age-related, environmental, and clinical correlates (Glahn et al., 2010; Miller et al., 2016; Moser et al., 2018; Wen et al., 2016).
2.3. Person-Based similarity index for neuroimaging profiles
The PBSI (Doucet et al., 2019b) is a new and validated method that quantifies the similarity of the neuroimaging profile of each participant to that of the other members of a study sample (Supplementary Figure S2). The first step of the process involves the generation of a neuroimaging profile for each individual (Supplementary Figure S3); this procedure is independent of diagnosis or sample as it uses only the datasets of each individual. In each individual, the neuroimaging measures that correspond to each neuroimaging profile are concatenated into a single vector. Subsequently, Spearman’s correlation coefficient ρ are computed between the neuroimaging profile of each individual to the corresponding profiles of the other members of a group. This process generates n-1 coefficients per individual, where n is the total number of individuals in the group. The mean value of these coefficients for each individual represents their PBSI score for the respective profile. Higher scores indicate greater similarity between the individual’s profile to that of the other members of the group.
The PBSI is therefore not a fixed property of an individual’s neuroimaging profile but a measure of its similarity to the profiles of others. It can be used to quantify the similarity of a patient’s profile to that of other patients with the same disorder or to that of healthy individuals. The former provides estimates of within-diagnosis similarity in neuroimaging profiles and the latter yields estimates of the normativeness of a patient’s profile. Accordingly, we computed person-based-similarity indices for cortical thickness (PBSI-CT), subcortical volume (PBSI-SV), module cohesiveness (PBSI-MC) and module integration (PBSI-MI)to estimate within-group similarity for each diagnostic group from each site sample. Additionally, within each site sample, we computed the person-based similarity indices between patients and healthy individual to estimate the degree of normativeness of each patient’s profile for each of the 4 neuroimaging phenotypes (referred to as nPBSI-CT, nPBSI-SV, nPBSI-MC and nPBSI-MI) (details in Supplementary Material).
We used bootstrap resampling to examine whether the PBSI-CT, PBSI-SV, PBSI-MC and PBSI-MI scores were sensitive to the contribution of their constituent neuroimaging measures. To achieve this, we created cortical thickness and module integration profiles for each individual by randomly grouping their constituent measures in increments of 10; we then recalculated the PBSI-CT and PBSI-MI 100 times in each diagnostic group within each site sample. Similarly, we created subcortical volume profiles and module cohesion profiles for each individual by randomly grouping half of their constituent measures and recalculated the PBSI-SV and PBSI-MC 100 times for each individual.
Further, in each diagnostic group within each site sample, we quantified the contribution of each neuroimaging measure to their respective PBSI by using the leave-one-out approach; this entailed recalculating the PBSI-CT, PBSI-SV, PBSI-MC and PBSI-MI scores for each individual within each diagnostic group and site sample after leaving out one discrete neuroimaging measure at a time.
2.4. Variability in discrete brain regions and networks
We computed the coefficient of variation for each neuroimaging measure (i.e., each subcortical volume, each regional cortical thickness, the cohesiveness of each module and the connectivity measures of integration each module with all other modules) separately within each diagnostic group and in each site sample. The coefficient of variation provides information about the distribution of the values of any given measure in each group; a higher coefficient of variation for any measure indicates greater variability of that measure.
2.5. Statistical analyses
All statistical procedures were performed separately for the discovery ISMMS sample and Yale replication sample using identical procedures implemented in SPSS® v23.0. Across all the analyses discussed below, the results were considered significant following false-discovery-rate (FDR) correction for multiple testing (Benjamini and Yekutieli, 2001). We employed the Kolmogorov-Smirnov test to evaluate data normalcy and implemented parametric (Student’s t-test) or non-parametric tests (Mann Whitney U test), as appropriate, to identify case-control differences in continuous variables. Group differences in the distribution of categorical data were examined using chi-square tests. We assessed the association of PBSI scores with age, sex, and cortical thickness measures in all participants and, in patients with symptom ratings, and medication status. Lithium was modeled as a binarised variable (prescribed vs not-prescribed) and daily antipsychotic dose in CPZE was modeled as a continuous variable. Analyses were also repeated after regressing out age and sex on the raw morphometric measures and recomputing the PBSI scores. We confirmed the stability of the results for sample size and composition modeling samples, selecting subsamples from 50% to 150% in each diagnostic group, 1000 times, using bootstrap resampling with replacement which enables the generation of boostrap samples larger than the original and has the advantage of increasing the likelihood that all observations will be eventually included in the bootstrap samples.
Finally, in the patients only, we also tested the association between the PBSI scores and their respective normativeness scores for each profile, using Pearson correlation analyses.
3. Results
3.1. Person-based similarity indices of neuroimaging profiles in bipolar disorder
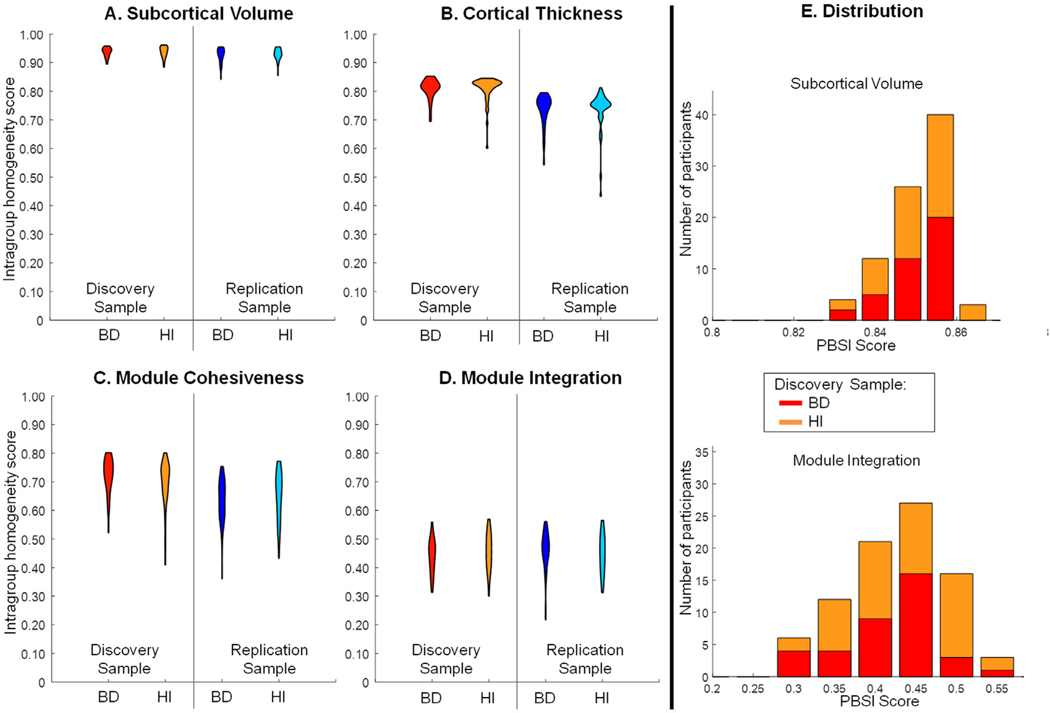
The PBSI-CT, PBSI-SV, PBSI-MC and PBSI-MI scores per diagnostic group and site are shown in Table 2. There was no significant effect of diagnosis (all punc>0.05) on any PBSI in either site sample (Fig. 1; Table 2; Supplementary Table S4; Supplementary Figure S4). There were no differences in any PBSI score when patients with psychosis were compared to those without psychosis (details in Supplemental Material). The daily antipsychotic dose or the symptom severity were not correlated with any PBSI in patients in either site sample (ρ range: −0.29, 0.24; punc>0.05). Both in the ISMMS discovery and the Yale replication sample, and within each diagnostic group, the PBSI-SV had the highest mean scores and the PBSI-MI had the lowest mean scores. Regardless of the diagnosis, age was positively correlated with the PBSI-MI (ISMMS: r = 0.21, punc=0.05) and the replication sample (r = 0.20, punc=0.03) (Supplementary Figure S4A). Regardless of the diagnosis, IQ was positively correlated with PBSI-CT (ISMMS: r = 0.33, punc=0.002; Yale: r = 0.20, punc=0.05) (Supplementary Figure S5B).
Table 2.
Person-Based Similarity Index (PBSI) Scores for Each Diagnostic Group and Each Site Sample.
| Sample | PBSI-SV | PBSI-CT | PBSI-MC | PBSI-MI |
|---|---|---|---|---|
| Discovery Sample | ||||
| Patients with Bipolar Disorder | 0.94 (0.02) | 0.81 (0.04) | 0.72 (0.06) | 0.44 (0.06) |
| Healthy Individuals | 0.94 (0.03) | 0.81 (0.04) | 0.68 (0.11) | 0.46 (0.06) |
| Replication Sample | ||||
| Patients with Bipolar Disorder | 0.93 (0.02) | 0.73 (0.05) | 0.63 (0.07) | 0.46 (0.07) |
| Healthy Individuals | 0.93 (0.02) | 0.73 (0.07) | 0.65 (0.08) | 0.45 (0.07) |
All variables are shown as mean (standard deviation); PBSI-SV=person-based similarity index for subcortical volume; PBSI-CT=person-based similarity index for cortical thickness; PBSI-MC= person-based similarity index for module cohesiveness; PBSI-MI=person-based similarity index for module integration.
Fig. 1. Person-Based Similarity Index scores for each neuroimaging phenotype.
Panels A-D: Violin plots of the person-based similarity index scores for cortical thickness (PBSI-CT), subcortical volume (PBSI-SV), module cohesiveness (PBSI-MC) and module integration (PBSI-MI) in patients with bipolar disorder (BD) and healthy individuals (HI). There was no statistically significant effect of diagnosis in either the ISMMS discovery or the Yale replication sample (all p>0.05). Panel E: Illustrative example of stacked distribution of the PBSI-SV and PBSI-MI in the ISMMS Discovery sample (also see Supplementary Figure S5).
We note that these results were robust to sample and remained unchanged when age and sex were regressed out of each imaging measure prior to recalculation the PBSI scores (Supplementary Material, Supplementary Figure S6).
3.2. Contributions t of regional morphometric and functional connectivity measures to the respective PBSI
Bootstrap resampling showed that no discrete measure appeared to drive any of these scores within each diagnostic group and site sample (Supplementary Figure S7); this was also confirmed by the leave-oneout analyses, in which each brain measure was removed and the PBSI scores were recalculated (Supplementary Table S4). The coefficients of variation for individual morphometric and functional connectivity measures did not differ by diagnostic group either in the discovery or replication samples (punc>0.05 for all, Supplementary Table S6).
3.3. Normativeness of neuroimaging profiles in bipolar disorder
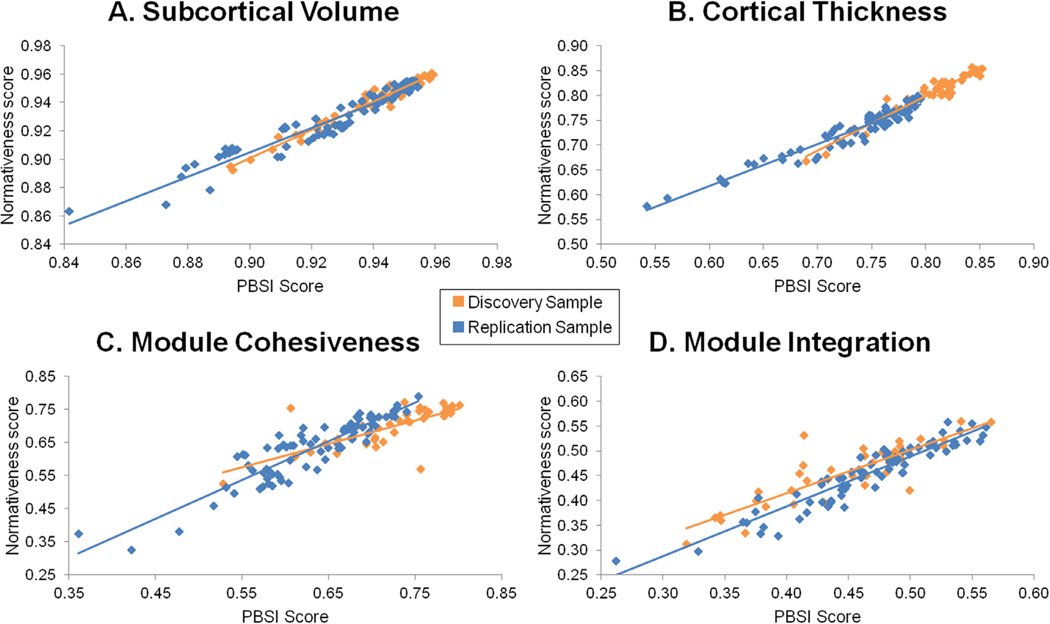
The nPBSI-CT, nPBSI-SV, nPBSI-MC and nPBSI-MI scores of patients, when referenced to healthy individuals, were generally very high (Table 3) denoting significant similarity between the neuroimaging profiles of patients and those of healthy individuals. In both site samples, the nPBSI scores were highly correlated with the PBSI scores (Fig. 2); with correlation coefficients ranging from 0.87–0.96 for morphometric measures and from 0.71–0.94 for functional connectivity measures (Supplementary Table S5).
Table 3.
Normative Person-Based Similarity Index (nPBSI) scores in Bipolar Disorder.
| Neuroimaging Measure | Normative PBSI Scores | |
|---|---|---|
| Discovery Sample | Replication Sample | |
| Subcortical Volume | 0.94 (0.02) | 0.83 (0.02) |
| Cortical Thickness | 0.77 (0.18) | 0.73 (0.05) |
| Module Cohesiveness | 0.71 (0.06) | 0.63 (0.09) |
| Module Integration | 0.45 (0.06) | 0.45 (0.07) |
All variables are shown as mean (standard deviation).
Fig. 2. Correlations between the normative person-based similarity (nPBSI) scores and the person-based similarity scores in patients with Bipolar Disorder.
(A) PBSI for subcortical volumes (ISMMS discovery sample: r = 0.91, p = 7 × 10−15; Yale replication Sample: r = 0.96, p = 4 × 10−45); (B) PBSI for cortical thickness (ISMMS discovery sample: r = 0.87, p = 4.10−12; Yale replication sample: r = 0.92, p = 9 × 10−33); (C) PBSI for module cohesiveness (ISMMS discovery sample: r = 0.71, p = 10−6; Yale replication sample: r = 0.90, p = 3 × 10−30); (B) PBSI for module integration (ISMMS discovery sample: r = 0.81, p = 10−9; Yale replication sample: r = 0.94, p = 10−38). Orange: ISMMS Discovery Sample, Blue: Yale Replication Sample.
In patients, the daily antipsychotic dose or the symptom severity were not associated with the normativeness of the patients’ profiles (ρ range: −0.27, 0.25; punc>0.05). In the ISMMS sample, the nPBSI-MI was higher in those patients who were prescribed Lithium compared to those that were not (t = 2.2, punc=0.03). This association was not detected in the Yale sample (punc=0.4), most likely due to insufficient power as only 14% of the patients in that sample were prescribed Lithium.
4. Discussion
We tested the degree of similarity in brain structure and resting-state functional connectivity amongst patients with bipolar disorder and between patients and healthy individuals. Similarity was quantified at the level of neuroimaging profiles and at the level of discrete brain regions and networks. Similarity in neuroimaging profiles was quantified using the PBSI, which is an individualized measure that informs about the degree of global similarity in neuroimaging profiles of each patient to other patients and the degree of similarity between patients and healthy individuals (normativeness). We found that patients’ profiles were very similar to each other and showed limited deviation from normative profiles derived from healthy individuals. We also found that the variability of discrete regional morphometric measures and resting-state networks was comparable between patients and healthy individuals. Importantly, these findings were reproducible across two independent samples.
Current diagnostic classifications emphasize the distinction between bipolar disorder Type I and Type II, based on the severity and duration of manic symptoms (American Psychiatric Association, 2013). These clinical subtypes may also vary in their heritability (Song et al., 2017) and genetic risk factors (Charney et al., 2017). Since the patients included here were all diagnosed with bipolar disorder Type I, our study does not address the issue of heterogeneity in neuroimaging phenotypes between subtypes. Nevertheless, evidence from large studies undertaken by the bipolar disorder working group of the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) Consortium points to a continuum of increasing severity from Type II to Type I in terms of subcortical (Hibar et al., 2016) and cortical morphology (Hibar et al., 2018). Similar large-scale comparisons between the two subtypes have not been undertaken for resting-state functional connectivity although individual studies more commonly suggest similarities rather than marked differences (Birur et al., 2017; Vargas et al., 2013). A further distinction in bipolar disorder, Type I, is often made based on the presence of psychotic features which have been considered as markers of etiological (Allardyce et al., 2018) and pathophysiological heterogeneity (Coryell et al., 2001). Prior neuroimaging studies have found that the presence of psychosis in bipolar disorder is associated with greater severity of case-control differences primarily in frontal and temporal cortical thickness (Hibar et al., 2018) and gray matter volume (Altamura et al., 2017; Maggioni et al., 2017). In our study, the presence of psychotic features did not seem to introduce greater heterogeneity in any of the neuroimaging measures considered suggesting that the effect of psychosis on global neuroimaging profiles is subtle.
Prior research has identified patients with bipolar disorder with different cognitive profiles, along a continuum of severity from those who are cognitively intact, to those having selective deficits, and those showing global impairment (Bora, 2017; Jensen et al., 2016; Karantonis et al., 2020). In their critical review of the literature, Green et al. (2019) proposed that differences in cognitive profiles amongst patients are dimensional rather than categorical and reflect normal variation in cognitive ability and other non-specific factors such as age. Here we found that IQ was positively correlated with PBSI scores for cortical thickness regardless of diagnosis. In other words, higher IQ was associated with greater similarity in cortical thickness profiles. Further, we found a diagnosis-independent correlation between age and PBSI-MI, which is likely to reflect age-related changes in functional integration (Meunier et al., 2009). Collectively, these findings suggest that disease-non-specific factors such as IQ and age may be more important for variability in neuroimaging profiles of patients than disease-related mechanisms.
We found no association between any PBSI and treatment with antipsychotic medication. By contrast, the PBSI-MI of patients who were prescribed Lithium, compared to those who were not prescribed Lithium, showed greater similarity with the profiles of healthy individuals. This is an intriguing but tentative finding since we did not obtain serum levels to confirm adherence. Moreover, medication was not randomly prescribed and consequently we cannot dissociate between medication effects and selection of patients based on response to Lithium. Our observations are aligned with proposals that Lithium treatment may normalize imaging phenotypes; such proposals are primarily based on the association between Lithium treatment and preservation or normalization of morphometric features in bipolar disorder (Abramovic et al., 2016; Hajek et al., 2014; Hibar et al., 2018, 2016; Lopez-Jaramillo et al., 2017; Phillips et al., 2008; Sarrazin et al., 2019).
4.1. Limitations
We have previously noted limitation regarding the inclusion of patients with bipolar disorder, Type 1. We focused specifically on morphometry and resting-state connectivity that shows relatively limited methodological variation across studies and is therefore relevant to much of the current literature. However, bipolar disorder is associated with abnormalities in task-related fMRI (Chen et al., 2011; Janiri et al., 2019) and in white matter integrity (Favre et al., 2019; Koshiyama et al., 2019; Pezzoli et al., 2018). Examination of disorder-related heterogeneity in these measures is an important aim for future studies. Although we demonstrated that PBSI is stable to sample size (Supplementary Figure S6), replication of the results in future studies with larger sample size would bolster their validity.
5. Conclusions
The findings reported here suggest the alterations in brain structure and functional connectivity associated with bipolar disorder are nested within the normal biological diversity. Patients in both study samples exhibited close correspondence between their imaging profiles and those of healthy individuals. These observations are in line with epidemiological evidence that suggest that bipolar-like symptoms are both prevalent and widely distributed in the general population (Papachristou et al., 2017; Tijssen et al., 2010a, 2010b) and that syndromal disorder per se may represent the most persistent or severe end of this distribution (Papachristou et al., 2017; Tijssen et al., 2010a).
Supplementary Material
Acknowledgments
This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Role of Funding Source
This work was supported by the National Institutes of Health (NIH) (R01MH104284; R01MH113619; R01MH116147; R01AG050345; R01MH078143; R01MH106324; R03AG064001; P20GM130447).
Footnotes
Data Sharing and Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2020.06.041.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Abramovic L, Boks MP, Vreeker A, Bouter DC, Kruiper C, Verkooijen S, van Bergen AH, Ophoff RA, Kahn RS, van Haren NE, 2016. The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. Eur Neuropsychopharmacol 26, 1741–1751. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E, 2013. Imaging structural co-variance between human brain regions. Nature reviews. Neuroscience 14, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allardyce J, Leonenko G, Hamshere M, Pardinas AF, Forty L, Knott S, Gordon-Smith K, Porteous DJ, Haywood C, Di Florio A, Jones L, McIntosh AM, Owen MJ, Holmans P, Walters JTR, Craddock N, Jones I, O’Donovan MC, Escott-Price V, 2018. Association Between Schizophrenia-Related Polygenic Liability and the Occurrence and Level of Mood-Incongruent Psychotic Symptoms in Bipolar Disorder. JAMA Psychiatry 75, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura AC, Maggioni E, Dhanoa T, Ciappolino V, Paoli RA, Cremaschi L, Prunas C, Orsenigo G, Caletti E, Cinnante CM, Triulzi FM, Dell’Osso B, Yatham L, Brambilla P, 2017. The impact of psychosis on brain anatomy in bipolar disorder: a structural MRI study. Journal of affective disorders. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM, 2009. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. The British journal of psychiatry: the journal of mental science 195, 194–201. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29, 1165–1188. [Google Scholar]
- Birur B, Kraguljac NV, Shelton RC, Lahti AC, 2017. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, 2017. Neurocognitive features in clinical subgroups of bipolar disorder: a meta-analysis. J Affect Disord 229, 125–134. [DOI] [PubMed] [Google Scholar]
- Charney AW, Ruderfer DM, Stahl EA, Moran JL, Chambert K, Belliveau RA, Forty L, Gordon-Smith K, Di Florio A, Lee PH, Bromet EJ, Buckley PF, Escamilla MA, Fanous AH, Fochtmann LJ, Lehrer DS, Malaspina D, Marder SR, Morley CP, Nicolini H, Perkins DO, Rakofsky JJ, Rapaport MH, Medeiros H, Sobell JL, Green EK, Backlund L, Bergen SE, Jureus A, Schalling M, Lichtenstein P, Roussos P, Knowles JA, Jones I, Jones LA, Hultman CM, Perlis RH, Purcell SM, McCarroll SA, Pato CN, Pato MT, Craddock N, Landen M, Smoller JW, Sklar, 2017. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry 7, e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET, 2011. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord 13, 1–15. [DOI] [PubMed] [Google Scholar]
- Coryell W, Leon AC, Turvey C, Akiskal HS, Mueller T, Endicott J, 2001. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J Affect Disord 67, 79–88. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S, 2017. The Role of Intrinsic Brain Functional Connectivity in Vulnerability and Resilience to Bipolar Disorder. Am J Psychiatry 174, 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Lee WH, Frangou S, 2019a. Evaluation of the spatial variability in the major resting-state networks across human brain functional atlases. Hum Brain Mapp 40, 4577–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Moser DA, Rodrigue A, Bassett DS, Glahn DC, Frangou S, 2019b. Person-Based Brain Morphometric Similarity is Heritable and Correlates With Biological Features. Cereb Cortex 29, 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S, 2018. Elevated Body Mass Index is Associated with Increased Integration and Reduced Cohesion of Sensory-Driven and Internally Guided Resting-State Functional Brain Networks. Cereb Cortex 28, 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abe C, Alda M, Alloza C, Alonso-Lana S, Andreassen OA, Baune BT, Benedetti F, Busatto GF, Canales-Rodriguez EJ, Caseras X, Chaim-Avancini TM, Ching CRK, Dannlowski U, Deppe M, Eyler LT, Fatjo-Vilas M, Foley SF, Grotegerd D, Hajek T, Haukvik UK, Howells FM, Jahanshad N, Kugel H, Lagerberg TV, Lawrie SM, Linke JO, McIntosh A, Melloni EMT, Mitchell PB, Polosan M, Pomarol-Clotet E, Repple J, Roberts G, Roos A, Rosa PGP, Salvador R, Sarro S, Schofield PR, Serpa MH, Sim K, Stein DJ, Sussmann JE, Temmingh HS, Thompson PM, Verdolini N, Vieta E, Wessa M, Whalley HC, Zanetti MV, Leboyer M, Mangin JF, Henry C, Duchesnay E, Houenou J, Group EBDW, 2019. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 44, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured clinical interview for DSM-5, research version, Arlington, VA. [Google Scholar]
- Frangou S, Dima D, Jogia J, 2017. Towards person-centered neuroimaging markers for resilience and vulnerability in Bipolar Disorder. Neuroimage 145, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ, 2010. International consensus study of antipsychotic dosing. Am J Psychiatry 167, 686–693. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM, Beckmann CF, Fox PT, Blangero J, 2010. Genetic control over the resting brain. Proc. Natl. Acad. Sci. U.S.A. 107, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quide Y, 2019. A Systematic Review of Studies Reporting Data-Driven Cognitive Subtypes across the Psychosis Spectrum. Neuropsychol Rev. [DOI] [PubMed] [Google Scholar]
- Hajek T, Bauer M, Simhandl C, Rybakowski J, O’Donovan C, Pfennig A, Konig B, Suwalska A, Yucel K, Uher R, Young LT, MacQueen G, Alda M, 2014. Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment response. Psychol Med 44, 507–517. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppa T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C, 2011. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol. Psychiatry 69, 326–335. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, Versace A, Bilderbeck AC, Uhlmann A, Mwangi B, Kramer B, Overs B, Hartberg CB, Abe C, Dima D, Grotegerd D, Sprooten E, Boen E, Jimenez E, Howells FM, Delvecchio G, Temmingh H, Starke J, Almeida JRC, Goikolea JM, Houenou J, Beard LM, Rauer L, Abramovic L, Bonnin M, Ponteduro MF, Keil M, Rive MM, Yao N, Yalin N, Najt P, Rosa PG, Redlich R, Trost S, Hagenaars S, Fears SC, Alonso-Lana S, van Erp TGM, Nickson T, Chaim-Avancini TM, Meier TB, Elvsashagen T, Haukvik UK, Lee WH, Schene AH, Lloyd AJ, Young AH, Nugent A, Dale AM, Pfennig A, McIntosh AM, Lafer B, Baune BT, Ekman CJ, Zarate CA, Bearden CE, Henry C, Simhandl C, McDonald C, Bourne C, Stein DJ, Wolf DH, Cannon DM, Glahn DC, Veltman DJ, Pomarol-Clotet E, Vieta E, Canales-Rodriguez EJ, Nery FG, Duran FLS, Busatto GF, Roberts G, Pearlson GD, Goodwin GM, Kugel H, Whalley HC, Ruhe HG, Soares JC, Fullerton JM, Rybakowski JK, Savitz J, Chaim KT, Fatjo-Vilas M, Soeiro-de-Souza MG, Boks MP, Zanetti MV, Otaduy MCG, Schaufelberger MS, Alda M, Ingvar M, Phillips ML, Kempton MJ, Bauer M, Landen M, Lawrence NS, van Haren NEM, Horn NR, Freimer NB, Gruber O, Schofield PR, Mitchell PB, Kahn RS, Lenroot R, Machado-Vieira R, Ophoff RA, Sarro S, Frangou S, Satterthwaite TD, Hajek T, Dannlowski U, Malt UF, Arolt V, Gattaz WF, Drevets WC, Caseras X, Agartz I, Thompson PM, Andreassen OA, 2018. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 23, 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, Haukvik UK, Hartberg CB, Doan NT, Agartz I, Dale AM, Gruber O, Kramer B, Trost S, Liberg B, Abe C, Ekman CJ, Ingvar M, Landen M, Fears SC, Freimer NB, Bearden CE, Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar, E., Sprooten E, Glahn DC, Pearlson GD, Emsell L, Kenney J, Scanlon C, McDonald C, Cannon DM, Almeida J, Versace A, Caseras X, Lawrence NS, Phillips ML, Dima D, Delvecchio G, Frangou S,Satterthwaite TD, Wolf D, Houenou J, Henry C, Malt UF, Boen E, Elvsashagen T, Young AH, Lloyd AJ, Goodwin GM, Mackay CE, Bourne C, Bilderbeck A, Abramovic L, Boks MP, van Haren NE, Ophoff RA, Kahn RS, Bauer M, Pfennig A, Alda M, Hajek T, Mwangi B, Soares JC, Nickson T, Dimitrova R, Sussmann JE, Hagenaars S, Whalley HC, McIntosh AM, Thompson PM, Andreassen OA, 2016. Subcortical volumetric abnormalities in bipolar disorder. Mol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, Murrough JW, Sani G, Eickhoff SB, Frangou S, 2019. Shared Neural Phenotypes for Mood and Anxiety Disorders: a Meta-analysis of 226 Task-Related Functional Imaging Studies. JAMA Psychiatry 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Knorr U, Vinberg M, Kessing LV, Miskowiak KW, 2016. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: associations with functional abilities. J Affect Disord 205, 378–386. [DOI] [PubMed] [Google Scholar]
- Karanti A, Kardell M, Joas E, Runeson B, Palsson E, Landen M, 2019. Characteristics of bipolar I and II disorder: a study of 8766 individuals. Bipolar disorders. [DOI] [PubMed] [Google Scholar]
- Karantonis JA, Rossell SL, Carruthers SP, Sumner P, Hughes M, Green MJ, Pantelis C, Burdick KE, Cropley V, Van Rheenen TE, 2020. Cognitive validation of cross-diagnostic cognitive subgroups on the schizophrenia-bipolar spectrum. J Affect Disord 266, 710–721. [DOI] [PubMed] [Google Scholar]
- Koshiyama D, Fukunaga, Okada M, Morita N, Nemoto K, Usui K, Yamamori K, Yasuda H, Fujimoto Y, Kudo M, Azechi N, Watanabe H, Hashimoto Y, Narita N, Kusumi H, Ohi I, Shimada K, Kataoka T, Yamamoto Y, Ozaki M, Okada N, Okamoto G, Harada Y, Matsuo K, Yamasue K, Abe H, Hashimoto O, Takahashi R, Hori T, Nakataki T, Onitsuka M, Holleran T, Jahanshad L, van Erp N, Turner TGM, Donohoe J, Thompson G, Kasai PM, Hashimoto K, Cocoro R, 2019. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima F, Rabelo-da-Ponte FD, Bucker J, Czepielewski L, Hasse-Sousa M, Telesca R, Sole B, Reinares M, Vieta E, Rosa AR, 2019. Identifying cognitive subgroups in bipolar disorder: a cluster analysis. J Affect Disord 246, 252–261. [DOI] [PubMed] [Google Scholar]
- Lopez-Jaramillo C, Vargas C, Diaz-Zuluaga AM, Palacio JD, Castrillon G, Bearden C, Vieta E, 2017. Increased hippocampal, thalamus and amygdala volume in long-term lithium-treated bipolar I disorder patients compared with unmedicated patients and healthy subjects. Bipolar Disord 19, 41–49. [DOI] [PubMed] [Google Scholar]
- Maggioni E, Altamura AC, Brambilla P, 2017. Exploring the neuroanatomical bases of psychotic features in bipolar disorder. Epidemiol Psychiatr Sci 26, 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Marengo E, Ibanez A, Scapola M, Igoa A, 2014. Toward the identification of neurocognitive subtypes in euthymic patients with bipolar disorder. J Affect Disord 167, 118–124. [DOI] [PubMed] [Google Scholar]
- Meda SA, Ruano G, Windemuth A, O’Neil K, Berwise C, Dunn SM, Boccaccio LE, Narayanan B, Kocherla M, Sprooten E, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Calhoun VD, Pearlson GD, 2014. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. In: Proceedings of the National Academy of Sciences of the United States of America. 111 pp. E2066–E2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E, 2009. Age-related changes in modular organization of human brain functional networks. Neuroimage 44, 715–723. [DOI] [PubMed] [Google Scholar]
- Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulos SN, Andersson JL, Griffanti L, Douaud G, Okell TW, Weale P, Dragonu I, Garratt S, Hudson S, Collins R, Jenkinson M, Matthews PM, Smith SM, 2016. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser DA, Doucet GE, Lee WH, Rasgon A, Krinsky H, Leibu E, Ing A, Schumann G, Rasgon N, Frangou S, 2018. Multivariate associations among behavioral, clinical and multimodal imaging phenotypes in psychosis. JAMA Psychiatry 75, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A, Schnack HG, Ching CRK, Agartz I, Akudjedu TN, Alda M, Alnaes D, Alonso-Lana S, Bauer J, Baune BT, Boen E, Bonnin CDM, Busatto GF, Canales-Rodriguez EJ, Cannon DM, Caseras X, Chaim-Avancini TM, Dannlowski U, Diaz-Zuluaga AM, Dietsche B, Doan NT, Duchesnay E, Elvsashagen T, Emden D, Eyler LT, Fatjo-Vilas M, Favre P, Foley SF, Fullerton JM, Glahn DC, Goikolea JM, Grotegerd D, Hahn T, Henry C, Hibar DP, Houenou J, Howells FM, Jahanshad N, Kaufmann T, Kenney J, Kircher TTJ, Krug A, Lagerberg TV, Lenroot RK, Lopez-Jaramillo C, Machado-Vieira R, Malt UF, McDonald C, Mitchell PB, Mwangi B, Nabulsi L, Opel N, Overs BJ, Pineda-Zapata JA, Pomarol-Clotet E, Redlich R, Roberts G, Rosa PG, Salvador R, Satterthwaite TD, Soares JC, Stein DJ, Temmingh HS, Trappenberg T, Uhlmann A, van Haren NEM, Vieta E, Westlye LT, Wolf DH, Yuksel D, Zanetti MV, Andreassen OA, Thompson PM, Hajek T, Group EBDW, 2018. Using structural MRI to identify bipolar disorders - 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou E, Oldehinkel AJ, Ormel J, Raven D, Hartman CA, Frangou S, Reichenberg A, 2017. The predictive value of childhood subthreshold manic symptoms for adolescent and adult psychiatric outcomes. J Affect Disord 212, 86–92. [DOI] [PubMed] [Google Scholar]
- Pezzoli S, Emsell L, Yip SW, Dima D, Giannakopoulos P, Zarei M, Tognin S, Arnone D, James A, Haller S, Frangou S, Goodwin GM, McDonald C, Kempton MJ, 2018. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci Biobehav Rev 84, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ, 2008. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry 165, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Rego V, Jogia J, Marquand AF, Mourao-Miranda J, Simmons A, Frangou S, 2014. Examination of the predictive value of structural magnetic resonance scans in bipolar disorder: a pattern classification approach. Psychol Med 44, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Poupon C, Teillac A, Mangin JF, Polosan M, Favre P, Laidi C, D’Albis MA, Leboyer M, Lledo PM, Henry C, Houenou J, 2019. Higher in vivo Cortical Intracellular Volume Fraction Associated with Lithium Therapy in Bipolar Disorder: a Multicenter NODDI Study. Psychother Psychosom 88, 171–176. [DOI] [PubMed] [Google Scholar]
- Song J, Kuja-Halkola R, Sjolander A, Bergen SE, Larsson H, Landen M, Lichtenstein P, 2017. Specificity in Etiology of Subtypes of Bipolar Disorder: evidence From a Swedish Population-Based Family Study. Biological psychiatry. [DOI] [PubMed] [Google Scholar]
- Tijssen MJ, van Os J, Wittchen HU, Lieb R, Beesdo K, Mengelers R, Krabbendam L, Wichers M, 2010a. Evidence that bipolar disorder is the poor outcome fraction of a common developmental phenotype: an 8-year cohort study in young people. Psychol Med 40, 289–299. [DOI] [PubMed] [Google Scholar]
- Tijssen MJ, van Os J, Wittchen HU, Lieb R, Beesdo K, Mengelers R, Wichers M, 2010b. Prediction of transition from common adolescent bipolar experiences to bipolar disorder: 10-year study. The British journal of psychiatry: the journal of mental science 196, 102–108. [DOI] [PubMed] [Google Scholar]
- Vargas C, Lopez-Jaramillo C, Vieta E, 2013. A systematic literature review of resting state network–functional MRI in bipolar disorder. J Affect Disord 150, 727–735. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP, 1993. Training and quality assurance with the Brief Psychiatric Rating Scale. Int J Methods Psychiatr Res 3, 221–244. [Google Scholar]
- Wallace ML, Simsek B, Kupfer DJ, Swartz HA, Fagiolini A, Frank E, 2016. An approach to revealing clinically relevant subgroups across the mood spectrum. J Affect Disord 203, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2011. Wechsler Abbreviated Scale of Intelligence-2nd edition. NCS Pearson, San Antonio, TX. [Google Scholar]
- Wen W, Thalamuthu A, Mather KA, Zhu W, Jiang J, de Micheaux PL, Wright MJ, Ames D, Sachdev PS, 2016. Distinct Genetic Influences on Cortical and Subcortical Brain Structures. Sci Rep 6, 32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T, Doan NT, Kaufmann T, Alnaes D, Moberget T, Agartz I, Buitelaar JK, Ueland T, Melle I, Franke B, Andreassen OA, Beckmann CF, Westlye LT, Marquand AF, 2018. Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry 75, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Wang XD, Zuo XN, Zang YF, 2016. DPABI: data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Zuo XN, Wang Z, Yuan C, Jiao Q, Chen H, Biswal BB, Lu G, Liu Y, 2011. Resting-state brain organization revealed by functional covariance networks. PLoS ONE 6, e28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.