Abstract
We describe a case of a 33-year-old-male with Mycoplasma pneumoniae-induced rash and mucositis and review the literature on this newly described syndrome.
Keywords: MIRM, Mycoplasma pneumoniae, Mycoplasma pneumoniae-induced rash and mucositis, Stevens-Johnson syndrome, SJS
Mycoplasma pneumoniae, a common cause of community-acquired upper respiratory tract infections and pneumonia, causes many extrapulmonary manifestations. Mycoplasma pneumoniae-induced rash and mucositis (MIRM) is a newly classified entity described in 2015, characterized by prominent mucositis with less cutaneous involvement. This disease is distinct from previously described erythema multiforme, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), all of which have been associated with M pneumoniae.
CASE
A 33-year-old-male with history of type 1 diabetes mellitus and prior alcohol and polysubstance use disorders presented to the hospital with a 10-day history of worsening productive cough, fever, myalgias, nasal congestion, and 3 days of painful oral ulcers, conjunctivitis, and a rash.
He initially presented to an urgent care clinic where he was prescribed doxycycline and valacyclovir. He continued to worsen with desquamation of the oral ulcers and new onset of sore throat. He was evaluated at an emergency room, where he was noted to have a right upper lobe infiltrate concerning for pneumonia, which was treated with oral doxycycline. Evaluation of the oral lesions for herpes simplex (polymerase chain reaction [PCR]), gonococcal/chlamydia (nucleic acid amplification test), and pharyngeal streptococcal cultures were all negative.
He then developed lip swelling and a pruritic, painful rash on his torso, extremities, and genitalia. He had no history of prior sexually transmitted infections, similar symptoms in the past, exposure to sick contacts, or exposure to new medications except for the antibiotics mentioned above and topical viscous lidocaine with Mylanta for mouth rinses. He reported being monogamous with his female partner. He reported poor oral intake secondary to odynophagia but denied other new symptoms.
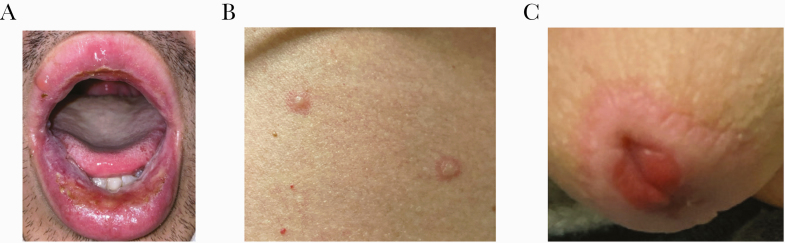
On admission, his exam showed bilateral conjunctival injection, oral ulcers with overlying hemorrhagic crusting on the lips (Figure 1A), painful cervical and occipital lymphadenopathy, as well as nontender inguinal lymphadenopathy; bilateral rhonchi were noted. Skin exam was notable: scattered on the trunk and proximal extremities were clear bullae atop ill-defined erythematous bases with a somewhat targetoid appearance (Figure 1B). A well delineated erythematous macule with a darker erythematous rim was surrounding the meatus of the penis (Figure 1C).
Figure 1.
Clinical photographs of the patient at time of admission. (A) Oral ulcers on the lips with mucosal sloughing and hemorrhagic crust. (B) Clear bullae atop ill-defined erythematous bases with a somewhat targetoid appearance on the trunk. (C) Well defined ulceration surrounding the penile meatus.
Chest x-ray revealed a persistent right upper lobe pneumonia. A respiratory pathogen panel, obtained by nasopharyngeal swab, was positive for M pneumoniae. He was diagnosed with MIRM and was started on intravenous (IV) azithromycin 500 mg. He was initially given IV methylprednisolone 125 mg for 5 days followed by prednisone 60 mg with subsequent slow taper over a 1-month period. The patient’s clinical course was complicated by poorly controlled pain, especially odynophagia, treated with oral oxycodone and IV hydromorphone. After several days of treatment he improved. One-month later, his condition greatly improved with near resolution of the oral ulcers and mild residual burning pain in his mouth and near resolution of the rash with residual erythematous macules on his arms.
DISCUSSION AND REVIEW OF THE LITERATURE
Mycoplasma pneumoniae is a common cause of upper and lower respiratory tract infections, which affects both children and adults [1]. Infection with M pneumoniae can cause a variety of extrapulmonary complications including pericarditis, myocarditis, hepatitis, thrombocytopenic purpura, autoimmune hemolytic anemia, and neurologic manifestations including acute cerebellar ataxia [2]. Dermatologic complications are particularly prominent and include urticaria, erythema nodosum, cutaneous leukocytoclastic vasculitis, maculopapular eruptions, and the spectrum of skin disease from erythema multiforme to SJS and TEN [2–4]. Mucocutaneous disease is estimated to occur in approximately 25% of patients with M pneumoniae [4, 5]. Erythema multiforme is characterized by a typical targetoid eruption often with associated mucositis [6, 7]. Stevens-Johnson syndrome is characterized by skin necrosis and sloughing involving less than 10 percent of body surface area with mucosal involvement and TEN when over 30 percent body surface area is observed [6].
In contrast to patients with erythema multiforme or SJS/TEN, multiple patients have also been described with prominent mucositis with no or limited skin involvement by a range of diagnoses including “Stevens-Johnson syndrome without skin lesions,” “Fuchs syndrome” and “M. pneumoniae- associated mucositis” [3, 8]. A 1996 study noting prominent mucositis, ocular and genital involvement with less skin involvement and good prognosis referred to the syndrome as “M. pneumoniae-associated SJS” [9]. It is interesting to note that a 2005 case report raised the question about whether SJS without skin lesions could exist in association with M pneumoniae [5]. A 2015 systematic review by Canavan et al [3] reclassified these cases as cases of MIRM, a unique clinical entity, distinct from SJS or TEN. Mycoplasma pneumoniae-induced rash and mucositis is characterized by pronounced mucositis with limited skin involvement, especially when compared with SJS, which is also on the differential diagnosis. This systematic review revealed that 47% of patients with M pneumoniae-related mucositis had limited skin involvement and 34% had mucositis without skin involvement, contrasting with SJS and TEN [3].
Mucocutaneous findings in MIRM are often preceded by a 1-week prodrome of cough, malaise, and fever, as in our patient [3]. Cutaneous findings are most commonly vesiculobullous lesions and targetoid lesions, that are atypical, usually not with the 3 zones of different coloration needed for a diagnosis of erythema multiforme, with other morphologies being less common [3]. Skin detachment is typically less than or equal to 10% body surface area [3]. Oral and urogenital involvement with erosions, ulcerations, vesicles, and bullae as well as ocular involvement most commonly inflammatory conjunctivitis are common in MIRM, with often 2 or more mucosal surfaces being involved [3, 7, 10]. Ophthalmic manifestations can be more severe and include conjunctival pseudomembrane formation and conjunctival ulceration [10]. Another aspect of the natural history of this disease is that recurrence with subsequent M pneumoniae or viral infections is possible [11, 12]. In addition, most of the patients in the literature were children and adolescents that recovered from MIRM over the course of days [13–17]; however, the adult patient we described had persistent symptoms at 1 month follow up. It is possible that this longer duration is related to age because another patient in the literature at 42 years old also had persistent pain at 1 month follow up; however, further data are needed to make a conclusion [18].
Descriptions of patients in case reports of MIRM are highlighted in Table 1, reports were obtained via PubMed searches for “Mycoplasma pneumoniae induced rash and mucositis” current through April 25, 2020.
Table 1.
A Summary of Cases Diagnosed as MIRM in the Literature
| Patient Age | Patient Sex | Systemic Features | Mucocutaneous Features | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|
| 46 | Male | Atypical pneumonia | Mucositis, conjunctivitis | Azithromycin, systemic corticosteroids, supportive care, gentamicin/ dexamethasone eye drops | Complete recovery | Zao et al [19] |
| 12 | Female | Sore throat, cough, fever | Oral lesions with mucosal sloughing, conjunctivitis, vesicular rash | Azithromycin, acyclovir, IVIG, supportive care | Recovered and re-presented 3 years later with similar symptoms and PCR positive for Chlamydophila pneumoniae | Brazel et al [20] |
| 8 | Female | Cough and dyspnea 1 week prior, fever | Oral, ocular and vulvar mucositis, mild rash <1% BSA (some atypical targetoid papules) | Levofloxacin, IVIG, supportive care | Gradual improvement followed by recurrence of mucositis 9 months later with positive Mycoplasma pneumoniae and 2 years later with influenza B | Mazori et al [11] |
| 13 | Male | Fevers, arthralgias, nonproductive cough, rhinorrhea | Pustules, targetoid lesions, pruritis, burning, macules on buccal mucosa, conjunctivitis | Systemic and topical corticosteroids, doxycycline | Significant improvement within 2 days of doxycycline | Chao et al [13] |
| 13 | Female | Rhinitis, cough, | Erosive oral and genital lesions, conjunctivitis, no cutaneous lesions | Azithromycin later changed to doxycycline, cyclosporine, supportive care | Had a past history of MIRM before this episode, near complete resolution after 6 days of treatment | Li et al [14] |
| 14 | Female | Sore throat, cough, dyspnea, orthopnea, malaise, diplopia, headache, nausea, vomiting | Oral and vaginal erosions, conjunctivitis | Cyclosporine, azithromycin, supportive care | Complete resolution of oral lesions by 1 week after hospital discharge | Li et al [14] |
| 4 | Male | Cough, wheezing, fever | Mucositis, erythematous papules with central hemorrhagic crusts as well as targetoid papulovesicles as rash, conjunctivitis | Cyclosporine, azithromycin, supportive care | Near complete resolution after 7 hospital days | Li et al [14] |
| 20 | Male | Fevers, leukocytosis | Conjunctivitis, oral ulcerations | Azithromycin, IV methylprednisolone transitioned to oral, supportive care | Full recovery in 10 days | Kheiri et al [21] |
| 10 | Male | Self-limited fever and cough 1 week prior | Ulcerative and hemorrhagic oral ulcers, mild serpiginous eruption on skin | Systemic steroids, clarithromycin, supportive care | Complete resolution in 1 week | Poddighe et al [15] |
| 11 | Male | Low grade fever, cough, atypical pneumonia | Oral ulcers, conjunctivitis, hemorrhagic crusts of nasal mucosa, genital skin sloughing, no rash | Azithromycin, systemic steroids, supportive care | Not noted | Bowling et al [22] |
| 15 | Male | Fever, decreased appetite 10 days prior, cough, diarrhea, atypical opacities on chest x-ray | Hemorrhagic conjunctivitis, diffuse oral and pharyngeal erosions, crust at urethra, rare papulovesicles on skin | Azithromycin, supportive care | Gradual improvement over days | Curtiss et al [16] |
| 6 | Male | Fever, cough, rhinorrhea, atypical pneumonia | Severe mucositis (oral and penile), small blisters on arms and legs, conjunctivitis | Clarithromycin | Full recover after 1 week | Chowdhury [23] |
| 8 | Male | Cough, fever, ill-defined opacity on chest x-ray, leukocytosis | Oral erosions, erythema and swelling of penis, scattered erythematous papules and targetoid lesions, conjunctivitis | Ocular care, supportive care, IVIG, clarithromycin | Improved and discharged in 8 days | Santos et al [17] |
| 42 | Male | Upper respiratory illness | Conjunctivitis, oral and genital erosions, atypical targetoid papules and plaques | Azithromycin, supportive care | Previous history of similar illness as a child, resolution after 1 month with continued oral pain and phimosis | Song et al [18] |
| 15 | Male | Fever, dry cough | Conjunctivitis, oral erosions, targetoid papules and vesicles on skin | Azithromycin, IVIG | Slow improvement over 1 month with recurrence that resolved with azithromycin, postinflammatory hyperpigmentation | Song et al [18] |
| 18 | Male | Fever, sore throat, cough | Conjunctival injection, targetoid vesicles on face, torso, and penis, and mucositis over lips and buccal mucosa | Azithromycin, systemic prednisone | Improved at 14-day follow up with only a few shallow erosions remaining in mouth | Sandhu et al [24] |
Abbreviations: BSA, body surface area; IV, intravenous; IVIG, intravenous immunoglobulin; MIRM, M pneumoniae-induced rash and mucositis; PCR, polymerase chain reaction.
Distinction between MIRM and SJS/TEN can have important implications for treatment and prognosis. A key differentiating feature between MIRM and SJS/TEN is the generally excellent prognosis of MIRM patients with 81% of patients making a full recovery and only a 3% mortality rate [3]. This contrasts with the prognosis of SJS and TEN with mortality rates ranging from 24% to 49%, respectively, at 1 year [25]. An additional reason why the distinction between MIRM and SJS/TEN is important is that when M pneumoniae can be identified as the culprit, this will avoid labeling patients with erroneous drug allergies, which can have long-standing adverse effects on future treatment options [4, 26]. Serologic testing (immunoglobulin M), culture, or PCR testing can assist in supporting the diagnosis of MIRM, which is important because SJS/TEN can also have an associated pneumonitis [3, 27].
The pathogenesis of MIRM is unknown but may involve (1) immune complex deposition or molecular mimicry via antibodies or (2) cytotoxic T cells leading to injury [4, 11]. As illustrated by our patient, M pneumoniae-induced mucocutaneous disease presents commonly in young males [3]. Mycoplasma pneumoniae-induced rash and mucositis is most commonly seen in patients who are on average approximately 12 years old; however, it can occur over a wide range of ages [3]. A question has been raised as to whether age should be used in diagnostic criteria for MIRM; however, it has been reported in older patients such as ours in the literature [18, 19, 27, 28].
In addition, there has been debate in the pediatric literature as to whether the term “reactive infectious mucocutaneous eruption” (RIME) better describes MIRM given the fact that other pathogens including Chlamydophila pneumoniae (Chlamydia pneumoniae) and viral pathogens, including influenza B, may induce a similar constellation of symptoms [11, 12, 20, 26, 29].
Mycoplasma pneumoniae-induced rash and mucositis may be a marker for more severe M pneumoniae sequelae. In a study of children with M pneumoniae, mucocutaneous involvement was associated with prolonged fever, higher C-reactive protein, increased chance of hospitalization, and need for oxygen [4]. It has been postulated that mucocutaneous phenomena from M pneumoniae infection are driven by an immune reaction to the pathogen, since the bacteria has rarely been isolated from lesions [4, 30].
The treatment for MIRM has not been established through randomized controlled trials or other forms of rigorous study. Antibiotics directed against M pneumoniae are most commonly used, and some patients receive corticosteroids or less commonly intravenous immunoglobulin as an adjunct [3, 19]. Cyclosporine has also been used for MIRM in a 3-patient case series with all patients quickly improving, although randomized controlled trials or larger studies are needed to further support efficacy [14]. Comprehensive supportive care is essential. Ophthalmic manifestations are extremely common, and it has been recommended for ophthalmologists to follow these patients similarly to their involvement in supporting patients with SJS/TEN [10].
CONCLUSIONS
Mycoplasma pneumoniae-induced rash and mucositis and/or RIME represent an often misdiagnosed clinical entity that is distinct from SJS/TEN. Future systematic studies are needed to elucidate the best treatment for this condition. Significant complications may result, although overall prognosis is excellent especially when compared with SJS/TEN.
Acknowledgments
We thank the patient for granting us permission to publish this case report to help future patients with Mycoplasma pneumoniae-induced rash and mucositis.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev 2017; 30:747–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol 2016; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015; 72:239–45. [DOI] [PubMed] [Google Scholar]
- 4. Meyer Sauteur PM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to Mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol 2020; 156:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schalock PC, Dinulos JG. Mycoplasma pneumoniae-induced Stevens-Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol 2005; 52:312–5. [DOI] [PubMed] [Google Scholar]
- 6. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993; 129:92–6. [PubMed] [Google Scholar]
- 7. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on erythema multiforme. Clin Rev Allergy Immunol 2018; 54:177–84. [DOI] [PubMed] [Google Scholar]
- 8. Li K, Haber RM. Stevens-Johnson syndrome without skin lesions (Fuchs syndrome): a literature review of adult cases with Mycoplasma cause. Arch Dermatol 2012; 148:963–4. [DOI] [PubMed] [Google Scholar]
- 9. Tay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol 1996; 35:757–60. [DOI] [PubMed] [Google Scholar]
- 10. Shah PR, Williams AM, Pihlblad MS, Nischal KK. Ophthalmic manifestations of mycoplasma-induced rash and mucositis. Cornea 2019; 38:1305–8. [DOI] [PubMed] [Google Scholar]
- 11. Mazori DR, Nagarajan S, Glick SA. Recurrent reactive infectious mucocutaneous eruption (RIME): insights from a child with three episodes. Pediatr Dermatol 2020; 37:545–7. [DOI] [PubMed] [Google Scholar]
- 12. Goyal A, Hook K. Two pediatric cases of influenza B-induced rash and mucositis: Stevens-Johnson syndrome or expansion of the Mycoplasma pneumoniae-induced rash with mucositis (MIRM) spectrum? Pediatr Dermatol 2019; 36:929–31. [DOI] [PubMed] [Google Scholar]
- 13. Chao K, Balin S, Sorenson E, Worswick S. Mycoplasma -induced pustulosis with perifollicular involvement. Dermatol Online J 2018; 24:13030. [PubMed] [Google Scholar]
- 14. Li HO, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg 2019; 23:608–12. [DOI] [PubMed] [Google Scholar]
- 15. Poddighe D, Bruni P. Mycoplasma pneumoniae-induced rash and mucositis (MIRM): an unusual mild skin rash associated with severe mucosal involvement. BMJ Case Rep 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtiss P, Melnick L, Sicco KL, Liebman TN. Mycoplasma pneumoniae, more than a lung disease. Dermatol Online J 2018; 24:13030. [PubMed] [Google Scholar]
- 17. Santos RP, Silva M, Vieira AP, Brito C. Mycoplasma pneumoniae-induced rash and mucositis: a recently described entity. BMJ Case Rep 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song H, Huang JT, Tan JK. Mycoplasma-induced rash and mucositis in a father and son: a case report. Pediatr Infect Dis J 2018; 37:e205–6. [DOI] [PubMed] [Google Scholar]
- 19. Zão I, Ribeiro F, Rocha V, et al. Mycoplasma pneumoniae-associated mucositis: a recently described entity. Eur J Case Rep Intern Med 2018; 5:000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brazel D, Kulp B, Bautista G, Bonwit A. Rash and mucositis associated with Mycoplasma pneumoniae and Chlamydophila pneumoniae: a recurrence of MIRM? J Pediatric Infect Dis Soc 2020. [DOI] [PubMed] [Google Scholar]
- 21. Kheiri B, Alhesan NA, Madala S, et al. Mycoplasma pneumoniae-associated Fuchs syndrome. Clin Case Rep 2018; 6:434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowling M, Schmutzler T, Glick S. Mycoplasma pneumoniae-induced mucositis without rash in an 11-year-old boy. Clin Case Rep 2018; 6:551–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhury S. Mycoplasma pneumoniae-induced rash and mucositis is a distinct entity that needs more recognition. J Paediatr Child Health 2020; 56:645–6. [DOI] [PubMed] [Google Scholar]
- 24. Sandhu R, Mareddy C, Itskowitz M, et al. Mycoplasma-induced rash and mucositis in a young patient with red eyes, oral mucositis, and targetoid cutaneous vesicles. Lancet Infect Dis 2017; 17:562. [DOI] [PubMed] [Google Scholar]
- 25. Sekula P, Dunant A, Mockenhaupt M, et al. ; RegiSCAR study group Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013; 133:1197–204. [DOI] [PubMed] [Google Scholar]
- 26. Ramien ML, Bruckner AL. Mucocutaneous eruptions in acutely Ill pediatric patients-think of mycoplasma pneumoniae (and other infections) first. JAMA Dermatol 2020; 156:124–5. [DOI] [PubMed] [Google Scholar]
- 27. Canavan TN, Mathes EF, Frieden IJ, Shinkai K. Reply to: “Diagnosing Mycoplasma pneumoniae-induced rash and mucositis (MIRM) in the emergency room”. J Am Acad Dermatol 2015; 73:e69. [DOI] [PubMed] [Google Scholar]
- 28. Norton SA. Diagnosing Mycoplasma pneumoniae-induced rash and mucositis (MIRM) in the emergency room. J Am Acad Dermatol 2015; 73:e67. [DOI] [PubMed] [Google Scholar]
- 29. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to Chlamydia pneumoniae infection: expanding the Mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017; 34:465–72. [DOI] [PubMed] [Google Scholar]
- 30. Amode R, Ingen-Housz-Oro S, Ortonne N, et al. Clinical and histologic features of Mycoplasma pneumoniae-related erythema multiforme: a single-center series of 33 cases compared with 100 cases induced by other causes. J Am Acad Dermatol 2018; 79:110–7. [DOI] [PubMed] [Google Scholar]