Abstract
Post-traumatic stress disorder (PTSD) is a psychiatric illness that results in an increased risk for a variety of inflammatory diseases. The exact etiology of this increased risk is unknown, and thus several animal models have been developed to investigate the neuroimmune interactions of PTSD. Repeated social defeat stress (RSDS) is an established preclinical model of psychological trauma that recapitulates certain behavioral and inflammatory aspects of human PTSD. Furthermore, RSDS has been utilized to subgroup animals into susceptible and resilient populations based on one specific behavioral phenotype (i.e., social interaction). Herein, we conducted an extensive investigation of circulating inflammatory proteins after RSDS and found significant elevations in various cytokines and chemokines after exposure to RSDS. When categorizing animals into either susceptible or resilient populations based on social interaction, we found no inflammatory or other behavioral differences between these subgroups. Furthermore, correlative analyses found no significant correlation between social interaction parameters and inflammation. In contrast, parameters from the elevated zero maze (EZM) demonstrated significant associations and clustering to five circulating cytokines. When animals were subdivided into susceptible and resilient populations solely based upon combined EZM performance, significant inflammatory differences were evident between these groups. Strikingly, these circulating inflammatory proteins displayed a stronger predictive ability of EZM performance compared to social interaction test performance. These findings provide new insights into inflammatory markers associated with RSDS, and the utility of EZM to effectively group RSDS-exposed mice into populations with differential levels of peripheral inflammation.
Keywords: Post-traumatic stress disorder, PTSD, Immune, Cytokine, Plasma, Social interaction, Anxiety, Susceptible, Resilient, Model
1. Introduction
Post-traumatic stress disorder (PTSD) is a devastating psychiatric illness characterized by exposure to a traumatic event, followed by flashbacks, avoidance, affective changes, and hyperarousal (American Psychiatric Association, 2013). Interestingly, only about 20% of those that experience a traumatic event develop clinical PTSD, which demonstrates trauma exposure is necessary, but not sufficient alone, to cause PTSD (Frans and Rimmö, 2005). Given this, there has been a continued interest in determining which psychological or biological factors could determine susceptibility or resilience to PTSD (Yehuda, 1999; Nasca, 2019). Understanding that patients with PTSD notably face an increased risk for a number of inflammation-driven diseases (Bisson, 2019; von Känel, 2007; Mikuls, 2013; O’Donovan, 2015; Edmondson and von Känel, 2017), investigations into the inflammatory contributions to PTSD have recently garnered significant attention (reviewed exceptionally by Sumner et al.) (Sumner et al., 2019).
In attempts to elucidate the pathophysiology of PTSD, many preclinical rodent models have emerged (Aspesi and Pinna, 2019; Deslauriers et al., 2018). Repeated social defeat stress (RSDS) is a well- characterized murine model that relies upon the aggressive and territorial nature of retired breeder CD-1 mice (Golden et al., 2011), while successfully recapitulating several behavioral and inflammatory aspects of PTSD (Aspesi and Pinna, 2019; Deslauriers et al., 2018). Furthermore, this model has been reported to produce both susceptible and resilient populations as defined by a single parameter on the social interaction behavioral test (i.e., social interaction ratio < 1.0 or ≥1.0, respectively) (Golden et al., 2011; Krishnan, 2007).
Recent work from our group utilized the model of RSDS to examine the autonomic and redox profiles of T-lymphocytes after RSDS exposure (Moshfegh et al., 2019). Interestingly, while we demonstrated significant and novel changes to T-lymphocyte-driven inflammation, these did not correlate well with behavior assessed by the social interaction test, despite individually showing differences between RSDS and control cohorts (Moshfegh et al., 2019). With this observation, we hypothesized that peripheral inflammation and social behavior are not tightly coupled following RSDS exposure.
In the present study, we sought to identify links between two behavioral tests to assess social and anxiety-like behavior (i.e., social interaction test and elevated zero maze, respectively) and peripheral inflammation using a large-scale assessment of circulating plasma inflammatory proteins following RSDS. In doing so across a large cohort of animals, we found little data to support any inflammatory differences between susceptible and resilient groups as determined by the social interaction ratio. However, we identified significant associations between select cytokines and the elevated zero maze (EZM) behavioral parameters. Sub-grouping animals into susceptible and resilient populations by an EZM-based definition, we found significant differences in circulating inflammatory proteins between the aforementioned EZM populations. Moreover, these circulating cytokines better predicted EZM susceptibility than social interaction susceptibility.
2. Methods
2.1. Mice
RSDS was performed as previously described (Moshfegh et al., 2019). In brief, control and RSDS animals were 8–12 week-old wild- type male mice of a C57BL/6J background (Jackson Laboratory #000664, Bar Harbor, ME, USA). Seventy-five total animals were used in this study. Standardized RSDS paradigms canonically utilize male mice only (Golden et al., 2011), thus biological sex as a variable was not examined herein. All experimental mice were bred in-house and group- housed (≤5 mice/cage) prior to stress induction to attenuate possible confounding physical, psychological, and social stressors. Aggressive mice used for RSDS induction were 4–8 month-old retired breeder male mice of a CD-1 background (Charles River #022, Wilmington, MA, USA). All mice were caged with corncob bedding, paper nesting material, and given access to standard chow (Teklad Laboratory Diet #7012, Harlan Laboratories, Madison, WI, USA) and water ad libitum. Experimental mice were sacrificed by intraperitoneal injection of pentobarbital (150 mg/kg, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA). Daily RSDS and terminal euthanasia occurred between 0700 and 0900 to eliminate the known effects of circadian rhythm on inflammation. Experimental mice were randomized to control or RSDS cohorts, and all efforts were made to blind experimenters during biological assays and data analysis. All procedures were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
2.2. RSDS
Stress induction was accomplished through a modified RSDS paradigm (Moshfegh et al., 2019). Briefly, retired breeder CD-1 mice were first pre-screened and selected for aggressive behavior (Golden et al., 2011). After singly inhabiting cages for 3 days, experimental mice were introduced into these cages for physical confrontation for 5 min. For the remaining 24 h, experimental and aggressor mice were co-housed but separated by a transparent barrier. The processes was repeated daily with a new CD-1 aggressive mouse for 10 consecutive days. Control mice were similarly pair-housed sans daily social defeat sessions. RSDS was completed in a separate procedure room away from general animal housing so as not to affect control animals by auditory, visual, or olfactory stimuli. All experimental mice were behavior tested on day 11. On day 12, all mice were sacrificed for biological analysis. While animals with visual wounding (> 1cm) (Golden et al., 2011) or lameness due to stress induction are excluded from further study, none of the 75 animals utilized herein met this threshold for exclusion.
2.3. Behavioral testing
All behavior testing was conducted as previously described (Moshfegh et al., 2019). Following RSDS or control exposure for 10 days, all animals were behaviorally assessed on the social interaction test and subsequently the EZM on day 11 between the hours of 0700 and 1400, with consistent timing between each behavior test for all mice.
For social interaction testing, an open field (40 × 40 cm, Noldus Information Technology, Leesburg, VA, USA) was outfitted with a transparent enclosure with mesh caging (6.5 × 10 cm). All mice were first tested in the open field with an empty enclosure, and then subsequently tested with a novel CD-1 mouse present within the enclosure. A ratio of the time spent in proximity to the enclosure (interaction zone) or distal corners of the maze in the presence versus absence of a novel CD-1 mouse defines the social interaction ratio and corner zone ratio, respectively (Golden et al., 2011; Krishnan, 2007). Each run lasted 2.5 min, and all sessions were recorded and digitally analyzed by Noldus Ethovision XT 13 software (Leesburg, VA, USA). Susceptible and resilient populations were defined by social interaction ratio < 1 or ≥1, respectively.
For EZM testing, all experimental mice were introduced into a circular elevated maze (50 cm diameter, 5 cm track width, Noldus Information Technology, Leesburg, VA, USA) with 50% enclosed (20 cm wall height) and 50% open arenas. Mice were allowed to freely explore the novel environment for 5 min. All sessions recorded and digitally analyzed by Noldus Ethovision XT 13 software (Leesburg, VA, USA).
2.4. Plasma inflammatory protein measurement
Blood was obtained by cardiac puncture immediately after sacrifice using ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Plasma was separated by centrifugation, and stored at −80 °C until use. Plasma inflammatory protein measurements were obtained by utilizing the Meso Scale Discovery V-Plex Mouse Cytokine 29-plex kit (#K15267D, Rockville, MD, USA). Meso Scale Discovery V-plex plates are tightly validated by the manufacturer to avoid antigenic cross-reactivity. The following inflammatory proteins were measured: IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-15, IL-16, IL-17A, IL-17C, IL-17F, IL-17A/F, IL-17E/IL-25, IL-21, IL-22, IL-23, IL-27p28/IL-30, IL-31, IL-33, CXCL10, CXCL1, CCL2, CCL3, CXCL2, CCL20, TNF-α. All experiments were conducted per the manufacturer’s instructions, with samples run on Meso Scale Discovery Quickplex SQ 120 and analyzed using the Mesoscale Discovery Workbench software.
2.5. Statistics
A total of 75 animals (35 control, 40 RSDS) were utilized for the following studies. Associations between behavioral assessments and inflammatory proteins were assessed by nonparametric Spearman correlation with two-tailed confidence intervals. Familywise error rate for the correlation matrix was conservatively corrected with the Bonferroni procedure for 30 inferential hypotheses (26 detectable inflammatory proteins and 4 behavioral outputs), with statistical significance thus established at p < 0.0017 (α/330 hypotheses tested). Receiver-operating characteristics (ROC) curves were assessed for classification ability by the area under the curve (AUC), with predicted probability for each subject set at 0.5. Goodness-of-fit was assessed by likelihood ratio test.
For inflammatory protein measurements, individual data are presented, with summary data displayed as mean ± SEM. Each data set was assessed for normality utilizing the Shapiro-Wilk test, followed by appropriate inferential hypothesis testing. For all inflammatory protein and behavioral measurements, Mann-Whitney U test was used to assess differences between control and RSDS. Susceptible and resilient populations by social interaction are subgroups of RSDS, and thus statistical tests were only conducted between the two subgroups. Susceptible and resilient populations by EZM includes both control and RSDS populations, and thus additionally only warrants comparative subgroup comparisons. All statistical analyses were completed in GraphPad Prism 8.4.2 (San Diego, CA, USA).
3. Results
3.1. Susceptibility and resilience by social interaction test does not differentiate inflammatory outcomes
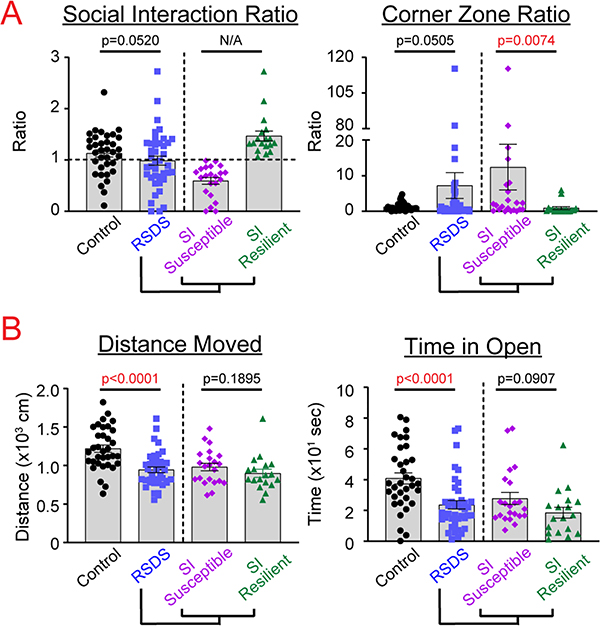
After RSDS, all mice were examined for behavioral changes by social interaction and EZM tests. Social interaction and corner zone ratios were highly variable, but showed near-significant differences between control and RSDS, respectively (p = 0.0520, p = 0.0505; Fig. 1). EZM parameters—distance moved and time in the open arm—showed significant and robust differences after RSDS (p < 0.0001, p < 0.0001; Fig. 1). When RSDS animals were subdivided into susceptible and resilient groups by social interaction ratio (susceptible = SI ratio < 1; resilient = SI ratio ≥ 1), there was a statistically significant difference between these subgroups in the corner zone ratio of the social interaction test (p = 0.0074), but no differences observed in distance moved or time in open on the EZM (p = 0.1895, p = 0.0907, respectively; Fig. 1).
Fig. 1.
Susceptible and resilient subgrouping as determined by social interaction ratio does not reflect additional behavioral phenotypes. Mice were run through the 10-day RSDS paradigm followed by behavioral testing, including A. social interaction test and B. elevated zero maze. RSDS mice were categorized into susceptible or resilient subgroups by a social interaction (SI) ratio < 1.0 or ≥1.0, respectively (A, left). N = 35 control, 40 RSDS, with susceptible and resilient as subgroups of RSDS. Statistical significance by parametric Student’s t- test or nonparametric Mann-Whitney U test where appropriate.
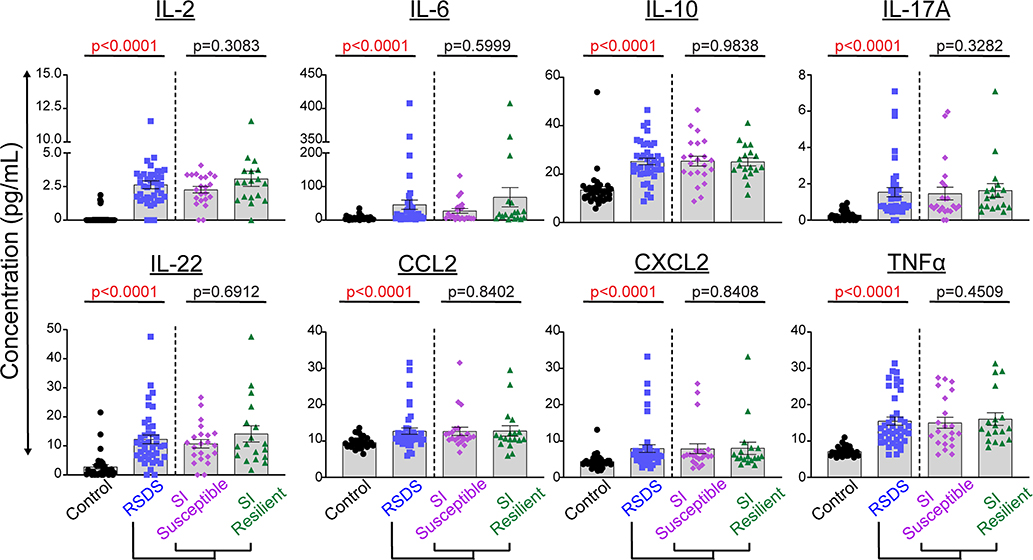
With peripheral inflammation as our primary biological outcome, we then investigated shifts in circulating inflammatory protein levels following RSDS. Of the 29 inflammatory proteins analyzed, 26 were found to have at least several mice consistently above the lower limit of detection. Furthermore, 8 of these proteins showed significant differences between control and RSDS animals after correction for multiple analyses (Fig. 2). Next, we further grouped the inflammatory proteins from RSDS animals into susceptible and resilient groups as determined by the social interaction ratio. However, no significant differences in peripheral inflammation were detected between susceptible and resilient groups (Fig. 2). Overall, RSDS results in complex and robust changes to peripheral inflammation. However, these changes do not group into susceptible or resilient populations when separated by social interaction ratio.
Fig. 2.
Susceptible and resilient subgrouping as determined by social interaction ratio does not reflect peripheral inflammation. Mice were run through the 10-day RSDS paradigm followed by behavioral testing and plasma extraction. RSDS mice were categorized into susceptible or resilient subgroups by a social interaction (SI) ratio < 1.0 or ≥1.0, respectively. Circulating levels of 29 cytokines were assessed by Meso Scale Discovery multiplex analysis; 26 were above the limit of detection, and 8 showed significant differences between control and RSDS animals. N = 35 control, 40 RSDS. Statistical significance by nonparametric Mann-Whitney U test.
3.2. Selected cytokines and EZM parameters demonstrate significant associations and hierarchical clustering
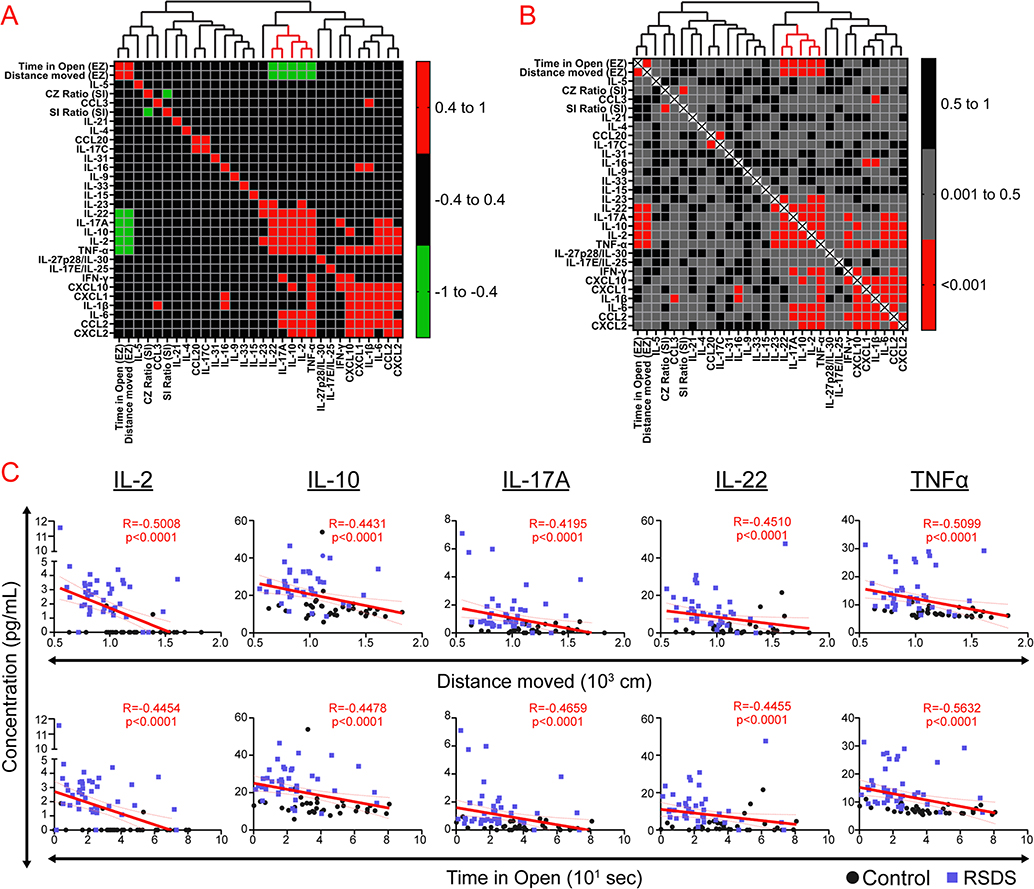
In order to conduct a more comprehensive examination of the associations between the social interaction test, EZM, and circulating inflammation, heat maps were generated which represent correlation coefficients (Fig. 3A) and p-values (Fig. 3B) between all detectable inflammatory proteins and the aforementioned behavioral parameters across all animals (controls and RSDS combined). Hierarchical clustering additionally provided qualitative associations between discrete clusters of variables. We found that social interaction test parameters (i.e., social interaction ratio and corner zone ratio) did not significantly correlate with any measured inflammatory proteins (Fig. 3A–B). However, EZM outputs (i.e., distance moved and time in open) associated significantly with 5 inflammatory proteins (Fig. 3C). Overall, social interaction test parameters do not appear to significantly associate with any measured variable of circulating inflammation, while EZM correlates significantly with 5 tightly clustered cytokines.
Fig. 3.
Selected cytokines and EZM parameters demonstrate significant associations and hierarchical clustering. Mice were run through the 10-day RSDS paradigm followed by behavioral testing, plasma extraction, and inflammatory assessment by Meso Scale Discovery multiplex analysis. Correlation matrices and cluster analyses were completed for all behavioral and detectable circulating inflammatory proteins within each animal. A Heat map representation of correlation matrix of all variables with hierarchical clustering above. Colors represent Spearman R coefficients (two-tailed test) with accompanying legend. B. Heat map representation of correlation matrix of all variables with hierarchical clustering above. Colors represent p-values by Spearman correlation (two-tailed test) with accompanying legend. C. Individual correlation plots of select significant cytokines and elevated zero maze parameters. N = 35 control, 40 RSDS used for all analyses. Significance by Spearman correlation (two-tailed test).
3.3. EZM-defined susceptible and resilient populations show significant differences in peripheral inflammation
In order to further assess the association between EZM and circulating inflammation, we developed a method for subgrouping all animals (controls and RSDS combined) by performance on the EZM. In order to generate a single metric which utilizes both distance moved and time spent in the open arm, each variable was normalized to the median (due to the non-gaussian distribution) seen across both control and RSDS animals. These normalized values were then summed. Animals with a sum greater than the overall median (median EZM = 2.12) were considered “resilient”, while those below were deemed “susceptible”. This can be represented mathematically as:
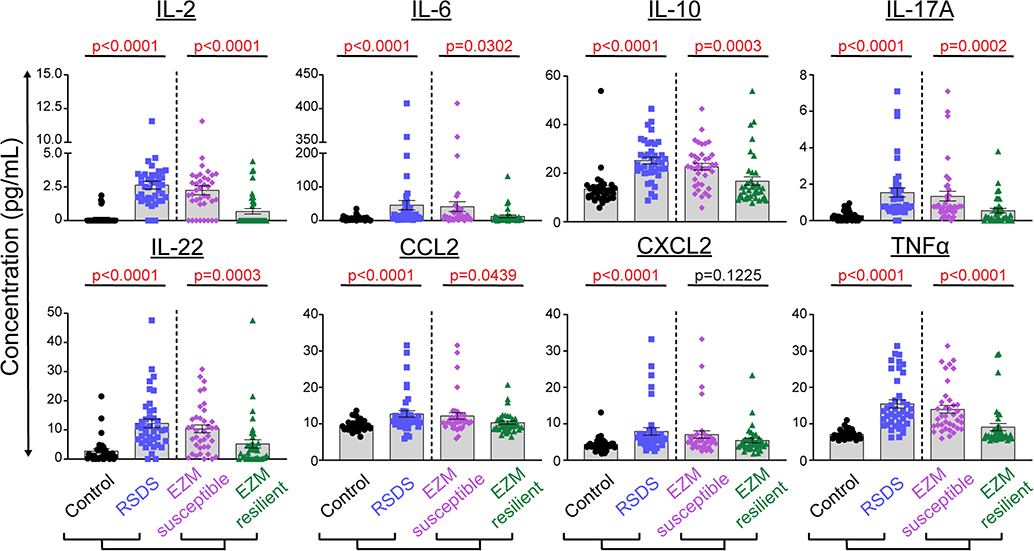
When peripheral inflammation was assessed based upon this EZM- defined susceptibility or resilience, 7 of the 8 original significant cytokines between control and RSDS demonstrated significant differences between EZM resilient and susceptible (Fig. 4): IL-2 (p < 0.0001), IL-10 (p < 0.0003), IL-17A (p < 0.0002), IL-22 (p = 0.0003), TNFα (p < 0.0001), IL-6 (p = 0.0302), and CCL2 (p = 0.0439).
Fig. 4.
Susceptible and resilient subgrouping as determined by elevated zero maze reflects peripheral inflammatory alterations. Mice were run through the 10-day RSDS paradigm followed by behavioral testing and plasma extraction. Both RSDS and control mice were categorized into susceptible or resilient groups by elevated zero maze (EZM; normalized distance moved + normlaized time in open) < median, or > median, respectively. Circulating levels of 29 cytokines were assessed by Meso Scale Discovery multiplex analysis; 26 were above the limit of detection, and 8 showed significant differences between control and RSDS animals. N = 35 control, 40 RSDS. Statistical significance by parametric Student’s t-test or nonparametric Mann-Whitney U test.
3.4. Selected circulating cytokines predict EZM-defined susceptibility but not social interaction test susceptibility
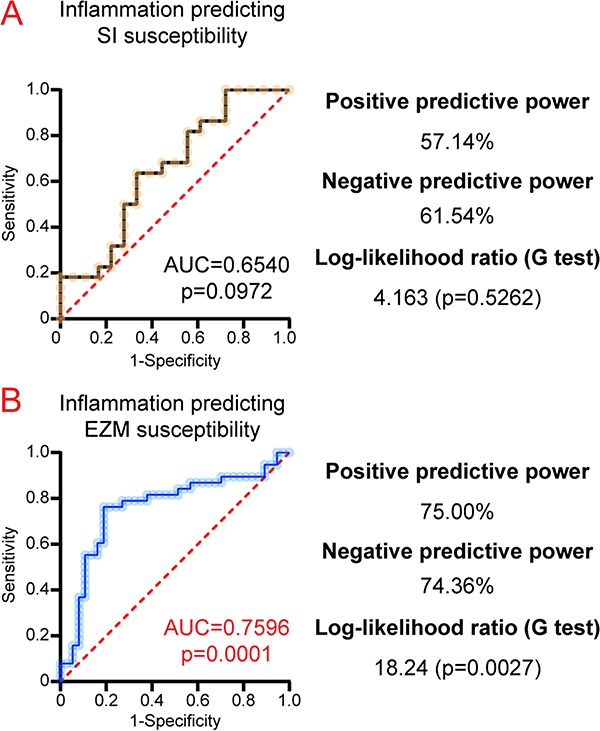
Next, multiple logistic regression was utilized to assess the predictive ability of inflammatory parameters in regard to behavioral outputs. From this regression model, ROC curves were generated. Social interaction-defined susceptibility was not significantly predicted by a regression model informed by circulating cytokines (IL-2, IL-10, IL-17A, IL-22, and TNFα; AUC = 0.6540, p = 0.0972; Fig. 5A). However, the aforementioned 5 circulating cytokines were significantly predictive of EZM-defined susceptibility (AUC = 0.7596, p = 0.0001; Fig. 5B). Overall, we demonstrate that peripheral inflammation is a better predictor of EZM susceptibility, while SI susceptibility is not predicted by inflammation.
Fig. 5.
A specific subset of peripheral inflammatory proteins are more predictive of EZM-determined susceptibility than SI-determined susceptibility. Mice were run through the 10-day RSDS paradigm followed by behavioral testing, plasma extraction, and inflammation assessment by Meso Scale Discovery multiplex analysis. A. Receiver-operating characteristics (ROC) curves were generated for select inflammatory parameters (IL-2, IL-10, IL-17A, IL-22, and TNFα) predicting social interaction (SI) susceptibility. B. ROC curves generated for select inflammatory parameters (IL-2, IL-10, IL-17A, IL-22, and TNFα together) predicting elevated zero maze (EZM) susceptibility. Prediction ability was calculated by area under curve, with p-value calculated by a two-tailed test with predicted probability of each subject set at 0.5. Goodness-of-fit additionally assessed by Log-likelihood ratio.
4. Discussion
In the current study, we confirm that RSDS results in specific behavioral and peripheral inflammatory alterations. When examining 29 inflammatory proteins in circulation, we found 8 that were differentially extant in the plasma of RSDS mice. However, grouping animals into susceptible and resilient subgroups by social interaction ratio did not meaningfully divide RSDS animals by any measured peripheral inflammatory changes. Furthermore, when categorizing RSDS into susceptible and resilient groups indexed by the social interaction ratio, we did not observe similar grouping by EZM outputs. Indeed, in the original categorization of susceptible and resilient by social interaction ratio by Krishnan et al., the authors also conceded this grouping did not reflect anxiety-like behavior, locomotor activity, stress-induced polydipsia, or despair behavior after RSDS (Krishnan, 2007). In relation to social interaction ratio and peripheral inflammation, previous work by Hodes et al. found significant associations between susceptibility and several circulating cytokines, with a potential causal role for IL-6 in social interaction behavior (Hodes, 2014). A major experimental difference between our two studies lies in the temporal relationship between RSDS and measurement of inflammation. Hodes et al. measured cytokines 20 min or 48 h after the first social defeat stress bout, whereas the inflammatory data herein was performed after 10 complete days of RSDS. While we recapitulated their observed differences in IL-6, IL-10, and CCL2 levels between control and RSDS, we did not find these to significantly relate to social interaction susceptibility. In our relatively large sample size (n = 75), we found no significant differences in inflammation when animals were grouped by the social interaction ratio. Furthermore, we demonstrated no significant relationship between social interaction or corner zone ratio and any measured circulating inflammatory proteins, dispelling the likelihood of any potential causal association. While the categorization of mice by the social interaction ratio has indeed uncovered biological differences between these social interaction ratio-derived populations, our findings suggest this grouping is not all-encompassing, and may be limited to only a specific behavioral manifestation that may or may not be related to human disease.
Importantly, in analyzing correlates between behavioral outcomes and inflammation, our approach combined both control and RSDS animals, with all analyses including both behavioral and inflammatory data points from every animal within this study. This approach provides a methodologically-different assessment of the interaction between behavior and peripheral inflammation—especially as it relates to the spectrum of behavior. These data support the sentiment of recommendations by the National Institute of Mental Health Research Domain Criteria (RDoC) (Sanislow, 2020), which seeks to represent all behavioral characterizations as continuous variables across multiple domains to allow for more definitive (and complex) analyses (Schmidt and Vermetten, 2018). In doing so, we were able to find significant associations between EZM parameters and select circulating cytokines (IL-2, IL-10, IL-17A, IL-22, and TNFα). Furthermore, when we subdivided animals by a metric that combined two outputs of the EZM (i.e., distance moved and time in open arm) we found significant differences in these newly defined EZ susceptible and resilient populations, as well as the ability of inflammation to accurately predict EZ susceptibility or resilience. Notably, there is prior significant work demonstrating a causal relationship between circulating inflammatory proteins differentially detected within our paradigm, such as CCL2, and anxiety-like behavior (Wohleb et al., 2013). This data prompts further query into the potential usage of the EZM to divide subpopulations of susceptible and resilient from RSDS, especially in showing differences in peripheral inflammation.
Based on our findings, an important aspect for discussion is how to appropriately segment traumatized animals into susceptible and resilient populations. While the social interaction ratio provides a convenient methodology for separating RSDS mice, we have presented data herein to shown that it does not effectively mirror changes in peripheral inflammation, while EZM behavior appears to be more strongly associated with this parameter. Additionally, recent work has shown that the social interaction test itself appears to involve conditioned learning based on prior exposures to an aggressive white CD-1 mice, versus true tests of antisocial behavior (Hodes, 2014). Importantly, RSDS has been shown to generalize to unfamiliar animals during the social interaction test, further complicating this interaction (Duque-Wilckens, 2018). Overall, these data should serve as unembellished reminders of the inherent complexity to which psychological and physiological factors might guide the development of susceptibility or resilience—even within our more reductionist preclinical models. The development of multifaceted approaches to assess the degree of psychological trauma will be paramount in further elucidation of the pathophysiology of this disease, as well as assessment of the efficacy of potential therapeutics.
The present study and its implications are of course not without limitations. The most crucial in its external validity is the lack of examination of sex as a biological variable. Due to the introduction of aggressive retired-breeder male mice in traditional RSDS, it cannot be used to induce psychological trauma similarly in female mice. There have been recent reports of modified female social defeat stress (Harris, 2018; Newman, 2019), which—while potentially effective insofar as stress induction—vary significantly enough in approach, intensity, and duration to serve as serious confounding variables reducing internal validity. For this reason, this was not addressed in the present study. Future examination should include these behavioral and inflammatory paradigms in the context of newly developed models of female social defeat stress.
Furthermore, while RSDS has shown robust and consistent peripheral inflammatory changes, one potentially problematic aspect of the model is the prospect of wounding. While we take care to exclude all animals with significant wounds or lameness, as previously discussed, this remains a possible confounder in RSDS and other models that incorporate physical stress. We estimate at least 75% of all animals in this study showed no visible signs of wounding, yet virtually all animals displayed a peripheral inflammatory response. These data are suggestive that wounding does not fully explain the inflammatory changes of these mice, but at this time cannot be completely ruled out. Importantly, previous research in preclinical models of PTSD has examined how witnessed social defeat stress can also result in inflammatory changes, which would definitionally be void of potential wounding (Hodes, 2014; Finnell, 2017). Additionally, it should be noted that psychological trauma in humans very often is accompanied by physical trauma. This is especially vital in the forms of trauma that are most likely to result in PTSD—rape, physical assault, and serious injury— and that may also directly impact the immune system (Kilpatrick, 2013).
In finding that peripheral inflammation in RSDS was most tightly- linked with the EZM—a test of anxiety-like behavior—it is worth noting the DSM-IV originally characterized PTSD under the umbrella of anxiety disorders (American Psychiatric Association, 2000). There is a breadth of clinical literature demonstrating differences in peripheral inflammation in patients with PTSD as well as other anxiety disorders (O’Donovan, 2015; Neigh and Ali, 2016; Boscarino, 2004; Kim et al., 2020; Renna et al., 2018). Importantly, many of the cytokines we found to be differentially elevated in control versus RSDS have also been shown to be elevated in human plasma from patients with PTSD, such as IL-2, IL-6, IL-17, and TNFα (summarized well by Wang et al) (Wang et al., 2017). While preclinical, our results provide potential new avenues for investigation into understanding how selected circulating cytokines could be involved mechanistically in the pathogenesis of disease or approached quantitatively as potential variables within a more complex biomarker panel.
In summary, this current study provides novel findings that suggest a more nuanced approach to determination of susceptibility and resilience only by use of the social interaction ratio. Further, we present a novel method of quantifying the results of EZM performance into newly defined susceptible and resilient subgroups. Moreover, this EZM-based definition strongly correlates with a select peripheral inflammatory panel, which was able to effectively predict EZM susceptibility. Together, these findings call for a more in-depth and mechanistic analysis of psychological trauma in the RSDS model, as well as translational examination of peripheral inflammation in trauma-exposed human individuals.
5. Ethics
This study was carried out in accordance with the recommendations of the University of Nebraska Medical Center Institutional Animal Care and Use Committee. The protocol was approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Acknowledgements
The authors would like to thank Dr. Christopher Wichman for their technical assistance and expertise in generating the heat maps and hierarchical cluster analyses. This work was supported by the National Institutes of Health (NIH) R00HL123471, NIH T32NS105594, and American Heart Association (AHA) 20PRE35080059.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- American Psychiatric Association. In: Diagnostic and statistical manual of mental disorders (4th edition) (American Psychiatric Publ, Washington, DC [u.a.], 2000). [Google Scholar]
- American Psychiatric Association. In: Diagnostic and Statistical Manual of Mental Disorders (ed American Psychiatric Association; ), Arlington, VA, USA, 2013). [Google Scholar]
- Aspesi D, Pinna G, 2019. Animal models of post-traumatic stress disorder and novel treatment targets. Behav. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Bisson JI, 2019. Stress related disorders and physical health. BMJ 367, l6036. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, 2004. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann. N. Y. Acad. Sci. 1032, 141–153. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, Risbrough VB, 2018. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatry 83, 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, et al. , 2018. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female california mice. Biol. Psychiatry 1969 (83), 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, von Känel R, 2017. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 4, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, et al. , 2017. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS ONE 12, e0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans O, Rimmö P, Aberg L, Fredrikson M, 2005. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr. Scand. 111, 291–299. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, et al. , 2018. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43, 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, et al. , 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U. S. A. 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick, et al. , 2013. National estimates of exposure to traumatic events and ptsd prevalence using dsm-iv and dsm-5 criteria. J. Trauma. Stress 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TD, Lee S, Yoon S, 2020. Inflammation in post-traumatic stress disorder (PTSD): a review of potential correlates of PTSD with a neurological perspective. Antioxidants 9, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, et al. , 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Mikuls TR, et al. , 2013. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 65, 227–234. [DOI] [PubMed] [Google Scholar]
- Moshfegh CM, Elkhatib SK, Collins CW, Kohl AJ, Case AJ, 2019. Autonomic and redox imbalance correlates with T-lymphocyte inflammation in a model of chronic social defeat stress. Front. Behav. Neurosci. 13, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, et al. , 2019. Multidimensional Predictors of Susceptibility and Resilience to Social Defeat Stress. Biol. Psychiatry 86, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Ali FF, 2016. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr. Opin. Pharmacol. 29, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, et al. , 2019. Fighting females: Neural and behavioral consequences of social defeat stress in female mice. Biol. Psychiatry 86 (9), 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, et al. , 2015. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol. Psychiatry 77, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna ME, O’Toole MS, Spaeth PE, Lekander M, Mennin DS, 2018. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress. Anxiety 35, 1081–1094. [DOI] [PubMed] [Google Scholar]
- Sanislow, et al. , 2020. The national institute of mental health research domain criteria: An alternative framework to guide psychopathology research. In: Geddes JR, Andreasen NC, Goodwin GM (Eds.), The New Oxford Textbook of Psychiatry. Oxford University Press, Oxford, UK: In press. [Google Scholar]
- Schmidt Vermetten, 2018. Integrating nimh research domain criteria (rdoc) into ptsd research. Curr. Top. Behav. Neurosci. 38, 69–91. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD, 2019. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol. Psychiatry 1969 (87), 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Känel R, et al. , 2007. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 41, 744–752. [DOI] [PubMed] [Google Scholar]
- Wang Z, Caughron B, Young MRI, 2017. Posttraumatic stress disorder: an immunological disorder? Front. Psychiatry 8, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF, 2013. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, 1999. Biological factors associated with susceptibility to posttraumatic stress disorder. Canad. J. Psychiatry, Rev. Canad. Psych. 44, 34–39. [DOI] [PubMed] [Google Scholar]