Abstract
Introduction
Severe pneumonia caused by multidrug-resistant Acinetobacter baumannii (MDR-AB) remains a difficult-to-treat infection. Considering the poor lung penetration of most antibiotics, the choice of the better antibiotic regimen is debated.
Methods
We performed a prospective, observational, multicenter study conducted from January 2017 to June 2020. All consecutive hospitalized patients with severe pneumonia due to MDR-AB were included in the study. The primary endpoint of the study was to evaluate risk factors associated with survival or death at 30 days from pneumonia onset. A propensity score for receiving therapy with fosfomycin was added to the model.
Results
During the study period, 180 cases of hospital-acquired pneumonia, including ventilator-associated pneumonia, caused by MDR-AB strains were observed. Cox regression analysis of factors associated with 30-day mortality, after propensity score, showed that septic shock, and secondary bacteremia were associated with death, while a fosfomycin-containing regimen was associated with 30-day survival. Antibiotic combinations with fosfomycin in definitive therapy for 44 patients were: fosfomycin + colistin in 11 (25%) patients followed by fosfomycin + carbapenem + tigecycline in 8 (18.2%), fosfomycin + colistin + tigecycline in 7 (15.9%), fosfomycin + rifampin in 7 (15.9%), fosfomycin + tigecycline in 6 (13.6%), fosfomycin + carbapenem in 3 (6.8%), and fosfomycin + aminoglycoside in 2 (4.5%).
Conclusions
This real-life clinical experience concerning the therapeutic approach to severe pneumonia caused by MDR-AB provides useful suggestions to clinicians, showing the use of different antibiotic regimens with a predominant role for fosfomycin. Further randomized clinical trials are necessary to confirm or exclude these observations.
Keywords: Acinetobacter, Fosfomycin, Multidrug-resistant, Pneumonia, Septic shock
Key Summary Points
| Severe pneumonia caused by multidrug-resistant Acinetobacter baumannii (MDR-AB) remains a difficult-to-treat infection. |
| Considering the poor lung penetration of most antibiotics, the choice of the better antibiotic regimen is debated. |
| During the study period, 180 cases of hospital-acquired pneumonia, including ventilator-associated pneumonia, caused by MDR-AB strains were observed. |
| A fosfomycin-containing regimen was associated with 30-day survival. |
| This real-life clinical experience provides useful suggestions to clinicians, showing the use of different antibiotic regimens with a predominant role for fosfomycin. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13056014.
Introduction
In recent years, severe pneumonia due to multidrug-resistant (MDR) gram-negative bacteria such as Acinetobacter baumannii (AB) has been increasingly observed among hospitalized patients admitted to the intensive care unit (ICU), surgical and medical wards [1, 2]. MDR-AB has been listed as one of the top priority pathogens by the World Health Organization [3, 4]; specifically, in Italy an increased incidence of MDR-AB was observed in the last years [5].
Acinetobacter baumannii bacteria are usually resistant to carbapenems and to β-lactams, aminoglycosides, rifampin, and fluoroquinolones, and there are limited therapeutic options, often resulting in inappropriate therapy and a subsequent negative impact on outcome. Early diagnosis and adequate administration of antimicrobials are essential for the management of critically ill patients with MDR-AB [6], and recent data reported in the literature compared monotherapy with combination therapy [7, 8]. Finally, a mortality rate > 60% has been reported for MDR-AB infections [9], particularly in patients with septic shock [10].
Recently, new agents with microbiologic activity against MDR-AB strains have been developed [11, 12]; however, the use of “old” antibiotics for these difficult-to-treat infections is mandatory, and antimicrobial combinations should be carefully evaluated. Many in vitro studies suggested a possible role for intravenous fosfomycin also for the treatment of MDR-AB [13–15]. Recently, a fosfomycin-containing regimen showed a more beneficial effect on all-cause mortality, with favorable effectiveness in clinical cure and microbiologic eradication [16].
The aim of the present study was to analyze the efficacy of antibiotic regimens and outcome of patients treated for hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), caused by MDR-AB.
Methods
Study Design and Patient Selection
This was a prospective, observational study conducted in Italy: three 300-bed hospitals in Rome and one 1200-bed tertiary hospital in Udine. From January 2017 to June 2020, all consecutive hospitalized patients with pneumonia caused by MDR-AB were included in the study. The inclusion criteria were: (1) age ≥ 18 years; (2) culture positive for MDR-AB; (3) clinical signs and symptoms consistent with pneumonia [17]. Polymicrobial etiology was excluded; only one episode of MDR-AB infection for each patient was reported in the study period. The prospective nature of the study was based on the consecutive enrollment of patients. However, all complete data were afterwards retrospectively extracted, and the Ethics Committee (Policlinico Casilino) waived the need for informed consent. The study was conducted according to the principles stated in the Declaration of Helsinki. Patient data were collected from medical charts and from hospital computerized databases or clinical charts according to a pre-established questionnaire. The following information was reviewed: demographics; clinical and laboratory findings; comorbid conditions; microbiologic data; duration of ICU and hospital stay; any MDR infection during hospitalization; treatment and procedures (e.g. non-invasive ventilation [NIV], mechanical ventilation, continuous renal replacement therapy [CRRT]) carried out during hospitalization and/or in the 30 days prior to infection; class of antibiotics received on admission and/or during admission before a positive culture of a biologic sample was obtained; the Simplified Acute Physiology Score (SAPS II); sequential organ failure assessment (SOFA) at time of infection; anamnestic MDR-AB colonization or during hospitalization; antibiotic regimens used for MDR-AB infection; development of septic shock; 30-day mortality.
Definitions
Infections were defined according to the standard definitions of the European Centers for Disease Control and Prevention (eCDC) [18].
Infection was defined as the presence of at least one positive culture from the lung for MDR-AB in individuals with signs and symptoms consistent with pneumonia [17–19]; concomitant isolation of MDR-AB in other sites such as the blood, urine, skin swabs or biopsies, or abdomen was also recorded. Infection onset was defined as the date of development of signs and symptoms of pneumonia.
HAP was considered pneumonia occurring 48 h or more after admission that did not appear to be incubating at the time of admission. VAP was considered HAP developing > 48 h after endotracheal intubation. The diagnosis of severe pneumonia was based on the Infectious Diseases Society of America/American Thoracic Society consensus guidelines, i.e., one major criterion (invasive mechanical ventilation or septic shock with the need for vasopressors) or three minor criteria (respiratory rate of > 30 breaths/min, partial pressure of arterial oxygen {PaO2]/fraction of inspired oxygen [FiO2] ratio of < 250, multilobar infiltrates, confusion/disorientation, uremia [blood urea nitrogen (BUN) level of > 20 mg/dl], leukopenia [white blood cell (WBC) count of < 4,000 cells/mm3}, thrombocytopenia [platelet count of < 100,000 cells/mm3], hypothermia [core temperature < 36 °C], or hypotension requiring aggressive fluid resuscitation) [20].
Septic shock was defined according to international definitions [21]. The severity of clinical conditions was determined by using SAPS II, and SOFA scores calculated at the time of infection onset. The length of hospital and ICU stay was calculated as the number of days from the date of admission to the date of discharge or death.
Microbiologic Identification
The identification of MDR-AB strains was based accordingly with local laboratory techniques. From positive cultures, gram staining and a rapid identification protocol were adopted. The bacterial pellet obtained directly from positive cultures was used for MALDI-TOF MS (Bruker Daltonics) identification and for molecular analysis. The SensiTitre™ system (Thermo Fisher Scientific) or the Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France) were used for isolate identification and antimicrobial susceptibility testing. Minimum inhibitory concentrations (MICs) were established according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [22].
Antimicrobial Treatment Evaluation
Empiric antibiotic regimens were selected according to clinical judgment by infectious disease specialists and were subsequently modified according to blood culture results. During the study period, the usual antimicrobial dosages, adopted for the most used antibiotics were the following: for colistin, a loading dose of 9 million IU followed by 4.5 million IU every 12 h; for tigecycline, a loading dose of 150–200 mg followed by 100 mg every 12 h; for gentamicin, a dosage of 5 mg/kg every 24 h; for rifampin, a dosage of 10 mg/kg/day; for meropenem, a dosage of 2 g every 8 h or 1.5 g every 6 h; for fosfomycin 12–24 g/day divided every 6–8 h; for ampicillin/sulbactam 3 g every 6 h; for trimethoprim/sulfamethoxazole 15–20 mg/kg/day divided every 6 h; for vancomycin 40 mg/kg/day divided every 12 h.
Depending on the number of drugs used (1 or > 1), treatment regimens were classified as either monotherapy or combination therapy. Initial antibiotic therapy, defined as antimicrobial chemotherapy implemented within 24 h after the onset of infection, was assessed along with definitive antibiotic therapy, defined as antimicrobial treatment based on in vitro MDR-AB isolate susceptibility. Drugs in definitive therapy must have been administered for at least 50% of the total duration of therapy (except for patients who died while on definitive therapy, who were included if they received at least 1 complete day of therapy). Time to initial definitive therapy was the period between the infection onset and initial definitive therapy.
Primary Endpoint and Statistical Analysis
The primary endpoint of the study was to evaluate risk factors associated with survival or death at 30 days from pneumonia onset.
To detect significant differences between groups, we used chi-square test or Fisher exact test for categorical variables, and the two-tailed t test or Mann-Whitney test for continuous variables, when appropriate. In a multivariate analysis of survival, the Cox regression model was tested using a proportional hazards model analysis with backward stepwise selection and p < 0.05 for all variables to determine the effects of all anamnestic, clinical, and therapeutic variables on 30-day survival. A propensity score for receiving therapy with fosfomycin was added to the model. The propensity score was calculated using a nonparsimonious multivariate logistic regression model in which the outcome variable was the treatment with fosfomycin. Kaplan-Meier curves were used to determine survival at 30 days in patients treated with either a fosfomycin-containing regimen or other antibiotic regimens. Survival curves for time-to-event variables, constructed using Kaplan-Meier estimates, were based on all available data and were compared with the use of the log-rank test. Wald confidence intervals and tests for the hazard ratio were computed based on the estimated standard errors. Possible confounding factors and interactions were weighted during analysis. Statistical significance was established at ≤ 0.05. All reported P values are two-tailed. The results obtained were analyzed using a commercially available statistical software package (SPSS, version 20.0; SPSS Inc, Chicago, IL).
Results
During the study period, 180 HAPs, including VAP, caused by MDR-AB strains were observed; 23 patients with polymicrobial etiology were excluded from the final analysis, as reported in Methods. Resistance rates of MDR-AB were the following: colistin 2.2%, gentamicin 88.1%, amikacin 90.2%, tigecycline 51.2%, fosfomycin 31.1% (assessed in 112/180 strains), and meropenem 100%. On these bases, 97.6% of AB strains were considered extensively drug-resistant (XDR) and 2.4% and pandrug-resistant (PDR). Wards of hospitalization at time of infection onset were ICU (79%), medical wards (19.2%), and surgical wards (1.8%). Finally, 122 (67.7%) cases were associated with development of septic shock, and 30-day mortality was reported in 101 (56.1%) patients.
Table 1 shows univariate analysis comparing survivors and non-survivors at 30 days from infection onset. Differences between survivors and non-survivors were reported for septic shock (54.4% vs. 75.2%, p = 0.004), secondary bacteremia (27.8% vs. 82.2%, p < 0.001), and cardiovascular events after infection onset (29.1% vs. 45.5%, p = 0.031).
Table 1.
Univariate analysis comparing survivors and non-survivors at 30 days from infection onset
| Variables | Survivors n = 79 (%) |
Non-survivors n = 101 (%) |
P |
|---|---|---|---|
| Anamnestic factors | |||
| Age, mean ± SD (years) | 62.5 ± 17.8 | 65.8 ± 14.6 | 0.175 |
| Male sex | 55 (69.6) | 67 (66.3) | 0.748 |
| Comorbidities | |||
| Chronic liver disease | 4 (5.1) | 4 (4) | 0.732 |
| Neoplasm | 10 (12.7) | 16 (15.8) | 0.67 |
| Diabetes | 26 (32.9) | 28 (27.7) | 0.513 |
| Chronic heart disease | 18 (22.8) | 33 (32.7) | 0.183 |
| Chronic renal disease/hemodialysis | 9 (11.4) | 15 (14.9) | 0.659 |
| COPD | 39 (49.4) | 37 (36.6) | 0.096 |
| Neurologic disease | 3 (3.8) | 8 (7.9) | 0.588 |
| > 2 comorbidities | 33 (41.8) | 43 (42.6) | 1.0 |
| Charlson Comorbidity Index, mean ± SD | 5.4 ± 3.1 | 7 ± 3.4 | 0.268 |
| Previous hospitalization (90 days) | 37 (46.8) | 42 (41.6) | 0.546 |
| Previous ICU admission (90 days) | 9 (11.4) | 12 (11.9) | 1.0 |
| Previous surgery (30 days) | 15 (19) | 27 (26.7) | 0.22 |
| Previous antibiotic therapy (30 days) | 47 (59.5) | 65 (64.4) | 0.538 |
| Previous Acinetobacter spp colonization/infection | 9 (11.4) | 13 (12.9) | 0.822 |
| Clinical and laboratory findings | |||
| Acinetobacter colonization prior infection | 9 (11.4) | 11 (10.9) | 1.0 |
| Fever | 40 (50.6) | 46 (45.5) | 0.549 |
| SAPS II at time of infection onset, mean ± SD | 41.9 ± 15.4 | 45.7 ± 14.2 | 0.089 |
| SOFA at time of infection onset, mean ± SD | 6.3 ± 3.5 | 7.4 ± 3.2 | 0.131 |
| Previous MDR infections during hospital stay | 22 (27.8) | 32 (31.7) | 0.625 |
| PCT at time of infection onset, mean ± SD | 7.6 ± 3.9 | 7.8 ± 5.9 | 0.96 |
| Lactate, mmol/l, mean ± SD | 1.4 ± 0.4 | 2.1 ± 2.2 | 0.441 |
| Endoscopy procedure | 9 (11.4) | 21 (20.8) | 0.109 |
| Steroid therapy | 46 (58.2) | 51 (50.5) | 0.366 |
| Septic shock | 43 (54.4) | 76 (75.2) | 0.004 |
| Secondary bacteremia | 22 (27.8) | 83 (82.2) | < 0.001 |
| Non-antibiotic therapies and outcomes | |||
| Cardiovascular events after infection onset | 23 (29.1) | 46 (45.5) | 0.031 |
| NIV | 26 (32.9) | 30 (29.7) | 0.746 |
| Mechanical ventilation | 18 (22.7) | 33 (32.6) | 0.156 |
| CRRT | 7 (8.8) | 13 (12.8) | 0.362 |
| Length of hospitalization, mean ± SD (days) | 33.5 ± 18.4 | 31.2 ± 24 | 0.483 |
| Length of ICU stay, mean ± SD (days) | 25.7 ± 17.8 | 25 ± 23.5 | 0.836 |
SD standard deviation, COPD chronic obstructive pulmonary disease, ICU intensive care unit, NIV non-invasive ventilation, CRRT continuous renal replacement therapy, MDR multidrug-resistant, PCT procalcitonin, CRP c-reactive protein, SAPS simplified acute physiology score, SOFA sequential organ failure assessment
Bold values indicate statistical significance (p ≤ 0.05)
Univariate analysis comparing antibiotic regimens as definitive therapy between survivors and non-survivors at 30 days from infection onset is reported in Table 2. No differences were observed between survivors and non-survivors related to the numbers of antibiotics used in definitive therapy. The fosfomycin-containing regimen was more frequently used in surviving patients (46.8% vs. 6.9%, p < 0.001) than in non-survivors. During the study period the usual antimicrobial dosages, adopted for the most used antibiotics, were the following: for colistin, a loading dose of 9 million IU followed by 4.5 million IU every 12 h (h); for tigecycline, a loading dose of 150 to 200 mg followed by 100 mg every 12 h; for gentamicin, a dosage of 5 mg/kg every 24 h; for rifampin, a dosage of 10 mg/kg/day; for meropenem, a dosage of 2 g every 8 h or 1.5 g every 6 h; for fosfomycin 12–24 g/day divided every 6–8 h; for ampicillin/sulbactam 3 g every 6 h; for trimethoprim/sulfamethoxazole 15–20 mg/kg/day divided every 6 h; for vancomycin 40 mg/kg/day divided every 12 h.
Table 2.
Univariate analysis comparing antibiotic regimens in definitive therapy between survivors and non-survivors at 30 days from infection onset
| Antibiotic therapy* | Survivors n = 79 (%) |
Non-survivors n = 101 (%) |
P |
|---|---|---|---|
| Use of only 1 antibiotic | 9 (11.4) | 14 (13.9) | 0.66 |
| Use of 2 antibiotics in combination | 40 (50.6) | 48 (47.5) | 0.764 |
| Use of 3 antibiotics in combination | 25 (31.6) | 30 (29.7) | 0.871 |
| Use of 4 antibiotics in combination | 4 (5.1) | 9 (8.9) | 0.656 |
| Use of 5 antibiotics in combination | 1 (1.3) | 0 | 0.439 |
| Colistin-containing regimen | 67 (84.8) | 84 (83.2) | 0.84 |
| Tigecycline-containing regimen | 14 (17.7) | 28 (27.7) | 0.155 |
| Aminoglycoside-containing regimen | 4 (5.1) | 7 (6.9) | 1.0 |
| Rifampin-containing regimen | 23 (29.1) | 30 (29.7) | 1.0 |
| Ampicillin/sulbactam-containing regimen | 0 | 2 (2) | 0.505 |
| Fosfomycin-containing regimen | 37 (46.8) | 7 (6.9) | < 0.001 |
| Trimethoprim/sulfamethoxazole-containing regimen | 1 (1.3) | 2 (2) | 1.0 |
| Vancomycin-containing regimen | 8 (10.1) | 3 (3) | 0.061 |
| Carbapenem-containing regimen | 44 (55.7) | 61 (60.3) | 0.765 |
| Use of colistin aerosol inhalation therapy | 13 (16.5) | 17 (16.8) | 1.0 |
| Length of definitive antibiotic therapy, mean ± SD (days) | 12.9 ± 9.4 | 9.5 ± 4.4 | 0.014 |
| Time to initial definitive therapy, mean ± SD (days) | 3.8 ± 1.8 | 3.6 ± 1.6 | 0.872 |
SD standard deviation
Bold values indicate statistical significance (p ≤ 0.05)
The antibiotics used in combination with fosfomycin in definitive therapy for 44 patients are reported in Fig. 1. The most used combination was fosfomycin + colistin in 11 (25%) patients, followed by fosfomycin + carbapenem + tigecycline in 8 (18.2%), fosfomycin + colistin + tigecycline in 7 (15.9%), fosfomycin + rifampin in 7 (15.9%), fosfomycin + tigecycline in 6 (13.6%), fosfomycin + carbapenem in 3 (6.8%), and fosfomycin + aminoglycoside in 2 (4.5%). Of these, 30-day mortality was observed in 2 patients treated with fosfomycin + colistin + tigecycline, 2 patients with fosfomycin + carbapenem, 2 patients with fosfomycin + rifampin, and 1 patient with fosfomycin + aminoglycoside.
Fig. 1.
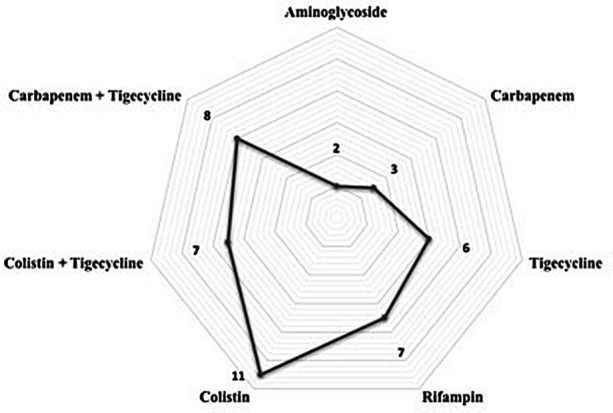
Antibiotics in combination with fosfomycin in definitive therapy (no. of patients treated)
Univariate analysis comparing patients treated with a fosfomycin-containing regimen or other antibiotic regimens in definitive therapy is reported in Table 3. In patients treated with the fosfomycin-containing regimen, COPD (61.4% vs. 36%, p = 0.005) and higher lactate values (3.3 ± 1.9 mmol/l vs. 1.8 ± 0.9 mmol/l, p = 0.001) were recorded more frequently; a previous MDR infection during hospital stay was more frequently observed in patients treated with other antibiotic regimens (36.8% vs. 9.1%, p < 0.001). Finally, 30-day mortality was reported in 7 (15.9%) patients on the fosfomycin-containing regimen compared to 94 (69.1%) treated with other antibiotic regimens (p < 0.001).
Table 3.
Univariate analysis comparing patients treated with a fosfomycin-containing regimen or other antibiotic regimens in definitive therapy
| Variables | Other antibiotic regimens n = 136 (%) |
Fosfomycin-containing regimen n = 44 (%) | P |
|---|---|---|---|
| Anamnestic factors | |||
| Age, mean ± SD (years) | 63.8 ± 16.2 | 66.3 ± 16.1 | 0.375 |
| Male sex | 91 (66.9) | 31 (70.5) | 0.714 |
| Comorbidities | |||
| Chronic liver disease | 6 (4.4) | 2 (4.5) | 1.0 |
| Neoplasm | 21 (15.4) | 5 (11.4) | 0.626 |
| Diabetes | 40 (29.4) | 14 (31.8) | 0.85 |
| Chronic heart disease | 37 (27.2) | 14 (31.8) | 0.568 |
| Chronic renal disease/hemodialysis | 18 (13.2) | 6 (13.6) | 1.0 |
| COPD | 49 (36) | 27 (61.4) | 0.005 |
| Neurologic disease | 10 (7.3) | 1 (2.2) | 0.489 |
| > 2 comorbidities | 60 (44.1) | 16 (36.4) | 0.386 |
| Charlson Comorbidity Index, mean ± SD | 5.6 ± 1.8 | 6.3 ± 1.6 | 0.76 |
| Previous hospitalization (90 days) | 58 (42.6) | 21 (47.7) | 0.602 |
| Previous ICU admission (90 days) | 16 (11.8) | 5 (11.4) | 1.0 |
| Previous surgery (30 days) | 31 (22.8) | 11 (25) | 0.838 |
| Previous antibiotic therapy (30 days) | 83 (61) | 29 (65.9) | 0.596 |
| Previous Acinetobacter spp colonization/infection | 16 (11.8) | 6 (13.6) | 0.792 |
| Clinical and laboratory findings | |||
| Acinetobacter colonization prior infection | 15 (11) | 5 (11.4) | 1.0 |
| Fever | 64 (47.1) | 22 (50) | 0.862 |
| SAPS II at time of infection onset, mean ± SD | 44.1 ± 15.3 | 43.9 ± 13.2 | 0.952 |
| SOFA at time of infection onset, mean ± SD | 7 ± 3.3 | 6.2 ± 3.4 | 0.398 |
| Previous MDR infections during hospital stay | 50 (36.8) | 4 (9.1) | < 0.001 |
| PCT at time of infection onset, mean ± SD | 6 ± 4.5 | 10.9 ± 7.3 | 0.229 |
| Lactate, mmol/l, mean ± SD | 1.8 ± 0.9 | 3.3 ± 1.9 | 0.01 |
| Endoscopy procedure | 27 (19.9) | 3 (6.8) | 0.061 |
| Steroid therapy | 69 (50.7) | 28 (63.6) | 0.165 |
| Septic shock | 90 (66.2) | 29 (65.9) | 1.0 |
| Secondary bacteremia | 84 (61.8) | 21 (47.7) | 0.115 |
| Non-antibiotic therapies and outcomes | |||
| Cardiovascular events after infection onset | 55 (40.4) | 14 (31.8) | 0.374 |
| NIV | 42 (30.9) | 14 (31.8) | 1.0 |
| Mechanical ventilation | 41 (30.1) | 10 (22.7) | 0.434 |
| CRRT | 17 (12.6) | 3 (6.8) | 0.623 |
| Length of hospitalization, mean ± SD (days) | 32.6 ± 23.2 | 31 ± 16.6 | 0.811 |
| Length of ICU stay, mean ± SD (days) | 26.5 ± 22.7 | 21.8 ± 15.6 | 0.212 |
| Time to initial definitive therapy, mean ± SD (days) | 4.1 ± 1.7 | 3.6 ± 1.9 | 0.092 |
| 30-day mortality | 94 (69.1) | 7 (15.9) | < 0.001 |
SD standard deviation, COPD chronic obstructive pulmonary disease, ICU intensive care unit, NIV non-invasive ventilation, CRRT continuous renal replacement therapy, MDR multidrug-resistant, PCT procalcitonin, CRP c-reactive protein, SAPS simplified acute physiology score, SOFA sequential organ failure assessment
Bold values indicate statistical significance (p ≤ 0.05)
As reported in Table 4, Cox regression analysis of factors associated with 30-day mortality showed that septic shock (HR 3.5, CI 95% 1.32–9.58, p = 0.012) and secondary bacteremia (HR 23.6, CI 95% 9.02–61.9, p < 0.001) were associated with death, while the fosfomycin-containing regimen (HR 0.04, CI 95% 0.01–0.13, p < 0.001) was associated with 30-day survival. After adjustment for the propensity score in the logistic regression model evaluating risk factors for mortality, all the variables remained in the model without significant differences.
Table 4.
Cox regression analysis about risk factors associated with 30-day mortality
| Variables | Without propensity score adjustment | With Propensity score adjustment | ||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p | HR | CI 95% | p | |
| Septic shock | 3.5 | 1.32–9.58 | 0.012 | 3.1 | 1.45–7.88 | 0.001 |
| Fosfomycin-containing regimen as definitive therapy | 0.04 | 0.01–0.13 | < 0.001 | 0.22 | 0.09–0.44 | < 0.001 |
| Secondary bacteremia | 23.6 | 9.02–61.9 | < 0.001 | 19.4 | 8.22–42.1 | < 0.001 |
HR hazard ratio, CI confidence interval
Finally, the Kaplan-Meier curve for 30-day survival of patients treated with a fosfomycin-containing regimen or other antibiotic regimens in definitive therapy is reported in Fig. 2.
Fig. 2.
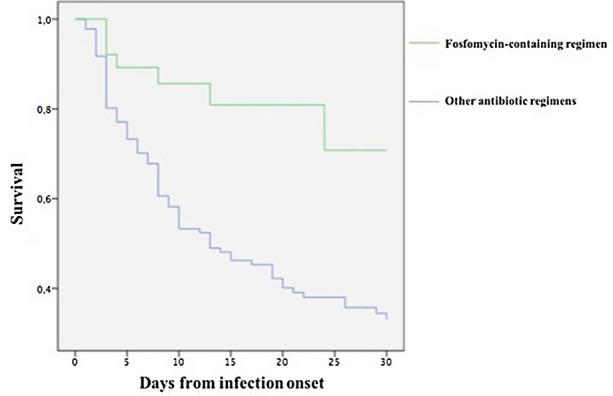
Kaplan-Meier curves about 30-day survival of patients treated with fosfomycin-containing regimen or other antibiotic regimens in definitive therapy
Discussion
In the present study, we evaluated the clinical features, therapeutic approach, and outcome of patients with pneumonia caused by MDR-AB. Our data confirmed previous observations about the very high rates of septic shock (67.7%) and 30-day (56.1%) mortality in the study population. Of importance, multivariate analysis after a propensity score for receiving therapy with fosfomycin confirmed the role of septic shock and bacteremia to determinate 30-day mortality; conversely, a fosfomycin-containing regimen was independently associated with survival at 30 days.
Recent studies confirmed data about mortality [2, 5], with rates > 90% in patients with septic shock [9, 10]. MDR-AB bloodstream infections remain a peculiar ICU-acquired infection, although recent data reported high rates of infection even in medical and surgical wards. Pneumonia is recognized as the primary source of infection caused by MDR-AB: in a multicenter Italian study about 281 patients with MDR-AB bloodstream infections, pneumonia was independently associated with a higher risk of septic shock [5]. As matter of fact, also in our analysis all strains of Acinetobacter baumannii were classified as XDR or PDR, reducing therapeutic options for treatment of this severe infection. However, this observation is in line with previous reports in Italy [5, 10].
Severe pneumonia remains a difficult-to-treat infection, and, considering the poor lung penetration of most antibiotics, the choice of the better antibiotic regimen is debated. As a matter of fact, some antibiotics such as colistin and aminoglycosides should probably be avoided for MDR-AB pneumonia, considering the poor lung penetration of these drugs [23–25]. Moreover, EUCAST recommendations [26] based on recent observations [27] advertised about potential false susceptibility to colistin in approximately 50% of Acinetobacter baumannii strains using automated systems or an E-test. Therefore, the very high rates of mortality observed in our population and in published studies [5, 10, 28] might also be attributed to a reported false susceptibility to colistin in patients for whom physicians were confident in prescribing a colistin-based regimen.
Different antibiotic combinations have been studied for the treatment of severe infections sustained by MDR-AB [29, 30]. In a randomized clinical trial [31], in patients with MDR-AB infections mortality was not reduced by addition of rifampicin to colistin; further in vitro studies explored the synergism of some drug combinations, especially colistin plus carbapenem for treatment of MDR-AB infections [32], suggesting the advantage of this combination based on high in vitro synergy rates. Combination of carbapenem plus colistin seems to be the first option for treatment of MDR-AB infections [33]. In a recent randomized trial comparing colistin alone versus colistin plus meropenem for the treatment of severe infections caused by carbapenem-resistant gram-negative bacteria, the authors concluded that combination therapy was not more efficient than monotherapy and that adding meropenem to colistin did not improve clinical failure in severe MDR-AB infections [7]. Of importance, Dickstein and coworkers performed a subgroup analysis on patients with Acinetobacter infections and reported that colistin monotherapy was associated with a better outcome compared to colistin-meropenem combination therapy [34]. It is important to underline that studies comparing the efficacy of monotherapy (mainly colistin) with combination regimens for Acinetobacter baumannii infections included a spectrum of different severe infections, such as ventilator-associated pneumonia, but not always associated with bacteremia. On this basis, our study confirms that comparative studies on MDR-AB therapy should include bacteremic patients, also in patients with pneumonia as the primary site of infection. Finally, a surprising finding of our analysis was that a previous MDR infection was observed in patients not treated with fosfomycin (36.8% vs. 9.1%).
Many in vitro studies suggested a possible role for intravenous fosfomycin also for the treatment of MDR-AB [13–15]. Recently, a fosfomycin-containing regimen showed a more beneficial effect on all-cause mortality, with favorable effectiveness in clinical cure and microbiologic eradication [16]. Fosfomycin may be an effective adjunctive therapy for pneumonia caused by MDR/XDR A. baumannii strains, considering the synergistic effect of colistin and fosfomycin reported in in vitro studies. A recent study showed fosfomycin achieved effective concentrations in infected lung tissue [35], and fosfomycin was introduced as a treatment option for infections caused by MDR-AB [36]. In a time-kill study, fosfomycin with colistin showed bactericidal and synergistic effects at 8 h, reducing the bacterial load in the lungs at 48 h compared with monotherapies and the combination of colistin plus minocycline [37]. In another recent study, a combination of colistin and fosfomycin had significantly better microbiologic responses with trends toward more favorable treatment outcomes and lower mortality compared with those treated with colistin alone [38].
Our study reveals some important limitations that should be acknowledged. First, the observational nature of the study and the relatively small sample size bring an intrinsic limitation to the analysis. Second, the underlying mechanisms of resistance in these strains were not routinely assessed, and in vitro synergistic combinations were not performed, except for a few cases. Third, this study was performed in a single geographical area of Europe (Italy) with a high incidence of MDR-AB infections, so these results may not necessarily be representative of other European or non-European centers. Finally, all conclusions about the efficacy of the therapeutic regimen, outside of randomized trials, should be validated also considering that 54 patients (30%) had been treated for previous MDR infections with similar antibiotic regimens.
Conclusions
In conclusion, this real-life clinical experience concerning the therapeutic approach to severe pneumonia caused by MDR-AB provides useful suggestions to clinicians about the management of this difficult-to-treat infection. Pneumonia caused by MDR-AB strains represents a challenge for physicians, considering the high rates of septic shock and mortality associated with this infection. Our data showed peculiar clinical features and use of different antibiotic regimens in this setting of infection, with a predominant role for fosfomycin. Further randomized clinical trials are mandatory to confirm or exclude these observations [39, 40].
Acknowledgements
We are grateful to Dr. Marco Alessi for statistical revision.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Conceived and designed study: Alessandro Russo, Matteo Bassetti, Alessandro D’Avino. Performed data collection: Alessandro Russo, Valeria Bellelli, Luigi Bianchi, Federica Marincola Cattaneo, Stefania Mazzocchetti, Elena Paciacconi, Fabrizio Cottini, Arcangelo Schiattarella, Giuseppe Tufaro, Francesco Sabetta and Alessandro D’Avino. Analyzed data: Alessandro Russo, Alessandro D’Avino. Wrote the paper: Alessandro Russo, Matteo Bassetti.
Disclosures
Alessandro Russo, Valeria Bellelli, Luigi Bianchi, Federica Marincola Cattaneo, Stefania Mazzocchetti, Elena Paciacconi, Fabrizio Cottini, Arcangelo Schiattarella, Giuseppe Tufaro, Francesco Sabetta, and Alessandro D’Avino have nothing to declare. Matteo Bassetti is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
The prospective nature of the study was based on the consecutive enrollment of patients. However, all complete data were afterwards retrospectively extracted, and the Ethics Committee (Policlinico Casilino) waived the need for informed consent. The study was conducted according to the principles stated in the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Perez F, Endimiani A, Ray AJ, Decker BK, Wallace CJ, Hujer KM, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65:1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti M, Righi E, Vena A, Graziano E, Russo A, Peghin M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug- resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr Opin Crit Care. 2018;24:385–393. doi: 10.1097/MCC.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 3.https://www.who.int/drugresistance/AMR_Importance/en. Accessed 6th July 2020.
- 4.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A, Bassetti M, Ceccarelli G, Carannante N, Losito AR, Bartoletti M, et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79:130–138. doi: 10.1016/j.jinf.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson JD, Kollef MH. Early and adequate antibiotic therapy in the treatment of severe sepsis and septic shock. Curr Infect Dis Rep. 2011;13:399–405. doi: 10.1007/s11908-011-0206-8. [DOI] [PubMed] [Google Scholar]
- 7.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero J, Dimopoulos G, Poulakou G, Akova M, Cisneros JM, De Waele J, et al. European Society of Intensive Care Medicine: Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41:2057–2075. doi: 10.1007/s00134-015-4079-4. [DOI] [PubMed] [Google Scholar]
- 9.Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, et al. Mortality in patients with septic shock by multidrug resistant bacteria. J Intensive Care Med. 2019;34:48–54. doi: 10.1177/0885066616688165. [DOI] [PubMed] [Google Scholar]
- 10.Russo A, Giuliano S, Ceccarelli G, Alessandri F, Giordano A, Brunetti G, et al. Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob Agents Chemother. 2018;62:e02562. doi: 10.1128/AAC.02562-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2018;63:e01110. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassetti M, Righi E, Russo A, Carnelutti A. New antibiotics for pneumonia. Clin Chest Med. 2018;39:853–869. doi: 10.1016/j.ccm.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Ku NS, Lee SH, Lim YS, Choi H, Ahn JY, Jeong SJ, Shin SJ, Choi JY, Choi YH, Yeom JS, Yong D, Song YG, Kim JM. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci Rep. 2019;9:17127. doi: 10.1038/s41598-019-53714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamm RK, Rhomberg PR, Lindley JM, Sweeney K, Ellis-Grosse EJ, Shortridge D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against gram-negative bacterial strains by using time-kill curves. Antimicrob Agents Chemother. 2019;63:e02549–e2618. doi: 10.1128/AAC.02549-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singkham-In U, Chatsuwan T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter Baumannii clinical isolates. Diagn Microbiol Infect Dis. 2018;91:169–174. doi: 10.1016/j.diagmicrobio.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Jung SY, Lee SH, Lee SY, Yang S, Noh H, Chung EK, et al. Antimicrobials for the treatment of drug-resistant Acinetobacter Baumannii pneumonia in critically Ill patients: a systemic review and bayesian network meta-analysis. Crit Care. 2017;21:319. doi: 10.1186/s13054-017-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone M, Russo A, Giannella M, Cangemi R, Scarpellini MG, Bertazzoni G, et al. Individualizing risk of multidrug-resistant pathogens in community-onset pneumonia. PLoS ONE. 2015;10:e0119528. doi: 10.1371/journal.pone.0119528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe, 2014. Stockholm, Sweden: European Centre for Disease Prevention and Control. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/AER-VPD-IBD-2014.pdf. Accessed 6 July 2020.
- 19.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 20.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2020. https://www.eucast.org/clinical_breakpoints. Accessed 6 July 2020.
- 23.Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest. 2005;128:545–552. doi: 10.1378/chest.128.2.545. [DOI] [PubMed] [Google Scholar]
- 24.Boselli E, Breilh D, Djabarouti S, Guillaume C, Rimmelé T, Gordien JB, et al. Reliability of mini- bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med. 2007;33:1519–1523. doi: 10.1007/s00134-007-0688-x. [DOI] [PubMed] [Google Scholar]
- 25.Boisson M, Jacobs M, Grégoire N, Gobin P, Marchand S, Couet W, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically Ill patients. Antimicrob Agents Chemother. 2014;58:7331–7339. doi: 10.1128/AAC.03510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.www.eucast.org/ast_of_bacteria/warnings. Accessed on 6 July 2020.
- 27.Matuschek E, Åhman J, Webster C, Kahlmeter G. Antimicrobial susceptibility testing of colistin: evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect. 2018;24:865–870. doi: 10.1016/j.cmi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Freire MP, de Oliveira GD, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri ASG, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect. 2016;22:352–358. doi: 10.1016/j.cmi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Bassetti M, Repetto E, Righi E, Boni S, Diverio M, Molinari MP, et al. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother. 2008;61:417–420. doi: 10.1093/jac/dkm509. [DOI] [PubMed] [Google Scholar]
- 30.Poulakou G, Bassetti M, Tsiodras S. "Excess mortality" and colistin-tigecycline for extensively drug-resistant Acinetobacter baumannii bacteremia. Crit Care Med. 2015;43:e470–e471. doi: 10.1097/CCM.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 31.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 32.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, et al. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med. 2015;43:1194–1204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 34.Dickstein Y, Lellouche J, Dalak Amar MB, Schwartz D, Nutman A, Daitch V, et al. Treatment outcomes of colistin and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis. 2019;69:769–776. doi: 10.1093/cid/ciy988. [DOI] [PubMed] [Google Scholar]
- 35.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, et al. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J Antimicrob Chemother. 2010;65:995–998. doi: 10.1093/jac/dkq070. [DOI] [PubMed] [Google Scholar]
- 36.Castanheira M, Mendes RE, Jones RN. Update on acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin Infect Dis. 2014;59:S367–S373. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 37.Ku NS, Lee SH, Lim YS, Choi H, Ahn JY, Jeong SJ, et al. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci Rep. 2019;9:17127. doi: 10.1038/s41598-019-53714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter Baumannii infections. Antimicrob Agents Chemother. 2014;58:5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paterson DL, Rogers BA. How soon is now? The urgent need for randomized, controlled trials evaluating treatment of multidrug-resistant bacterial infection. Clin Infect Dis. 2010;51:1245–1247. doi: 10.1086/657243. [DOI] [PubMed] [Google Scholar]
- 40.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


