Abstract
Background
Based on current evidence, recent guidelines of the National Institute of Health, USA indicated the use of remdesivir and dexamethasone for the treatment of COVID-19 patients with mild-moderate disease, not requiring high-flow oxygen. No therapeutic agent directed against the immunologic pathogenic mechanisms related to the cytokine release syndrome complicating the disease was indicated.
Objectives
The purpose of this review was to assess the clinical impact of different therapies for COVID-19; thus, helping to identify the optimal management of the disease. To explain the rationale for the different therapeutic approaches, the characteristics of SARS-CoV-2, the pathogenesis of COVID-19, and the immune response triggered by SARS-CoV-2 infection were reported.
Methods
The efficacy assessment of the different treatments was performed by a systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Available English language published articles including randomised controlled trials, open-label trials of antivirals and immune therapies extracted from Medline, Google Scholar, and MedRxiv databases were analysed. For inclusion, the primary end point of the trials had to be the efficacy as measured by the improvement of clinical features, or mortality, or the Intensive Care Unit Admission rate, or the discharge number. Case reports, paediatric studies, and studies without control group were excluded. The literature search was extended up to August 15, 2020.
Results
After the removal of duplicate articles, and the exclusion of studies not meeting the eligibility criteria, 2 trials of lopinavir/ritonavir, 1 of favipiravir, 3 of remdesivir, 1 of dexamethasone, 3 of hydroxychloroquine, 2 of colchicine, 6 of tocilizumab, 1 of sarilumab, 1 of siltuximab, 2 of anakinra, 3 of baricitinib, 1 of ruxolitinib, 1 of mavrilimumab, and 1 of itolizumab were suitable for the review. Among antivirals, only remdesivir significantly reduced the time to recovery, and mortality. Data for chloroquine and hydroxychloroquine were largely inconclusive. In a large trial, dexamethasone 6 mg/day reduced mortality by one-third. Trials of tocilizumab and sarilumab did not definitively demonstrate efficacy. Anakinra significantly reduced the mortality in 2 trials. Three retrospective trials on a cumulative number of 145 patients, reported the efficacy of baricitinib, with significant reduction of intensive care unit admission, and deaths. These results were recently confirmed by the ACTT-2 trial. Due to paucity of studies and to the small size clinical series, the results of other immune therapies were not conclusive.
Conclusions
Beyond the supportive therapy, up to now the best therapeutic approach for COVID-19 may be a three-step combination therapy, including remdesivir 100 mg/day (200 mg loading dose on first day) in the first stage of the disease, and combined dexamethasone 6 mg/day plus baricitinib 4 mg/day to target the immune dysregulation triggered by the SARS-CoV-2 infection. The promising results of anakinra should be confirmed by the ongoing RCTs.
Electronic supplementary material
The online version of this article (10.1007/s40265-020-01421-w) contains supplementary material, which is available to authorized users.
Key Points
| The effectiveness of antiviral and immune therapies was inconsistent in most cases of COVID-19. |
| To date, remdesivir, dexamethasone, and baricitinib represent the best therapeutic option. |
| The promising results of efficacy of anakinra need confirmation by the ongoing RCTs. |
Introduction
COronaVIrus Disease 19 (COVID-19), caused by the severe acute respiratory syndromes Corona Virus (SARS)-CoV-2, has spread all over the inhabited world, and at the end of March 2020 the World Health Association declared COVID-19 as a pandemic [1]. SARS-CoV-2 belongs to RNA virus family β-Coronaviridae, and probably is a recombinant virus originating from bats [2].
The critical first step for SARS-CoV-2 infectivity and pathogenesis is entry into the susceptible host cells binding to a specific receptor, the human ACE2 (hACE2) [3, 4]. SARS-CoV-2 is transmitted human to human by respiratory droplets [5], aerosols [6], and possibly by faecal-oral contact [7]. Most of SARS-CoV-2-infected individuals are asymptomatic or present mild symptoms [8]. Symptoms of COVID-19 disease are fever, cough, dyspnoea, repeated chills, muscle pain, headache, sore throat, loss of smell (anosmia) and/or taste (ageusia) [9] Approximately 2–10% of patients with COVID-19 present with diarrhoea, and SARS-CoV-2 RNA has been detected in blood, intestine, stool, and liver [10–12] (Fig. 1). About 20% of patients become seriously ill, with dyspnoea, reduction of peripheral capillary oxygen saturation (defined as PaO2/FiO2 < 300 mmHg) and supplemental oxygen requirement [11].
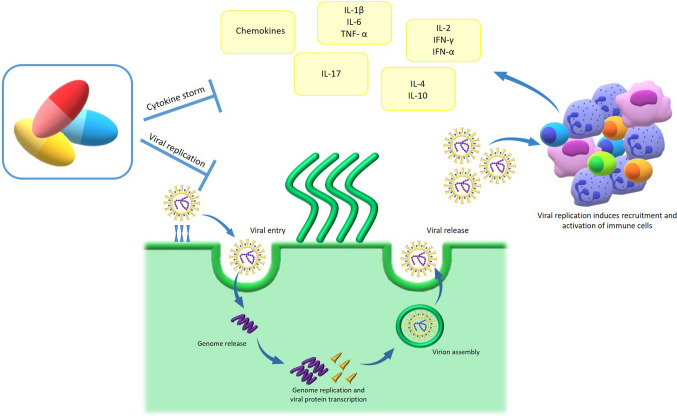
Fig. 1.
Most of SARS-CoV-2-infected individuals are asymptomatic or present mild symptoms. According to the CDC, people with the following symptoms may have COVID-19: fever, cough, dyspnoea, repeated chills, muscle pain, sore pain head, sore throat, loss of smell (anosmia) and/or taste (ageusia) and diarrhoea. About 25% percent of these patients will have a seriously ill disease. A small proportion may develop a very severe pneumonia, which may progress to acute respiratory distress syndrome (ARDS) or end-organ failure that may be associated with a cytokine storm syndrome. ESR erythrocyte sedimentation rate, CRP C-reactive protein, LDH lactate dehydrogenase, G-CSF granulocyte colony-stimulating factor, MIP-1a macrophage inflammatory protein 1-a, PT prolonged prothrombin time, TNF-α tumour necrosis factor-α
This second clinical stage is characterised by pulmonary disease, viral multiplication and localised inflammation in the lung. Cytokine storm may occur after 7–8 days from symptoms onset and refers to an excessive and uncontrolled release of pro-inflammatory cytokines, which can initiate viral sepsis and the inflammatory-induced lung injury leading to other complications including pneumonia, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure and potentially death [13] (Fig. 1). Imaging with chest X-ray or computed tomography shows bilateral infiltrates or ground glass opacities [14]. Blood tests may reveal lymphopenia, increased markers of systemic inflammation, and cytokines such as interleukin (IL)-2, IL-6, IL-7, granulocyte colony-stimulating factor (GC-SF), macrophage inflammatory protein 1-a (MIP-1a), tumour necrosis factor-a (TNF-α) [14]. A minority of patients will transit into the third stage, which is characterised by an extrapulmonary systemic hyperinflammation syndrome respiratory failure, shock, cardiopulmonary collapse that can lead to death [8, 15, 16].
Both innate and adaptive immune responses may play crucial roles in protective or destructive responses. Active viral replication leads to production of type I interferon (IFN) and influx of neutrophils and macrophages, which are the major sources of pro-inflammatory cytokines [17], as well as massive activation and dysregulation of T cells [18] (Fig. 2). Increased total neutrophils, lymphopenia, selective loss of CD4+ T cells, CD8+ T cells, and NK cells, excessive T cell activation (defined by CD38+ and HLA-DR+), and high expression of T-cell inhibitory molecules (e.g. PD-1) are more prominent in severe cases than in those with mild disease [19–21]. Post-mortem histochemical studies of lung tissue showed sub-anatomical distributions of SARS-CoV-2 RNA and massive infiltration of CD4+ and CD8+ T cells and macrophages [20]. The inflammatory cytokines and chemokines (IL-1β, IFN-γ, IP-10, and MCP-1), which may lead to activated T-helper-1 (Th1) cell responses have been described as upregulated [14]. However, these patients have excessive IL-4 and IL-10 levels that may attempt suppression of the viral-induced hyper-inflammation [14]. Several cytokines appear to be involved in the severity of COVID-19. In patients with ARDS, increased IL-6 has been found at baseline in those with a poor survival [22, 23]. IL-17 has been associated with high viral load and disease severity [21, 24–26]. IL‐17, mainly produced by Th17 cells, recruits monocytes and neutrophils to the site of infection with inflammation and activates other downstream cytokine and chemokine cascades, such as IL‐1, IL‐6, IL‐8, IL‐21, TNF‐β, and MCP-1 [25, 26].
Fig. 2.
SARS-CoV-2 infects mucous membranes expressing high levels of ACE2 as nasal and larynx mucosa, then may pass into the lungs through the respiratory tract. After receptor recognition and viral entry into the ciliated epithelial cells, SARS-CoV-2 replicates the viral genome and encodes structural and non-structural viral proteins. Therefore, new virions are assembled, and released. Active viral replication leads to production of type I interferon (IFN) and influx of neutrophils and macrophages. These cells are the major cell sources of pro-inflammatory cytokines and chemokines as interleukin (IL)-1β, IFN-γ, inducible protein-10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1), which may result in activation of T-helper-1 (Th1) cells. Moreover, IL-17, produced by Th17 cells recruits monocytes and neutrophils to the site of infection contributing to the inflammation. Finally, Th2 cytokines such as IL-4 and IL-10 are also produced with the attempt to suppress the hyper-inflammation. This cytokine storm, as well as the several stages of viral replication, are the target of the current therapies for COVID-19
The impressive number of infected individuals, of hospital admissions, and the high mortality rate lead most countries to adopt restrictive measures to limit or avoid contagions. Meanwhile, exceptional efforts to develop a specific vaccine are ongoing.
The managing strategies for COVID-19 symptomatic subjects were oriented toward providing symptomatic respiratory assistance if needed, in parallel with three main directions. First, once the aetiologic agent SARS-CoV-2 was detected, the objective was to reduce the viral load by using antiviral drugs that target the essential steps of viral entry and replication. Experimental data, hydroxychloroquine was demonstrated to inhibit the viral reproduction, and the drug was employed alone or in association with antivirals [27]. Similarly, as anti-calcineurin immunosuppressants cyclosporin A, and alisporivir (an analogue of cyclosporin) were detected to exert antiviral action, some authors suggested their use [28, 29]. Second, since the more severe clinical manifestations were found to be mainly related to massive cytokine outbreak from immune cells, thus resembling an autoinflammatory condition [30], anti-cytokine agents, including anti-IL-6 tocilizumab, sarilumab, and siltuximab, anti-IL-1 anakinra, Janus kinase (JAK) inhibitors ruxolitinib, and baricitinib, anti-granulocyte–macrophage colony-stimulating factor receptor-alpha monoclonal antibody (anti-GM-CSF) mavrilimumab, and anti-CD6 itolizumab were used [31, 32]. Clazakizumab, another anti-IL-6 is currently in clinical evaluation.
Third, in most trials both antivirals and anti-cytokine agents were employed concomitantly.
The objective of the present systematic review was to assess the optimal therapeutic strategy based on the results of these different therapeutic approaches in patients with COVID-19 disease, in terms of intensive care unit (ICU) admission rate, mortality, respiratory function improvement, and hospital discharges.
Methods
Search strategy. Medline, Google Scholar, and MedRxiv databases were used to perform the review. Studies were identified by combining the name coronavirus and COVID-19 with the following key terms: infection, virus characteristics, immune response, cytokines, cytokine storm, treatment, clinical trials, prevention, antivirals, lopinavir/ritonavir (LPV/RTV), remdesivir, favipiravir, chloroquine, hydroxychloroquine, colchicine, tumour necrosis factor inhibitors, anti-IL-1 anakinra, anti-IL-6 tocilizumab, sarilumab, and siltuximab, inhibitors of JAK baricitinib, ruxolitinib, anti-GM-CSF mavrilimumab, anti-CD6 itolizumab, corticosteroids.
We included data from English language articles. The efficacy assessment of the different treatments was performed by a systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [33]. To be eligible, the primary end point of the trials needed to be the efficacy as measured by (i) the improvement of clinical features, (ii) mortality, (iii) ICU admission rate, (iv) discharge number. Case reports, paediatric studies, studies without control group, and meeting abstracts not yet published as full articles were excluded. The literature search was extended up to August 15, 2020.
Selected Trials
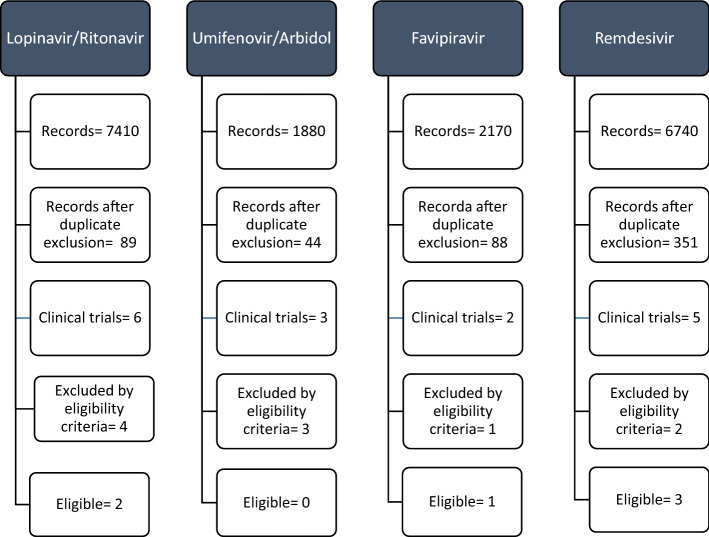
After the removal of duplicates, 56 trials, 16 of antivirals and 40 of non-antivirals were extracted. Of these, 10 trials of antivirals and 18 of non-antivirals were excluded by the eligibility criteria (Electronic supplementary materials). Therefore, the following trials were suitable for review: lopinavir/ritonavir (2), favipiravir (1), remdesivir (3), dexamethasone (1), hydroxychloroquine (3), colchicine (2), tocilizumab (6), sarilumab (1), siltuximab (1), anakinra (2), baricitinib (3), ruxolitinib (1), mavrilimumab (1), and itolizumab (1). Figures 3 and 4 show the PRISMA flow diagram for antiviral and non-antiviral drugs selected for the review.
Fig. 3.
Systematic review of efficacy of antivirals in COVID-19: PRISMA flow diagram. All extracted trials, and the reasons for exclusion, are reported in the supplementary material file
Fig. 4.
Systematic review of efficacy of anti-inflammatory and immune therapies in COVID-19: PRISMA flow diagram*. *All extracted trials, and the reason for exclusion, are reported in the supplementary material file
Antivirals
Overall, six clinical trials investigated the efficacy and safety of antivirals lopinavir/ritonavir (2 trials), favipiravir (1 trial), and remdesivir (3 trials) for the treatment of COVID-19 (Table 1).
Table 1.
Published clinical trials of antiviral drug efficacy for the treatment of COVID-19
| Author (Ref.) | Design | Dose | Days from symptom onset | Combo | Patients | Controls | ICU admitted | Non-ICU admitted | Primary end point | Outcome measure | Results | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | ||||||||
| Lopinavir (HIV-1 protease inhibitor)/ritonavir (inhibitor of cytochrome P450) | ||||||||||||
| Ye et al. [34] | Retrosp. | 500 mg/bid | NR | No | 42 | SOC 5 pts | 0 | 47 | Efficacy | Days to fever resolutions | 4.8 ± 1.94 vs 7.3 ± 1.53 | 0.0364 |
| Cao et al. [35] | RCT | 500 mg/bid | 13 | SOC | 99 | SOC 100 pts | 1 | 198 | Clinical improvement | Time to improvement (days) | NS | NS* |
| Deaths | Death N | NS | NS* | |||||||||
| Favipiravir (RNA polymerase inhibitor) | ||||||||||||
| Chen et al. [37] | Rand. OL | 600 mg/bid † | NR | SOC | 116 |
Umifenovir 600 mg/day 120 pts |
0 | 236 | Recovery rate | N (%) | 71/116 (61.2) vs 62/120 (51.6) | 0.139 |
| Pneumonia improvement |
CT score N (%) |
32/35 (91.4) vs 28/45 (62.2) | 0.004 | |||||||||
| Remdesivir (RNA polymerase inhibitor) | ||||||||||||
| Wang et al. [40] | RCT | 100 mg/day/IV‡ | 11 | SOC | 158 | Placebo 78 pts | 0 | 236 | Efficacy | Days to 6-point scale improvement | 21 (13–28) vs 23 (15–28) | 0.24 |
| Mortality | Percentage | 15% vs 13% | NS* | |||||||||
| Beigel et al. [41] | RCT | 100 mg/day/IV‡ | 9 | Supportive therapy | 538 | Placebo 521 pts | 272 | 791 | Recovery | Time to recovery | 11 vs 15 | < 0.001 |
| Mortality | N (HR) | 32 vs 54 (0.70) | NS* | |||||||||
| Olender et al. [42] | Rand. OL | 100 mg/day/IV/5 or 10 days‡ | 8 | SOC | 312 | SOC 818 pts | 312 | Recovery | % | 74% vs 59% | OR 2.03 (95% CI 1.34–3.08) p < 0.001 | |
| Mortality | % | 7.6% vs 12.5% | OR 0.38, (95% CI 0.22–0.68) p 0.001 | |||||||||
bid twice daily, Combo combined therapy, HR hazard risk, ICU intensive care unit, IV intravenous, N number, NA not applicable, NR not reported, NS not significant, pts patients, Rand. randomised, Ref. reference, RCT randomised controlled trial, Retrosp. retrospective, SOC standard of care
*p value not reported, †1600 mg/bid loading dose at day 1; ‡200 mg/day/IV loading dose at day 1
Lopinavir/Ritonavir
The pharmacological association lopinavir/ritonavir is composed by lopinavir, an inhibitor of human immunodeficiency virus (HIV) type 1 aspartate protease, and ritonavir, which increases the half-life of lopinavir through the inhibition of cytochrome P450. The drug, approved for the treatment of HIV, was found to exert an antiviral effect on SARS-CoV-2 virus in vitro [34], and this represented the rationale to treat COVID-19 patients. The efficacy and safety of lopinavir/ritonavir was evaluated in a cumulative number of 245 patients in one retrospective study [35], and one randomised controlled trial (RCT) [36]. No significant differences resulted between lopinavir/ritonavir treatment and the standard of care therapy (SOC) in terms of fever resolution, discharges, time to clinical improvement, deaths, and viral load reduction.
Favipiravir
Favipiravir, an inhibitor of RNA polymerase, was demonstrated active against SAR-COV-2 in vitro [37]. In a randomised, open-label trial of 236 patients, 116 allocated to favipiravir and 120 to umifenovir (another antiviral drug), the efficacy of favipiravir did not differ significantly from controls (p: 0.139) [38].
Remdesivir
Preclinical studies suggested that remdesivir (GS5734)—an inhibitor of RNA polymerase with in vitro activity against multiple RNA viruses, including Ebola—can play a therapeutic and preventive role in COVID-19 [39]. One open-label trial, and two RCTs evaluated the efficacy and safety of remdesivir in COVID-19 pneumonia.
Two RCTs are available: one from China on 237 patients, 158 allocated to remdesivir and 79 to placebo [40]; the second from the USA [41], enrolling 1059 patients, 538 randomised to remdesivir and 521 to placebo. Conflicting results were obtained. Indeed, in the first study no differences in 6-point severity scale improvement resulted between remdesivir group and controls (p: 0.24), whereas in the second RCT a significant reduction of the time to recovery was recorded in remdesivir receivers (p: 0.001), although the mortality rate did not significantly differ. In both RCTs serious adverse events (SAEs) occurred in around 20% of the patients. However, the frequency of adverse events (AEs) was not significantly different between the remdesivir arms and controls.
In a recent open-label, randomized trial [42], 312 patients receiving remdesivir added to the SOC for 5 or 10 days were compared with 818 matched controls treated with SOC therapy. The recovery rate was significantly higher in the remdesivir arm compared with controls (74.4% vs 59%; adjusted OR: 2.03; 95% CI 1.34–3.08; p < 0.001). A significant reduction of mortality at day 14 was recorded in the remdesivir cohort (7.6% vs 12.5%, OR 0.38; 95% CI 0.22–0.68, p = 0.001). Data on safety were not available.
Non-antiviral Drugs
Overall, 22 trials of anti-inflammatory and immune therapies were eligible for the review (Table 2).
Table 2.
Non-anti-viral and immune targeted agent trials for the treatment of COVID-19
| Author (Ref.) | Design | Dose | Days from symptom onset N |
Combo | Patient N |
Controls | ICU N |
Non-ICU N |
Primary end point | Outcome measure | Results | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticosteroids | ||||||||||||
| Horby et al. [43] | RCT | DEXA 6 mg/day | 8 | SOC | 2104 | SOC 4321 pts | 1007 | 5418 | Deaths | N (%) | 454 (21.6) vs 1065 (24.6) | < 0.001 |
| Discharge | N (%) | 1360 (64.6) vs 2639 (61.1) | 0.002 | |||||||||
| Hydroxychloroquine | ||||||||||||
| Time to death | Probability of being event-free | HR 1.03 | NS | |||||||||
| Rosenberg et al. [47] | Retrosp. | 200 mg to 600 mg/day | 3 | AZT 500 mg/day | 1438 |
HCLR alone 271 pts HCLR + AZT 735 pts AZT 211 pts SOC 221 |
0 | 1438 | Deaths | N (%) |
54/271 (19.9) 189/735 (25.7) 21/211 (10) 28/221 (12.7) |
NS |
| Magagnoli et al. [48] | Retrosp. | 400 mg/day 198 pts | NR | HCLR + AZT 500 mg 214 pts | 412 |
No HCLR 395 pts |
0 | 807 | Deaths | N (%) | 38 (19.2), 49 (22.9) vs 37 (9.4) | < 0.001 |
| Cavalcanti et al. [49] | RCT | 800 mg or 800 mg + AZT 500 mg | 7 | SOC | 438 | SOC 227 pts | 92 | 573 | Clinical improvement | 7 points ordinal scale OR |
1.21 (0.69–2.11) 0.99 (0.57–1.73) |
1 1 |
| Colchicine | ||||||||||||
| Deftereos et al. [51] | OL rand. | 1.0 mg/day* | NR† | SOC | 55 | SOC 50 pts | 0 | 105 | Time to deterioration | 7 points ordinal scale N (%) | 1 (1.8) vs 7 (14) | 0.02 |
| Scarsi et al. [52] | OL | 1.0 mg/day | NR | SOC | 122 | SOC 140 pts | 0 | 262 | Deaths | N (%) | 20 (16.3) vs 52 (37.1) | 0.001 |
| Tocilizumab (anti-il-6) | ||||||||||||
| Capra et al. [53] | Retrosp. | 400 mg/IV or 324/SC | NR | LPV//RTV 500 mg/bid + HCLR 400 mg/day | 62 | SOC 23 pts | 0 | 85 | Deaths | N (%) | 2/62 (3.2) vs 11/23 (47.8) | 0.004 |
| Campochiaro et al. [54] | Retrosp. | 400 mg/IV | 11 | SOC | 32 | SOC 33 pts | 0 | 65 | Discharge | N (%) | 20 (63) vs 16 (49) | 0.32 |
| Deaths | N (%) | 5 (16) vs 11 (33) | 0.150 | |||||||||
| ICU admission | N (%) | 4 (13) vs 2 (6) | 0.43 | |||||||||
| Improvement from BL | N (%) | 22 (69) vs 20 (61) | 0.61 | |||||||||
| Perrone et al. [55] | Single arm Phase II | 8 mg/kg/IV | NR | SOC | 331 | Validation cohort 920 pts | Deaths | < 10% of expected 20% and 35% at day 14 and 30 |
Day 14: 18.4% Day 30: 11.4% |
0.52 0.001 |
||
| Klopfenstein et al. [56] | Retrosp. | 8 mg/kg/IV | 13 | SOC | 20 | SOC 21 pts | 0 | 41 | Deaths | N (%) | 5 (25 vs 12 (48) | 0.066 |
| ICU admission | N (%) | 0 vs 11(44) | < 0.001 | |||||||||
| Rojas-Marte et al. [57] | Retrosp. | 8 mg/kg/IV | NR | SOC | 96 | SOC 97 pts | 121 | 72 | Deaths (overall) | N (%) | 43 (44.8) vs 55 (56.7) | 0.09 |
| Deaths (intubated) | N (%) | 41 (67.2) vs 45 (75) | 0.34 | |||||||||
| Deaths (non-intubated) | N (%) | 2 (6.1) vs 9 (26.5) | 0.024 | |||||||||
| Guaraldi et al. [58] | Retrosp. | 8 mg/kg/IV or 324 mg/SC | 7 | SOC | 179 | SOC 365 pts | 90 | 454 | Deaths | N (%) | 13 (7) vs 73 (20) | 0.0007 |
| Sarilumab (anti-IL-6) | ||||||||||||
| Della-Torre et al. [59] | OL | 400 mg/IV | 7 | SOC | 28 | SOC 28 pts | NR | NR | Deaths | N (%) | 2 (7) vs 5 (18) | 0.42 |
| Clinical improvement | 6-point scale N (%) | 17 (60) vs 18 (64) | 0.99 | |||||||||
| Time to clinical improvement | Days N | 16 vs 19 | 0.89 | |||||||||
| Siltuximab (anti-IL-6) | ||||||||||||
| Gritti et al. [60] | OL | 11 mg/kg/IV | NR | SOC | 30 | SOC 30 pts | 5 | 55 | Deaths | HR | 0.462 (0.221–0.965) | 0.0399 |
| Anakinra (anti-IL-1) | ||||||||||||
| Cavalli et al. [62] | Retrosp. | 200 mg/bid/SC or 10 mg/kg/day/IV | NR | LPV//RTV 500 mg/bid + HCLR 400 mg/day | 36 | SOC 16 pts | 35 | 17 | Outcome | Discharge N (%) | 13/36 (45) vs 7/16 (44) | NS |
| Death N (%) | 3/36 (8.3) vs 7/16 (43.7) | 0.021 | ||||||||||
| Survival (%) | 90% vs 56% | 0.009 | ||||||||||
| Huet et al. [63] | Retrosp. | 100 mg/bid/SC/3 days + 100 mg/day/SC/7 days | 8 | NA | 52 | SOC 44 pts | 0 | 96 | ICU admission | N (%) |
13 (35) vs 32 (73) HR: 0.22 (0.11–0.41) |
0.009 |
| Deaths | HR | HR 0.30 (0.12–0.71) | 0.0063 | |||||||||
| Baricitinib (anti-JAK 1/2) | ||||||||||||
| Cantini et al. [66] | Retrosp. | 4 mg/day | 6 | LPV//RTV 500 mg/bid | 12 | SOC 12 pts | 0 | 24 | ICU admission | N (%) |
0/12 (0) vs 4/12 (33) |
0.093 |
| Discharge | N (%) |
7/12 (58) vs 1/12 (8) |
0.027 | |||||||||
| Cantini et al. [67] | Retrosp. | 4 mg/day | 7 | LPV//RTV 500 mg/bid | 113 | SOC 78 | 0 | 191 | Deaths | N (%) | 0 (0) vs 7 | 0.010 |
| ICU admission | N (%) | 1 (0.8) vs 14 (17.9) | 0.019 | |||||||||
| Discharge | N (%) | 88 (77.8) vs 10 (12.8) | < 0.0001 | |||||||||
| Bronte et al. [68] | Retrosp. | 4 mg/day | NR | LPV//RTV 500 mg/bid + HCLR 400 mg/day | 20 | SOC 56 | 0 | 56 | Deaths | N (%) | 1 (5) vs 25 (45) | < 0.001 |
| Ruxolitinib (anti-JAK 1/2) | ||||||||||||
| Cao et al. [69] | RCT | 5 mg/bid | 20 | SOC | 20 | SOC 21 pts | 0 | 41 | Time to improve | Days N | 12 vs 15 | 0.147 |
| Mavrilimumab (anti granulocyte–macrophage colony-stimulating factor receptor-alpha monoclonal antibody) | ||||||||||||
| De Luca et al. [72] | OL Prosp. | 6 mg/kg/IV | NR | SOC | 13 | SOC 26 pts | 0 | 39 | Clinical improvement | WHO 7-point scale | 13(100) vs 17 (65) | 0.030 |
| Days to discharge | N | 10 vs 20 | 0.030 | |||||||||
| Deaths | N (%) | 0 vs 7 (27) | 0.086 | |||||||||
| ICU admission | N (%) | 1 (8) vs 9 (35) | 0.14 | |||||||||
| Itolizumab (anti-CD6) | ||||||||||||
| Ramos-Suzarte et al. [73] | OL | 200 mg/IV | NR | SOC | 19 | SOC 53 pts | 0 | 72 | ICU admission | (%) | 28.6% vs 60.6% | 0.042 |
| Deaths | (%) | 7.1% vs 42.4% | 0.020 | |||||||||
AZT azithromycin, bid twice daily, BL baseline, CIS COVID-19 Inflammation Score, Combo combined therapy, DEXA dexamethasone, HCLR hydroxychloroquine, ICU intensive care unit, IV intravenous, LPV/RTV lopinavir/ritonavir, MPDN methylprednisolone, N number, NA not applicable, NR not reported, OL open-label trial, OR odds ratio, PaO2/FiO2 ratio arterial oxygen partial pressure/fractional inspired oxygen, pts patients, Prosp. prospective, Retrosp. retrospective, Rand. randomised, RCT randomised controlled trial, SC subcutaneous, SOC standard of care therapy, HR hazard risk, ICU intensive care unit, N number, NA not applicable, NR not reported, NS not significant, pts patients, Rand. randomised, Ref. reference, RCT randomised controlled trial, Retrosp. retrospective, SOC standard of care
*Loading dose of 1.5 mg followed by 0.5 mg after 60 min; †The time from hospital admission to is reported, but not the interval from symptom onset
Corticosteroids
The therapeutic role of corticosteroids in COVID-19 is controversial. WHO recommended to avoid the routine use of corticosteroids in COVID-19 in absence of additional reasons [44], based on a systematic review and meta-analysis on the impact of CS in SARS-CoV-2, SARS-CoV and MERS-CoV, showing delayed virus clearance, no significant reduction of deaths or of ICU admissions [45].
Recently, the results of the randomised RECOVERY trial on the effects of dexamethasone in patients with COVID-19 pneumonia have been published [43]. In this study, 2104 patients receiving dexamethasone 6 mg/day + SOC were compared with 4321 patients treated with SOC alone. The primary outcome measure was the death rate at day 28. Dexamethasone reduced the mortality by one-third in ventilated patients [rate ratio 0.65 (95% CI 0.51–0.882; p < 0.001), and by one-fifth in other patients receiving oxygen only (rate ratio 0.80 95% CI 0.70–0.92); p = 0.002], while there were no differences in patients not requiring respiratory support [1.22 (95% CI 0.93–1.61); p = 0.14]. The discharge rate in patients receiving dexamethasone was 64.6 and 61.1% in those patients receiving SOC, with a significant difference (p: 0.002). The Authors reported no new safety alerts related to dexamethasone therapy.
Hydroxychloroquine
Hydroxychloroquine acts on immune response through the interference with the macrophage antigen processing, and T-cells response, and in in vitro studies, hydroxychloroquine has been found to prevent the viral entry into the cells by inhibiting its binding with the ACE-2 receptor [46].
Hydroxychloroquine for the treatment of COVID-19 patients was investigated giving the drug in association with lopinavir/ritonavir, or with azithromycin, or alone in comparison with SOC (Table 2). Two open-label, retrospective studies, and one RCT evaluated the efficacy and safety of hydroxychloroquine alone or in combination with azithromycin in comparison with SOC.
In a retrospective study from the USA on a large clinical series of 1438 COVID-19 patients with mild-to-moderate disease, 735 received hydroxychloroquine plus azithromycin, 271 hydroxychloroquine alone, 211 azithromycin, and 221 SOC, without hydroxychloroquine or azithromycin [47]. The primary outcome measure was in-hospital mortality, and secondary the abnormal electrocardiographic abnormalities in terms of arrhythmia or prolonged Q–T fraction. Regarding the mortality rate, no significant differences were shown between the individual treatment group in comparison with the SOC group, while in patients receiving combined hydroxychloroquine and azithromycin, and hydroxychloroquine alone, a significantly higher occurrence of arrhythmias resulted (22.7 and 18.5% vs 14.8%; p: 0.001). In the report by Magagnoli et al. [48], the death rate was significantly lower in 395 controls treated with SOC in comparison with 198 and 214 patients receiving hydroxychloroquine and hydroxychloroquine plus azithromycin, respectively (p < 0.001 for both comparisons). Data on safety were not reported. Finally, in a recent RCT of 665 patients from Brazil [49], neither hydroxychloroquine alone, nor hydroxychloroquine combined with azithromycin significantly improved the clinical status evaluated with a seven-point ordinal scale [odds ratio (OD); 1.21 (95% CI 0.69–2.11) and 0.99 (95 CI 0.57–1.73), respectively]. With regard to safety, prolongation of Q–T interval at electrocardiogram occurred in 16.5% of the hydroxychloroquine plus azithromycin group, in 14.3% of hydroxychloroquine alone group, and in 1% of controls, with significant differences (p: 0.009).
Colchicine
Colchicine exerts its anti-inflammatory action through several mechanisms, including inhibition of neutrophil chemotaxis and of the release of IL-1β and IL-18 by blocking the caspase-1 activation [50]. Based on these properties, two studies evaluated the efficacy of colchicine in COVID-19. In an open-label, randomised trial, 55 patients received colchicine, which was added to the SOC treatment at a loading dose of 2 mg in the first day followed by 1 mg/day for a maximum of 21 days; 50 patients treated with SOC therapy served as controls. Disease deterioration, measured by a 7-grade ordinal scale, occurred in 1 (1.8%) of colchicine-exposed patients and in 7 (14%) of controls, with a significant difference (p: 0.02) [51]. In the second study [52], a significant reduction of mortality was recorded in 122 COVID-19 patients treated with colchicine 1 mg/day in combination with SOC therapy, in comparison with 140 control patients receiving SOC alone (16.3% vs 37.1%; p: 0.001). No new safety alerts emerged in colchicine receivers in either trial.
Anti-IL-6
As mentioned above, the severe COVID-19 disease is characterised by a cytokine storm [14]. IL-6 seems to play a pivotal role, hence anti-IL-6 biological drugs tocilizumab, sarilumab, and, recently, siltuximab have been used to treat patients with COVID-19 pneumonia.
Tocilizumab
Based on the eligibility criteria of this review, six clinical of tocilizumab efficacy have been included. However, more than 30 RCTs are ongoing worldwide. The drug was given in single intravenous (IV) infusion at a dose of 8 mg/kg, with a possible second infusion after 24 h if required.
In a retrospective trial of 85 patients with moderate-severe COVID-19 pneumonia, 62 patients were allocated to receive tocilizumab 400 mg/IV (33 patients), tocilizumab 800 mg/IV (2 patients), and tocilizumab 324 mg subcutaneously (SC) (27 patients) associated with SOC (hydroxychloroquine 400 mg/day and lopinavir/ritonavir 1000 mg/day, while 23 patients treated with SOC served as controls [53]. Primary end point was the survival rate at Day 14 from hospitalisation. In the tocilizumab group, a significantly greater survival rate was recorded (p: 0.004), with 2/62 (3.22%) deaths in comparison with 11/23 (47.8%) in the SOC group. Tocilizumab was well tolerated, with no relevant AEs.
The efficacy and safety of tocilizumab was investigated in a retrospective, open-label trial of 65 patients, 32 of whom received one or two tocilizumab 400 mg/IV infusions combined with SOC, and 33 were treated with SOC (lopinavir/ritonavir 1000 mg/day, hydroxychloroquine 400 mg/day, and azithromycin 500 mg/day) [54]. No significant differences were observed in terms of deaths (16% vs 33%; p: 0.150), ICU admission (13% vs 6%; p: 0.43), discharge from hospital (63% vs 49%; p: 0.32). Serious AEs were recorded in 8/32 (25%) tocilizumab-exposed and in 9/33 (27%) in SOC group (p: 0.94).
Primary end point of a Phase II, single-arm trial from Italy was the reduction of 10% of expected lethality at days 14 and 30 in a large cohort of 331 patients with COVID-19 pneumonia treated with 1 or 2 infusions of tocilizumab 8 mg/kg associated with SOC therapy [55]. A validation cohort of 920 COVID-19 patients constituted the control group. Initially, to calculate the sample size, the estimated 30-day lethality was 15%, and the hypothesis was that tocilizumab would halve the mortality to 15%. However, the protocol was amended and the expected lethality rate at 14 and 30 days was redefined at 20 and 35%, respectively. Therefore, the primary outcome measure was readjusted to a 10% reduction of mortality at both time points. The 14-day end point was not reached with a lethality rate of 18.4% (p: 0.52), while at day 30 the lethality rate was 22.4% with a significant reduction with respect to the expected 35% (p: < 0.001). No significant differences in mortality rate were recorded in a retrospective study of 20 patients treated with tocilizumab in comparison with 21 controls receiving SOC [56]. In this trial, allergic reactions, and a severe increase of transaminases attributable to tocilizumab were recorded in 3% of patients. In the retrospective trial of Rojas-Marte et al. [57], the efficacy of tocilizumab was evaluated in 96 COVID-19 patients compared with 97 controls. The overall percentage of deaths was not significantly different, even if a significantly higher number of survivors resulted in non-intubated patients of tocilizumab group. Tocilizumab safety was good, and notably, bacteraemia was significantly more frequent in controls (23.7% vs 12.5%; p: 0.04).
Finally, in a large retrospective study of 179 patients receiving tocilizumab in addition to SOC, a significant reduction of mortality was found in comparison with 365 treated with SOC (7% vs 20%; p: 0.0007) [58]. Of note, in the latter 2 studies, the number of deaths were not significantly different in critical patients who required intubation. New infections occurred in 13% of tocilizumab receivers as compared with 4% of controls treated with SOC (p: 0.0001).
Sarilumab
This IL-6 inhibitor was employed in a clinical series of 28 patients with COVID-19 pneumonia in comparison with 28 controls receiving SOC [59]. The drug was administered at a loading dose of 400 mg/IV at day 1. No significant differences resulted between sarilumab receivers and controls in terms of deaths (7% vs 18%; p: 0.42), clinical improvement (60% vs 64%; p: 0.99), and days to clinical improvement (16 vs 19; p: 0.89). No significant differences in terms of frequency of AEs were recorded between the two treatment groups.
Siltuximab
In an open-label study, 30 patients with moderate COVID-19 pneumonia received 1 or 2 siltuximab infusions at a dose of 11 mg/kg combined with SOC [60], and 30 patients treated with SOC constituted the control group. The 30-day mortality hazard risk was significantly lower in the siltuximab arm [HR 0.462 (95% CI 0.221–0.965); p: 0.0399]. Data on safety were not reported.
Anti-IL-1
All available studies detected high levels of pro-inflammatory cytokine IL-1, particularly IL-1β, in serum of COVID-19 patients [61]. These findings constituted the rationale to employ the IL-1 inhibitor anakinra.
Anakinra is an anti-IL-1 receptor agent blocking the release if IL-1α and IL-1β. The same rationale is behind the testing of canakinumab, and rilonacept, but no trial results are yet available.
In a retrospective study of 52 patients with pneumonia, 29 received anakinra at a high dose of 10 mg/kg/day, 7 received 100 mg twice daily (bid) subcutaneously, in association with hydroxychloroquine 400 mg/day and lopinavir/ritonavir 1000 mg/day, while 16 patients were treated with SOC (hydroxychloroquine and lopinavir/ritonavir) [62]. The outcome measures were the rate of discharge, death, and the percentage of patients with respiratory improvement at day 21. The rate of discharge was not different between the two groups (45% vs 44%), and 7/16 (44%) patients in the SOC group and 3/29 (10%) deaths occurred in the high-dose anakinra group. The difference was statistically significant (Fisher test: p = 0.021). Seven cases of sepsis occurred in the anakinra-exposed group, leading to drug discontinuation, and 4 (14%) had bacteraemia. A significantly lower rate of ICU admission was reported in a retrospective French study of 52 patients treated with anakinra in comparison with 44 controls treated with SOC (35% vs 73%; p: 0.009) [63]. In anakinra-exposed patients, 7 (13%) developed pulmonary embolism, 3 (6%) deep vein thrombosis of the legs, and 1 (2%) arterial thrombosis.
Janus Kinase Inhibitors
The Janus kinase (JAK) family consists of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), and the different JAK inhibitors are targeted against one or more of these JAK members. To date, three anti-JAK drugs have been marketed: tofacitinib, inhibiting JAK1, JAK2, and JAK3, and baricitinib and ruxolitinib, both acting against JAK1 and JAK2. JAK enzymes regulate gene transcription through the phosphorylation of seven STAT factors (STAT-1/2/3/4/5A/5B/6), with consequent T-cell activation and cytokine release from immune cells, including IL-2, IL-6, IL-7, IL-12, IL-15, IL-21, IL-22, IL-23 and IFN-γ [64]. Recently, it has been showed that baricitinib, at therapeutic doses, has a dual action, including the inhibition of cytokine release, and, through its high affinity for AP2-associated protein kinase 1 (AAK1), which is an important endocytosis regulator, the drug inhibits viral cell entry [65]. Such affinity for AAK1 was not seen for tofacitinib and ruxolitinib.
Baricitinib
In the first trial of baricitinib in mild-to-moderate COVID-19 pneumonia, 12 patients receiving the drug in association with lopinavir/ritonavir were compared with 12 controls treated with lopinavir/ritonavir. Co-primary outcomes were safety after 2 weeks of treatment, the 2-week ICU admission rate, and the number of discharges [66].
At week 2, no serious AEs were observed, and baricitinib therapy significantly improved all clinical parameters, with no ICU admissions. The number of discharges was significantly higher in the baricitinib group as compared with controls [7/12 (58%) vs 1/12 (8%); p: 0.027].
A multicentre retrospective trial conducted in seven Italian hospitals evaluated the efficacy of baricitinib in patients with moderated COVID-19 pneumonia [67]. Primary objective was to evaluate the 2-week effectiveness and safety of baricitinib combined with antivirals (lopinavir/ritonavir) in comparison with the SOC, which was hydroxychloroquine and lopinavir/ritonavir. The primary outcome measure was the mortality rate, and the secondary outcome measures were the rate of ICU transfer, rate of hospital discharge, improvement of respiratory parameters, and AE occurrences. Between February and May 2020, 113 consecutive, hospitalised patients treated with baricitinib 4 mg/day, and 78 controls were recruited. At week 2, the death rate was significantly lower in the baricitinib arm compared with controls [0% (0/113) vs 6.4% (5/78) (p-value: 0.010; 95% CI 0.0000–0.4569)]. ICU admission was necessary in 0.88% (1/113) baricitinib treated patients versus 17.9% (14/78) controls [p-value: < 0.0001; (95% CI 0.0038–0.2624)]. Discharge rate was significantly higher in the baricitinib arm [77.8% (88/113) vs 12.8% (10/78) p: < 0.0001; (95% CI 10.79–51.74)]. All clinical and respiratory parameters improved significantly in the baricitinib group, and a significant reduction of positive nasopharyngeal swabs was observed in the baricitinib cohort at discharge, with only 12.5% positive patients compared to 40% (4/10) in the control group. Baricitinib was well tolerated with no AES.
In another retrospective study of 20 patients receiving baricitinib combined with lopinavir/ritonavir, a significant reduction of mortality in comparison with 56 controls treated with SOC was found (5% vs 45%; p: < 0.001) [68]. No safety data are available for this study.
Ruxolitinib
Up to now, one small sample size RCT evaluated the efficacy and safety of ruxolitinib for the treatment of COVID-19 pneumonia [69]. In this trial, 20 patients were allocated to receive ruxolitinib 5 mg/bid/orally associated with SOC, and 21 patients treated with SOC plus placebo constituted the control group. Median time from symptom onset and randomisation was 20 days. At day 28, there were no significant differences between the ruxolitinib arm and controls in terms of time to clinical improvement (primary outcome), mortality rate, and virus clearance (p: 0.147, p: 0.232, p: 0.649, respectively). However, a significant improvement of pulmonary CT findings and of lymphopenia resulted in ruxolitinib-exposed patients. AEs in the ruxolitinib group and controls did not differ significantly.
Other Immune Therapies
Anti-Granulocyte–Macrophage Colony-Stimulating Factor Receptor-Alpha Monoclonal Antibody (Anti-GM-CSF) Mavrilimumab
GM-CSF is a cytokine that activates macrophages and neutrophils to release pro-inflammatory cytokines, including TNF, IL-1, IL-6, IL-23, and IL-12. Moreover, it stimulates the JAK2 signal with consequent cytokine outbreak [70].
Mavrilimumab, a monoclonal antibody, interrupts the hyperinflammation status by blocking the GM-CSF [71]. Based on this rationale, the drug was tested in a single-centre, prospective, open-label trial of 13 non-mechanically ventilated patients with COVID-19 pneumonia in comparison with 26 controls treated with SOC therapy [72]. Mavrilimumab, combined with SOC therapy was administered at the dose of 6 mg/kg in single IV infusion. At day 28, a significant improvement of clinical parameters, and of time to improvement resulted in the active treatment arm (p: 0.030; p: 0.0001, respectively), while no differences were recorded in terms of ICU admission. The drug was well tolerated with no serious AEs.
Anti-CD6 Itolizumab
A small sample size study of 19 patients treated with the anti-CD6 monoclonal antibody itolizumab at the dose of 200 mg/IV (1 or 2 infusions), was recently posted [73]. Admission to ICU and mortality resulted significantly lower in itolizumab cohort in comparison with 53 controls receiving SOC therapy (ICU admission: 28.6% vs 60.6; p = 0.042; deaths: 7.1% vs 42.4%; p = 0.020). No serious AEs were observed.
Discussion
During the past 6 months, COVID-19 pandemic represented a challenging disease for clinicians who faced a new viral infection characterised by an elevated spread in the community, and by a severe clinical course leading to lethal pneumonia in up to 15–20% of the cases. With the diagnostic ability to detect SARS-CoV-2 in the biological samples, the therapeutic strategies were oriented toward the use of antivirals agents in association with standard supportive care.
As a general comment, the overall level of evidence is low, due to the paucity of published randomised controlled trials (Tables 1 and 2). The retrospective, open-label design of most trials was likely due to the urgency of treating patients with the new disease, thus avoiding the long procedures for approval of RCTs. Moreover, the different dosages of employed drugs, the variability of the interval between the onset of symptoms and the therapy starting, the different disease severity of the patients enrolled among the different trials, the absence of standardised controls, and the different outcome measures, may explain the conflicting results.
In the present systematic review, trials with comparable primary end points were included, but the homogeneity of the clinical series was reduced by the association of the tested drug with other therapies, and by several differences in the therapeutic regimen of controls.
Nevertheless, the results of efficacy of antivirals lopinavir/ritonavir and favipiravir, were largely disappointing, with no significant differences as compared with controls in terms of symptoms improvement, ICU admissions, deaths, and viral clearance.
Remdesivir was recently authorised for emergency use by the U.S. Food and Drug Administration (FDA), according to the results of a recent RCT [41], and of an open-label trial [42], based on its effectiveness to significantly reduce the time to recovery, the recovery rate, and the mortality in patients with moderate-to-severe COVID-19 pneumonia [74]. Moreover, remdesivir has been included in the most recent guidelines of National Institute of Health (NIH), USA, for the treatment of COVID-19 patients with mild-moderate disease needing supplemental oxygen, but not requiring high-flow oxygen [75]. On the contrary, an expert panel from Canada recommended remdesivir only for patients with severe disease [76]. However, according to NIH guidelines, remdesivir seems more appropriate during the early phase of COVID-19 disease, preceding the massive cytokine release (Fig. 1). Indeed, this phase is characterised by the highest viral peak [77–79]; hence, the antiviral action of the drug may be better exploited.
Around 20–30% of the symptomatic patients experience a worsening of their condition, with an important release of pro-inflammatory cytokine (so called cytokine storm) [14]. In this phase of COVID-19 the clinical manifestations are mainly related to the activation of T cells (CD4+, cytotoxic granules CD8 T cells) with a massive outbreak of cytokines [21]. Autopsy findings, characterised by abundant CD4+ T-lymphocytes and giant cells, with variable amounts of viral inclusions, seem to confirm the T cell-mediated nature of the alveolar damage, quite similar to that of a pulmonary vasculitis [80, 81].
These findings may explain the limited benefit of the antiviral agents during the second and the third phases of infection, and constitute the rationale to add non-antiviral drugs including corticosteroids, hydroxychloroquine, and cytokine-targeted drugs to the supportive treatment, to reduce the inflammatory phase of the disease (Fig. 1).
Hydroxychloroquine had no beneficial effects when given alone, and combined with azithromycin [47].
Based on the positive results obtained in a large trial of dexamethasone 6 mg/day associated with SOC therapy, with a significant reduction of mortality and a higher number of discharges in 2104 COVID-19 patients with more severe disease [43], the drug was included in the NIH guidelines [75]. These results may constitute the rationale to design a controlled trial testing the efficacy of combined therapy with remdesivir and dexamethasone.
The demonstration of elevated levels of IL-6 in severe COVID-19 represented the rationale to employ monoclonal antibodies that inhibit IL-6, including tocilizumab, sarilumab, siltuximab, and clazakizumab, to reduce the detrimental effects of cytokine storm and mortality. Six clinical trials of clazakizumab are ongoing (ClinicalTrials.gov), but, to date, no results have been published. The available data on tocilizumab are controversial, and the drug seems more effective in non-critical patients. Similarly, disappointing results were obtained with sarilumab. Consequently, Roche and Sanofi released press comments on the failure of trials of tocilizumab and sarilumab to meet the primary end points [82, 83]. However, more recently, in a subsequent press release focused on the Phase III EMPACTA trial, Roche highlighted the significant efficacy of tocilizumab to reduce the likelihood to progress to mechanical ventilation in COVID-19 patients with pneumonia [84].
Two retrospective trials evaluated the efficacy of anakinra. Both studies demonstrated a significant efficacy of the drug in comparison with controls in terms of ICU admission and mortality rate. However, the safety profile of anakinra raises some concerns, as seen in the study of Cavalli et al. [62], where 7 (19.4%) of 36 patients were required to discontinue treatment due to serious AEs (bacterial infections), and 10/52 (19.2%) anakinra-exposed patients developed thromboembolism in the study of Huet et al. [63]. Six trials of canakinumab, an anti-IL-1, are ongoing, but, to date, no data are available. The other anti-IL-1 rilonacept was no longer authorised by the European Medicine Agency.
Most cytokines released in the hyperinflammation phase of COVID-19 act via the JAK-2 and JAK-2 signal transducers with subsequent activation of STAT pathway. Hence, JAK-inhibitors ruxolitinib and baricitinib have been employed to treat moderate-to-severe COVID-19 clinical manifestations.
Up to now, only one RCT of ruxolitinib has been published [69]. The results showed no significant differences between 20 patients treated with the drug and 21 controls receiving SOC regarding the time to improvement (12 vs 15 days; p: 0.147) and the mortality rate (0 vs 3; p: 0.232). Ongoing trials with larger numbers of patients would clarify the efficacy and safety of ruxolitinib for the treatment of COVID-19.
In contrast to other tested anti-JAKs, baricitinib 4 mg/day has a dual action characterised by the inhibition of the cytokine release and the inhibition of SARS-CoV-2 entry into the cells [65].
Three retrospective, open-label trials were included in this review, and showed a promising impact of baricitinib on the clinical course and outcome in patients with moderate COVID-19 pneumonia, with significant reduction of ICU admissions, and mortality rate, with an excellent safety profile after 14 days of treatment [66–68]. Notably, in one study, at hospital discharge, the number of patients with positive nasopharyngeal swabs was significantly lower in baricitinib-treated compared with controls receiving SOC (12.5% vs 40%; p: 0.043) [67]. This finding seems to confirm the dual action of baricitinib on viral endocytosis and on cytokine outbreak. However, due to the low grade of evidence, NIH guidelines did not recommend baricitinib for the treatment of COVID-19. The same was true for ruxolitinib. Nevertheless, in contrast to other therapeutic agents, all three retrospective trials of baricitinib demonstrated comparable results of efficacy, and we are confident that the ongoing RCTs (ClinicalTrials.gov) would definitively clarify if these compounds are useful in the management of COVID-19. Confirming the previous retrospective studies, a recent press release of Lilly on the preliminary results of the National Institute of Allergy and Infectious Diseases ACTT-II trial on more than 1000 COVID-19 patients reported that baricitinib combined with remdesivir significantly reduced the time to recovery (primary end point) [85]. Based on the ACTT-II trial of baricitinib, Lilly will discuss the potential for emergency use authorisation with the FDA.
Conclusion
Based on the results of present review, beyond the supportive therapy, up to now the best therapeutic approach for COVID-19 may be a three-step combination therapy, including dexamethasone 6 mg/day, remdesivir 100 mg/day (200 mg loading dose at first day), and, to target the immune dysregulation triggered by the SARS-CoV-2 infection, baricitinib 4 mg/day, or tocilizumab. The promising results of anakinra should be confirmed by the ongoing RCTs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Declarations
Funding
This research received partial support from the Italian Ministry of Health, Ricerca corrente Linea 1 and 4. No other specific grant from additional public, commercial, or not-for-profit sectors was obtained.
Conflicts of interest/Competing interests
Authors have nothing to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Fabrizio Cantini had the conceptual idea for the review. Fabrizio Cantini and Delia Goletti equally contributed to the manuscript. The literature search was performed by Fabrizio Cantini, Delia Goletti, Laura Niccoli, and Rosario Foti. The PRISMA flow diagrams, and figures were prompted by Fabrizio Cantini, Delia Goletti, Linda Petrone and Saied Najafi Fard. Data analysis was done by Fabrizio Cantini, Delia Goletti, and Rosario Foti. The first draft of the manuscript was written by Fabrizio Cantini and Delia Goletti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.WHO. Coronavirus 2019. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Accessed 14 Mar 2020.
- 2.Ji W, Wang W, Zhao X, Zai J, Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci USA. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention, Atlanta, USA, May 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 20 July 2020.
- 10.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/s2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/s2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical characteristics of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prompetchara F, Ketloy C, Tanapat Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/ap-200220-0772. [DOI] [PubMed] [Google Scholar]
- 18.Cossarizza A, De Biasi S, Guaraldi G, Girardis M, Mussini C, Modena Covid-19 Working Group SARS-CoV-2, the virus that causes COVID-19: cytometry and the new challenge for global health. Cytometry A. 2020;97(4):340–343. doi: 10.1002/cyto.a.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song JW, Zhang C, Fan X, Meng FP, Zhu X, Xia P, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11(1):3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quartuccio L, Sonaglia A, Pecori D, Peghin M, Fabris M, Tascini C, et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J Med Virol. 2020 doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol. 2020;20(6):345–346. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2020. Features, evaluation, and treatment of coronavirus (COVID-19) [PubMed] [Google Scholar]
- 28.Cure E, Kucuk A, Cumhur MC. Cyclosporine therapy in cytokine storm due to coronavirus disease 2019 (COVID-19) Rheumatol Int. 2020 doi: 10.1007/s00296-020-04603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Softic L, Brillet R, Berry F, Ahnou N, Nevers Q, Morin-Dewaele M, et al. Inhibition of SARS-CoV-2 infection by the cyclophilin inhibitor alisporivir (Debio 025) Antimicrob Agents Chemother. 2020 doi: 10.1128/aac.00876-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez Y, Novelli L, Rojas M, De Santis M, Acosta-Ampudia Y, Monsalve DM, et al. Autoinflammatory and autoimmune conditions at the crossroads of COVID-19. J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu YF, Chien CS, Yarmishyn AA, Lin YY, Luo YH, Lin YT, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21:2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye XT, Luo YL, Xia SC, Sun QF, Ding JG, Zhou Y, et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24(6):3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 35.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 38.Choy KT, Wong AYL, Kaewpreedee P, Sia SF, Chen D. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;29:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;16(395):1569–1578. doi: 10.1016/s0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beigel JH, Tomashek KM, Dodd LE, Metha AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PubMed] [Google Scholar]
- 42.Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horby P, Lim WS, Emberson J, Mafham M, Bell JL, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. Clinical management of COVID-19. Interim guidance. 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 3 June 2020.
- 45.Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–1511. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastick KA, Okafor EC, Wang F, Lofgren SM, Skipper CP, Nicol, et al. Review: Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open Forum Infect Dis. 2020;7(4):ofaa130. doi: 10.1093/ofid/ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg ES, Dufort EM, Tomoko U, Wilbeschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magagnoli J, Narendran S, Pereira F, Cumming TH, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. MED. 2020 doi: 10.1101/2020.04.16.20065920v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/nejmoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57(suppl_1):i4–i11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarsi M, Piantoni S, Colombo E, Airò P, Richini D, Miclini M, et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrone F, Piccirillo MC, Ascierto P, Salvarani C, Parrella R, Marata AM, et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase II trial. MedRxiv, 2020 prep-print-medrxiv.org. 10.1101/2020.06.01.20119149.
- 56.Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine et Maladies Infectieuses. 2020 doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rojas-Marte GR, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab—a case-controlled study. QJM. 2020 doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. http://www.thelancet.com/rheumatology. Published online June 24, 2020. 10.1016/S2665-9913(20)30173-9.
- 59.Della-Torre E, Campochiaro C, Cavalli G, De Luca G, Napolitano A, La Marca S, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. medRxiv preprint. 10.1101/2020.04.01.200. Posted June 20, 2020.
- 61.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavalli G, De Luca G, Campochiaro C, Della Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19 acute distress respiratory syndrome and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/s2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:117–127. doi: 10.1177/1759720X18776224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020 doi: 10.1016/51473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantini F, Niccoli L, Nannini C, Matarrese D, Di Natale ME, Lotti P, et al. Retrospective, multicenter study on the impact of baricitinib in COVID-19 moderate pneumonia. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, et al. Baricitinib restrains the immune dysregulation in COVID-19 patients. medRxiv preprint. 2020. 10.1101/2020.06.26.20135319. [DOI] [PMC free article] [PubMed]
- 69.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiomi A, Usui T. Pivotal role of GM-CSF in autoimmunity and inflammation. Mediat Inflamm. 2015;2015:568543. doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinblatt ME, McInnes IB, Kremer JM, Miranda P, Vencovsky J, Guo X, et al. A randomized phase IIb study of mavrilimumab and golimumab in rheumatoid arthritis. Arthritis Rheumatol. 2018;70:49–59. doi: 10.1002/art.40323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Luca G, Cavalli G, Campochiaro C, Della Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatology. 2020 doi: 10.1016/s2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramos-Suzarte M, Diaz Y, Martin Y, Calderon NA, William Santiago, Vinet O, et al. Use of a humanized anti-CD6 monoclonal antibody (itolizumab) in elderly patients with moderate COVID-19. medRxiv preprint. 10.1101/2020.07.24.20153833. Posted July 30, 2020. [DOI] [PMC free article] [PubMed]
- 74.Food and drug administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. May 1, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed 3 July 2020.
- 75.NIH July 2020. Disease 2019 (COVID-19) treatment guidelines. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf. Accessed 2 Aug 2020.
- 76.Rochwerg B, Agarwal A, Zeng L, Leo YS, Appiah JA, Agoritsas T, et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020;370. 10.1136/bmj.m2924(Published 30 July 2020). Accessed 2 Aug 2020. [DOI] [PubMed]
- 77.Huang JT, Ran RX, Lv ZH, Feng LN, Ran CY, Tong YQ, et al. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan. China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 79.Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 81.Ackermann M, Verleden SE, Kuehnel M, Averich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29, 2020. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. Accessed 12 Sept 2020.
- 83.Sanofi provides update on Kevzara (sarilumab) Phase III trial in severe and critically ill COVID-19 patients outside the U.S. September 1, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-09-01-07-00-00. Accessed 12 Sept 2020.
- 84.Roche’s phase III EMPACTA study showed Actemra/Roactemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. September 18, 2020. https://www.roche.com/media/releases/med-cor-2020-09-18.htm. Accessed 20 Sept 2020.
- 85.Lilly press release archives. Baricitinib in combination with remdesivir reduces time to recovery in hospitalized patients with COVID-19 in NIAID-sponsored ACTT-II trial. September 14, 2020. http://lilly.mediaroom.com/2020-09-14-Baricitinib-in-Combination-with-Remdesivir-Reduces-Time-to-Recovery-in-Hospitalized-Patients-with-COVID-19-in-NIAID-Sponsored-ACTT-2-Trial. Accessed 14 Sept 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.