Abstract
This study assessed the disinfection using 70% ethanol; H2O2-quaternary ammonium salt mixture; 0.1% sodium hypochlorite and autoclaving of four 3D-printed face shields with different designs, visor materials; and visor thickness (0.5-0.75 mm). We also investigated their clinical suitability by applying a questionnaire to health workers (HW) who used them.
Each type of disinfection was done 40 times on each type of mask without physical damage. In contrast, autoclaving led to appreciable damage.
Key Words: Autoclaving, 3D printer face shield
The World Health Organization (WHO) established that the impact on the healthcare system due to the additional clinical and operational demands was substantial during the COVID-19 pandemic, which could lead to failure to prevent and protect Health Workers (HW).1 , 2
The facial protector is one of the essential PPE for HW in the management of COVID-19 patients.3 , 4 The Health Care Infection Control Practices Advisory Committee(HICPAC) recommend the face/eye protection used as an adjunct to other facial protection for preventing transmission of infectious agents in health care settings.5
The restricted access to PPE supplies such as medical masks, respirators, goggles, face shields, aprons, and gloves are leaving frontline HW at risk to develop COVID-19 during the pandemic.1 , 6 Under the circumstances, to provide HW with sufficient PPE to increase facial protection, face shields could be 3D printed.7 Regardless of not featuring high productivity as injection molding processes, 3D-printing allows fast-response, on-demand manufacturing of face shields by a broad spectrum of producers, including 3D-printer equipped laboratories in universities, schools, companies, and even at home.
For this reason, the aim of this study was to evaluate the face shields obtained by 3D-printing technology, test chemical disinfectants and autoclaving to disinfecting the models and to assess the comfort, visibility, and feasibility on real life.
Methods
Setting
The present study was conducted at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo(HC-FMUSP), a public, tertiary and teaching hospital, affiliated to the Unified Health System with 2,000 beds, of which a building with 900 beds, including 250 intensive care beds dedicated to COVID-19. About 6,000 HW dedicated to taking care of COVID-19 as well.
Face shield visor visual integrity after chemical disinfection. The face shields produced using a 3D printer were kindly donated from Makers contra covid, InovaUSP- University of São Paulo Innovation Center, FAU USP - School of Architecture and Urbanism of the University of São Paulo and INSPER. Different thickness and materials were tested: Polyethylene glycol of 0.5 mm and 0.75 mm, polycarbonate of 0.75 mm (Makers contra COVID), polyethylene terephthalate (PET) of 0.5 mm (Facens) and glycol-modified polyethylene terephthalate (PETG) of 0.5 mm (InovaUSP and FAU USP). The visual integrity, such as crack and visibility were examined after several chemical disinfections with 70% ethanol (Farmax-Brazil), H2O2-quaternary ammonium salt mixture (3M-Brazil), 0.1% sodium hypochlorite (Proaction-Brazil) or water (negative control). For disinfection, a gauze soaked with the test solution was used for one minute and allowing for spontaneous drying before using. Postdecontamination vapors were evaluated at the laboratory, one physician and one technician wore the face shields 1 minute, 3 minutes, and 5 minutes after disinfection.
Headbands for face shield after chemical and autoclave disinfection. The supports printed using a 3D printer were donated from IMO.3D for disinfection tests. Different materials (Tritan HT, PLA easyfill, ASA WP, ABS PT and PETG XT), and layer thickness (0.15 mm, 0.30 mm, 0.60 mm) were tested for chemical substances such as 70% ethanol (Farmax-Brazil), H2O2-quaternary ammonium salt mixture (3M-Brazil), 0.1% sodium hypochlorite (Proaction) or water (negative control). Other disinfection method using autoclave (121°C for 15 minutes) was evaluated. The headbands and visors disinfection was done 30 and 40 times, respectively.
Face shields use and disinfection training. The infection control committee team composed of 5 physicians and 5 nurses evaluated all 3-D printed design face shield; They were responsible for the PPE training that included don and doff PPE and face shield disinfection using 70% alcohol. The HW was trained in face-to-face sessions and with videos and posters. Use this video link: https://youtu.be/yksZT9TvKFg and https://youtu.be/zeku1WRbl_s. Face shield was recommended for all HW as an additional protection; HW were instructed to use N95 respirators or surgical masks plus face shield according to the type of contact with patients. HW who provided direct patient care wore N95 masks and scrubs during their entire shifts. When examining or touching patients they added disposable gloves, a gown and a face shield. The face shields were communal and reused after 70% alcohol disinfection. Health workers wore the same face shield during duty, and disinfection was done after each patient contact.
Questionnaire. An online SurveyMonkey questionnaire comprised questions regarding comfort, visibility and feasibility of the 3D face shields was applied by WhatsApp for physicians were working at the Intensive Care Units, Emerging Department and Infectious Diseases wards.
Results
At the moment, 3,343 COVID-19 patients were hospitalized; a total of 2,778 HW were personally trained and 30,000 face shields were used during the COVID-19 pandemic in our hospital.
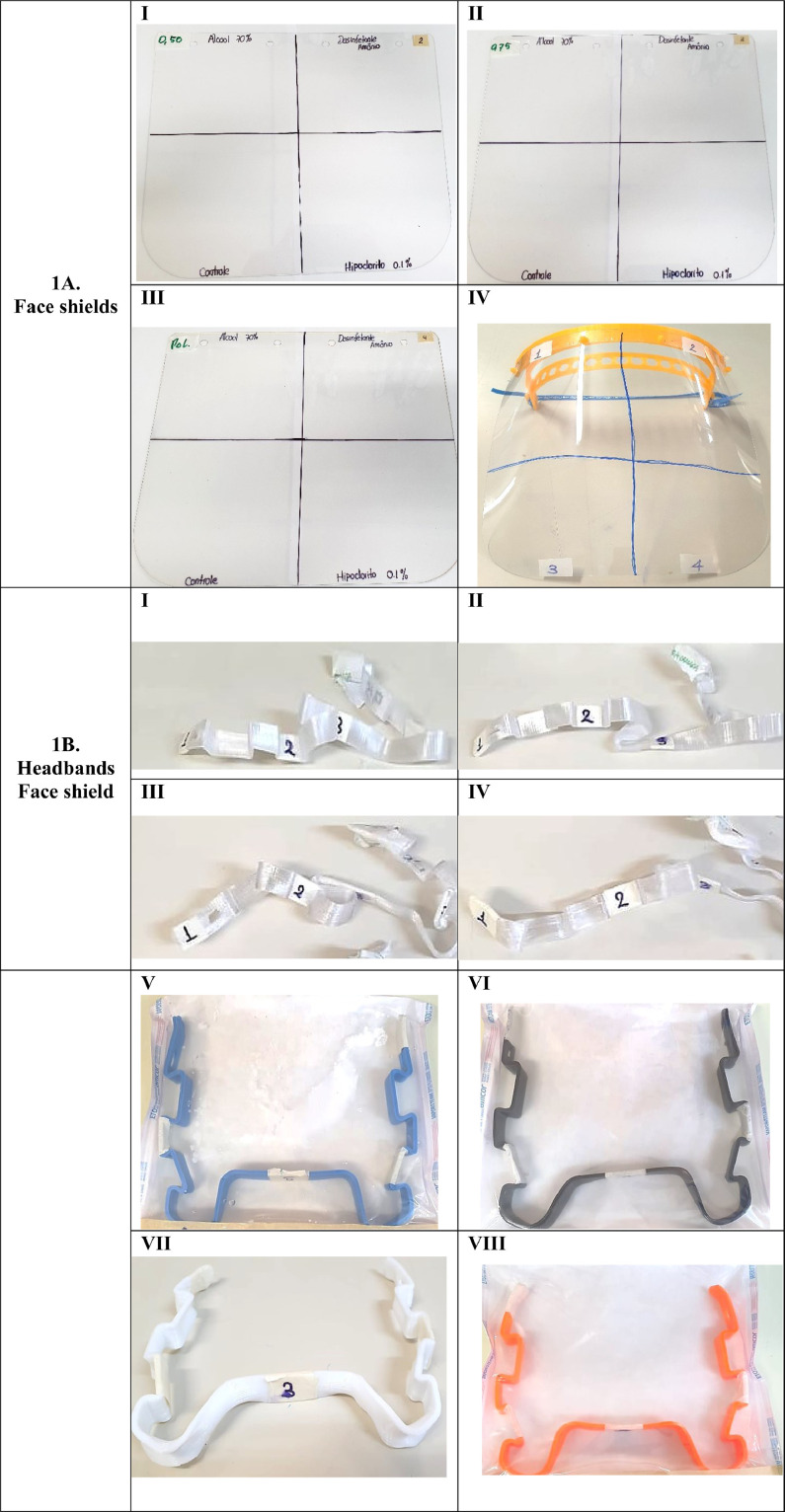
Face shield visor visual integrity after chemical disinfection. We assessed the potential reduction in the visual quality of the shield after cleaning in a subset of interventions. None of the face shields materials and layer thickness presented damages after up to 40 disinfection with ethanol 70%, H2O2-quaternary ammonium salt mixture or 0.1% sodium hypochlorite (Fig 1A). To reduce potential vapor damage, we recommend waiting 3-5 minutes after each disinfection, as 1 minute after 70% alcohol disinfection vapors can cause eye redness.
Fig 1.
(A) Face shields of different materials cleaned 40 times with ethanol 70% (1), H2O2-quaternary ammonium salt mixture (2), H2O (3) or 0.1% sodium hypochlorite (4). Polyethylene glycol sheets of 0.5 mm (I), or 0.75 mm (II), polycarbonate sheet of 0.75 mm (III) and design of glycol-modified polyethylene terephthalate (PETG) of 0.5 mm (IV). (B) Headbands of different materials and thickness were tested for chemical substances and sterilized in autoclave, (I) Tritan HT of 0.3 mm (II) of 0.15 mm (III) of 0.6 mm (smooth model) (IV) of 0,6 mm (rolled model) (V) PLA easyfill (VI) ASA WP (VII) ABS PT and (VIII) PETG XT.
Headbands for face shield after chemical and autoclave decontamination. We observed that after 30 times of chemical disinfection, none of the face shields headbands show any alteration in the visible physical structure, as occurred with the face shields visors. However, after autoclave decontamination, the supports of PETG XT and TRITAN HT suffered appreciable damage (Fig 1B). It was observed reduction in size, material wrapping, and some cracking after the effect of temperature and pressure of the autoclave; this leading to strength reduction, through triggering fibers micro buckling.
The questionnaire about 3D-printing face shield. The first column described the variables assessed. The answers were classified using four categories: Very good, good, regular or bad. Most of the evaluations showed very good answers about mobility, visibility, mask removal, and disinfection (Table 1 ). All designs were considered suitable, and there was no important difference between them. Nevertheless, two of the designs (GRU and INSPER) received higher user assessment grades. InovaUSP design was significantly lighter, demanding less material to be produced.
Table 1.
Summary of 4 models of 3D-printed face shield evaluation in real life
| InovaUSP Design model I (n = 4) |
VivaSUS Design model II (n = 2) |
GRU Design model III (n = 1) |
INSPER Design model IV (n = 1) |
|
|---|---|---|---|---|
| Print volume requirement | 120 × 135 × 16.5 mm | 200 × 200 × 20 mm | 200 × 250 × 20 mm | 200 × 250 × 20 mm |
| Filament weight (headband) | 12.8 g |
25g | 27.60g | 42.58g |
| Total weight | 68g | ND | 98.50g | 145.30g |
| Printing time | 1 h 20 min* | 1 h 20 min | 1 h 40 min* | 1 h 50 min* |
| Tools for assembling | Manual assembly | Manual assembly | Manual assembly | Manual assembly |
| Comfort | Very good Good |
Good | Very good | Very good |
| Mobility | Very good Good Regular |
Very good | Very good | Very good |
| Stability | Good | Very good Good |
Very good | Very good |
| Condensation | Good Regular |
Very good Regular |
Good | Good |
| Compatibility use glasses | Very good Good |
Very good | Very good | NA |
| Visibility | Very good Good |
Very good Good |
Very good | Very good |
| Lateral protection | Very good Good |
Very good Good |
Regular | Very good |
| Mask removal | Very good Good |
Very good Good |
Very good | Very good |
| Disinfection | Very good Good |
Very good Good |
Very good | Very good |
ND, not done.
Estimated weight: calculated from the density of the PETG sheet.
Discussion
The present study evaluated different 3D-printed face shield designs using an online questionnaire as well. We observed excellent stability, comfort, visibility, and feasibility after disinfection. The design usual made up 3 elements: A visor made from optically clear material, an interlocking headband, and a head strap that ties the two together.7 The National Institute for Occupational Safety and Health guidelines detail that face/eye protection must allow for appropriate peripheral vision, comfortable, and adjustable to ensure a secure fit.8
Studies that used cough simulation demonstrated that face shields reduce the risk of inhalation exposure up to 95% immediately following aerosol production.9 However, it protection decreased with smaller aerosol particles and 30 minutes after cough simulation, due to persistence of airborne particles and particle flow around the sides of the mask.9 Therefore, the face shields should not be used as primary protection for preventing respiratory disease transmission, but they can be used as an adjunct to other facial protection such as surgical mask or N95 respirators.10 , 11 In addition, the face shield can be useful in a scenario of N95 respirators shortage and need for reuse, as a supplementary protection to avoid respirators contamination during patient care.
Although, the number of survey participants was a limitation of our study. The HW pointed out that face shields were preferred by them than glasses because they were more comfortable and fogged less easily, and the perceived protection was higher.2 Currently, reuse and disinfection of these face protectors are highly needed due to an imminent shortage of supply.
Disinfection of face shields is needed for reuse of them with safety, but improper decontamination could damage the blocking structure of this PPE. We evaluated the appearance of the 3D-printed face shields pre and postdisinfection, but there was no damage due to cleaning products (ethanol 70%, sodium hypochlorite 0.1% and H2O2-quaternary ammonium salt mixture). On the other hand, autoclaving was not useful and led to important physical damage.
In conclusion, we observed that chemical disinfection with ethanol 70%, sodium hypochlorite 0.1%, and H2O2-quaternary ammonium salt mixture of the 3D-printed face shields, made by different material is suitable and can be performed repeatedly without demonstrating physical alterations. In contrast, it seems that autoclaving is not an ideal method to decontaminate 3D-printed face shields as it led to appreciable damage.
Personal Protective Equipment COVID-19 Hospital das Clinicas da Faculdade de Medicina University of São Paulo - Task Force: Sueli Izaki; Maura Saraloli de Oliveira.
Acknowledgments
The authors would like to acknowledge Denilton Donizetti, Giuliana Barajas, Dayrin Vanessa Tarazona Carvajal, Yuri Spuras, Daniel Santos Souza and Emilio Leocadio for participating in the face shield production and assembly and Personal Protective Equipment COVID-19 Hospital das Clinicas da Faculdade de Medicina University of São Paulo - Task Force: Sueli Izaki; Ana Catarina Nastri; Pedro Mendes.
Footnotes
Conflicts of interest: None to report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.10.008.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.WHO Infection Prevention and Control Guidance for COVID-19. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-andcontrol. Accessed May 29, 2020.
- 2.WHO Shortage of personal protective equipment endangering health workers worldwide. Available at:https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. Accessed May 20, 2020.
- 3.Roberge RJ. Face shields for infection control: a review. J Occup Environ Hyg. 2016;13:239–246. doi: 10.1080/15459624.2015.1095302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapoval M, Gaultier AL, Del Giudice C, et al. 3D-printed face protective shield in interventional radiology: evaluation of an immediate solution in the era of COVID-19 pandemic. Diagn Interv Imaging. 2020;101:413–415. doi: 10.1016/j.diii.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Heal. 2020;5 doi: 10.1136/bmjgh-2020-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MM, Parab SR. Simple economical solution for personal protection equipment (face mask/shield) for health care staff during COVID 19. Indian J Otolaryngol Head Neck Surg. 2020;2019:1–5. doi: 10.1007/s12070-020-01863-4. http://link.springer.com/10.1007/s12070-020-01863-4 Available at. Accessed October 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesemann C, Pieralli S, Fretwurst T, et al. 3-D printed protective equipment during COVID-19 pandemic. Materials (Basel) 2020;13:1997. doi: 10.3390/ma13081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 SUPPL. 2) doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godoy LRG, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19 related supply shortages. Am J Med. 2020 doi: 10.1016/j.amjmed.2020.04.002. http://www.ncbi.nlm.nih.gov/pubmed/32330492%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7172844 Available at. Accessed October 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koven S. They Call Us and We Go. N Engl J Med. 2020;382:1978–1979. doi: 10.1056/NEJMp2009027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.