Abstract
Healthcare expenditure is continually increasing and projected to accelerate in the future, with an increasing proportion being spent on interventional radiology. The role of cost effectiveness studies in ensuring the best allocation of resources is discussed, and the role of National Institute of Health and Care Excellence (NICE) in determining this. Issues with demonstrating cost effectiveness have been discussed, and it has been found that there is significant scope for improving cost effectiveness, with suggestions made for how this can be achieved. In this way, more patients can benefit from better treatment given limited healthcare budgets.
Importance of evaluation of cost effectiveness in healthcare
Pre-COVID-19, approximately 10% of UK gross domestic product (GDP) was spent on healthcare, with planned spending for Department of Health and Social Care for 2019/20 being £140 billion: contrast this with the 3.5% of GDP spent for the first full year of the NHS in 1948 (£373 million; approximately £13 billion in today's money).1 The impact of COVID-19 to the national economy has been estimated to add at least £70 billion to government borrowing in 2020 alone.2 Clearly, there is currently extreme pressure on spending on public finance, so it is important to spend on healthcare as effectively as possible, and demonstrate that this has been done. NICE (the National Institute of Health and Care Excellence) was set up to help with this (Box 1 ).
Box 1. Role of NICE.
National Institute for Health and Care Excellence (NICE).
The Department of Health proposed the formation of NICE in 1997 with the aim of creating consistent evidence based guidelines and end unevenly distributed local rationing of treatment (“postcode lottery”), brought about by several issues:
-
1.
There had been increasingly rapid growth in the breadth and depth of medical knowledge, making it difficult for clinicians, patients, and commissioning groups to draw meaningful comparisons and conclusions;
-
2.
There were multiple conflicting and incomplete local guidelines for management of clinical problems, e.g., prostatism, heavy uterine bleeding;
-
3.
There were no agreed consensus criteria to compare different technologies, e.g. angioplasty versus vascular surgery, or open hysterectomy versus uterine artery embolisation versus laparoscopic surgery;
-
4.
There was no future horizon scanning of medical technologies. NICE was launched originally as the National Institute for Clinical Excellence in April 1999, by the Health Secretary, Frank Dobson. The first Chair was Sir Michael Rawlins, a Professor of Clinical Therapeutics.
NICE's remit in IR is to assess the efficacy of procedures, and conduct cost-effectiveness assessment with a view to formulating guidelines for management of clinical conditions. Where the data are sparse or insufficient, NICE will recommend further studies, registries, and ongoing audits to gather further information.
NICE carried out its first health technology appraisal in 2000, and set up the Interventional Procedures Advisory Committee in 2002; the first meeting of which considered uterine artery embolisation for fibroids. Committees to consider new medical technologies and devices were set up in 2009, and in 2015 for highly specialised technologies, which particularly include interventional radiology. By 2018, NICE had published its 500th technology appraisal.
Alt-text: Box 1
Cost-effectiveness studies (CES) can be helpful 3 in guiding how we should spend public resources, as1: CES provide an objective system to compare the complete range of relevant alternatives, from invasive treatments to conservative management. Costs for the same procedure can vary widely, e.g., the “Getting it Right First Time” (GIRFT) Vascular Surgery report noted that reported cost for elective endovascular aortic repair (EVAR) varied between £2,251 and £19,690 for no apparent reason and with no indication that lower cost procedures were less effective 2 , 4; CES encompass a wider societal perspective than just the clinician's or patient's point of view alone, helping demonstrate equitable resource allocation in a publicly funded service3; CES allow evaluation of short- and long-term costs and benefits, which are often under- or overestimated; and4 CES provide an explicit and accountable framework for decision making, which can be re-examined as data accumulate, particularly important with evolving techniques and experience as in interventional radiology (IR).
Increasing costs of healthcare
Healthcare costs continue to grow faster than the economy as measured by GDP: since 1978 public spending on health in the UK has increased by 3.8% per annum on average while GDP has grown by 2.2%, with similar trends in other European countries ,5 , 6 due to: demographic change, chronic medical conditions and rising cost of medical infrastructure and medical technology.
Demographic change
As mean population age has risen due to increasing life expectancy and falling fertility rates, so has healthcare expenditure. The precise reasons for this are complex, but broadly divide into the “Sisyphus effect” 7 (more elderly expect to be fit and independent into older age, requiring more medical resources, creating more elderly), and the “multimorbidity hypothesis” (decreasing mortality rates create a larger pool of less fit multimorbid elderly 8). Most costs arise in the last 6 months of life regardless of age,9 , 10 where IR may be a highly acceptable substitute for surgery; for example, embolisation for gastrointestinal bleeding, or an useful option for symptom palliation, such as placement of ascitic or pleural drains.11 , 12
Chronic medical conditions
Accelerating rates of obesity and diabetes worldwide are significantly increasing the incidence of vascular disease and cancer. The number of adults with diabetes is projected to increase worldwide by 48% by 2045.13 The risk of peripheral arterial disease in patients with diabetes is increased by a factor of 2.72, more than smoking (1.88),14 with a prevalence of 30% in those aged >50 yearshttps://paperpile.com/c/e2SMlw/2hzYS,15 and more diffuse and infrapopliteal disease, which is challenging to treat.16 The increasing demand for rapid diagnosis, treatment, and palliation means interventional oncology is now regarded as a vital and highly cost-effective component of cancer care.17
Rising cost of medical infrastructure and medical technology
About half of the increased overall healthcare expenditure in high-income countries is due to increasing costs of medical technology, including equipment and drugs.6 , 18, 19, 20, 21 When analysed in more detail, costs have actually fallen in some conditions but increased disproportionately in conditions with high-technology interventions, including IR.22
These factors indicate increased demand for, and cost of, IR in the future.
CES
CES can consider not only costs to the healthcare system, but also costs incurred by the patient, such as loss of ability to look after family, and wider societal costs. The benefits calculated have developed from unadjusted life years to quality adjusted life years (QALYs) gained, where quality of life is gauged using questionnaires such as EQ-5D or SF-6D,23 , 24 and multiplied by years of survival, so that 1 QALY= 1 year of perfect health, reducing to 0.5 for poor health and zero for death. QALYs provide a consistent and transparent means of comparing the outcomes of different surgical, IR, or medical procedures.
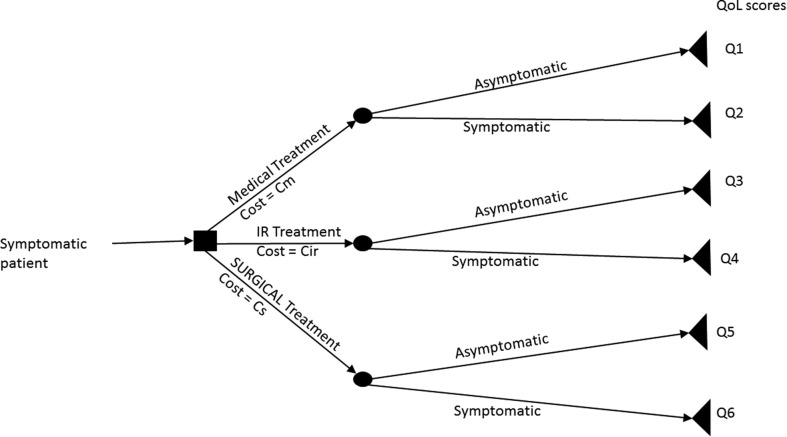
CES often show states of health using decision trees (Fig 1 ). These represent the points of treatment decision or choice (with probability of choice) as decision nodes, leading to various outcomes represented by branches, which each lead to a chance node with probabilities of different clinical outcomes, repeating until the patient has no further decisions or changes in risk, represented by a terminal node. Costs and health outcomes are ascribed to each branch. The tree thus generated can be “rolled back” to calculate the overall costs and outcomes for each treatment option.25
Figure 1.
Illustration of the decision tree used in health economics assessments. The patient's treatment journey is mapped out using various decision points, with costs and Illustration of a Markov model showing the probability of transitioning between health states, over fixed periods of time outcomes assigned to each branch.
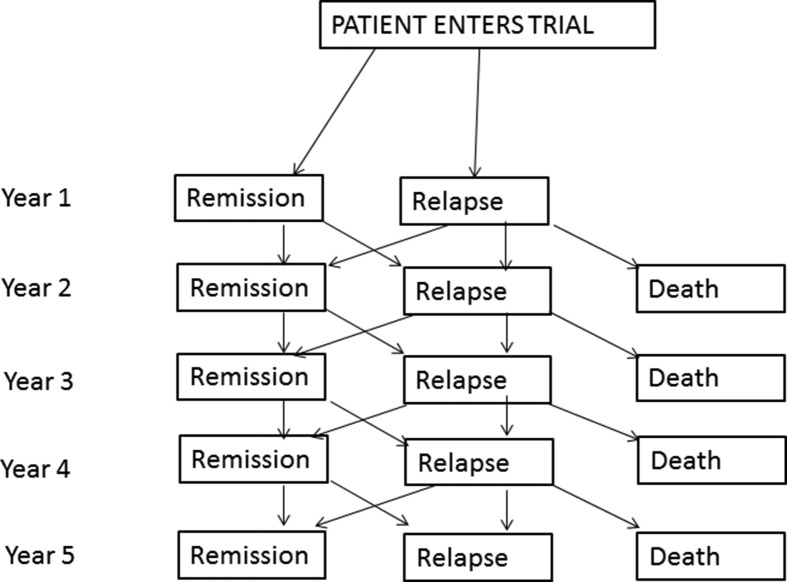
In the healthcare scenario, the patient may have a relapsing or recurrent condition and can transition back and forth from different health states, e.g., remission and active disease, so decision trees that only allow one-way progression become unmanageable; these are better demonstrated by Markov models (Fig 2 ), which allow two-way progression and map the health states and the probability of transitioning between these states after an intervention during a given time cycle. The time spent in each health state is associated with a cost and outcome, and these can be aggregated to calculate overall costs and QALYs for each treatment option.26
Figure 2.
Illustration of a Markov model showing the probability of transitioning between health states, over fixed periods of time.
The incremental cost effectiveness ratio (ICER) is calculated as the difference in cost divided by difference in QALYs between different strategies, compared to an alternative. For example, the EVAR1 and DREAM trials showed an ICER of £8,579 per QALY for EVAR in patients unfit for surgical repair.27 In the National Health Service (NHS), a ICER threshold of £20,000 to £30,000 per QALY (and up to £50,000 for end-of-life treatments) has been considered reasonable to decide whether a treatment is cost effective versus baseline, and although it has been emphasised by NICE that thresholds are not fixed,28 they have attracted attention for seeming to put an arbitrary price on health, or being associated with rationing of resources.29 There is no explicit rationale for the cost per QALY, although some commentators relate it to the share of GDP per person in an economy, or average household income. The threshold can cause issues with new IR technologies where initial prices may be higher reflecting development costs or low volume production runs.30 Notably the Affordable Care Act in the USA forbad the use of cost per QALY as a threshold, to counter accusations of enacting “big-government healthcare” or setting up “death panels”.31
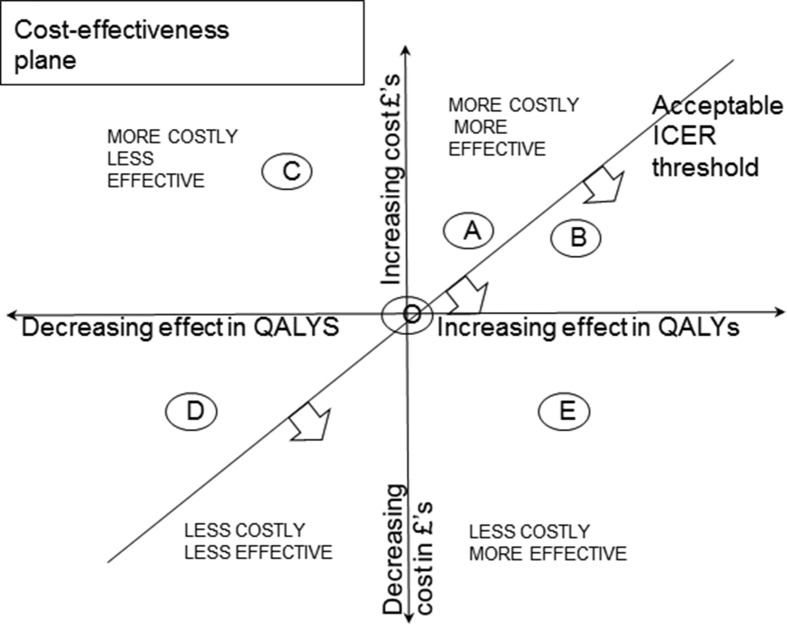
Fig 3 illustrates the ICER cost-effectiveness plane, which plots cost in pounds sterling against effect in QALYs. The ICER threshold represents the acceptable threshold to decide whether the increased effect of a procedure justifies its cost. Compared to the baseline procedure O at the origin, treatments A and B are more effective but more costly, C is more costly and less effective, D is less costly and less effective, but above the threshold, and E is less costly and more effective, so the clear winner. The usual situation is A or B: in this case, it can be seen that B would be preferable as it is below the ICER threshold.
Figure 3.
Illustration of ICER (incremental cost-effectiveness plane) and derivation of ICER threshold.
Sensitivity analyses (SA) vary key inputs (e.g., probability of treatment efficacy or price of equipment) where there is uncertainty of real or estimated parameters and can determine which factors in the model are the main drivers in cost effectiveness.3 If the ICER varies little when inputs are varied, the findings can be considered more robust. SA can yield unexpected insights into cost effectiveness and are thus a powerful tool in designing pathways to maximise benefits gained for a given budget and improve a procedure's ICER. For example, contrast-enhanced follow-up post-EVAR with abdominal radiography and ultrasound has been shown to be more cost-effective than computed tomography (CT).32
Although widely used, QALYs are inherently subjective as they are based on valuing the patient's quality of life (QoL), raising ethical, methodological, and disease-specific issues.33 34 Ethical issues include valuing another's QoL when one can have no experience of having the specific physical disorder. Methodological issues involve relating this subjective judgement to one or more of many different health utility scores, which introduces further intra- and interobserver variability (e.g., varying among different age groups, nationalities). Disease-specific issues arise in less common conditions where estimates of the impairment caused vary widely. Uncertainties also arise in costing items, such as time off work after surgery or an interventional procedure is highly variable between patients and difficult to quantify.
The rapid growth in health CES has created the need for a checklist to ensure that different CES are complete and contain enough information to be compared and combined with each other for meta-analysis. The CHEERS (Consolidated Health Economic Evaluation Reporting Standards) checklist 35 is recommended when planning CES to ensure quality and completeness. Many recent radiological studies assessed by this checklist are incomplete.36
The quality of the conclusions drawn from CES are only as robust as the data used, and if these are drawn from a wide range of disparate studies, or are biased or not generalisable, this will limit the strength of the study. Choice of control groups or comparison strategies will influence the ICER and sensitivity results. The time horizon will also have a large effect, especially because of compound discounting.
Cost effectiveness in IR
There are already some conditions where IR has been shown to provide a more cost-effective solution (Table 1 ). Areas where IR can improve and better demonstrate its cost effectiveness are discussed below.
Table 1.
Examples of interventional radiology procedures proven more cost effective than surgery.
| Tumour ablation | Liver metastases https://paperpile.com/c/e2SMlw/BJe9+Ctuh+YPj9+5VMv37, 38, 39, 40 |
| Hepatocellular carcinoma (single or multiple nodules <3 cm) https://paperpile.com/c/e2SMlw/O4BE+Vszk+5VMv40, 41, 42 | |
| Renal cell carcinoma <4 cm https://paperpile.com/c/e2SMlw/PaUH+tCl143,44 | |
| Embolisation | Uterine fibroids initially although equipoise after ∼ 5 years https://paperpile.com/c/e2SMlw/tkkc45 |
| Endovascular aortic repair | Ruptured abdominal aortic aneurysm https://paperpile.com/c/e2SMlw/CrHpz46 |
Improving evidence base
Robust trials with clear outcomes are the basis of CES, and many trials comparing IR are underpowered or incomplete for the following reasons: study power, recruiting team factors, and non-robust outcome measures.
Study power
Recruitment into trials is often expensive and difficult,47 and many fail to achieve planned recruitment.48 NIHR found that less than one third of clinical trials achieved their recruitment target. From 2000 to 2013, 11% of cardiovascular trials registered on ClinicalTrials.gov terminated early,49 mainly due to poor recruitment or high patient dropout. The latter often arises as some patients prefer a specific option, often what they perceive as more “active” treatments such as angioplasty or stenting over medical therapy, e.g. in the EXACT (Exercise versus Angioplasty) only 6% of screened patients agreed to be randomised and the trial was terminated early.50 Other reasons are unwillingness to undergo treatment regarded as “experimental”, and non-compliance with follow-up.51, 52, 53 Complex trial protocols increase non-completion rate. Recruitment may also prove prohibitively expensive: a 2016 study54 on peripheral arterial disease demonstrated a wide range of recruitment cost from $4 (at a Community event) to more than $18,000 (radio advertising) per randomised participant: the same study spent more than $340,000 on recruiting 171 participants, a mean of approximately $2,000. A business model approach can help to improve recruitment, retention, and trial completion55 (Table 2 ). Specialist research nurses can make a significant contribution in recruiting, retaining, and following up patients.
Table 2.
Improving the evidence base: a marketing approach.
| Building brand value and defining purpose of the trial | Gain legitimacy and prestige (coordinated by an academic centre, funding by a non-commercial body, signal worthiness (that benefits to trial participants will outweigh costs) |
| Marketing and product planning | Adopt an explicit marketing plan with stakeholder engagement, local and regional champions, and strategies for overcoming resistance (address concerns), providing a complete administrative process with easy data collection and transfer |
| “Making the sale” | Deliver a targeted multi-level approach to multiple different audiences (with appropriate language) and achieving “buy-in” (confirmed commitment), through websites and communications back to trial participants |
| Maintaining engagement | Especially important when follow-up or supplementary studies are envisaged. Key points: deal with feedback constructively, continue to provide reinforcement, and communicate findings and positive learning points |
Recruiting team factors
Research teams often have to balance research and clinical commitments. Clinician participation may be suboptimal where the clinician feels options are not in equipoise or is unfamiliar with, unconvinced by, or actively resistant to a novel technique. Pseudorandomisation may occur whereby sicker patients with more comorbidities are referred for IR procedures and fitter ones for surgery, thereby introducing bias into the long-term outcomes and decreasing generalisability of the results to the general population.56 A whole team approach should be used, with wide involvement in auditing, presenting, and publishing the results of the work.
Non-robust outcome measures
Clinical endpoints need to be objective, robust, and verifiable, or bias may be introduced. There needs to be clear guidance of who assesses, when and how. Non-blinded observers have been shown to exaggerate treatment effect by up to 68% versus blinded.57 Lack of definition of “best supportive care” in oncology studies has been shown to distort interpretation of outcomes.58
Lack of long-term follow-up is a common issue in interventional procedures where patients revert back to the original referring consultant for ongoing review. Some EVAR studies found only 50% of patients having complete surveillance as per the protocol.59 , 60 IR involvement in follow-up would not only improve the evidence base for long-term efficacy, but would also afford IR the opportunity to advise or intervene if late complications arise.
Improving procedure performance
Learning curve and procedure volume effects
Medical outcomes improve as teams complete a “learning curve” and start performing larger volumes of procedures regularly.61 , 62 Interventional radiologists perform a wide variety of emergency and elective procedures, which are being continually developed and elaborated. It is challenging to master and maintain competence in these different procedures, which may be infrequently performed. These factors can reduce outcomes and cause delayed recognition of complications, which may require expensive retreatment and further surveillance,63, 64, 65, 66 thus reducing cost effectiveness.
Two strategies can help here. First, increasingly sophisticated simulation training (ST) has been developed, which provide detailed real time audiovisual and haptic feedback. Simulation training has already been embraced by cardiologists (for arterial puncture and coronary artery catheterisation), neurosurgeons (for neurovascular procedures such as aneurysm coiling and stroke thrombectomy) and vascular surgeons (for renal, carotid and peripheral vascular procedures),67, 68, 69, 70, 71, 72, 73 and ST has been explicitly incorporated into their curricula. ST allows the trainee to experience a standardised set of scenarios designed to cover a range of teaching points and embed useful skills in the most efficient way, without relying on random caseload in a particular centre, at a convenient time and setting conducive to learning, with objective and supportive feedback. It has been shown to reduce procedure time and radiation dose, improve outcomes and patient safety, and operator confidence.66 The operator can learn at their own pace, and pay attention to areas where they need more training, or where they feel less confident. ST can be particularly helpful to teach the basic skills rapidly, such as arterial puncture and selective catheterisation, allowing the trainee to concentrate on the more advanced aspects of the procedure. It reduces the burden on trainers,74 , 75 and increases patient safety. ST can also be valuable for experienced operators to maintain and update their skills, with objective feedback. ST is still expensive, but resources can be shared at a regional or college level, as most trainees only require 1–2 days of practice initially with shorter further sessions as required.
Secondly, hub and spoke models with concentration of more advanced services in a hub with higher volumes have been noted by the GIRFT report on Vascular Surgery to achieve better outcomes with better use of healthcare resources and opportunities for cost saving due to improved procurement.4 , 61 There is already a tendency for hub and spoke working to provide 24-h IR availability, and increasing use of this model will improve outcomes at relatively minor increased cost, thus improving cost-effectiveness.
Increased use of day-case and shorter-stay procedures
Interventional procedures lend themselves to shorter length of stay, due to smaller incisions, decreased use of general anaesthetic, and faster recovery times, versus surgical alternatives. This leads to shorter operating time, length of stay in hospital, time away from home and back to work and therefore significant cost savings,22 especially if one considers an overnight stay costs £400 on a ward, and £1,150 in a critical care unit (NHS reference costs 76). Thus moving from EVAR with open surgical femoral exposure to percutaneous radiological femoral access was shown to reduce operating theatre time by 19%, length of stay by 50% and overall cost by 23%.77 The Audit Commission noted in 1990 that switching to day-case surgery nationwide would allow up to 200,000 patients more to be treated annually without extra expenditure,78 and similar calculations would show significant cost savings from increased use of IR. Furthermore, in the current COVID era, many IR units have been able to continue doing day-case and outpatient treatments without cancellations due to ward or critical care shortages,79 also helping to reduce waiting lists, which have recently risen sharply.80
Kit and technique used
A substantial proportion of IR procedure cost is the kit used, which may be substantially more than the surgical equivalent (e.g., average price of EVAR graft and wires £6,945 versus surgical aortic graft and consumables £429), due to small production volumes and requirement to recoup development costs.81 The GIRFT Vascular Surgery report noted a nearly 10-fold variation nationwide in cost of EVAR grafts with no apparent difference in effectiveness,4 implying a massive opportunity to reduce costs with judicious procurement. Equipment prices tend to fall with increasing production and commercial competition (as with coronary angioplasty balloons and stents) so it is important to continually review the market to see if more cost-effective kit or cheaper alternatives become available. With increasing experience, less kit is generally used, which underlines the value of ST as above. Costs vary significantly between countries, e.g., coronary stents cost six-times more in the USA versus UK and Germany,82 so local costs should be obtained and compared.
Complications, re-intervention, and long term-benefits
Complications can massively increase the cost of a routine procedure, and therefore relatively small additions to a procedure may prove highly cost effective if they reduce complication incidence. This has been an important factor in EVAR, especially in those patients with relatively long lifespan post-procedure, and may easily tip the balance of cost-effectiveness between IR and surgery. Some radiological interventions provide short-term benefit but not long-term durability: for example, in uterine artery embolisation (UAE), the REST trial showed the initial cost benefit of UAE over surgery at 12 months was eroded by a higher rate of treatment failure rate in the embolisation group (32%) versus surgery (4%), which reduced the cost effectiveness to equipoise by 5 years.83 Similarly, the HOPEFUL study showed improved quality of life initially but with erosion of benefits over time.84 For aortic aneurysm, EVAR was noted to have short-term benefits, but lack of benefit in the long term (as well as increasing costs from re-intervention) leading NICE to approve it only for acute rupture 46 , 85 and not elective repair of uncomplicated aortic aneurysms.86 , 87
The reasons for complications and lack of durability need to be acknowledged and addressed. With increasing experience and training, complications can be recognised and mitigated earlier and more cost effectively. Developments in technique frequently occur: for example, in UAE, the role of anastomotic vessels causing regrowth of fibroids has been recognised and can be treated primarily or with re-intervention.88 Long-term studies can also help define which subgroup of patients are likely to benefit most, so patients can be better informed of the options most likely to suit them and therefore prove cost-effective.89
Sometimes a different treatment paradigm may prove more suitable. For example, magnetic resonance imaging (MRI)-focussed ultrasound may prove more effective for certain groups of patients with fibroids,90 and this is currently being trialled.91 Similarly, the EVAR strategy of internal graft fixation by radial force and non-abolition of the aneurysm sac may be superseded.92 So far, different approaches such as endovascular aneurysm sealing (for example with Nellix) have not proved successful, although eventually a hybrid technique with EVAR graft plus sac filling may prove the best solution.93
Follow-up
Imaging follow-up adds significant costs, especially if cross sectional, such as CT, which costs three times the cost of ultrasound.22 Follow-up consultations can often be done more conveniently for patients and IR teams by telephone rather than face to face, and by different team members such as specialist nurses,22 , 94 and large societal cost savings can be obtained due to reduced hospital usage, fewer missed appointments, and decreased transport costs.95 The duration of follow-up for all procedures should be continually under review as better and longer-term data becomes available, and tailored to where re-intervention can make a significant difference.
Conclusion
IR is a rapidly evolving field with many potential clinical advantages for patients and cost-effectiveness advantages for the whole healthcare system. IR can demonstrate and promote its value with high-quality robust long-term data, using criteria developed by NICE, which is recognised worldwide as a leader in objective evaluation of healthcare effectiveness, working in the best interests of patients. The methodology of cost-effectiveness analysis and areas where improvements could be made have been discussed. It is hoped that IR teams will use these and work together to expand this evidence base, which can potentially be of great benefit to all.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Appleby J., Thorlby R. Data briefing. Why budgets have always been a bugbear. Health Serv J. 2008 Feb 14:23. [PubMed] [Google Scholar]
- 2.Emmerson C., Stockton I. Insitute for Fiscal Studies; 2020. The economic response to coronavirus will substantially increase government borrowing.https://www.ifs.org.uk/publications/14771 Available at: [Google Scholar]
- 3.Drummond M.F., Sculpher M.J., Claxton K. Oxford University Press; Oxford: 2015. Methods for the economic evaluation of health care programmes. [Google Scholar]
- 4.Horrocks M. 2018. Vascular surgery GIRFT programme national specialty report. GIRFT (getting it Right first time)https://gettingitrightfirsttime.co.uk/vascular-surgery-report/ Available at: [Accessed 1 July 2020] [Google Scholar]
- 5.Licchetta M., Stelmach M. OBR Office for budget Responsibility; 2016 Sep. Fiscal sustainability and public spending on health.https://obr.uk/docs/dlm_uploads/Health-FSAP.pdf Available at: [Accessed 1 July 2020] [Google Scholar]
- 6.Willemé P., Dumont M. Machines that go “ping”: medical technology and health expenditures in OECD countries. Health Econ. 2015 Aug;24(8):1027–1041. doi: 10.1002/hec.3089. [DOI] [PubMed] [Google Scholar]
- 7.Zweifel P., Ferrari M. Is there a Sisyphus Syndrome in health care? In: Zweifel P., Frech H.E., editors. Health economics worldwide. Springer Netherlands; Dordrecht: 1992. pp. 311–330. [DOI] [PubMed] [Google Scholar]
- 8.Bähler C., Huber C.A., Brüngger B. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res. 2015 Jan 22;15:23. doi: 10.1186/s12913-015-0698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zweifel P., Felder S., Werblow A. Population ageing and health care expenditure: new evidence on the “red herring. Geneva Pap Risk Insurance Issue Pract. 2004 Oct 1;29(4):652–666. [Google Scholar]
- 10.Seshamani M., Gray A.M. A longitudinal study of the effects of age and time to death on hospital costs. J Health Econ. 2004 Mar;23(2):217–235. doi: 10.1016/j.jhealeco.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Desai K.R., Chen R.I. Endovascular therapy for palliative care of cancer patients. Semin Intervent Radiol. 2007 Dec;24(4):382–390. doi: 10.1055/s-2007-992326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell T.C., Roenn J.H.V. Palliative care for interventional radiology: an oncologist’s perspective. Semin Intervent Radiol. 2007 Dec;24(4):375–381. doi: 10.1055/s-2007-992325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Diabetes Federation . 9th edn. International Diabetes Federation; 2019. IDF diabetes atlas. [Google Scholar]
- 14.Fowkes F.G.R., Rudan D., Rudan I. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013 Oct 19;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 15.Marso S.P., Hiatt W.R. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006 Mar 7;47(5):921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 16.Jude E.B., Oyibo S.O., Chalmers N. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001 Aug;24(8):1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberg S.O., Attenberger U.I., Solomon S.B. Developing a roadmap for interventional oncology. Oncologist. 2018 Oct;23(10):1162–1170. doi: 10.1634/theoncologist.2017-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Affairs E.A.F. European Commission; 2015. The 2015 ageing report: economic and budgetary projections for the 28 EU member states (2013-2060) [Google Scholar]
- 19.Martins J.O., de la Maisonneuve C. The drivers of public expenditure on health and long-term care: an integrated approach. SSRN Electron J. 2006 doi: 10.2139/ssrn.917782. [Accessed 1 July 2020] [DOI] [Google Scholar]
- 20.Newhouse J.P. Medical care costs: how much welfare loss? J Econ Perspect. 1992;6(3):3–21. doi: 10.1257/jep.6.3.3. [DOI] [PubMed] [Google Scholar]
- 21.Sorenson C., Drummond M., Bhuiyan Khan B. Medical technology as a key driver of rising health expenditure: disentangling the relationship. Clinicoecon Outcome. Res. 2013 May 30;5:223–234. doi: 10.2147/CEOR.S39634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scitovsky A.A. Changes in the costs of treatment of selected illnesses, 1971–1981. Med Care. 1985 Dec;23(12):1345–1357. doi: 10.1097/00005650-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Noyes J., Edwards R.T. EQ-5D for the assessment of health-related quality of life and resource allocation in children: a systematic methodological review. Value Health. 2011 Dec;14(8):1117–1129. doi: 10.1016/j.jval.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Han Y., Zhao F.-L. Validation and comparison of EuroQoL-5 dimension (EQ-5D) and Short Form-6 dimension (SF-6D) among stable angina patients. Health Qual Life Outcome. 2014 Oct 25;12:156. doi: 10.1186/s12955-014-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.YHEC - York Health Economics Consortium Decision tree. https://yhec.co.uk/glossary/decision-tree/ Available at:
- 26.YHEC - York Health Economics Consortium Markov model. https://yhec.co.uk/glossary/markov-model/ Available at:
- 27.NICE Abdominal aortic aneurysm endovascular stentgrafts final appraisal determination. 2008. https://www.nice.org.uk/guidance/ta167/documents/abdominal-aortic-aneurysm-endovascular-stentgrafts-final-appraisal-determination2 Available at: [Accessed 1 July 2020]
- 28.Rawlins M.D., Culyer A.J. National Institute for clinical excellence and its value judgments. BMJ. 2004 Jul 24;329(7459):224–227. doi: 10.1136/bmj.329.7459.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weintraub W.S., Cohen D.J. The limits of cost-effectiveness analysis. Circ Cardiovasc Qual Outcome. 2009 Jan;2(1):55–58. doi: 10.1161/CIRCOUTCOMES.108.812321. [DOI] [PubMed] [Google Scholar]
- 30.Xcenda . 29 October 2019. The cost of cures: what does zolgensma teach us about the use of cost-effectiveness assessments?https://www.xcenda.com/insights/htaq-fall-2019-the-cost-of-cures-zolgensma-cea Available at: [Google Scholar]
- 31.Neumann P.J., Weinstein M.C. Legislating against use of cost-effectiveness information. N Engl J Med. 2010 Oct 14;363(16):1495–1497. doi: 10.1056/NEJMp1007168. [DOI] [PubMed] [Google Scholar]
- 32.Gray C., Goodman P., Herron C.C. Use of colour duplex ultrasound as a first line surveillance tool following EVAR is associated with a reduction in cost without compromising accuracy. Eur J Vasc Endovasc Surg. 2012 Aug;44(2):145–150. doi: 10.1016/j.ejvs.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Pettitt D.A., Raza S., Naughton B. The limitations of QALY: a literature review. J Stem Cell Res Ther. 2016;6(4) DOI: 0.4172/2157.7633.1000334. [Google Scholar]
- 34.Lipscomb J., Drummond M., Fryback D., Gold M. Retaining, and enhancing, the QALY. Value Health. 2009 Mar;12(Suppl 1):S18–S26. doi: 10.1111/j.1524-4733.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 35.Husereau D., Drummond M., Petrou S. Consolidated health economic evaluation reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013 Mar;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhou A., Yousem D.M., Alvin M.D. Cost-effectiveness analysis in radiology: a systematic review. J Am Coll Radiol. 2018 Nov;15(11):1536–1546. doi: 10.1016/j.jacr.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Loveman E., Jones J., Clegg A.J. The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol Assess. 2014 Jan;18(7):1–283. doi: 10.3310/hta18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazelle G.S., McMahon P.M., Beinfeld M.T. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology. 2004 Dec;233(3):729–739. doi: 10.1148/radiol.2333032052. [DOI] [PubMed] [Google Scholar]
- 39.Abramson R.G., Rosen M.P., Perry L.J. Cost-effectiveness of hepatic arterial chemoembolization for colorectal liver metastases refractory to systemic chemotherapy. Radiology. 2000 Aug;216(2):485–491. doi: 10.1148/radiology.216.2.r00au26485. [DOI] [PubMed] [Google Scholar]
- 40.Shetty S.K., Rosen M.P., Raptopoulos V. Cost-effectiveness of percutaneous radiofrequency ablation for malignant hepatic neoplasms. J Vasc Interv Radiol. 2001 Jul;12(7):823–833. doi: 10.1016/s1051-0443(07)61507-3. [DOI] [PubMed] [Google Scholar]
- 41.Cucchetti A., Piscaglia F., Cescon M. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013 Aug;59(2):300–307. doi: 10.1016/j.jhep.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda K., Kobayashi M., Saitoh S. Cost-effectiveness of radiofrequency ablation and surgical therapy for small hepatocellular carcinoma of 3cm or less in diameter. Hepatol Res. 2005 Nov 1;33(3):241–249. doi: 10.1016/j.hepres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Pandharipande P.V., Gervais D.A., Mueller P.R. Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology. 2008 Jul;248(1):169–178. doi: 10.1148/radiol.2481071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larcher A., Sun M., Dell’Oglio P. Mortality, morbidity and healthcare expenditures after local tumour ablation or partial nephrectomy for T1A kidney cancer. Eur J Surg Oncol. 2017 Apr;43(4):815–822. doi: 10.1016/j.ejso.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Moss J.G. Trials update: UFE versus myomectomy versus hysterectomy. Cardiovasc Intervent Radiol. 2012;35(Suppl. 1):S86–S87. [Google Scholar]
- 46.IMPROVE Trial Investigators Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017 Nov 14;359:j4859. doi: 10.1136/bmj.j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDermott M.M. The importance and challenge of recruitment for peripheral artery disease randomized clinical trials. Vasc Med. 2016 Aug;21(4):352–354. doi: 10.1177/1358863X16651506. [DOI] [PubMed] [Google Scholar]
- 48.Campbell M.K., Snowdon C., Francis D. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007 Nov;11(48):105. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]
- 49.Bernardez-Pereira S., Lopes R.D., Carrion M.J.M. Prevalence, characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: insights from the ClinicalTrials.gov registry. Am Heart J. 2014 Aug;168(2):213–219.e1. doi: 10.1016/j.ahj.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Hobbs S.D., Bradbury A.W. The EXercise versus Angioplasty in Claudication Trial (EXACT): reasons for recruitment failure and the implications for research into and treatment of intermittent claudication. J Vasc Surg. 2006 Aug;44(2):432–433. doi: 10.1016/j.jvs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Schain W.S. Barriers to clinical trials: part II: knowledge and attitudes of potential participants. Cancer. 1994;74(Suppl):2666–2671. doi: 10.1002/1097-0142(19941101)74:9+<2666::aid-cncr2820741814>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 52.Mansour E.G. Barriers to clinical trials: part III: knowledge and attitudes of health care providers Cancer. 1994;74(Suppl):2672–2675. doi: 10.1002/1097-0142(19941101)74:9+<2672::aid-cncr2820741815>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 53.Fleming I.D. Barriers to clinical trials. Part I: reimbursement problems. Cancer. 1994;74(Suppl):2662–2665. doi: 10.1002/1097-0142(19941101)74:9+<2662::aid-cncr2820741813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Love B., Nwachokor D., Collins T. Recruiting African Americans with peripheral artery disease for a behavioral intervention trial. Vasc Med. 2016 Aug;21(4):345–351. doi: 10.1177/1358863X16628646. [DOI] [PubMed] [Google Scholar]
- 55.McDonald A.M., Treweek S., Shakur H. Using a business model approach and marketing techniques for recruitment to clinical trials. Trials. 2011 Mar 11;12:74. doi: 10.1186/1745-6215-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antman K., Amato D., Wood W. Selection bias in clinical trials. J Clin Oncol. 1985 Aug;3(8):1142–1147. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- 57.Hróbjartsson A., Thomsen A.S.S., Emanuelsson F. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ. 2013 Mar 5;185(4):E201–E211. doi: 10.1503/cmaj.120744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zafar S.Y., Currow D., Abernethy A.P. Defining best supportive care. J Clin Oncol. 2008 Nov 1;26(31):5139–5140. doi: 10.1200/JCO.2008.19.7491. [DOI] [PubMed] [Google Scholar]
- 59.Kret M.R., Azarbal A.F., Mitchell E.L. Compliance with long-term surveillance recommendations following endovascular aneurysm repair or type B aortic dissection. J Vasc Surg. 2013 Jul;58(1):25–31. doi: 10.1016/j.jvs.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 60.Waduud M.A., Choong W.L., Ritchie M. Endovascular aneurysm repair: is imaging surveillance robust, and does it influence long-term mortality? Cardiovasc Intervent Radiol. 2015 Feb;38(1):33–39. doi: 10.1007/s00270-014-0890-5. [DOI] [PubMed] [Google Scholar]
- 61.Hannan E.L., Wu C., Walford G. Volume-outcome relationships for percutaneous coronary interventions in the stent era. Circulation. 2005 Aug 23;112(8):1171–1179. doi: 10.1161/CIRCULATIONAHA.104.528455. [DOI] [PubMed] [Google Scholar]
- 62.Kimmel S.E., Berlin J.A., Laskey W.K. The relationship between coronary angioplasty procedure volume and major complications. JAMA. 1995 Oct 11;274(14):1137–1142. [PubMed] [Google Scholar]
- 63.Das R., Lucatelli P., Wang H. Identifying the learning curve for uterine artery embolisation in an interventional radiological training unit. Cardiovasc Intervent Radiol. 2015 Aug;38(4):871–877. doi: 10.1007/s00270-014-1040-9. [DOI] [PubMed] [Google Scholar]
- 64.Dias T.R., Alves Junior J de D da C., Abdala N. Learning curve of radiology residents during training in fluoroscopy-guided facet joint injections. Radiol Bras. 2017 May;50(3):162–169. doi: 10.1590/0100-3984.2015.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iezzi R., Posa A., Merlino B. Operator learning curve for transradial liver cancer embolization: implications for the initiation of a transradial access program. Diagn Interv Radiol. 2019 Sep;25(5):368–374. doi: 10.5152/dir.2019.18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandal I., Ojha U. Training in interventional radiology: a simulation-based approach. J Med Educ Curric Dev. 2020 Jan;7 doi: 10.1177/2382120520912744. 2382120520912744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amin A., Salsamendi J., Sullivan T. High-fidelity endovascular simulation. Tech Vasc Interv Radiol. 2019 Mar;22(1):7–13. doi: 10.1053/j.tvir.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Zaika O., Boulton M., Eagleson R. Simulation reduces navigational errors in cerebral angiography training. Adv Simul (Lond) 2020 Jun 12;5:10. doi: 10.1186/s41077-020-00125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirza S., Athreya S. Review of simulation training in interventional radiology. Acad Radiol. 2018 Apr;25(4):529–539. doi: 10.1016/j.acra.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Ramjeeawon A., Sharrock A.E., Morbi A. Using fully-immersive simulation training with structured debrief to improve nontechnical skills in emergency endovascular surgery. J Surg Educ. 2020;77(5):1300–1311. doi: 10.1016/j.jsurg.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Koyama J.-I., Hanaoka Y., Kiuchi T. Development of simulator system using endovascular evaluator for catheter intervention training. J Neuroendovasc Ther. 2018;12:1–5. [Google Scholar]
- 72.Matsumura Y., Taudorf M., Søvik E. Endovascular resuscitation and trauma management: education and simulation. In: Hörer T., DuBose J.J., Rasmussen T.E., editors. Endovascular resuscitation and trauma management: bleeding and haemodynamic control. Springer International Publishing; Cham: 2020. pp. 253–262. [Google Scholar]
- 73.Voelker W., Petri N., Tönissen C. Does simulation-based training improve procedural skills of beginners in interventional cardiology?—a stratified randomized study. J Interv Cardiol. 2016;29(1):75–82. doi: 10.1111/joic.12257. [DOI] [PubMed] [Google Scholar]
- 74.Klass D., Tam M.D.B.S., Cockburn J. Training on a vascular interventional simulator: an observational study. Eur Radiol. 2008 Dec;18(12):2874–2878. doi: 10.1007/s00330-008-1090-y. [DOI] [PubMed] [Google Scholar]
- 75.McGaghie W.C., Issenberg S.B., Cohen E.R. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011 Jun;86(6):706–711. doi: 10.1097/ACM.0b013e318217e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NHS Improvement . 2017. National tariff payment system 2017/18 and 2018/19.https://improvement.nhs.uk/resources/national-tariff/ Available at: [Google Scholar]
- 77.Thurston J.S., Camara A., Alcasid N. Outcomes and cost comparison of percutaneous endovascular aortic repair versus endovascular aortic repair with open femoral exposure. J Surg Res. 2019 Aug;240:124–129. doi: 10.1016/j.jss.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Audit Commission . H.M. Stationery Office; London: 1990. A short cut to better services: day surgery in England and Wales. [Google Scholar]
- 79.Rostampour S., Cleveland T., White H. Response of UK interventional radiologists to the COVID-19 pandemic — survey findings. CVIR Endovasc. 2020 Jun 26;3(1):41. doi: 10.1186/s42155-020-00133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iacobucci G. Covid-19: NHS leaders braced for longer waiting times as service deals with fallout. BMJ. 2020 Jul 1;370:m2639. doi: 10.1136/bmj.m2639. [DOI] [PubMed] [Google Scholar]
- 81.Patel R., Powell J.T., Sweeting M.J. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess. 2018 Jan;22(5):1–132. doi: 10.3310/hta22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wenzl M., Mossialos E. Prices for cardiac implant devices may be up to six times higher in the US than in some european countries. Health Aff. 2018 Oct;37(10):1570–1577. doi: 10.1377/hlthaff.2017.1367. [DOI] [PubMed] [Google Scholar]
- 83.Moss J.G., Cooper K.G., Khaund A. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-Year results. BJOG. 2011;118(8):936–944. doi: 10.1111/j.1471-0528.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 84.Wu O., Briggs A., Dutton S. Uterine artery embolisation or hysterectomy for the treatment of symptomatic uterine fibroids: a cost–utility analysis of the HOPEFUL study. BJOG. 2007;114(11):1352–1362. doi: 10.1111/j.1471-0528.2007.01525.x. [DOI] [PubMed] [Google Scholar]
- 85.Stroupe K.T., Lederle F.A., Matsumura J.S. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg. 2012 Oct;56(4):901–909.e2. doi: 10.1016/j.jvs.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 86.Hinchliffe R.J., Earnshaw J.J. Endovascular treatment of abdominal aortic aneurysm: a NICE U-turn. Br J Surg. 2020;107:940–942. doi: 10.1002/bjs.11054. [DOI] [PubMed] [Google Scholar]
- 87.NICE . 2020. Abdominal aortic aneurysm: diagnosis and management NICE guideline. NICE guideline.https://www.nice.org.uk/guidance/ng156 Available at: [PubMed] [Google Scholar]
- 88.Worthington-Kirsch R.L. Uterine artery embolization: state of the art. Semin Intervent Radiol. 2004 Mar;21(1):37–42. doi: 10.1055/s-2004-831403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chrisman H., Md M., Basu P. The positive effect of targeted marketing on an existing uterine fibroid embolization practice. J Vasc Interv Radiol. 2006;17(3):577–581. doi: 10.1097/01.rvi.0000204854.35429.eb. [DOI] [PubMed] [Google Scholar]
- 90.Peregrino P.F.M., de Lorenzo Messina M., Dos Santos Simões R. Review of magnetic resonance-guided focused ultrasound in the treatment of uterine fibroids. Clinics. 2017 Oct;72(10):637–641. doi: 10.6061/clinics/2017(10)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov The FIRSTT: comparing MRgFUS (MR-guided focused ultrasound) versus UAE (uterine artery embolization) for uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00995878 Available at:
- 92.Uberoi R., Jenkins M. Is this the end for EVAR? Cardiovasc Intervent Radiol. 2020 Feb;43(2):169–171. doi: 10.1007/s00270-019-02361-z. [DOI] [PubMed] [Google Scholar]
- 93.Verhoeven E.L.G., Mani K. New technology failures: who to blame or time to be cautious? Eur J Vasc Endovasc Surg. 2018 Sep;56(3):318–319. doi: 10.1016/j.ejvs.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 94.Salamanca-Balen N., Seymour J., Caswell G. The costs, resource use and cost-effectiveness of clinical nurse specialist-led interventions for patients with palliative care needs: a systematic review of international evidence. Palliat Med. 2018 Feb;32(2):447–465. doi: 10.1177/0269216317711570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Midlands and Lancashire Commissioning Support Unit . 2018 Aug. The potential economic impact of virtual outpatient appointments in the West Midlands — a scoping study.https://www.strategyunitwm.nhs.uk/publications/potential-economic-impact-virtual-outpatient-appointments-west-midlands-scoping-study Available at: [Google Scholar]