Abstract
Introduction
In the current COVID-19 pandemic, disease diagnosis is essential for optimal management and timely isolation of infected cases in order to prevent further spread. The aim of this study was to systematically review the assessment of risk and model the predictors of mortality in COVID-19 patients.
Methods
A systematic search was conducted of PubMed, Scopus, Embase, Google Scholar, and Web of Science databases. Variables associated with hospital mortality using bivariate analysis were included as potential independent predictors associated with mortality at the p < 0.05 levels.
Results
We included 114 studies accounting for 310,494 patients from various parts of the world. For the purpose of this analysis, we set a cutoff point of 10% for the mortality percentages. High mortality rates were defined as higher than 10% of confirmed positive cases and were given a score of two, while low mortality (<10%) was assigned the score of one. We then analyzed the associations between 72 variables and the observed mortality rates. These variables included a large range of related variables such as demographics, signs and symptoms and related morbidities, vital signs, laboratory findings, imaging studies, underlying diseases, and the status of countries' income, based on the United Nation's classifications.
Conclusion
Findings suggest that older age, hypertension, and diabetes mellitus conferred a significant increased risk of mortality among patients with COVID-19. In the multivariate analysis, only diabetes mellitus demonstrated an independent relationship with increased mortality. Further studies are needed to ascertain the relationship between possible risk factors with COVID-19 mortality.
Keywords: COVID-19, Modeling, Mortality, Risk factors, Predictors, Systematic review
1. Introduction
Coronavirus Disease 2019 (COVID-19) attracted worldwide attention as an international public health emergency as the first pandemic caused by a coronavirus [1,2]. The global number of cases and deaths has reached almost 11,000,000 and 404,396 [3], imposing an unavoidable burden and pressure on the healthcare systems in all countries as well as their economies [4], [5], [6]. This inflicted pressure requires careful strategies as well as their implementation. Actions should be guided by scientific facts to minimize the imposed harms, and this has created an urgent need to examine studies and model outcomes [7].
Miscellaneous COVID-19 mortality rates have been reported as determining an accurate mortality rate is still a challenge and might not be achievable [8]. Mortality rates show an increase in older populations having underlying diseases [9,10]. Characteristic signs and symptoms raise clinical suspicions and are vital for detecting an infected individual [11,12]. In the current pandemic settings, diagnosing the disease is essential for providing the best management to the infected people and avoiding further spreading the disease to others through timely isolation [13,14]. All this outlines the vast importance of understanding signs and symptoms and their role in the disease's pathogenesis and clinical manifestations. Estimating the patterns of signs, symptoms, comorbidities, and other variables, and their association with mortality rates might be the key to the management of COVID-19. Understanding these issues helps us to provide adequate and in-time personalized care based on individual's conditions. This article aimed to provide evidence-based modeling of COVID-19 by addressing variables that might be related to an alteration in Severe Acute Respiratory Syndrome- Coronavirus-2 (SARS-CoV-2) mortality rates. Variables were divided into six major categories: demographics, signs and symptoms and related morbidities, laboratory findings and vital signs, imaging studies, underlying conditions, and countries’ income. The focus was on increasing knowledge about the disease for better prevention, diagnosis, and treatment.
2. Methods
This systematic review conducted in 2020 aimed to provide a risk analysis and model predictors of mortality in COVID-19 patients.
2.1. Data sources
This systematic review was conducted using PubMed, Scopus, Embase, Google Scholar, and Web of Science databases between January 1, 2020, and June 27, 2020. The literature search was done using the keywords in combination with the following search strategy:
-
A
"Coronavirus OR COVID-19 OR SARS-CoV-2 OR Novel Coronavirus OR 2019-nCoV" [Title/Abstract]
-
B
"Clinical characteristics OR clinical feature OR clinical manifestation" [Title/Abstract]
-
C
"Death OR died OR fatal OR mortality OR deceased OR non survivor OR non survival" [All fields]
-
D
"Recovered OR discharged OR alive OR survivor OR survival" [All fields]
-
E
[A] AND [B] AND [C] AND [D]
2.2. Study selection
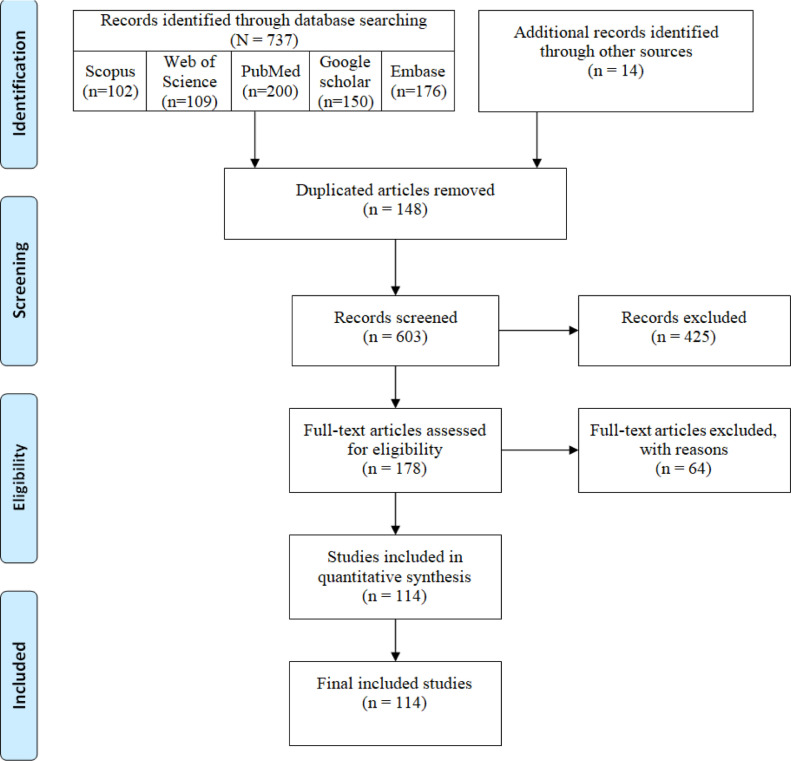
For study selection, we followed the PRISMA guidelines ( Fig. 1 ). The selection of the studies was performed by assessing relevance by titles and abstracts by three independent investigators. The full texts were reviewed for the eligibility criteria. The English-written peer-reviewed original papers published from January 1, 2020, to June 27, were included. The exclusion criteria were as follows:
-
−
Different types of studies, such as ongoing protocols, abstracts, reports, and letters to the editor.
-
−
No access to the full-text document.
-
−
Duplicated results in databases.
-
−
Papers addressing non-human studies, or discussing COVID-19 in general, without reference to the disease's mortality.
Fig. 1.
PRISMA flow diagram of the articles selection process.
The title and abstract of each manuscript were evaluated, and the most relevant manuscripts were selected based on the previously mentioned inclusion and exclusion criteria. Two independent investigators conducted the screening, and disagreement was resolved through discussion.
2.3. Data extraction
We used the data extraction forms, including information on the authors, year of publication, country, sample size, age, gender, clinical symptoms (e.g., fever, chills). This information was obtained independently by two investigators, and disagreement was resolved through discussion.
2.4. Quality assessment
To ensure the quality assessment of selected articles, a checklist ( Table 1 ) with 15 items was developed based on the relevant studies [15], [16], [17]. The quality of articles was evaluated by the scoring of checklist items and estimated the mean score and rated on a three-point scale: low quality (0–5), medium quality (6–10), and high quality (11–15). The full text of selected articles was then thoroughly studied to extract the essential findings. The qualified full-text articles were included, and their results were discussed to make the final selection. After reading the full text of all eligible papers, the researchers decided to include/exclude each study.
Table 1.
Quality assessment checklist.
| NO. | Question |
|---|---|
| 1 | Does the study address any research question(s) or objective(s)? |
| 2 | Does the study provide any theoretical framework for the evaluation method? |
| 3 | Does the theoretical framework of the study include any health promotion theory? |
| 4 | Does the study provide a timeframe for the data collection? |
| 5 | Does the study identify the country where the search was conducted? |
| 6 | Does the study mention that the reviewed current evidence was downloaded for evaluation? |
| 7 | Does the study discuss the selection criteria for current evidence to be included or excluded for review? |
| 8 | Does the study provide a clear description of the evaluation method? |
| 9 | Are there at least two independent data extractors with a consensus procedure in place in case of disagreement? |
| 10 | Is a list of the reviews current evidence provided? |
| 11 | Does the study discuss the findings of the evaluation? |
| 12 | Does the study look at the reviewed evidence to promote or enable behavioral change? |
| 13 | Does the study discuss any limitations? |
| 14 | Does the study provide any future recommendations in general? |
| 15 | Does the study state any conflict of interest? |
2.5. Data synthesis
A quantitative synthesis of the identified studies was carried out according to the search strategy and the identified characteristics; these were later analyzed according to frequencies (n) to facilitate data interpretation. We used SPSS software (version 26) for data analysis and data extraction. The data was analyzed and categorized using the following variables listed in Table 2 . These factors were extracted after thorough and careful reading of the articles to include them as efficiently as possible.
Table 2.
List of the variables included in the data analysis.
| Demographics | |
|---|---|
| Male (%) | Mean age |
| Signs, symptoms, and related morbidities | |
| Fever | Chest pain |
| Cough | Dyspnea |
| Hemoptysis | Sputum expectoration |
| Throat pain (Pharyngalgia) | Nose discharge |
| Diarrhea | Nasal obstruction |
| Nausea | ARDS |
| Vomiting | Shock |
| Abdominal pain | Cardiac involvement |
| Dizziness | Abnormal liver function |
| Headache | Acute kidney injury |
| Myalgia | Pneumothorax |
| Malaise (Fatigue)(Asthenia) | Asymptomatic |
| Laboratory findings and vital signs | |
| Hyperglycemia | Heart rate (average) |
| Lymphopenia | Respiratory rate |
| Elevated CRP | Mean arterial pressure |
| Elevated d-dimer | SpO2 |
| Imaging studies | |
| Chest CT changes | Chest XR changes |
| Comorbidities | |
| COPD | Immunosuppression |
| Diabetes Mellitus | Liver disease |
| Hypertension | Asthma |
| Cardiovascular disease | Obesity |
| Cerebrovascular disease | Smoking history |
| Chronic kidney disease (CKD) | Country income rate |
| Malignancy | |
Variables associated with hospital mortality in the bivariate analysis were included as potential independent predictors at the p < 0.05 levels. The final model retained those variables associated with the mortality at the p < 0.05 level.
3. Results
In this study, using the applied systematic search strategies, 751 sources were identified and retrieved. After an initial review of retrieved articles, 148 duplicates were removed, and the title and abstract of the remaining 603 articles were reviewed. Applying the selection criteria, 489 articles were excluded, and only 114 articles met inclusion criteria and were included in the final review ( Fig. 1 ). The mean quality score of the selected articles was 13 (=range: 11 to 15), indicating the high quality of these articles.
We included 114 studies accounting for 310,494 patients from various parts of the world. For this analysis, we set a cutoff point of 10% for the mortality percentages. High mortality rates were defined as higher than 10% of confirmed positive cases and were given a score of two, while low mortality percentage (<10%) was assigned to the score of one. We then analyzed the associations between 72 variables and the observed mortality rates. These variables included a wide range of related conditions such as demographics, signs and symptoms and related morbidities, vital signs, laboratory findings, imaging studies, underlying diseases, and the status of countries' income based on the United Nation's classifications. To categorize countries based on their incomes, we used the United Nation's classification of least developed countries, developing countries, and developed countries and gave them one, two, and three points, respectively. For instance, China, the country reporting the first cases of COVID-19, scores two in our classification. Iran also scored two in this classification. USA and Western European countries were among those achieving a score of three.
3.1. The most common signs and symptoms
The 10 most common observed signs and symptoms with the highest mean percentage were fever (68.48 (SD: 23.40)), cough (58.37 (SD: 18.16)), olfactory dysfunction (56.96 (SD: 26.56)), postnasal drip (49.18 (SD: 1.68)), gustatory dysfunction (46.96 (SD: 24.77)), face pain or heaviness (39.69 (SD: 16.19)), dyspnea (37.40 (SD: 25.46)), malaise (36.84 (SD: 22.72)), arthralgia (33.17 (SD: 22.73)), and nasal obstruction (32.09 (SD: 22.77)). Detailed information is illustrated in Table 3 .
Table 3.
Different variables and their associations with the hospital mortality of COVID-19 in the bivariate analysis, 2020.
| Characteristics | Mean (SD) | Odds ratio (95% CI OR) | p-value |
|---|---|---|---|
| Demographics | |||
| Male (%) | 51.59 (13.68) | 1.04 (1.00–1.09) | 0.06 |
| Mean age* | 54.16 (12.33) | 1.18 (1.08–1.29) | <0.001 |
| Signs, symptoms, and related morbidities | |||
| Fever | 68.48 (23.40) | 1.01 (0.98–1.04) | 0.47 |
| Cough | 58.37 (18.16) | 1.01 (0.97–1.04) | 0.79 |
| Hemoptysis | 2.29 (1.96) | 3.71 (0.23–61.05) | 0.36 |
| Throat pain (Pharyngalgia) | 22.33 (16.15) | 0.78 (0.60–1.01) | 0.06 |
| Diarrhea | 16.47 (12.56) | 1.04 (0.96–1.12) | 0.34 |
| Nausea | 11.45 (9.06) | 1.08 (0.92–1.27) | 0.34 |
| Vomiting | 8.11 (6.72) | 1.25 (0.92–1.71) | 0.16 |
| Abdominal pain | 9.37 (12.58) | 1.03 (0.93–1.13) | 0.57 |
| Dizziness | 9.82 (14.91) | 1.02 (0.68–1.55) | 0.93 |
| Headache | 22.19 (20.83) | 0.93 (0.84–1.03) | 0.18 |
| Myalgia | 22.58 (18.32) | 0.94 (0.88–1.00) | 0.07 |
| Malaise (Fatigue)(Asthenia) | 36.84 (22.72) | 0.98 (0.94–1.02) | 0.36 |
| Chest pain | 9.41 (8.33) | 0.97 (0.83–1.14) | 0.71 |
| Dyspnea | 37.40 (25.46) | 1.03 (0.99–1.07) | 0.06 |
| Sputum expectoration | 23.00 (11.48) | 0.97 (0.89–1.06) | 0.49 |
| Nose discharge | 24.25 (19.29) | 0.00 (0.00–0.00) | 0.99 |
| Nasal obstruction | 32.09 (22.77) | 0.97 (0.83–1.13) | 0.65 |
| ARDS | 39.68 (30.85) | 1.02 (0.96–1.08) | 0.64 |
| Shock | 25.55 (1.59) | 1.01 (0.96–1.06) | 0.73 |
| Cardiac involvement | 24.57 (24.92) | 1.01 (0.93–1.11) | 0.75 |
| Abnormal liver function | 18.48 (15.80) | 0.94 (0.64–1.39) | 0.76 |
| Acute kidney injury | 14.60 (16.62) | 2.40 (0.65–8.85) | 0.19 |
| Pneumothorax | 1.91 (1.90) | 0.92 (0.34–2.45) | >0.9 |
| Asymptomatic | 11.77 (11.77) | 2.34 (0.03–170.73) | 0.70 |
| Laboratory findings and vital signs | |||
| Hyperglycemia | 33.91 (1.00) | - | >0.9 |
| Lymphopenia | 54.37 (20.51) | 1.09 (0.95–1.24) | 0.22 |
| Elevated CRP | 72.57 (43.47) | 1.02 (0.96–1.08) | 0.48 |
| Elevated d-dimer | 33.13 (30.83) | 1.01 (0.93–1.10) | 0.77 |
| Heart rate (average) | 89.72 (4.49) | 1.12 (0.73–1.72) | 0.60 |
| Respiratory rate | 21.46 (2.43) | 1.18 (0.59–2.37) | 0.64 |
| Mean arterial pressure | 93.16 (4.00) | 1.24 (0.00–0.00) | 0.99 |
| SpO2 | 95.09 (1.59) | - | 0.99 |
| Imaging studies | |||
| Chest CT changes | 87.44 (14.67) | 1.00 (0.94–1.07) | 0.94 |
| Chest XR changes | 67.09 (29.70) | 5.06 (0.00–0.00) | 0.99 |
| Comorbidities | |||
| COPD | 5.15 (5.56) | 3.93 (0.89–17.30) | 0.07 |
| Diabetes Mellitus* | 17.49 (12.46) | 1.34 (1.10–1.64) | 0.003 |
| Hypertension* | 32.17 (21.82) | 1.11 (1.03–1.20) | 0.006 |
| Cardiovascular disease | 12.24 (10.83) | 1.12 (0.98–1.30) | 0.11 |
| Cerebrovascular disease | 5.54 (3.67) | 1.77 (0.88–3.57) | 0.11 |
| Chronic kidney disease | 7.61 (9.32) | 1.63 (1.01–2.64) | 0.05 |
| Malignancy | 10.90 (20.41) | 1.51 (0.99–2.31) | 0.06 |
| Immunosuppression | 4.84 (5.59) | 1.00 (0.78–1.29) | 0.99 |
| Liver disease | 7.61 (19.38) | 1.04 (0.87–1.23) | 0.67 |
| Asthma | 8.32 (6.15) | 1.07 (0.83–1.38) | 0.62 |
| Obesity | 30.86 (13.00) | 1.85 (0.58–5.89) | 0.30 |
| Smoking history | 14.53 (12.49) | 1.09 (0.96–1.24) | 0.17 |
| Country income rate: | - | 2.46 (0.71–8.54) | 0.16 |
*p < 0.05.
3.2. Bivariate analysis
In the bivariate analysis applying a cut point of p <0.05 for significance, three variables, mean age (p < 0.001, OR of 1.18; 95% CI: 1.08–1.29), hypertension (p = 0.006, OR of 1.11; 95% CI:1.03–1.20), and Diabetes Mellitus (p = 0.003, OR of 1.34; 95% CI: 1.10–1.64) had a significant relationship with increased mortality.
We defined borderline association as 0.05<p < 0.10. Concerning the demographics, the male gender (p = 0.06, OR of 1.04; 95% CI: 1.00–1.09) had a borderline relationship with the mortality rate. In the signs, symptoms and related morbidities, factors having a borderline association (direct or inverse) with mortality rates were chills (p = 0.05), throat pain (p = 0.06, OR of 0.78; 95% CI: 0.6–1.01), dyspnea (p = 0.06, OR of 1.03; 95% CI:0.99–1.07), olfactory dysfunction (p = 0.07), and myalgia (p = 0.07, OR of 0.94; 95% CI:0.88–1.00). After analyzing lab data results, we found that an increased lactate dehydrogenase (LDH) had borderline association (p = 0.05). Other than hypertension and diabetes mellitus which demonstrated significant associations with increased mortality, three other comorbidities, CKD (p = 0.05, OR of 1.63; 95% CI: 1.01–2.64), malignancy (p = 0.06, OR of 1.51; 95% CI: 0.99–2.31), and COPD (p = 0.07, OR of 3.93; 95% CI: 0.89–17.30), showed a borderline correlation. Other factors, such as countries’ income and positive smoking history, did not exhibit either a significant or borderline correlation with mortality of COVID-19.
The detailed relationship of each variable with mortality rates is thoroughly presented in Table 3 .
3.3. Multivariate regression analysis
Taking a step forward, we then conducted a multivariate regression analysis of variables that were found to be significantly associated with COVID-19, with a similar threshold of significance (p <0.05). Independent association with the mortality was observed with Diabetes Mellitus (P = 0.038, the adjusted OR of 1.24; 95% CI:1.01–1.52).
4. Discussion
COVID-19 is caused by SARS-CoV-2 initially detected in Wuhan, China, in December 2019 and currently is the greatest challenge of the health care system all over the world. Preliminary molecular studies have shown that bats can be the potential reservoir of the virus [18]. To date, however, no specific treatment has been approved for this disease, and the core of current therapies is symptomatic and supportive care [19]. Therefore, it is crucial to study the rate and the factors affecting mortality in this disease. During this short time-period, many studies have been conducted in this field. Based on these studies, the predictors of mortality can be divided into five categories [20].
4.1. Demographics
Among the demographic factors, age is one of the most important factors affecting mortality, and in our study, age was significantly associated with increased mortality. Studies have shown that the age-related defects in immune cell function and increased production of inflammatory cytokines may play a role [21]. Studies have shown that the male gender is also a risk factor for severity and higher mortality [22].
4.2. Clinical signs and symptoms
According to the findings, the first ten most common observed signs and symptoms with the highest mean percentage affecting COVID-19 mortality were fever, cough, olfactory dysfunction, postnasal drip, gustatory dysfunction, face pain or heaviness, dyspnea, malaise, arthralgia, and nasal obstruction. Our large sample of 310,494 patients included from 114 studies showed that fever was the most common disease sign. Our findings are consistent with those of others [20,23].
4.3. Laboratory findings
This study does not support the findings of recent studies that have highlighted the role of laboratory results in predicting mortality. For example, a recent study of 485 patients in Wuhan, China, noted the role of LDH and CRP in increasing mortality. The study, conducted on 4659 patients, also found CRP, LDH, Troponin, Creatinine, and Albumin as predictors of mortality [23]. Nevertheless, we only found the increased level of LDH as a relative predictor of mortality. So it seems important to investigate this more thoroughly.
4.4. Imaging studies
Imaging plays a key role in the diagnosis of COVID-19. Among these, are chest X-Rays and Chest CT Scans. Common findings from Chest X-Rays include multifocal peripheral consolidation and multifocal rounded opacities and nodules [23], and in chest CTs there are ground-glass opacities (86% ), consolidation (29%), and Crazy-paving (19%) [24]. In contrast, this study does not show any significant correlation between radiologic findings and mortality rate.
4.5. Comorbidities
Endothelial dysfunction is one of the very first changes in diseases such as hypertension, diabetes, Coronary heart disease (CHD), and CKD. Numerous studies have also shown that SARS-COV2 tends to bind to the host Angiotensin-converting enzyme-2 (ACE2) receptor in vascular endothelial cells, which could well justify the role of underlying diseases in increasing mortality [23], [24], [25]. Infection-related demand ischemia and direct viral infection of the myocardium have also been reported in studies among the etiologies of increased mortality in patients with COVID-19 with a prior history of cardiovascular disease. The role of underlying diseases alongside age in increasing mortality has been strongly suggested in related articles, which has also been shown in our study, especially in diabetes and hypertension [26]. The pathogenesis of increased mortality of COVID-19 in patients with diabetes is still unknown. Immune dysregulation in diabetic patients such as phagocytic cell dysfunction, inhibition of neutrophil chemotaxis and impaired T-cell mediated immune response can be one of the probable mechanisms [27]. In addition, type 2 diabetes mellitus and coronavirus infection have common pathogenic pathways. So that two receptors of coronavirus, ACE2 and Dipeptidy-l Peptidase-4 (DPP4), have also a role in regulating glucose homeostasis[28]. Diabetes was also a strong predictor of mortality among patients suffering Middle-Eastern Respiratory Syndrome (MERS) and SARS in previous studies [29]. Therefore, all diabetic patients with COVID-19, should be taken as being at high risk, even though he or she may present with only mild or no symptoms. These patients will need extra monitoring, and so that their threshold for hospitalization and ICU admission also are lowered.
About hypertension, regular use of medications, including Angiotensin II receptor blockers (ARB) and Angiotensin-converting enzyme inhibitors (ACEI) upregulates ACE2 expression, therefore facilitating the entry of SARS-CoV-2 into pneumocytes which ultimately increases the severity and fatality of infection. But there is no evidence showing that the termination of ACEI and ARB in patients with COVID-19 infection would be beneficial [30]. Interestingly, no association was found between smoking and increased mortality. This finding is unlike several previous studies that showed association between smoking and high mortality rate in patients with COVID-19 [31].
5. Conclusion
Our findings support that older age, hypertension and diabetes mellitus might increase the risk of mortality among patients with COVID-19. In the multivariate analysis, only Diabetes Mellitus demonstrated an independent relationship with increased mortality. Further studies may be needed to ascertain the relationship of possible risk factors with COVID-19 mortality.
6. Limitations and strengths
This is an extensive systematic review of COVID-19 mortality and its associated factors. We screened a large number of available articles in several databases, assessed their quality, and extracted relevant data to run regression analysis. However, we were not able to obtain adequate information to run weighted analysis and draw forest plots. As many of our studies comprised of those with small populations, it was not feasible to analyze according the population density. Given that the studies are of case series, cross-sectional design, it was also not possible to pool the data together to estimate the heterogeneity between the studies. We attempted to categorize the countries based on income and present a map, demonstrating the differences between countries in presented cases in the literature. Owing to the circumstances of the pandemic, published data on some potentially suitable factors were limited. Therefore, these factors were not included in our study. Stability and availability of medical service and the role of lack of facility, treatment protocols, economic situations, and ethnicity of the patients are some of these missing variables.
7. Clinical implications
Health-care providers should pay special attention to comorbidities such as diabetes and hypertension because these conditions, if not controlled, can increase mortality in patients with COVID-19.
8. Research implications
By conducting further and more complete studies on patients with COVID-19, a more appropriate and complete model can be found for factors related to patient mortality. Also, based on the sample size, different weights can be given to these studies to consider the effect of sample size.
Author contributions
Esmaeil Mehraeen: The conception and design of the study, Drafting the article, Final approval of the version to be submitted; Amirali Karimi: Acquisition of data; Alireza Barzegary: Analysis and interpretation of data; Farzin Vahedi: Acquisition of data; Amir Masoud Afsah: Drafting the article; Omid Dadras: Final approval of the version to be submitted; Banafsheh Moradmand-Badie: Acquisition of data; Seyed Ahmad Seyed Alinaghi: The conception and design of the study, Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be submitted, Shayesteh Jahanfar: Revising it critically for important intellectual content.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The authors state that all information provided in this article can be obtained from the author on request.
Declaration of Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Acknowledgements
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences, and Department of Global Health and Socioepidemiology, Kyoto University.
References
- 1.Organization WH. Rolling updates on coronavirus disease (COVID-19) 2020, April 24 [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 2.Ghiasvand F., Ghadimi M., Ghadimi F., Safarpour S., Hosseinzadeh R., SeyedAlinaghi S., Symmetrical polyneuropathy in coronavirus disease 2019 (COVID-19), IDCases 21 (2020) e00815. [DOI] [PMC free article] [PubMed]
- 3.Organization WH. Coronavirus Disease 2019 (COVID-19) Situation Report–141 2020 [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 4.Cutler D. How will COVID-19 affect the health care economy? JAMA. 2020;323(22):2237–2238. doi: 10.1001/jama.2020.7308. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M., Abdelmaksoud A., Jafferany M., Lotti T., Sadoughifar R., Goldust M. COVID-19 and economy. Dermatol. Ther.. 2020:e13329. [DOI] [PMC free article] [PubMed]
- 6.Seyed Alinaghi S., Ghadimi M., Hajiabdolbaghi M., Rasoolinejad M., Abbasian L., Nezhad MH. Prevalence of COVID-19-like Symptoms among People Living with HIV, and Using Antiretroviral Therapy for Prevention and Treatment. Curr HIV Res. 2020;18(5):373–380. doi: 10.2174/1570162X18666200712175535. [DOI] [PubMed] [Google Scholar]
- 7.Bowie C., Hill T. Exit strategy to control covid-19 and relaunch the economy. BMJ. 2020;369:m1851. doi: 10.1136/bmj.m1851. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L., Ting S., Yufei H., Wendong L., Yubo F., Jing Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Moghadam S.R.J., Bayrami S., Moghadam S.J., Golrokhi R., Pahlaviani F.G., SeyedAlinaghi S. Zika virus: a review of literature. Asian Pac. J. Trop. Biomed. 2016;6(12):989–994. [Google Scholar]
- 13.Lee A. Wuhan novel coronavirus (COVID-19): why global control is challenging? Public Health. 2020;179:A1–A2. doi: 10.1016/j.puhe.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BinDhim N.F., Hawkey A., Trevena L. A systematic review of quality assessment methods for smartphone health apps. Telemed. e-Health. 2015;21(2):97–104. doi: 10.1089/tmj.2014.0088. [DOI] [PubMed] [Google Scholar]
- 16.Zapata B.C., Fernández-Alemán J.L., Idri A., Toval A. Empirical studies on usability of mHealth apps: a systematic literature review. J. Med. Syst. 2015;39(2):1. doi: 10.1007/s10916-014-0182-2. [DOI] [PubMed] [Google Scholar]
- 17.McKay F.H., Cheng C., Wright A., Shill J., Stephens H., Uccellini M. Evaluating mobile phone applications for health behaviour change: a systematic review. J. Telemed. Telecare. 2018;24(1):22–30. doi: 10.1177/1357633X16673538. [DOI] [PubMed] [Google Scholar]
- 18.Salata C., Calistri A., Parolin C., Palu G. Coronaviruses: a paradigm of new emerging zoonotic diseases. Pathog. Dis. 2019;77(9):ftaa006. doi: 10.1093/femspd/ftaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X., Wei F., Hu L., Wen L., Chen K. Epidemiology and clinical characteristics of COVID-19. Arch. Iran. Med. 2020;23(4):268–271. doi: 10.34172/aim.2020.09. [DOI] [PubMed] [Google Scholar]
- 20.Baay M., Lina B., Fontanet A., Marchant A., Saville M., Sabot P. SARS-CoV-2: virology, epidemiology, immunology and vaccine development. Biol.: J. Int. Assoc. Biol. Stand. 2020;66:35–40. doi: 10.1016/j.biologicals.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opal S.M., Girard T.D., Ely E.W. The immunopathogenesis of sepsis in elderly patients. Clin. Infect. Dis. 2005;41(Supplement_7):S504–SS12. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 22.Jin J.-.M., Bai P., He W., Wu F., Liu X.-.F., Han D-M. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J. Med. Virol. 2020. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed]
- 24.Bonow R., Fonarow G., O'Gara P., Yancy C. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality JAMA Cardiol. Published online March. 2020;27. [DOI] [PubMed]
- 25.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanne J.P. China: Key Points For the Radiologist. Radiological Society of North America; 2020. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelidi A.M., Belanger M.J., Mantzoros C.S. Commentary: COVID-19 and diabetes mellitus: what we know, how our patients should be treated now, and what should happen next. Metab. Clin. Exp. 2020;107 doi: 10.1016/j.metabol.2020.154245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J. Renin-Angiotensin-Aldosterone Syst.: JRAAS. 2020;21(2) doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all information provided in this article can be obtained from the author on request.