Abstract
Background
Opioid overdose deaths remain high in the U.S. Despite having effective interventions to prevent overdose deaths, there are numerous barriers that impede their adoption. The primary aim of the HEALing Communities Study (HCS) is to determine the impact of an intervention consisting of community-engaged, data-driven selection, and implementation of an integrated set of evidence-based practices (EBPs) on reducing opioid overdose deaths.
Methods
The HCS is a four year multi-site, parallel-group, cluster randomized wait-list controlled trial. Communities (n = 67) in Kentucky, Massachusetts, New York and Ohio are randomized to active intervention (Wave 1), which starts the intervention in Year 1 or the wait-list control (Wave 2), which starts the intervention in Year 3. The HCS will test a conceptually driven framework to assist communities in selecting and adopting EBPs with three components: 1) a community engagement strategy with local coalitions to guide and implement the intervention; 2) a compendium of EBPs coupled with technical assistance; and 3) a series of communication campaigns to increase awareness and demand for EBPs and reduce stigma. An implementation science framework guides the intervention and allows for examination of the multilevel contexts that promote or impede adoption and expansion of EBPs. The primary outcome, number of opioid overdose deaths, will be compared between Wave 1 and Wave 2 communities during Year 2 of the intervention for Wave 1. Numerous secondary outcomes will be examined.
Discussion
The HCS is the largest community-based implementation study in the field of addiction with an ambitious goal of significantly reducing fatal opioid overdoses.
Keywords: Opioid use disorder (OUD), Overdose, Community engagement, Medications for opioid use disorder (MOUD), Naloxone, Opioid prescribing, Helping to End Addiction Long-term, HEALing Communities Study
1. Introduction
The current U.S. opioid overdose crisis has been ongoing for two decades, but was only officially declared a national emergency in 2017 (Gostin et al., 2017). However, it is important to note that, since the 1960’s, a less well publicized and often neglected opioid crisis has been underway in the U.S., driven largely by heroin use in primarily urban areas and disproportionately impacting minority populations. Overdose deaths from prescription opioids, heroin, and illicit synthetic opioids contributed to more than 450,000 deaths from 1999 to 2018 and nearly 47,000 deaths in 2018 alone (Seth et al., 2018). In recent years, the availability and use of illicit fentanyl and fentanyl analogs have accounted for an increasing proportion of opioid overdose deaths (NIDA, 2019). One driver of the opioid crisis is the recognized gap between the number of individuals who could benefit from evidence-based treatment and prevention interventions to reduce opioid misuse, opioid use disorder (OUD) and associated medical consequences, including overdose deaths, versus those engaged in care. The 2018 National Survey on Drug Use and Health estimated conservatively that 2.1 million Americans have OUD; however, this excludes several highly affected populations (e.g., incarcerated individuals, homeless)(SAMHSA, 2019). Fewer than 20% of those with OUD receive addiction care in a given year (SAMHSA, 2019; Wu et al., 2016). In a cohort of opioid overdose survivors, fewer than one-third received any medication for opioid use disorder (MOUD) within a year of the overdose event (Larochelle et al., 2018).
A number of evidence-based practices (EBPs) exist, including: opioid overdose education and naloxone distribution programs (e.g., Walley et al., 2013); prescription drug monitoring programs and improved professional guidelines to reduce inappropriate opioid prescribing (e.g., Bonert et al., 2018); Food and Drug Administration–approved MOUD including methadone, buprenorphine, and naltrexone; treatment engagement and retention interventions; and recovery support services (e.g., Strang et al., 2020). Many of these EBPs have largely failed to penetrate community settings where opioid misuse and OUD could be addressed, including general medical care, the justice system, social support services, and addiction treatment programs. This failure is, in part, due to a lack of evidence-based approaches to assist communities in the development and deployment of data-driven, community-specific strategies to adopt, deliver, and use integrated EBPs.
In the US, the substance use treatment gap is a result of at least three major challenges: 1) many individuals with OUD do not perceive a need for treatment; 2) there is insufficient treatment capacity; and 3) there is suboptimal treatment retention (Williams et al., 2018). National data indicate that, among individuals with substance use disorders who are not in treatment, a lack of recognition of their disorder is a major impediment to seeking help (Ali et al., 2015; Olsen and Sharfstein, 2014). A recent report indicated that, of those respondents with opioid misuse, nearly 87% did not perceive a need for treatment (Choi et al., 2019). Furthermore, many individuals have internalized stigma about OUD that prevents them from seeking treatment (Corrigan et al., 2017). In addition to these challenges, there is limited capacity for delivering MOUD in many communities. Most of the nation’s 1,782 Opioid Treatment Programs (OTPs; e.g., federally licensed methadone programs) are located in urban communities, are not integrated into traditional health care settings, and growth in the number has been modest in the past decade (Jones et al., 2015). Buprenorphine, in contrast to methadone, is more widely delivered in office-based addiction treatment in the U.S. and requires a unique license designation known as a waiver (Kraus et al., 2011). The number of U.S. buprenorphine-waivered physicians has increased (Knudsen et al., 2017) along with buprenorphine dispensing (Alderks, 2017), but both remain insufficient to meet the need for treatment even with the new provision to allow nurse practitioners and physician assistants to prescribe buprenorphine. For example, according to the DEA prescriber database as of September 2020, there were a total of 1,740,770 civilian physicians, nurse practitioners, and physician assistants actively licensed to prescribe controlled substances but, of those, only 85,317 or 4.9% also possessed the waiver to prescribe buprenorphine for OUD. In addition, for patients receiving MOUD, overall treatment retention is poor, and the percentage of those receiving treatment has declined from 25% in 2010 to 16% in 2014 due to increasing numbers of affected individuals (Morgan et al., 2018). Finally, access to treatment and ongoing care are further limited by policy barriers, such as required prior authorizations and arbitrary time limits for care.
In addition to MOUD-related challenges, both underutilization of overdose prevention strategies and the opioid-prescribing behaviors of medical professionals that can increase the risk of overdose are significant drivers of the national epidemic. Naloxone effectively reverses opioid overdose, thus preventing fatalities (Walley et al., 2013). Although demonstration projects have shown that community distribution of naloxone can reduce opioid fatality rates, national data show very limited prescribing of naloxone (including distribution through standing orders at pharmacies)(Sohn et al., 2019; Xu et al., 2018). Opioid overdose deaths also reflect continuing patterns of risky prescribing, such as concurrent benzodiazepine and opioid prescribing and high opioid doses (i.e., >90 morphine milligram equivalents per day). These prescribing behaviors increase the risk of overdose even among individuals without OUD and heighten the risk of developing OUD.
For these reasons, a study that addresses barriers and facilitators of EBPs and their implementation is imperative to change the course of the overdose crisis, particularly for EBPs most likely to have an immediate impact on overdose deaths. The primary aim of the HEALing Communities Study (HCS) is to evaluate the effectiveness of a community-engaged intervention on reducing opioid overdose fatalities by deploying an integrated set of EBPs through a community-driven process in an array of settings, including behavioral health, healthcare and criminal justice to reach populations vulnerable to opioid overdose. The experimental design also offers an opportunity to extend our understanding of the multi-level factors that mediate or moderate implementation success in heterogenous communities, which vary considerably in their current resources and ongoing response to the opioid crisis. By adapting the Reach, Effectiveness, Adoption, Implementation, and Maintenance model and Practical, Robust Implementation and Sustainability Model (RE-AIM/PRISM; described below) (Aarons et al., 2011; Damschroder et al., 2009; Greenhalgh et al., 2004; Kitson et al., 2008; Raghavan et al., 2008; Tabak et al., 2012), the HCS can define system-level interrelationships as well as dynamics within the intervention itself that facilitate successful attainment of reducing opioid overdose deaths.
1.1. Objective
The HCS is a four-year multi-site study with the overarching objective of implementing EBPs to significantly reduce opioid-related overdose fatalities in 67 urban and rural communities. The HCS will test the Communities That Heal (CTH) intervention, a conceptually driven framework built upon the evidence-based Communities That Care model, that assists communities in adopting EBPs to prevent drug use and other risky behaviors (Oesterle et al., 2018). The CTH intervention seeks to promote a common vision, shared goals, and tailored strategies to mobilize HCS communities to adopt and implement EBPs. CTH uses a stepwise process to engage community members to implement system- and practice-level changes, while at the same time delivering communication campaigns to increase awareness and demand for EBPs and reduce stigma. Secondary outcomes will assess the impact of the CTH intervention on increasing naloxone distribution, expansion of MOUD utilization and reduction of high-risk opioid prescribing practices. The HCS will also examine conceptually driven internal and external contexts for implementation of the CTH intervention, including policy; resources at the community, state and national level; treatment guidelines; fidelity of the HCS implementation; and overall cost-effectiveness of the intervention.
2. Methods
2.1. Trial design
The HCS is a multi-site, parallel group, cluster randomized wait-list controlled trial testing the impact of the CTH intervention. The HCS enrolled 67 communities from four states. This cluster randomized controlled trial treats communities as clusters, allocating each to either the CTH intervention or the wait-list comparison group. Communities randomized to the CTH intervention during the first two years are referred to as Wave 1 communities. Communities randomized to the wait-list comparison group (Wave 2 communities) continue usual care during the first 2 years and begin the CTH intervention thereafter. Due to the time needed to work with communities and organizations to expand delivery of EBPs, a lag is expected from when the CTH intervention is introduced into a community to when its effect on key outcomes will be observed. Therefore, the primary study outcome compares the randomized groups during Year 2 of the intervention in Wave 1 Communities, referred to as the evaluation period. This study is registered on Clinical Trials.gov (https://clinicaltrials.gov/ct2/show/NCT04111939), registered on October 1, 2019 with first enrollment on Oct 23, 2019.
2.2. Ethics and informed consent
This study is being conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The protocol was approved by a central Institutional Review Board (IRB; Advarra Inc.) for all sites on October 16, 2019. The strategy for obtaining informed consent was considered across three domains: consent for participating communities, administrative data (population-level), and de novo data collection from individuals (e.g., surveys), with the process for each reviewed and approved by the IRB.
1) HCS Communities: HCS communities have a population of approximately 10,144,261 people. Because no one person or group of people possess(es) the authority to give consent on behalf of all community members, investigators sought expert consultation and applied guidelines from the Ottawa Statement (Taljaard et al., 2013). Because the CTH intervention poses no more than minimal risk to community members and the research could not be carried out otherwise (see 45.CFR.46.116), a waiver of informed consent was obtained for all community members who may be affected by the CTH intervention. Additionally, key stakeholders/participants provided letters of support for community participation prior to funding, and community coalitions completed charters outlining roles and responsibilities of coalition members following randomization.
2) Administrative Data Records: To examine study outcomes, numerous administrative data records will be collected. Given the time frame of the study and study population, there will likely be millions of individual health records included, but no individuals will be contacted. Thus, a waiver of informed consent and Health Insurance Portability and Accountability Act waiver were obtained.
3) Individual Data Collection: Individuals who provide de novo data (e.g., surveys, interviews) provide informed consent via phone, online or in writing depending on the measure and data collection process. A partial waiver of consent was obtained for baseline individual data collection.
2.3. Participants
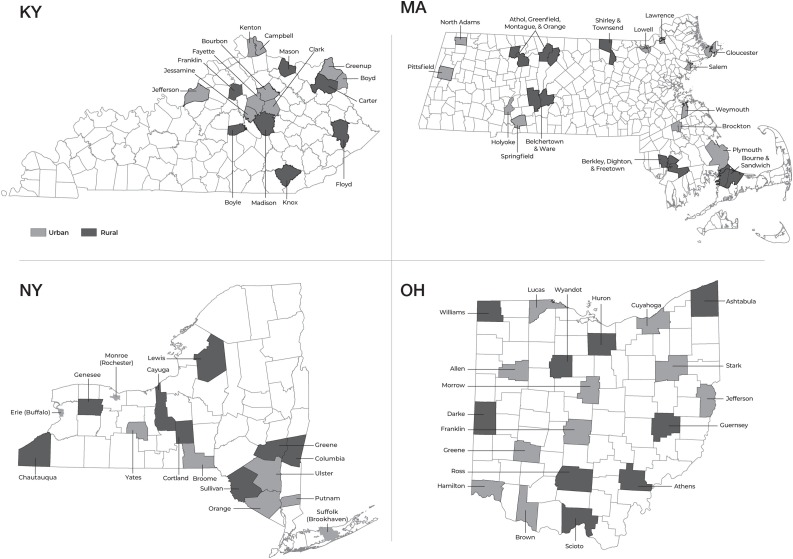
Communities across four states (Kentucky, Massachusetts, New York, Ohio) were selected to participate based on the following eligibility criteria established by the National Institute on Drug Abuse: 1) expressed willingness to address the implementation of MOUD and overdose education and naloxone distribution; 2) expressed willingness to develop partnerships across health care, behavioral health, and justice settings for EBPs to address opioid misuse, OUD, and overdoses; 3) within each state, ≥30% of selected communities were rural; 4) Across the HCS communities in each state, ≥150 opioid-related overdose fatalities (at least 15% occurring in rural communities) and a rate of ≥25 opioid-related overdose fatalities per 100,000 people, based on 2016 data. The communities are either counties (NY, KY, OH) or cities/towns (MA; see Fig. 1 ). Additional state-specific criteria were applied to further refine selection. KY selected counties with: 1) a syringe service program (marker of community readiness); 2) a jail; 3) ≥1 buprenorphine-waivered provider; and 4) ≥5 opioid overdose deaths in 2017. MA selected non-adjacent communities to minimize proximity and contamination, favored communities with an anchor office-based addiction treatment (OBAT) program and a pre-existing substance use coalition. NY included three urban or semi-urban communities (Buffalo, Rochester, Brookhaven) to ensure geographic diversity and the comparability of population sizes in Wave 1 and Wave 2 communities. OH selected counties stratified by urban/rural that: 1) were not contiguous and 2) did not share an existing Alcohol, Drug and Mental Health Board. KY excluded three counties that were actively engaged in two National Institutes of Health–funded community-level addiction intervention studies. OH excluded 10 counties for lack of available opioid-related data.
Fig. 1.
Map of rural and urban communities participating in the HEALing Communities Study. Communities (n = 67) from the four participating states are shown with gray shading representing urban communities and black shading representing rural communities.
2.4. Interventions
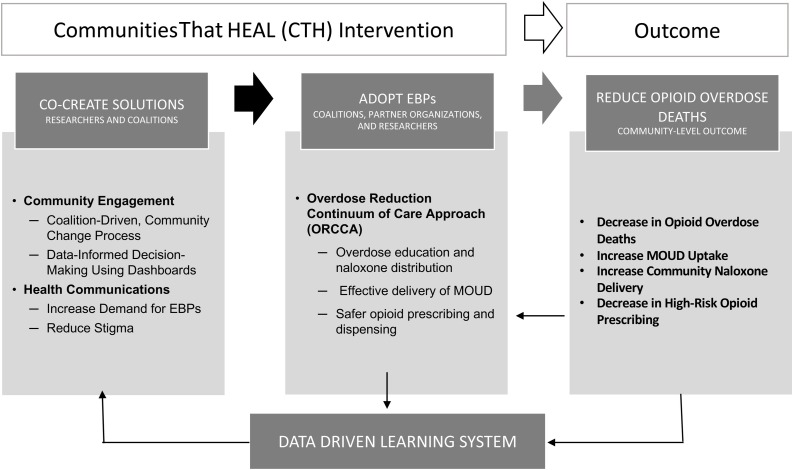
The CTH intervention has three elements: 1) a community engagement strategy designed to facilitate implementation of EBPs (Sprague Martinez et al., 2020); 2) a compendium of EBPs coupled with technical assistance guides (Winhusen et al., 2020); and 3) a series of communication campaigns aimed at reducing stigma and raising awareness about EBPs (Lefebvre et al., 2020) (see Fig. 2 ).
Fig. 2.
The Communities That Heal intervention core components.
2.4.1. Community engagement strategy
The CTH intervention is guided by principles of community engagement to build capacity and support the adoption of EBPs that are described below. Community engagement and partnering with local stakeholders provides researchers with nuanced understandings of community needs, resources, priorities and community norms, thus allowing for implementation of EBPs best suited for the community (Wallerstein and Duran, 2010; Wallerstein et al., 2015).
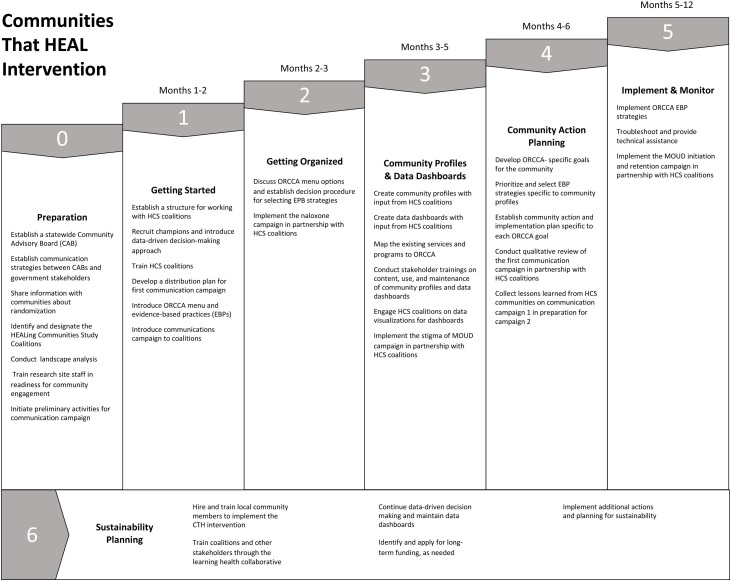
The CTH intervention (see Fig. 3 ) consists of an iterative, 7 phase change process for communities that is led by a diverse, multi-sector community coalition. This coalition-led, data-driven, and non-linear planning process is adapted from the Communities That Care model (Hawkins et al., 2009). The Preparatory Phase of the CTH intervention (Phase 0) begins with each HCS research team establishing a multisectoral community advisory board (CAB), separate from local coalitions, whose role is to serve in an advisory capacity to the research team. CABs are state-specific and include representation from state agencies and HCS communities. Research teams identify existing coalitions or establish new coalitions to partner with in the CTH intervention. Research teams present an overview of the project including the goals, randomization, and timeline to community coalitions in both Wave 1 and 2 of the study. A landscape analysis conducted by the research teams identifies local resources to address the opioid crisis and inform community planning, which is done in Phase 3.
Fig. 3.
The seven phases of the Communities That Heal community-engagement strategy.
Phase 1 launches intervention activities in Wave 1 communities. Research teams lay the groundwork for collaboration by co-creation of a partnership agreement/charter to outline key milestones, clarifying roles for coalitions and the research teams, and introducing the Opioid-overdose Reduction Continuum of Care Approach (ORCCA; a compendium of EBPs and EBP strategies (Winhusen et al., 2020). Coalition Champions within each coalition are identified, who serve as liaisons for implementation in three key areas: ORCCA menu, collection and understanding of data, and communication campaigns.
Phase 2 includes an in-depth review of the ORCCA. EBPs are discussed within the context of community need and readiness by engaging coalition members, people at high risk of overdose and their family/friends and local experts from partner organizations. Coalitions develop a shared vision for implementing the EBPs, consider decision-making processes for selections, and plan for the launch of the communication campaigns. Phase 3 involves an iterative process of co-creating a community profile of local resources and a data dashboard to inform community action planning. Research staff and/or data champions lead coalitions through a series of workshops and/or training to review, gather, and/or display available data from a landscape analysis (defined above) and other sources in a way that meaningfully guides decision-making. The community profile summarizes baseline community data to help identify resources needed to address the opioid crisis. The data dashboard is a web-based platform designed to visualize data related to community goals and the overarching HCS outcomes.
In Phase 4, each coalition synthesizes data from its community profile to develop an overall community action plan that matches its selected EBP strategies. Action plans require coalitions to incorporate insights from Phase 3 to reach consensus and prioritize EBP strategies to address vulnerable populations in healthcare, behavioral health, and justice settings and suggest partnerships with emphasis on impact and feasibility. Communities set goals, brainstorm, and weigh the benefits of specific EBPs and strategies. All research sites are using consensus discussions throughout Phase 4 to guide the selection of EBPs. The community engagement training protocol for community engagement facilitators includes a module about meeting facilitation and inclusive consensus building that prepares facilitators for these discussions. The Action Plan includes monitoring and feedback opportunities, and, in some cases, direction for community implementation teams and ORCCA champions to engage community partners in Partner Implementation Agreements, defining steps and resources needed for a given strategy. In Phase 5, research staff and community coalition members meet with partner agencies to develop and support their implementation plans. These plans allow community partners to articulate goals and the resources needed, including technical assistance and funding, to implement the ORCCA menu selections successfully. Monitoring and feedback of implementation activities includes reporting of technical assistance events and EBP selections for specific target populations and venues, and implementation strategies from the compilation by Powell (Powell et al., 2012, 2015). Throughout Phase 5, coalitions support community stakeholders’ progress, problem solve and suggest further plans as needed with new and existing partners. Coalitions may revisit earlier CTH phases based on emerging data.
Phase 6 focuses on sustainability planning and is ongoing throughout the intervention. Sustainability activities include: 1) Increasing community capacity through hiring and training of community members; 2) Implementing Learning Health Collaboratives to enable local communities and coalitions to learn from each other, share strategies and problem solve; and 3) Developing a culture of data-driven decision-making with the use of data dashboards that can be customized and maintained throughout the project and beyond. Coalitions receive support to create sustainability plans as they move through each of the phases to ensure CTH activities can be maintained when research funding ends.
2.4.2. The Opioid-overdose Reduction Continuum of Care Approach (ORCCA)
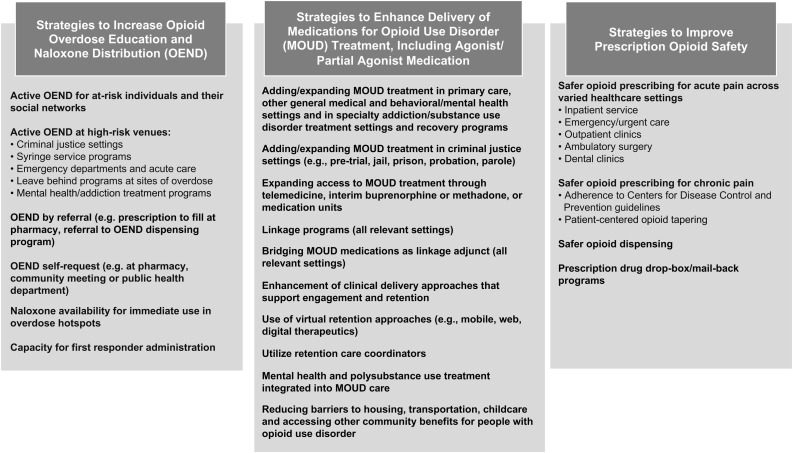
The ORCCA organizes EBPs and strategies, primarily focused on individuals 18 and older, that are most likely to reduce opioid overdose deaths into menus: 1) overdose education and naloxone distribution; 2) effective delivery of MOUD; and 3) safer opioid prescribing and dispensing. Multiple options are available within each menu. In addition to EBPs and strategies, each menu includes priority groups at highest risk of overdose and required community settings for implementation. Fig. 4 provides an overview of the ORCCA.
Fig. 4.
Opioid-overdose Reduction Continuum of Care Approach (ORCCA) Menu.
Communities have flexibility in the EBPs they select, and selection is based on multifactorial data (e.g., infrastructure and resources, existing services and delivery organizations, gaps in service provision). At least five strategies must be selected for implementation from the three EBP menus: one for overdose education and naloxone distribution, three in different areas that will enhance the care cascade for MOUD (i.e., linkage to care, initiation of treatment, retention on MOUD), and one for safer opioid prescribing/dispensing. Across the study, if new EBPs/strategies emerge (e.g., newly approved medication), they can be added by consensus to the ORCCA. As the CTH intervention seeks to support communities in developing and implementing a community-wide integrated approach, they are also required to implement at least one EBP strategy within each of three required community settings: 1) healthcare (e.g., emergency medical services, primary care, emergency departments); 2) behavioral health (e.g., substance use treatment programs, mental health treatment centers); and 3) criminal justice (e.g., jails, parole and probation). The HCS research teams developed a Technical Assistance Guide that provides resources (e.g., toolkits, publications, websites) about each EBP, including examples of successful national, state, and local programs.
2.4.3. Community-based communication campaigns
The CTH intervention includes a series of communication campaigns built on the foundations of health communication, social marketing, and mass communication for behavior change (Robinson et al., 2014; Snyder, 2007; Wakefield et al., 2010). The campaigns goals are: 1) increase demand for and access to naloxone; 2) increase demand, access, and retention for MOUD; and 3) reduce stigma of OUD, MOUD, and naloxone. Each campaign targets three priority groups: community leaders, health care providers and individuals at high risk of overdose as well as their family and social networks. These campaigns are tailored to local context and translated to languages other than English as needed; coalitions develop the distribution plans.
The HCS website serves an important role in the communication campaigns as a place where community leaders, advocates, policymakers, administrators, providers, people at risk for overdose and their families and friends, and others can learn about and become engaged in the study (https://healingcommunitiesstudy.org/). The website also includes: 1) contact information for coalitions; 2) education resources supporting campaign topics; 3) links to local resources for naloxone and MOUD; 4) communication campaign resources; and 5) announcements and events planned by coalitions. Website analytics help determine what information on the website is being used by members of the community.
The communication campaigns occur within the phases of the CTH intervention (see Fig. 2). Communication specialists from the four sites develop materials for the campaigns, including print materials and manuals to guide coalitions in distributing these materials via social media and other channels. Extensive message testing is conducted with priority groups to finalize content and images used in print materials. Coalitions are also given the option to add other components to their campaigns (e.g., materials for specific groups or social media platforms, use of paid targeted advertising on social media and other outlets). The campaigns are designed to use only locally accessible media resources to avoid spillover effects into Wave 2 communities.
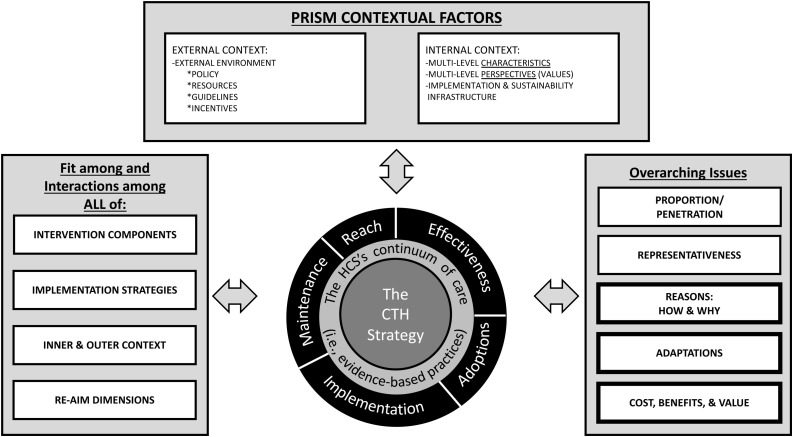
2.5. Implementation science framework: applying RE-AIM and PRISM to the HCS
The use of RE-AIM as an outcome framework – Reach, Effectiveness, Adoption, Implementation, and Maintenance (Glasgow et al., 2019, 1999) – offers a means by which the HCS can identify patterns among outcomes that are more generalizable (Knudsen et al., 2020) (see Fig. 5 ). We define Reach in the HCS as the number and proportion of individuals who are engaged in the CTH intervention, and that is conceptualized as three discrete components described above (i.e., community engagement, the ORCCA, and the communications campaign). Reach also focuses on equity in access to these three components by race/ethnicity, rural/urban geographic status, gender and age. Effectiveness encompasses the impacts of the intervention on the primary and other important secondary outcomes (Glasgow et al., 2019, 1999).
Fig. 5.
HEALing Communities Study implementation science framework. This figure is adapted from the revised, enhanced RE-AIM/PRISM 2019 model (Glasgow et al., 2019, p. 6).
Adoption, implementation, and maintenance in RE-AIM shifts attention to the uptake and use of the CTH intervention in a community (Glasgow et al., 2019, 1999). Adoption refers to the number and proportion of settings that are willing to initiate use of the intervention. For HCS, adoption is relevant both for coalitions, in terms of the number of coalitions that are willing to participate in the CTH intervention and for the number of settings in communities that are willing to begin delivering EBPs from the ORCCA menu. Implementation in RE-AIM focuses on fidelity to the elements of an intervention. For the HCS, fidelity to the community-engaged activities contained in the CTH phases are measured by adherence, quality of delivery, dosage and output. Finally, Maintenance in RE-AIM adds a longitudinal design, as it is concerned with assessing the sustained use of an intervention over time and whether the intervention becomes “institutionalized” in routine practice and policy within a given setting. The HCS includes a sustainability phase focused on building the infrastructure necessary to address the ongoing maintenance of the CTH intervention and sustainment of the EBPs in partner organizations after the research team withdraws.
At a broader level, PRISM – the “Practical, Robust Implementation and Sustainability Model” – provides insight into how internal and external contexts may come to impact implementation processes and subsequently outcomes (Feldstein and Glasgow, 2008). Integrating constructs from earlier frameworks on implementation and diffusion of innovations (Berwick, 2003; Green and Kreuter, 1999; Green et al., 1996; Rogers, 2003), PRISM’s conceptualization of the internal context includes perspectives about the interventions to be implemented as well as the characteristics of those engaged in the intervention. Because the HCS is focused on both a community-engaged process with coalitions as well as implementing EBPs within the communities, perspectives of coalition members on both elements are likely to impact the RE-AIM outcomes. Implementation success across the RE-AIM outcomes may be influenced by a coalition’s characteristics, including whether they were developed de novo or existed prior to the start of the study as well as the sectors represented in the coalition. Constructs in the PRISM model are being measured using a mixed methods approach that includes both longitudinal surveys and interviews with coalition members and key stakeholders.
The external context will likely play a significant role in the implementation process. Key factors in the external context for the HCS include policy and resources at the community, state and national level that may help or hinder efforts to expand the reach of the CTH intervention and, more recently, the COVID-19 pandemic. Further, stigma in the community and in partnering agencies about persons with OUD as well as stigma regarding the ORCCA EBP strategies may pose potent barriers to scaling up these practices over the course of the study. The communications campaigns within the CTH intervention are envisioned to mitigate forms of stigma, and thus, increase community support for the ORCCA EBPs and reduce the stigma-related barriers to accessing these services.
2.6. Outcomes and measures
The HCS has numerous outcomes tied to hypothesis testing for the primary aim (i.e., reduction of opioid overdose deaths), implementation outcomes (e.g., surveys and interviews on attitudes and access to EBPs within communities, agency surveys to assess capacity and barriers), health economics costing of the intervention, and an evaluation of the effectiveness of the communications campaigns. Most of these are described in detail in companion papers (e.g., Aldridge et al., 2020; Knudsen et al., 2020; Slavova et al., 2020). Here we report only on the primary outcome meaures and three secondary outcomes (each mapping onto one of the three ORCCA menus) that will examine differences between Wave 1 and Wave 2 communities during the evaluation period.
2.6.1. Primary outcome and measure
Number of opioid overdose deaths among HCS residents as measured by deaths with an underlying cause of drug overdose according to state death certificate data where opioids, alone or in combination with other drugs, were determined to be contributing to the drug overdose death.
2.6.2. Secondary outcome measures
(a) Number of naloxone units distributed in an HCS community as measured by the sum of the number of naloxone units sold by retail pharmacies located within the community, and units distributed in the community with support from state and federal funding (including dedicated HCS funding). Data are captured from IQVIA Xponent database and state departments of health.
(b) Number of HCS residents receiving buprenorphine products that are FDA-approved for treatment of OUD as measured by HCS residents with dispensed prescriptions for these buprenorphine products using state prescription monitoring program (PMP) data.
(c) Number of HCS residents with new incidents of high-risk opioid prescribing as measured by the number of HCS residents who met at least 1 of the following 4 criteria for a new high-risk opioid prescribing episode after a washout period of at least 45 days (from records of dispensed controlled substance prescriptions reported to state PMP):
-
i)
Incident opioid prescribing episode >30 days duration (continuous opioid receipt with no more than a seven-day gap)
-
ii)
Starting an incident opioid prescribing episode with extended-release or long-acting opioid formulation
-
iii)
Incident high dose opioid prescribing defined as ≥ 90 mg morphine milligram equivalents over three calendar months, and
-
iv)
Incident overlapping opioid and benzodiazepine prescriptions >30 days over three calendar months.
2.7. Health economics analysis
The HCS will conduct health economics research to determine startup and ongoing costs and the overall cost-effectiveness of the CTH intervention (Aldridge et al., 2020). Using an activity-based costing approach, the costs of the core components of the CTH intervention are estimated, including time spent in coalition meetings, planning and implementing the communication campaign, facilitating the intervention in communities, and other costs such as staff training and increases in MOUD dispensed. These cost estimates combine data on the reduced number of opioid overdose deaths attributable to the CTH intervention to estimate the cost-effectiveness of the intervention by calculating the additional cost per averted opioid overdose death. Simulation models will be developed to evaluate the short- and long-term health and economic impacts of the CTH intervention and inform decision-makers about optimal resource allocation to achieve reductions in opioid overdose deaths.
2.8. Statistical methods
2.8.1. Sample size
A total of 67 communities (16 from KY, MA, and NY, and 19 from OH) were randomized to either Wave 1 (n = 34) or Wave 2 (n = 33). The HCS as designed has greater than 99% power to detect a 40% reduction (i.e., relative risk of 0.60) in opioid overdose deaths for Wave 1 relative to Wave 2 communities. However, power remains high even if the intervention effect is smaller than anticipated (e.g., 88% power for a 20% reduction).
Simulation studies were used to calculate power, accounting for the study design and planned statistical approach. Simulation study parameters (i.e., the marginal parameters for the negative binomial regression model and the model’s dispersion term which is analogous to the intra-cluster correlation coefficient (ICC) (Eldridge et al., 2009) were estimated based on data obtained from the 67 communities during 2016 and 2017, including community population size and number of opioid overdose deaths. These estimates were assumed to be the true parameter values for Wave 2 communities at the time of the evaluation period. Each simulation study was conducted using 20,000 replications to ensure negligible error in calculated powers. A two-sided test with an alpha of 0.05 was used.
2.8.2. Randomization
Stratified, covariate-constrained randomization (Ivers et al., 2012; Moulton, 2004) was used to assign communities to either the CTH intervention or waitlist comparison arm and stratified by research site (i.e., the state). Within sites, covariate-constrained randomization was used to balance arms on three baseline community characteristics: 1) opioid overdose deaths (i.e., rate), 2) population size, and 3) urban/rural status. The following constraints were applied: 1) urban/rural status will be equal for sites with even numbers, otherwise a difference of 1 will be allowed; 2) community population; and 3) opioid death rate will be constrained to <0.2 standard deviation difference.
Allocation was carried out by the Data Coordinating Center and concealed from all others, including the research sites and the communities. Communities were the unit of randomization. Once randomization was complete, the resulting community assignments were communicated electronically to the principal investigators at research sites as well as the study sponsors simultaneously. The research site investigators then informed their communities.
The communities (clusters) were enrolled by representatives at their respective research sites. Random numbers were generated for each site and incorporated into a SAS macro (Greene, 2017) used to implement the covariate-constrained randomization. All steps in the randomization process were documented and reviewed by an independent statistician for quality control. Due to the nature of the CTH intervention, the HCS is an open, unblinded study.
2.8.3. Statistical Approach
The primary analysis will be based on the intention-to-treat (ITT) principle, including all randomized HCS communities according to their assigned group. The primary outcome, number of opioid overdose deaths, and all other secondary count outcomes, will be analyzed using a marginal negative binomial regression model, utilizing small-sample adjusted empirical standard error estimates and degrees of freedom equal to the number of communities minus the number of regression parameters (Ford and Westgate, 2017; Li et al., 2017; Mancl and DeRouen, 2001; SAS Institute, 2013; Westgate, 2013). The model will include trial arm as the main independent variable and control for the community-level variables included in the stratified, covariate-constrained randomization (i.e., research site, rural/urban status, and baseline opioid overdose death rate) in order to increase statistical power as each of these factors was included in the constrained randomization scheme. The reported natural log of the population size for each community will be utilized as the offset of the regression model, such that the proposed model is a model for the probability of an opioid overdose death in the population. Interpretation from the resulting model can be either with respect to changes in the population probabilities (i.e., risk ratios for opioid overdose death for Wave 1 vs. Wave 2 communities) or in terms of opioid overdose death rates (i.e., rate ratios). Reported p-values will be based on two-sided tests at an α = 0.05. A sensitivity analysis will be conducted using a permutation test based on the implementation of covariate-constrained randomization. A secondary per-protocol analysis will also be conducted that excludes any community that withdraws early from the HCS and any community that has the intervention halted early based on recommendations from the Data Safety and Monitoring Board.
3. Discussion
The HCS, a highly complex study in 67 communities across four states, is the largest community-engaged, implementation science addiction research study ever conducted. Achieving the study goal to reduce opioid overdose deaths hinges on the engagement of participating communities. Each site has an active CAB at the state-level to provide guidance on the study design, while local community coalitions serve as the vehicle for implementation. The CTH intervention employs a multi-stage, data-driven, community engagement strategy to facilitate the uptake of and access to EBPs proven to reduce opioid overdose deaths. Community coalitions develop action plans to address the local opioid overdose crisis that include selection of EBPs, strategies for implementation, and partner agencies to facilitate implementation. Coalitions use data to drive decision-making on the selection and deployment of EBPs and to revise response plans as changes occur within the communities. This coalition planning process allows diverse stakeholders, including individuals personally impacted by OUD and their family members, to address complex health problems through collaborative decision-making, which increases likelihood of EBP uptake and sustainability. The public health communication campaigns are intended to reduce stigma surrounding the EBPs and increase demand for them within the target audiences in the communities (e.g., individuals with OUD, healthcare providers). While the 67 HCS communities are heterogenous in many characteristics, all are located in Medicaid expansion states, so it is unknown whether the impacts of the CTH would generalize to communities in non-expansion states.
The HCS study was launched prior to the onset of the COVID-19 pandemic. Mitigation efforts have affected many aspects of the study (e.g., stay-at-home orders have impacted the provision of OUD treatment, the capacity of communities to respond to COVID-19 while participating in the study, and the ability to have in-person meetings with communities and key stakeholders). Research sites and communities are adapting to meet these challenges. Most notably, community coalition planning and stakeholder meetings switched to web-based formats.
While the early advent of this current opioid crisis impacted primarily white communities, more recent data show dramatic increases in rates of opioid overdose deaths in minority populations, especially in Black individuals, with an alarming increase attributable to synthetic opioids (Drake et al., 2020). These epidemiological findings coupled with ongoing lack of equitable access to treatment for OUD (e.g., Lagisetty et al., 2019) highlight the importance of addressing these inequities to ensure equal access to EBPs for treating OUD and reducing opioid overdose in the HCS. The HCS design and execution are envisioned to ensure inclusivity and diversity in planning and community engagement and to ensure that diverse and marginalized populations are reached with the intervention. With regard to planning and community engagement, there are intentional efforts to ensure CABs and community coalitions include diverse representatation with attention to race, ethnicity, gender, sexual orientation and ensuring participation of those in recovery. With regard to reach of the intervention, communities are actively targeting marginalized populations for intervention often through careful selection of sites or agency partnerships providing services to those populations (e.g., criminal justice populations, homeless, etc.). HCS data analyses will directly evaluate the impact of various demographics, including race and ethnicity, and other social determinants of health on study outcomes. Community facing HCS materials are developed to reflect different racial and ethnic groups, including, for example, the communication campaign advertisements, while study tools, such as overdose education training materials, are developed in multiple languages. The CTH intervention with its strong community engagement, diverse stakeholders, and data-driven decision-making provides a way to document and address racial and ethnic inequities in access to EBPs.
4. Conclusions
The HCS will collect a rich data set to understand uptake and sustainability of the CTH including cost and cost-effectiveness. This will be accomplished within an established implementation science framework that will identify barriers and opportunities (examining both internal and external context) and engage community agencies to enhance delivery of EBPs, expand agency access to resources, facilitate change in policy to enhance availability of EBPs as needed, and understand how community characteristics (e.g., rural/urban, community-specific social determinants of health) underlie effective uptake of, fidelity to, and sustainability of the CTH intervention. If effective, the CTH intervention will provide a generalizable intervention that can be applied in other communities to prevent opioid overdose fatalities and related consequences.
Trial registration
Clinical Trials.gov http://www.clinicaltrials.gov; Identifier: NCT04111939
Contributors
The following individuals contributed to the conception of the HCS Study including the experimental design, the CTH intervention components (community engagement strategy, Opioid-overdose Reduction Continuum of Care Approach, communication strategy), implementation science framework, the health economics analysis, the identification of data elements, overdose simulation modeling, community dashboard design, and/or statistical analysis plan: APA, TB, CB, JAB, TAB, D. Beers, RBS, CB, JLB, HMB, JB, AB, AC (NY), MC, RKC, DMC, JC, TC, KPC, ELC, AC (NIDA), JLD, MLD, NEB, LCF, DF (NY), SF, DF (OH), PERF, BF, LG, DGE, DG, KH, DWH, T. Huang, T. Huerta, T. Hunt, AH, RDJ, RK, KK, CK, HKK, MK, MRL (MA), FL, BPL, MRL (KY), DL, MSL, SM, AM, KEM, TM, CM, DN, EN, EAO, CBO, TP, BR, SR, ER, LRM, MS, NS, RS, PS, JHS, BS, ES, MDS (OH), SS, LFS, MDS (MA), HLS, JCT, JV, DW, AYW, SLW, TW, EW, AMY, GAZ. The following individuals contributed to the design of the HCS Study including the experimental design, the CTH intervention components (community engagement strategy, Opioid-overdose Reduction Continuum of Care Approach, communication strategy), implementation science framework, the health economics analysis, the identification of data elements, overdose simulation modeling, community dashboard design, and/or statistical analysis plan: MA, APA, CB, JAB, TAB, D. Bernson, RBS, CB, JLB, HMB, JB, AB, AC (NY), MC, RKC, DMC, JC, TC, KPC, ELC, JLD, MLD, NEB, LCF, DF (NY), SF, DF (OH), PERF, LG, DGE, DG, KH, DWH, T. Huang, T. Huerta, T. Hunt, AH, RDJ, RK, KK, CK, HKK, MK, MRL (MA), RCL, FL, NL, BPL, DL, MSL, SM, KRM, AMA, KEM, TMC, CM, DN, EN, EAO, CBO, TP, BR, SR, ER, LRM, MS, NS, RS, PS, JHS, CS, BS, ES, MDS (OH), DS, LFS, MDS (MA), DJS, KLT, KT, NAV, JV, DW, AYW, SLW, SW, PMW, TW, EW, GY, AMY, GAZ. The following individuals made substantial contributions to the drafted work and/or substantively revised it: APA, CB, JAB, TAB, JLB, JB, RKC, JC, TC, KPC, JLD, DF (NY), SF, DF (OH), BF, LMG, DG, KH, T. Huerta, T. Hunt, AH, RDJ, HKK, MK, MRL (MA), RCL, MSL, SM, AM, KEM, JM, EN, EAO, TP, ER, RS, PS, JHS, BS, ES, SS, LSM, LFS, HLS, KT, NAV, JV, DW, SLW, SW, PMW, TW, GY, AMY, GAZ. All authors read and approved the final manuscript.
Role of the funding source
This research was supported by the National Institutes of Health through the NIH HEAL Initiative under award numbers UM1DA049394, UM1DA049406, UM1DA049412, UM1DA049415, UM1DA049417. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Declaration of Competing Interest
Competing interest disclosures include the following: DMC Serves on Data Safety Monitoring Boards for Janssen Research & Development; the studies have no relation to HCS work. MRL (MA) has research funds paid to their institution from OptumLabs for research on OUD treatment pathways. RS has received medication at no charge from Alkermes in support of a study testing naltrexone for alcohol use disorder. MDS (MA) reviewed grants for Alkermes in 2019. EN is an investigator on a study sponsored and funded by Braeburn-Camurus, served as a consultant without compensation to Alkermes, Braeburn-Camurus, and Pear Therapeutics, and has been an investigator on studies receiving in kind medication from Alkermes and Indivior, and in kind digital therapeutic from Pear Therapeutics. FL receives grant support from the NIDA, SAMHSA and US World Meds, consults for Major League Baseball, serves as an unpaid member of a Scientific Advisory Board for Alkermes, Novartis and US WorldMeds. KK has been compensated for providing consultation and reports for ongoing opioid litigation. SLW serves as a scientific advisor to Opiant and has served as a scientific consultant to Otsuka, Brainsway Therapeutics, Astra Zeneca and Biosciences Summit. All other authors have no conflicts to declare.
Acknowledgments
The Consortium is grateful to many individuals who have provided guidance and meaningful contributions to the development of the protocol, including Dr. David Murray, Director of the Office of Disease Prevention at the National Institutes of Health for consulting on the study design; Professor Karla Hemming from the University of Birmingham, England for her guidance on statistical power, analysis and design; Professor Charles Weijer from London University, Ontario Canada for his critical consultation on informed consent at the community level; Jennifer Reynolds, MPH, CHES from Oak Ridge Associated Universities for her role in the development of the communication campaign; and Dr. Chris Jones, U.S. Public Health Service, Centers for Disease Control and Prevention for his early contributions in guiding the design elements for inclusion in the Funding Opportunity Announcement. We are also indebted to all of the site research staff and community members who have dedicated their time and passion to this project. IQVIA data are not reported in this paper, but is mentioned as a data source; therefore, the manuscript was reviewed and approved by IQVIA.
Contributor Information
The HEALing Communities Study Consortium:
Sharon L. Walsh, Nabila El-Bassel, Rebecca D. Jackson, Jeffrey H. Samet, Maneesha Aggarwal, Arnie P. Aldridge, Trevor Baker, Carolina Barbosa, Joshua A. Barocas, Tracy A. Battaglia, Donna Beers, Dana Bernson, Rachel Bowers-Sword, Carly Bridden, Jennifer L. Brown, Heather M. Bush, Joshua L. Bush, Amy Button, Aimee N.C. Campbell, Magdalena Cerda, Debbie M. Cheng, Jag Chhatwal, Thomas Clarke, Kevin P. Conway, Erika L. Crable, Andrea Czajkowski, James L. David, Mari-Lynn Drainoni, Laura C. Fanucchi, Daniel J. Feaster, Soledad Fernandez, Darcy Freedman, Bridget Freisthler, Louisa Gilbert, LaShawn M. Glasgow, Dawn Goddard-Eckrich, Damara Gutnick, Kristin Harlow, Donald W. Helme, Terry Huang, Timothy R. Huerta, Timothy Hunt, Ayaz Hyder, Robin Kerner, Katherine Keyes, Charles E. Knott, Hannah K. Knudsen, Michael Konstan, Marc R. Larochelle, R. Craig Lefebvre, Frances Levin, Nicky Lewis, Benjamin P. Linas, Michelle R. Lofwall, David Lounsbury, Michael S. Lyons, Sarah Mann, Katherine R. Marks, Ann McAlearney, Kathryn E. McCollister, Tara McCrimmon, Jennifer Miles, Cortney C. Miller, Denis Nash, Edward Nunes, Emmanuel A. Oga, Carrie B. Oser, Tracy Plouck, Bruce Rapkin, Patricia R. Freeman, Sandra Rodriguez, Elisabeth Root, Lisa Rosen-Metsch, Nasim Sabounchi, Richard Saitz, Pamela Salsberry, Caroline Savitsky, Bruce R. Schackman, Eric E. Seiber, Michael D. Slater, Svetla Slavova, Drew Speer, Linda Sprague Martinez, Leyla F. Stambaugh, Michele Staton, Michael D. Stein, Danelle J. Stevens-Watkins, Hilary L. Surratt, Jeffery C. Talbert, Katherine L. Thompson, Kim Toussant, Nathan A. Vandergrift, Jennifer Villani, Daniel M. Walker, Alexander Y. Walley, Scott T. Walters, Philip M. Westgate, Theresa Winhusen, Elwin Wu, April M. Young, Greg Young, Gary A. Zarkin, and Redonna K. Chandler
References
- Aarons G.A., Hurlburt M., Horwitz S.M. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm. Policy Ment. Health. 2011;38:4–23. doi: 10.1007/s10488-010-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderks C.E. Center for Behavioral Health Statistics and Quality National Survey of Substance Abuse Treatment Services; 2017. Trends in the Use of Methadone, Buprenorphine, and Extended-release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (update)https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.pdf (access date November 15, 2018) [PubMed] [Google Scholar]
- Aldridge A.P., Barbosa C., Barocas J.A., Bush J.L., Chhatwal J., Harlow K.J., Hyder A., Linas B.P., McCollister K.E., Morgan J.R., Murphy S.M., Savitzky C., Schackman B.R., Seiber E.E., Starbird L.E., Villani J., Zarkin G.A. Health economic design for cost, cost-effectiveness and simulation analyses in the HEALing Communities Study. Drug Alcohol Depend. 2020;217:108336. doi: 10.1016/j.drugalcdep.2020.108336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.M., Teich J.L., Mutter R. The role of perceived need and health insurance in substance use treatment: implications for the Affordable Care Act. J. Subst. Abuse Treat. 2015;54:14–20. doi: 10.1016/j.jsat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Berwick D.M. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- Bonert A.S.B., Guy G.P., Losby J.L. Opioid prescribing in the United States before and after the centers for disease control and prevention’s 2016 opioid guideline. Ann. Intern. Med. 2018;169:367–375. doi: 10.7326/M18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N.G., DiNitto D.M., Marti C.N., Choi B.Y. Adults who misuse opioids: substance abuse treatment use and perceived treatment need. Subst. Abus. 2019;40:247–255. doi: 10.1080/08897077.2019.1573208. [DOI] [PubMed] [Google Scholar]
- Corrigan P., Schomerus G., Shuman V., Kraus D., Perlick D., Harnish A., Kulesza M., Kane-Willis K., Qin S., Smelson D. Developing a research agenda for understanding the stigma of addictions Part I: lessons from the mental health stigma literature. Am. J. Addict. 2017;26:59–66. doi: 10.1111/ajad.12458. [DOI] [PubMed] [Google Scholar]
- Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement. Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J., Charles C., Bourgeois J.W., Daniel E.S., Kwende M. Exploring the impact of the opioid epidemic in Black and Hispanic communities in the United States. Drug Sci Policy Law, epub ahead of print. 2020 doi: 10.1177/2050324520940428. [DOI] [Google Scholar]
- Eldridge S.M., Ukoumunne O.C., Carlin J.B. The intra‐cluster correlation coefficient in cluster randomized trials: a review of definitions. Int. Stat. Rev. 2009;77:378–394. [Google Scholar]
- Feldstein A.C., Glasgow R.E. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Comm. J. Qual. Patient Saf. 2008;34:228–243. doi: 10.1016/s1553-7250(08)34030-6. [DOI] [PubMed] [Google Scholar]
- Ford W.P., Westgate P.M. Improved standard error estimator for maintaining the validity of inference in cluster randomized trials with a small number of clusters. Biom. J. 2017;59:478–495. doi: 10.1002/bimj.201600182. [DOI] [PubMed] [Google Scholar]
- Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Harden S.M., Gaglio B., Rabin B., Smith M.L., Porter G.C., Ory M.G., Estabrooks P.A. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front. Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin L.O., Hodge J.G., Jr., Noe S.A. Reframing the opioid epidemic as a national emergency. JAMA. 2017;318:1539–1540. doi: 10.1001/jama.2017.13358. [DOI] [PubMed] [Google Scholar]
- Green L.W., Kreuter M.W. Mayfield Pub. Co.; Mountain View, CA: 1999. Health Promotion Planning : an Educational and Ecological Approach. [Google Scholar]
- Green L.W., Richard L., Potvin L. Ecological foundations of health promotion. Am. J. Health Promot. 1996;10:270–281. doi: 10.4278/0890-1171-10.4.270. [DOI] [PubMed] [Google Scholar]
- Greene E.J. A SAS macro for covariate-constrained randomization of general cluster-randomized and unstratified designs. J. Stat. Softw. 2017:77. doi: 10.18637/jss.v077.c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Robert G., Macfarlane F., Bate P., Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J.D., Oesterle S., Brown E.C., Arthur M.W., Abbott R.D., Fagan A.A., Catalano R.F. Results of a type 2 translational research trial to prevent adolescent drug use and delinquency: a test of Communities that Care. Arch. Pediatr. Adolesc. Med. 2009;163:789–798. doi: 10.1001/archpediatrics.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers N.M., Halperin I.J., Barnsley J., Grimshaw J.M., Shah B.R., Tu K., Upshur R., Zwarenstein M. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials. 2012;13:120. doi: 10.1186/1745-6215-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Campopiano M., Baldwin G., McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Public Health. 2015;105:e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson A.L., Rycroft-Malone J., Harvey G., McCormack B., Seers K., Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement. Sci. 2008;3:1. doi: 10.1186/1748-5908-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H.K., Havens J.R., Lofwall M.R., Studts J.L., Walsh S.L. Buprenorphine physician supply: relationship with state-level prescription opioid mortality. Drug Alcohol Depend. 2017;173:S55–S64. doi: 10.1016/j.drugalcdep.2016.08.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H.K., Drainoni M.-L., Gilbert L., Huerta T.R., Oser C.B., Aldrich A.M., Campbell A.N.C., Crable E.L., Garner B.R., Glasgow L.M., Goddard-Eckrich D., Marks K.R., McAlearney A.S., Oga E.A., Scalise A.L., Walker D.M. Model and approach for assessing implementation context and fidelity in the HEALing Communities Study. Drug Alcohol Depend. 2020;217:108330. doi: 10.1016/j.drugalcdep.2020.108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M.L., Alford D.P., Kotz M.M., Levounis P., Mandell T.W., Meyer M., Salsitz E.A., Wetterau N., Wyatt S.A., American Society Of Addiction, M Statement of the American Society of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addiction. J. Addict. Med. 2011;5:254–263. doi: 10.1097/ADM.0b013e3182312983. [DOI] [PubMed] [Google Scholar]
- Lefebvre R.C., Chandler R.K., Helme D.W., Kerner R., Mann S., Stein M.D., Reynolds J., Slater M.D., Anakaraonye A.R., Beard D., Burrus O., Frkovich J., Hedrick H., Lewis N., Rodgers E. Health communication campaigns to drive demand for evidence-based practices and reduce stigma in the HEALing Communities Study. Drug Alcohol Depend. 2020;217:108338. doi: 10.1016/j.drugalcdep.2020.108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty P.A., Ross R., Bonhert A., Clay M., Maust D.T. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psych. 2019;26:979–981. doi: 10.1001/jamapsychiatry.2019.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M.R., Bernson D., Land T., Stopka T.J., Wang N., Xuan Z., Bagley S.M., Liebschutz J.M., Walley A.Y. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med. 2018;169:137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Turner E.L., Heagerty P.J., Murray D.M., Vollmer W.M., DeLong E.R. An evaluation of constrained randomization for the design and analysis of group-randomized trials with binary outcomes. Stat. Med. 2017;36:3791–3806. doi: 10.1002/sim.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancl L.A., DeRouen T.A. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57:126–134. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- Morgan J.R., Schackman B.R., Leff J.A., Linas B.P., Walley A.Y. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J. Subst. Abuse Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton L.H. Covariate-based constrained randomization of group-randomized trials. Clin. Trials. 2004;1:297–305. doi: 10.1191/1740774504cn024oa. [DOI] [PubMed] [Google Scholar]
- NIDA . National Institute on Drug Abuse. 2019. Overdose death rates.https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates url. [Google Scholar]
- Oesterle S., Kuklinski M.R., Hawkins J.D., Skinner M.L., Guttmannova K., Rhew I.C. Long-term effects of the Communities that Care Trial on substance use, antisocial behavior, and violence through age 21 years. Am. J. Public Health. 2018;108:659–665. doi: 10.2105/AJPH.2018.304320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen Y., Sharfstein J.M. Confronting the stigma of opioid use disorder--and its treatment. JAMA. 2014;311:1393–1394. doi: 10.1001/jama.2014.2147. [DOI] [PubMed] [Google Scholar]
- Powell B.J., McMillen J.C., Proctor E.K., Carpenter C.R., Griffey R.T., Bunger A.C., Glass J.E., York J.L. A compilation of strategies for implementing clinical innovations in health and mental health. Med. Care Res. Rev. 2012;69:123–157. doi: 10.1177/1077558711430690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B.J., Waltz T.J., Chinman M.J., Damschroder L.J., Smith J.L., Matthieu M.M., Proctor E.K., Kirchner J.E. A refined compilation of implementation strategies: results from the Expert Recommendations for implementing Change (ERIC) project. Implement. Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R., Bright C.L., Shadoin A.L. Toward a policy ecology of implementation of evidence-based practices in public mental health settings. Implement. Sci. 2008;3:26. doi: 10.1186/1748-5908-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.N., Tansil K.A., Elder R.W., Soler R.E., Labre M.P., Mercer S.L., Eroglu D., Baur C., Lyon-Daniel K., Fridinger F., Sokler L.A., Green L.W., Miller T., Dearing J.W., Evans W.D., Snyder L.B., Kasisomayajula Viswanath K., Beistle D.M., Chervin D.D., Bernhardt J.M., Rimer B.K., Community Preventive Services Task, F Mass media health communication campaigns combined with health-related product distribution: a community guide systematic review. Am. J. Prev. Med. 2014;47:360–371. doi: 10.1016/j.amepre.2014.05.034. [DOI] [PubMed] [Google Scholar]
- Rogers E.M. Free Press; New York: 2003. Diffusion of Innovations. [Google Scholar]
- SAMHSA . Center for Behavioral Health Statistics and Quality, HHS Publication No. PEP19-5068, NSDUH Series H-54. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2019. Key Substance Use and Mental Health Indicators in the United States: Results From the 2018 National Survey on Drug Use and Health. [Google Scholar]
- SAS Institute, I . 2013. SAS/STAT 12.3 User’s Guide. Cary, N.C. [Google Scholar]
- Seth P., Scholl L., Rudd R.A., Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:349–358. doi: 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavova S., LaRochelle M.R., Root E., Feaster D.J., Villani J., Knott C.E., Talbert J., Mack A., Crane D., Bernson D., Booth A., Walsh S.L. Operationalizing and selecting outcome measures for the HEALing Communities Study. Drug Alcohol Depend. 2020;217:108328. doi: 10.1016/j.drugalcdep.2020.108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L.B. Health communication campaigns and their impact on behavior. J. Nutr. Educ. Behav. 2007;39:S32–40. doi: 10.1016/j.jneb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sprague Martinez L., Rapkin B.D., Young A., Freisthler B., Glasgow L., Hunt T., Salsberry P., Oga E.A., Bennet-Fallin A., Plouck T.J., Drainoni M.-L., Freeman P.R., Surratt H., Gulley J., Hamilton G.A., Bowman P., Roeber C.A., El-Bassel N., Battaglia T. Community engagement to implement evidence-based practices in the HEALing Communities Study. Drug Alcohol Depend. 2020;217:108326. doi: 10.1016/j.drugalcdep.2020.108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M., Talbert J.C., Huang Z., Lofwall M.R., Freeman P.R. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J., Volkow N.D., Degenhardt L., Hickman M., Johnson K., Koob G.F., Marshall B.D.L., Tyndall M., Walsh S.L. Opioid use disorder. Nat. Rev. Dis. Primers. 2020;6:3. doi: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- Tabak R.G., Khoong E.C., Chambers D., Brownson R.C. Bridging research and practice: models for dissemination and implementation research. Am. J. Prev. Med. 2012;43:337–350. doi: 10.1016/j.amepre.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taljaard M., Weijer C., Grimshaw J.M., Eccles M.P., Ottawa Ethics of Cluster Randomised Trials Consensus, G The Ottawa Statement on the ethical design and conduct of cluster randomised trials: precis for researchers and research ethics committees. BMJ. 2013;346:f2838. doi: 10.1136/bmj.f2838. [DOI] [PubMed] [Google Scholar]
- Wakefield M.A., Loken B., Hornik R.C. Use of mass media campaigns to change health behaviour. Lancet. 2010;376:1261–1271. doi: 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein N., Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am. J. Public Health. 2010;100:S40–S46. doi: 10.2105/AJPH.2009.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein N., Minkler M., Carter-Edwards L., Avila M., Sánchez V. In: Health Behavior: Theory, Research, and Practice Jossey-Bass. Glanz K., Rimer B.K., Viswanath K.V., editors. 2015. Improving health through community engagement, community organization, and community building; pp. 277–300. [Google Scholar]
- Walley A.Y., Xuan Z., Hackman H.H., Quinn E., Doe-Simkins M., Sorensen-Alawad A., Ruiz S., Ozonoff A. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. Br. Med. J. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate P.M. On small‐sample inference in group randomized trials with binary outcomes and cluster‐level covariates. Biom. J. 2013;55:789–806. doi: 10.1002/bimj.201200237. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Nunes E.V., Bisaga A., Pincus H.A., Johnson K.A., Campbell A.N., Remien R.H., Crystal S., Friedmann P.D., Levin F.R., Olfson M. Developing an opioid use disorder treatment cascade: a review of quality measures. J. Subst. Abuse Treat. 2018;91:57–68. doi: 10.1016/j.jsat.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen, T., Walley, A., Fanucchi, L.C., Hunt, T., Lyons, M., Lofwall, M., Brown, J.L., Freeman, P.R., Nunes, E., Beers, D., Saitz, R., Stambaugh, L., Oga, E., Herron, N., Baker, T., Cook, C.D., Roberts, M.F., Alford, D.P., Starrels, J.L., Chandler, R., The Opioid-overdose Reduction Continuum of Care Approach (ORCCA): Evidence-based Practices in the HEALing Communities Study. Drug Alcohol Depend. 2020; 217: 108325. 10.1016/j.drugalcdep.2020.108325. [DOI] [PMC free article] [PubMed]
- Wu L.T., Zhu H., Swartz M.S. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Davis C.S., Cruz M., Lurie P. State naloxone access laws are associated with an increase in the number of naloxone prescriptions dispensed in retail pharmacies. Drug Alcohol Depend. 2018;189:37–41. doi: 10.1016/j.drugalcdep.2018.04.020. [DOI] [PubMed] [Google Scholar]