Abstract
Perinatal cardiovascular care has evolved considerably to become its own multidisciplinary field of care. Despite advancements, there remain significant gaps in providing optimal care for the fetus, child, mother, and family. Continued advancement in detection and diagnosis, perinatal care and delivery planning, and prediction and improvement of morbidity and mortality for fetuses affected by cardiac conditions such as heart defects or functional or rhythm disturbances requires collaboration between the multiple types of specialists and providers. The Fetal Heart Society was created to formalize and support collaboration between individuals, stakeholders, and institutions. This article summarizes the challenges faced to create the infrastructure for advancement of the field and the measures the FHS is undertaking to overcome the barriers to support progress in the field of perinatal cardiac care.
Keywords: Fetal echocardiography, Perinatal cardiology, Prenatal cardiology, Congenital heart disease, Multidisciplinary collaboration, Fetal therapy
Highlights
-
•
Progress in perinatal cardiology is challenged by the rarity of fetal cardiac disease, care variation, and barriers to collaboration.
-
•
The Fetal Heart Society was founded to formalize collaboration between the multiple disciplines in perinatal cardiac care.
-
•
The FHS facilitates interdisciplinary multicenter research, education and advocacy to provide optimal perinatal cardiac care.
1. Introduction
Perinatal cardiovascular care including imaging of the fetal heart has evolved considerably over the past few decades and is now established as its own multidisciplinary field of care “bridging the gap and providing continuity of care for the fetus, child, and mother.” [1,2]. Fetal cardiac imaging has benefited from significant technological advancements in two-dimensional imaging and Doppler echocardiography in obstetrics and pediatric cardiology since its inception [[3], [4], [5]]. Acquisition and interpretation of serial fetal imaging have enhanced the understanding of the natural history of cardiac lesions in fetal life [[6], [7], [8], [9]]. More recently this knowledge has led to efforts to critically evaluate our ability to affect the natural history of some types of fetal cardiac disease [10]. Advances have also allowed pediatric cardiologists and maternal fetal medicine specialists to work together to better understand cardiac physiology in high risk fetal or maternal conditions that affect pregnancy [11,12].
The main goals of perinatal cardiac care are to optimize care on many fronts including improving the accuracy of detection and diagnosis, predicting in utero and postnatal outcomes, risk stratifying fetal patients to inform perinatal care and delivery planning, supporting and counseling affected families, and altering disease course if applicable, with fetal treatment. While significant advances have been made in these areas, there are still many gaps in achieving and optimizing these goals. Since the field of fetal cardiology spans multiple specialties and provider types, multidisciplinary collaboration is essential to achieve continued progress towards these goals. Such a collaborative approach has resulted in the most recent consensus American Heart Association (AHA) Statement on Diagnosis and Treatment of Fetal Cardiac Disease [13], the Joint Opinion of the International Fetal Medicine and Surgical Society and the North American Fetal Therapy Network [14], and recent American Institute of Ultrasound in Medicine (AIUM) Practice Parameter for the Performance of Fetal Echocardiography. [15] The benefit of formalizing such efforts with a multidisciplinary organization has gained recognition [16], and it is clear that an infrastructure facilitating continued collaborative efforts is important to address the challenges and gaps to providing optimal prenatal diagnosis and care, and move the field further forward.
1.1. Challenges to advancements in fetal cardiovascular care
A primary challenge to optimizing diagnostic capabilities and care of the in utero cardiac patient is the lack of rigorous studies and therefore the lack of strong evidence-based data on which to base counseling and care. Multiple factors contribute to this, including the fact that heart disease in the fetus is relatively rare [17], care varies by center, multicenter collaboration in fetal cardiology has been uncommon, and fetal cardiology as a field has often not been able to obtain sufficient research funding to support multi-center studies. The Pediatric Heart Network is an NIH-funded national research collaborative that is one of the few groups that has successfully conducted funded multicenter pediatric cardiology studies in the United States. This group serves as an excellent example that rigorous studies can be successfully performed in congenital cardiology. However, performing a query of indexed PubMed studies 2015–2019 including “prenatal” or “fetal” and “echocardiography” or “cardiology” show that to date less than 3% of published studies in fetal cardiology are multicenter [18]. In addition, it should be noted that most of the recommendations and practice protocols in the AHA Fetal Cardiac Diagnosis and Treatment Statement are based on single center studies, case studies, or expert consensus (level of evidence B or C) and not multicenter studies or randomized case-control investigations [13]. In response to this limited data, a handful of single centers have initiated collaborative multicenter studies for defects such as fetal Ebstein anomaly or tricuspid valve dysplasia [19] and isolated complete atrioventricular block [20]. These studies, by including multiple centers, have been able to enroll a larger number of fetuses to identify key fetal predictors of morbidity and mortality while accounting for known confounders. While most such multicenter collaborations have been retrospective, there have also been a smaller number of prospective studies undertaken such as that investigating the use of home monitoring for the surveillance of SSA related complete atrioventricular block [21]. Further, collaborative registries have organized prospective collection of fetal cardiac data. The International Fetal Cardiac Intervention Registry (IFCIR), in particular, is collecting data on maternal-fetal dyad referred for fetal cardiac interventions from 39 centers in 18 countries, pooling procedural and maternal and fetal outcome data [[22], [23], [24], [25]]. Similarly, the Fetal Atrial Flutter and Supraventricular Tachycardia (FAST) registry is collecting data on tachyarrhythmia treatments and outcomes from 43 centers in 13 countries [26,27]. The FAST trial importantly also encompasses the first international randomized controlled multicenter fetal trial on therapy for fetal tachycardia. Harnessing the power of prospective data collection at multiple centers allows for more robust and adequately powered observational studies in our field.
Despite these significant accomplishments, the many challenges to collaborative research have likely prevented many other such studies from being undertaken. Individual centers establishing multicenter collaborations must navigate challenging and often variable regulatory requirements or “paperwork” and cope with often complex technical aspects for data transfer and sharing across institutions. The infrastructure to support these efforts is lacking at many centers. An additional concern is that individual or center driven efforts may not always be open to equitable participation and be based only on established relationships without an open playing field where centers can network and communicate. Furthermore, academic structures do not always reward multicenter collaborative efforts, taking note of only “first” or “senior” author publications. Finally, even when rigorous research exists, unified multidisciplinary dissemination of knowledge and advocacy to ensure its incorporation into clinical care is often lacking.
2. The Fetal Heart Society
To address these challenges, the Fetal Heart Society, Inc. (FHS) was established and incorporated as a 501c non-profit organization in October of 2014. The mission of FHS as outlined in its bylaws is:
-
•
To advance the cause of research and education relating to the field of fetal cardiology and other reasonably related medical or scientific pursuits
-
•
To promote and encourage the development and advancement of the field of fetal cardiovascular diagnosis, management, and therapy
-
•
To promote the establishment of mutually beneficial relationships among the FHS members to enable the sharing of ideas and research collaboration
-
•
To foster and facilitate multicenter research and collaboration, and
-
•
To advance the field of fetal cardiovascular science and clinical practice by the establishment of a Fetal Cardiovascular Research Collaborative within the Society's auspices.
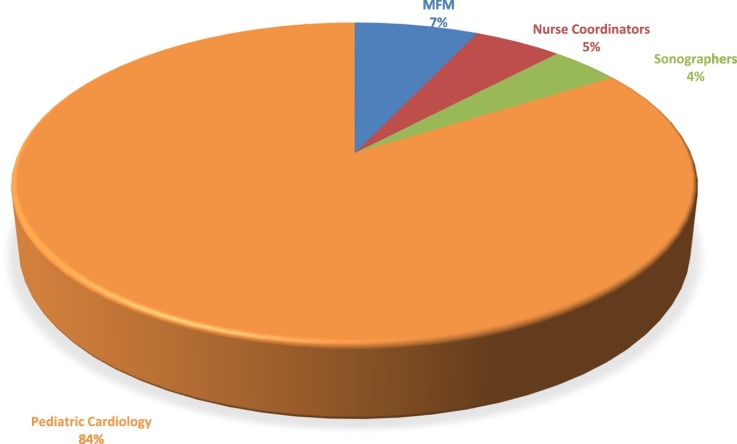
Since its inception, membership has grown to include 285 members across specialties and provider types representing 100 institutions and 12 countries (Fig. 1 ). Institutional sponsorship of the FHS was initiated to enable individual centers the opportunity to support the efforts of the Society including collaborative fetal cardiovascular research, multidisciplinary education, and advocacy efforts. Currently, 16 institutions have joined, giving their staff, including physicians, nurses, sonographers, and trainees the advantages of membership, including access to senior members and experts in the field (Table 1 ).
Fig. 1.
Distribution of Fetal Heart Society (FHS) members by field and specialty of practice. MFM – Maternal fetal medicine specialist.
Table 1.
Fetal Heart Society institutional sponsors as of August 2020.
| Institution⁎ |
|---|
| Children's National Hospital, Washington DC |
| University of California San Francisco Benioff Children's Hospital |
| University of Utah |
| Sick Kids, Toronto |
| Stanford Children's Health |
| Mount Sinai Hospital |
| Ann and Robert H. Lurie Children's Hospital of Chicago |
| Children's Minnesota |
| Columbia University Medical Center |
| Texas Children's Hospital |
| Children's Health, Dallas |
| Phoenix Children's Hospital |
| Arkansas Children's |
| Cincinnati Children's |
| Kentucky Children's Hospital |
| Nemours Cardiac Center |
Institutions are listed in the general order in which they joined as sponsors.
In pursuit of their mission, the FHS leadership and its members have identified several priorities/gaps in the field of fetal cardiovascular care which can be summarized as follows:
-
1.
Improving diagnosis and detection
-
2.
Improving the understanding of fetal cardiovascular hemodynamics, the progression of disease, and factors that predict outcomes
-
3.
Standardizing protocols for fetal cardiac imaging and management across disciplines and
-
4.
Advancing fetal therapy
The pursuit of these priority objectives is supported by a collaborative approach within the three main pillars of the Society - Research, Education, and Advocacy (Fig. 2 ).
Fig. 2.
Fetal Heart Society (FHS) Pillars. The three pillars of Research, Education, and Advocacy are central to achieving the FHS missions.
2.1. Improving diagnosis and detection
The accuracy of fetal echocardiography, for those referred due to maternal or fetal risk factors or suspicion for CHD on screening ultrasound, has improved greatly in diagnosing most complex CHD in the second trimester [28,29]. Diagnoses of more challenging defects such as total pulmonary venous return and coarctation remain limited with room for progress [30,31]. In addition, providing earlier diagnosis of CHD in the fetus (particularly for high risk mothers) is an active area of study as is improving the ability to assess fetal cardiac anatomy and function with advanced techniques such as tissue Doppler, strain and three-dimensional imaging [[32], [33], [34], [35], [36], [37]].
However, the goal of “advancing the art and science of fetal cardiovascular medicine” continues to be hampered by continued low rates of prenatal detection of CHD on screening ultrasounds in low risk mothers [[38]]. Understanding the prenatal progression of disease, establishing appropriate perinatal care and delivery planning, and risk stratifying patients to optimize outcomes all depend on early detection of fetal CHD. While prenatal detection rates have been slowly improved over time, detection of CHD before birth remains low both nationally and internationally [[39], [40], [41]]. Reducing the inadequacy in prenatal detection is a key priority of the FHS and requires engagement and interface with frontline providers who perform obstetric screening ultrasounds. To address this, the FHS membership has recognized the need to address this issue on multiple fronts at both ends of the spectrum: from research studying the socioeconomic barriers to prenatal detection to supporting efforts to develop novel interventions to improve detection (Table 2 ). The FHS has also developed educational lectures on protocols and performance of screening ultrasounds and is playing a key role in advocacy efforts to emphasize the importance of recognizing this most common birth defect in utero to improve outcomes after birth [[42], [43], [44]].
Table 2.
Active Fetal Heart Society research studies.
| Project title | Lead institution | Study design | Summary |
|---|---|---|---|
| Prenatal predictors of postnatal outcome in pulmonary atresia with intact ventricular septum | University of Utah/Primary Children's Hospital | Retrospective cohort with core imaging analysis | Aims to identify longitudinal growth of structures and fetal echocardiographic predictors of post-natal outcomes in pulmonary atresia |
| Mitral valve regurgitation in the fetus | Texas Children's Hospital | Retrospective cohort with core imaging analysis | Aims to describe longitudinal changes and outcomes in fetuses with congenital moderate to severe mitral valve regurgitation, either in isolation or in combination with other left-sided obstructive lesions/conditions. Also aims to evaluate predictors of fetal and infant survival, and to describe fetal cardiac interventions |
| Impact of socioeconomic and geographic factors on diagnosis of HLHS and dTGA | Children's National Hospital | Retrospective cohort | Aims to determine whether sociodemographic or geographic characteristics are associated with lower rates of prenatal diagnosis of hypoplastic left heart syndrome and transposition of the great arteries |
| Risk stratification in twin-twin transfusion syndrome: the importance of mitral regurgitation | Lucile Packard Children's Hospital and University of California San Francisco | Retrospective cohort with core imaging analysis | Aims to examine the association between mitral regurgitation and perinatal mortality and morbidity in monochorionic, diamniotic twin fetuses with advanced (Stage III-IV) twin-twin transfusion syndrome |
| Fetal echocardiographic Z-score project: Feasibility study | Lucile Packard Children's Hospital and Primary Children's Hospital, and Children's National Hospital | Retrospective cohort with core imaging analysis | Aims to determine evaluate measurements and calculations in a normal fetal echocardiogram obtained in a clinical setting in order to determine the appropriate sample size needed for a larger Fetal Echocardiographic Z-Score Project |
| Normal fetal strain values – Ancillary study to Z-score project | University of Utah/Primary Children's Hospital | Retrospective cohort with core imaging analysis | Aims to determine the feasibility and reproducibility of measuring right and left ventricular strain in healthy fetuses from a normal echocardiogram obtained in a clinical setting in order to determine the appropriate sample size needed for a larger study aimed at establishing normal strain values in the fetal population |
| Fetal anatomy and physiology and associations with fetal and perinatal outcomes in DTGA with intact ventricular septum | Lurie Children's Hospital, Children's National Hospital, Vanderbilt University Medical Center, Children's Hospital of Philadelphia | Prospective observational cohort | Anatomic arm: Aims to identify associations between prenatal characteristics and fetal/postnatal outcomes Hyperoxia arm aim: Aims to describe the physiologic and anatomic response to maternal hyperoxygenation and its associations with fetal and postnatal outcomes Strain arm aim: Aims to evaluate fetal myocardial strain patterns and associations with fetal/postnatal outcomes |
| Pre and postnatal outcome following fetal diagnosis of congenitally corrected transposition of the great arteries and impact of associated lesions on outcome | Mount Sinai Hospital | Retrospective cohort with core imaging analysis | Aims to assess outcomes in patients with a prenatal diagnosis of corrected transposition of the great arteries and determine if fetal echocardiographic findings are associated with outcome |
| Fetal hemodynamics in coarctation of aorta by fetal echocardiography and computational fluid dynamics simulation | St. Christopher's Hospital for Children | Retrospective cohort with core imaging analysis | Aims to pursue computational fluid dynamics simulation of aortic arch and isthmus using images from fetal echocardiography, and aims to investigate fetal hemodynamic determinants of coarctation of aorta |
| Computational analysis of fetal echocardiogram | University of California San Francisco | Retrospective cohort with core imaging analysis | Aims to refine a computer model that distinguishes normal fetal hearts from fetal hearts with CHD and to train a model that can distinguish among CHD lesions |
| Perinatal outcomes of prenatally diagnosed truncus arteriosus | Lurie Children's Hospital of Chicago | Retrospective cohort | Aims to report perinatal outcomes of patients prenatally diagnosed with truncus arteriosus |
CHD – congenital heart disease, HLHS – hypoplastic left heart syndrome, dTGA – d-transposition of the great arteries.
2.2. Improving understanding of fetal cardiovascular hemodynamics, the progression of disease and factors that predict outcomes
The goals of fetal cardiac assessment include enhancing the understanding of fetal hemodynamics, predicting outcomes in utero such as fetal demise, identifying requirements for a successful delivery room transition including the need for postnatal interventions, and minimizing postnatal morbidity and mortality [[45], [46], [47], [48]]. Accurate risk stratification and outcome prediction has been achieved with varying success depending on the lesion often due to limited numbers [45,49,50] and may not always be generalizable from single center studies due to variations in practice. By prioritizing multicenter research and establishing an infrastructure for its conduct (see Research below), the FHS hopes to overcome these limitations.
2.3. Standardizing fetal cardiovascular imaging and management
The indications for referral, the technical requirements for imaging, and protocols for the performance of fetal echocardiography have varied across published guidelines from individual professional organizations that support professions that perform prenatal ultrasound. These organizations include the American Institute of Ultrasound in Medicine (AIUM) [51], the American Society of Echocardiography (ASE) [52], the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) [53] and the Association for European Pediatric Cardiology [54]. Since fetal echocardiography can be performed by a variety of providers with different training experiences including pediatric cardiologists, radiologists, obstetricians, and maternal fetal medicine specialists, it is critical to strive towards protocols which standardize the technical requirements, imaging views, sweeps and components, and reporting practices to ensure the provision of equal and optimal care.
Appropriate monitoring and delivery planning for the fetus with CHD are critical to providing optimal care; however, protocols are not standard across institutions [10,55,56]. Center-specific delivery room protocols have been shown to improve transitions from fetal to neonatal life as well as communication among multispecialty teams involved in the care of these families [57]. Appropriate surveillance and timing of delivery can be especially challenging when competing conditions coexist in fetuses with CHD including additional non-cardiac defects, placental insufficiency, or growth restriction. No clear guidelines for perinatal surveillance in particular currently exist resulting in wide variation in practices [13]. As a multidisciplinary society, the FHS can work with key stakeholders across other vested organizations to address these gaps in care standards (see Advocacy below).
2.4. Advancing fetal therapy
Increased understanding of the natural history and evolution of cardiac disease in the fetus has also led to efforts to alter the in utero course of CHD in certain conditions. In utero invasive interventions for severe pulmonary stenosis or pulmonary atresia with intact ventricular septum to prevent single ventricle physiology or for HLHS with intact atrial septum to improve mortality have been studied but are limited by relatively small numbers and experience to make robust conclusions regarding utility [24,[58], [59], [60]]. Fetal aortic valvuloplasty for aortic stenosis with evolving HLHS, the most extensively studied [61,62], has been associated with an increased likelihood of biventricular repair compared to those with no intervention or an unsuccessful intervention, but impact on long term outcomes is still variably reported [22,[63], [64], [65]]. The FHS and its members are committed to supporting continued multicenter research collaboratives as well using its infrastructure to facilitate collaborative multicenter prospective studies into novel therapies such as the use of maternal nonsteroidal anti-inflammatory drugs to promote ductal restriction in severe fetal Ebstein anomaly [18,66].
3. Research infrastructure
To address the above gaps in knowledge, a primary mission of the FHS is to support continued collaborative research in fetal cardiovascular hemodynamics, the progression of disease, and factors that predict outcome in CHD. Housed within the infrastructure of the FHS is the Research Collaborative Committee whose purpose is to oversee a formalized process to solicit, review, and provide feedback for fetal cardiovascular study protocols (Fig. 3 ). To streamline the process for multicenter studies, the FHS utilizes a Data Coordinating Center (the University of Utah DCC) to provide consistent and reliable support for research. The DCC provides comprehensive project management including overseeing regulatory requirements such as Business Use Agreements, Data Use Agreements, and Institutional Review Boards for participating institutions and coordinating the preparation of regulatory documents that can be used across multiple studies. With centralized data management and informatics expertise, the DCC serves as the repository for clinical and imaging data, aids in the creation of databases and forms, performs data queries and quality checks, and provides online training for data entry. The DCC is also able to provide clinical research design and statistical expertise to aid study investigators with study design and implementation. Currently, the FHS supports multiple investigator initiated multicenter studies (11 studies to date, Table 2) both retrospective and prospective in design. Another important benefit is that the opportunities for research funding are likely to be increased with multicenter studies, due to increased patient numbers, and the support of seasoned investigators with an established track record in obtaining grant funding. Thus far, funding has been successfully obtained for a prospective study of fetal d-TGA initiated by three institutions working together to approach the diagnostic challenges of predicting outcomes from multiple vantage points (Mend a Heart Foundation).
Fig. 3.
The Fetal Heart Society (FHS) research review process. The research review process is overseen by the Research Collaborative Committee (RCC). The process steps include submission of a concept proposal, review by the RCC, convening of a collaborative study group from FHS members, preparation and submission of a full proposal responsive to RCC feedback with the study group and upon approval, working with the FHS Data Coordinating Center (DCC) on the preparation of regulatory documents and database creation.
4. Education
The educational mission of the FHS is essential for continued advancement in the field of fetal cardiovascular medicine. Given the multidisciplinary nature of the field, educational efforts must span current providers and trainees across disciplines and provider types. These include but are not limited to sonographers, physicians, nurses, and other allied providers in pediatric cardiology, maternal fetal medicine, general obstetrics, and radiology who screen for and provide care to families affected by fetal cardiac disease. While educational offerings exist to different extents from professional organizations within these disciplines, the FHS has embarked on creating a core curriculum on prenatal cardiology for all levels of practice including screening for CHD, basic fetal echocardiography, and advanced fetal echocardiography and perinatal cardiac care.
For sonographers and obstetricians, the screening curriculum prioritizes advancing knowledge and skills to increase the detection of CHD in the low risk patient. The education initiatives for specialists in fetal cardiology are broader and encompass a large variety of subjects including 1) detailing the technical aspects of cardiovascular imaging, 2) assessing critical imaging features that predict outcomes for specific CHD, 3) creating content and a standardized approach to counseling families, 4) detailing strategies to reduce sociodemographic disparities, and 5) optimizing communication and collaborative care between specialties. In addition, the FHS Education Committee has expanded on the efforts of a sponsoring institution (Stanford University) to create CHD specific provider information sheets that collate the most recent data on incidence, fetal imaging predictors of outcome, available fetal interventions, prognosis, and associated problems [67]. The FHS has pursued these education efforts with the assistance of several educational grants (GE Healthcare).
More recently, the FHS began a monthly webinar to supplement the website lecture series. The content of the webinar is directed towards all levels of practice and covers multiple topics including a review of guidelines for the performance of fetal echocardiography, a lecture on advanced arrhythmia management, a journal club discussion of genetics, and presentation of a series of interesting and challenging fetal cases. Building on its collaborative foundation, the FHS has had the opportunity to partner with several organizations to plan the fetal educational content for national and international conferences including the first fetal track in the upcoming World Congress in Pediatric Cardiology and Cardiac Surgery in 2021.
5. Advocacy
The FHS has worked with key stakeholders across institutional organizations to decrease variation in the practice and performance of fetal echocardiography and perinatal care of fetuses affected by cardiovascular conditions. Collaborative input from across these organizations was sought and incorporated into the recent AIUM Practice Parameters for Fetal Echocardiography [15]. To ensure the quality of care in the transition period from fetus to neonate, the FHS is collaborating with the Neonatal Heart Society, an international society dedicated to the care of neonates with cardiac disease, in the creation of fetal/neonatal cardiac guidelines for care. Finally, the FHS has worked with ASE and the Society of Pediatric Echocardiography to generate guidelines in response to COVID-19 that included pandemic modifications for indication, timing, and performance of fetal echocardiograms to reduce exposure and assure the safety of staff and patients.
Advocating for the multidisciplinary resources required to provide optimal care to fetuses and families affected by fetal cardiovascular disease has been critical in recent years. It has led to the inclusion of genetic counselors, social workers, palliative care teams, and psychologists in the cadre of providers supporting and caring for these families. Continued advocacy for the need for psychosocial support for families is required across disciplines in our field as our understanding increases of how such interventions improve both mental and physical health [68,69]. Including these as metrics for centers of excellence will raise the bar for practice in our field. The FHS will serve to coalesce and lend weight to such crucial requests as an institutional single voice.
6. Conclusion
The field of perinatal cardiac care has achieved significant advances since its inception more than four decades ago. Within this history, tremendous opportunity for further progress exists in the pursuit of optimal care of patients and families. The continued evolution of the field of fetal cardiology will proceed much faster with multidisciplinary collaboration in investigation, education, and advocacy. The Fetal Heart Society is at the forefront of the effort to foster such collaboration and provide the infrastructure and support to succeed.
Funding sources
Work for this publication was not supported by any funding sources. The Fetal Heart Society is supported by the institutional sponsors listed in Table 1 and has received support from the Mend a Heart Foundation for a study on d-Transposition of the Great Arteries and from GE Healthcare to support educational initiatives described herein.
Acknowledgments
Acknowledgement
The work of the FHS is supported by its members and committees. The authors would like to acknowledge current and past members of the FHS Board of Directors including David Schidlow, Lisa Hornberger, Alfred Abuhamad, Jack Rychik, Bhawna Arya, Michael Puchalski, Bettina Cuneo, Edgar Jaeggi, and Wayne Tworetzky.
Declaration of competing interest
The authors have no conflicts of interest to report. The Fetal Heart Society is supported by the institutional sponsors listed in Table 1 and has received support from the Mend a Heart Foundation for a study on d-Transposition of the Great Arteries and from GE Healthcare to support educational initiatives described herein.
References
- 1.Huhta J.C. What is a perinatal cardiologist? Ultrasound Obstet Gynecol. 1995;5:145–147. doi: 10.1046/j.1469-0705.1995.05030145.x. [DOI] [PubMed] [Google Scholar]
- 2.Moon-Grady A.J., Hirose S., Kesby G., Menahem S., Tworetzky W. The fetus as a cardiac patient: assessment and therapy of cardiovascular pathology before birth. Int J Pediatr. 2010;2010:974520. doi: 10.1155/2010/974520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riis A., Jensen C.E., Bro F., Maindal H.T., Petersen K.D., Bendtsen M.D. A multifaceted implementation strategy versus passive implementation of low back pain guidelines in general practice: a cluster randomised controlled trial. Implement Sci. 2016;11:143. doi: 10.1186/s13012-016-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan L.D., Tynan M.J., Campbell S., Wilkinson J.L., Anderson R.H. Echocardiographic and anatomical correlates in the fetus. Br Heart J. 1980;44:444–451. doi: 10.1136/hrt.44.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange L.W., Sahn D.J., Allen H.D., Goldberg S.J., Anderson C., Giles H. Qualitative real-time cross-sectional echocardiographic imaging of the human fetus during the second half of pregnancy. Circulation. 1980;62:799–806. doi: 10.1161/01.cir.62.4.799. [DOI] [PubMed] [Google Scholar]
- 6.Allan L.D., Sharland G., Tynan M.J. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–343. doi: 10.1016/0167-5273(89)90226-X. [DOI] [PubMed] [Google Scholar]
- 7.Simpson J.M., Sharland G.K. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77:205–210. doi: 10.1136/hrt.77.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paladini D., Palmieri S., Lamberti A., Teodoro A., Martinelli P., Nappi C. Characterization and natural history of ventricular septal defects in the fetus. Ultrasound Obstet Gynecol. 2000;16:118–122. doi: 10.1046/j.1469-0705.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 9.Davey B.T., Rychik J. The natural history of atrioventricular valve regurgitation throughout fetal life in patients with atrioventricular canal defects. Pediatr Cardiol. 2016;37:50–54. doi: 10.1007/s00246-015-1237-y. [DOI] [PubMed] [Google Scholar]
- 10.Simpson J.M. Impact of fetal echocardiography. Ann Pediatr Cardiol. 2009;2:41–50. doi: 10.4103/0974-2069.52806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Mieghem T., Gucciardo L., Doné E., Van Schoubroeck D., Graatsma E.M., Visser G.H.A. Left ventricular cardiac function in fetuses with congenital diaphragmatic hernia and the effect of fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:424–429. doi: 10.1002/uog.7340. [DOI] [PubMed] [Google Scholar]
- 12.Bahtiyar M.O., Copel J.A. Cardiac changes in the intrauterine growth-restricted fetus. Semin Perinatol. 2008;32:190–193. doi: 10.1053/J.SEMPERI.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Donofrio M.T., Moon-Grady A.J., Hornberger L.K., Copel J.A., Sklansky M.S., Abuhamad A. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the american heart association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 14.Moon-Grady A.J., Baschat A., Cass D., Choolani M., Copel J.A., Crombleholme T.M. Fetal treatment 2017: the evolution of fetal therapy centers - a joint opinion from the international fetal medicine and surgical society (IFMSS) and the North American fetal therapy network (NAFTNet) Fetal Diagn Ther. 2017;42:241–248. doi: 10.1159/000475929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AIUM Practice parameter for the performance of fetal echocardiography. J Ultrasound Med. 2020;39:E5–16. doi: 10.1002/jum.15188. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs J.P., Rychik J., Tulzer G., Fouron J.C., Maulik D., Tworetzky W. A vision for an international society for fetal and perinatal cardiovascular disease. Curr Opin Pediatr. 2008;20:532–537. doi: 10.1097/MOP.0b013e328311d344. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman J.I. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- 18.Freud L.R., Wilkins-Haug L.E., Beroukhim R.S., Lafranchi T., Phoon C.K., Buzzard C.J. Prenatal NSAID therapy to mitigate circular shunt physiology in fetuses with severe Ebstein anomaly [abstract] Circulation. 2018;A14979 [Google Scholar]
- 19.Freud L.R., Escobar-Diaz M.C., Kalish B.T., Komarlu R., Puchalski M.D., Jaeggi E.T. Outcomes and predictors of perinatal mortality in fetuses with Ebstein anomaly or tricuspid valve dysplasia in the current era: a multicenter study. Circulation. 2015;132:481–489. doi: 10.1161/CIRCULATIONAHA.115.015839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson H., Sonesson S.-E., Sharland G., Granath F., Simpson J.M., Carvalho J.S. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 21.Cuneo B.F., Moon-Grady A.J., Sonesson S.-E., Levasseur S., Hornberger L., Donofrio M.T. Heart sounds at home: feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J Perinatol. 2017;37:226–230. doi: 10.1038/jp.2016.220. [DOI] [PubMed] [Google Scholar]
- 22.Moon-Grady A.J., Morris S.A., Belfort M., Chmait R., Dangel J., Devlieger R. International fetal cardiac intervention registry. J Am Coll Cardiol. 2015;66:388–399. doi: 10.1016/j.jacc.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Jantzen D.W., Moon-Grady A.J., Morris S.A., Armstrong A.K., Berg C., Dangel J. Hypoplastic left heart syndrome with intact or restrictive atrial septum: a report from the international fetal cardiac intervention registry. Circulation. 2017;136:1346–1349. doi: 10.1161/CIRCULATIONAHA.116.025873. [DOI] [PubMed] [Google Scholar]
- 24.Hogan W.J., Grinenco S., Armstrong A., Devlieger R., Dangel J., Ferrer Q. Fetal cardiac intervention for pulmonary atresia with intact ventricular septum: international fetal cardiac intervention registry. Fetal Diagn Ther. 2020:1–9. doi: 10.1159/000508045. [DOI] [PubMed] [Google Scholar]
- 25.IFCIR: International fetal cardiac invention registry - home n.d. http://www.ifcir.org/ (accessed August 17, 2020).
- 26.FAST Therapy trial of fetal tachyarrhythmia - full text view - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT02624765 (accessed August 12, 2020).
- 27.FAST Therapy trial n.d. https://www.fasttherapytrial.com/ (accessed August 12, 2020).
- 28.Gottliebson W.M., Border W.L., Franklin C.M., Meyer R.A., Michelfelder E.C. Accuracy of fetal echocardiography: a cardiac segment-specific analysis. Ultrasound Obstet Gynecol. 2006;28:15–21. doi: 10.1002/uog.2795. [DOI] [PubMed] [Google Scholar]
- 29.van Velzen C.L., Clur S.A., Rijlaarsdam M.E.B., Pajkrt E., Bax C.J., Hruda J. Prenatal diagnosis of congenital heart defects: accuracy and discrepancies in a multicenter cohort. Ultrasound Obstet Gynecol. 2016;47:616–622. doi: 10.1002/uog.15742. [DOI] [PubMed] [Google Scholar]
- 30.Tegnander E., Williams W., Johansen O.J., Blaas H.-G.K., Eik-Nes S.H. Prenatal detection of heart defects in a non-selected population of 30 149 fetuses-detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27:252–265. doi: 10.1002/uog.2710. [DOI] [PubMed] [Google Scholar]
- 31.Pinto N.M., Keenan H.T., Minich L.L., Puchalski M.D., Heywood M., Botto L.D. Barriers to prenatal detection of congenital heart disease: a population-based study. Ultrasound Obstet Gynecol. 2012;40 doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 32.McAuliffe F.M., Trines J., Nield L.E., Chitayat D., Jaeggi E., Hornberger L.K. Early fetal echocardiography—a reliable prenatal diagnosis tool. Am J Obstet Gynecol. 2005;193:1253–1259. doi: 10.1016/J.AJOG.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho J.S., Moscoso G., Tekay A., Campbell S., Thilaganathan B., Shinebourne E.A. Clinical impact of first and early second trimester fetal echocardiography on high risk pregnancies. Heart. 2004;90:921–926. doi: 10.1136/hrt.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pike J.I., Krishnan A., Donofrio M.T. Early fetal echocardiography: congenital heart disease detection and diagnostic accuracy in the hands of an experienced fetal cardiology program. Prenat Diagn. 2014;34:790–796. doi: 10.1002/pd.4372. [DOI] [PubMed] [Google Scholar]
- 35.Nii M., Roman K.S., Kingdom J., Redington A.N., Jaeggi E.T. Assessment of the evolution of normal fetal diastolic function during mid and late gestation by spectral Doppler tissue echocardiography. J Am Soc Echocardiogr. 2006;19:1431–1437. doi: 10.1016/J.ECHO.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Ishii T., McElhinney D.B., Harrild D.M., Marcus E.N., Sahn D.J., Truong U. Circumferential and longitudinal ventricular strain in the normal human fetus. J Am Soc Echocardiogr. 2012;25:105–111. doi: 10.1016/J.ECHO.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chelliah A., Dham N., Frank L.H., Donofrio M., Krishnan A. Myocardial strain can be measured from first trimester fetal echocardiography using velocity vector imaging. Prenat Diagn. 2016;36:483–488. doi: 10.1002/pd.4813. [DOI] [PubMed] [Google Scholar]
- 38.van Velzen C.L., Ket J.C.F., van de Ven P.M., Blom N.A., Haak M.C. Systematic review and meta-analysis of the performance of second-trimester screening for prenatal detection of congenital heart defects. Int J Gynecol Obstet. 2017 doi: 10.1002/ijgo.12373. [DOI] [PubMed] [Google Scholar]
- 39.Quartermain M.D., Pasquali S.K., Hill K.D., Goldberg D.J., Huhta J.C., Jacobs J.P. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136:e378–e385. doi: 10.1542/peds.2014-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoo NS, Van Essen P, Richardson M, Robertson T. Effectiveness of prenatal diagnosis of congenital heart defects in South Australia: a population analysis 1999–2003. Aust N Z J Obs Gynaecol 2008;48:559–63. doi:AJO915 [pii] 10.1111/j.1479-828X.2008.00915.x. [DOI] [PubMed]
- 41.Marek J, Tomek V, Skovranek J, Povysilova V, Samanek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart n.d.;97:124–30. doi:97/2/124 [pii] 10.1136/hrt.2010.206623. [DOI] [PubMed]
- 42.Khoshnood B., Lelong N., Houyel L., Bonnet D., Ballon M., Jouannic J.-M. Impact of prenatal diagnosis on survival of newborns with four congenital heart defects: a prospective, population-based cohort study in France (the EPICARD study) BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-018285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey A., Glickstein J.S., Kleinman C.S., Levasseur S.M., Chen J., Gersony W.M. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr Cardiol. 2010;31:587–597. doi: 10.1007/s00246-010-9648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyvandi S., De Santiago V., Chakkarapani E., Chau V., Campbell A., Poskitt K.J. Association of prenatal diagnosis of critical congenital heart disease with postnatal brain development and the risk of brain injury. JAMA Pediatr. 2016;170 doi: 10.1001/jamapediatrics.2015.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divanovic A., Bowers K., Michelfelder E., Jaekle R., Newman T., Marcotte M. Intrauterine fetal demise after prenatal diagnosis of congenital heart disease: assessment of risk. Prenat Diagn. 2016;36:142–147. doi: 10.1002/pd.4755. [DOI] [PubMed] [Google Scholar]
- 46.Wieczorek A., Hernandez-Robles J., Ewing L., Leshko J., Luther S., Huhta J. Prediction of outcome of fetal congenital heart disease using a cardiovascular profile score. Ultrasound Obstet Gynecol. 2008;31:284–288. doi: 10.1002/uog.5177. [DOI] [PubMed] [Google Scholar]
- 47.Davey B.T., Donofrio M.T., Moon-Grady A.J., Fifer C.G., Cuneo B.F., Falkensammer C.B. Development and validation of a fetal cardiovascular disease severity scale. Pediatr Cardiol. 2014;35:1174–1180. doi: 10.1007/s00246-014-0911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donofrio M.T., Skurow-Todd K., Berger J.T., McCarter R., Fulgium A., Krishnan A. Risk-stratified postnatal care of newborns with congenital heart disease determined by fetal echocardiography. J Am Soc Echocardiogr. 2015;28:1339–1349. doi: 10.1016/J.ECHO.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Salvin J.W., McElhinney D.B., Colan S.D., Gauvreau K., del Nido P.J., Jenkins K.J. Fetal tricuspid valve size and growth as predictors of outcome in pulmonary atresia with intact ventricular septum. Pediatrics. 2006;118:e415–e420. doi: 10.1542/peds.2006-0428. [DOI] [PubMed] [Google Scholar]
- 50.Moon-Grady A.J., Tacy T.A., Brook M.M., Hanley F.L., Silverman N.H. Value of clinical and echocardiographic features in predicting outcome in the fetus, infant, and child with tetralogy of Fallot with absent pulmonary valve complex. Am J Cardiol. 2002;89:1280–1285. doi: 10.1016/S0002-9149(02)02326-3. [DOI] [PubMed] [Google Scholar]
- 51.AIUM Practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32:1083–1101. doi: 10.7863/ultra.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 52.Rychik J., Ayres N., Cuneo B., Gotteiner N., Hornberger L., Spevak P.J. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J Am Soc Echocardiogr. 2004;17:803–810. doi: 10.1016/j.echo.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Lee W., Allan L., Carvalho J.S., Chaoui R., Copel J., Devore G. ISUOG consensus statement: what constitutes a fetal echocardiogram? Ultrasound Obstet Gynecol. 2008;32:239–242. doi: 10.1002/uog.6115. [DOI] [PubMed] [Google Scholar]
- 54.Allan L., Dangel J., Fesslova V., Marek J., Mellander M., Oberhänsli I. Recommendations for the practice of fetal cardiology in Europe. Cardiol Young. 2004;14:109–114. doi: 10.1017/S1047951104001234. [DOI] [PubMed] [Google Scholar]
- 55.Morris S.A., Ethen M.K., Penny D.J., Canfield M.A., Minard C.G., Fixler D.E. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation. 2014;129:285–292. doi: 10.1161/CIRCULATIONAHA.113.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanapo L., Moon-Grady A.J., Donofrio M.T. Perinatal and delivery management of infants with congenital heart disease. Clin Perinatol. 2016;43 doi: 10.1016/j.clp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Donofrio M.T., Levy R.J., Schuette J.J., Skurow-Todd K., Sten M.-B., Stallings C. Specialized delivery room planning for fetuses with critical congenital heart disease. Am J Cardiol. 2013;111:737–747. doi: 10.1016/J.AMJCARD.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Jantzen D.W., Moon-Grady A.J., Morris S.A., Armstrong A.K., Berg C., Dangel J. Hypoplastic left heart syndrome with intact or restrictive atrial septum: a report from the international fetal cardiac intervention registry. Circulation. 2017;136:1346–1349. doi: 10.1161/CIRCULATIONAHA.116.025873. [DOI] [PubMed] [Google Scholar]
- 59.Galindo A., Gutiérrez-Larraya F., Velasco J.M., De la Fuente P. Pulmonary balloon valvuloplasty in a fetus with critical pulmonary stenosis/atresia with intact ventricular septum and heart failure. Fetal Diagn Ther. 2006;21:100–104. doi: 10.1159/000089058. [DOI] [PubMed] [Google Scholar]
- 60.Tulzer A., Arzt W., Gitter R., Prandstetter C., Grohmann E., Mair R. Immediate effects and outcome of in-utero pulmonary valvuloplasty in fetuses with pulmonary atresia with intact ventricular septum or critical pulmonary stenosis. Ultrasound Obstet Gynecol. 2018;52:230–237. doi: 10.1002/uog.19047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tworetzky W., Wilkins-Haug L., Jennings R.W., van der Velde M.E., Marshall A.C., Marx G.R. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 62.McElhinney D.B., Marshall A.C., Wilkins-Haug L.E., Brown D.W., Benson C.B., Silva V. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freud L.R., McElhinney D.B., Marshall A.C., Marx G.R., Friedman K.G., del Nido P.J. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014;130:638–645. doi: 10.1161/CIRCULATIONAHA.114.009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman K.G., Sleeper L.A., Freud L.R., Marshall A.C., Godfrey M.E., Drogosz M. Improved technical success, postnatal outcome and refined predictors of outcome for fetal aortic valvuloplasty. Ultrasound Obstet Gynecol. 2018;52:212–220. doi: 10.1002/uog.17530. [DOI] [PubMed] [Google Scholar]
- 65.Kovacevic A., Öhman A., Tulzer G., Herberg U., Dangel J., Carvalho J.S. Fetal hemodynamic response to aortic valvuloplasty and postnatal outcome: a European multicenter study. Ultrasound Obstet Gynecol. 2018;52:221–229. doi: 10.1002/uog.18913. [DOI] [PubMed] [Google Scholar]
- 66.Torigoe T., Mawad W., Seed M., Ryan G., Marini D., Golding F. Treatment of fetal circular shunt with non-steroidal anti-inflammatory drugs. Ultrasound Obstet Gynecol. 2019;53:841–846. doi: 10.1002/uog.20169. [DOI] [PubMed] [Google Scholar]
- 67.Provider Counseling Sheets | Fetal Heart Society n.d. https://fetalheartsociety.org/education/provider-counseling-sheets/ (accessed August 12, 2020).
- 68.Wu Y., Kapse K., Jacobs M., Niforatos-Andescavage N., Donofrio M.T., Krishnan A. Association of maternal psychological distress with in utero brain development in fetuses with congenital heart disease. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2019.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCusker C.G., Doherty N.N., Molloy B., Rooney N., Mulholland C., Sands A. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. 2010;36:110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]