Highlights
-
•
20 % of state Medicaid programs operate monthly prescription fill limit policies.
-
•
Prescription fill limits may pose barriers to Medicaid-covered naloxone fills.
-
•
Naloxone exemptions may improve naloxone access and prevent opioid overdose deaths.
Keywords: Naloxone, Medicaid, Pharmaceutical policy, Opioid overdose, Access
Abstract
Background
Expanding access to and utilization of naloxone is a vitally important harm reduction strategy for preventing opioid overdose deaths, particularly in vulnerable populations like Medicaid beneficiaries. The objective of this study was to characterize the landscape of monthly prescription fill limit policies in Medicaid programs and their potential implications for expanding naloxone use for opioid overdose harm reduction.
Methods
A cross-sectional, multi-modal online and telephonic data collection strategy was used to identify and describe the presence and characteristics of monthly prescription fill limit policies across state Medicaid programs. Contextual characteristics were described regarding each state’s Medicaid enrollment, opioid prescribing rates, and overdose death rates. Data collection and analysis occurred between February and May 2020.
Results
Medicaid-covered naloxone fills are currently subject to monthly prescription fill limit policies in 10 state Medicaid programs, which cover 20 % of the Medicaid population nationwide. Seven of these programs are located in states ranking in the top 10 highest per-capita opioid prescribing rates in the country. However, 8 of these programs are located in states with opioid overdose death rates below the national average.
Conclusions
Medicaid beneficiaries at high risk of opioid overdose living in states with monthly prescription fill limits may experience significant barriers to obtaining naloxone. Exempting naloxone from Medicaid prescription limit restrictions may help spur broader adoption of naloxone for opioid overdose mortality prevention, especially in states with high opioid prescribing rates. Achieving unfettered naloxone coverage in Medicaid is critical as opioid overdoses and Medicaid enrollment increase amid the COVID-19 pandemic.
1. Introduction
Opioid overdoses caused 46,802 deaths in the United States in 2018 (Wilson et al., 2020). The rate of fatal and non-fatal opioid overdose has surged in the midst of the COVID-19 pandemic (Glober et al., 2020; Ochalek et al., 2020; Rodda et al., 2020; Slavova et al., 2020). Expanding the accessibility and use of naloxone—an opioid antagonist that reverses acute opioid overdose—has emerged as a key harm reduction strategy for preventing opioid overdose mortality (Giroir, 2018). While naloxone dispensing has increased in recent years as states relaxed naloxone prescribing authority (Roberts et al., 2019) and naloxone co-prescribing practices gained momentum (Giroir, 2018), naloxone’s penetration into high-risk communities remains low (Guy et al., 2019).
Accelerating the uptake of naloxone is particularly important in state Medicaid programs. Low-income adults are more likely to engage in risky opioid use behaviors that increase their risk of overdose than higher income populations (Han et al., 2019). Medicaid also provides health insurance coverage to a disproportionately high share of adults with opioid use disorder across the United States compared to other payers (Medicaid and CHIP Payment and Access Commission (MACPAC), 2017).
However, Medicaid prescription drug benefits vary from state-to-state, introducing potential barriers to life-saving naloxone for high-risk beneficiaries living in states with more restrictive prescription coverage policies. One such policy is monthly prescription fill limits. Prescription fill limits impose additional restrictions—such as prior authorization—on Medicaid beneficiaries attempting to fill a Medicaid-covered prescription if they have already filled a predetermined number of allowable monthly prescriptions set by the Medicaid program. Little is known about the current landscape of prescription fill limit policies across Medicaid programs and its potential implications for inhibiting the accessibility of naloxone among Medicaid patients. The purpose of this study was to identify state Medicaid programs with prescription fill limit policies that could pose potential barriers to the accessibility of naloxone for Medicaid beneficiaries.
2. Materials and methods
A multi-modal online and telephonic data collection strategy was used to identify the presence and characteristics of monthly prescription fill limit policies in Medicaid programs in all 50 states and Washington, DC. First, we conducted structured internet searches for each state using relevant combinations of key terms and phrases in Google. An example search for determining the presence of a monthly prescription fill limit policy was, “[state name] AND Medicaid AND prescription AND (limit* OR cap*).” An example search for determining applicability of a Medicaid monthly prescription fill limit to naloxone was, “[state name] AND Medicaid AND naloxone AND (coverage OR exception).” We prioritized online data collection first from official state government Medicaid program websites, then from external websites accessed from hyperlinks shared on official state government Medicaid program websites, and then from other reputable external websites (e.g. Kaiser Family Foundation). After online data collection was completed, a member of the study team contacted an appropriate representative of the state’s Medicaid prescription benefit program to confirm or correct the accuracy of the online data we collected or to provide necessary study data that was not found through the online search process. Data collection and analysis occurred between 2/20/2020 to 5/7/2020. We describe characteristics of prescription fill limit policies for the states currently operating these policies. We also report state-level Medicaid enrollment numbers (Centers for Medicare and Medicaid Services, 2020), rates of opioid prescribing (Centers for Disease Control and Prevention, 2018), and prescription opioid overdose deaths (Kaiser Family Foundation, 2018) to provide additional context about the size and opioid-related risks of the Medicaid population subject to the monthly prescription fill limit policy. This study was considered non-human subjects research and was exempt from IRB review.
3. Results
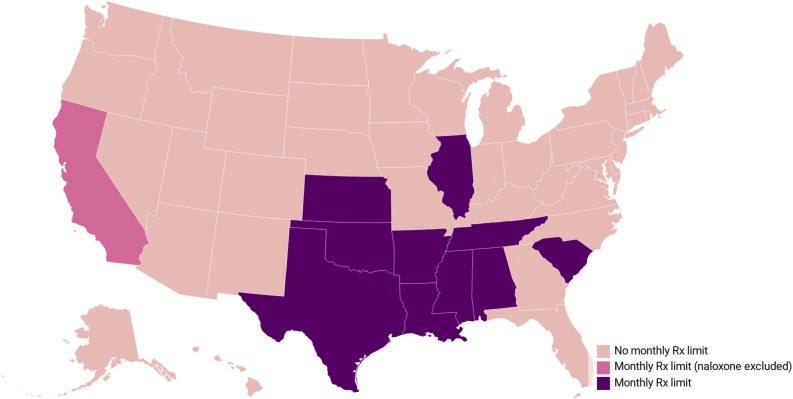
Our search strategy identified 10 state Medicaid programs currently operating monthly prescription fill limit policies (Fig. 1 ) where naloxone fills are subject to monthly fill limit restrictions. These states were: Alabama, Arkansas, Illinois, Kansas, Louisiana, Mississippi, Oklahoma, South Carolina, Tennessee, and Texas. California’s Medicaid program also operates a monthly fill limit policy, but it excludes naloxone from its monthly prescription fill limit restrictions by carving out naloxone coverage. Of note, the Kansas Medicaid program excludes fills of “preferred drugs” from applying toward its monthly prescription fill limit, but naloxone is not listed as a preferred drug in Kansas.
Fig. 1.
Location of state Medicaid programs operating a monthly prescription limit policy, as of May 2020. Note: Rx = Prescription. Drugs designated as preferred products in Kansas Medicaid do not count toward its monthly prescription limit, but naloxone is not designated a preferred drug.
As of March 2020, there were over 12.7 million Medicaid beneficiaries living in one of the 10 states where naloxone is subject to monthly prescription fill limit policies in the state’s Medicaid program (Table 1 ). This represents 20 % of the national Medicaid beneficiary population. The number of allowable Medicaid-covered prescriptions per month ranged from 3 (Arkansas and Texas) to 6 prescription fills (Oklahoma).
Table 1.
Characteristics of state Medicaid programs with monthly prescription limit policies.
| State | Medicaid enrollees (1000s) | Monthly prescription fill limit | Naloxone exemption | Opioid prescribing rate/100 people | National rank - opioid prescribing rate | Opioid overdose death rate/100,000 people | National rank - opioid overdose death rate |
|---|---|---|---|---|---|---|---|
| United States | 64094.7 | 51.4 | 14.6 | ||||
| Alabama | 757.4 | 5 | No | 97.5 | 1 | 8.3 | 34 |
| Arkansas | 757.8 | 3 | No | 93.5 | 2 | 7.4 | 38 |
| California | 10146.5 | 6 | Yes | 35.1 | 48 | 5.8 | 44 |
| Illinois | 2535.3 | 4 | No | 45.2 | 35 | 17 | 21 |
| Kansas | 320.5 | 4 | No | 64.3 | 11 | 5.7 | 45 |
| Louisiana | 1387.6 | 4 | No | 79.4 | 5 | 10 | 30 |
| Mississippi | 527.3 | 5 | No | 76.8 | 7 | 6.1 | 43 |
| Oklahoma | 614.1 | 6 | No | 79.1 | 6 | 7.8 | 37 |
| South Carolina | 920.2 | 4 | No | 69.2 | 9 | 17.1 | 20 |
| Tennessee | 1305.8 | 5 | No | 81.8 | 3 | 19.9 | 16 |
| Texas | 3585.4 | 3 | No | 47.2 | 32 | 4.8 | 48 |
Notes: Drugs designated as preferred products in Kansas Medicaid do not count toward its monthly prescription limit, but naloxone is not designated a preferred drug. Medicaid enrollee counts reflect the total Medicaid-enrolled population in the state in March 2020, as reported by Centers for Medicare and Medicaid Services. Opioid prescribing and overdose death rates were ascertained using 2018 data, as reported by the Centers for Disease Control and Prevention.
Out of these 10 states, 7 ranked in the top 10 nationally for the rate of opioid prescriptions filled-per-100 residents in 2018 (Table 1). Notably, Alabama, Arkansas, and Tennessee had the Top 3 highest opioid prescribing rates nationally in 2018 with 97.5, 93.5, and 81.8 opioid prescription fills per 100 residents, respectively, compared to a national average of 51.4 opioid prescriptions per 100 residents. Most states with Medicaid programs operating monthly prescription fill limits were below the national average opioid overdose death rate of 14.6 opioid overdose fatalities-per-100,000 residents based on 2018 data. Only Tennessee, South Carolina, and Illinois exceeded the national opioid overdose death rate at 19.9, 17.1, and 17.0 deaths/100,000 residents, respectively.
4. Discussion
In our review of Medicaid prescription drug coverage policies, we found Medicaid-covered naloxone prescriptions are subject to monthly prescription fill limit policies in 10 Medicaid programs. This means, for example, that a high-risk Medicaid beneficiary in Texas who already filled 3 prescriptions during the calendar month may experience substantial barriers to filling a naloxone prescription, such as delays in obtaining naloxone while prior authorizations are processed. This delay may leave some high-risk individuals without naloxone during a peak period of opioid overdose risk. Patients may be able to avoid these barriers posed by Medicaid prescription fill limit restrictions by purchasing naloxone without their Medicaid coverage at the pharmacy’s full out-of-pocket price. Patients may also be able to access community-based naloxone dispensing programs at no cost. However, out-of-pocket naloxone purchases place undue financial burden on low-income Medicaid patients, and prior studies have shown naloxone costs are a leading reason for not filling naloxone prescriptions (Bessen et al., 2019). Moreover, community-based naloxone dispensing programs may not be readily and consistently accessible for all Medicaid enrollees, particularly in under-resourced areas.
While we found that 1-in-5 Medicaid beneficiaries are subject to monthly prescription limits—most of whom live in states with the highest opioid prescribing rates—it remains unclear the extent to which prescription fill limit policies restrict naloxone accessibility in these states. Prior research suggests that a large portion of the adult Medicaid population exhibit prescription utilization patterns that would trigger monthly prescription fill limit restrictions. One study found that over 50 % of adult Medicaid beneficiaries in two states with at least one chronic condition used 5 or more classes of prescription medications simultaneously (Feng et al., 2018). Among those patients that reach their state’s monthly prescription fill limit, prior research also indicated that Medicaid monthly prescription fill limits significantly reduce utilization of preventive medications (Lieberman et al., 2016).
For these reasons, we recommend that state Medicaid programs operating monthly prescription fill limit policies implement explicit exceptions for Medicaid-covered naloxone fills. Failing to do so will likely result in patients at high risk of opioid overdose deferring or delaying receipt of naloxone for opioid overdose harm reduction. Enacting this policy change is made all the more urgent by the ongoing surge in Medicaid enrollment (Frenier et al., 2020; Rudowitz et al., 2020) and preventable opioid overdose deaths brought on by the COVID-19 pandemic (Glober et al., 2020; Ochalek et al., 2020; Rodda et al., 2020; Slavova et al., 2020).
4.1. Limitations
Our study is subject to limitations. We relied on information collected online and through phone calls with Medicaid program representatives; our study findings may be inaccurate if states did not accurately or fully represent their current Medicaid prescription fill limit policies. However, the large variation in these policies state-to-state and the challenge of collecting accurate information about these policies underscores the perils of our current system in advancing a unified strategy for expanding naloxone access to our most vulnerable populations. Second, our study was not designed to determine the prevalence of Medicaid beneficiaries who experience monthly fill limit restrictions or estimate the policy’s effects on naloxone utilization. Future research is needed to quantify these outcomes, ideally in individual-level Medicaid claims data. Third, the most recent state-level opioid prescribing and overdose rate data reported in Table 1 were from 2018. It is possible our reported state rankings for opioid prescribing and overdose rates changed from 2018 to March 2020, the date of our reported state-level Medicaid enrollment totals. Fourth, we did not assess the availability of community-based naloxone distribution programs for Medicaid enrollees facing potential barriers to pharmacy-based naloxone coverage. While the goal of our study was to examine the potential impact of Medicaid prescription fill limit policies on Medicaid-covered naloxone prescription access, future research should examine the role of alternative naloxone distribution programs in mitigating the potential impacts of naloxone coverage restrictions in Medicaid.
5. Conclusions
Monthly prescription fill limit policies threaten to restrict naloxone access for opioid overdose harm reduction in 20 % of the adult Medicaid population. Exempting naloxone from these policies may help increase naloxone uptake and prevent opioid overdose death among these patients, whom are particularly vulnerable to opioid overdose.
Author disclosures
Dr. Carpenter received funding from the NIH National Institute on Drug Abuseto support this work (#R34 DA046598-01A1), and Dr. Roberts received support from the NIH National Center for Advancing Translational Science (#KL2TR002367).
Role of funding source
The funders had no role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the study for publication.
Contributors
Data collection was conducted by Roberts and Trull; Data analysis was performed by Roberts; Manuscript drafting was led by Roberts with contributions from all authors; All authors contributed to study design and interpretation of study data. All authors approved the final article submitted for peer review.
Declaration of Competing Interest
None.
Acknowledgments
This research was supported by a research grant from the National Institute on Drug Abuse (#R34 DA046598-01A1); and the National Center for Advancing Translational Science via the Frontiers: University of Kansas Clinical and Translational Science Institute (#KL2TR002367).
References
- Bessen S., Metcalf S.A., Saunders E.C. Barriers to naloxone use and acceptance among opioid users, first responders, and emergency department providers in New Hampshire, USA. Int. J. Drug Policy. 2019;74:144–151. doi: 10.1016/j.drugpo.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2018. Centers for Disease Control and Prevention.https://www.cdc.gov/drugoverdose/maps/rxstate2018.html [Google Scholar]
- Centers for Medicare and Medicaid Services . 2020. March 2020 Medicaid & CHIP Enrollment Data Highlights.https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html [Google Scholar]
- Feng X., Tan X., Riley B., Zheng T., Bias T., Sambamoorthi U. Polypharmacy and multimorbidity among medicaid enrollees: a multistate analysis. Popul. Health Manag. 2018;21(2):123–129. doi: 10.1089/pop.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundation K.F. 2018. State Health Facts: Opioid Overdose Death Rates and All Drug Overdose Death Rates Per 100,000 Population (Age-Adjusted)https://www.kff.org/other/state-indicator/opioid-overdose-death-rates/?currentTimeframe=0&selectedDistributions=opioid-overdose-death-rate-age-adjusted&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D [Google Scholar]
- Frenier C., Nikpay S.S., Golberstein E. COVID-19 has increased medicaid enrollment, but short-term enrollment changes are unrelated to job losses. Health Aff. 2020;39(10):1822–1831. doi: 10.1377/hlthaff.2020.00900. [DOI] [PubMed] [Google Scholar]
- Giroir B. 2018. Naloxone: the Opioid Reversal Drug That Saves Lives: How Healthcare Providers and Patients Can Better Utilize This Life-saving Drug.https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf [Google Scholar]
- Glober N., Mohler G., Huynh P. Impact of COVID-19 pandemic on drug overdoses in Indianapolis. J. Urban Health. 2020:1–6. doi: 10.1007/s11524-020-00484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G.P., Jr., Haegerich T.M., Evans M.E., Losby J.L., Young R., Jones C.M. Vital Signs: Pharmacy-Based Naloxone Dispensing - United States, 2012-2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68(31):679–686. doi: 10.15585/mmwr.mm6831e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B.H., Sherman S.E., Palamar J.J. Prescription opioid misuse among middle-aged and older adults in the United States, 2015-2016. Prev. Med. 2019;121:94–98. doi: 10.1016/j.ypmed.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D.A., Polinski J.M., Choudhry N.K., Avorn J., Fischer M.A. Medicaid prescription limits: policy trends and comparative impact on utilization. BMC Health Serv. Res. 2016;16(1):15. doi: 10.1186/s12913-016-1258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicaid and CHIP Payment and Access Commission (MACPAC) 2017. Report to Congress on Medicaid and CHIP June 2017: Chapter 2- Medicaid and the Opioid Epidemic. [Google Scholar]
- Ochalek T.A., Cumpston K.L., Wills B.K., Gal T.S., Moeller F.G. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.W., Carpenter D.M., Smith A., Look K.A. Reviewing state-mandated training requirements for naloxone-dispensing pharmacists. Res. Social Adm. Pharm. 2019;15(2):222–225. doi: 10.1016/j.sapharm.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.N., West K.L., LeSaint K.T. Opioid overdose-related emergency department visits and accidental deaths during the COVID-19 pandemic. J. Urban Health. 2020:1–6. doi: 10.1007/s11524-020-00486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudowitz R., Corallo B., Artiga S. 2020. Analysis of Recent National Trends in Medicaid and CHIP Enrollment.https://www.kff.org/coronavirus-covid-19/issue-brief/analysis-of-recent-national-trends-in-medicaid-and-chip-enrollment/ [Google Scholar]
- Slavova S., Rock P., Bush H.M., Quesinberry D., Walsh S.L. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend. 2020;214 doi: 10.1016/j.drugalcdep.2020.108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith Ht, Davis N.L. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb. Mortal. Wkly. Rep. 2020;69(11):290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]