Abstract
Placental macrophages are a heterogenous population of immune cells present throughout pregnancy. They are essential for maintenance of the homeostatic placenta environment and host defense against infections. The characterization of placental macrophages as well as their activation have been limited for a long time by the lack of convenient tools. The emergence of unbiased methods makes it possible to reappraise the study of placental macrophages. In this review, we discuss the diversity and the functions of placental macrophages to better understand their dysfunctions during placental infections.
Keywords: Macrophages, Placenta, M1/M2 polarization, Infectious pregnancy
Graphical abstract
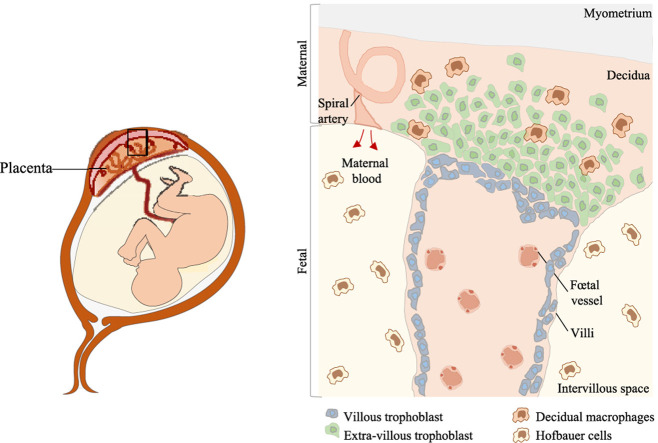
Schematic representation of the maternal and fetal parts of the placenta villi. Decidual macrophages are localized in the decidual at the maternal part, whereas Hofbauer cells are present in the fetal part in the intervillous space.
1. Revisit placental macrophages?
The placenta is a chimeric, rapidly growing organ in which fetal and maternal tissues are in close contact [1]. The maternal part of the placenta is composed of the decidua basalis which is directly related to the uterus; this constitutes an intimate connection between the mother and her developing fetus as fetal membranes that are composed of extravillous trophoblasts. The fetal part is covered by the amnion, which is involved in the secretion of the amniotic fluid. Under the amnion, the chorion, a membrane in continuity with the lining of the uterine wall, is required for supplying nutriments to the fetus and preventing fetus rejection by the maternal immune system [[1], [2], [3]]. The placenta is characterized by the presence of an immune system supporting the immune tolerance toward the fetus and the ability of mother to prevent infections. The placental immune system comprises natural killer (NK) cells, macrophages (~20%), T cells (~10–20%) and rarer cell types, such as dendritic cells, B cells, NKT cells and mast cells [[3], [4], [5]]. Although the NK population is the largest during the first trimester (~70%), its number steadily decreases to reach 20% of total immune cells at the end of the third trimester [6]. The number of macrophages follows same kinetics 50%–~20% before delivery based on immunohistochemical technique [7,8]. In contrast, the use of flow cytometry reveals that macrophage population remains stable during the first trimesters [8]. In contrast, T cell number gradually remains stable thought pregnancy [8].
Placental macrophages are composed of two distinct populations, i.e. decidual macrophages and Hofbauer cells; they are detected as early as day 10 of pregnancy and are present during the three trimesters of pregnancy [9]. The lack of convenient tools has limited the characterization of human placental macrophages as well as their functional studies. The diversity of these macrophages has been greatly underestimated, and most studies have been limited to immunohistochemical characterization and inferences from what has been reported in other resident macrophages. The application of single-cell RNA sequencing (scRNA-Seq) to placenta investigation has paved the way for a novel atlas of placental populations, including macrophages [10]. The analysis of placental macrophages publications revealed that the number of reports focusing on placental macrophages has increased steadily since 2000 but the number of publications on the role of placental macrophages and infection has not significantly increased (PubMed database).
2. Placental macrophage investigation: breaking through the barriers
The study of tissue macrophages has been revolutionized by the development of unbiased methods such as multiparametric flow cytometry and mass cytometry. Surprisingly, placental macrophages have been overlooked for several reasons. First, placentation is distinct in humans and rodents, and human and mouse placental macrophages are phenotypically different, thus restricting the use of murine models [11]. Second, their location in a complex and rapidly evolving tissue has favored in situ investigations, which precluded functional studies. An ex vivo placental perfusion assay might be an alternative to study placental macrophages in their natural microenvironment [12,13]. However, despite its initial description some 50 years ago, placental perfusion assay has been mainly limited to pharmacological investigations [14]. In contrast, the combination of laser microdissection and scRNA-Seq has permitted the characterization of the signature of macrophage populations in their microenvironment [10].
The isolation of human macrophages from placenta has appeared as the most convenient approach for macrophage characterization and functional studies. Different methods have been used to isolate placental macrophages [[15], [16], [17], [18]]. The location of macrophages in a complex tissue such as placenta and the lack of pertinent animal models make their study particularly challenging. The investigation of human placental macrophages has mostly been performed on immunohistochemical sections, which excludes functional studies. An ex vivo placental perfusion assay might be useful to study placental macrophages in their natural microenvironment in murine models and humans [12,13], but is to-day limited to pharmacological investigations [14]. Isolating macrophages from human placentas is the most convenient approach for functional studies. Different methods have been used: they vary in the use of enzymes (collagenase, DNase and/or trypsin), density gradient type (Ficoll or Percoll), positive or negative selection using anti-CD68, -CD10 or -CD14 antibodies or adhesive properties [[15], [16], [17], [18]]. We have developed a method using Ficoll procedure and anti-CD14 antibodies to isolate human placental macrophages with high purity and yield [18], but this does not discriminate their fetal/maternal origin.
We recommend here a method to isolate human placental macrophages using CD14 antibodies with high purity and yield [17]. Placental cells obtained by CD14 positive selection exhibit typical macrophage features; despite the fact that they share CD14, the canonical marker of monocytes, they are morphologically, phenotypically and functionally distinct from circulating monocytes. They are of both maternal (30%) and fetal origin (70%) as determined by sex chromosome staining [19]. The introduction of scRNA-Seq on one hand and multicolor flow cytometry or mass cytometry on another hand would have a direct impact on the characterization of placental macrophages. Given the reported differences between Hofbauer cells and decidual macrophages, we will use the term “placental macrophages” for both Hofbauer cells and decidual macrophages, except when their origin is specified.
3. Ontogeny of placental macrophages: an emerging field
It is largely established that resident macrophages in most tissues appear during the pre-natal period, and self-renewal rather than replenishment with monocytes supports their maintain throughout life. In response to aggression, monocytes become the major source of tissue macrophages [20]. The ontogeny of placental macrophages must be analyzed according to the heterogeneity of their origin. Initially, Hofbauer cells were thought to be in the chorionic villi, suggesting a fetal origin, while deciduous macrophages were found in the decidua basalis in contact with the maternal myometrium, suggesting a maternal origin (Graphical abstract). The sex chromatin staining in the placenta of a newborn boy has shown that X and Y chromosomes are found in macrophages from the fetal part, but only X chromosomes in decidual macrophages [21]. We recently found that CD14+ macrophages isolated from at term human placentas are of both maternal (30%) and fetal origin (70%) [19].
The lack of convenient animal models for genetic fate mapping methods does not permit to update the knowledge regarding placental macrophage ontogeny contrary to other tissue macrophages. Only fragmentary information concerning the ontogeny of human placental macrophages is available. Some authors proposed that Hofbauer cells originate from mesenchymal cells within stroma of developing chorionic villi at the early stage of gestation [22,23]. For others, Hofbauer cells were originate from monocyte progenitors of yolk sac and migrate to the chorionic villi, whereas decidual macrophages derive from hematopoietic pluripotent stem cells that differentiate into monocyte progenitors; these latter migrate from bone marrow to the bloodstream of maternal side of the placenta where they maturate [24]. It is noteworthy that transitional forms between monocytes and macrophages exist during the second and third trimesters, suggesting a differentiation of macrophages from fetal circulating monocytes [25]. The question of self-renewal of placental macrophages is warranted by placenta microenvironment. First, type 2 cytokines such as interleukin (IL)-4 and macrophage-colony-stimulating factor (M-CSF)-1 that are known to stimulate macrophage proliferation are over-represented in placenta [26]. Second, placental macrophages - and also extravascular trophoblasts - likely communicate with placental NK cells through interaction of M-CSF-1 produced by NK cells and M-CSF-1 receptor present on placental macrophages and extravascular trophoblasts [27]. Recently, the study of single cell transcriptomic atlas of maternal-fetal interface has shown that macrophages are able to self-renew [10]. In addition, placental macrophages proliferate in pathological conditions including Zika virus infection [28]. In contrast, Hofbauer cells did not exhibit mitotic activity or expression of Ki-67 marker [29]. In summary, placental macrophages represent a heterogeneous population of embryonic and hematopoietic origins but their ability to renew macrophage placental compartment is debated.
4. Placental macrophage heterogeneity: an increase in complexity
Besides ontogenic heterogeneity, placental macrophages change their phenotype with gestational age (Table 1 ). The phenotypic analysis of CD14+ macrophages reveal that seventy percent of them express CD209 (dendritic cell-specific intercellular molecule adhesion (ICAM)-3-grabbing non-integrin or DC-SIGN) and CD206 (mannose receptor), considered as M2 markers (see below). The study of CD209 expression during first-trimester gestation has shown the existence of two subsets of decidual macrophages. The major subset expresses CD209 following CSF-1 stimulation [30] or combined action of CSF-1 and IL-10 [31]. These CD209+ cells also express high levels of CD163, CD206, CD304 (neuropilin-1) and CD50 (ICAM-3), but low levels of CD11c, suggesting that they rather are M2 macrophages. The minor subset of decidual macrophages that does not express CD209, highly expresses CD11c and class II major histocompatibility complex (MHC) proteins, but not CD163, CD206, CD304 [[32], [33], [34]], suggesting that they are M1 macrophages. Although phenotypically distinct from blood monocytes, the transcriptional profile of these CD209- macrophages is close to that of circulating monocytes [35].
Table 1.
Phenotype of placental macrophages during gestation.
| Markers | Functions | 1st trimester | 2nd trimester | 3rd trimester | Refs |
|---|---|---|---|---|---|
| CD1 | •Antigen presentation to T lymphocytes | Yes | Yes | Nr | [105,106] |
| CD4 | •Interacts with antigen-presenting cells | Yes | Yes | Yes | [71,105,107] |
| CD11c | •Antigen uptake and presentation | Yes | Yes | Nr | [105,106] |
| CD14 | •Co-receptor for bacterial LPS detection •Cooperates with TLR-4 •Microbicidal functions |
Yes | Nr | Yes | [86,105,108] |
| CD16 | •Involved in phagocytosis •Degranulation •Oxidative burst: ROI production •Protective function against fetal antibodies |
Yes | Yes | No | [105,109] |
| CD68 | •Binds lectins and/or selectins •Crawling |
Yes > than 3rd trimester | Yes > than 3rd trimester | Yes | [57,82,110,111] |
| CD80 | •Co-stimulatory molecule •T cell priming |
Yes > than 3rd trimester | Nr | Yes | [[112], [113], [114]] |
| CD86 | •Co-stimulatory molecule •T cell priming |
Yes > than 3rd trimester | Nr | Yes | [[112], [113], [114]] |
| CD163 | •Recognizes and binds bacteria •Host defense •Immunosuppressive barrier between mother and fetus •Innate immune sensor for bacteria |
Nr | Nr | Yes | [108,110,115,116] |
| CD206 | •Pattern recognition receptor •Antigen processing •Endocytosis •Phagocytosis •Innate immune response |
Yes | Yes | Yes | [[112], [113], [114]] |
| CD209 | •Pattern recognition receptor •Binds CD50 (ICAM-3) •Bind microorganisms through envelope mannose •Viral receptor •Innate immune response through TLR modulation •Immune tolerance |
Yes | Yes | Yes > than 1st trimester | [117] |
| CCR5 | •Co-receptor for CD4 receptor •Used by macrophage-tropic (R5) HIV-1 for virus entry |
Yes | Nr | Yes | [96,[117], [118], [119], [120]] |
| CR3 | •Pattern recognition receptor •Binds microorganisms •Phagocytosis •Destruction of cells/microorganisms |
Yes | Yes | Nr | [105] |
| CXCR4 | •Binds SDF-1 •Chemoattractant for T-lymphocytes •Receptor for HIV |
Yes | Nr | Yes | [117] |
| HLA-DQ | •Binds and presents antigens to T cells •Immune tolerance •Its absence during the 1st trimester leads to the generation of cytotoxic cells •Cooperates with HLA-DR |
No | Nr | Yes | [105,[121], [122], [123]] |
| HLA-DP | •Receptor for self-antigens | Yes (low expression) | Nr | Yes > than 1st trimester | [105,121,123] |
| HLA-DR | •Binds microorganism peptides •Antigen uptake and presentation to T cells •Cooperates with HLA-DQ |
Yes (low expression) | Nr | Yes > than 1st trimester | [105,121,123] |
| TLR-2 | •Microorganism recognition •Activation of innate immunity •Regulation of innate immune function at the maternal-fetal interface |
Nr | Nr | Yes | [124] |
| TLR-4 | •Microorganism recognition •Activation of innate immunity •Cooperates with CD14 |
Nr | Nr | Yes | [108,124,125] |
CC and CXC: chemokines; CD: cluster of differentiation; CR3: complement receptor 32; HIV: human immunodeficiency virus; HLA: human leukocyte antigen; ICAM: intercellular adhesion molecule; LPS: lipopolysaccharide; Nr: not reported; ROI: reactive oxygen intermediates; SDF-1: stromal-derived-factor-1; TLR: toll-like receptor.
The recent use of scRNA-Seq has added an alternative degree in the heterogeneity of placental macrophage populations. Tsang et al. identified two clusters of macrophage-like cells that express activation markers of monocytes and Hofbauer cells. The monocyte signature varies during the pregnancy [36]. Vento-Torno et al. identified three new macrophage subsets. Two of them are discriminated by the level of expression of integrin subunit alpha X (ITGAX) [10]. Pique-Regi et al. identified different myeloid clusters, some of them matching with Hofbauer-type cells [37]. It is likely that the single cell transcriptome approach will allow to identify non-previously identified populations of placental macrophages beyond the classical dichotomy between Hofbauer cells and decidual cells.
5. Placental macrophage polarization: the limits of such classification
Besides the different subsets of placental macrophages described above, the functional properties of placental macrophages also change during pregnancy. The M1/M2 dichotomy has been largely used to characterize activation changes of macrophages including placental macrophages. Macrophage polarization is crucial for maintaining tissue homeostasis, even in pathological conditions. These two polarization categories lead to the expression of specific surface markers and to the secretion of several key cytokines to respond to microenvironmental stimuli such as placenta tissue modulation throughout pregnancy. When macrophages are stimulated with inflammatory cytokines or bacterial ligands such as lipopolysaccharide, they acquire inflammatory and microbicidal properties. Immunoregulatory cytokines, such as IL-4, IL-10 or IL-13 render macrophages poorly inflammatory and microbicidal, but competent for healing [38]. These polarization profiles, called M1 and M2, respectively, correspond to specific transcriptional, epigenetic and proteomic signatures [38,39]. Throughout pregnancy, a balance of polarization between M1 and M2 placental macrophages is necessary for the placenta plasticity and adaptation to the progression of gestation [40].
During the first and early second trimesters, placental macrophages exhibit an M1 profile characterized by the expression of pro-inflammatory cytokines including tumor necrosis factor (TNF), IL-12, IL-23, interferon (IFN)-γ and IL-18 [41]. However, Houser et al. showed that two subsets of placental macrophages, CD11clow and CD11chigh, do not fit a conventional M1/M2 categorization based on the secretion of both pro- and anti-inflammatory cytokines duting the first trimester [35]. At the end of the second trimester and during the early third trimester, placental macrophages exhibit an M2 profile characterized by the production of vascular endothelial growth factor (VEGF), IL-6 and IL-10 [42]. At the end of gestation, placental macrophages still exhibit an M1 profile. Indeed, at term, CD14+ placental macrophages express a program including the transcriptional expression of several members of TNF superfamily, the expression of chemokine receptors and the secretion of immune cytokines (IL-6, IL-10 and IL-1) [17,41,42]. The M1/M2 dichotomy to characterize placental macrophage populations deserves some criticisms and requires to be rethought. M1/M2 markers were found vary according to the studies and are not enough robust to allow characterization of cell subsets. This is illustrated by conflicting results concerning macrophage polarization in preeclampsia, a major inflammatory disease of pregnancy in which we could expect an M1 signature [43]. We recommended to assess macrophage activation by considering agonist and cell types however with a combination of several markers [38]. When this latter approach was used, we were unable to detect M1/M2 polarization in at term placental macrophages [17].
6. Placental macrophages and multinucleated giant cells: a continuum
The originality of placental macrophages is to form multinuclear giant cells (MGCs). Their formation is associated with down-modulation of CD14 and up-regulation of CD68 and CD163, which suggests a maturation process [17]. Placental MGCs exhibit features reminiscent of osteoclasts and foreign body giant cells (FBGCs), other types of myeloid MGCs. Their cytoskeleton is reorganized with podosomes in peripheral ring as in osteoclasts and placental MGCs contain small number of nuclei randomly distributed as in osteoclasts and FBGCs [44]. The ability of placental macrophages to form MGCs may be related to the fusion of cytotrophoblasts into syncytiotrophoblasts, a step required for placenta function [45]. Although placental macrophages have an intrinsic ability to fuse, it is likely that trophoblasts create a microenvironment prone to favor macrophage fusion. Several molecules produced by trophoblasts are candidate to affect differentiation of placental macrophages into MGCs. They include syncytins [46,47], E-cadherin and IL-4, a cytokine that mediates macrophage fusion in an E-cadherin-dependent manner [44,48]. The functions of placental MGCs are still obscure relying on indirect evidence. They exhibit enrichment of genes implicated in cytoskeleton organization, adhesion and immune response, thus highlighting functional diversity [17]. They possess macrophage properties such as phagocytosis and production of reactive oxygen intermediates (ROIs). In the light of what is known in myeloid MGCs, it is likely that placental MGCs are involved in the engulfment of large particles resulting from remodeling of placenta during pregnancy [49]. The profile of cytokine and chemokine production by MGCs rules out a polarized phenotype, but they possess both inflammatory and anti-inflammatory features. We propose that placental MGCs are fully competent macrophages that play a role in host defense and placental homeostasis.
7. Placental macrophages and infections
Placental macrophages are armed to fight microbial pathogens. They express microbial sensors (Toll-like receptors, lectins, complement receptor of the immunoglobulin superfamily) [[50], [51], [52]]. They are competent to ingest inert particles and microorganisms, produce ROIs, but are poor antigen-presenting cells [17,53]. This latter point may be useful to limit deleterious effect of adaptive immunity on pregnancy. As a consequence, placental macrophages are likely involved in the occurrence of placental infections during pregnancy (Fig. 1 ), a major cause of obstetric complications, fetal pathologies and preterm deliveries [54].
Fig. 1.
Number of publications associated with “Placenta macrophage” an “placenta macrophage and infection” (Pubmed database).
7.1. Bacterial infection
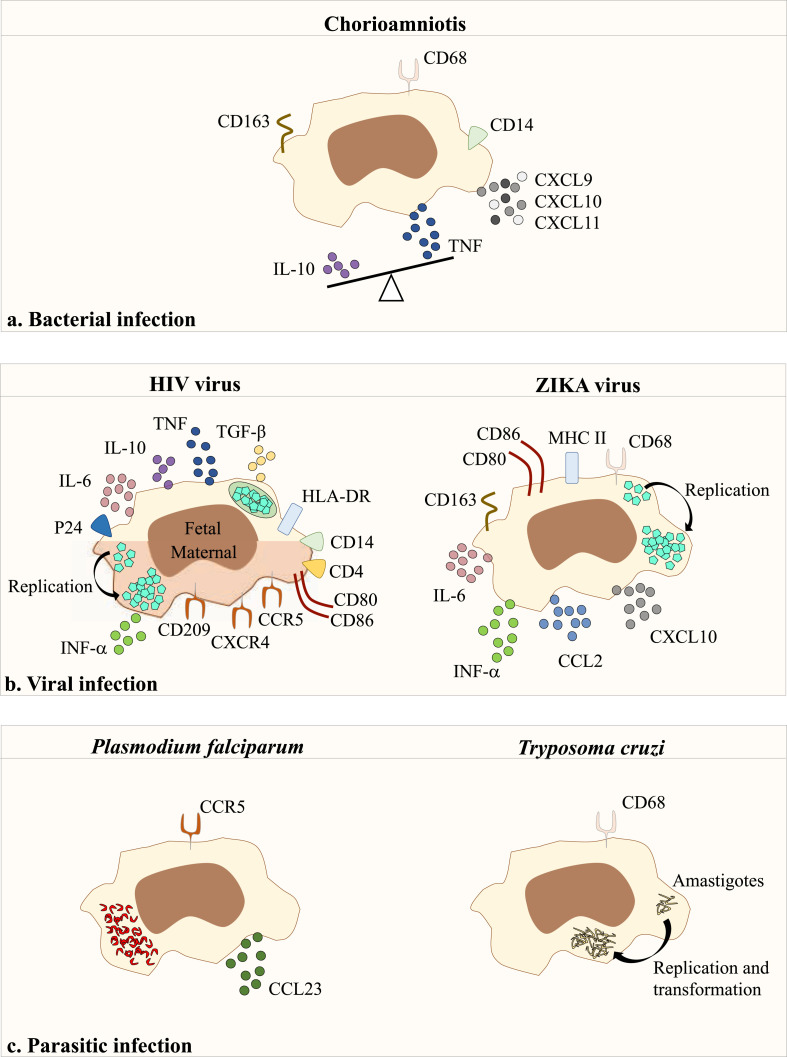
Chorioamnionitis or intra-amniotic infection is an acute or chronic inflammation of fetal membranes [55] causing premature rupture of the membranes that allows the direct introduction of microorganisms during chorionic villi sampling or via amniocentesis or fetoscopy. The bacteria mainly found include Escherichia coli, group B Streptococcus, Hemophilus sp. and Staphylococcus sp. [56]. Conflicting results have been reported concerning the presence of placental macrophages in chorioamnionitis lesions: decreased number [17,57] and increased number [58] as compared to controls. The activation level of placental macrophages varies according to pregnancy trimesters. In the third trimester, they over-express T cell chemokines (CXCL9, CXCL10, CXCL11) associated with altered villous architecture [59,60]. We found that the balance between TNF and IL-10, as pro- and anti-inflammatory cytokines respectively, is reoriented toward inflammatory response in chorioamnionitis. The expression of CD163, an M2 marker, on placental macrophages is higher in grade III than in grade II chorioamnionitis. We also showed that CD14+ placental macrophages from patients with chorioamnionitis are unable to form MGCs. This defect is partially corrected by incubating placental macrophages with control trophoblast supernatants [17], thus demonstrating the role of the placenta microenvironment in MGC formation. Placental macrophages may be also pathogenic through the release of extracellular traps (ET) in group B Streptococcus infection [61]. The release of placenta ET depends on actin polymerization and reactive oxygen species. Placenta ETs contain MMPs which are released during infection and lead to breakdown of extracellular matrix and placenta lesions [62]. This way of response of placenta macrophages to infection is shared with other placenta cells. Indeed, we reported the expression of cytonemes, actin-based structures, by placenta mast cells [63,64] in response to infection with anti-bacterial properties [5]. The role in infections by intracellular bacteria that present a placenta tropism is less well documented. During Listeria monocytogenes infection, a ubiquitous intracellular gram-positive bacterium responsible of listeriosis macrophages from placenta were found permissive [65,66]. It has been also provided evidence that during Brucella sp. Or C. burnetii infection, placental macrophages were found infected [19,67,68]. We recently showed that CD14+ macrophages from at term healthy placentas infected ex vivo by C. burnetii eliminate bacteria within 9 days. This elimination is associated with their polarization in M1 cells and is related to the production of IFN-γ [19]. The microbicidal activity of macrophages from at term placentas may account for the fact that the transmission from mother to fetus mainly occurs during first and second trimesters, not during the third trimester. But to date, the factors that govern the mechanisms of infection, the resistance and/or the susceptibility to these bacteria in decidual macrophages are still unknown.
7.2. Viral infections
Viral infections increase the risk of pregnancy disorders and fetal pathologies, as demonstrated by attention of media for Zika outbreaks. The role of placental macrophages during viral infections has been extensively studied in human immunodeficiency virus (HIV) infection. In placenta from non-emitting mothers, the immune response is effective, but placenta from surrogate mothers have an inflammatory response associated with chorioamnionitis [69]. HIV is detected in placental macrophages that express receptors for HIV including CD4, CCR5, CXCR4 and CD209 [70,71]. CD14+ placental macrophages are less permissive to HIV replication than monocyte-derived macrophages [72]. It has been shown that HIV transiently replicates within isolated CD14+/CD68+ placental macrophages [73]. In contrast, Johnson et al. showed that Hofbauer cells assemble and sequester HIV-1 without replication in compartments rich in endosomal/lysosomal markers, such as CD9, CD81, CD63 and lysosomal-associated membrane protein (LAMP)-1 [74]. Other placental partners are able to control placental macrophage permittivity to HIV. Hence, decidual NK cells inhibit infection of decidual macrophages by HIV through direct contact and IFN-γ release [75]. These findings highlight the key role of placental macrophages in the mediation of protection of placental tissue during HIV infection (Fig. 2 ). The spotlights have recently turned their attention to the ZIKA virus. The ZIKA virus is transmitted by mosquito bites (Aedes) that causes ZIKA fever in humans. During pregnancy, ZIKA virus is vertically transmitted from mother to fetus [76]. ZIKA infection in pregnant women is at the origin of adverse pregnancy and birth outcomes causing essentially severe brain malformations [77]. Pregnant women infected by ZIKA virus present a chronic placentitis with a chronic villous inflammation, edema and trophoblastic lesions [78]. There is evidence that ZIKA infection compromises mesenchymal and Hofbauer cells in human villi [79,80]. Immunohistochemistry approaches also reveal that ZIKA virus stimulates the proliferation of placental macrophages within the chorionic villous stroma [81]. CD163 or CD68 positive cells are colocalized with ZIKA virus antigens in vivo [82,83]. Ex vivo models show a higher permittivity of placental macrophages to ZIKA virus than trophoblasts [84]. Recently it has been shown that Abs directed against dengue virus increase ZIKA virus infection of Hofbauer cells, suggesting that pre-existing immunity to dengue affects host response to ZIKA virus [85]. The replication of ZIKA virus within Hofbauer cells induces the production of type I IFN, IL-6, CCL3 and inducible protein (IP)-10 [86]. The blockade of IFN production using Janus Kinase (JAK) inhibitors inhibits the production and the replication of the ZIKA virus in human Hofbauer cells in vitro [87]. Additionally, the expression of the co-stimulatory molecules CD80 and CD86 by Hofbauer cells is increased, suggesting that they are potentially APCs in vivo. All these studies suggest that the primary tropism of the ZIKA virus for placental macrophages enables the virus to cross the placental barrier and to eventually access to the fetal compartment. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to the coronavirus disease in late 2019 (Covid-19) has been associated with a large debate concerning the occurrence of transplacental transmission. Although no transplacental transmission was reported from China, Hosier. H et al., presented for the first time the case of woman with Covid-19 with a SARS-CoV-2 invasion of the placenta and local tissue inflammation and fibrin deposition [88]. They reported an inflammatory infiltrate of immune cells composed of CD3+ lymphocytes and CD68+ macrophages with only infection of syncytiotrophoblast cells by SARS-CoV-2 virus. The infiltration of CD68+ placenta macrophages in SARS-CoV-2 infected placenta was next confirmed [89,90] associated with a M2 phenotype in a case report of an asymptomatic Covid-19 positive woman [91]. Interestingly, Facchetti. F et al., reported a strong expression of S-protein in areas with dense monocytes-macrophage inflammation, which suggests a local activation of these cells [90]. Thus, to date, although the presence of placental macrophage into placenta lesions was clearly established, their role in SARS-CoV-2 infection of the placenta remains to elucidate.
Fig. 2.
Responses of placental macrophages to infection.
7.3. Parasitic and fungal infections
The place of placental macrophages in pathophysiology of parasitic or fungal infections during pregnancy is less documented than bacterial and viral infections. Among them, Plasmodium falciparum represents the most virulent of the plasmodial species in pregnancy and is associated with poor birth outcomes and low birth weight [92]. Only P. falciparum among plasmodial species is found in decidual macrophages [93,94]. Intravital microscopy has shown that infected erythrocytes accumulate in maternal blood, interact with trophoblasts in a stable manner and are engulfed by placental macrophages [95]. The accumulation of infected erythrocytes in human placenta causes an inflammatory response characterized by the expression of CCR5 [96] and the release of CCL3 [97] by placental macrophages. If the role of placental macrophages in parasite clearance remains uncertain, their contribution to pathogenesis of inflammatory lesions is well admitted. Same conclusions were also attributed to Tryposoma cruzi infection an obligate intracellular parasite responsible for the Chagas disease. Chagasic villitis is characterized by inflammatory infiltrates in which CD68+ macrophages and CD8+ T cells are prominent [98]. In addition, there is evidence of the multiplication of T. cruzi in CD68+ macrophages from placental chorionic villi [99,100]. However, the pattern of inflammatory reaction mediated by infected placental macrophages is so far unknown.
Although they represent a lower prevalence of chorioamnionitis with 0.3–0.5% [101], fungal infection are ascending infections that may severely compromise pregnancy rarely observed in neonates with preterm birth or neonatal death [102]. Congenital infections leading to preterm infants are mainly due to Candida species, including C. albicans, C. grablata, C. kyfer and C. parapsilosis. Immunohistological examination of placentas infected by C. guilliermondii shows the presence of hypersegmented neutrophils and large macrophages with a filled cytoplasm by several 3–6 μm oval bodies [103]. In addition, these infected placentas present edema of the lamina propria and macrophage infiltration. Although the role of macrophages in Candida infection is well documented in host defense against deeply invasive candidiasis [104], their role in placental infection has not been investigated to date.
8. Concluding remarks
Through the focus on pregnancy-associated infections, this review pointed that it is necessary to break down the technical barriers that have long hindered the study of placental macrophages. The results of scRNA-Seq have questioned the dichotomy between Hofbauer cells and decidual macrophages and paved the way for a re-writing of placenta macrophage diversity. The study of placental macrophages in their tissue environment will require the development of ex vivo placenta perfusion coupled with intravital microscopy and multiplexed-single-molecule fluorescent in situ hybridization. The question of whether placental macrophages are competent to combat microbial pathogens or rather whether they are involved in pathogenicity has not been sufficiently studied. Similarly, the role of placental macrophages in pregnancy-associated infectious diseases is poorly understood and will be a major issue for future research. Nevertheless, placental macrophage characterization could help in prediction of obstetric complications. It might help obstetricians in tricky situations where prolongation of pregnancy generally improves neonatal outcomes but increase infection/inflammation materno-fetal risks.
Authorship
S.M and J.L.M conceived and wrote the manuscript. M.K., A.B.A and F.B provided critical revision of the manuscript.
Conflict of competing interest
The authors declare no competing interests.
Infection with (a) bacteria, (b) virus or (c) parasites results in the expression of membrane receptors and the secretion of several key proteins by placental macrophages. CC and CXC: chemokines; CD: Cluster of differentiation; HIV: Human Immunodeficiency virus; HLA: Human leukocyte antigen; IL: Interleukin; IFN: Interferon; MHC: Major Histocompatibility complex; TGF: Transforming growth factor; TNF: Tumor necrosis factor.
Acknowledgments
Soraya Mezouar was supported by a “Fondation pour la Recherche Médicale” postdoctoral fellowship (reference: SPF20151234951). This work was supported by the French Government under the “Investissements d'avenir” (Investments for the future) program managed by the “Agence Nationale de la Recherche” (reference: 10-IAHU-03).
References
- 1.Erlebacher A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 2.Gerson K.D., Modest A.M., Hecht J.L., Young B.C. Persistent amnion-chorion membrane separation. J. Obstet. Gynaecol. Res. 2019;45:352–357. doi: 10.1111/jog.13852. [DOI] [PubMed] [Google Scholar]
- 3.Gobert M., Lafaille J.J. Maternal-fetal immune tolerance, block by block. Cell. 2012;150:7–9. doi: 10.1016/j.cell.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulmer J.N., Morrison L., Johnson P.M., Meager A. Immunohistochemical localization of interferons in human placental tissues in normal, ectopic, and molar pregnancy. Am. J. Reprod. Immunol. N. Y. N. 1990;1989(22):109–116. doi: 10.1111/j.1600-0897.1990.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 5.Mezouar S., Vitte J., Gorvel L., Ben Amara A., Desnues B., Mege J.-L. Mast cell cytonemes as a defense mechanism against Coxiella burnetii. MBio. 2019;10 doi: 10.1128/mBio.02669-18. e02669–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffett-King A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 7.Williams P.J., Searle R.F., Robson S.C., Innes B.A., Bulmer J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009;82:24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Yang F., Zheng Q., Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019;10:2317. doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mor G., Abrahams V.M. Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol. RBE. 2003;1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vento-Tormo R., Efremova M., Botting R.A., Turco M.Y., Vento-Tormo M., Meyer K.B., Park J.-E., Stephenson E., Polański K., Goncalves A., Gardner L., Holmqvist S., Henriksson J., Zou A., Sharkey A.M., Millar B., Innes B., Wood L., Wilbrey-Clark A., Payne R.P., Ivarsson M.A., Lisgo S., Filby A., Rowitch D.H., Bulmer J.N., Wright G.J., Stubbington M.J.T., Haniffa M., Moffett A., Teichmann S.A. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox B., Kotlyar M., Evangelou A.I., Ignatchenko V., Ignatchenko A., Whiteley K., Jurisica I., Adamson S.L., Rossant J., Kislinger T. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol. Syst. Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conings S., Amant F., Annaert P., Van Calsteren K. Integration and validation of the ex vivo human placenta perfusion model. J. Pharmacol. Toxicol. Methods. 2017;88:25–31. doi: 10.1016/j.vascn.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 13.D'Errico J.N., Fournier S.B., Stapleton P.A. Ex vivo perfusion of the rodent placenta. JoVE. 2019:59412. doi: 10.3791/59412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ala-Kokko T.I., Myllynen P., Vähäkangas K. Ex vivo perfusion of the human placental cotyledon: implications for anesthetic pharmacology. Int. J. Obstet. Anesth. 2000;9:26–38. doi: 10.1054/ijoa.1999.0312. [DOI] [Google Scholar]
- 15.Tang Z., Tadesse S., Norwitz E., Mor G., Abrahams V.M., Guller S. Isolation of hofbauer cells from human term placentas with high yield and purity, Am. J. Reprod. Immunol. 2011;66:336–348. doi: 10.1111/j.1600-0897.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z., Niven-Fairchild T., Tadesse S., Norwitz E.R., Buhimschi C.S., Buhimschi I.A., Guller S. Glucocorticoids enhance CD163 expression in placental Hofbauer cells. Endocrinology. 2013;154:471–482. doi: 10.1210/en.2012-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Amara A., Gorvel L., Baulan K., Derain-Court J., Buffat C., Vérollet C., Textoris J., Ghigo E., Bretelle F., Maridonneau-Parini I., Mege J.-L. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J. Immunol. Baltim. Md. 2013;1950(191):5501–5514. doi: 10.4049/jimmunol.1300988. [DOI] [PubMed] [Google Scholar]
- 18.Mezouar S., Ben Amara A., Chartier C., Gorvel L., Mege J.-L. A fast and reliable method to isolate human placental macrophages. Curr. Protoc. Immunol. 2019;125:e77. doi: 10.1002/cpim.77. [DOI] [PubMed] [Google Scholar]
- 19.Mezouar S., Benammar I., Boumaza A., Diallo A.B., Chartier C., Buffat C., Boudjarane J., Halfon P., Katsogiannou M., Mege J.-L. Full-term human placental macrophages eliminate Coxiella burnetii through an IFN-γ autocrine loop. Front. Microbiol. 2019;10:2434. doi: 10.3389/fmicb.2019.02434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassler K., Schulte-Schrepping J., Warnat-Herresthal S., Aschenbrenner A.C., Schultze J.L. The myeloid cell compartment-cell by cell. Annu. Rev. Immunol. 2019;37:269–293. doi: 10.1146/annurev-immunol-042718-041728. [DOI] [PubMed] [Google Scholar]
- 21.Wynn R.M. Derivation and ultrastructure of the so-called Hofbauer cell. Am. J. Obstet. Gynecol. 1967;97:235–248. doi: 10.1016/0002-9378(67)90546-7. [DOI] [PubMed] [Google Scholar]
- 22.Vacek Z. Derivation and ultrastructure of the stroma cells of the human chorionic villus. Folia Morphol. 1970;18:1–13. [PubMed] [Google Scholar]
- 23.Kaufmann P., Stark J., Stegner H.E. The villous stroma of the human placenta. I. The ultrastructure of fixed connective tissue cells, Cell Tissue Res. 1977;177:105–121. doi: 10.1007/bf00221122. [DOI] [PubMed] [Google Scholar]
- 24.Reyes L., Wolfe B., Golos T. Hofbauer cells: placental macrophages of fetal origin, Results Probl. Cell Differ. 2017;62:45–60. doi: 10.1007/978-3-319-54090-0_3. [DOI] [PubMed] [Google Scholar]
- 25.Zulu M.Z., Martinez F.O., Gordon S., Gray C.M. The elusive role of placental macrophages: the Hofbauer cell. J. Innate Immun. 2019;11:447–456. doi: 10.1159/000497416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramhorst R., Grasso E., Paparini D., Hauk V., Gallino L., Calo G., Vota D., Pérez Leirós C. Decoding the chemokine network that links leukocytes with decidual cells and the trophoblast during early implantation. Cell Adhes. Migr. 2016;10:197–207. doi: 10.1080/19336918.2015.1135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlino C., Stabile H., Morrone S., Bulla R., Soriani A., Agostinis C., Bossi F., Mocci C., Sarazani F., Tedesco F., Santoni A., Gismondi A. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. 2008;111:3108–3115. doi: 10.1182/blood-2007-08-105965. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg A.Z., Yu W., Hill D.A., Reyes C.A., Schwartz D.A. Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch. Pathol. Lab. Med. 2017;141:43–48. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 29.Grigoriadis C., Tympa A., Creatsa M., Bakas P., Liapis A., Kondi-Pafiti A., Creatsas G. Hofbauer cells morphology and density in placentas from normal and pathological gestations. Rev. Bras. Ginecol. E Obstet. Rev. Fed. Bras. Soc. Ginecol. E Obstet. 2013;35:407–412. doi: 10.1590/s0100-72032013000900005. [DOI] [PubMed] [Google Scholar]
- 30.Tagliani E., Shi C., Nancy P., Tay C.-S., Pamer E.G., Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med. 2011;208:1901–1916. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svensson J., Jenmalm M.C., Matussek A., Geffers R., Berg G., Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. Baltim. Md. 2011;1950(187):3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 32.Cupurdija K., Azzola D., Hainz U., Gratchev A., Heitger A., Takikawa O., Goerdt S., Wintersteiger R., Dohr G., Sedlmayr P. Macrophages of human first trimester decidua express markers associated to alternative activation. Am. J. Reprod. Immunol. 2004;51:117–122. doi: 10.1046/j.8755-8920.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 33.Heikkinen J., Möttönen M., Komi J., Alanen A., Lassila O. Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soilleux E.J., Morris L.S., Lee B., Pöhlmann S., Trowsdale J., Doms R.W., Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 35.Houser B.L., Tilburgs T., Hill J., Nicotra M.L., Strominger J.L. Two unique human decidual macrophage populations. J. Immunol. Baltim. Md. 2011;1950(186):2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang J.C.H., Vong J.S.L., Ji L., Poon L.C.Y., Jiang P., Lui K.O., Ni Y.-B., To K.F., Cheng Y.K.Y., Chiu R.W.K., Lo Y.M.D. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:E7786–E7795. doi: 10.1073/pnas.1710470114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pique-Regi R., Romero R., Tarca A.L., Sendler E.D., Xu Y., Garcia-Flores V., Leng Y., Luca F., Hassan S.S., Gomez-Lopez N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. ELife. 2019;8 doi: 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.-L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benoit M., Desnues B., Mege J.-L. Macrophage polarization in bacterial infections. J. Immunol. Baltim. Md. 2008;1950(181):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y., Xu X.-H., Jin L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mezouar S., Mege J.-L. Gene expression profiling of placenta from normal to pathological pregnancies. In: Ahmed R.G., editor. Placenta, IntechOpen. 2018. [DOI] [Google Scholar]
- 42.Gustafsson C., Mjösberg J., Matussek A., Geffers R., Matthiesen L., Berg G., Sharma S., Buer J., Ernerudh J. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y., Ye Y., Zhang J., Ruan C.-C., Gao P.-J. Immune imbalance is associated with the development of preeclampsia. Medicine (Baltim.) 2019;98:e15080. doi: 10.1097/MD.0000000000015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks P.J., Glogauer M., McCulloch C.A. An overview of the derivation and function of multinucleated giant cells and their role in pathologic processes. Am. J. Pathol. 2019;189:1145–1158. doi: 10.1016/j.ajpath.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Gerbaud P., Pidoux G. Review: an overview of molecular events occurring in human trophoblast fusion, Placenta. 2015. 36, 1, S35-S42. [DOI] [PubMed]
- 46.Møller A.M.J., Delaissé J.-M., Søe K. Osteoclast fusion: time-lapse reveals involvement of CD47 and syncytin-1 at sifferent stages of nuclearity. J. Cell. Physiol. 2017;232:1396–1403. doi: 10.1002/jcp.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langford M.B., Outhwaite J.E., Hughes M., Natale D.R.C., Simmons D.G. Deletion of the syncytin A receptor Ly6e impairs syncytiotrophoblast fusion and placental morphogenesis causing embryonic lethality in mice. Sci. Rep. 2018;8:3961. doi: 10.1038/s41598-018-22040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devaux C.A., Mezouar S., Mege J.-L. The E-cadherin cleavage associated to pathogenic bacteria infections can favor bacterial invasion and transmigration, dysregulation of the immune response and cancer induction in humans. Front. Microbiol. 2019;10:2598. doi: 10.3389/fmicb.2019.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milde R., Ritter J., Tennent G.A., Loesch A., Martinez F.O., Gordon S., Pepys M.B., Verschoor A., Helming L. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep. 2015;13:1937–1948. doi: 10.1016/j.celrep.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helmy K.Y., Katschke K.J., Gorgani N.N., Kljavin N.M., Elliott J.M., Diehl L., Scales S.J., Ghilardi N., van M. Lookeren Campagne, CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 51.Pudney J., He X., Masheeb Z., Kindelberger D.W., Kuohung W., Ingalls R.R. Differential expression of toll-like receptors in the human placenta across early gestation. Placenta. 2016;46:1–10. doi: 10.1016/j.placenta.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y., Krikun G., Abrahams V.M., Mor G., Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28:1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belhareth R., Mezouar S., Ben Amara A., Chartier C., Azzouz E.B., Chabrière E., Amri M., Mege J.-L. Cigarette smoke extract interferes with placenta macrophage functions: a new mechanism to compromise placenta functions? Reprod. Toxicol. Elmsford N. 2018;78:120–129. doi: 10.1016/j.reprotox.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg R.L., Hauth J.C., Andrews W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 55.Kim C.J., Romero R., Chaemsaithong P., Chaiyasit N., Yoon B.H., Kim Y.M. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015;213:S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redline R.W. Inflammatory response in acute chorioamnionitis. Semin. Fetal Neonatal Med. 2012;17:20–25. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Vinnars M.-T.N., Rindsjö E., Ghazi S., Sundberg A., Papadogiannakis N. The number of CD68(+) (Hofbauer) cells is decreased in placentas with chorioamnionitis and with advancing gestational age. Pediatr. Dev. Pathol. 2010;13:300–304. doi: 10.2350/09-03-0632-OA.1. [DOI] [PubMed] [Google Scholar]
- 58.Bae G.-E., Hong J.-S., Kim J.-S., Park H.Y., Jang J.Y., Kim Y.S., Choi S.-J., Oh S.-Y., Roh C.-R. Differential immunophenotype of macrophages in acute and chronic chorioamnionitis. J. Perinat. Med. 2017;45:483–491. doi: 10.1515/jpm-2015-0353. [DOI] [PubMed] [Google Scholar]
- 59.Kim J.-S., Romero R., Kim M.R., Kim Y.M., Friel L., Espinoza J., Kim C.J. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Förger F., Villiger P.M. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat. Rev. Rheumatol. 2020;16:113–122. doi: 10.1038/s41584-019-0351-2. [DOI] [PubMed] [Google Scholar]
- 61.Doster R.S., Sutton J.A., Rogers L.M., Aronoff D.M., Gaddy J.A. Streptococcus agalactiae induces placental macrophages to release extracellular traps loaded with tissue remodeling enzymes via an oxidative burst-dependent mechanism. MBio. 2018;9 doi: 10.1128/mBio.02084-18. e02084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doster R.S., Sutton J.A., Rogers L.M., Aronoff D.M., Gaddy J.A. Streptococcus agalactiae induces placental macrophages to release extracellular traps loaded with tissue remodeling enzymes via an oxidative burst-dependent mechanism. MBio. 2018;9 doi: 10.1128/mBio.02084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mezouar S., Ben Amara A., Vitte J., Mege J.-L. Isolation of human placental mast cells. Curr. Protoc. Cell Biol. 2018;80:e52. doi: 10.1002/cpcb.52. [DOI] [PubMed] [Google Scholar]
- 64.Mezouar S., Vitte J., Mege J.L. A role for placental mast cells in normal and complicated pregnancy. Reprod. Immunol. 2019;3:37. [Google Scholar]
- 65.Robbins J.R., Skrzypczynska K.M., Zeldovich V.B., Kapidzic M., Bakardjiev A.I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeldovich V.B., Clausen C.H., Bradford E., Fletcher D.A., Maltepe E., Robbins J.R., Bakardjiev A.I. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meador V.P., Hagemoser W.A., Deyoe B.L. Histopathologic findings in Brucella abortus-infected, pregnant goats. Am. J. Vet. Res. 1988;49:274–280. [PubMed] [Google Scholar]
- 68.Osburn B.I., Kennedy P.C. Pathologic and immunologic responses of the fetal lamb to Brucella ovis. Pathol. Vet. 1966;3:110–136. doi: 10.1177/030098586600300202. [DOI] [PubMed] [Google Scholar]
- 69.Patterson B.K., Behbahani H., Kabat W.J., Sullivan Y., O'Gorman M.R.G., Landay A., Flener Z., Khan N., Yogev R., Andersson J. Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J. Clin. Invest. 2001;107:287–294. doi: 10.1172/JCI11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis S.H., Reynolds-Kohler C., Fox H.E., Nelson J.A. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet Lond. Engl. 1990;335:565–568. doi: 10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- 71.Lairmore M.D., Cuthbert P.S., Utley L.L., Morgan C.J., Dezzutti C.S., Anderson C.L., Sedmak D.D. Cellular localization of CD4 in the human placenta. Implications for maternal-to-fetal transmission of HIV. J. Immunol. Baltim. Md. 1993;1950(151):1673–1681. [PubMed] [Google Scholar]
- 72.Johnson E.L., Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology. 2012;9:101. doi: 10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesson A.M., Fear W.R., Williams L., Chang J., King N.J., Cunningham A.L. HIV infection of placental macrophages: their potential role in vertical transmission. J. Leukoc. Biol. 1994;56:241–246. doi: 10.1002/jlb.56.3.241. [DOI] [PubMed] [Google Scholar]
- 74.Johnson E.L., Chu H., Byrareddy S.N., Spearman P., Chakraborty R. Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies. J. Int. AIDS Soc. 2015;18 doi: 10.7448/IAS.18.1.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quillay H., El Costa H., Duriez M., Marlin R., Cannou C., Madec Y., de Truchis C., Rahmati M., Barré-Sinoussi F., Nugeyre M.T., Menu E. NK cells control HIV-1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology. 2016;13:39. doi: 10.1186/s12977-016-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz D.A. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch. Gynecol. Obstet. 2017;295:1361–1368. doi: 10.1007/s00404-017-4361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rather I.A., Lone J.B., Bajpai V.K., Park Y.-H. Zika virus infection during pregnancy and congenital abnormalities. Front. Microbiol. 2017;8:581. doi: 10.3389/fmicb.2017.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simoni M.K., Jurado K.A., Abrahams V.M., Fikrig E., Guller S. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol. N. Y. N. 2017;1989:77. doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayer A., Lennemann N.J., Ouyang Y., Bramley J.C., Morosky S., Marques E.T.D.A., Cherry S., Sadovsky Y., Coyne C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Zhao S. Morgan & Claypool Life Sciences; San Rafael (CA): 2010. Vascular Biology of the Placenta.http://www.ncbi.nlm.nih.gov/books/NBK53247/ PMID: 21452443. [PubMed] [Google Scholar]
- 81.de Noronha L., Zanluca C., Azevedo M.L.V., Luz K.G., Santos C.N.D.D. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz. 2016;111:287–293. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabata T., Petitt M., Puerta-Guardo H., Michlmayr D., Wang C., Fang-Hoover J., Harris E., Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jurado K.A., Simoni M.K., Tang Z., Uraki R., Hwang J., Householder S., Wu M., Lindenbach B.D., Abrahams V.M., Guller S., Fikrig E. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adibi J.J., Zhao Y., Cartus A.R., Gupta P., Davidson L.A. Placental mechanics in the zika-microcephaly relationship. Cell Host Microbe. 2016;20:9–11. doi: 10.1016/j.chom.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Zimmerman M.G., Quicke K.M., O'Neal J.T., Arora N., Machiah D., Priyamvada L., Kauffman R.C., Register E., Adekunle O., Swieboda D., Johnson E.L., Cordes S., Haddad L., Chakraborty R., Coyne C.B., Wrammert J., Suthar M.S. Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe. 2018;24:731–742.e6. doi: 10.1016/j.chom.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quicke K.M., Bowen J.R., Johnson E.L., McDonald C.E., Ma H., O'Neal J.T., Rajakumar A., Wrammert J., Rimawi B.H., Pulendran B., Schinazi R.F., Chakraborty R., Suthar M.S. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gavegnano C., Bassit L.C., Cox B.D., Hsiao H.-M., Johnson E.L., Suthar M., Chakraborty R., Schinazi R.F. Jak inhibitors modulate production of replication-competent Zika virus in human hofbauer, trophoblasts, and neuroblastoma cells. Pathog. Immun. 2017;2:199–218. doi: 10.20411/pai.v2i2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B.F., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Cruz C.D., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS–CoV-2 infection of the placenta. J. Clin. Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V., Papaccio M., Castellani R., Casola S., Boniotti M.B., Cavadini P., Lavazza A. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schoenmakers S., Snijder P., Verdijk R., Kuiken T., Kamphuis S., Koopman L., Krasemann T., Rousian M., Broekhuizen M., Steegers E., Koopmans M., Fraaij P., Reiss I. SARS-CoV-2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman, Infectious Diseases (except HIV/AIDS) 2020. [DOI] [PMC free article] [PubMed]
- 92.Fried M., Muga R.O., Misore A.O., Duffy P.E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J. Immunol. Baltim. Md. 1998;1950(160):2523–2530. [PubMed] [Google Scholar]
- 93.Abrams E.T., Brown H., Chensue S.W., Turner G.D.H., Tadesse E., Lema V.M., Molyneux M.E., Rochford R., Meshnick S.R., Rogerson S.J. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated chemokine expression. J. Immunol. 2003;170:2759–2764. doi: 10.4049/jimmunol.170.5.2759. [DOI] [PubMed] [Google Scholar]
- 94.Mayor A., Moro L., Aguilar R., Bardají A., Cisteró P., Serra-Casas E., Sigaúque B., Alonso P.L., Ordi J., Menéndez C. How hidden can malaria Be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin. Infect. Dis. 2012;54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 95.de Moraes L.V., Tadokoro C.E., Gómez-Conde I., Olivieri D.N., Penha-Gonçalves C. Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of plasmodium-infected erythrocytes. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tkachuk A.N., Moormann A.M., Poore J.A., Rochford R.A., Chensue S.W., Mwapasa V., Meshnick S.R. Malaria enhances expression of CC chemokine receptor 5 on placental macrophages. J. Infect. Dis. 2001;183:967–972. doi: 10.1086/319248. [DOI] [PubMed] [Google Scholar]
- 97.Dudley D.J., Hunter C., Mitchell M.D., Varner M.W. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet. Gynecol. 1996;87:94–98. doi: 10.1016/0029-7844(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 98.Altemani A.M., Bittencourt A.L., Lana A.M. Immunohistochemical characterization of the inflammatory infiltrate in placental Chagas' disease: a qualitative and quantitative analysis. Am. J. Trop. Med. Hyg. 2000;62:319–324. doi: 10.4269/ajtmh.2000.62.319. [DOI] [PubMed] [Google Scholar]
- 99.Díaz-Luján C., Triquell M.F., Castillo C., Hardisson D., Kemmerling U., Fretes R.E. Role of placental barrier integrity in infection by Trypanosoma cruzi. Acta Trop. 2016;164:360–368. doi: 10.1016/j.actatropica.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 100.Diaz-Lujn C., Fernanda M., Mezzano L., Fretes R.E. Placental infection by trypanosome cruzi, the causal agent of congenital Chagas' disease. In: Zheng J., editor. Recent Adv. Res. Hum. Placenta, InTech. 2012. [DOI] [Google Scholar]
- 101.Maki Y., Fujisaki M., Sato Y., Sameshima H. Candida chorioamnionitis leads to preterm birth and adverse fetal-neonatal outcome. Infect. Dis. Obstet. Gynecol. 2017;2017:1–11. doi: 10.1155/2017/9060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benirschke K., Raphael S.I. Candida albicans infection of the amniotic sac, Am. J. Obstet. Gynecol. 1958;75:200–202. doi: 10.1016/0002-9378(58)90572-6. [DOI] [PubMed] [Google Scholar]
- 103.Stefanetti V., Marenzoni M.L., Lepri E., Coletti M., Casagrande Proietti P., Agnetti F., Crotti S., Pitzurra L., Del Sero A., Passamonti F. A case of Candida guilliermondii abortion in an Arab mare. Med. Mycol. Case Rep. 2014;4:19–22. doi: 10.1016/j.mmcr.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roilides E., Walsh T. Recombinant cytokines in augmentation and immunomodulation of host defenses against Candida spp. Med. Mycol. 2004;42:1–13. doi: 10.1080/13693780310001631341. [DOI] [PubMed] [Google Scholar]
- 105.Goldstein J., Braverman M., Salafia C., Buckley P. The phenotype of human placental macrophages and its variation with gestational age, Am. J. Pathol. 1988;133:648–659. [PMC free article] [PubMed] [Google Scholar]
- 106.Houser B.L., Tilburgs T., Hill J., Nicotra M.L., Strominger J.L. Two unique human decidual macrophage populations. J. Immunol. Baltim. Md. 2011;1950(186):2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maury W., Potts B.J., Rabson A.B. HIV-1 infection of first-trimester and term human placental tissue: a possible mode of maternal-fetal transmission, J. Infect. Dis. 1989;160:583–588. doi: 10.1093/infdis/160.4.583. [DOI] [PubMed] [Google Scholar]
- 108.Young O.M., Tang Z., Niven-Fairchild T., Tadesse S., Krikun G., Norwitz E.R., Mor G., Abrahams V.M., Guller S. Toll-like receptor-mediated responses by placental Hofbauer cells (HBCs): a potential pro-inflammatory role for fetal M2 macrophages. Am. J. Reprod. Immunol. N. Y. N. 2015;1989(73):22–35. doi: 10.1111/aji.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson P.M., Brown P.J. Review article: Fc gamma receptors in the human placenta. Placenta. 1981;2:355–370. doi: 10.1016/s0143-4004(81)80031-8. [DOI] [PubMed] [Google Scholar]
- 110.Tang Z., Buhimschi I.A., Buhimschi C.S., Tadesse S., Norwitz E., Niven-Fairchild T., Huang S.-T.J., Guller S. Decreased levels of folate receptor-β and reduced numbers of fetal macrophages (Hofbauer cells) in placentas from pregnancies with severe pre-eclampsia. Am. J. Reprod. Immunol. N. Y. N. 2013;1989(70):104–115. doi: 10.1111/aji.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ingman K., Cookson V.J.K.W., Jones C.J.P., Aplin J.D. Characterisation of Hofbauer cells in first and second trimester placenta: incidence, phenotype, survival in vitro and motility. Placenta. 2010;31:535–544. doi: 10.1016/j.placenta.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 112.Reyes L., Golos T.G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 2018;9:2628. doi: 10.3389/fimmu.2018.02628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schliefsteiner C., Peinhaupt M., Kopp S., Lögl J., Lang-Olip I., Hiden U., Heinemann A., Desoye G., Wadsack C. Human placental hofbauer cells maintain an anti-inflammatory M2 phenotype despite the presence of gestational diabetes mellitus. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joerink M., Rindsjö E., van Riel B., Alm J., Papadogiannakis N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta. 2011;32:380–385. doi: 10.1016/j.placenta.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Tang Z., Abrahams V.M., Mor G., Guller S. Placental Hofbauer cells and complications of pregnancy. Ann. N. Y. Acad. Sci. 2011;1221:103–108. doi: 10.1111/j.1749-6632.2010.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fabriek B.O., Dijkstra C.D., van den Berg T.K. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 117.Soilleux E.J., Morris L.S., Lee B., Pöhlmann S., Trowsdale J., Doms R.W., Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 118.Behbahani H., Popek E., Garcia P., Andersson J., Spetz A.L., Landay A., Flener Z., Patterson B.K. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 2000;157:1811–1818. doi: 10.1016/S0002-9440(10)64819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Torres G., García V., Sánchez E., Segarra A., Patterson B.K., Meléndez-Guerrero L.M. Expression of the HIV-1 Co-receptors CCR5 and CXCR4 on placental macrophages and the effect of IL-10 on their expression. Placenta. 2001;22:S29–S33. doi: 10.1053/plac.2001.0652. [DOI] [PubMed] [Google Scholar]
- 120.Joubert B.R., Franceschini N., Mwapasa V., North K.E., Meshnick S.R. Regulation of CCR5 expression in human placenta: insights from a study of mother-to-child transmission of HIV in Malawi. PLoS One. 2010;5:e9212. doi: 10.1371/journal.pone.0009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cupurdija K., Azzola D., Hainz U., Gratchev A., Heitger A., Takikawa O., Goerdt S., Wintersteiger R., Dohr G., Sedlmayr P. Macrophages of human first trimester decidua express markers associated to alternative activation. Am. J. Reprod. Immunol. N. Y. N. 2004;1989(51):117–122. doi: 10.1046/j.8755-8920.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 122.Bulmer J.N., Johnson P.M. Macrophage populations in the human placenta and amniochorion. Clin. Exp. Immunol. 1984;57:393–403. [PMC free article] [PubMed] [Google Scholar]
- 123.Sutton L., Mason D.Y., Redman C.W. HLA-DR positive cells in the human placenta. Immunology. 1983;49:103–112. [PMC free article] [PubMed] [Google Scholar]
- 124.Ma Y., Krikun G., Abrahams V.M., Mor G., Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28:1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumazaki K., Nakayama M., Yanagihara I., Suehara N., Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum. Pathol. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]