Abstract
Neurodegenerative diseases, including Alzheimer disease (AD), Parkinson disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington disease, are characterized by the loss of neurons as well as neuronal function in multiple regions of the central and peripheral nervous systems. Several studies in animal models have shown that androgens have neuroprotective effects in the brain and stimulate axonal regeneration. The presence of neuronal androgen receptors in the peripheral and central nervous system suggests that androgen therapy might be useful in the treatment of neurodegenerative diseases. To illustrate, androgen therapy reduced inflammation, amyloid-β deposition, and cognitive impairment in patients with AD. As well, improvements in remyelination in MS have been reported; by comparison, only variable results are observed in androgen treatment of PD. In ALS, androgen administration stimulated motoneuron recovery from progressive damage and regenerated both axons and dendrites. Only a few clinical studies are available in human individuals despite the safety and low cost of androgen therapy. Clinical evaluations of the effects of androgen therapy on these devastating diseases using large populations of patients are strongly needed.
Keywords: androgens, testosterone, Alzheimer’s disease, Parkinson’s disease, Multiple sclerosis, neuroregeneration, remyelination
Neurodegenerative diseases including Alzheimer disease (AD), Parkinson disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington disease (HD) are characterized by progressive alterations of neuronal function in the brain and spinal cord. Progression of these diseases causes the loss of cognitive and motor functions and the consequent death of patients. The pathogenesis of neurodegenerative diseases is multifactorial and not completely understood. The most intensively investigated contributing factor is that of epigenetic factors, which showed the potential role of deoxyribonucleic acid (DNA) and histone modifications in the etiology of these disorders [1]. The incidence of neurodegenerative diseases and dementia is high, affecting more than 47.4 million people worldwide in 2015, with projected patient numbers of 75.63 million in 2030 and 135.46 million in 2050 [2]. Although there is a significant gender difference in the incidence of neurological disorders, the specific contributing substrates remain unknown [3]. AD and MS are more prevalent in women [4] when compared with the disproportionate incidence of PD and ALS in men [5]. Circulating levels of androgens and estrogens could have a role in determining gender differences in some neurodegenerative diseases.
It has been calculated that an intervention that reduces risk factors by 10% to 25% could potentially decrease the incidence of AD cases by 184 000 to 492 000 cases in the United States alone and up to 1.1 million to 3.0 million cases worldwide [6]. Emerging clinical evidence shows that androgens are essential not only in maintaining sexual function, mood, and cognition [7] but also in brain efficiency and reducing the incidence of dementia [8]. However, the pleiotypic effects of androgens on brain function are complex and not fully elucidated. This article aims to consider potential preventive and therapeutic effects of androgens on neurodegenerative diseases.
1. Androgens and Brain Function
The classical androgens, testosterone (T), dihydrotestosterone (DHT), and dehydroepiandrosterone (DHEA) act on cells through 2 independent genomic and nongenomic pathways. The classical genomic pathway is activated by androgen receptor (AR) binding to specific DNA response elements in target gene promoters, and subsequent regulation of messenger ribonucleic acid (mRNA) transcription and protein synthesis. The nongenomic pathway can give rise to a response within seconds to minutes, and the mechanism of action is independent of DNA binding because the AR does not need to translocate into the nucleus [9]. The nongenomic effects of androgens are mediated not only by interactions with classical nuclear ARs, but also by putative membrane androgen receptors (mARs). Despite the limited demonstration of the nongenomic effects of androgens, the most evident one is a rapid rise in intracellular calcium [10]. GPRC6A is a pertussis toxin-sensitive G-protein–coupled receptor that allows extracellular T to activate a rapid, nongenomic signal to cells lacking ARs [11]. Among mARs, GPRC6A is a membrane protein involved in physiological responses to hormones and environmental stimuli regulating the effect of androgens in various tissues [12]. However, androgens interact also with other different mARs that are unrelated to nuclear receptors (extensively reviewed by Thomas, [13]). Androgens regulate neuronal growth, differentiation, survival, or death through both genomic and nongenomic signaling pathways [14].
T activates several intracellular signal transduction pathways, including the growth hormone/insulin-like growth factor (GH/IGF)-1 axis [15], the phosphatidylinositol 3-kinase/protein kinase B pathway [16], the Wnt/b-catenin signaling pathway [17], and Notch signaling [18].
In aging men, plasma total T levels decline progressively [19] at an average rate of 110 ng/dL per decade [20]; by comparison, free T levels decrease at a higher rate (2%-3% per year) than total T [20]. The decrease in plasma T levels is correlated with functional alterations in the brain [21] and cognitive dysfunction in aging [22], classical symptoms of androgen deficiency syndrome [23]. Rosario et al [24], during a postmortem neuropathological investigation on patients more than 80 years old, found that decreased levels of brain T were correlated with advancing age; in comparison, no changes in brain 17β-estradiol levels were observed. The brain is greatly influenced by changes in circulating T levels; however, its actions are integrated with those of other essential hormones, such as 17β-estradiol, progesterone, and IGF-1 [15]. T protects neurons from different types of insults, increasing the level of neuroglobin [25] secreted by astrocytes and microglia in critical conditions, that is, after injury [26], glucose deprivation [27], and kainic acid toxicity [28]. T also stimulates neuronal differentiation, thereby maintaining neuronal plasticity [29], promoting synaptic density [30], increasing connectivity of hypothalamic neurons [31], as well as stimulating neurite outgrowth [32]. It reduces the reactivity of astrocytes following brain injury, gliosis reactivity [33], and retards the aging process [34]. T improves the survival of human neurons and astrocytes, acting directly on the mitochondrial membrane, inhibiting the generation of reactive oxygen [35, 36] and nitrogen species [27], as well as sirtuin-1 expression [37]. Conversely, the absence of T may contribute to the neurodegenerative process [38] as observed in animal models of PD [39] and AD [40] and may decrease the density of spinal synapses in the hippocampus [30]. These pathological effects were reversed following treatment with T or DHT; 17β-estradiol, likely, partially mediates these effects.
In light of these observations, it is not surprising that the protective effect of T is dose dependent. Dosages of T that induced physiological plasma concentrations (10 nM) exerted significant neuroprotective influences; by contrast, dosages 10-fold greater were ineffective [41]. This neuroprotective effect is due to T inhibition of caspase-3, an effector of the apoptotic cascade [27]. T also exerted a neuroprotective effect in experimental autoimmune encephalomyelitis, a disease model used to study the inflammatory process in MS [42]. The disease was correlated with altered immunoregulation [43] including activation of CD8+ T cells [44], and CD4+ T lymphocytes [45].
In order to exert its effects on the brain, plasma T must cross the blood-brain barrier (BBB). It is generally believed that steroid hormones freely diffuse from plasma into the brain by passing through the BBB [46]. However, this concept has been challenged recently by the demonstration that in Drosophila, a membrane transporter expressed on the surface of glial cells, the Ecdysone Importer, is necessary to allow steroid hormones to cross the BBB and permit T entry into the brain [47]. The Ecdysone Importer belongs to the family of organic anion transporting polypeptides that could have potential functions in transmembrane transport of mammalian steroid hormones [48]. Another transporter, the MDR1-type P-gp is involved in transporting these hormones out of the brain, providing a kinetic barrier. The intracerebral concentrations of sex hormones are at least in part regulated by the activity of MDR1-type P-gp [49].
2. The Neuroactive Steroids
A group of steroids, including T, deoxycorticosterone, progesterone, the bound sulfate form of DHEA, (DHEAS), and their metabolites, are also called neurosteroids because they can regulate neuronal excitability and function [50]. Neurosteroids are synthesized within the brain or derived from peripheral precursors. They act on neurons by a rapid nongenomic pathway, primarily interacting with a diversity of receptors, including glutamate and gamma-aminobutyric acid (GABA)-A receptors [51], N-methyl-D-aspartic acid (NMDA), and 5-hydroxytryptamine type 3 receptors [52, 53]. The various types of neurotransmitter receptors in the brain are responsible for different clinical effects such as potent anxiolytic [54], antidepressant [55, 56], and antiepileptic activities [57]. Furthermore, neurosteroids modulate learning and memory processes in the young and elderly [58] and are also involved in the pathophysiology of schizophrenia [59].
The principal neurosteroids are progesterone and deoxycorticosterone, which are precursors for the endogenous allopregnanolone (5α-pregnane-3α-ol-20-one) and THDOC (5α-pregnane-3α, 21-diol-20-one), respectively [58], and both hormones stimulate GABA-A receptors. Neurosteroids modulating GABA-A activity present significant anxiolytic, antidepressant [55], and antiepileptic effects [60].
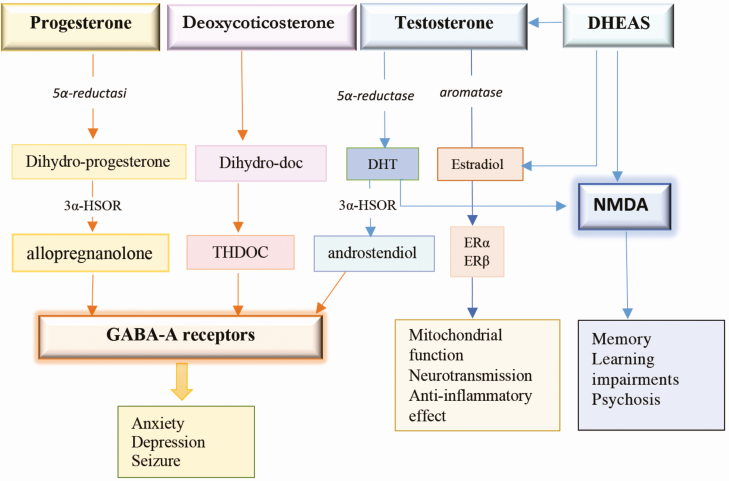
T acts indirectly after its aromatization to 17β-estradiol and to DHT as a consequence of 5α-reductase activity. DHT can be metabolized by 3alpha-hydroxysteroid oxide reductase and 3beta-hydroxysteroid oxide reductase, to 5α-androstan-3α,17β-diol (3α-Diol) and 5α-androstane-3β,17β-diol (3β-Diol), respectively [61]. Both steroids, 3α-Diol and 3β-Diol, have little activity on the AR, while 3β-Diol exerts a moderate activation of estrogen receptor (ER)β [62]. 3α-Diol exerts a positive allosteric action on GABA-A receptors [63], while 17β-estradiol stimulates mitochondrial activity and neuroprotection [64] (Fig. 1).
Figure 1.
Neurosteroids: biosynthesis in the human brain and their function. Progesterone, testosterone, and deoxycorticosterone are converted by 5α-reductase into 5-dihydro-reduced steroids, which are then reduced further to 3α-hydroxylated neurosteroids by 3-HSOR in allopregnenolone, THDOC, and androstendiol. All these metabolites activate GABA-A receptors that regulate anxiety, depression, and seizure. Testosterone is converted by aromatase into 17β-estradiol that activates ERα and ERβ improving mitochondrial function, anti-inflammatory effect, and neurotransmission. DHEAS and testosterone activate NMDA and can converted in testosterone and estradiol. NMDA activation is involved in memory, learning impairments, and psychosis. DHEAS, dehydroepiandrosterone sulfate; DHT, dihydrotestosterone; ER, estrogen receptor; GABA, gamma-aminobutyric acid; HSOR, 3alpha-hydroxysteroid oxide reductase; NMDA, N-methyl-D-aspartate; THDOC, 5α-pregnane-3α, 21-diol-20-one.
DHEA and DHEAS are both partially converted to either T, DHT, or estradiol. DHEA has a neuroprotective effect on the brain [65] and is involved in the regulation of depression and seizures [66] through the activation of GABA-A [67] and NMDA receptors [53] (Fig. 1). The activation of NMDA receptors stimulates brain development, working memory [68, 69], and learning performance and regulates psychoses [70]. The modulation of NMDA receptors in the prefrontal cortex plays a fundamental part of the neurobiology of cognition and is positively correlated with sex hormone levels [71].
Although DHEAS levels showed a positive correlation with cognitive function, randomized clinical trials did not provide clear evidence for the usefulness of DHEA treatment in improving cognitive function in elderly participants with dementia [72, 73]. Neurosteroids are likely to be more effective in prophylactic roles, and endogenous hormones are more effective than exogenous.
3. ARs and Neurodegenerative Diseases
An early study demonstrated the expression of ARs and ERs in the brain [74], and a detailed distribution map of ARs [75] and ERs [76] was generated. Simerly et al [77] found widespread AR and ER mRNA in neurons of the rat brain. ARs are mostly expressed in the hypothalamus [78], telencephalon, and amygdala and also in the majority of the brainstem and spinal cord areas associated with sensory functions [77], as well as in Purkinje cells of the cerebellar cortex. At the cellular level, ARs were found on axons and dendrites, suggesting that androgens may have an essential and novel extranuclear role in neuronal function [79]. ARs are distributed in many brain areas, especially in those regions involved in learning and memory such as the hippocampus and amygdala [78].
The loss of AR function could contribute to motor neuron degeneration [80, 81]. During spinal cord motor neuron growth in vitro, ARs facilitated the growth of cell bodies and elongation of neurites [32, 82]. An AR signal plays an essential role in the development and trophism of motor neurons as well as in the maintenance of their morphology and survival [83]. Motor neuron vulnerability increases when AR expression is reduced [84] (Fig. 2).
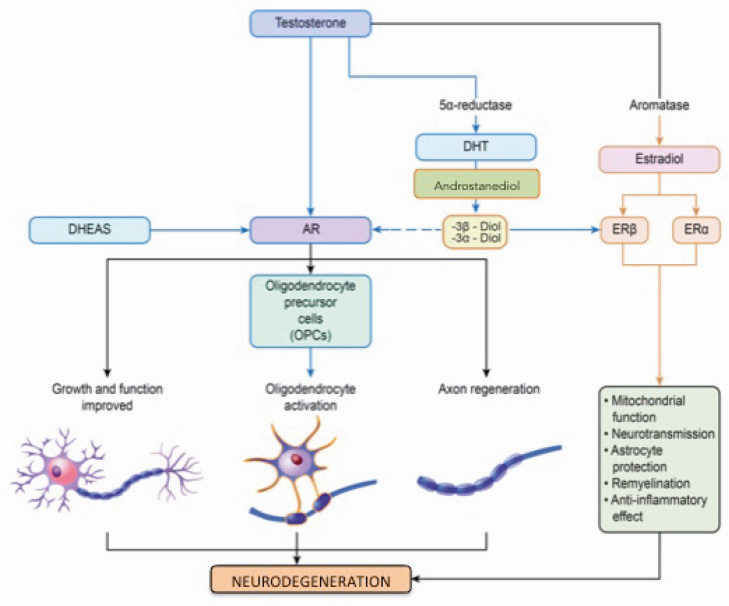
Figure 2.
Effect of androgens on neuron and myelin regeneration. Androgens (testosterone, DHT, and DHEAS) activate ARs, which are located on the membrane of neurons and mitochondria. The activation of ARs promotes the trophism and growth of neurons and the formation of new myelin following the OPCs activation and axonal regeneration. Testosterone, after its aromatization in 17β-estradiol, activates the ERα and Erβ, improving mitochondrial function, anti-inflammatory and neurotransmission effects and protecting astrocytes. Testosterone is converted in DHT by 5α-reductase and then in androstendiol for which the metabolites 3α and 3β-diol then have a weak effect on ARs but are more active on ERβ. AR, androgen receptor; DHEAS, dehydroepiandrosterone sulfate; DHT, dihydrotestosterone; ER, estrogen receptor; OPC, oligodendrocyte precursor cell.
The activation of ARs exerts direct trophic effects on motor neurons of the spinal cord anterior horn as manifested in larger cell bodies, broader dendritic processes, extended life spans [82, 85], and increased synaptic densities in the cervical ganglion [86]. Effects of T on the hippocampus in maintaining spine synaptic density is direct and not mediated by 17β-estradiol [29]. AR signaling has a neuroprotective effect in neuroblastoma cells [87], as shown in vitro by the administration of T, which stimulated the secretion of the non-amyloidogenic amyloid-β (Aβ ) precursor protein and decreased the secretion of Aβ peptides from neuronal cells and rat primary cerebrocortical neurons [88, 89].
AR signaling is associated with various trophic factors acting on motor neuron biology. AR activation increases the expression of vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and neurogenesis in the brain [90] and promotes IGF-1 secretion in various tissues [91]. Androgens stimulate BDNF expression, which activates brain neurogenesis [92], dendritic spine maturation [93], and motor neuron morphology [94]. On the other hand, BDNF has an autocrine effect, stimulating steroidogenesis in Leydig cells [95]. Neuronal health and survival are associated with the number of ARs in the motor neuron. Nguyen et al [96] demonstrated an AR-dependent androgen activation of mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling that activates a specific neuroprotective pathway in neurons.
Alterations in AR function may produce various pathologies as observed in spinal and bulbar muscle atrophy (SBMA), a degenerative disease of lower motor neurons with consequent atrophy of muscle limbs [97]. SBMA is comparable with ALS but differs in that only lower motor neurons are affected in ALS, whereas both upper and lower motor neurons degenerate in SBMA. SBMA is characterized by the expansion of a polymorphic trinucleotide (cytosine-adenine-guanine, CAG) that encodes a polyglutamine (polyQ) tract in the AR gene altering the normal protein function [98]. The expansion of the polyQ tract to a length of 36 to 40 glutamines favors modifications in folding and structure of the amino terminal domain of the receptor that is responsible of AR aggregation and has been reported in 9 neurodegenerative disorders, including HD and other types of spinocerebellar ataxia [99]. Changes involving interaction amino-N-terminal FXXLF motif and carboxyl-C- terminal AF-2 domain interaction are a determinant of the mutant AR aggregation and toxicity [100]. An AR mutation is also a determinant in SBMA pathogenesis; however, although patients present clinical signs of androgen insensitivity (eg, gynecomastia and infertility), loss of AR function is not the primary mechanism of the disease [101]. Neuronal loss is due to binding of the polyQ-AR to T or DHT, which induces toxicity, while T reduction prevents the phenotypic expression of SBMA [102, 103]. Although the reduction of T levels by leuprorelin showed a significant reduction in polyQ-AR, no beneficial effects on the swallowing reflex in patients with SBMA were observed [104]. In patients with SBMA, the endocrine alterations of the polyQ expansion contributes only partially to the pathogenesis of the disease by increasing misfolded protein toxicity and the loss of normal protein function [80].
Interdomain interactions of AR transcription factors involve a variety of nuclear receptors [100]. In SBMA animal models, a downregulation of genes that regulate the expression of VEGF, IGF-1, glial cell line-derived neurotrophic factor [105], and transforming growth factor-beta type II receptor [106] has been reported. Importantly, IGF-1 mediates the inactivation of mutant AR, reducing muscle and spinal cord pathology in SBMA. The activation of IGF-1/Akt signaling reduced histopathological abnormalities and extended life span in SBMA mice [107]. These data suggest that therapy to improve skeletal muscle tropism is a helpful strategy in SBMA intervention. However, the pathogenesis that leads to AR toxicity remains unknown [101]. An enhanced understanding of AR signaling in neurons may provide the conceptual basis for therapeutic strategies to mitigate, or perhaps inhibit, the progress of neurodegenerative diseases [84].
4. Androgens and Neurodegenerative Diseases
A. Alzheimer Disease
Alzheimer disease (AD) is responsible for about 70% of the global incidence of dementia, which involves 24 million people and has been predicted to increase 4-fold by the year 2050 [108]. The etiology of AD is complex but is allegedly the consequence of an interaction between genetic and environmental factors. Aging is the preponderant factor: the incidence of AD at 80 years of age is 4-fold greater than that at age 70 years and 9-fold greater than that at age 65 years [109, 110]. The influence of genetic [108, 111] nutritional [112, 113], and hormonal components; low serum T levels in men [112]; and low 17β-estradiol in females [114] are involved. Furthermore, high serum levels of free T in males and females have a protective effect against AD incidence and its progression [115].
The neuropathology of AD is characterized by the formation of extracellular amyloid plaques in the brain, and the accumulation of cellular neurofibrillary tangles [108]. In neuron cell cultures with genetic induction of AD, T administration significantly reduced Aβ neurotoxicity [116], and the anti-Aβ effect was potentiated in association with high estrogen levels [89]. Aβ is derived from amyloid precursor protein (APP), which is a glycoprotein found ubiquitously in the cells. APP may be metabolized in 2 metabolic pathways: the amyloidogenic and non-amyloidogenic [117]. The amyloidogenic pathway produces Aβ by the activity of the secretases β and γ. The β-secretase, also called BACE1, is a type-1 membrane protein essential for the generation of Aβ and AD. In BACE1 knockout mice, neither the production of Aβ nor development of AD was observed [118]. Therefore, inhibition of BACE1 represents an exciting area of research for the treatment of AD [119]. Recently, it has been suggested that BACE1 levels in plasma may serve a useful biomarker of latent AD [120]. Aβ deposition originates from APP [121, 122] after a proteolytic catalyzation by β- and γ-secretase [123] as observed in the brain cortex of patients with AD [124]. In the non-amyloidogenic pathway, APP is cleaved by secretase-α and removed from the brain [125]. Physiologic androgen levels decrease the incidence of AD related to Aβ by a classic genomic pathway activating AR and neprilysin gene expression, as well as by a nongenomic activation of MAPK/ERK that can represent a therapeutic strategy against AD and other age-related neurodegenerative diseases [126]. Low serum T levels in men have been associated with increased Aβ deposition in brain tissue, favoring the progression of AD [8, 114, 127-130], while high T levels reduced the onset and development of AD [131]. T administration increased the amount of APP secretion of the non-amyloidogenic pathway; this effect is due to the 17β-estradiol derived from T aromatization [132]. 17β-estradiol plays a significant role in reducing amyloid formation in the brain by downregulating BACE1 mRNA [133] and consequent levels of the APP mRNA isoform [134] by activation of the MAPK signaling pathway, independently of the ER [135]. It is conceivable that T could interfere in APP metabolism through an estrogen-dependent pathway. Non-aromatizable androgens, such as DHT, did not show any effect on α-APP levels but reduced Aβ deposition in the brain [136]. However, T has a direct beneficial effect by increasing Aβ degradation and downregulating BACE1 expression; these activities are independent of T conversion into estradiol [137] and are consistent with the effects of DHT (a non-aromatizable androgen). The deposition of Aβ seems to be synergistically regulated by the activation of both ARs and ERs [138].
DHEAS also presents neuroprotective effects in a variety of experimental models by antagonizing NMDA-induced excitotoxicity [139], Aβ peptide toxicity [140], and oxygen-glucose deprivation [141].
Although there is evidence for positive effects of T in the treatment of AD in experimental models, clinical studies conducted on aging male patients with AD present conflicting results. To illustrate, T administration improved cognition [142], and visual-spatial skills [143] in some cases, whereas other studies showed no positive effects on memory or mental impairment [144-147]. In a recent meta-analysis, Buskbjerg et al [147] showed that T therapy in men with normal plasma T levels had no significant clinical effect on cognitive function; T effects on men with hypogonadism remain to be evaluated.
These contradictory findings may be a consequence of different methodologies of investigation; to illustrate, some studies administered T via a subcutaneous gel (5 mg/day) [146, 148] whereas other studies administered T by intramuscular injection (100/200 mg weekly or biweekly). Furthermore, if androgens are considered primary regulators in the maintenance of cognition, other important hormones are implicated as well, including insulin and IGF-1 [149]. High serum cortisol levels favor a low sex steroid production and may play a critical indirect action in the pathogenesis of AD [150].
Moreover, in patients with AD, high plasma levels of luteinizing hormone had functional consequences on vulnerable neurons, and in women with AD not taking estrogens, these levels might be involved in the pathogenesis of Aβ plaque formations [151]. Another essential aspect may be related to the stage of the disease at which therapy starts. If the neuroanatomical condition of neurons is severely compromised, the probability of reversing disease progression is inconsistent. Nonetheless, the potential utility of androgen and estrogen therapy for AD is significant, although the mechanisms are not clearly understood [152].
B. Parkinson Disease
Parkinson disease (PD) is the second most common neurodegenerative disease after AD and is mainly characterized by a progressive and selective depletion of dopamine neurons in the substantia nigra [153]. The selective degeneration of the dopamine neurons in the pars compacta of the substantia nigra and the progressive buildup of intracellular alpha-synuclein protein deposits and Lewy bodies [154-156] are recognized as the crucial features of the pathology [157]. The classical motor signs including rest tremors, bradykinesia, rigidity, and postural instability are accompanied by a variety of nonmotor symptoms such as cognitive decline and neuropsychiatric deficits, sleep, autonomic and sensory problems that deeply affect the quality of life [157, 158]. Only in few familial events PD is accounted for by sporadic genetic mutations [159]; however, in most cases, the underlying causes are currently unknown, and a combination of genetic, epigenetic, and environmental risk factors have been proposed as triggers of PD onset [160]. Over the past years, considerable data have come to light concerning the susceptibility to the disease development, the age at onset, and the phenotype of the symptoms that support the existence of a sex difference. Indeed, PD affects males and females differently [161, 162]. Numerous reports have proposed a lower incidence and more benign phenotype in women, which appear to be mediated by neuroprotective effects of estrogens and genetic factors [161-164].
In PD, neuropathology is characterized by the reduction of dopaminergic, cholinergic, and other nondopaminergic neurotransmitters, with consequent neuron atrophy in the hippocampus and brain cortex [165]. In patients with PD, dementia is particularly prevalent with advancing age and is responsible for higher morbidity and mortality. The pathogenesis of PD is not clearly explained, but recently, the role of insulin resistance has been proposed [166, 167], suggesting a correlation between the alteration of glucose metabolism and neurodegeneration. The mechanisms of steroid hormone actions are complex and multifaceted.
PD is substantially an idiopathic, multifactorial disease caused by the interplay between genetic and environmental factors. Genetic studies have identified increasing numbers of risk polymorphisms. Heinzel et al recently highlighted the sex-related differences in prodromal PD. They concluded that women and men show distinctive prodromal markers of PD (subthreshold parkinsonism, constipation, olfactory loss, depression, probable rapid eye movement sleep behavior disorder, nonsmoking, abstinence from caffeine, nigral hyperechogenicity), suggesting that these differences should be taken into account in order to guarantee the diagnostic accuracy of PD [168]. Sex hormones (estrogens, androgens) can influence oxidative stress generation, and it is known that estrogens are protective against oxidative stress insults [169]. While the neuroprotective role of estrogens has been studied in many models, potential roles of progesterone have received less attention. This steroid exerts functions in the brain other than reproduction, including neuroprotection, enhanced cognitive function, and stimulation of neurogenesis [170].
In male patients with PD, lower plasma T levels were observed compared with age-matched control individuals [171]. Sex hormones are active in the basal ganglia and dopaminergic neurons and may be involved with different mechanisms in the pathogenesis of PD [172]. In a case report study, it was found that T therapy improved motor symptoms, handwriting, and general well-being [173]. For these reasons, some studies have focused on the therapeutic potential of T and its metabolite DHT, which have been shown, in preclinical settings, to improve cognitive performance [174].
T replacement therapy significantly improved motor and nonmotor symptoms [173, 175]. The impact of androgen deficits in increasing PD risk onset or exacerbating the incidence and/or severity of nonmotor and extrapyramidal symptoms was also observed in men affected by prostate cancer and subjected to androgen deprivation therapy [176, 177]. In these patients, the withdrawal of anticancer therapy reversed the cognitive and motor deficits [178]. However, the androgen protective role in neurodegenerative conditions is still a matter of debate as other studies did not detect a clear association between androgen deprivation therapy and PD [179].
Although the etiology of PD remains elusive, oxidative stress has been linked to PD development and progression [180]. Diminished neurotransmitter level, oxidative stress, mitochondrial dysfunction, and perturbed protein homeostasis over time worsen the disease manifestations in elderly people.
At the moment, no pharmacological breakthrough has been made to protect dopaminergic neurons and associated motor circuitry components, and current management strategies aim to provide symptomatic relief and to retard disease progression.
C. Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune inflammatory disease of the CNS that causes neuronal demyelination with subsequent loss of axonal function and paralysis. MS manifests itself with varied symptoms ranging from loss of vision, to neuromuscular disorders. Sex hormones influence the incidence and progression of MS, and for relapsing-remitting multiple sclerosis (RRMS), a clear predominance of women (ratio 3:1 women to men) has been reported [181].
The neurologic decline in patients with MS is associated with an irreversible degeneration of axons and neurons that have lost their capacity for remyelination [182]. There are 2 clinical variants of MS: the RRMS in 85% of cases and the primary progressive MS [183]. MS starts as a severe immune disease mediated by the CD4+ T-helper 1 cell. However, other cells, such as CD8+ T cells, B cells, macrophages, dendritic cells, astrocytes, and oligodendrocytes [184], are all variously involved in the progression of the disease. The pathogenesis of MS is manifold, and genetic susceptibility cannot in itself explain this autoimmune disease [185]. Although significant advances in immunotherapy have been made, the actual therapy for MS shows few benefits to remediating the pathology [186], and many needs remain unresolved [187].
The brain has the endogenous capability to repair myelin damage, a process depending at least in part on AR activation [188]. In animal models of MS, it has been demonstrated that T exerts a strong stimulation on remyelination (even in cases where spontaneous remyelination would not have been possible [189] with a protective role against the progression of the disease [190]. Furthermore, there is evidence for androgen protective effects in experimental autoimmune encephalomyelitis [190, 191], with immunomodulatory actions mediated by ARs on CD4+ T lymphocytes [42, 45, 192].
Without functional AR, T fails to stimulate remyelination and recruitment of new oligodendrocytes in the chronically demyelinated corpus callosum [193]. Low plasma androgen levels (T, DHEA, or DHEAS) were found both in men and women affected by MS [194-196]. In men with MS at different ages, low plasma levels of T were found in about 40% of patients and correlated with the worst progression of the disease [197]. Also, low T levels in women with MS were associated with tissue damage revealed by magnetic resonance imaging and accompanied by clinical disability [196]. The demyelination process is also a consequence of aging, but myelin regeneration can be restored by neurosteroids [198]. Only a few studies on the effect of androgen therapy in neurodegenerative diseases in humans have been conducted. T therapy for 12 months in patients with MS increased gray matter volume [199], improved cognitive performance [200], and increased the production of BDNF and platelet-derived growth factor BB [201].
Even though some positive effects of T on myelin regeneration have been demonstrated, only a few clinical studies have investigated the impact of androgen therapy in patients with MS, and no definitive conclusions can be drawn from these data.
Myelin regeneration represents the primary therapeutic goal in demyelinating diseases [202]. Although the remyelination process exists naturally in the brain, it is often inadequate in conditions such as MS [202]. The remyelination process is mediated by the oligodendrocyte precursor cells (OPCs), which are widely distributed throughout the adult CNS. Myelin regeneration depends on the efficiency of oligodendrocytes and OPC differentiation [202, 203] that remyelinate the axon. Oligodendrocytes are responsible for the maintenance of healthy axons [204] and neuron survival [205]. In MS, demyelination plays a primary role, but in recent years, it has become increasingly apparent that in the pathology there is also substantial axonal and neuronal loss [202] (Fig. 2). Findings in postmortem brains of patients with MS showed that oligodendrocyte activity never stops, even during the illness, and the presence of new active oligodendrocytes and OPCs in the cortex, compared with controls, was found to be more evident in gray matter than white matter in patients with primary progressive than in those with RRMS [206, 207]. In the early stage of MS, neurons can repair myelin loss, but with the progression of the disease, this ability decreases. Sicotte et al [200] showed that in male patients with RRMS presenting chronically demyelinated brain lesions, T therapy induced a significant improvement in cognitive performance and reduced brain atrophy. Kurth et al [199], in a pilot clinical trial in 10 men with RRMS, demonstrated a neurotrophic effect of T on cerebral gray matter and reduced brain atrophy associated with increased gray matter in the right frontal cortex. These data are of significant importance because cortical lesions and brain atrophy are correlated with mental disorders in RRMS [208]. Both aromatizable and non-aromatizable androgens [209], such as 7-methyl-19-nortestosterone [209] and oxandrolone, effectively stimulated myelin repair [210].
Estrogens contribute substantially to the remyelination process [211], leading to axon repair [212]. The mechanism of neuroprotection is sustained by the activation of ERα and ERβ, which are also implicated in significantly decreasing inflammation in the CNS [213]. Estriol showed a protective action as an immunomodulator in patients with MS [214] and pregnant patients with MS. Estrogen and androgen therapy should be further investigated in future clinical trials as possible adjunctive therapy in MS, given the encouraging results that have been provided [38]. In conclusion, in patients with MS, AR and ER activation may represent important therapeutic strategies for myelin recovery and a viable strategy in future clinical trials.
D. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is characterized by the degeneration of the motor neurons of the corticospinal tract, brainstem, and spinal cord causing progressive muscle atrophy and paralysis [215]. Death results from progressive respiratory failure; the average survival time is 2 to 4 years, and only 10% to 20% of patients survive longer than 10 years [216]. ALS has an incidence of approximately 3.9 to 8 per 100 000 individuals in the United States, and an increase of 69% is expected mainly in developing nations [217]. ALS is a multifactorial interacting disease, but a genetic cause has been identified in approximately 30% of familial ALS cases [218]. Genes and environmental factors may interact in the genesis of ALS [219], but also a viral etiology is gaining increasing credence [220]. Family history, age, and sex are the main risk factors, with a male to female ratio of 1:3 [221]. In the pathogenesis of ALS, the toxicity of mutant SOD1 may be involved in reducing the nuclear protection from the active enzymes [222]. In ALS, motor neurons have metabolic alterations in the mitochondria with a change in lipid oxidation, leading to increased glycolysis, which suggests that metabolic intervention may represent a viable therapeutic strategy [223]. There is increasing interest in the impact of nutrition on the pathogenesis and development of ALS [224, 225]. Lower body mass index is correlated with an increased risk of ALS [226], whereas patients who are overweight and obese have a more prolonged survival [227]. Weight loss in patients with ALS has an adverse prognostic index that may be evident even when patients eat regularly [228]. In ALS, the most suspected risk factors are sports activity, exposure to chemicals, smoking, and nutrition [229, 230]. In patients with ALS, the anabolic condition is reduced and is associated with a low level of free T [231]. Spinal motor neurons degenerate when the production of muscle trophic factors is decreased [232-234]. Alterations in muscle tissue can contribute to increasing axonal vulnerability as trophic muscle factors such as IGF-1 [235] and androgen levels are supportive of muscle maintenance [236]. Interestingly, interactions between androgens and IGF-1 have been described in animal models and in humans [237]. Elevated levels of IGF-1 delay disease progression [238], extend survival time, and decrease disease progression in the ALS mouse model [239]. Muscle atrophy and strength loss are responsible for physical disability in ALS, and androgen therapy should be considered for the treatment of ALS with the aim to stimulate motor neuron recovery from regressive damage and restore normal neuromuscular function [240, 241]. In a murine model of familial ALS, the administration of nandrolone improved the structural preservation of mitochondria in the neuromuscular junction and modestly increased the innervation of diaphragm muscle fibers [242]. However, Galbiati et al [243], in a mouse model of ALS expressing mutant human SOD1, found that nandrolone altered the muscle gene expression and exacerbated the alterations induced by SOD1. Nandrolone administration induced muscle fiber hypertrophy but caused motor neuron death. The activation of endogenous AR protein aggregation revealed the involvement of AR dysfunction in the pathogenesis of ALS [244]. However, in this study, the dose of nandrolone administered was extremely high (10 mg/kg body weight/week), and there was no comparison with the effects of physiologic doses (1 mg/kg body weight/week). Consequently, it is difficult to discern if, in this study, motor neuron death was an artifact of the excessive pharmacologic dose of nandrolone.
The effect of androgens on motor neuron regeneration results from interactions with other hormones. AR has the ability to “crosstalk” with other growth factors, such as IGF-1, as observed in prostate cancer [245]. Therefore, both the evaluation of plasma androgens and IGF-1 levels should be taken into account. In patients with ALS, an appropriate anabolic program including nutrition, exercise, and hormonal therapy may provide positive results [232].
E. Huntington Disease
Huntington disease (HD) is a dominant inherited neurodegenerative disease with a brain pathology located in the basal ganglia with hypothalamic involvement [246]. The neuropathology of HD arises from the expansion of a polymorphic trinucleotide repeat (CAG) in the huntingtin gene [247]. HD is characterized by severe motor dysfunction, chorea, psychosis, and dementia, which have an insidious progression, with a fatal outcome in about 15 to 20 years following onset [248]. Furthermore, it was found that the dysfunction and death of neocortical and basal ganglia neurons responsible for severe motor symptoms, psychosis, and dementia [249] worsened with disease progression and were manifested by progressive cognitive impairment [250]. Various clinical studies have shown that in patients with HD [251-254], symptoms were associated with a progressive alteration of the hypothalamic-pituitary-gonadal axis and changes in neuroendocrine systems [255]. Hypothalamic neurons are reduced in number accompanied by a reduction in gonadotropin-releasing hormone production [256]. Saleh et al [254], evaluated the 5 hypothalamic-pituitary axes in 219 patients with genetically documented HD and found that GH, IGF-1, and cortisol levels were significantly higher than in controls. In contrast, thyroid-stimulating hormone, free triiodothyronine, and T were lower compared with healthy individuals. Endocrine alterations were correlated with the severity of the disease. Markianos et al [250] demonstrated that in male patients with HD, hypothalamic dysfunction caused hypogonadism and low T levels. Interestingly, it was demonstrated that patients with HD had a specific testicular pathology with reduced numbers of germ cells and abnormal seminiferous tubule morphology [257]. This testicular atrophy, characterized by low plasma T levels and reduced spermatogenesis, is negatively correlated with the CAG expansion tract, a direct toxic effect of mutant AR [257]. In R6/HD mice, T administration rescued AR density and functionality in the dentate gyrus [249] and in testes improved testicular function and spermatogenesis [258]. Considering that in patients with HD, therapies are symptomatic and limited to antidopaminergic neuroleptics and antidepressant drugs to regulate motor dysfunctions and mood disorders [259, 260], androgen therapy may represent a valuable tool not yet explored. At this time, there is no documented evidence arising from human clinical trials of T treatment of patients with HD. In light of the emerging evidence of neuroendocrine compromise in males affected with HD, the potential benefits associated with T replacement therapy suggest a novel and promising therapeutic paradigm [249]. Recently, the effects of gene therapy for HD has been evaluated in vitro using primary fibroblasts from patients with HD; it consisted of excision of the excessive CAG repeat tract in the huntingtin gene and its repair using the CRISPR-Cas9n method with insertion of a correct DNA sequence [261]. If this gene therapy is successful, it will open the horizon for new therapeutic approaches to HD in humans.
Conclusions
In animal models and in humans, the potent neuroprotective and trophic effects exerted by androgens have been demonstrated. Low serum T levels represent a predisposing risk factor involved in various neurodegenerative diseases. Androgens could play an essential role in the prevention and treatment of neurodegenerative diseases because of their anti-inflammatory effects, which include the inhibition of proinflammatory cytokines production [262], protection from oxidative stress (thereby increasing Cu-Zn SOD activity in adipocytes [263], and modulation of Aβ formation). However, clinical studies conducted in patients with AD have provided conflicting results.
Better prospective long-term studies are necessary to assess the correlation between androgen deprivation and dementia; confounding factors, such as chronic pain or other treatments such as chemotherapy, may interfere with the selection of appropriate candidates.
In preclinical models of the neurodegenerative diseases considered above, androgens have been shown to exert an essential role in remyelination and axon regeneration; consequently, future clinical studies should better investigate androgen therapy as a promising avenue of treatment for demyelinating diseases and the aging process, both in male and female patients [198]. In women, androgen therapy at low doses is well tolerated and appears to be safe in the short term [264], but long-term treatment should be carefully monitored [265, 266]. Considering the limited number of clinical trials published, no definitive conclusion can be drawn on the effect of androgens on neurodegenerative diseases. Accordingly, new clinical trials will require (a) large numbers of patients, (b) prolonged therapeutic durations, (c) appropriate and comprehensive brain imaging, coupled with (d) accurate and cumulative assessment of cognitive function.
Glossary
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- AR
androgen receptor
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- CAG
cytosine-adenine-guanine
- CNS
central nervous system
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- DHT
dihydrotestosterone
- DNA
deoxyribonucleic acid
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- GABA
gamma-aminobutyric acid
- GH
growth hormone
- HD
Huntington disease
- IGF
insulin-like growth factor
- MAPK
mitogen-activated protein kinase
- mAR
membrane AR
- mRNA
messenger ribonucleic acid
- MS
multiple sclerosis
- NMDA
N-methyl-D-aspartic acid
- OPC
oligodendrocyte precursor cell
- PD
Parkinson disease
- polyQ
polyglutamine
- RRMS
relapsing-remitting MS
- SBMA
spinal and bulbar muscle atrophy
- T
testosterone
- VEGF
vascular endothelial growth factor
Additional Information
Disclosure Summary: The authors have nothing to disclose and no conflicts of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Lardenoije R, van den Hove DLA, Havermans M, et al. Age-related epigenetic changes in hippocampal subregions of four animal models of Alzheimer’s disease. Mol Cell Neurosci. 2018;86:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin Prince A. World Alzheimer report 2015 the global impact of dementia an analysis of prevalence, incidence, cost and trends. 2015. https://www.alz.co.uk/sites/default/files/conf2016/pl12-martin-prince-the-global-impact-of-dementia.pdf. [Google Scholar]
- 3. Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34(9):989-998. [DOI] [PubMed] [Google Scholar]
- 5. Elbaz A, Bower JH, Maraganore DM, et al. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55(1):25-31. [DOI] [PubMed] [Google Scholar]
- 6. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kische H, Gross S, Wallaschofski H, et al. Associations of androgens with depressive symptoms and cognitive status in the general population. PLoS One. 2017;12(5):e0177272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5(4):267-269. [DOI] [PubMed] [Google Scholar]
- 9. Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29(2):169-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benten WP, Lieberherr M, Sekeris CE, Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407(2):211-214. [DOI] [PubMed] [Google Scholar]
- 11. Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285(51):39953-39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas P. Membrane androgen receptors unrelated to nuclear steroid receptors. Endocrinology. 2019;160(4):772-781. [DOI] [PubMed] [Google Scholar]
- 14. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knapczyk-Stwora K, Grzesiak M, Duda M, Koziorowski M, Slomczynska M. Effect of flutamide on folliculogenesis in the fetal porcine ovary–regulation by Kit ligand/c-Kit and IGF1/IGF1R systems. Anim Reprod Sci. 2013;142(3-4):160-167. [DOI] [PubMed] [Google Scholar]
- 16. Lee SH, Johnson D, Luong R, Sun Z. Crosstalking between androgen and PI3K/AKT signaling pathways in prostate cancer cells. J Biol Chem. 2015;290(5):2759-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kretzschmar K, Cottle DL, Schweiger PJ, Watt FM. The androgen receptor antagonizes Wnt/β-catenin signaling in epidermal stem cells. J Invest Dermatol. 2015;135(11):2753-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarulli GA, Butler LM, Tilley WD, Hickey TE. Bringing androgens up a NOTCH in breast cancer. Endocr Relat Cancer. 2014;21(4):T183-T202. [DOI] [PubMed] [Google Scholar]
- 19. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413. [DOI] [PubMed] [Google Scholar]
- 20. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589-598. [DOI] [PubMed] [Google Scholar]
- 21. Atwi S, McMahon D, Scharfman H, MacLusky NJ. Androgen modulation of hippocampal structure and function. Neuroscientist. 2016;22(1):46-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57(1):80-88. [DOI] [PubMed] [Google Scholar]
- 23. Morley JE. Androgens and aging. Maturitas. 2001;38(1):61-71; discussion 71. [DOI] [PubMed] [Google Scholar]
- 24. Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292(12):1431-1432. [DOI] [PubMed] [Google Scholar]
- 25. De Marinis E, Fiocchetti M, Acconcia F, Ascenzi P, Marino M. Neuroglobin upregulation induced by 17β-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013;4:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gürer B, Kertmen H, Kasim E, et al. Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury. 2015;46(2):240-248. [DOI] [PubMed] [Google Scholar]
- 27. Toro-Urrego N, Garcia-Segura LM, Echeverria V, Barreto GE. Testosterone protects mitochondrial function and regulates neuroglobin expression in astrocytic cells exposed to glucose deprivation. Front Aging Neurosci. 2016;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122(3):573-578. [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997;44(4):539-547. [DOI] [PubMed] [Google Scholar]
- 30. Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Brain Res Dev Brain Res. 1997;98(1):74-81. [DOI] [PubMed] [Google Scholar]
- 32. Marron TU, Guerini V, Rusmini P, et al. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92(1):10-20. [DOI] [PubMed] [Google Scholar]
- 33. Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25(10):3039-3046. [DOI] [PubMed] [Google Scholar]
- 34. Zárate S, Stevnsner T, Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci. 2017;9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meydan S, Kus I, Tas U, et al. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J Chem Neuroanat. 2010;40(4):281-285. [DOI] [PubMed] [Google Scholar]
- 36. Son SW, Lee JS, Kim HG, Kim DW, Ahn YC, Son CG. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J Neurochem. 2016;136(1):106-117. [DOI] [PubMed] [Google Scholar]
- 37. Ota H, Akishita M, Akiyoshi T, et al. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PLoS One. 2012;7(1):e29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. 2009;175:239-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khasnavis S, Ghosh A, Roy A, Pahan K. Castration induces Parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem. 2013;288(29):20843-20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed). 2012;4:976-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Orlando R, Caruso A, Molinaro G, et al. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21-29. [DOI] [PubMed] [Google Scholar]
- 42. Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;146(1-2):144-152. [DOI] [PubMed] [Google Scholar]
- 43. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313-3318. [DOI] [PubMed] [Google Scholar]
- 44. Page ST, Plymate SR, Bremner WJ, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290(5):E856-E863. [DOI] [PubMed] [Google Scholar]
- 45. Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060-2067. [DOI] [PubMed] [Google Scholar]
- 46. Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153(9):4111-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okamoto N, Yamanaka N. Steroid hormone entry into the brain requires a membrane transporter in Drosophila. Curr Biol. 2020;30(2):359-366.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Green SM, Kaipainen A, Bullock K, et al. Role of OATP transporters in steroid uptake by prostate cancer cells in vivo. Prostate Cancer Prostatic Dis. 2017;20(1):20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Uhr M, Holsboer F, Müller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14(9):753-759. [DOI] [PubMed] [Google Scholar]
- 50. Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37(3):395-403. [DOI] [PubMed] [Google Scholar]
- 51. Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S59-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sedlácek M, Korínek M, Petrovic M, et al. Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol Res. 2008;57(Suppl 3):S49-S57. [DOI] [PubMed] [Google Scholar]
- 53. Monnet FP, Mahé V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci U S A. 1995;92(9):3774-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48(1):14-24. [DOI] [PubMed] [Google Scholar]
- 55. Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55-77. [DOI] [PubMed] [Google Scholar]
- 57. Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003;15(3-4):197-234. [DOI] [PubMed] [Google Scholar]
- 59. Marx CE, Stevens RD, Shampine LJ, et al. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31(6):1249-1263. [DOI] [PubMed] [Google Scholar]
- 60. Palma E, Ruffolo G, Cifelli P, Roseti C, Vliet EAV, Aronica E. Modulation of GABAA receptors in the treatment of epilepsy. Curr Pharm Des. 2017;23(37):5563-5568. [DOI] [PubMed] [Google Scholar]
- 61. Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15(1):79-94. [DOI] [PubMed] [Google Scholar]
- 62. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863-870. [DOI] [PubMed] [Google Scholar]
- 63. Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334(3):1031-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chakrabarti M, Das A, Samantaray S, et al. Molecular mechanisms of estrogen for neuroprotection in spinal cord injury and traumatic brain injury. Rev Neurosci. 2016;27(3):271-281. [DOI] [PubMed] [Google Scholar]
- 65. Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol. 2015;145:254-260. [DOI] [PubMed] [Google Scholar]
- 66. Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009;30(1):65-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Greaves RF, Wudy SA, Badoer E, et al. A tale of two steroids: the importance of the androgens DHEA and DHEAS for early neurodevelopment. J Steroid Biochem Mol Biol. 2019;188:77-85. [DOI] [PubMed] [Google Scholar]
- 68. Farooqi NAI, Scotti M, Lew JM, et al. Role of DHEA and cortisol in prefrontal-amygdalar development and working memory. Psychoneuroendocrinology. 2018;98:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. do Vale S, Selinger L, Martins JM, et al. The relationship between dehydroepiandrosterone (DHEA), working memory and distraction–a behavioral and electrophysiological approach. PLoS One. 2014;9(8):e104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Newcomer JW, Farber NB, Olney JW. NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci. 2000;2(3):219-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Locklear MN, Bhamidipaty S, Kritzer MF. Local N-methyl-d-aspartate receptor antagonism in the prefrontal cortex attenuates spatial cognitive deficits induced by gonadectomy in adult male rats. Neuroscience. 2015;288:73-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maggio M, De Vita F, Fisichella A, et al. DHEA and cognitive function in the elderly. J Steroid Biochem Mol Biol. 2015;145:281-292. [DOI] [PubMed] [Google Scholar]
- 73. Huppert FA, Van Niekerk JK. WITHDRAWN: Dehydroepiandrosterone (DHEA) supplementation for cognitive function. Cochrane Database Syst Rev. 2007;(2):CD000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J Comp Neurol. 1976;170(3):279-293. [DOI] [PubMed] [Google Scholar]
- 75. Sar M, Stumpf WE. Androgen concentration in motor neurons of cranial nerves and spinal cord. Science. 1977;197(4298):77-79. [DOI] [PubMed] [Google Scholar]
- 76. Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151(2):121-158. [DOI] [PubMed] [Google Scholar]
- 77. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76-95. [DOI] [PubMed] [Google Scholar]
- 78. Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213-3221. [DOI] [PubMed] [Google Scholar]
- 79. DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144(8):3632-3638. [DOI] [PubMed] [Google Scholar]
- 80. Thomas PS Jr, Fraley GS, Damian V, et al. Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy. Hum Mol Genet. 2006;15(14):2225-2238. [DOI] [PubMed] [Google Scholar]
- 81. Lieberman AP, Harmison G, Strand AD, Olson JM, Fischbeck KH. Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum Mol Genet. 2002;11(17):1967-1976. [DOI] [PubMed] [Google Scholar]
- 82. Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70(3):1054-1060. [DOI] [PubMed] [Google Scholar]
- 83. Cary GA, La Spada AR. Androgen receptor function in motor neuron survival and degeneration. Phys Med Rehabil Clin N Am. 2008;19(3):479-494, viii. [DOI] [PubMed] [Google Scholar]
- 84. Perera ND, Sheean RK, Crouch PJ, White AR, Horne MK, Turner BJ. Enhancing survival motor neuron expression extends lifespan and attenuates neurodegeneration in mutant TDP-43 mice. Hum Mol Genet. 2016;25(18):4080-4093. [DOI] [PubMed] [Google Scholar]
- 85. Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77(5):1319-1326. [DOI] [PubMed] [Google Scholar]
- 86. Wright AF, Goedert M, Hastie ND. Familial Alzheimer’s disease. Beta amyloid resurrected. Nature. 1991;349(6311):653-654. [DOI] [PubMed] [Google Scholar]
- 87. Chisu V, Manca P, Lepore G, Gadau S, Zedda M, Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch Ital Biol. 2006;144(2):63-73. [PubMed] [Google Scholar]
- 88. Gouras GK, Xu H, Gross RS, et al. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97(3):1202-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24(23):5315-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Louissaint A Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945-960. [DOI] [PubMed] [Google Scholar]
- 91. Wu Y, Zhao W, Zhao J, et al. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology. 2007;148(6):2984-2993. [DOI] [PubMed] [Google Scholar]
- 92. Fanaei H, Karimian SM, Sadeghipour HR, et al. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014;1558:74-83. [DOI] [PubMed] [Google Scholar]
- 93. Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76-84. [DOI] [PubMed] [Google Scholar]
- 94. Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151(1):253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gao S, Chen S, Chen L, et al. Brain-derived neurotrophic factor: a steroidogenic regulator of Leydig cells. J Cell Physiol. 2019;234(8):14058-14067. [DOI] [PubMed] [Google Scholar]
- 96. Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94(6):1639-1651. [DOI] [PubMed] [Google Scholar]
- 97. Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671-680. [DOI] [PubMed] [Google Scholar]
- 98. La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77-79. [DOI] [PubMed] [Google Scholar]
- 99. Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575-621. [DOI] [PubMed] [Google Scholar]
- 100. Orr CR, Montie HL, Liu Y, et al. An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy. J Biol Chem. 2010;285(46):35567-35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pinsky L, Trifiro M, Kaufman M, et al. Androgen resistance due to mutation of the androgen receptor. Clin Invest Med. 1992;15(5):456-472. [PubMed] [Google Scholar]
- 102. Chevalier-Larsen ES, O’Brien CJ, Wang H, et al. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24(20):4778-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Katsuno M, Adachi H, Kume A, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35(5):843-854. [DOI] [PubMed] [Google Scholar]
- 104. Katsuno M, Banno H, Suzuki K, et al. ; Japan SBMA Interventional Trial for TAP-144-SR (JASMITT) study group Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(9):875-884. [DOI] [PubMed] [Google Scholar]
- 105. Halievski K, Nath SR, Katsuno M, et al. Disease affects Bdnf expression in synaptic and extrasynaptic regions of skeletal muscle of three SBMA mouse models. Int J Mol Sci. 2019;20(6):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Katsuno M, Adachi H, Minamiyama M, et al. Disrupted transforming growth factor-beta signaling in spinal and bulbar muscular atrophy. J Neurosci. 2010;30(16):5702-5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Palazzolo I, Stack C, Kong L, et al. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63(3):316-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(8):a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Niu H, Alvarez-Alvarez I, Guillen-Grima F, Al-Rahamneh MJ, Aguinaga-Ontoso I. Trends of mortality from Alzheimer’s disease in the European Union, 1994-2013. Eur J Neurol. 2017;24(6):858-866. [DOI] [PubMed] [Google Scholar]
- 111. Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73(4):371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Droogsma E, van Asselt D, De Deyn PP. Weight loss and undernutrition in community-dwelling patients with Alzheimer’s dementia: from population based studies to clinical management. Z Gerontol Geriatr. 2015;48(4):318-324. [DOI] [PubMed] [Google Scholar]
- 113. Lv W, Du N, Liu Y, et al. Low testosterone level and risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Mol Neurobiol. 2016;53(4):2679-2684. [DOI] [PubMed] [Google Scholar]
- 114. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32(4):604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee JH, Byun MS, Yi D, et al. ; KBASE Research Group Sex-specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol Aging. 2017;58:34-40. [DOI] [PubMed] [Google Scholar]
- 116. Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919(1):160-165. [DOI] [PubMed] [Google Scholar]
- 117. Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115(5):1121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vassar R, Kuhn PH, Haass C, et al. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem. 2014;130(1):4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cervellati C, Trentini A, Rosta V, et al. Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. Geroscience. 2020;42(1):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Matsui T, Ingelsson M, Fukumoto H, et al. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116-123. [DOI] [PubMed] [Google Scholar]
- 123. Hajjari SN, Mehdizadeh M, Sadigh-Eteghad S, Shanehbandi D, Teimourian S, Baradaran B. Secretases-related miRNAs in Alzheimer’s disease: new approach for biomarker discovery. Neurol Sci. 2017;38(11):1921-1926. [DOI] [PubMed] [Google Scholar]
- 124. Preece P, Virley DJ, Costandi M, et al. Amyloid precursor protein mRNA levels in Alzheimer’s disease brain. Brain Res Mol Brain Res. 2004;122(1):1-9. [DOI] [PubMed] [Google Scholar]
- 125. Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53(5):693-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Verdile G, Laws SM, Henley D, et al. ; AIBL Research Group Associations between gonadotropins, testosterone and β amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry. 2014;19(1):69-75. [DOI] [PubMed] [Google Scholar]
- 128. Gandy S, Almeida OP, Fonte J, et al. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285(17):2195-2196. [DOI] [PubMed] [Google Scholar]
- 129. Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29(8):1071-1081. [DOI] [PubMed] [Google Scholar]
- 130. Lei Y, Renyuan Z. Effects of androgens on the amyloid beta protein in Alzheimer’s disease. Endocrinology. 2018;159(12):3885-3894. [DOI] [PubMed] [Google Scholar]
- 131. Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res Rev. 2008;57(2):444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett. 2000;296(1):49-52. [DOI] [PubMed] [Google Scholar]
- 133. Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y. Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol. 2013;47(3):857-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thakur MK, Mani ST. Estradiol regulates APP mRNA alternative splicing in the mice brain cortex. Neurosci Lett. 2005;381(1-2):154-157. [DOI] [PubMed] [Google Scholar]
- 135. Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268(15):4285-4291. [DOI] [PubMed] [Google Scholar]
- 136. Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ. Androgens regulate neprilysin expression: role in reducing beta-amyloid levels. J Neurochem. 2008;105(6):2477-2488. [DOI] [PubMed] [Google Scholar]
- 137. McAllister C, Long J, Bowers A, et al. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30(21):7326-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Grimm A, Biliouris EE, Lang UE, Götz J, Mensah-Nyagan AG, Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell Mol Life Sci. 2016;73(1):201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mao X, Barger SW. Neuroprotection by dehydroepiandrosterone-sulfate: role of an NFkappaB-like factor. Neuroreport. 1998;9(4):759-763. [DOI] [PubMed] [Google Scholar]
- 140. Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. Proc Soc Exp Biol Med. 1999;222(2):145-149. [DOI] [PubMed] [Google Scholar]
- 141. Kaasik A, Kalda A, Jaako K, Zharkovsky A. Dehydroepiandrosterone sulphate prevents oxygen-glucose deprivation-induced injury in cerebellar granule cell culture. Neuroscience. 2001;102(2):427-432. [DOI] [PubMed] [Google Scholar]
- 142. Wahjoepramono EJ, Asih PR, Aniwiyanti V, et al. The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol Disord Drug Targets. 2016;15(3):337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6(1):13-17. [PubMed] [Google Scholar]
- 144. Sun M, Cole AP, Hanna N, et al. Cognitive impairment in men with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol. 2018;199(6):1417-1425. [DOI] [PubMed] [Google Scholar]
- 145. Deka R, Simpson DR, Bryant AK, et al. Association of androgen deprivation therapy with dementia in men with prostate cancer who receive definitive radiation therapy. JAMA Oncol. 2018;4(11):1616-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA. 2017;317(7):717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Buskbjerg CR, Gravholt CH, Dalby HR, Amidi A, Zachariae R. Testosterone supplementation and cognitive functioning in men-a systematic review and meta-analysis. J Endocr Soc. 2019;3(8):1465-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Huang G, Wharton W, Bhasin S, et al. Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. 2016;4(8):657-665. [DOI] [PubMed] [Google Scholar]
- 149. Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci. 2017;18(11):2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bowen RL, Smith MA, Harris PL, et al. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. J Neurosci Res. 2002;70(3):514-518. [DOI] [PubMed] [Google Scholar]
- 151. Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76(9):906-909. [DOI] [PubMed] [Google Scholar]
- 152. Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hornykiewicz O. Ageing and neurotoxins as causative factors in idiopathic Parkinson’s disease–a critical analysis of the neurochemical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(3-4):319-328. [DOI] [PubMed] [Google Scholar]
- 154. Lang AE. The progression of Parkinson disease: a hypothesis. Neurology. 2007;68(12):948-952. [DOI] [PubMed] [Google Scholar]
- 155. Siderowf A, Stern M. Update on Parkinson disease. Ann Intern Med. 2003;138(8):651-658. [DOI] [PubMed] [Google Scholar]
- 156. Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13-24. [DOI] [PubMed] [Google Scholar]
- 157. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896-912. [DOI] [PubMed] [Google Scholar]
- 158. Park A, Stacy M. Non-motor symptoms in Parkinson’s disease. J Neurol. 2009;256(Suppl 3):293-298. [DOI] [PubMed] [Google Scholar]
- 159. Olanow CW, Kordower JH. Modeling Parkinson’s disease. Ann Neurol. 2009;66(4):432-436. [DOI] [PubMed] [Google Scholar]
- 160. Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48-R59. [DOI] [PubMed] [Google Scholar]
- 161. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Heller J, Mirzazade S, Romanzetti S, et al. Impact of gender and genetics on emotion processing in Parkinson’s disease - a multimodal study. Neuroimage Clin. 2018;18:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):905-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Burn DJ. Sex and Parkinson’s disease: a world of difference? J Neurol Neurosurg Psychiatry. 2007;78(8):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60(12):1745-1748. [DOI] [PubMed] [Google Scholar]
- 166. Ashraghi MR, Pagano G, Polychronis S, Niccolini F, Politis M. Parkinson’s disease, diabetes and cognitive impairment. Recent Pat Endocr Metab Immune Drug Discov. 2016;10(1):11-21. [DOI] [PubMed] [Google Scholar]
- 167. Bosco D, Plastino M, Cristiano D, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315(1-2):39-43. [DOI] [PubMed] [Google Scholar]
- 168. Heinzel S, Kasten M, Behnke S, et al. Age- and sex-related heterogeneity in prodromal Parkinson’s disease. Mov Disord. 2018;33(6):1025-1027. [DOI] [PubMed] [Google Scholar]
- 169. Regan JC, Partridge L. Gender and longevity: why do men die earlier than women? Comparative and experimental evidence. Best Pract Res Clin Endocrinol Metab. 2013;27(4):467-479. [DOI] [PubMed] [Google Scholar]
- 170. Brinton RD, Thompson RF, Foy MR, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Okun MS, Crucian GP, Fischer L, et al. Testosterone deficiency in a Parkinson’s disease clinic: results of a survey. J Neurol Neurosurg Psychiatry. 2004;75(1):165-166. [PMC free article] [PubMed] [Google Scholar]
- 172. Smith KM, Dahodwala N. Sex differences in Parkinson’s disease and other movement disorders. Exp Neurol. 2014;259:44-56. [DOI] [PubMed] [Google Scholar]
- 173. Mitchell E, Thomas D, Burnet R. Testosterone improves motor function in Parkinson’s disease. J Clin Neurosci. 2006;13(1):133-136. [DOI] [PubMed] [Google Scholar]
- 174. Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci. 2006;26(51):13384-13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Okun MS, McDonald WM, DeLong MR. Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol. 2002;59(5):807-811. [DOI] [PubMed] [Google Scholar]
- 176. Elshaikh MA, Underwood W, Soto DE. Androgen deprivation therapy for patients with prostate carcinoma and Parkinson’s disease: case report and review of literature. Can J Urol. 2009;16(1):4495-4497. [PubMed] [Google Scholar]
- 177. Young JWS, Sutradhar R, Rangrej J, Marras C, Fleshner N, Alibhai SMH. Androgen deprivation therapy and the risk of parkinsonism in men with prostate cancer. World J Urol. 2017;35(9):1417-1423. [DOI] [PubMed] [Google Scholar]
- 178. Abbate C, Caputo L, Damanti S, et al. Reversible Parkinson’s dementia associated with withdrawal of androgen-deprivation therapy for prostate cancer. J Am Geriatr Soc. 2016;64(10):e115-e117. [DOI] [PubMed] [Google Scholar]
- 179. Chung SD, Lin HC, Tsai MC, Kao LT, Huang CY, Chen KC. Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology. 2016;4(3):481-485. [DOI] [PubMed] [Google Scholar]
- 180. Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kalincik T, Vivek V, Jokubaitis V, et al. ; MSBase Study Group Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609-3617. [DOI] [PubMed] [Google Scholar]
- 182. Kotelnikova E, Kiani NA, Abad E, et al. Dynamics and heterogeneity of brain damage in multiple sclerosis. PLoS Comput Biol. 2017;13(10):e1005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913-919. [DOI] [PubMed] [Google Scholar]
- 185. Wu H, Zhao M, Yoshimura A, Chang C, Lu Q. Critical link between epigenetics and transcription factors in the induction of autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2016;50(3):333-344. [DOI] [PubMed] [Google Scholar]
- 186. Gholamzad M, Ebtekar M, Ardestani MS, et al. A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res. 2019;68(1):25-38. [DOI] [PubMed] [Google Scholar]
- 187. Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225-240. [DOI] [PubMed] [Google Scholar]
- 188. Bielecki B, Mattern C, Ghoumari AM, et al. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc Natl Acad Sci U S A. 2016;113(51):14829-14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159(1):3-6. [PubMed] [Google Scholar]
- 191. Bebo BF Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162(1):35-40. [PubMed] [Google Scholar]
- 192. Giatti S, Rigolio R, Romano S, et al. Dihydrotestosterone as a protective agent in chronic experimental autoimmune encephalomyelitis. Neuroendocrinology. 2015;101(4):296-308. [DOI] [PubMed] [Google Scholar]
- 193. Hussain R, Ghoumari AM, Bielecki B, et al. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136(Pt 1): 132-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Foster SC, Daniels C, Bourdette DN, Bebo BF Jr. Dysregulation of the hypothalamic-pituitary-gonadal axis in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol. 2003;140(1-2):78-87. [DOI] [PubMed] [Google Scholar]
- 195. Téllez N, Comabella M, Julià E, et al. Fatigue in progressive multiple sclerosis is associated with low levels of dehydroepiandrosterone. Mult Scler. 2006;12(4):487-494. [DOI] [PubMed] [Google Scholar]
- 196. Tomassini V, Onesti E, Mainero C, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76(2):272-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chitnis T. The role of testosterone in MS risk and course. Mult Scler. 2018;24(1):36-41. [DOI] [PubMed] [Google Scholar]
- 198. Ibanez C, Shields SA, El-Etr M, et al. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog Neurobiol. 2003;71(1):49-56. [DOI] [PubMed] [Google Scholar]
- 199. Kurth F, Luders E, Sicotte NL, et al. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 2014;4:454-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sicotte NL, Giesser BS, Tandon V, et al. Testosterone treatment in multiple sclerosis: a pilot study. Arch Neurol. 2007;64(5):683-688. [DOI] [PubMed] [Google Scholar]
- 201. Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation. 2008;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839-855. [DOI] [PubMed] [Google Scholar]
- 203. Piaton G, Williams A, Seilhean D, Lubetzki C. Remyelination in multiple sclerosis. Prog Brain Res. 2009;175:453-464. [DOI] [PubMed] [Google Scholar]
- 204. Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333(6049):1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chang A, Staugaitis SM, Dutta R, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72(6):918-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Strijbis EMM, Kooi EJ, van der Valk P, Geurts JJG. Cortical remyelination is heterogeneous in multiple sclerosis. J Neuropathol Exp Neurol. 2017;76(5):390-401. [DOI] [PubMed] [Google Scholar]
- 208. Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144-1150. [DOI] [PubMed] [Google Scholar]
- 209. Anderson RA, Wallace AM, Sattar N, Kumar N, Sundaram K. Evidence for tissue selectivity of the synthetic androgen 7 alpha-methyl-19-nortestosterone in hypogonadal men. J Clin Endocrinol Metab. 2003;88(6):2784-2793. [DOI] [PubMed] [Google Scholar]
- 210. Bianchi V, Marbini A. Neuroregenerative effect of oxandrolone: a case report. Am J Case Rep. 2015;16:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Raghava N, Das BC, Ray SK. Neuroprotective effects of estrogen in CNS injuries: insights from animal models. Neurosci Neuroecon. 2017;6:15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Collongues N, Patte-Mensah C, De Seze J, Mensah-Nyagan AG, Derfuss T. Testosterone and estrogen in multiple sclerosis: from pathophysiology to therapeutics. Expert Rev Neurother. 2018;18(6):515-522. [DOI] [PubMed] [Google Scholar]
- 213. Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29-60. [DOI] [PubMed] [Google Scholar]
- 214. Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171(11):6267-6274. [DOI] [PubMed] [Google Scholar]
- 215. Oh J, An JW, Oh SI, et al. Socioeconomic costs of amyotrophic lateral sclerosis according to staging system. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):202-208. [DOI] [PubMed] [Google Scholar]
- 216. del Aguila MA, Longstreth WT Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813-819. [DOI] [PubMed] [Google Scholar]
- 217. Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016;7:12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):616-630. [DOI] [PubMed] [Google Scholar]
- 219. Riancho J, Bosque-Varela P, Perez-Pereda S, Povedano M, de Munaín AL, Santurtun A. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. Int J Biometeorol. 2018;62(8):1361-1374. [DOI] [PubMed] [Google Scholar]
- 220. Celeste DB, Miller MS. Reviewing the evidence for viruses as environmental risk factors for ALS: a new perspective. Cytokine. 2018;108:173-178. [DOI] [PubMed] [Google Scholar]
- 221. Manjaly ZR, Scott KM, Abhinav K, et al. The sex ratio in amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler. 2010;11(5):439-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sau D, De Biasi S, Vitellaro-Zuccarello L, et al. Mutation of SOD1 in ALS: a gain of a loss of function. Hum Mol Genet. 2007;16(13):1604-1618. [DOI] [PubMed] [Google Scholar]
- 223. Joardar A, Manzo E, Zarnescu DC. Metabolic dysregulation in amyotrophic lateral sclerosis: challenges and opportunities. Curr Genet Med Rep. 2017;5(2):108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Okamoto K, Kihira T, Kondo T, et al. Nutritional status and risk of amyotrophic lateral sclerosis in Japan. Amyotroph Lateral Scler. 2007;8(5):300-304. [DOI] [PubMed] [Google Scholar]
- 226. O’Reilly ÉJ, Wang H, Weisskopf MG, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(1):20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ahmed RM, Caga J, Devenney E, et al. Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J Neurol. 2016;263(8):1593-1603. [DOI] [PubMed] [Google Scholar]
- 229. Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Arch Neurol. 2000;57(8):1171-1176. [DOI] [PubMed] [Google Scholar]
- 230. Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14(4):248-264. [DOI] [PubMed] [Google Scholar]
- 231. Militello A, Vitello G, Lunetta C, et al. The serum level of free testosterone is reduced in amyotrophic lateral sclerosis. J Neurol Sci. 2002;195(1):67-70. [DOI] [PubMed] [Google Scholar]
- 232. Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57(5):649-655. [DOI] [PubMed] [Google Scholar]
- 233. Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53(6):804-807. [DOI] [PubMed] [Google Scholar]
- 234. Deforges S, Branchu J, Biondi O, et al. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2009;587(Pt 14):3561-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Horm Res. 2004;62(Suppl 1):50-58. [DOI] [PubMed] [Google Scholar]
- 236. Serra C, Bhasin S, Tangherlini F, et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152(1):193-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7(6):591-604. [DOI] [PubMed] [Google Scholar]
- 238. Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839-842. [DOI] [PubMed] [Google Scholar]
- 239. Wen D, Cui C, Duan W, et al. The role of insulin-like growth factor 1 in ALS cell and mouse models: a mitochondrial protector. Brain Res Bull. 2019;144:1-13. [DOI] [PubMed] [Google Scholar]
- 240. Fargo KN, Foecking EM, Jones KJ, Sengelaub DR. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol. 2009;30(2):130-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Fargo KN, Galbiati M, Foecking EM, Poletti A, Jones KJ. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008;53(5):716-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Cappello V, Vezzoli E, Righi M, et al. Analysis of neuromuscular junctions and effects of anabolic steroid administration in the SOD1G93A mouse model of ALS. Mol Cell Neurosci. 2012;51(1-2):12-21. [DOI] [PubMed] [Google Scholar]
- 243. Galbiati M, Onesto E, Zito A, et al. The anabolic/androgenic steroid nandrolone exacerbates gene expression modifications induced by mutant SOD1 in muscles of mice models of amyotrophic lateral sclerosis. Pharmacol Res. 2012;65(2):221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Aggarwal T, Polanco MJ, Scaramuzzino C, et al. Androgens affect muscle, motor neuron, and survival in a mouse model of SOD1-related amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35(8):1929-1938. [DOI] [PubMed] [Google Scholar]
- 245. Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15(4):841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kassubek J, Gaus W, Landwehrmeyer GB. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2004;62(3):523-524; author reply 524. [DOI] [PubMed] [Google Scholar]
- 247. Andrew SE, Goldberg YP, Kremer B, et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet. 1993;4(4):398-403. [DOI] [PubMed] [Google Scholar]
- 248. Tabrizi SJ, Scahill RI, Owen G, et al. ; TRACK-HD Investigators Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637-649. [DOI] [PubMed] [Google Scholar]
- 249. Ransome MI. Androgen function in the pathophysiology and treatment of male Huntington’s disease patients. J Neuroendocrinol. 2012;24(10):1275-1283. [DOI] [PubMed] [Google Scholar]
- 250. Markianos M, Panas M, Kalfakis N, Vassilopoulos D. Plasma testosterone in male patients with Huntington’s disease: relations to severity of illness and dementia. Ann Neurol. 2005;57(4):520-525. [DOI] [PubMed] [Google Scholar]
- 251. Aziz NA, Pijl H, Frölich M, van der Graaf AW, Roelfsema F, Roos RA. Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94(4):1223-1228. [DOI] [PubMed] [Google Scholar]
- 252. Aziz NA, Swaab DF, Pijl H, Roos RA. Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington’s disease: clinical consequences and therapeutic implications. Rev Neurosci. 2007;18(3-4):223-251. [DOI] [PubMed] [Google Scholar]
- 253. Heuser IJ, Chase TN, Mouradian MM. The limbic-hypothalamic-pituitary-adrenal axis in Huntington’s disease. Biol Psychiatry. 1991;30(9):943-952. [DOI] [PubMed] [Google Scholar]
- 254. Saleh N, Moutereau S, Durr A, et al. Neuroendocrine disturbances in Huntington’s disease. PLoS One. 2009;4(3):e4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Petersén A, Hult S, Kirik D. Huntington’s disease - new perspectives based on neuroendocrine changes in rodent models. Neurodegener Dis. 2009;6(4):154-164. [DOI] [PubMed] [Google Scholar]
- 256. Papalexi E, Persson A, Björkqvist M, et al. Reduction of GnRH and infertility in the R6/2 mouse model of Huntington’s disease. Eur J Neurosci. 2005;22(6):1541-1546. [DOI] [PubMed] [Google Scholar]
- 257. Van Raamsdonk JM, Murphy Z, Selva DM, et al. Testicular degeneration in Huntington disease. Neurobiol Dis. 2007;26(3):512-520. [DOI] [PubMed] [Google Scholar]
- 258. Hannan AJ, Ransome MI. Deficits in spermatogenesis but not neurogenesis are alleviated by chronic testosterone therapy in R6/1 Huntington’s disease mice. J Neuroendocrinol. 2012;24(2):341-356. [DOI] [PubMed] [Google Scholar]
- 259. Bachoud-Lévi AC, Ferreira J, Massart R, et al. International guidelines for the treatment of Huntington’s disease. Front Neurol. 2019;10:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Venuto CS, McGarry A, Ma Q, Kieburtz K. Pharmacologic approaches to the treatment of Huntington’s disease. Mov Disord. 2012;27(1):31-41. [DOI] [PubMed] [Google Scholar]
- 261. Dabrowska M, Olejniczak M. Gene therapy for Huntington’s disease using targeted endonucleases. Methods Mol Biol. 2020;2056:269-284. [DOI] [PubMed] [Google Scholar]
- 262. Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. 2019;3(1):91-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Pérez-Torres I, Guarner-Lans V, Zúñiga-Muñoz A, et al. Effect of cross-sex hormonal replacement on antioxidant enzymes in rat retroperitoneal fat adipocytes. Oxid Med Cell Longev. 2016;2016:1527873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Seaberg P, Jadoon-Khamash E, Vegunta S. Managing recurrent urinary tract infections in postmenopausal women. J Womens Health (Larchmt). 2018;27(7):856-858. [DOI] [PubMed] [Google Scholar]
- 265. Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10): 3489-3510. [DOI] [PubMed] [Google Scholar]
- 266. Diem SJ, Greer NL, MacDonald R, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020;172(2):105-118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.