Abstract
Sepsis is a leading cause of morbidity and mortality in intensive care units. Previously, we identified Protein Kinase C-delta (PKCδ) as an important regulator of the inflammatory response in sepsis. An important issue in development of anti-inflammatory therapeutics is the risk of immunosuppression and inability to effectively clear pathogens. In this study, we investigated whether PKCδ inhibition prevented organ dysfunction and improved survival without compromising pathogen clearance. Sprague Dawley rats underwent sham surgery or cecal ligation and puncture (CLP) to induce sepsis. Post-surgery, PBS or a PKCδ inhibitor (200μg/kg) was administered intra-tracheally (IT). At 24 hrs post-CLP, there was evidence of lung and kidney dysfunction. PKCδ inhibition decreased leukocyte influx in these organs, decreased endothelial permeability, improved gas exchange, and reduced blood urea nitrogen/Creatinine ratios indicating organ protection. PKCδ inhibition significantly decreased bacterial levels in the peritoneal cavity, spleen and blood but did not exhibit direct bactericidal properties. Peritoneal chemokine levels, neutrophil numbers, or macrophage phenotypes were not altered by PKCδ inhibition. Peritoneal macrophages isolated from PKCδ inhibitor-treated septic rats demonstrated increased bacterial phagocytosis. Importantly, PKCδ inhibition increased survival. Thus, PKCδ inhibition improved survival and improved survival was associated with increased phagocytic activity, enhanced pathogen clearance, and decreased organ injury.
Keywords: inflammation, organ injury, cecal ligation and puncture, macrophages, phagocytosis
Introduction
Sepsis is a clinical syndrome that is now defined as life-threatening organ dysfunction caused by dysregulated host response to infection (1). Sepsis is one of the leading causes of death in ICUs with greater than 250,000 deaths/year in the US despite appropriate antimicrobial therapies (2–4). Sepsis is characterized by an intense systemic inflammatory response that develops in response to pathogen associated molecular patterns that are released during infection. This systemic inflammation activates a cascade of inflammatory events that can damage the vascular endothelium resulting in increased permeability and neutrophil migration into critical organs such as the lung and kidneys. While neutrophils are critical to host defense, neutrophil dysregulation has a critical role in the early course of organ damage through release of proteases, neutrophil extracellular traps, and oxygen radicals. This increased neutrophil recruitment is associated with tissue damage, multiple organ dysfunction syndrome (MODS), and increased mortality (5–8).
To date, therapeutic approaches to the treatment of sepsis are largely supportive and there are no specific pharmacologic therapies available that target the dysregulated host response to infection (2, 9). Immunoregulatory therapies focused on targeting individual mediators, such as specific cytokine therapies, have met with little success in the treatment of sepsis (3, 6, 8, 10). The inflammatory response is composed of multiple overlapping and redundant mechanisms and recent research has shifted the focus to common control signaling points that are activated by diverse signals. We identified Protein Kinase C-delta (PKCδ) as a critical regulator of the inflammatory response (10–20). Inflammatory mediators involved in the septic response including LPS, TNF and IL-1 activate PKCδ (21–23). Studies with PKCδ−/− mice and PKCδ inhibitors indicate a role for PKCδ in regulating neutrophil trafficking to the lung in response to inflammation triggered by asbestos, stroke/reperfusion injury, LPS, or pancreatitis (24–27). Recently, we demonstrated that cecal ligation and puncture (CLP) activated PKCδ in the lungs of septic rats and selective PKCδ inhibition attenuated neutrophil influx into the lung, reduced alveolar-capillary permeability, decreased pulmonary edema, and preserved lung architecture suggesting targeting PKCδ as a potential strategy for preserving lung function and possibly other organs (14–18, 20).
It is now becoming evident that sepsis is not solely the result of excessive activation of the proinflammatory response but is coupled with immune dysfunction and the inability to effectively clear pathogens through impaired phagocytic cell function (3). Thus, an issue in the development of anti-inflammatory therapeutics is the risk of immunosuppression and inability to effectively clear pathogens. To address these issues, we investigated whether PKCδ inhibition provided organ protection and improved survival without compromising pathogen clearance.
Materials and Methods
Reagents
All reagents, analytical grade, were obtained from Thermo Fisher Scientific (Waltham, MA) unless stated otherwise.
PKCδ Inhibitor Peptide Synthesis
As previously described (12–19), PKCδ activity was selectively inhibited by a peptide antagonist that consisted of a peptide derived from the first unique region (V1) of PKCδ (SFNSYELGSL: amino acids 8–17) coupled to a membrane permeant peptide sequence in the HIV TAT gene product (YGRKKRRQRRR: amino acids 47–57 of TAT) (28). Extensive in vitro and in vivo studies demonstrate that, when taken up by cells, the PKCδ TAT peptide produces a unique dominant-negative phenotype that effectively inhibits activation of PKCδ, but not other PKC isotypes (12, 28, 29). Further studies have demonstrated that the TAT peptide alone is nontoxic and does not alter PKCδ activity (11–13, 29–31). The peptide was synthesized by Mimotopes (Melbourne, Australia) by 9-fluorenylmethoxycarbonyl solid-phase chemistry. Peptides were purified to >95% by preparative reverse-phase HPLC.
Animal Protocols
All animal handling and care adhered to National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at the Lewis Katz School of Medicine at Temple University. Male Sprague-Dawley rats (250–300g) (Charles River, Boston, MA, USA) were used in all experiments. Rats were acclimated for at least 1 week in a climate-controlled facility and given free access to food and water.
Cecal Ligation and Puncture Model
Sepsis was induced by the cecal ligation and puncture (CLP) method as described previously (14–19). Sham controls underwent a laparotomy without cecal ligation or puncture. Following CLP or Sham surgery, the abdominal incision was closed and the animals were orally intubated with a 16-gauge intravenous cannula and randomized to receive either the PKCδ TAT inhibitory peptide (200 μg/kg in 200 μl of PBS) or a like volume of PBS (vehicle). Post-operative pain was managed by injection of 2 mg/kg bupivacaine (Marcaine) at the incision site following surgery, then every 8–12 hours post-operatively until euthanasia. Normal saline solution (50 ml/kg) was injected subcutaneously in all groups for fluid resuscitation. Animals were studied in subgroups, differentiated by measurements performed at 24 hours post-surgery.
Sample Collection
At 24 hours post-surgery, oxygenation saturation was measured by tail pulse oximetry (SurgiVet V3304: Smiths Medical, Dublin, OH) in a subgroup of animals serially exposed in a temperature-controlled environmental chamber to FIO2 = 1 and then 0.40 for 5 min intervals, respectively. The final SpO2 was used for analyses. The difference between SpO2 between the oxygen conditions was used to non-invasively assess oxygen compensatory reserve under resting conditions (32). All animals were then euthanized and blood, peritoneal cavity fluid and organs were collected. Heparinized blood samples were obtained by cardiac puncture. Plasma was obtained after centrifugation of heparinized blood. Samples were aliquoted and frozen at –70°C. Peritoneal cavity fluid was collected by lavage under sterile conditions. Briefly the abdomen was wiped with ethanol and the skin carefully removed exposing the intact peritoneum. Sterile PBS (20 ml) was injected into the cavity using a 21-guage needle and the abdomen massaged to distribute the PBS. The peritoneal fluid was then collected in sterile polypropylene tubes and stored on ice prior to bacteriological studies and cell analysis. For peritoneal fluid cell analysis, cells were counted using the Hemavet® Multispecies Hematology System (Drew Scientific, Inc. Oxford, CT). Peritoneal cavity lavage fluid samples were centrifuged and supernatant samples were collected and frozen at −70°C. The lungs, livers, kidneys and spleen were collected and frozen immediately in liquid nitrogen. In one group of rats, bronchoalveolar lavage fluid (BALF) was obtained by inserting a cannula into the trachea and instilling 1.5 ml room temperature sterile PBS until the lungs were fully distended as described previously(14, 18). The fluid was withdrawn and saved. This process was repeated until a total of 4.5 ml PBS had been instilled. The samples were pooled for each animal and the volumes recorded. The percent BALF recovered was calculated and BALF volumes normalized. White blood cell (WBC) counts in the BALF were measured using the Hemavet® Multispecies Hematology System.
Myeloperoxidase (MPO) activity assay
Units of MPO enzymatic activity in tissue homogenates were measured as we described previously (16). Briefly, lung or kidney tissue was homogenized in freshly prepared lysis buffer (0.5% hexadecyltrimethyl ammonium bromide in 50 mM potassium phosphate buffer) at a ratio of 0.1 grams of wet tissue weight per milliliter of lysis buffer. Homogenates were cleared by centrifugation at 13,362 X g for 15 minutes at 4 °C and placed on ice. On a per well basis, the reaction mixture contained 284 μl of 50 mM potassium phosphate buffer, 3 μl of H2O2, and 3 μl of 20 mg/ml o-dianisidine added to 10 μl of sample to initiate the reaction (300 μl total volume per well). Absorbance (460nm) was measured over a 5-minute time course and units of MPO activity were quantified using a standard curve.
Evans blue permeability assay
Alterations of lung vascular permeability were investigated by tissue accumulation of Evans blue as previously described (33, 34). Under anesthesia, CLP and sham-operated animals were administered 160 mg/kg Evans blue (Sigma) by jugular vein injection 30 min prior to termination of the experiment. Lungs were then perfused with PBS, removed, weighed and homogenized in 3 ml PBS. Evans blue was extracted from lung homogenates by incubating samples in 2 ml formamide (Sigma) at 60°C for 14–18 h. The supernatant was separated by centrifugation at 5000 g for 30 min. The concentration of Evans blue in lung homogenate supernatants was quantified by a dual wavelength spectrophotometric method (19) at absorptions of 620 and 740 nm, that allows for correction of contaminating heme pigments using the following formula: E620 (corrected) = E620 − (1.426×E740 + 0.030). Data are expressed as micrograms per milligram lung weight.
Organ Processing for histology and injury scoring
Lung histology. At 24 post surgery, rats were euthanized and the lungs were gravity-fixed with 10% neutral buffered formalin instillation into the airways; the trachea was then be tied off, to maintain inflation during fixation. After fixation, lungs were paraffin-embedded, sectioned (5 to 10 μm thick), and stained with hematoxylin-eosin (H&E) as we described previously (14–16, 18). Kidney histology: At 24 post-surgery, kidney tissue sections were obtained and formalin-fixed, paraffin embedded, sectioned and stained with H & E and assessed for the kidney morphology and fibrosis (35).
Clinical chemistry analysis
Plasma levels of BUN and creatinine were determined by Charles River Clinical Pathology Services (Shrewsbury, MA).
Determination of colony forming units (CFU) in peritoneal cavity fluid and blood
For aerobic bacteria detection, peritoneal cavity lavage fluid and spleen tissue homogenates were serially diluted with PBS. Each diluted sample was plated on blood-agar plates and incubated overnight at 37°C. Colony- forming units (CFU) were then counted. For anaerobic bacteria detection, peritoneal cavity lavage fluid was collected and serially diluted with PBS. Peritoneal cavity lavage fluid and blood samples were then plated on blood-agar plates and added to an anaerobic jar. Two Gaspak EZ Campy sachets (Becton Dickinson and Company, Franklin Lakes, NJ) were opened and placed in the container. The plates were stored in the jar for 48 hours at 37°C. CFU were then counted and results expressed as CFU/ml of fluid (PCF and blood) or as CFU/g tissue (spleens).
Determination of direct in vitro effects of PKCδ-TAT inhibitor on Pathogens
Single colonies of Escherichia Coli Nissle (ST221) and Salmonella typhimurium (ST1) bacteria were grown overnight. Aliquots (20L) of the bacteria cultures were then diluted 1:200 and plated in a 96-well plate. Bacteria were then incubated overnight at 37°C with different concentrations of the PKCδ-TAT peptide inhibitor (0, 1.25, 2.5, 5, and 10 M). PBS was used as control. Plates were visually inspected for inhibition of growth.
Flow cytometric analysis of peritoneal cells
Cells recovered from the peritoneal cavity were blocked with rat Fc block (BD Biosciences; clone 2.4G2; San Jose, CA) in FACS staining buffer (BD Biosciences) for 30 minutes at 4°C. Cells were pelleted, the supernatant removed, and resuspended in FACS buffer containing fluorescent-labeled antibodies including CD45-BV421 (BD Bioscience; clone OX-1), CD68-Alexa700 (Bio-Rad; clone ED1), CD86-Alexa488 (Bio- Rad; clone 24F), CD163-Alexa647 (Bio-Rad; clone ED2), CCR2-PE (R&D Systems; clone #890231) PE anti-rat granulocyte (BD Pharmigen, Clone RP-1) and CD11b-FITC (BD Pharmigen, Clone WT.5). In parallel, cells were stained with isotype control antibodies as background controls. Cells were incubated for 30 minutes at 4°C with the antibody cocktail followed by washing with FACS buffer. Cells were fixed with 2% paraformaldehyde for 10 minutes at 4°C followed by centrifugation and resuspension in FACS buffer. At least 250,000 events per sample were acquired using an LSRII flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software (version 10.1; FlowJo, LLC; Ashland, OR). Spectral overlap was compensated with the FlowJo software using data acquired from compensation beads that were individually stained with each fluorochrome-labelled antibody. Gates for each marker were set based on isotype control antibody staining. A diagram showing the gating strategy is shown in Figure 1S.
Peritoneal fluid cytokine level determinations
The levels of CCL2, CCL3, CCL5, and CX3CL1 in the peritoneal fluid were determined using the Rat Cytokine/Chemokine magnetic bead panel kit (EMD Millipore; RECYTMAR-65K; Burlington, MA) according to manufacturer’s instructions. A five-parameter logistical curve fit (MILLIPLEX Analysis 5.1 software; EMD Millipore) of the standard curves was used to calculate the concentrations of each analyte in the peritoneal fluid. All samples were measured within the linear region of the standard curves.
IL-10 Determination
IL-10 levels were determined in the peritoneal cavity fluid and plasma from sham, CLP+vehicle and CLP+ PKCδ TAT at 24hr post-surgery using a Rat IL-10- Quantikine ELISA kit (R1000, R & D Systems) according to manufacturer’s instructions.
Phagocytosis assay
Peritoneal cells were collected 4 hrs post-surgery from CLP+vehicle and CLP+ PKCδ TAT treated animals. Macrophages were isolated from the peritoneal cavity fluid by plating the cells on gelatin-coated flasks for macrophage adherence, and incubated for 90 min in 5% CO2 at 37°C. The flasks were washed with DMEM to remove lymphocytes and other nonadherent cells. Adherent macrophages were then detached by incubation with 10 mM EDTA in DMEM containing 20% FCS for 15 min at 37°C. The cells were washed, counted and plated in 24 well plates at a concentration of 0.5–1 X 106 cells/well and allowed to adhere for 1 hr at 37°C. To determine bacterial phagocytosis, macrophages were infected with E. coli for 1 h at 37°C at an MOI of 2 bacteria per cell. Cells were then washed three times with 0.5 ml PBS. RPMI medium (0.5 ml) containing 0.1 mg/ml gentamicin (Gibco) was then added to the cells for 60 min. The cells were then washed 3 times with 0.5 ml PBS and lysed in 0.5 ml of 0.1% Triton-X in PBS for 30 minutes. The recovery of bacteria from macrophages was quantified by spreading serial 10-fold dilutions on LB agar plates containing the appropriate antibiotics.
Survival Studies
Rats underwent CLP surgery and were then treated IT with either PBS vehicle or PKCδ inhibitor (200g/kg) as described above. Antibiotic therapy (imipenem 25mg/kg) was administered i.p. following surgery and two times per day until euthanasia. Survival after CLP surgery was assessed three times a day for 4 days. All surviving rats were euthanized at the end of the fourth day.
Statistical Analysis
Results are expressed as mean values ± SEM. Data were analyzed by analysis of variance (ANOVA) for multiple comparisons, followed by Student’s t test for individual comparisons. The Tukey-Kramer multiple comparisons post-test was used to evaluate the significance between experimental groups if analysis of variance indicated a significant difference; differences were considered significant when P<0.05. Survival was monitored for 96 hrs and Kaplan-Meier survival curves constructed with statistical analysis by the log-rank test.
Results
Effect of PKCδ inhibition on neutrophil influx and organ function
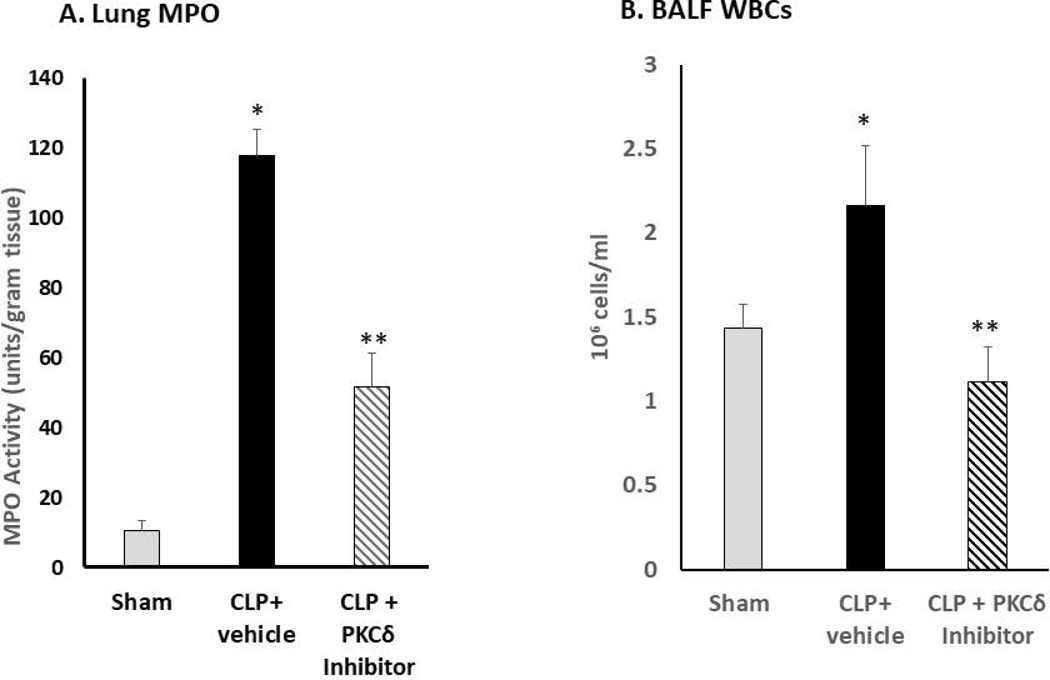
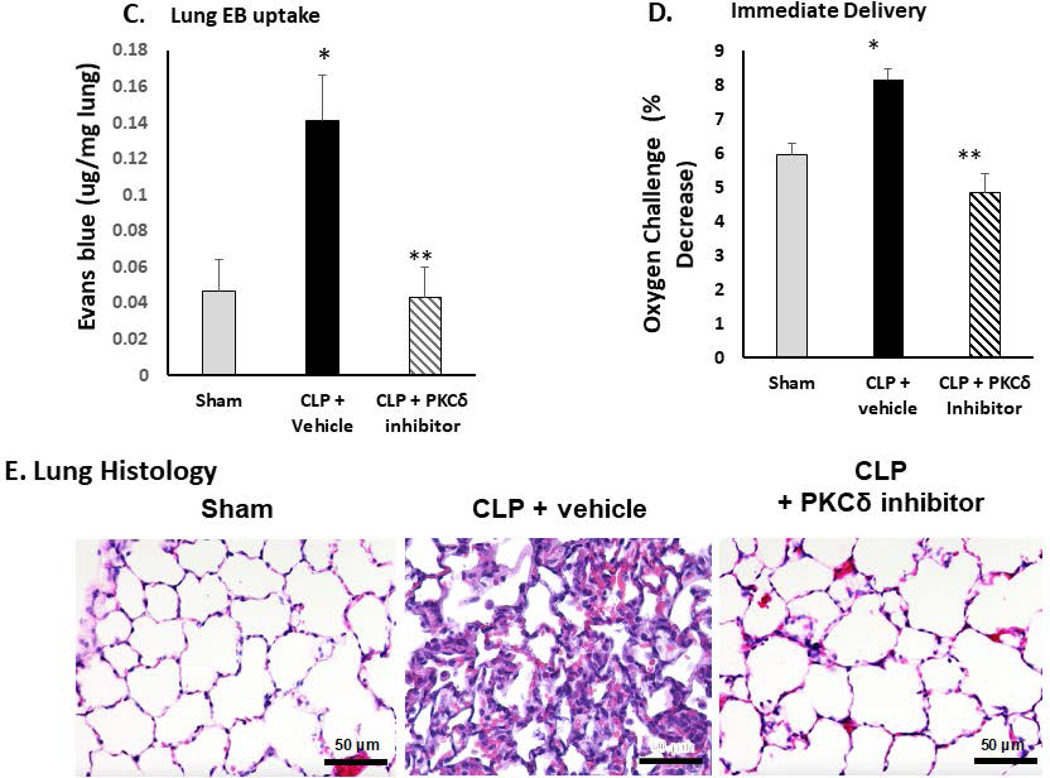
Acute lung and kidney injury develop early in sepsis and are associated with increased mortality (36). In our rat model of sepsis, CLP leads to significant inflammation and damage to the lungs that is evident at 24 hr post-surgery (14–18). At this time, there is significant influx of neutrophils into the lung (Figure 1A), increased WBCs in the BALF (Figure 1B), increased permeability (Figure1C), decreased oxygenation reserve (Figure 1D), and increased lung injury (Figure 1E) as compared to sham surgery indicating lung injury and decreased lung function. IT administration of the PKCδ inhibitor following CLP surgery decreased inflammation and lung injury as evidenced by decreased neutrophil influx, decreased WBCs in the BALF, decreased uptake of Evans blue dye, and decreased lung injury associated with improved oxygen reserve (Figure 1A–E).
Figure 1: PKCδ inhibition reduces neutrophil influx and attenuates acute lung injury in sepsis.
A. PKCδ inhibition attenuates pulmonary Myeloperoxidase activity in sepsis. Pulmonary myeloperoxidase activity was measured 24 hr post sham or CLP surgery in lung tissue homogenates obtained from rats administered IT vehicle or PKCδ inhibitor (200ug/kg). *P< 0.01 Sham vs. CLP+ Vehicle, **P<0.05 CLP+ vehicle vs. CLP+ PKCδ inhibitor, n= 4 rats/group. B. PKCδ inhibition decreases WBC in the BALF in sepsis. White blood cell counts were analyzed in the BAL fluid at 24 hr post sham or CLP surgery in rats administered with the vehicle or the PKCδ inhibitor. BALF cells were counted using a Hemavet® Multispecies Hematology System (*p < 0.05 Sham vs. CLP+Vehicle, ** P<0.03 CLP+Vehicle vs. CLP+PKCδ inhibitor, n=5 rats/group). C. PKCδ inhibition decreases lung permeability in sepsis. Lung vascular permeability was determined by Evans blue dye uptake into the lung at 24 hr post sham or CLP surgery in rats administered with the vehicle or the PKCδ inhibitor. *P< 0.05 Sham vs. CLP+ Vehicle, **P<0.05 CLP+ vehicle vs. CLP+ PKCδ inhibitor, n= 3–4 rats/group. D. PKCδ inhibition improves lung function in sepsis. Oxygen Reserve was determined by performing an oxygen challenge and measuring oxygen saturation 24 hr post-surgery in Sham, CLP+ vehicle and CLP+PKCδ inhibitor treated rats who were serially exposed to FiO2 = 1 and then 0.40 for 5 min intervals with the difference between the final SpO2 between these oxygen conditions representing oxygen reserve under resting conditions. * P≤ 0.001 Sham vs. CLP+ Vehicle, ** P<0.001 CLP+ vehicle vs. CLP+ PKCδ inhibitor, n= 5 rats/group. E. PKCδ inhibition is lung protective and attenuated histologic changes in the lung associated with CLP-induced sepsis. H&E staining in representative lung tissue sections from 24 hours after surgery (minimum of 4 animals per group). Sham-surgery; lung architecture was normal, with open alveoli and thin alveolar walls. CLP+vehicle; sepsis-induced severe indirect lung injury evidenced by a loss of lung architecture, marked inflammatory infiltrate, thickening of alveolar walls and septa, visible hemorrhaging and proteinaceous exudate filling alveoli. CLP + PKCδ inhibitor; PKCδ inhibition attenuated sepsis-induced lung injury, evidenced by preservation of lung architecture, reduced inflammatory infiltrate, maintenance of alveolar wall thickness, and reduction of hemorrhaging and proteinaceous exudate induced by sepsis. Scale bars: 50 μm. Original magnification: 400x.
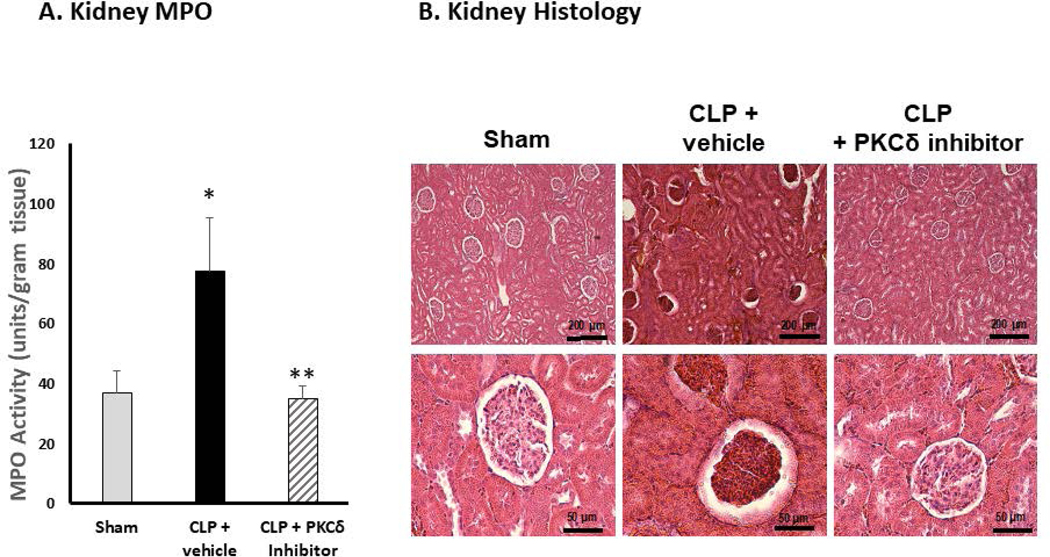
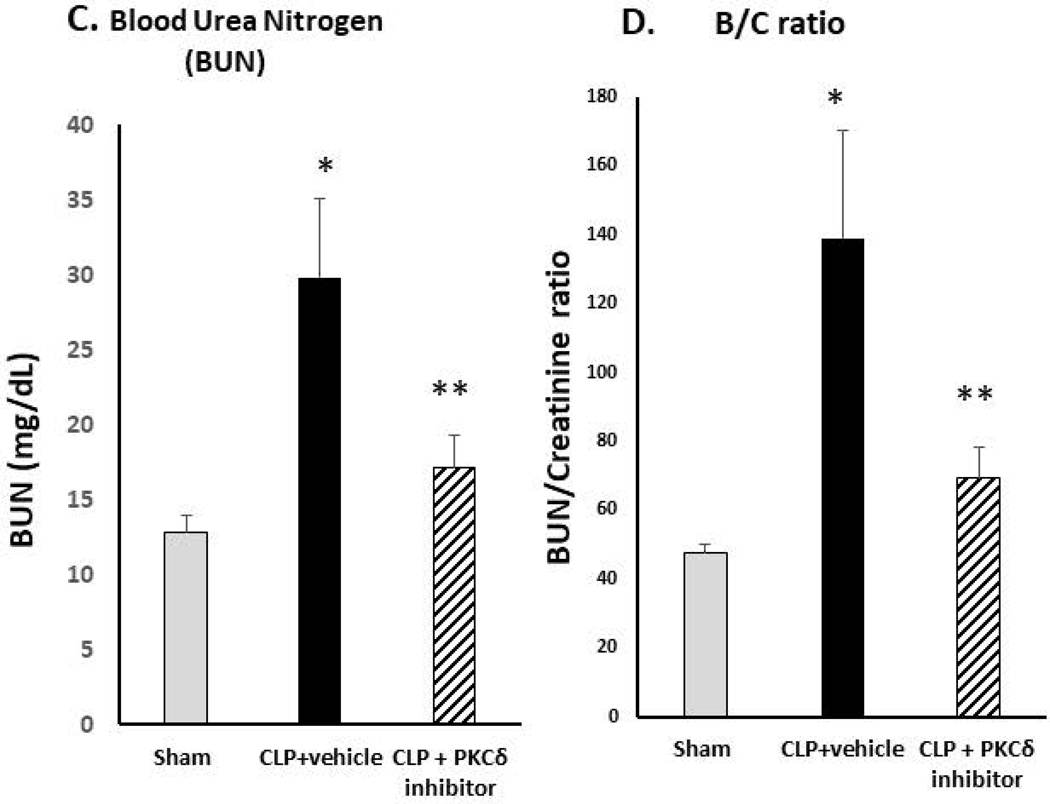
Kidney damage and development of acute kidney injury (AKI) is a frequent complication in patients with sepsis and other critical illnesses (5, 37). Studies indicate that neutrophils play a critical role in the development of AKI (38). While IT administration of the PKCδ inhibitor was lung protective, it is not clear whether this treatment would also protect other distal organs. To investigate this possibility, we performed histopathologic analysis of kidney tissues from each group (Figure 2). At 24 hr post-CLP surgery, there is significant influx of neutrophils into the kidney as compared to sham surgery rats (Figure 2A). As shown in Figure 2B, there were no overt signs of AKI visible in the sham-operated group. By contrast, CLP surgery caused marked acute kidney injury (AKI) as evidenced by the presence of acute tubular lesions and apparent necrosis leading to apparent destruction of kidney architecture. Notably, we observed widespread hemorrhaging indicative of microvascular dysfunction. Further evidence of severe AKI was seen in the form of glomerular atrophy. Plasma indicators of kidney injury were also elevated in the CLP+ vehicle group demonstrating increased levels of blood urea nitrogen (BUN) and increased BUN/creatinine ratios (Figure 2C–D). Importantly, IT delivery of the PKCδ inhibitor resulted in a significant decrease in neutrophil influx into the kidneys, preserved tubular architecture and prevented the severe hemorrhaging and glomerular atrophy observed in untreated septic animals (Figure 2A–B). BUN and BUN/Creatinine ratios were also reduced following administration of the PKCδ inhibitor indicating improved kidney function (Figure 2C–D).
Figure 2: PKCδ inhibition reduces neutrophil influx and attenuates acute kidney dysfunction in sepsis.
A. PKCδ inhibition attenuates renal MPO activity in sepsis. MPO activity was measured in kidney tissue homogenates from Sham, CLP+ vehicle or CLP+PKCδ inhibitor treated rats at 24 hr post-surgery. *P< 0.05 Sham vs. CLP+ Vehicle, **P<0.05 CLP+ vehicle vs. CLP+ PKCδ inhibitor, n=5–6 rats/group. B. PKCδ inhibition is protective in sepsis-induced acute kidney injury. H&E staining in representative kidney tissue sections from 24 hours after surgery (3–4 animals per group). In the sham-surgery group, kidney histology was normal with typical architecture of the tubular epithelium and glomeruli. In the CLP + vehicle group, histologic evidence of acute kidney injury was observed including acute tubular lesions with apparent necrosis, widespread hemorrhaging, inflammatory infiltrates and glomerular atrophy. CLP + PKCδ inhibitor; PKCδ inhibition partially protected against sepsis-induced acute kidney injury. While evidence of tubular injury is still apparent, tubular architecture was preserved with reduced inflammatory infiltrate. PKCδ inhibition also preserved glomerular architecture and prevented widespread hemorrhaging and glomerular atrophy observed in the CLP + vehicle group. Scale bars: 200 μm (top row), 50 μm (bottom row). Original magnification: 100x (top row), 400x (bottom row). C. Blood urea nitrogen (BUN) and D. creatinine/BUN ratios were determined 24 hr post-surgery in Sham, CLP+ vehicle and CLP+PKCδ inhibitor treated rats. *P< 0.01 Sham vs. CLP+ Vehicle, **P<0.05 CLP+ vehicle vs. CLP+ PKCδ inhibitor, n=5–6 rats/group
PKCδ inhibition enhances bacterial clearance in sepsis
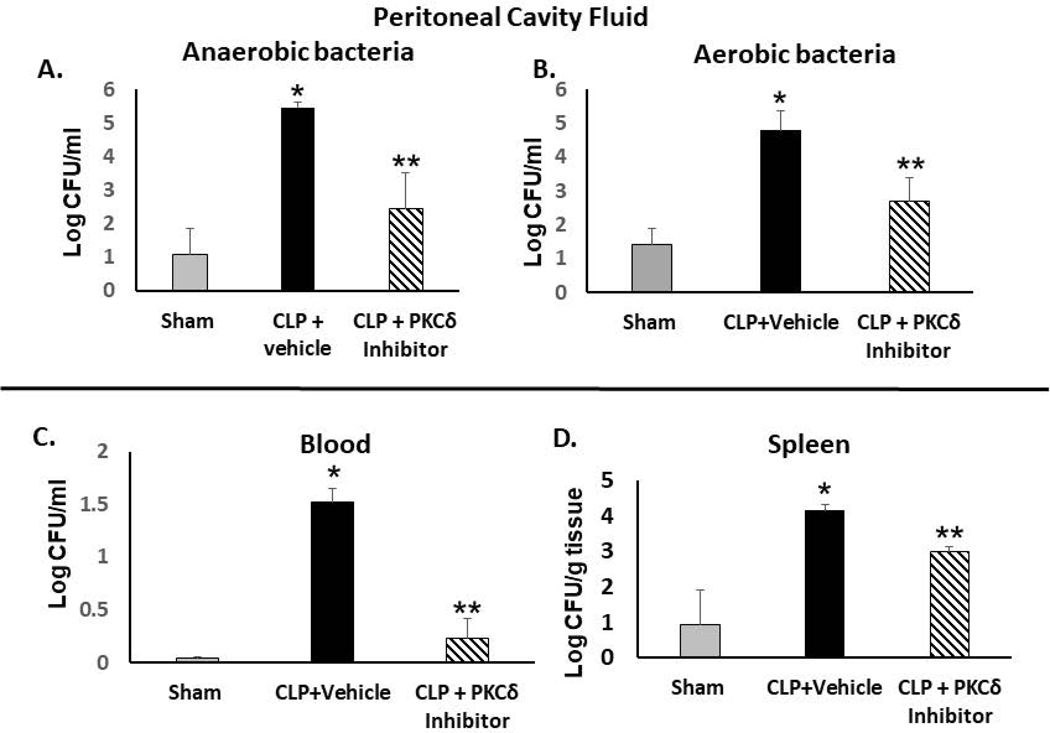
Effective host immunity against pathogens is compromised in sepsis patients leading to sustained infections (39). What role PKCδ plays in host immunity and pathogen clearance in sepsis is not known. To ascertain the involvement of PKCδ in bacterial clearance, the effect of PKCδ inhibition on the bacterial burden in the peritoneal cavity, spleen, and blood were determined in septic rats as compared to sham controls. At 24 hr post-CLP, the bacteria burden was significantly increased in the peritoneal cavity of septic rats compared with the sham control (Figure 3A and 3B). In the vehicle-treated septic rats, both anaerobic and aerobic bacterial CFU levels were significantly increased as compared to sham levels. However, in response to administration of the PKCδ inhibitor, there was a significant reduction in aerobic and anaerobic pathogens in the peritoneal cavity fluid (Figure 3A and 3B). Similar beneficial effects were observed in the blood indicating decreased bacteremia in response to administration of the PKCδ inhibitor (Figure 3C). CFU levels were also significantly decreased in spleens from septic rats who had been treated with the PKCδ inhibitor (Figure 3D). Of interest, in vitro addition of the PKCδ-TAT peptide inhibitor (1–10uM) directly to cultures of Escherichia Coli or Salmonella Thyphirium for 24hr did not significantly inhibit growth of either bacterial strain as compared to cultures treated with PBS (vehicle) (data not shown). Thus, the PKCδ inhibitor does not exhibit direct bactericidal activity indicating an indirect effect either on phagocyte function and/or on the inflammatory milieu.
Figure 3: PKCδ inhibition improves bacterial clearance in septic rats.
Bacterial clearance was analyzed in the peritoneal cavity fluid (PCF), peripheral blood and spleens from Sham, CLP+vehicle and CLP +PKCδ inhibitor-treated rats at 24 hr post-surgery. In the peritoneal cavity fluid, bacterial colony counts were determined in serially diluted samples for 48 hrs (anaerobic, A) and 24 hr (aerobic, B). (*P< 0.01 Sham vs. CLP+ Vehicle, **p<0.05 CLP+ vehicle vs CLP+PKCδ inhibitor, n=6 rats/group). C: Peripheral blood from Sham, CLP+vehicle and CLP +PKCδ inhibitor treated rats at 24 hr post-surgery (*P< 0.001 Sham vs. CLP+ Vehicle, **p<0.001 CLP+vehicle vs CLP +PKCδ inhibitor, n=6 rats/group). D: Spleens collected from Sham, CLP+ vehicle and CLP +PKCδ inhibitor rats (*P< 0.05 Sham vs. CLP+ Vehicle, **p<0.05 CLP+ vehicle vs CLP+PKCδ inhibitor, n=3 rats/group. Bacterial spreading was quantified as CFU (colony-forming unit) per ml of fluid (PCF and blood) or as CFU/g tissue (spleens).
Effect of PKCδ inhibition on peritoneal immune cell numbers and cell phenotype
To examine possible mechanisms for increased pathogen clearance in rats treated with the PKCδ inhibitor, we next examined whether PKCδ inhibition altered the environmental milieu and/or number of immune cells recruited to the peritoneal cavity at the site of infection. At 24 hr post CLP surgery, there were significant increases in multiple chemokines important for recruitment of phagocytic cells. As shown in Table I, PCF concentrations of MIP1α (CCL3), MCP (CCL2), and Fractalkine (CX3CL1) increased in septic animals. In contrast, sepsis induced a 9-fold decrease in the concentration of RANTES (CCL5) in the PCF. There were no statistically significant differences in these chemokine levels in the PKCδ inhibitor-treated animals as compared to vehicle (PBS)-treated septic animals.
Table 1:
Peritoneal Cavity fluid Chemokine Concentrations
| MIP1α | MCP1 | Fractalkine | RANTES | |
|---|---|---|---|---|
| pg/ml | pg/ml | pg/ml | pg/ml | |
| Sham | 5.2±0.4 | 341.1±64.1 | 2.1±0.5 | 35.9±9.4 |
| CLP + vehicle | 386.6±70.7* | 29210.9±4479.6* | 14.2±6.0* | 3.5±0.0*# |
| CLP+PKCδ Inhibitor | 471.6±189.2* | 25416.7±5610.4* | 23.9±9.1* | 3.5±0.0*# |
P<0.01 sham vs CLP+vehicle and sham vs. CLP+ PKCδ inhibitor. P=NS CLP+vehicle vs. CLP+ PKCδ inhibitor
Lower limit of detection (LLOD) (n=12 rats/group)
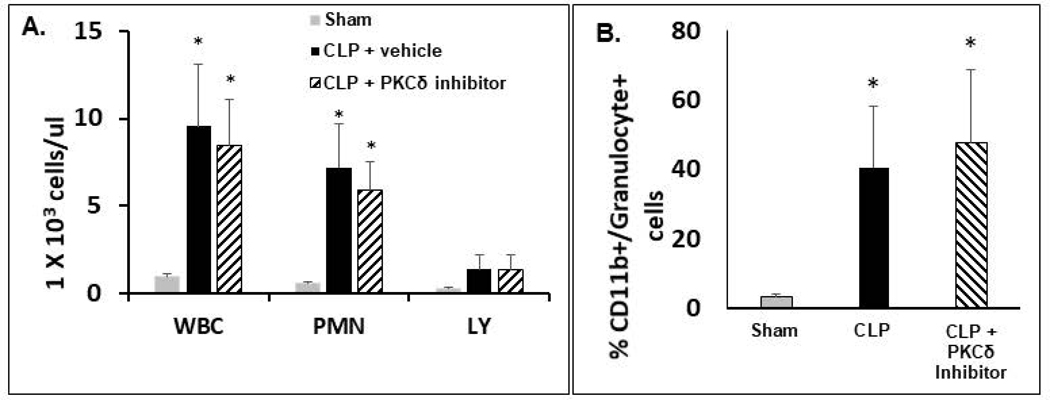
At 24 hrs post-surgery, the number of white blood cells (WBCs) in the peritoneal cavity of septic rats significantly increased as compared to sham surgery animals (Figure 4A). This increase was primarily the result of increased neutrophil influx into the peritoneal cavity. Lymphocyte numbers were not significantly different in the septic rats compared to shams. Further, there were no significant differences in peritoneal cavity WBCs or neutrophil counts between the septic animals treated with the PKCδ inhibitor as compared to treatment with the vehicle (PBS). The peritoneal neutrophil population was further analyzed by flow cytometry for neutrophil populations positive for the rat neutrophil antigen, RP-1 and the integrin CD11b, which regulates leukocyte adhesion and migration. As shown in Figure 4B, 24hr post CLP surgery there was a significant increase in peritoneal CD11b+RP1+ cells as compared to sham surgery rats. There was no significant differences between septic animals treated with the PKCδ inhibitor as compared to treatment with vehicle.
Figure 4: PKCδ inhibition does not alter sepsis-induced immune cell recruitment to the peritoneal cavity.
A.-B.: Peritoneal cells were isolated from the PCF of Sham, CLP+ Vehicle and CLP+ PKCδ inhibitor treated rats 24 hr post-surgery. A: Total WBCs, neutrophil, and lymphocyte counts in peritoneal cavity fluid. Values are expressed as 1×103 cells/μL. *p<0.05 Sham vs. CLP+ Vehicle and Sham vs. CLP+ PKCδ inhibitor mice, n = 5–6 rats/group. B: Cells harvested from the peritoneal cavity were analyzed by flow cytometry. Activated neutrophils are shown as the percentage of cells double positive for CD11b and the rat granulocyte marker (clone RP-1). *P<0.05 Sham vs. CLP+ vehicle and Sham vs. CLP+ PKCδ inhibitor, n= 3 rats/group.
We next analyzed rat peritoneal cells by flow cytometry to determine the impact of sepsis and PKCδ inhibition on macrophage phenotypes. As shown in Table II, we used a flow cytometry gating strategy to examine the impact of sepsis on specific subsets of CD45 positive cells (Figure 1S). The proportion of CD68+/CD86+ macrophages (as a percentage of total CD45+ cells) decreased by almost 63% 24hr post CLP surgery as compared to Sham. This is most likely a reflection of the increased number of neutrophils in the PCF (Figure 4). There was a modest, but statistically significant, increase in the proportion of macrophages comparing the CLP+PKCδ inhibitor with CLP + vehicle groups. The percentage of CD163+ (M2-like) macrophages significantly decreased in both of the CLP groups, and there was no difference in the CD163 expression comparing the CLP + vehicle and CLP+PKCδ inhibitor groups (9.59±1.96 vs 6.62±0.71, respectively). Finally, the expression of CCR2, a proinflammatory chemokine receptor, increased on both the CD163-positive and negative cells, but there was no significant difference between the CLP+ vehicle and CLP+PKCδ inhibitor groups.
Table II.
Peritoneal Cavity Fluid Macrophage Phenotypes
| Cell population | Sham (n=5) | CLP + Vehicle (n=8) | CLP + PKCδ Inhibitor (n=6) |
|---|---|---|---|
| CD68+CD86+(% of CD45+ cells) | 48.6 ± 9.74 | 17.8 ± 1.32 *** | 22.7 ± 2.15 *§ |
| CD163+(% of CD68+CD86+ cells) | 19.2 ± 2.33 | 9.59 ± 1.96 ** | 6.62 ± 0.71 *** |
| CCR2+(% of CD68+CD86+CD163+ cells) | 2.68 ± 0.63 | 9.75 ± 2.25 * | 6.27 ± 1.79 |
| CD163-(% of CD68+CD86+ cells) | 73.5 ± 3.14 | 86.5 ± 2.45 ** | 91.1 ± 1.27 *** |
| CCR2+ (% of CD68+CD86+CD163- cells) |
2.32 ± 0.37 | 6.53 ± 1.90 | 4.25 ± 1.48 |
p < 0.05 relative to Sham
p < 0.01 relative to Sham
p < 0.001 relative to Sham
p < 0.05 relative to CLP + vehicle
Effect of PKCδ inhibition on blood and peritoneal fluid IL-10 levels
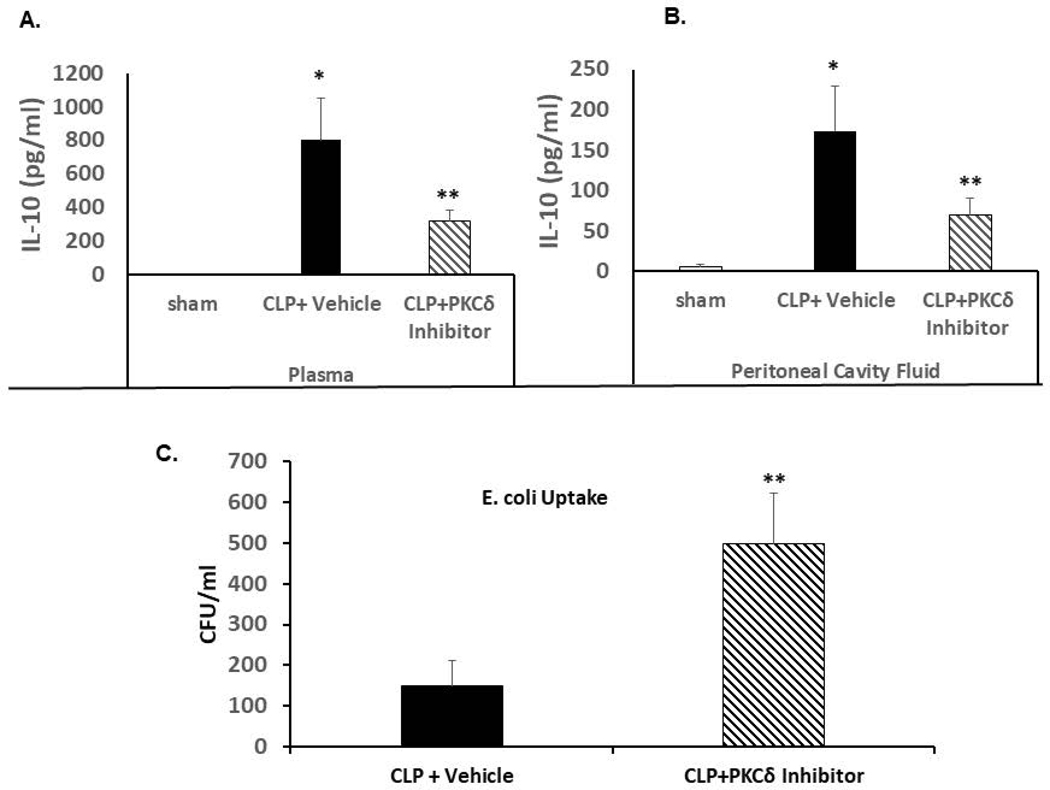
At 24 hrs post-CLP surgery, both plasma and peritoneal cavity fluid had significantly elevated levels of IL-10 as compared to sham surgery animals (Figure 5A and 5B). The administration of the PKCδ inhibitor significantly decreased IL-10 levels both systemically and in the peritoneal cavity.
Figure 5: PKCδ inhibition attenuates sepsis-increased levels of IL-10 in the plasma and peritoneal cavity fluid and increases peritoneal macrophage phagocytosis ex vivo.
A. IL-10 levels were determined in plasma isolated from Sham, CLP+ Vehicle and CLP+ PKCδ inhibitor treated rats 24 hrs post-surgery. *P<0.001 Sham vs. CLP+ vehicle, **P<0.03 CLP+ vehicle vs. CLP+ PKCδ inhibitor (n=12). B. IL-10 levels in the peritoneal cavity fluid *P<0.001 Sham vs. CLP+ vehicle, **P<0.03 CLP+ vehicle vs. CLP+ PKCδ inhibitor (n=6). C: Peritoneal macrophages were isolated 4 hrs post CLP surgery in rats treated with vehicle (PBS) or the PKCδ inhibitor after CLP surgery. Isolated macrophages were incubated with E. coli and bacterial phagocytosis was determined as described in Methods. Bacteria levels are expressed as colony forming units (CFU)/ml. *P<0.05 CLP+ vehicle vs. CLP+ PKCδ inhibitor (n=3 rats/group)
Effect of PKCδ inhibition on macrophage phagocytosis ex vivo
We next determined whether PKCδ inhibition affects macrophage phagocytosis. Peritoneal macrophages were isolated 4 hrs post CLP surgery from rats treated with PBS or the PKCδ inhibitor. Incubation of the isolated peritoneal macrophages from septic rats with E. coli resulted in increased uptake of bacteria within 1 hour as compared to Sham controls. Animals treated with the PKCδ inhibitor demonstrated significantly increased peritoneal macrophage phagocytosis ex vivo as compared to those treated with PBS (Figure 5C).
Survival studies
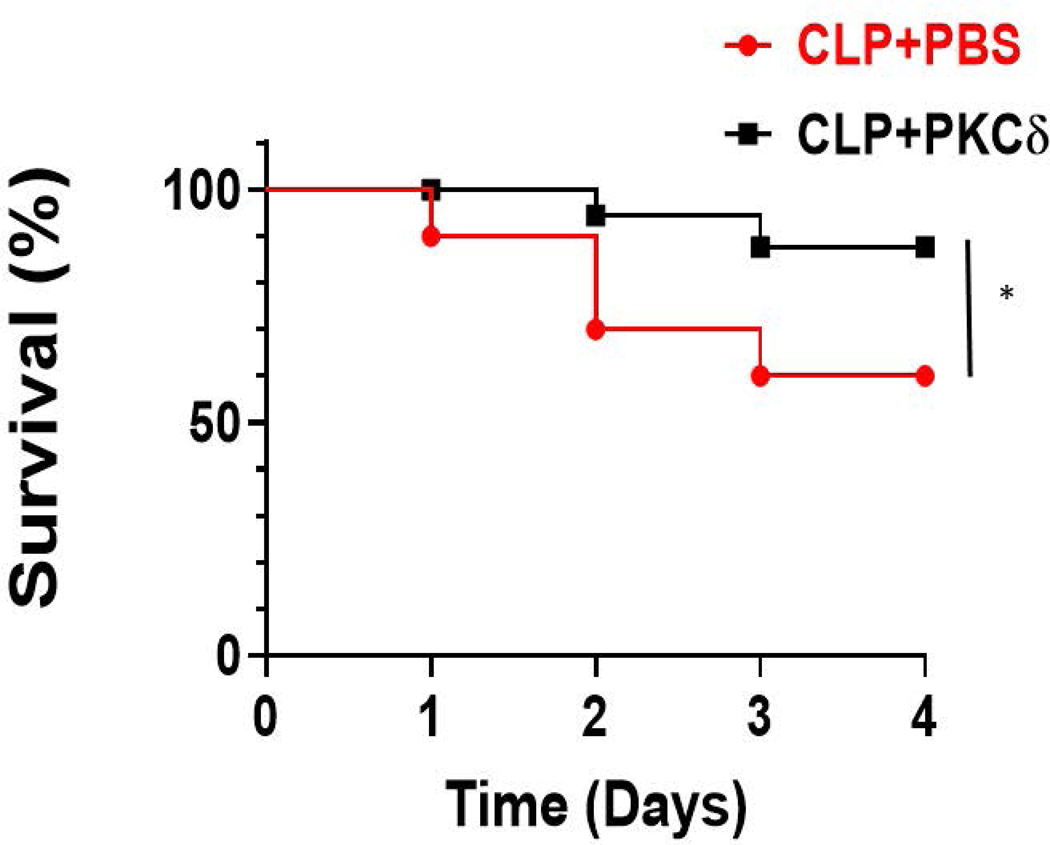
To examine whether administration of this inhibitor decreased mortality in this CLP model of sepsis, we analyzed survival rate in rats treated IT with a single dose of either the vehicle (PBS) or the PKCδ inhibitor following CLP surgery. Following surgery, all animals received fluids and antibiotic therapy to provide a more clinically relevant model. The broad-spectrum antibiotic, Imipenem-Cilastatin Sodium (25mg/kg) was administrated IP following surgery and then twice a day until euthanasia. The rats were monitored for 4 days and the results are shown in a Kaplan-Meier survival plot of study groups (Figure 6). In the presence of antibiotic therapy, at day 4 there was a survival rate of approximately 60% in septic animals that received the PBS vehicle. Treatment with the PKCδ inhibitor significantly improved survival as compared to vehicle-treated animals to 90% (P<0.05).
Figure 6: PKCδ inhibition improves survival in sepsis.
Survival was measured for 4 days in septic rats receiving antibiotics (Imipenem-Cilastatin Sodium-25mg/kg) and fluid resuscitation. Septic rats received either a single dose of vehicle (PBS) or PKCδ inhibitor following CLP surgery. Data are shown in a Kaplan-Meier survival plot of study groups. (*p < 0.05; CLP+ vehicle vs CLP +PKCδ inhibitor, n=20 rats/group).
Discussion
According to the Sepsis 3 definition (1), sepsis is now defined as life-threatening organ dysfunction caused by dysregulated host response to infection. In this study, we demonstrate for the first time that administration of a PKCδ inhibitor significantly improved survival in a rat model of sepsis where animals received appropriate antibiotics and fluid resuscitation. We further demonstrate that inhibition of PKCδ limited organ damage as evidenced by reduced acute lung and kidney damage, critical complications in sepsis. Our results indicate that recruitment of neutrophils to the site of infection and to distal organs (lung and kidney) are regulated differently and PKCδ regulation of neutrophil migration is selective targeting the systemic inflammatory response rather than recruitment to the primary foci of infection. Thus, PKCδ inhibition may promote distal organ protection from inflammatory damage. Importantly we establish that the modification of the inflammatory response by PKCδ inhibition was not associated with immunosuppression and increased infection, rather pathogen clearance and phagocytic cell function was enhanced indicating that PKCδ inhibition improved host immunity and decreased organ dysfunction leading to improved survival.
In this CLP model of sepsis, the lung and the kidney show earliest signs of dysfunction (40, 41). In these studies, we demonstrate both lung and kidney dysfunction by 24hr post CLP surgery. Our studies show that IT administration of the PKCδ inhibitor was lung protective. In agreement with our previous studies (14–18), IT administration of the PKCδ-TAT peptide inhibitor following the induction of sepsis inhibited PKCδ activation, was lung protective, evidenced by reduced neutrophil influx and pulmonary vascular permeability. In addition, in the current study, we expanded our previous observations of the impact of the inhibitor to improve lung mechanics and oxygenation, to include preserved oxygen reserve in the face of oxygen challenge, suggesting salutary effect to support oxygenation in the presence of stress.
While targeted pulmonary delivery of the PKCδ inhibitory peptide clearly protects against sepsis-induced indirect lung injury (Figure 1), it was unclear if our therapeutic approach could be an effective treatment for multiple organ failure. In this CLP model of sepsis, we found significant signs of renal injury as evidenced by increased neutrophil influx into the kidney, organ damage, and elevated plasma BUN and BUN/creatinine levels (Figure 2). IT administration of the PKCδ-TAT peptide inhibitor attenuated neutrophil influx, decreased organ injury, and reduced biochemical indicators of kidney dysfunction. Thus, we demonstrate for the first time that treatment with the PKCδ TAT peptide inhibitor reduced acute lung injury and acute kidney injury, critical complications in sepsis. This key observation implies a possible functional connection between acute lung injury and acute kidney injury in the setting of experimental sepsis.
The mechanism by which IT administration of the PKCδ inhibitor reduces kidney dysfunction has not been delineated. There may be a direct effect on the kidney through release of the PKCδ-TAT peptide into the circulation. The PKCδ inhibitor is coupled to a cell permeant TAT carrier peptide. These permeable carrier peptides provide a method to deliver intracellular acting peptides to multiple cell types and organs (29). It is possible that the peptide is released into the circulation through alveolar capillary barrier disruption to act directly on the kidney. Alternatively, the inhibitor may have an indirect effect through modulation of systemic inflammation. In previous studies, we demonstrated that IT administration of the PKCδ inhibitor decreased BALF and systemic levels of key rat neutrophil chemokines (CINC-1and MIP-2) indicating decreased systemic inflammation (14). In sepsis, the lung is a significant source of inflammatory mediators that can cross the injured alveolar-capillary barrier and exacerbate the systemic inflammatory response. The decrease in systemic chemokines could reflect local pulmonary action of the δ‐PKC TAT inhibitor, resulting in preservation of the alveolar capillary barrier and decreased release of pulmonary‐derived proinflammatory mediators into the circulation (29). Furthermore, the protection of lung function by administration of the PKCδ inhibitor can reduce progression to MODS and mortality (42, 43). The lung has a critical role in initiating and/or controlling the development of MODS (42–44). In support of this concept, animal studies have shown that a reduction in lung injury is associated with decreased mortality ((42, 43, 45) and this study). Thus, PKCδ inhibition is protective in sepsis and attenuates lung and kidney dysfunction.
As noted by Hotchkiss et al (39), autopsy studies have revealed that sepsis patients often have unresolved septic foci at post mortem indicating a failure of effective pathogen clearance and suggest that therapeutic approaches should not only limit organ damage but also improve host immunity and pathogen clearance to improve survival. Thus, a critical issue in the development of anti-inflammatory therapeutics is the risk of immunosuppression and inability to effectively clear pathogens. Interestingly, the modification of the inflammatory response by PKCδ inhibition was not associated with immunosuppression and increased infection, rather bacterial clearance was enhanced following PKCδ inhibition suggesting that PKCδ activation impairs pathogen clearance. Further studies demonstrated that the PKCδ-TAT peptide inhibitor itself was not cytotoxic and had no bactericidal effects when added directly to cultures of Escherichia Coli or Salmonella Thyphirium in vitro suggesting that PKCδ inhibition may impact immune cell anti-infective function either directly by impacting phagocyte function or indirectly by altering the environmental milieu. Impaired innate immune cell function has been associated with increased mortality in sepsis (46).
To address this question, we examined immune cell recruitment to the peritoneal cavity and phagocytic capabilities of peritoneal macrophages. As phagocytic cell recruitment to the site of infection is the first line of defense, we examined the impact of PKCδ inhibition on recruitment of neutrophils and monocyte/macrophages. In contrast to decreased neutrophil recruitment to organs distal to the site of infection, we found PKCδ inhibition had no impact on sepsis-induced neutrophil recruitment to the peritoneal cavity. Consistent with this observation, PKCδ inhibition had no effect on the levels of chemokines in the peritoneal cavity of sepsis animals. These results indicate that recruitment of neutrophils to the site of infection and to distal organs (lung and kidney) are regulated differently and PKCδ regulation of neutrophil migration is selective targeting the systemic inflammatory response rather than recruitment to the primary foci of infection. Thus, PKCδ inhibition may promote distal organ protection from inflammatory damage. This concept is in line with recent studies demonstrating a separation of infection control from distal organ damage in sepsis (47–51).
Analysis of the effect of PKCδ inhibition on peritoneal macrophage phenotypes also showed minimal alterations. We observed a reduction in the percentage of CD163 expression on the macrophages in both of the CLP groups, suggesting a decrease in the M2-like macrophages in this compartment. However, there was no significant difference in the percentage of CD163+ cells with the PKCδ inhibitor treatment. Moreover, we also speculated that the expression of the highly pro-inflammatory chemokine receptor CCR2 might increase with the PKCδ inhibitor treatment, but there was no significant difference in this case. These results suggest that the PKCδ inhibitor does not substantially alter inflammatory cell recruitment to the peritoneal cavity.
Phagocytic activity significantly increased in peritoneal macrophages isolated from septic rats treated with the PKCδ-TAT peptide inhibitor compared to septic rats treated with vehicle, suggesting that PKCδ may be a negative regulator of phagocytosis in sepsis. Thus, increased pathogen clearance by PKCδ inhibition is most likely the result of increased phagocytic activity. IL-10 is a known inhibitor of macrophage function including phagocytic activity and IL-10−/− mice demonstrate significantly greater bacterial phagocytosis in sepsis as compared to wild type mice (52–54). Sepsis induced by CLP significantly increased both peritoneal and blood levels of IL-10. To investigate possible mechanisms by which PKCδ may regulate phagocytosis, we determined the impact of PKCδ inhibition on IL-10 levels. Administration of the PKCδ inhibitor significantly reduced IL-10 levels both systemically and in the peritoneal cavity suggesting a possible mechanism of increased pathogen clearance. In support of this concept, PKCδ has also been shown to play an important proinflammatory role in infection of macrophages with the Leishmania major parasite whereby PKCδ regulates macrophage IL-10 production and an immune response favoring parasite growth (54). However, our findings that PKCδ inhibition increased isolated peritoneal macrophage phagocytic capacity ex vivo indicate other IL-10 independent mechanisms that directly impact the macrophages are also involved. In support of this concept, PKCδ has been shown to be a negative regulator of FcγR- mediated phagocytosis (55) and PKCδ associates with the TIRAP/Mal, a TLR2 and TLR4 specific adaptor in vitro (56). Further studies are needed to identify specific PKCδ-mediated pathways regulating phagocytosis in sepsis.
A critical finding in this study is that administration of the PKCδ TAT-peptide inhibitor after CLP surgery significantly improved survival under clinically relevant conditions where the animals received fluid resuscitation and antibiotics. This increased survival was associated with increased phagocytic activity, enhanced pathogen clearance, and decreased organ injury. Thus, PKCδ inhibition may be a novel therapeutic strategy used in conjunction with antibiotics.
Supplementary Material
Cell isolated from the peritoneal cavity were stained with markers for CD45, CD68, CD86, CD163, and CCR2. The gating strategy was sequential and first identified singlets followed by forward- vs side-scatter discrimination. Next, these cells were gated for CD45 expression. CD68 was used to identify the macrophages within the CD45+ population and these CD68+ cells were also CD86+. Finally, the CD68+CD86+ cells were gated for CD163 expression. CCR2+ cells were measured in the CD68+CD86+CD163+ and CD68+CD86+CD163- cell populations and these data are shown in Table II. Isotype controls were used to set the gates for each of the markers.
Acknowledgements
Supported, in part, by the National Institute of General Medical Sciences and National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) Grant No. HL111552, GM114359, GM134701 (LEK), DA040619 and P30DA13429 (TJR), AI132996 and AI125429 (CT)
Abbreviations
- AKI
Acute kidney injury
- BALF
Bronchoalveolar lavage fluid
- BUN
Blood urea nitrogen
- CCL
C-C motif chemokine ligand
- CX3CL1
Chemokine (C-X3-C motif) ligand 1 also known as fractalkine
- CFU
Colony forming units
- CLP
Cecal ligation and puncture
- FACS
Fluorescence-activated cell sorting
- FcγR
Fc gamma receptor
- FITC
Fluorescein isothiocyanate
- H & E
Hematoxylin and Eosin
- IL-1
Interleukin-1
- IT
Intra-tracheally
- LPS
Lipopolysaccharide
- MODS
Multiple organ dysfunction syndrome
- MOI
Multiplicity of infection
- MPO
Myeloperoxidase
- PBS
Phosphate buffered saline
- PCF
peritoneal cavity fluid
- PKCδ
Protein Kinase C-delta
- SpO2
Peripheral capillary oxygen saturation
- TAT
Transactivator of transcription
- TIRAP/Mal
TIR domain containing adaptor protein/MyD88 adaptor-like
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
Footnotes
Disclosures: L.E.K. is listed as an inventor on US patent #8,470,766 entitled “Novel Protein Kinase C Therapy for the Treatment of Acute Lung Injury” that is assigned to Children’s Hospital of Philadelphia and the University of Pennsylvania.”
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, and Angus DC (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)Consensus Definitions for Sepsis and Septic ShockConsensus Definitions for Sepsis and Septic Shock. JAMA 315, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutschman CS, and Tracey KJ (2014) Sepsis: Current Dogma and New Perspectives. Immunity 40, 463–475 [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, and van der Poll T (2013) Severe Sepsis and Septic Shock. New England Journal of Medicine 369, 840–851 [DOI] [PubMed] [Google Scholar]
- 4.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, and Walkey AJ (2014) Two Decades of Mortality Trends Among Patients With Severe Sepsis: A Comparative Meta-Analysis*. Critical Care Medicine 42, 625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craciun FL, Iskander KN, Chiswick EL, Stepien DM, Henderson JM, and Remick DG (2014) Early murine polymicrobial sepsis predominantly causes renal injury. Shock 41, 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, and Treacher DF (2006) Neutrophils in development of multiple organ failure in sepsis. The Lancet 368, 157–169 [DOI] [PubMed] [Google Scholar]
- 7.Aldridge AJ (2002) Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg 168, 204–214 [DOI] [PubMed] [Google Scholar]
- 8.Williams AE, and Chambers RC (2014) The mercurial nature of neutrophils: still an enigma in ARDS? American Journal of Physiology - Lung Cellular and Molecular Physiology 306, L217–L230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, and Remick DG (2013) Sepsis: Multiple Abnormalities, Heterogeneous Responses, and Evolving Understanding. Physiological Reviews 93, 1247–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondrinos MJ, Kennedy PA, Lyons M, Deutschman CS, and Kilpatrick LE (2013) Protein Kinase C and Acute Respiratory Distress Syndrome. Shock 39, 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick LE, Sun S, and Korchak HM (2004) Selective regulation by delta-PKC and PI 3-kinase in the assembly of the antiapoptotic TNFR-1 signaling complex in neutrophils. Am J Physiol Cell Physiol 287, C633–642 [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick LE, Sun S, Mackie D, Baik F, Li H, and Korchak HM (2006) Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of {delta}-PKC and ERK1/2. J Leuk Biol 80, 1512–1521 [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick LE, Sun S, Li H, Vary TC, and Korchak HM (2010) Regulation of TNF-induced oxygen radical production in human neutrophils: role of d-PKC. Journal of Leukocyte Biology 87, 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick LE, Standage SW, Li H, Raj NR, Korchak HM, Wolfson MR, and Deutschman CS (2011) Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-d (d-PKC). Journal of Leukocyte Biology 89, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondrinos MJ, Zhang T, Sun S, Kennedy PA, King DJ, Wolfson MR, Knight LC, Scalia R, and Kilpatrick LE (2014) Pulmonary Endothelial Protein Kinase C-Delta (PKCd) Regulates Neutrophil Migration in Acute Lung Inflammation. The American Journal of Pathology 184, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondrinos MJ, Knight LC, Kennedy PA, Wu J, Kauffman M, Baker ST, Wolfson MR, and Kilpatrick LE (2015) Biodistribution and Efficacy of Targeted Pulmonary Delivery of a Protein Kinase C-d Inhibitory Peptide: Impact on Indirect Lung Injury. Journal of Pharmacology and Experimental Therapeutics 355, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soroush F, Zhang T, King DJ, Tang Y, Deosarkar S, Prabhakarpandian B, Kilpatrick LE, and Kiani MF (2016) A novel microfluidic assay reveals a key role for protein kinase C delta in regulating human neutrophil-endothelium interaction. J Leukoc Biol 100, 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liverani E, Mondrinos MJ, Sun S, Kunapuli SP, and Kilpatrick LE (2018) Role of Protein Kinase C-delta in regulating platelet activation and platelet-leukocyte interaction during sepsis. PLOS ONE 13, e0195379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Soroush F, Sun S, Liverani E, Langston JC, Yang Q, Kilpatrick LE, and Kiani MF (2018) Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. Journal of Neuroinflammation 15, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soroush F, Tang Y, Guglielmo K, Engelmann A, Liverani E, Patel A, Langston J, Sun S, Kunapuli S, Kiani MF, and Kilpatrick LE (2019) Protein Kinase C-Delta (PKCδ) Tyrosine Phosphorylation is a Critical Regulator of Neutrophil-Endothelial Cell Interaction in Inflammation. Shock 51, 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilpatrick LE, Lee JY, Haines KM, Campbell DE, Sullivan KE, and Korchak HM (2002) A role for PKC-delta and PI 3-kinase in TNF-alpha-mediated antiapoptotic signaling in the human neutrophil. Am J Physiol Cell Physiol 283, C48–57 [DOI] [PubMed] [Google Scholar]
- 22.Vancurova I, Miskolci V, and Davidson D (2001) NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J Biol Chem 276, 19746–19752 [DOI] [PubMed] [Google Scholar]
- 23.Page K, Li J, Zhou L, Iasvovskaia S, Corbit KC, Soh JW, Weinstein IB, Brasier AR, Lin A, and Hershenson MB (2003) Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol 170, 5681–5689 [DOI] [PubMed] [Google Scholar]
- 24.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, and Messing RO (2004) Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest 114, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI, Nakayama K, Steele C, and Mossman BT (2007) Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol 170, 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramnath R, Sun J, and Bhatia M (2010) PKC δ mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. Journal of Molecular Medicine 88, 1–9 [DOI] [PubMed] [Google Scholar]
- 27.Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, Ayala A, Rounds S, Klinger JR, and Harrington EO (2012) Genetic disruption of protein kinase Cdelta reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303, L880–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW 2nd, and Mochly-Rosen D (2001) Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A 98, 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begley R, Liron T, Baryza J, and Mochly-Rosen D (2004) Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem Biophys Res Commun 318, 949–954 [DOI] [PubMed] [Google Scholar]
- 30.Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, and Mochly-Rosen D (2004) Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci 24, 6880–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki K, Hahn HS, Dorn GW 2nd, and Mochly-Rosen D (2003) Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation 108, 869–875 [DOI] [PubMed] [Google Scholar]
- 32.Mestry N, Thirumaran M, Tuggey JM, Macdonald W, and Elliott MW (2009) Hypoxic challenge flight assessments in patients with severe chest wall deformity or neuromuscular disease at risk for nocturnal hypoventilation. Thorax 64, 532–534 [DOI] [PubMed] [Google Scholar]
- 33.Neumann B, Zantl N, Veihelmann A, Emmanuilidis K, Pfeffer K, Heidecke CD, and Holzmann B (1999) Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int Immunol 11, 217–227 [DOI] [PubMed] [Google Scholar]
- 34.Wagner EM, Karagulova G, Jenkins J, Bishai J, and McClintock J (2006) Changes in lung permeability after chronic pulmonary artery obstruction. J Appl Physiol (1985) 100, 1224–1229 [DOI] [PubMed] [Google Scholar]
- 35.Toledo-Rodriguez M, Loyse N, Bourdon C, Arab S, and Pausova Z (2012) Effect of prenatal exposure to nicotine on kidney glomerular mass and AT1R expression in genetically diverse strains of rats. Toxicology Letters 213, 228–234 [DOI] [PubMed] [Google Scholar]
- 36.Bhargava R, Altmann CJ, Andres-Hernando A, Webb RG, Okamura K, Yang Y, Falk S, Schmidt EP, and Faubel S (2013) Acute Lung Injury and Acute Kidney Injury Are Established by Four Hours in Experimental Sepsis and Are Improved with Pre, but Not Post, Sepsis Administration of TNF-α Antibodies. PLOS ONE 8, e79037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poston JT, and Koyner JL (2019) Sepsis associated acute kidney injury. BMJ 364, k4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi ÊJ, Correa-Silva R, Elias RM, Salvador F, Moraes-Vieira PM, Cenedeze MA, Reis MA, Hiyane MI, Pacheco-Silva Á, Gonçalves GM, and Câmara NOS (2012) TLR2, TLR4 and the MYD88 Signaling Pathway Are Crucial for Neutrophil Migration in Acute Kidney Injury Induced by Sepsis. PLOS ONE 7, e37584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Monneret G, and Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The Lancet Infectious Diseases 13, 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, and Stewart DJ (2010) Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am. J. Respir. Crit. Care Med. 182, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 41.Haskó G, Csóka B, Koscsó B, Chandra R, Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virág L, Gergely P, Rolandelli RH, and Németh ZH (2011) Ecto-5′-Nucleotidase (CD73) Decreases Mortality and Organ Injury in Sepsis. The Journal of Immunology 187, 4256–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perl M, Chung C-S, Perl U, Thakkar R, Lomas-Neira J, and Ayala A (2010) Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury Critical Care Medicine 38, 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss YG, Maloyan A, Tazelaar J, Raj N, and Deutschman CS (2002) Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest 110, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slutsky AS (2002) Hot new therapy for sepsis and the acute respiratory distress syndrome. The Journal of Clinical Investigation 110, 737–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta N, Su X, Popov B, Lee JW, Serikov V, and Matthay MA (2007) Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. The Journal of Immunology 179, 1855–1863 [DOI] [PubMed] [Google Scholar]
- 46.Tao X, Song Z, Wang C, Luo H, Luo Q, Lin X, Zhang L, Yin Y, and Cao J (2017) Interleukin 36α Attenuates Sepsis by Enhancing Antibacterial Functions of Macrophages. The Journal of Infectious Diseases 215, 321–332 [DOI] [PubMed] [Google Scholar]
- 47.Figueiredo N, Chora A, Raquel H, Pejanovic N, Pereira P, Hartleben B, Neves-Costa A, Moita C, Pedroso D, Pinto A, Marques S, Faridi H, Costa P, Gozzelino R, Zhao, Jimmy L, Soares, Miguel P, Gama-Carvalho M, Martinez J, Zhang Q, Döring G, Grompe M, Simas JP, Huber, Tobias B, Baltimore D, Gupta V, Green, Douglas R, Ferreira, João A, and Moita, Luis F (2013) Anthracyclines Induce DNA Damage Response-Mediated Protection against Severe Sepsis. Immunity 39, 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castanheira F. V. e. S., Borges V, Sônego F, Kanashiro A, Donate PB, Melo PH, Pallas K, Russo RC, Amaral FA, Teixeira MM, Ramalho FS, Cunha TM, Liew FY, Alves-Filho JC, Graham GJ, and Cunha FQ (2018) The Atypical Chemokine Receptor ACKR2 is Protective Against Sepsis. Shock 49, 682–689 [DOI] [PubMed] [Google Scholar]
- 49.Villa P, Saccani A, Sica A, and Ghezzi P (2002) Glutathione Protects Mice from Lethal Sepsis by Limiting Inflammation and Potentiating Host Defense. The Journal of Infectious Diseases 185, 1115–1120 [DOI] [PubMed] [Google Scholar]
- 50.Medzhitov R (2013) Septic Shock: On the Importance of Being Tolerant. Immunity 39, 799–800 [DOI] [PubMed] [Google Scholar]
- 51.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora Â, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, and Soares MP (2010) A Central Role for Free Heme in the Pathogenesis of Severe Sepsis. Science Translational Medicine 2, 51ra71–51ra71 [DOI] [PubMed] [Google Scholar]
- 52.Couper KN, Blount DG, and Riley EM (2008) IL-10: The Master Regulator of Immunity to Infection. The Journal of Immunology 180, 5771–5777 [DOI] [PubMed] [Google Scholar]
- 53.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G, and DeMatteo RP (2011) Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. Journal of Leukocyte Biology 89, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudan R, Srivastava N, Pandey SP, Majumdar S, and Saha B (2012) Reciprocal Regulation of Protein Kinase C Isoforms Results in Differential Cellular Responsiveness. The Journal of Immunology 188, 2328–2337 [DOI] [PubMed] [Google Scholar]
- 55.Hazeki K, Inoue K, Nigorikawa K, and Hazeki O (2009) Negative Regulation of Class IA Phosphoinositide 3-kinase by Protein Kinase Cδ Limits Fcγ Receptor-Mediated Phagocytosis in Macrophages. The Journal of Biochemistry 145, 87–94 [DOI] [PubMed] [Google Scholar]
- 56.Kubo-Murai M, Hazeki K, Sukenobu N, Yoshikawa K, Nigorikawa K, Inoue K, Yamamoto T, Matsumoto M, Seya T, Inoue N, and Hazeki O (2007) Protein kinase Cδ binds TIRAP/Mal to participate in TLR signaling. Molecular Immunology 44, 2257–2264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell isolated from the peritoneal cavity were stained with markers for CD45, CD68, CD86, CD163, and CCR2. The gating strategy was sequential and first identified singlets followed by forward- vs side-scatter discrimination. Next, these cells were gated for CD45 expression. CD68 was used to identify the macrophages within the CD45+ population and these CD68+ cells were also CD86+. Finally, the CD68+CD86+ cells were gated for CD163 expression. CCR2+ cells were measured in the CD68+CD86+CD163+ and CD68+CD86+CD163- cell populations and these data are shown in Table II. Isotype controls were used to set the gates for each of the markers.