Abstract
This study aimed to investigate whether the initial red cell distribution width (RDW) at the emergency department (ED) is associated with poor neurological outcomes in out-of-hospital cardiac arrest (OHCA) survivors. We performed a prospective observational analysis of patients admitted to the ED between October 2015 and June 2018 from the Korean Cardiac Arrest Research Consortium registry. We included OHCA patients who visited the ED and achieved return of spontaneous circulation. Initial RDW values were measured at the time of the ED visit. The primary outcome was a poor neurological (Cerebral Performance Category, or CPC) score of 3–5. A total of 1008 patients were ultimately included in this study, of whom 712 (70.6%) had poor CPC scores with unfavorable outcomes. Higher RDW quartiles (RDW 13.6–14.9%, RDW ≥ 15.0%), older age, female sex, nonshockable initial rhythm at the scene, unwitnessed cardiac arrest, bystander cardiopulmonary resuscitation (CPR), medical history, low white blood cell counts and high glucose levels were associated with poor neurological outcomes in univariate analysis. In multivariate analysis, the highest RDW quartile was independently associated with poor neurological outcomes (odds ratio 2.04; 95% confidence interval 1.12–3.69; p = 0.019) at hospital discharge after adjusting for other confounding factors. Other independent factors including age, initial rhythm, bystander CPR and high glucose were also associated with poor neurological outcomes. These results show that an initial RDW in the highest quartile as of the ED visit is associated with poor neurological outcomes at hospital discharge among OHCA survivors.
Subject terms: Biomarkers, Medical research
Introduction
Out-of-hospital cardiac arrest (OHCA) is becoming an issue worldwide, and a considerable amount of research is being conducted on factors related to survival1–3. Among patients with OHCA, return of spontaneous circulation (ROSC) occurs in a maximum of approximately 10%; furthermore, even OHCA survivors with ROSC often die in the emergency department (ED) within 24 h, and the survival-to-discharge rate at the hospital is known to be only approximately 10%. Additionally, many OHCA survivors show poor neurological outcomes at discharge1,2.
High red cell distribution width (RDW) values are used as a predictor of poor prognosis in many diseases, including cardiovascular diseases, perioperative stroke, malignancies and respiratory diseases4–8. Elevated RDW values are associated with adequate collateral development in patients with stable coronary artery disease and perioperative stroke in patients undergoing cardiac valve surgery9,10. Additionally, high RDW is associated with inflammatory conditions in various diseases, such as Hashimoto’s thyroiditis, rheumatoid arthritis, inflammatory bowel disease and gastrointestinal disorders11–14. In addition, a previous study found that RDW is strongly associated with prognostic factors reflecting severe inflammation, and changes in RDW within 72 h of ED admission are associated with 90-day mortality in critical patients with septic shock15,16. Moreover, Kim et al. reported that changes in the RDW value are an independent risk factor for all-cause mortality in OHCA patients17.
For the critical care management of OHCA survivors in EDs, the early prediction of neurological outcomes and mortality is also important for decision making by emergency medicine (EM) physicians in active and intensive care. Poor neurological outcomes in OHCA survivors are associated with ischemic reperfusion injury of the brain and can cause an increase in various inflammatory markers associated with sepsis-like physiological mechanisms of OHCA18. Studies indicate that C-reactive protein, procalcitonin, and the neutrophil-to-lymphocyte ratio can be used as inflammatory markers of cardiac arrest to predict neurologic outcomes19–21. However, few studies have used multicenter research data to investigate whether RDW is a prognostic factor predicting poor neurological outcomes21.
RDW can be easily checked from an initial blood sample in the ED, and the results can be obtained rapidly. Therefore, the aim of this study was to investigate whether initial RDW in the ED is associated with not only mortality but also poor neurological outcomes in OHCA survivors who visited the ED.
Materials and methods
Study design and setting
We conducted a multicenter prospective observational study based on the Korean Cardiac Arrest Research Consortium (KoCARC) registry between October 2015 and June 2018. The KoCARC is a multicenter collaborative research network of hospitals in the Republic of Korea3. The present study involved nontraumatic OHCA patients who were resuscitated. This study also included patients who had ROSC after cardiopulmonary resuscitation (CPR) by emergency medical services (EMS) on the scene. The exclusion criteria of this registry were patients with a history of terminal illness in their medical charts, patients who were pregnant, and patients with a previously documented “Do Not Resuscitate” order. The participating hospitals shared a standard registry form, and the investigators at each participating hospital collected EMS records and reviewed hospital medical charts for the clinical characteristics of the patients. The KoCARC registry was registered at clinicaltrials.gov as protocol NCT03222999.
Study population and data collection
The study population included OHCA patients in the KoCARC registry who visited the ED and had ROSC at the scene or at the ED. We excluded OHCA patients under 15 years old and patients with incomplete or missing RDW data. We analyzed several variables from the KoCARC registry: patient demographic data (age and sex); initial prehospital rhythm; presence of witnesses to cardiac arrest; bystander CPR; prehospital ROSC; comorbidities (hypertension, diabetes mellitus, dyslipidemia); initial hospital rhythm; and white blood cells (WBCs), total bilirubin, glucose and RDW evaluated from the initial blood sample in the ED.
Neurological outcomes were measured with the Cerebral Performance Category (CPC) scale at hospital discharge. A CPC score of 1 (good cerebral performance) or 2 (moderate cerebral disability) was considered a favorable neurological outcome, and a score of 3 (severe cerebral disability), 4 (coma or vegetative state), or 5 (death) was considered a poor neurological outcome. Additionally, 24-h mortality and 30-day mortality in OHCA survivors were analyzed.
Statistical analysis
We explored demographic characteristics, comorbidities and laboratory findings to analyze the predictors of poor outcomes. The Kolmogorov–Smirnov test was applied to continuous variables to test whether they followed the normal distribution; for those that were not normally distributed, the results are presented as the medians and interquartile ranges. RDW was divided into 4 quartiles, defined by ranges of ≤ 12.7%, 12.8–13.5%, 13.6–14.9% and ≥ 15.0%. Demographic and laboratory data of OHCA survivors were analyzed according to RDW quartiles, and the continuous variables were compared using the Kruskal–Wallis test. The categorical variables were compared and analyzed using the chi-square test or Fisher's exact test.
Univariate and multivariate Cox regression analyses were used for the prediction of 30-day mortality; they are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariate logistic analysis was used to identify independent predictors of poor neurological outcome at hospital discharge. The predictors are presented with their odds ratios (ORs) and 95% CIs, and statistical significance is defined at a p value < 0.05. Receiver operating characteristic (ROC) curves were drawn to estimate the sensitivity and specificity of the RDW level in terms of predicting poor CPC; we also calculated the areas under the ROC curves (AUCs). All analyses were performed using SPSS software version 24.0 (SPSS, Inc., Chicago, IL, USA).
Human ethical approval and informed consent
The KoCARC data collection protocol of this study was approved by the Institutional Review Boards (IRBs) of 34 participating hospitals. In a recent study, KoCARC investigators described the conceptualization, development, and implementation processes of the KoCARC registry to improve OHCA outcomes in the Republic of Korea3. The IRBs of most of the participating institutions (Seoul National University Hospital, Konkuk University Medical Center, Kyung Hee University Hospital, Korea University Guro Hospital, Korea University Anam Hospital, SMG-SNU Boramae Medical Center, Yonsei University Severance Hospital, Yonsei University Gangnam Severance Hospital, Hallym University Kangdong Sacred Heart Hospital, Hallym University Kangnam Sacred Heart Hospital, Hanyang University Seoul Hospital, Kyungpook National University Hospital, Chosun University Hospital, Seoul National University Bundang Hospital, Myongji Hospital, Korea University Ansan Hospital, Dongguk University Ilsan Hospital, Bundang Jesaeng Hospital, Wonkwang University Sanbon Hospital, Hallym University Dongtan Sacred Heart Hospital, Chungbuk National University Hospital, Soonchunhyang University Cheonan Hospital, Jeju National University Hospital, Hanyang University Guri Hospital, Gyeongsang National University Hospital, Ajou University Hospital, Pusan National University Yangsan Hospital, Ewha Womans University Mokdong Medical Center, Inha University Hospital, and Hallym University Sacred Heart Hospital) waived the requirement for informed consent3. However, Samsung Medical Center, Asan Medical Center, Wonju Severance Christian Hospital and Incheon St. Mary’s Hospital received informed consent from individual participants for the follow-up survey of neurological outcomes and death. (The requirement for informed consent was waived for participants who did not need a telephone interview for the follow-up survey.) This study was approved by the Ethics Committee of Incheon St. Mary’s Hospital (OC15OIMI0134). All methods were performed in accordance with the relevant guidelines and regulations.
Results
Characteristics of the study population
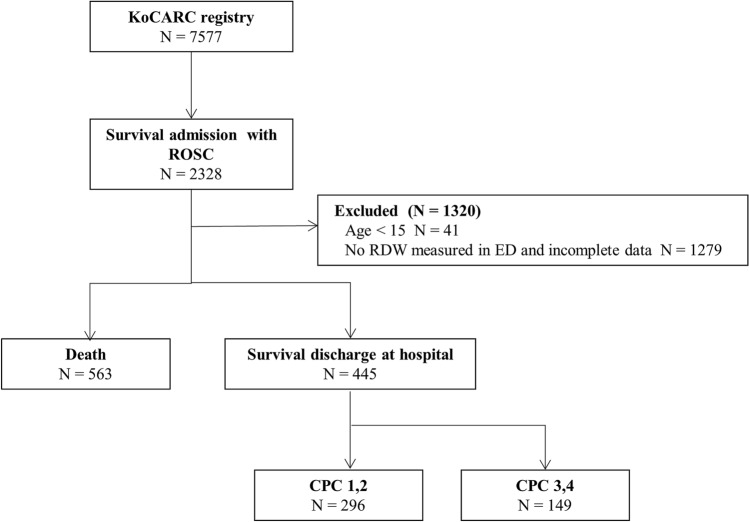
Of the 7577 OHCA patients examined during this research period, 5249 were excluded because they did not survive to hospital admission. In addition, of the 2328 patients who survived admission, 41 patients were excluded because they were under 15 years old. Moreover, 1279 patients were excluded due to incomplete data or missing initial RDW at the ED. We ultimately included a total of 1008 nontraumatic OHCA survivors (Fig. 1).
Figure 1.
Flow diagram of out-of-hospital cardiac arrest survivors in this study.
The mean age of the patients was 61.9 (SD 15.3) years, the median (IQR) age was 62 (52, 74) years, and 717 (71.1%) patients were male. Two hundred and fifty-three patients (25.1%) had high RDW values (≥ 15%) on arrival in the ED. After ROSC, a total of 285 (28.3%) patients died within 24 h, and 550 (54.6%) patients died within 30 days. A total of 712 (70.6%) patients had poor CPC scores (indicating unfavorable outcomes) at hospital discharge (Table 1). The clinical characteristics of the patients according to their initial RDW quartiles after ROSC were analyzed and are shown in Table 2. The factors that significantly differed among the four RDW quartile groups included age, initial shockable rhythm at the scene, bystander CPR, prehospital ROSC, history of diabetes, total bilirubin and glucose. Additionally, 24-h mortality, 30-day mortality and poor CPC outcomes at hospital discharge were significantly different across the four RDW quartiles (p < 0.001, p < 0.001, p < 0.001) (Table 2).
Table 1.
Baseline characteristics of the study population.
| Total | |
|---|---|
| N = 1008 | |
| Age (years) | |
| Mean ± SD | 61.9 ± 15.3 |
| Median (IQR) | 62 (52, 74) |
| Sex | |
| Male | 717 (71.1) |
| Female | 291 (28.9) |
| Initial shockable rhythm at the scene | |
| Nonshockable | 552 (59.9) |
| Shockable | 369 (40.1) |
| Initial shockable rhythm in the hospital | |
| Nonshockable | 927 (92) |
| Shockable | 81 (8) |
| Bystander CPR | |
| No | 423 (45.9) |
| Yes | 499 (54.1) |
| Prehospital ROSC | |
| No | 616 (61.1) |
| Yes | 392 (38.9) |
| Hypertension | |
| No | 490 (53.5) |
| Yes | 426 (46.5) |
| Diabetes mellitus | |
| No | 636 (70.6) |
| Yes | 265 (29.4) |
| Dyslipidemia | |
| No | 814 (93.2) |
| Yes | 59 (6.8) |
| Witnessed arrest | |
| No | 241 (24.5) |
| Yes | 742 (75.5) |
| RDW (quartile) | |
| < 25% (≤ 12.7%) | 238 (23.6) |
| 25–50% (12.8–13.5%) | 243 (24.1) |
| 50–75% (13.6–14.9%) | 274 (27.2) |
| > 75% (≥ 15%) | 253 (25.1) |
| White blood cell count (109/L) | |
| Mean ± SD | 13 ± 5.6 |
| Median (IQR) | 12 (9.3, 15.5) |
| Total bilirubin (mg/dL) | |
| Mean ± SD | 0.7 ± 1.2 |
| Median (IQR) | 0.5 (0.3, 0.7) |
| Glucose (mg/dL) | |
| Mean ± SD | 279.4 ± 143.9 |
| Median (IQR) | 264 (190, 339) |
| Survival discharge | |
| No | 563 (55.9) |
| Yes | 445 (44.2) |
| 24-h mortality | |
| No | 723 (71.7) |
| Yes | 285 (28.3) |
| 30-day mortality | |
| No | 458 (45.4) |
| Yes | 550 (54.6) |
| Poor CPC | |
| No | 296 (29.4) |
| Yes | 712 (70.6) |
CPR cardiopulmonary resuscitation, RDW red cell distribution width, ROSC return of spontaneous circulation, CPC Cerebral Performance Category.
Table 2.
Patient characteristics stratified according to red cell distribution width quartiles.
| ≤ 12.7% | 12.8–13.5% | 13.6–14.9% | ≥ 15% | p value | |
|---|---|---|---|---|---|
| n = 238 | n = 243 | n = 274 | n = 253 | ||
| Age | < 0.001 | ||||
| Median (IQR) | 57.5 (49, 69) | 61 (51, 73) | 65 (55, 76) | 66 (55, 76) | |
| Sex | 0.075 | ||||
| Male | 178 (74.8) | 180 (74.1) | 194 (70.8) | 165 (65.2) | |
| Female | 60 (25.2) | 63 (25.9) | 80 (29.2) | 88 (34.8) | |
| Initial shockable rhythm at the scene | < 0.001 | ||||
| Nonshockable | 111 (51.4) | 115 (52.5) | 150 (59.8) | 176 (74.9) | |
| Shockable | 105 (48.6) | 104 (47.5) | 101 (40.2) | 59 (25.1) | |
| Initial shockable rhythm in the hospital | 0.076 | ||||
| Nonshockable | 219 (92) | 223 (91.8) | 244 (89.1) | 241 (95.3) | |
| Shockable | 19 (8) | 20 (8.2) | 30 (11) | 12 (4.7) | |
| Witnessed arrest | 0.306 | ||||
| No | 68 (29.1) | 55 (23.1) | 64 (23.9) | 54 (22.2) | |
| Yes | 166 (70.9) | 183 (76.9) | 204 (76.1) | 189 (77.8) | |
| Bystander CPR | < 0.001 | ||||
| No | 84 (37.7) | 86 (39.5) | 126 (50.2) | 127 (55.2) | |
| Yes | 139 (62.3) | 132 (60.6) | 125 (49.8) | 103 (44.8) | |
| Prehospital ROSC | < 0.001 | ||||
| No | 136 (57.1) | 118 (48.6) | 172 (62.8) | 190 (75.1) | |
| Yes | 102 (42.9) | 125 (51.4) | 102 (37.2) | 63 (24.9) | |
| Hypertension | 0.078 | ||||
| No | 128 (59.3) | 125 (56.6) | 124 (49.4) | 113 (49.6) | |
| Yes | 88 (40.7) | 96 (43.4) | 127 (50.6) | 115 (50.4) | |
| Diabetes mellitus | < 0.001 | ||||
| No | 170 (79.1) | 167 (77.3) | 168 (68.6) | 131 (58.2) | |
| Yes | 45 (20.9) | 49 (22.7) | 77 (31.4) | 94 (41.8) | |
| Dyslipidemia | 0.390 | ||||
| No | 199 (93.9) | 192 (91) | 227 (95) | 196 (92.9) | |
| Yes | 13 (6.1) | 19 (9) | 12 (5) | 15 (7.1) | |
| Witnessed arrest | 0.306 | ||||
| No | 68 (29.1) | 55 (23.1) | 64 (23.9) | 54 (22.2) | |
| Yes | 166 (70.9) | 183 (76.9) | 204 (76.1) | 189 (77.8) | |
| WBC count (109/L) | 0.053 | ||||
| Median (IQR) | 12.3 (9.8, 15.5) | 12.4 (9.5, 15.5) | 12.2 (9.6, 16.1) | 11.3 (8.6, 15.3) | |
| Total bilirubin (mg/dL) | < 0.001 | ||||
| Median (IQR) | 0.5 (0.3, 0.6) | 0.5 (0.3, 0.7) | 0.4 (0.3, 0.7) | 0.6 (0.3, 1) | |
| Glucose (mg/dL) | < 0.001 | ||||
| Median (IQR) | 285 (214, 346) | 267 (209, 342) | 265.5 (193, 350) | 234 (150, 321) | |
| Survival discharge | < 0.001 | ||||
| No | 117 (49.2) | 108 (44.4) | 158 (57.7) | 180 (71.2) | |
| Yes | 121 (50.8) | 135 (55.6) | 116 (42.3) | 73 (28.9) | |
| 24-h mortality | < 0.001 | ||||
| No | 190 (79.8) | 178 (73.3) | 198 (72.3) | 157 (62.1) | |
| Yes | 48 (20.2) | 65 (26.8) | 76 (27.7) | 96 (37.9) | |
| 30-day mortality | < 0.001 | ||||
| No | 124 (52.1) | 139 (57.2) | 120 (43.8) | 75 (29.6) | |
| Yes | 114 (47.9) | 104 (42.8) | 154 (56.2) | 178 (70.4) | |
| Poor CPC | < 0.001 | ||||
| No | 85 (35.7) | 103 (42.4) | 72 (26.3) | 36 (14.2) | |
| Yes | 153 (64.3) | 140 (57.6) | 202 (73.7) | 217 (85.8) |
Values are shown as the number(percentage) for categorical variables and the mean (SD) or median (IQR) for other variables.
p values are calculated using the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables.
CPR cardiopulmonary resuscitation, RDW red cell distribution width, ROSC return of spontaneous circulation, WBC white blood cell, CPC Cerebral Performance Category.
Prediction of 30-day mortality in OHCA survivors
Regarding 30-day mortality, univariate analysis showed that the predictive factors included RDW in the highest quartile group, older age, female sex, initial rhythm at the scene, witnesses to cardiac arrest and a history of diabetes; moreover, WBC count and glucose level were also found to be predictive factors. In the multivariate analysis, RDW in the highest quartile (≥ 15.0%) was shown to be a predictive factor of 30-day mortality (HR 1.39; 95% CI 1.05–1.82; p = 0.020) (Table 3). Other significant factors included age, initial rhythm at the scene, WBC count, and glucose level (p < 0.001, p < 0.001, p = 0.039 and p = 0.016).
Table 3.
Hazard ratios of risk factors for mortality within 30 days.
| Univariate hazard ratio (95% CI) | p value | Multivariate hazard ratio (95% CI) | p value | |
|---|---|---|---|---|
| RDW (quartile) | ||||
| < 25% (≤ 12.7%) | (reference) | (reference) | ||
| 25–50% (12.8–13.5%) | 0.91 (0.70–1.19) | 0.483 | 0.92 (0.68–1.24) | 0.564 |
| 50–75% (13.6–14.9%) | 1.26 (0.99–1.60) | 0.063 | 1.13 (0.85–1.48) | 0.402 |
| > 75% (≥ 15%) | 1.73 (1.36–2.18) | < 0.001 | 1.39 (1.05–1.82) | 0.020 |
| Age | 1.02 (1.02–1.03) | < 0.001 | 1.02 (1.01–1.02) | < 0.001 |
| Female | 1.47 (1.24–1.76) | < 0.001 | 1.13 (0.93–1.39) | 0.225 |
| Initial shockable rhythm at the scene | 0.36 (0.29–0.44) | < 0.001 | 0.46 (0.36–0.58) | < 0.001 |
| Hypertension | 1.18 (0.98–1.40) | 0.074 | ||
| Diabetes mellitus | 1.43 (1.19–1.73) | < 0.001 | 1.14 (0.93–1.39) | 0.225 |
| Dyslipidemia | 0.74 (0.50–1.10) | 0.131 | ||
| Witness arrest | 0.74 (0.61–0.89) | 0.002 | 0.84 (0.68–1.04) | 0.106 |
| Bystander CPR | 0.85 (0.72–1.02) | 0.078 | ||
| WBC count (109/L) | 0.98 (0.96–0.99) | 0.004 | 0.98 (0.96–1.00) | 0.039 |
| Total bilirubin (mg/dL) | 1.02 (0.95–1.09) | 0.597 | ||
| Glucose (mg/dL) | 1.00 (1.00–1.00) | 0.009 | 1.00 (1.00–1.00) | 0.016 |
Hazard ratios were calculated by using univariate and multivariate Cox regression analysis.
RDW red cell distribution width, CPR cardiopulmonary resuscitation, WBC white blood cell.
Prediction of poor neurological outcomes in OHCA survivors
In the univariate analysis, RDW values in the ranges of 13.6–14.9% and ≥ 15.0% were associated with CPC scores indicating poor neurological outcomes at hospital discharge (p = 0.021, p < 0.001). After adjustment for demographic, prehospital and laboratory findings with p values < 0.05 in univariate analysis, multivariate logistic analysis was performed. Of all the independent prognostic factors, RDW ≥ 15% at ED admission had the highest OR for poor neurological outcomes at ED admission (OR 2.04, 95% CI 1.12–3.69, p = 0.019) (Table 4).
Table 4.
Odds ratios for poor Cerebral Performance Category at hospital discharge.
| Univariate analysis (95% CI) | p value | Multivariate analysis (95% CI) | p value | |
|---|---|---|---|---|
| RDW (quartile) | ||||
| < 25% (≤ 12.7%) | (reference) | (reference) | ||
| 25–50% (12.8–13.5%) | 0.76 (0.52–1.09) | 0.134 | 0.60 (0.35–1.01) | 0.053 |
| 50–75% (13.6–14.9%) | 1.56 (1.07–2.27) | 0.021 | 1.32 (0.78–2.24) | 0.308 |
| > 75% (≥ 15%) | 3.35 (2.15–5.21) | < 0.001 | 2.04 (1.12–3.69) | 0.019 |
| RDW (value) | 1.03 (1.00–1.06) | 0.082 | ||
| Age | 1.05 (1.04–1.06) | < 0.001 | 1.04 (1.02–1.05) | < 0.001 |
| Female | 2.30 (1.65–3.22) | < 0.001 | 1.42 (0.89–2.25) | 0.138 |
| Initial shockable rhythm at the scene | 0.09 (0.06–0.12) | < 0.001 | 0.12 (0.08–0.18) | < 0.001 |
| Hypertension | 1.45 (1.09–1.92) | 0.011 | 0.82 (0.53–1.27) | 0.383 |
| Diabetes mellitus | 2.46 (1.74–3.50) | < 0.001 | 1.45 (0.89–2.37) | 0.135 |
| Dyslipidemia | 0.48 (0.28–0.81) | 0.006 | 0.50 (0.24–1.06) | 0.071 |
| Witness arrest | 0.53 (0.37–0.75) | < 0.001 | 0.70 (0.44–1.13) | 0.149 |
| Bystander CPR | 0.57 (0.43–0.76) | < 0.001 | 1.55 (1.03–2.34) | 0.037 |
| WBC count (109/L) | 0.98 (0.95–1.00) | 0.044 | 0.97 (0.94–1.01) | 0.123 |
| Total bilirubin (mg/dL) | 1.00 (0.89–1.12) | 0.997 | ||
| Glucose (mg/dL) | 1.00 (1.00–1.00) | < 0.001 | 1.00 (1.00–1.01) | < 0.001 |
RDW red cell distribution width, CPR cardiopulmonary resuscitation, WBC white blood cell.
Odds ratios were calculated by logistic regression analysis.
RDW at the ED after ROSC had an AUC of 0.630 (95% CI 0.593–0.666) for predicting poor CPC at hospital discharge (p < 0.001). The group with the highest RDW (≥ 15.0%) showed a sensitivity of 29.8%, a specificity of 88.2%, a positive predictive value (PPV) of 85.8% and a negative predictive value (NPV) of 34.3% for poor CPC outcomes (Fig. 2).
Figure 2.
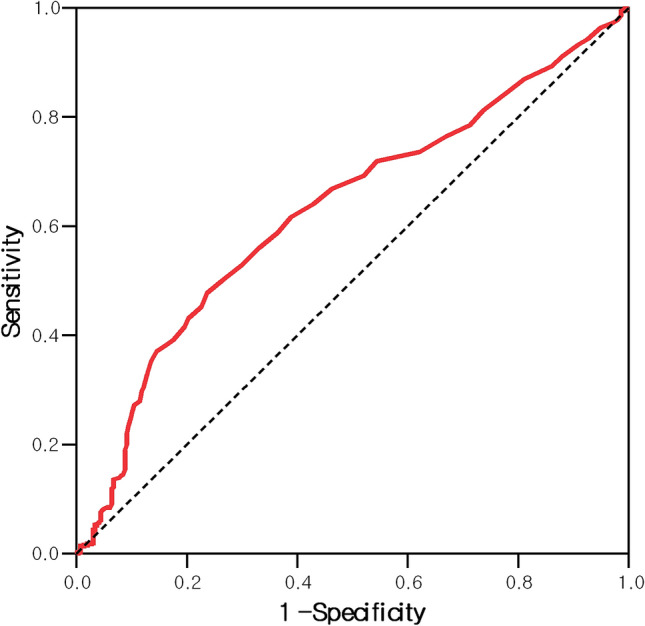
Receiver operating characteristic curve of the initial red cell distribution width for the prediction of poor neurological outcomes.
Discussion
The present study showed that RDW ≥ 15% was an independent predictor of poor CPC scores at hospital discharge in OHCA survivors. Significant predictive factors other than RDW included age and initial rhythm at the scene. RDW was an independent marker predicting 30-day mortality and poor neurological outcomes.
Ideal prognostic markers for use in the ED should be inexpensive and rapidly available, with rapidly confirmable results, to facilitate the decision making of ED physicians about the critical care of OHCA patients. RDW, a measurement of the range of variation in erythrocyte size and volume, can be obtained from a complete blood count from routine laboratory testing at the ED22. RDW levels may be increased or decreased according to OHCA patients’ initial clinical conditions at the ED17,21. In a previous study, RDW was an independent risk factor predicting the severity and progression of cardiovascular disease, inflammation and oxidative stress23–29. The pathophysiology of the association between the outcome of cardiovascular disease and RDW is unclear. However, elevated RDW may be related myocardial ischemia, as heart failure caused by atherosclerosis of the coronary vessel walls is stimulated by inflammation9,23,24. Some studies have shown that RDW is an effective prognostic factor for outcomes in critically ill patients and septic patients28–32. In Tonelli’s study, RDW levels were associated with the risk of adverse outcomes and mortality in patients with coronary disease33. Felker’s study showed an association between increased RDW and mortality in congestive heart failure (CHF) patients (HR 1.17)24. Additionally, among 219 OHCA patients who had ROSC, the highest RDW quartile (> 15.4%) in OHCA patients admitted to the ED was independently associated with 30-day all-cause mortality (HR 1.95)17. Our study yielded similar results. Among the 1008 OHCA patients who had ROSC, the highest RDW (≥ 15.0%) quartile in admitted ED patients was independently associated with an increased risk of mortality during the 30-day period (HR 1.39). Additionally, RDW showed significantly elevated values in subarachnoid hemorrhage (SAH) patients and cerebral infarction patients with poor outcomes34,35. Although cardiovascular disease is known to be the main cause of OHCA, stroke may also be a cause. Studies on the association between increased RDW and poor outcomes in cardiovascular disease and stroke have already been proposed23–26,34,35. Therefore, the poor outcome of OHCA patients may be related to RDW.
Few studies have focused on the association between RDW and neurological outcomes in patients with ROSC following OHCA21. In a previous single-center study, high RDW levels on admission were associated with poor CPC outcomes (scores of 3–5) among cardiac arrest (CA) survivors admitted to the intensive care unit (390 patients)21. They showed that an RDW threshold of 13.4% on admission had a sensitivity of 64% and a specificity of 43% for predicting poor CPC (3–5) after 3 months of survival21. The present study showed that an initial RDW value of ≥ 15.0% at the time of the ED visit had a sensitivity of 29.8% and a specificity of 88.2% for predicting unfavorable outcomes at hospital discharge. Although the sensitivity of RDW in predicting neurological outcomes is not high, it showed relatively high specificity for predicting poor CPC scores in the ED. RDW combined with other prognostic factors may be useful in predicting the neurological prognosis of OHCA survivors in the ED. Our investigation is a multicenter study that is based on ED visits. The study included all patients who died within 24 h after ROSC, and we analyzed the prediction of poor CPC at hospital discharge. This may be why the present study has higher specificity than previous studies.
RDW is measured as part of a complete blood count, which is a standard routine laboratory test in the ED22; thus, RDW is easily obtainable and simple for EM physicians to use as a prognostic marker. However, the direct association between the prognostic variable of RDW and the outcome variable of CA is unclear. The presence of increased RDW in OHCA survivors with poor outcomes may be mediated by other factors rather than a single prognostic factor. A previous study reported the predictive value of RDW and N-terminal pro-brain natriuretic peptide (NT-proBNP) for the mortality of CHF patients. The authors indicated that the RDW value was a predictor of mortality but had lower predictive value than NT-proBNP. They suggested that RDW be used along with other biomarkers as a complementary prognostic factor to improve the accuracy of prognosis25. Therefore, further prospective studies on OHCA survivors are needed to validate the predictive association between RDW and poor outcomes.
This study has several limitations. First, our study has some selection bias. Although prospectively collected KoCARC registry data were analyzed, 1279 patients were excluded from the analysis because of incomplete or missing data, as their RDW values had not been measured in the ED. Second, we could not analyze the relationship between RDW and long-term neurological outcomes. Further studies should examine 6-month neurological outcomes, activities of daily living, and return to work as elements of health-related quality of life in OHCA survivors. Additionally, although this study included only OHCA survivors who met the inclusion criteria, there are various confounding factors because the baseline characteristics of each CA patient are different. However, this study is meaningful because it suggested that RDW could be used as an early prognostic marker for poor neurological outcomes in OHCA survivors.
In conclusion, high initial RDW values in the ED were associated with poor neurological outcomes at hospital discharge and with 30-day mortality in OHCA survivors. Thus, these results suggest that initial RDW can be a useful predictive marker of poor outcomes in OHCA survivors who visit the ED.
Acknowledgements
We would like to acknowledge and thank to investigators from all participating hospitals of KoCARC: Do Kyun Kim (Seoul National University Hospital), Sang Kuk Han, Phil Cho Choi (Kangbuk Samsung Medical Center), Sang O Park, Jong Won Kim (Konkuk University Medical Center), Han Sung Choi, Jong Seok Lee (Kyung Hee University Hospital), Sung Hyuk Choi, Young Hoon Yoon (Korea University Guro Hospital), Su Jin Kim (Korea University Anam Hospital), Min Seob Sim, Gun Tak Lee (Samsung Medical Center), Shin Ahn (Asan Medical Center), Jong Whan Shin (SMG-SNU Boramae Medical Center), Sang Hyun Park, Keun Hong Park (Seoul Medical Center), In Cheol Park, Yoo Seok Park (Yonsei University Severance Hospital), Tae Young Kong (Yonsei University Gangnam Severance Hospital), Kyoung Won Lee, Chu Hyun Kim (Inje University Seoul Paik Hospital), Youngsuk Cho (Hallym University Kangdong Sacred Heart Hospital), Gu Hyun Kang, Yong Soo Jang (Hallym University Kangnam Sacred Heart Hospital), Tai Ho Im, Jae Hoon Oh (Hanyang University Seoul Hospital), Seok Ran Yeom, Sang Kyoon Han (Pusan National University Hospital), Jae Hoon Lee (Dong-A University Hospital), Jeong Bae Park, Hyun Wook Ryoo (Kyungpook National University Hospital), Kyung Woo Lee, Tae Chang Jang (Daegu Catholic University Medical Center), Jae-hyug Woo (Gachon University Gil Medical Center), Woon Jeong Lee, Seon Hee Woo (The Catholic University of Korea Incheon St. Mary's Hospital), Sung Hyun Yun, Tae Jin Cho (Catholic Kwandong University International St. Mary's Hospital), Sun Pyo Kim, Yong Jin Park (Chosun University Hospital), Jin Woong Lee, Wonjoon Jeong (Chungnam National University Hospital), Sung Soo Park, Jae Kwang Lee (Konyang University Hospital), Ryeok Ahn, Wook Jin Choi (Ulsan University Hospital), Young Gi Min, Eun Jung Park (Ajou University Hospital), You Hwan Jo, Joong Hee Kim (Seoul National University Bundang Hospital), In Byung Kim, Ki Ok Ahn (Myongji Hospital), Han Jin Cho (Korea University Ansan Hospital), Seung Cheol Lee, Sang Hun Lee (Dongguk University Ilsan Hospital), Young Sik Kim, Young Rock Ha (Bundang Jesaeng Hospital), Jin Sik Park, Myoung Woo Lee (Sejong Hospital), Dai Han Wi (Wonkwang University Sanbon Hospital), Ok Jun Kim, Tae Nyoung Chung (Cha University Bundang Medical Center), Soon Joo Wang, Hang A Park (Hallym University Dongtan Sacred Heart Hospital), Jun Hwi Cho, Chan Woo Park (Kangwon National University Hospital), An Mu Eob, Tae Hun Lee (Hallym University Chuncheon Sacred Heart Hospital), Sang Chul Kim, Hoon Kim (Chungbuk National University Hospital), Han Joo Choi, Chan Young Koh (Dankook University Hospital), Jung Won Lee, Dong Wook Lee (Soonchunhyang University Cheonan Hospital), Tae Oh Jung, Jae Chol Yoon (Chonbuk National University Hospital), Dai Hai Choi, Jung Tae Choi (Dongguk University Gyeongju Hospital), Jin Hee Jeong, Soo Hoon Lee (Gyeongsang National University Hospital), Ji Ho Ryu, Maeng Real Park (Pusan National University Yangsan Hospital ), Won Kim (Cheju Halla General Hospital), Sung Wook Song, Woo Jung Kim (Jeju National University Hospital), Joon-myoung Kwon, Eui Hyuk Kang (Mediplex Sejong Hospital), Sang Chan Jin, Tae-kwon Kim (Keimyung University Dongsan Medical Center), Hyuk Joong Choi (Hanyang University Guri Hospital), Seong Chun Kim (Gyeongsang National University Changwon Hospital).
To steering committee, comprised of following individuals: Sung Oh Hwang (Chair, Wonju Severance Christian Hospital), Sang Do Shin (Chair of Steering Committee, Seoul National University hospital), Hyuk Jun Yang (Advisory Committee, Gachon University Gil hospital), Sung Phil Chung (Data Safety and Management Board, Gangnam Severance Hospital), Sung Woo Lee (Security and Monitoring Board, Korea University Anam hospital), Kyung Jun Song (Secretariat, SMG-SNU Boramae Medical Center), Seung Sik Hwang (Epidemiology and Prevention Research Committee, Seoul National University), Gyu Chong Cho (Community Resuscitation Research Committee, Hallym University Kangdong Sacred Heart Hospital), Sung Woo Moon (Emergency Medical Service Resuscitation Research Committee, Korea University Ansan Hospital), Kyoung Chul Cha (Hospital Resuscitation Research Committee, Wonju Severance Christian Hospital), Won Young Kim (Hypothermia and Post-Resuscitation Care Research Committee, Asan Medical Center), Sang Hoon Na (Cardiac Care Resuscitation Research Committee, Seoul National University Hospital), Young Ho Kwack (Pediatric Resuscitation Research Committee, Seoul National University hospital).
To member of Secretariat: Joo Yeong Kim (Korea University Ansan hospital), Jeong Ho Park (Seoul National University hospital), Sun Young Lee (Seoul National University hospital), and Jung Eun Kim (Seoul National University hospital).
And to National Fire Agency for providing prehospital EMS data. Statistical consultation was supported by the Department of Biostatistics of the Catholic Research Coordinating Center. This research was supported by a Grant of Translational R&D Project through Institute for Bio-Medical convergence, Incheon St. Mary’s Hospital, The Catholic University of Korea (IBC2018-26).
Author contributions
S.H.W. and W.J.L. performed data analysis and drafted the manuscript. D.H.K., G.C.C. and Y.C. acquired data and critical revisions to the manuscript. SHW managed the data and revisions to the manuscript. All authors have read and approved the final manuscript.
Data availability
All data used to support the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan PS, McNally B, Tang F, Kellermann A, CARES Surveillance Group Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130:1876–1882. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes C, et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation. 2017;110:133–140. doi: 10.1016/j.resuscitation.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, et al. Korean Cardiac Arrest Research Consortium (KoCARC): rationale, development, and implementation. Clin. Exp. Emerg. Med. 2018;5:165–176. doi: 10.15441/ceem.17.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun E, Kheir J, Mashiach T, Naffaa M, Azzam ZS. Is elevated red cell distribution width a prognostic predictor in adult patients with community acquired pneumonia? BMC Infect. Dis. 2014;14:129. doi: 10.1186/1471-2334-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SM, et al. The clinical significance of changes in red blood cell distribution width in patients with community-acquired pneumonia. Clin. Exp. Emerg. Med. 2016;3:139–147. doi: 10.15441/ceem.15.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit. Care Med. 2011;39:1913–1921. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen LA, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J. Card. Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aktas G, et al. Could red cell distribution width be a marker of thyroid cancer? J. Coll. Phys. Surg. Pak. 2017;27:556–558. [PubMed] [Google Scholar]
- 9.Sincer I, Gunes Y, Mansiroglu AK, Cosgun M, Aktas G. Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Postep. Kardiol. Int. 2018;14:263–269. doi: 10.5114/aic.2018.78329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchnowski P, Hryniewiecki T, Kusmierczyk M, Szymanski P. Red cell distribution width is a prognostic marker of perioperative stroke in patients undergoing cardiac valve surgery. Interact. Cardiovasc. Thorac. Surg. 2017;25:925–929. doi: 10.1093/icvts/ivx216. [DOI] [PubMed] [Google Scholar]
- 11.Aktas G, et al. Could red cell distribution width be a marker in Hashimoto's thyroiditis? Exp. Clin. Endocrinol. Diabetes. 2014;122:572–574. doi: 10.1055/s-0034-1383564. [DOI] [PubMed] [Google Scholar]
- 12.Bellan M, et al. Association between red cell distribution width and response to methotrexate in rheumatoid arthritis. Reumatismo. 2020;72:16–20. doi: 10.4081/reumatismo.2020.1243. [DOI] [PubMed] [Google Scholar]
- 13.Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M, Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz. Gastroenterol. 2014;9:160–163. doi: 10.5114/pg.2014.43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song CS, et al. Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig. Dis. Sci. 2012;57:1033–1038. doi: 10.1007/s10620-011-1978-2. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 16.Kim CH, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care. 2013;17:R282. doi: 10.1186/cc13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, et al. Red blood cell distribution width as an independent predictor of all-cause mortality in out of hospital cardiac arrest. Resuscitation. 2012;83:1248–1252. doi: 10.1016/j.resuscitation.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Adrie C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.CIR.0000023891.80661.AD. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, et al. Association between the neutrophil-to-lymphocyte ratio and neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. J. Crit. Care. 2018;47:227–231. doi: 10.1016/j.jcrc.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Pekkarinen PT, et al. Procalcitonin and presepsin as prognostic markers after out-of-hospital cardiac arrest. PLoS ONE. 2017;12:e0188180. doi: 10.1371/journal.pone.0188180. [DOI] [PubMed] [Google Scholar]
- 21.Fontana V, et al. Can red blood cell distribution width predict outcome after cardiac arrest? Minerva Anestesiol. 2018;84:693–702. doi: 10.23736/S0375-9393.17.12102-4. [DOI] [PubMed] [Google Scholar]
- 22.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis. Markers. 2017;2017:7089493. doi: 10.1155/2017/7089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felker GM, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Wang P, Shen PP, Zhou JH. Predictive values of red blood cell distribution width in assessing severity of chronic heart failure. Med. Sci. Monit. 2016;22:2119–2125. doi: 10.12659/MSM.898103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinho J, et al. Red cell distribution width as a predictor of 1-year survival in ischemic stroke patients treated with intravenous thrombolysis. Thromb. Res. 2018;164:4–8. doi: 10.1016/j.thromres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Luo R, Hu J, Jiang L, Zhang M. Prognostic value of red blood cell distribution width in non-cardiovascular critically or acutely patients: a systematic review. PLoS ONE. 2016;11:e0167000. doi: 10.1371/journal.pone.0167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Lu H, Gong Y, Ying B, Cheng B. The association between red blood cell distribution width and mortality in critically Ill patients with acute kidney injury. Biomed. Res. Int. 2018;2018:9658216. doi: 10.1155/2018/9658216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Gong Y, Ying B, Cheng B. Relation between red cell distribution width and mortality in critically Ill patients with acute respiratory distress syndrome. Biomed. Res. Int. 2019;2019:1942078. doi: 10.1155/2019/1942078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunziker S, Celi LA, Lee J, Howell MD. Red cell distribution width improves the simplified acute physiology score for risk prediction in unselected critically ill patients. Crit. Care. 2012;16:R89. doi: 10.1186/cc11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Lee K, Kim I, Jung S, Kim MJ. Red cell distribution width and early mortality in elderly patients with severe sepsis and septic shock. Clin. Exp. Emerg. Med. 2015;2:155–161. doi: 10.15441/ceem.15.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo YH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am. J. Emerg. Med. 2013;31:545–548. doi: 10.1016/j.ajem.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, for the Cholesterol and Recurrent Events (CARE) Trial Investigators Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 34.Chugh C, et al. Red blood cell distribution width is associated with poor clinical outcome after subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2015;23:217–224. doi: 10.1007/s12028-015-0117-x. [DOI] [PubMed] [Google Scholar]
- 35.Siegler JE, et al. Elevated red cell distribution width is associated with cerebral infarction in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26:26–33. doi: 10.1007/s12028-016-0306-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request.