Abstract
Background
The Barthel index (BI) is a widely used assessment tool for evaluating physical performance in activities of daily living (ADL). The association between BI scores and mortality in hospital and during follow-up of acute coronary syndrome (ACS) patients remains unclear. The present study investigated whether the BI score could be used as a predictor for mortality of ACS.
Methods
We investigated ACS patients from the multi-center Retrospective Evaluation of Acute Chest Pain (REACP) study. The association between BI scores and all-cause mortality of patients with ACS was analyzed by Cox proportional hazards models. The primary endpoint was all-cause death and the secondary endpoint was cardiac death during follow-up.
Results
Among 2908 patients with ACS enrolled, 277 (9.5%) patients died within a median follow-up time of 10.6 months. Patients with lower BI had higher risks of mortality, compared with those with higher BI in ACS patients. Kaplan–Meier analysis revealed that patients with lower BI had worse survival rates than patients with higher BI (P < 0.001). After adjustment for potential influencing factors, multivariate Cox regression analysis showed that the BI was independently associated with all-cause mortality and cardiac mortality, respectively.
Conclusion
The BI at admission has the powerful potential to provide useful prognostic information of early risk stratification, and routine recording of the BI at the ED visit may help in decision-making and health care planning for patients with ACS.
Keywords: Barthel index, activities of daily living, acute coronary syndrome, prognosis, mortality
Introduction
Acute coronary syndrome (ACS) is one of the fatal diseases with high morbidity and mortality.1 The emergence of advanced antithrombotic therapy and percutaneous coronary intervention (PCI) has dramatically reduced acute and long-term deaths.2 However, despite this, the one-year mortality of ACS still remains around 10%.3 The identification of high-risk patients with ACS is important for therapeutic decision-making and benefits prognosis-improving.4 Activities of daily living, as a basic functional capacity, refers to the ability to carry out daily activities in a normal or accepted way.5,6 ADL is correlated with aging, frailty, inflammation and other cardiovascular risk factors.7–13 Therefore, patients with ADL deficiency are more prone to have poor prognosis and mortality when exposed to acute severe diseases, such as ACS. Moreover, a prior study showed that low ADL was related to increased risk of mortality in elderly patients with acute myocardial infarction.14 Thus, early assessment of ADL and identification of high-risk patients with ADL deficiency may be significant in improving prognosis in ACS patients.
The Barthel index (BI) is a widely used assessment tool for evaluating physical performance in ADL.15 It comprises 10 items: feeding, toilet use, bathing, grooming, dressing, bowel and bladder control, chair transferring, stair climbing and ambulating. With sufficient validity and reliability,16,17 the BI is a standard routine evaluation program for inpatients and can be performed repeatedly. The BI score can be assessed orally by inquiring the patient or a family member. Simple and rapid, the BI is quite suitable for early assessment of ACS patients in the emergency room. However, few studies have evaluated the prognostic value of the BI in ACS patients.
Previous studies showed the BI score to be a prognostic predictor of heart failure18 and stroke.19 A recent study showed that the BI at discharge may be related to one-year mortality of ACS patients >85 years old undergoing PCI.20 We hypothesized that BI scores at admission would provide additional prognostic information for ACS patients. Thus, we conducted this retrospective multi-center study to determine whether BI scores at admission could predict all-cause death and cardiac death in patients with ACS and identify high-risk ACS patients, with the aim of providing appropriate therapeutic and management strategies.
Materials and Methods
Study Design
This was a multi-center retrospective cohort study that included patients who took part in the multi-center Retrospective Evaluation of Acute Chest Pain (REACP) study. The REACP study was registered at www.chictr.org.cn (Identifier: ChiCTR1900024657) and included patients with acute chest pain admitted to the emergency department of seven tertiary hospitals in China from January 2017 to December 2018. The present study investigated whether pre-hospital ADL could be used as a predictor of mortality in patients with ACS. The study was conducted in agreement with the Declaration of Helsinki and was approved by the Human Ethical Committee of West China Hospital, Sichuan University. And other researchers who need the REACP study data can contact our corresponding author.
Study Population
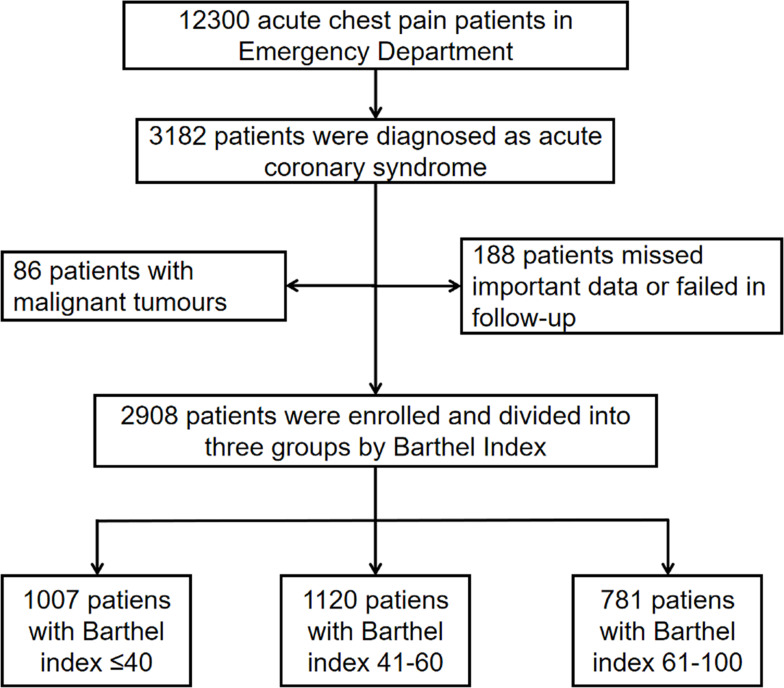
We investigated the prognostic value of pre-hospital ADL for ACS patients in this present study. The inclusion criteria were: >18 years of age; diagnosed with ACS including ST-segment elevated myocardial infarction (STEMI), non-STEMI and unstable angina for the first time. The exclusion criteria were patients with malignant tumors, missed important data or lost at follow-up. A diagram outlining patient selection is shown in Figure 1.
Figure 1.
Flow chart of the enrollment of participants in the study.
Data Collection and Measures
We obtained data from the database of the REACP study. Demographic data, characteristic information and clinical features of the patient, including vital signs, medical histories, echocardiography, laboratory examination, coronary angiography, inpatient complications, prehospital treatment, in-hospital treatment and discharge medication, were collected using standard case report forms.
We calculated the Global Registry of Acute Coronary Events (GRACE) score and Gensini score in this study to assess the risk level and the severity of patients with ACS. The GRACE score is guideline-recommended and the main basis for risk stratification in ACS patients, which is widely used in clinical practice. GRACE scores were determined using data including age, heart rate, systolic blood pressure, creatinine, cardiac enzyme, ST-segment deviation, Killip class and cardiac arrest in the emergency department.21 Gensini scores, indicating the severity of coronary artery disease lesions, were calculated by accumulative effect of multiple obstructions, geometric severity of the coronary artery and importance of vessel location after the first PCI.22
Barthel Index
The BI is a widely used assessment tool performed by nurses for evaluating physical performance in ADL. It comprises 10 items: feeding, toilet use, bathing, grooming, dressing, bowel and bladder control, chair transferring, stair climbing and ambulating.15 Each item is scored proportionally and a given number of points are assigned to each level or rank. The score ranges from 0 (completely dependent patient) to 100 (independent patient) in 5-point intervals. The score for each item is determined by verbal or physical help needed to perform each task. Higher BI scores reflect a higher level of ADL of patients. The BI score is grouped into three standard diagnostic categories according to the standard BI grouping method:15 high disability of ADL (BI score 0 to 40), moderate disability of ADL (BI score 41 to 60), low disability of ADL (BI score 61 to 100). In the present study, patients were divided into three groups according to the BI scores. Patients with BI scores ≤ 40 were assigned to group 1 (G1), with BI scores of 41–60 were assigned to group 2 (G2) and with BI scores > 60 were assigned to group 3 (G3). The BI values of ACS patients were assessed at the time of admission by a trained physician according to responses from the patient or a family member.
Endpoint and Follow-Up
The primary endpoint was all-cause death and the secondary endpoint was cardiac death during follow-up. The follow-up period started from admission of the patient with ACS until the date of an event or the last follow-up, the median time of which was 10.6 (8.2–14.1) months. Post-discharge follow-up was accomplished by specific physicians with emergency department and cardiology training using structured telephone questionnaire forms.
Statistical Analysis
The mean ± standard deviation or median with interquartile range was used to report continuous variables according to their distribution type. Parametric patient characteristics were compared by one-way analysis of variance and non-parametric characteristics by Kruskal–Wallis H-test. Frequencies with percentages were used to report categorical data. The chi-square test or Fisher’s exact test was performed to compare categorical data. The correlation between BI score and GRACE score, and between BI score and Gensini score was analyzed by Spearman correlation analysis. The Kaplan–Meier method and log rank tests were performed to calculate and compare the cumulative survival of ACS patients in different groups. The Cox regression model was used to identify whether variables were related to time to mortality. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Only variables determined to be significant (P < 0.05) following univariate Cox regression models were entered into the multivariate Cox regression model. Subgroup analysis was performed to test whether the association between the BI scores and the all-cause mortality was robust. Medians were taken as the cutoff points for BMI, SBP, DBP, heart rate, WBC, platelet count, hemoglobin, troponin T, GRACE and NTproBNP. The cutoff point for age was according to the definition of the elderly (65 years old). The cutoff point for Killip class was having congestive heart failure (≥II). Two-tailed P-values of <0.05 were considered significant. Statistical analysis was performed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline Patient Characteristics
A total of 2908 patients with ACS were recruited. The average age of the 2908 patients was 65.9 ± 13.1 years and 2192 (75.4%) were male. The median follow-up time was 10.6 (8.2–14.1) months. Patients were divided into three groups: G1 (n = 1007, 34.6%), G2 (n = 1120, 38.5%) and G3 (n = 781, 26.9%) according to BI score. A total of 277 (9.5%) patients died, of whom 228 (7.8%) died due to cardiac causes. The characteristics of the patients in the three groups are shown in Table 1. Patients in G1, with the lowest BI values, were older, more likely to have Killip class ≥ 2, had lower body mass index (BMI), SBP, diastolic blood pressure (DBP), left ventricular ejection fraction (LVEF) and triglycerides, and had higher heart rate, WBC count, D-dimer, blood glucose, creatinine, BUN, N-terminal pro-B-type natriuretic peptide (NT-proBNP), cardiac troponin T, creatinine kinase and CK-MB levels.
Table 1.
Relationships Between Baseline Clinical Characteristics and Barthel Index in Patients with Acute Coronary Syndrome
| Variables | G1 (n = 1007) | G2 (n = 1120) | G3 (n = 781) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 67.20 ± 13.40 | 64.95 ± 13.40 | 65.48 ± 12.17 | <0.001 |
| ≥75 years, n (%) | 337 (33.5%) | 323 (28.8%) | 178 (22.8%) | <0.001 |
| Males, n (%) | 741 (73.6%) | 863 (77.1%) | 588 (75.3%) | 0.179 |
| BMI, kg/m2 | 23.88 ± 3.44 | 24.26 ± 3.35 | 24.97 ± 13.08 | 0.010 |
| Medical histories | ||||
| Smoking, n (%) | 551 (54.7%) | 657 (58.7%) | 425 (54.4%) | 0.097 |
| Drinking, n (%) | 315 (31.3%) | 377 (33.8%) | 258 (33.2%) | 0.460 |
| Hypertension, n (%) | 550 (54.6%) | 585 (52.2%) | 435 (55.7%) | 0.291 |
| Diabetes, n (%) | 287 (28.5%) | 278 (24.8%) | 210 (26.9%) | 0.157 |
| Hyperlipidemia, n (%) | 106 (10.5%) | 139 (12.4%) | 99 (12.7%) | 0.281 |
| COPD, n (%) | 30 (3.7%) | 35 (4.1%) | 22 (3.5%) | 0.818 |
| Admission SBP, mmHg | 125.92 ± 25.12 | 128.76 ± 24.11 | 130.83 ± 23.07 | <0.001 |
| Admission DBP, mmHg | 77.45 ± 16.33 | 78.54 ± 15.97 | 79.28 ± 15.20 | 0.048 |
| Heart rate,/min | 83.20 ± 20.60 | 80.82 ± 17.69 | 77.61 ± 15.16 | <0.001 |
| Killip class ≥ 2, n (%) | 504 (50.0%) | 453 (40.4%) | 310 (39.7%) | <0.001 |
| LVEF, (%)C | 52.01 ± 11.95 | 53.77 ± 11.14 | 57.52 ± 12.05 | <0.001 |
| Laboratory findings | ||||
| WBC, ×109/L | 10.18 ± 3.89 | 9.91 ± 3.69 | 7.98 ±2.89 | <0.001 |
| Platelet count, ×109/L | 182.38 ± 69.71 | 187.79 ± 75.56 | 185.1 ± 76.45 | 0.244 |
| Fibrinogen, g/L | 9.47 ± 4.49 | 9.29 ±4.13 | 9.04 ± 3.95 | 0.103 |
| D-dimer, mg/L | 1.72 (1.35–2.08) | 0.99 (0.80–1.17) | 0.70 (0.57–0.83) | <0.001 |
| Blood glucose, mmol/L | 9.23 ± 4.82 | 8.61 ± 3.70 | 8.31 ± 3.75 | <0.001 |
| Creatinine, μmol/L | 105 (99–111) | 93 (89–97) | 92 (87–97) | 0.001 |
| BUN, mmol/L | 7.42 ± 4.72 | 6.65 ± 3.99 | 6.33 ± 3.41 | <0.001 |
| Triglycerides, mmol/L | 1.62 ± 1.44 | 1.73 ± 1.58 | 2.00 ± 1.66 | <0.001 |
| Total cholesterol, mmol/L | 4.38 ± 1.26 | 4.49 ± 1.21 | 4.34 ± 1.27 | 0.022 |
| HDL, mmol/L | 1.15 ± 0.36 | 1.16 ± 0.33 | 1.12 ± 0.34 | 0.096 |
| LDL, mmol/L | 2.72 ± 1.10 | 2.80 ± 1.07 | 2.63 ± 1.09 | 0.002 |
| NT-proBNP, pg/mL | 3576 (3141–4010) | 2447 (2167–2728) | 1757 (1480–2035) | <0.001 |
| CTnT, pg/mL | 2474 (2271–2677) | 2062 (1885–2240) | 837 (714–960) | <0.001 |
| Creatinine kinase, IU/L | 1031(933–1129) | 938(849–1026) | 393 (339–447) | <0.001 |
| CK-MB, U/L | 73.2 (66.9–79.5) | 65.1 (59.5–70.8) | 24.7 (20.8–28.7) | <0.001 |
| Culprit coronary vessel | ||||
| Left main, n (%) | 190 (18.9%) | 190 (17.0%) | 139 (17.8%) | 0.519 |
| LAD, n (%) | 818 (81.2%) | 947 (84.6%) | 642 (82.2%) | 0.065 |
| Left circumflex, n (%) | 656 (65.1%) | 730 (65.2%) | 494 (63.3%) | 0.154 |
| RCA, n (%) | 735 (73.0%) | 847 (75.6%) | 570 (73.0%) | 0.288 |
| Risk score | ||||
| GRACE score | 155.22 ± 43.70 | 143.11 ± 37.84 | 137.04 ± 35.65 | <0.001 |
| Gensini score | 79 (75–82) | 72 (69–75) | 59 (55–63) | <0.001 |
| Type of ACS | ||||
| STEMI | 663 (33.1%) | 724 (35.1%) | 248 (31.8%) | <0.001 |
| NSTEMI | 225 (40.6%) | 238 (44.2%) | 216 (15.2%) | <0.001 |
| UA | 119 (20.0%) | 158 (26.6%) | 317 (53.4%) | <0.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; COPD, chronic obstructive pulmonary disease; WBC, white blood cell count; BUN, blood urea nitrogen; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CTnT, cardiac troponin T; NT-proBNP, N-terminal pro-brain natriuretic peptide; CK-MB, creatinine kinase-myocardial band isoenzyme; CRP, C-reactive protein; LVEF, left ventricular ejection fraction; LAD, left anterior descending; RCA, right coronary artery; GRACE score, Global Registry of Acute Coronary Events score; ACS, acute coronary syndrome; STEMI, ST-segment elevated myocardial infarction; NSTEMI, non ST-segment elevated myocardial infarction; UA, unstable angina.
The Correlation of Barthel Index with GRACE Score and Gensini Score
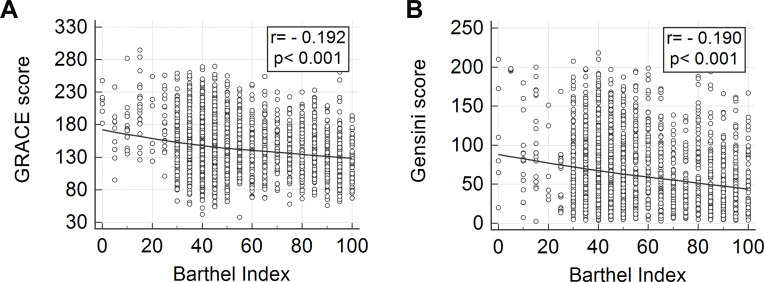
There were significant differences in the GRACE score and Gensini score between the three groups with different BI values. G1, with lower BI values, showed higher GRACE scores and Gensini scores. The GRACE scores for groups G1, G2 and G3 were 155.2 ± 43.7, 143.1 ± 37.8 and 137.0 ± 35.7, respectively (P < 0.001). The Gensini scores for groups G1, G2 and G3 were 79 (75–82), 72 (69–75) and 59 (55–63), respectively (P < 0.001) (Figure 2). Spearman correlation analysis showed that the GRACE score and Gensini score were correlated with BI score (r=﹣0.192, p <0.001; r=﹣0.190, p <0.001) (Figure 2).
Figure 2.
Scatter plot of GRACE score and BI (A); Gensini score and BI (B).
Abbreviations: BI, Barthel index; GRACE score, Global Registry of Acute Coronary Events score.
Discrimination of Barthel Index Compared with the Other Risk Scores
The ROC curves of BI, GRACE score and Gensini score in relation to all-cause mortality and cardiac mortality are shown in Figure 4. The AUC values of BI, GRACE score and Gensini score were 0.648, 0.774 and 0.624 in relation to all-cause mortality, and 0.646, 0.798 and 0.637 in relation to cardiac mortality, respectively.
Figure 4.
ROC curves for GRACE score, Gensini score and BI in relation to all-cause mortality (A) and cardiac mortality (B).
Abbreviations: BI, Barthel index; GRACE score, Global Registry of Acute Coronary Events score; ROC, receiver operating characteristic.
Barthel Index and Clinical Outcomes
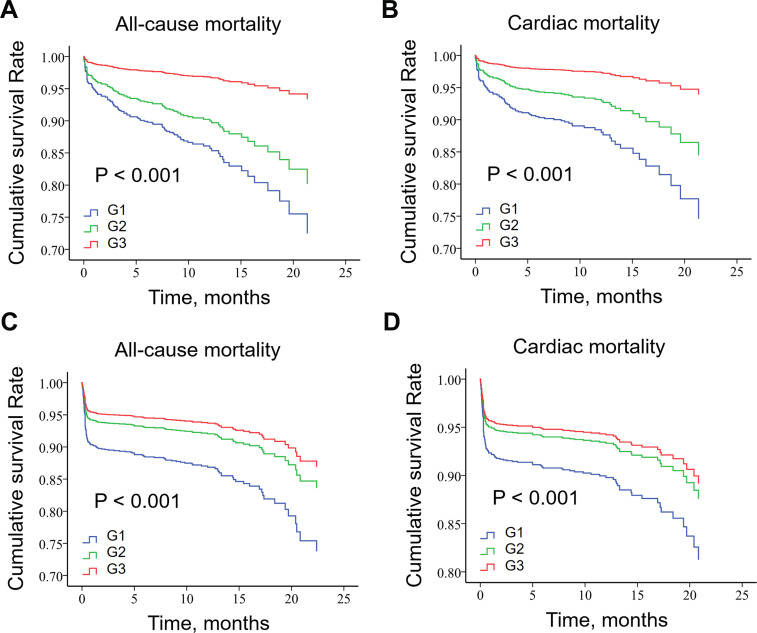
Patients in G1 had the highest all-cause mortality and cardiac death both during hospitalization and during follow-up (P < 0.001 for all) (Table 2). Kaplan–Meier analysis (Figure 3) showed that patients with higher BI values had better cumulative survival than those with lower BI values, regardless of STEMI and non-ST segment elevation acute coronary syndrome (NST-ACS), in both all-cause mortality and cardiac mortality (P < 0.001 for all).
Table 2.
In-Hospital and Long-Term Adverse Outcome of Patients Categorized by Barthel Index
| Outcomes | G1 (n = 1007) | G2 (n = 1120) | G3 (n = 781) | P-value | |
|---|---|---|---|---|---|
| In-hospital clinical outcomes, n (%) | |||||
| All-cause mortality, n (%) | 70 (7.0%) | 32 (2.9%) | 7 (0.9%) | <0.001 | |
| Cardiac death, n (%) | 68 (6.8%) | 28 (2.5%) | 7 (0.9%) | <0.001 | |
| Clinical outcomes during follow-up, n (%) | |||||
| All-cause mortality, n (%) | 141 (14.4%) | 101 (9.2%) | 35 (4.7%) | <0.001 | |
| Cardiac death, n (%) | 115 (11.5%) | 81 (7.3%) | 32 (4.1%) | <0.001 | |
Figure 3.
Kaplan–Meier survival curve of all-cause death for NST-ACS patients (A), cardiac death for NST-ACS patients (B), all-cause death for STEMI patients (C) and cardiac death for STEMI patients (D) by Barthel index score.
Abbreviations: G1, group 1; G2, group 2; G3, group 3.
Multivariate Cox regression analysis adjusted for confounders, including sex, age, drinking, smoking, hypertension, type 2 diabetes mellitus, GRACE score and Gensini score, showed that a lower BI value was an independent predictive factor of all-cause mortality (continuous BI: HR, 0.983, 95% CI, 0.975–0.990, P < 0.001; categorized BI: G1 vs G3: HR, 2.147, 95% CI, 1.372–3.359, P = 0.001; G2 vs G3: HR, 1.747, 95% CI: 1.105–2.761, P = 0.017) and cardiac mortality (continuous BI: HR, 0.984, 95% CI: 0.976–0.993, P < 0.001; categorized BI: G1 vs G3: HR, 1.887, 95% CI: 1.163–3.062, P = 0.010; G2 vs G3: HR, 1.478, 95% CI: 0.898–2.435, P = 0.125) (Table 3).
Table 3.
Cox Regression Analysis Regarding Correlations Between All-Cause Mortality and Barthel Index
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| BI | <0.001 | 0.004 | ||||
| G1 vs G3 | 3.392 | 2.342–4.912 | <0.001 | 2.147 | 1.372–3.359 | 0.001 |
| G2 vs G3 | 2.109 | 1.436–3.099 | <0.001 | 1.747 | 1.105–2.761 | 0.017 |
| Age | 1.050 | 1.040–1.061 | <0.001 | 1.017 | 1.002–1.033 | 0.023 |
| Sex | 1.641 | 1.281–2.101 | <0.001 | 1.066 | 0.721–1.575 | 0.749 |
| Smoking | 1.287 | 1.098–1.437 | 0.005 | 1.194 | 0.819–1.739 | 0.357 |
| Drinking | 1.352 | 1.148–1.507 | 0.002 | 0.881 | 0.614–1.265 | 0.493 |
| Hypertension | 1.108 | 0.875–1.405 | 0.045 | 1.145 | 0.848–1.546 | 0.378 |
| Diabetes | 1.404 | 1.094–1.803 | 0.008 | 1.305 | 0.959–1.776 | 0.091 |
| Gensini score | 1.006 | 1.004–1.008 | <0.001 | 1.004 | 1.001–1.006 | 0.002 |
| GRACE score | <0.001 | <0.001 | ||||
| Mediuma vs lowb risk | 2.707 | 1.121–6.538 | 0.027 | 1.850 | 0.748–4.576 | 0.183 |
| Highc vs low risk | 14.439 | 6.419–32.482 | <0.001 | 6.191 | 2.611–14.679 | <0.001 |
Notes: aMedium risk: 108–140; bLow risk: ≤108; cHigh risk: >140.
Abbreviations: BI, Barthel index; GRACE score, Global Registry of Acute Coronary Events score; HR, hazard ratio; CI, confidence interval.
Subgroup Analysis
Subgroup analysis was performed by grouping patients according to gender, age, BMI, SBP, DBP, heart rate, WBC, platelet count, hemoglobin, cardiac troponin T, NT-proBNP, Killip class, GRACE score, ACS type and polypharmacy (defined as the number of medications in chronic use ≥ 5 drugs). Patients in G1 had the lowest cumulative survival rates of all-cause mortality in each subgroup except for the Killip class I subgroup (P = 0.102) and the GRACE score ≤ 142 subgroup (P = 0.136) (Table 4).
Table 4.
Kaplan–Meier Survival Analysis of Mortality in Acute Coronary Syndrome Patients
| Subgroups | Cumulative Survival Rate | Log Rank χ2 | P-value | ||
|---|---|---|---|---|---|
| G1 | G2 | G3 | |||
| Gender | |||||
| Male (n = 2192) | 0.787 | 0.850 | 0.938 | 33.237 | <0.001 |
| Female (n = 716) | 0.645 | 0.726 | 0.851 | 14.665 | 0.001 |
| Agea | |||||
| ≤65 (n = 1396) | 0.820 | 0.887 | 0.961 | 13.162 | 0.001 |
| >65 (n = 1512) | 0.601 | 0.764 | 0.830 | 25.670 | <0.001 |
| BMIb, kg/m2 | |||||
| ≤24 (n = 1501) | 0.625 | 0.708 | 0.856 | 21.713 | <0.001 |
| >24 (n = 1407) | 0.829 | 0.909 | 0.955 | 24.125 | <0.001 |
| SBPb, mmHg | |||||
| ≤127 (n = 1480) | 0.690 | 0.821 | 0.869 | 32.309 | <0.001 |
| >127 (n = 1428) | 0.791 | 0.824 | 0.964 | 20.087 | <0.001 |
| DBPb, mmHg | |||||
| ≤77 (n = 1468) | 0.722 | 0.797 | 0.877 | 24.376 | <0.001 |
| >77 (n = 1440) | 0.750 | 0.849 | 0.957 | 25.177 | <0.001 |
| Heart rateb/min | |||||
| ≤78 (n = 1430) | 0.759 | 0.889 | 0.919 | 14.196 | 0.001 |
| >78 (n = 1478) | 0.706 | 0.766 | 0.910 | 28.688 | <0.001 |
| WBCb, 109/L | |||||
| ≤9 (n = 1412) | 0.747 | 0.830 | 0.943 | 22.445 | <0.001 |
| >9 (n = 1496) | 0.731 | 0.816 | 0.848 | 15.209 | <0.001 |
| Platelet countb,109/L | |||||
| ≤180 (n = 1415) | 0.695 | 0.810 | 0.898 | 32.474 | <0.001 |
| >180 (n = 1493) | 0.790 | 0.836 | 0.938 | 15.776 | <0.001 |
| Hemoglobinb, g/L | |||||
| ≤137 (n = 1448) | 0.682 | 0.779 | 0.864 | 24.597 | <0.001 |
| >137 (n = 1460) | 0.814 | 0.871 | 0.967 | 16.112 | <0.001 |
| Troponin Tb, pg/mL | |||||
| ≤247 (n = 1472) | 0.753 | 0.805 | 0.949 | 0.969 | <0.001 |
| >247 (n = 1436) | 0.744 | 0.841 | 0.860 | 0.982 | <0.001 |
| NT-proBNPb, pg/mL | |||||
| ≤813 (n = 1429) | 0.876 | 0.885 | 0.973 | 7.866 | 0.020 |
| >813 (n = 1479) | 0.619 | 0.741 | 0.803 | 24.622 | <0.001 |
| Killip classc | |||||
| I (n = 1267) | 0.943 | 0.920 | 0.955 | 4.557 | 0.102 |
| II–IV (n = 1641) | 0.522 | 0.662 | 0.856 | 36.032 | <0.001 |
| GRACE scoreb | |||||
| ≤142 (n = 1421) | 0.921 | 0.943 | 0.979 | 3.994 | 0.136 |
| >142 (n = 1487) | 0.600 | 0.682 | 0.810 | 25.727 | <0.001 |
| ACS type | |||||
| STEMI (n = 1635) | 0.727 | 0.760 | 0.938 | 36.614 | <0.001 |
| NST-ACS (n = 1273) | 0.741 | 0.850 | 0.874 | 15.721 | <0.001 |
| Polypharmacy (≥5 drugs) | |||||
| Yes (n = 1318) | 0.762 | 0.812 | 0.923 | 14.018 | 0.001 |
| No (n = 1590) | 0.716 | 0.837 | 0.903 | 36.596 | <0.001 |
Notes: aThe cutoff point for age was according to the definition of the elderly (65 years old); bThe cutoff points for these variates were medians; cThe cutoff point for Killip class was having congestive heart failure (≥II).
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; GRACE score, Global Registry of Acute Coronary Events score. ACS, acute coronary syndrome; STEMI, ST-elevation myocardial infarction; NST-ACS, non-ST elevation acute coronary syndrome
Discussion
The present study investigated the prognostic usefulness of the BI score at admission to predict mortality in ACS patients. Our findings showed that the BI score at admission may be a reliable prognostic index for patients with ACS. Compared with lower BI scores, higher BI scores were correlated with a decreased risk of all-cause mortality and cardiac mortality. Higher BI values at admission independently predicted better prognosis of ACS patients. Subgroup analysis indicated that the BI score had a stable prognostic value in patients with different levels of cardiovascular risk factors. The BI score at admission was significant as a factor of risk stratification for patients with ACS in the early phase.
To the best of our knowledge, this study is the largest one till now to verify the association between BI scores at admission and mortality both in-hospital and during follow-up for ACS patients. One study20 did research on BI scores and prognosis for very elderly ACS patients. They enrolled 91 very elderly (≥85 years) ACS patients who underwent PCI, showing that there was correlation between BI scores assessed at discharge and one-year mortality of these patients. Our study is carried out in multiple centers with a much larger population and further demonstrated that BI scores at admission were correlated with mortality both in-hospital and during follow-up in ACS patients.
It is well established that a maladaptive immune system and overactive inflammatory reaction are involved in the process of atherosclerosis.23,24 A study has shown that low levels of ADL may affect the immune system and facilitate chronic inflammatory processes.25 Favorable ADL may induce anti-inflammatory pathways and is associated with better cardiovascular health.26 Apart from that, ADL reflects the aging status of the human body.12 And older ACS patients are more likely to have worse prognosis, considering their poor physical condition and functional decline.27 Moreover, studies indicate that ADL can predict the occurrence of frailty.13 Patients with a low level of ADL are more vulnerable to frailty.28 Frailty leads to a decreased homeostasis reserve and difficulty in responding adequately to stressful events. The manifestations of frailty are associated with damage to the vital functional reserve systems that control hormones, immune, inflammatory and neural processes.29 Previous studies have shown that frailty has predictive value of mortality in ACS patients.13,30 And our previous studies demonstrated that indicators associated with multiple pathophysiological states involved in the pathogenesis of cardiovascular disease might provide more prognostic information.31–33 The BI score as a validated assessment tool for ADL is highly likely to predict prognosis of ACS patients, which well explains our findings.
The GRACE score is a guideline-recommended risk stratification tool for ACS patients, which includes several factors: demographics, heart damage and damage to other organs related to ACS. However, there are no geriatric factors, such as frailty, involved in the GRACE score. The proportion of ACS patients combined with geriatric conditions is increasing, which should be paid attention to.34 It has been reported that geriatric conditions may predict outcomes beyond age and standard risk factors.35 A study showed that worse frailty was associated with higher GRACE scores.36 A recent study reported that frailty may enhance the prognostic properties of the GRACE score.37 The BI, which is associated with the occurrence of frailty, may provide additional prognostic information beyond the GRACE score.
Our results underscore the importance of assessing BI scores at admission of ACS patients. The BI is a simple and rapid assessment tool with high reliability.17 It takes approximately five minutes for a physician to carry out, and it is easy to interpret in clinical practice. In the conversation with patients and their families, the information about dependency in ADL is of particular importance with regard to specific nursing care plans and intervention strategies. Patients with disability in ADL are more likely to develop to be bedridden and have disuse syndrome, which may easily cause decubitus ulcer and infections, such as pneumonia and urinary tract infection.20 These morbidities may lead to a worse outcome of disease development. Undertaking vigorous timely rehabilitation can help patients recover from disability in ADL and improve the prognosis of patients.38
From the results of our study, risk stratification can be performed at an early stage for ACS patients according to the BI score at admission. For high-risk patients, personalized nursing care, specific rehabilitation, appropriate intervention and management should be carried out as early as possible, as well as conducting reasonable follow-up and disease management after discharge with the purpose of helping improve prognosis of ACS patients.
Limitations
The present study has some limitations. First, this was a retrospective study, and large, multi-center, prospective studies should be conducted to further verify the validity of results. Second, the BI score was only evaluated at admission, and it is unclear whether subsequent changes in this index could provide additional prognostic value. Third, the median follow-up time was 10.63 months, which is relatively short. Thus, further studies are needed to assess the long-term prognostic value of the BI score.
Conclusions
The present study demonstrated that the BI at admission was an independent prognostic factor in patients with ACS, predicting both all-cause mortality and cardiac mortality of ACS patients. The BI at admission has powerful potential to provide useful prognostic information of early risk stratification, and routine recording of the BI at the ED visit may help in decision-making and health care planning for patients with ACS.
Funding Statement
This work was supported financially by grants from Sichuan Science and Technology Programme (No. 2019YFSY0030, 2019JDRC0105, 2018RZ0139, and 20ZDYF2813), 1•3•5 Project for Disciplines of Excellence-Clinical Research Incubation Project, Sichuan University West China Hospital (Nos. 2018HXFH001, 2018HXFH027), and Sichuan University West China Nursing Discipline Development Special Fund Project (HXHL19023).
Abbreviations
ACS, acute coronary syndrome; ADL, activities of daily living; AUC, area under the curve; BI, Barthel index; BUN, blood urea nitrogen; CI, confidence interval; CRP, C-reactive protein; cTnT, cardiac troponin T; ED, emergency department; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; SA, serum albumin; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; WBC, white blood cell count.
Ethics Approval and Informed Consent
The study was conducted in agreement with the Declaration of Helsinki and was approved by the Human Ethical Committee of West China Hospital, Sichuan University.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Roth GA. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covic A. Practical issues in clinical scenarios involving CKD patients requiring antithrombotic therapy in light of the 2017 ESC guideline recommendations. BMC Med. 2018;16(1):158. doi: 10.1186/s12916-018-1145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83(5):1085–1196. [DOI] [PubMed] [Google Scholar]
- 4.Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–2945. [DOI] [PubMed] [Google Scholar]
- 5.M N. Comprehensive geriatric assessment and team intervention. JMAJ. 2007;50(6):461–466. [Google Scholar]
- 6.Finlayson M, Mallinson T, Barbosa VM. Activities of daily living (ADL) and instrumental activities of daily living (IADL) items were stable over time in a longitudinal study on aging. J Clin Epidemiol. 2005;58(4):338–349. [DOI] [PubMed] [Google Scholar]
- 7.Wei L, Wu B. Racial and ethnic differences in obesity and overweight as predictors of the onset of functional impairment. J Am Geriatr Soc. 2014;62(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na L, Streim JE. Psychosocial well-being associated with activity of daily living stages among community-dwelling older adults. Gerontol Geriatric Med. 2017;3:2333721417700011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koizumi Y, Hamazaki Y, Okuro M, et al. Association between hypertension status and the screening test for frailty in elderly community-dwelling Japanese. Hypertension Res. 2013;36(7):639–644. [DOI] [PubMed] [Google Scholar]
- 10.Unnanuntana A, Jarusriwanna A, Nepal S. Validity and responsiveness of Barthel index for measuring functional recovery after hemiarthroplasty for femoral neck fracture. Arch Orthop Trauma Surg. 2018;138(12):1671–1677. doi: 10.1007/s00402-018-3020-z [DOI] [PubMed] [Google Scholar]
- 11.Schloss MJ, Swirski FK, Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res. 2020;126(9):1242–1259. doi: 10.1161/CIRCRESAHA.120.315936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bier N, Belchior Pda C, Paquette G, et al. The instrumental activity of daily living profile in aging: a feasibility study. J Alzheimer’s Dis. 2016;52(4):1361–1371. doi: 10.3233/JAD-150957 [DOI] [PubMed] [Google Scholar]
- 13.Axelle Costenoble VK, Vermeiren S, Azzopardi Vella R, Debain A, Rossi G, Bautmans I. A comprehensive overview of activities of daily living in existing frailty instruments: a systematic literature search. The Gerontologist. 2019;15(3):178–183. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi: 10.1186/1471-2318-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 16.Bouwstra H, Smit EB, Wattel EM, et al. Measurement properties of the barthel index in geriatric rehabilitation. J Am Med Dir Assoc. 2019;20(4):420–425.e421. doi: 10.1016/j.jamda.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 17.P K. Measures of adult general functional status: the Barthel Index, Katz Index of activities of daily living, Health Assessment Questionnaire (HAQ), MACTAR patient preference disability questionnaire,and Modified Health Assessment Questionnaire (MHAQ). Arthritis Care Res. 2003;49(5):S15–S27. doi: 10.1002/art.11415 [DOI] [PubMed] [Google Scholar]
- 18.Takabayashi K, Kitaguchi S, Iwatsu K, et al. A decline in activities of daily living due to acute heart failure is an independent risk factor of hospitalization for heart failure and mortality. J Cardiol. 2019;73(6):522–529. doi: 10.1016/j.jjcc.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 19.Li QX, Zhao XJ, Fan HY, et al. Application values of six scoring systems in the prognosis of stroke patients. Front Neurol. 2019;10:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoshi Higuchi YK, Matsushita K, Taguchi H, Ishiguro H, Kohshoh H, Yoshino H. Barthel Index as a predictor of 1-year mortality in very elderly patients who underwent percutaneous coronary intervention for acute coronary syndrome: better activities of daily living, longer life. Clin Cardiol. 2016;39(2):83–89. doi: 10.1002/clc.22497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CB GR G, Dabbous O. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 22.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 23.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science (New York, NY). 2013;339(6116):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Zhao L, Yu J, et al. Lipoprotein-associated phospholipase A2 in coronary heart disease: review and meta-analysis. Clin Chim Acta. 2017;465:22–29. [DOI] [PubMed] [Google Scholar]
- 25.Noz MP, Hartman YAW, Hopman MTE, et al. Sixteen-week physical activity intervention in subjects with increased cardiometabolic risk shifts innate immune function towards a less proinflammatory state. J Am Heart Assoc. 2019;8(21):e013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19(9):563–572. [DOI] [PubMed] [Google Scholar]
- 27.Veerasamy M, Edwards R, Ford G, et al. Acute coronary syndrome among older patients: a review. Cardiol Rev. 2015;23(1):26–32. [DOI] [PubMed] [Google Scholar]
- 28.OHV LPM. The ability of four frailty screening instruments to predict mortality, hospitalization and dependency in (instrumental) activities of daily living. Eur J Ageing. 2019;31(5):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianluca Campo EM, Tonet E, Biscaglia S, Albert A-S. The assessment of scales of frailty and physical performance improves prediction of major adverse cardiac events in older adults with acute coronary syndrome. J Gerontol a Biol Sci Med Sci. 2019;5(10):146–151. [DOI] [PubMed] [Google Scholar]
- 30.Uchmanowicz I, Lisiak M, Wleklik M, Gurowiec P, Kałużna-Oleksy M. The relationship between frailty syndrome and quality of life in older patients following acute coronary syndrome. Clin Interv Aging. 2019;14:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Ye L, Yu J, et al. Significance of the thrombo-inflammatory status-based novel prognostic score as a useful predictor for in-hospital mortality of patients with type B acute aortic dissection. Oncotarget. 2017;8(45):79315–79322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia Y, Li D, Cao Y, et al. Inflammation-based Glasgow Prognostic Score in patients with acute ST-segment elevation myocardial infarction: a prospective cohort study. Medicine. 2018;97(50):e13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du R, Li D, Yu J, et al. Association of platelet to lymphocyte ratio and risk of in-hospital mortality in patients with type B acute aortic dissection. Am J Emerg Med. 2017;35(2):368–370. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55(4):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168(5):784–791. [DOI] [PubMed] [Google Scholar]
- 36.White HD, Westerhout CM, Alexander KP, et al. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care. 2016;5(3):231–242. [DOI] [PubMed] [Google Scholar]
- 37.Anand A, Cudmore S, Robertson S, et al. Frailty assessment and risk prediction by GRACE score in older patients with acute myocardial infarction. BMC Geriatr. 2020;20(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues P, Santos M, Sousa MJ, et al. Cardiac rehabilitation after an acute coronary syndrome: the impact in elderly patients. Cardiology. 2015;131(3):177–185. [DOI] [PubMed] [Google Scholar]