Controlled human infection models (CHIMs) are invaluable tools utilized to understand the human response to infection, potentially leading to protective immune mechanisms and allowing efficacy testing of enteric countermeasures, including vaccines, antibiotics, and other products. The development of an improved Shigella CHIM for both Shigella sonnei and Shigella flexneri is consistent with international efforts, supported by international donors and the World Health Organization, focused on standardizing Shigella CHIMs and using them to accelerate Shigella vaccine development. The use of lyophilized Shigella challenge strains rather than plate-grown inoculum preparations is considered an important step forward in the standardization process. Furthermore, the results of studies such as this justify the development of lyophilized preparations for additional epidemiologically important S. flexneri serotypes, including S. flexneri 3a and S. flexneri 6.
KEYWORDS: controlled human infection models, shigellosis, Shigella, diarrhea, enteric disease, human challenge
ABSTRACT
Controlled human infection models (CHIMs) are useful for vaccine development. To improve on existing models, we developed a CHIM using a lyophilized preparation of Shigella sonnei strain 53G produced using current good manufacturing practice (cGMP). Healthy adults were enrolled in an open-label dose-ranging study. Following administration of a dose of rehydrated S. sonnei strain 53G, subjects were monitored for development of disease. The first cohort received 500 CFU of 53G, and dosing of subsequent cohorts was based on results from the previous cohort. Subjects were administered ciprofloxacin on day 5 and discharged home on day 8. Subjects returned as outpatients for clinical checks and sample collection. Attack rates increased as the dose of S. sonnei was increased. Among those receiving the highest dose (1,760 CFU), 70% developed moderate to severe diarrhea, 50% had dysentery, and 40% had fever. Antilipopolysaccharide responses were observed across all cohorts. An S. sonnei CHIM using a lyophilized lot of strain 53G was established. A dose in the range of 1,500 to 2,000 CFU of 53G was selected as the dose for future challenge studies using this product. This model will enable direct comparison of study results between institutions and ensure better consistency over time in the challenge inoculum.
IMPORTANCE Controlled human infection models (CHIMs) are invaluable tools utilized to understand the human response to infection, potentially leading to protective immune mechanisms and allowing efficacy testing of enteric countermeasures, including vaccines, antibiotics, and other products. The development of an improved Shigella CHIM for both Shigella sonnei and Shigella flexneri is consistent with international efforts, supported by international donors and the World Health Organization, focused on standardizing Shigella CHIMs and using them to accelerate Shigella vaccine development. The use of lyophilized Shigella challenge strains rather than plate-grown inoculum preparations is considered an important step forward in the standardization process. Furthermore, the results of studies such as this justify the development of lyophilized preparations for additional epidemiologically important S. flexneri serotypes, including S. flexneri 3a and S. flexneri 6.
INTRODUCTION
As has been highlighted by recent advances in diagnostics, Shigella species are significant pathogens in people living in low- and middle-income countries (LMICs) (1–6). In 2016, Global Burden Disease investigators estimated Shigella to be the second leading cause of diarrheal mortality, accounting for ∼212,440 annual deaths globally, including ∼64,000 in children <5 years of age (2). Moreover, Shigella-attributable mortality in children may be 28% higher if indirect deaths are included (7). Thus, there have been renewed efforts targeting primary prevention, including vaccination (8).
Controlled human infections have proven extremely valuable in gaining insights into disease pathogenesis for enteric pathogens and mechanism of immunological protection (9, 10). CHIMs are increasingly valuable in the development of candidate vaccines, as they permit an early assessment of vaccine efficacy and facilitate down-selection of less promising candidates. Recently, CHIMs have also contributed to late-stage enteric vaccine licensure and policy recommendations (9). The Shigella CHIM previously has been utilized to study candidate Shigella vaccines; however, concerns have been raised, including those regarding reproducibility, small sample sizes, differing outcome definitions, and limited characterization of the host response (9, 11). Current models use freshly harvested cells in a bacterial suspension for inoculation which may be a further barrier to standardization and reproducibility (11). Therefore, we looked to address concerns about Shigella CHIM standardization by using a lyophilized preparation of Shigella sonnei 53G produced using current good manufacturing practice (cGMP).
RESULTS
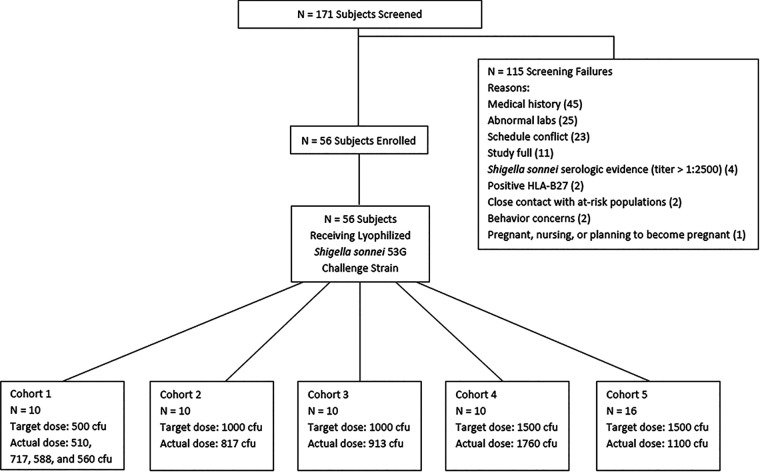
Of the 171 subjects enrolled, 56 met eligibility criteria to receive a dose of S. sonnei 53G (Fig. 1). Ten subjects were enrolled in each of the first four dose-ranging cohorts while 16 subjects were enrolled in the dose confirmation cohort (cohort 5) (Table 1). There were no differences in demographic characteristics among subjects across study groups.
FIG 1.
Screening and disposition of study subjects.
TABLE 1.
Demographics of subjects participating in the open-label dose-finding study of a lyophilized strain of S. sonnei 53G
| Characteristic | Value for cohort (n) |
||||
|---|---|---|---|---|---|
| Cohort 1 (10) | Cohort 2 (10) | Cohort 3 (10) | Cohort 4 (10) | Cohort 5 (16) | |
| Mean age (SD) (yr) | 35.7 (10.7) | 32.3 (9.9) | 34.3 (9.9) | 32.2 (8.8) | 30.1 (7.9) |
| Gender [n (%)] | |||||
| Male | 3 (30) | 7 (70) | 4 (40) | 3 (30) | 6 (37.5) |
| Female | 7 (70) | 3 (30) | 6 (60) | 7 (70) | 10 (62.5) |
| Race [n (%)] | |||||
| Caucasian | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (12.5) |
| Black | 9 (90.0) | 9 (90) | 10 (100) | 10 (100) | 14 (87.5) |
| Multirace | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity [n (%)] | |||||
| Hispanic or Latino | 1 (10) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-Hispanic or Latino | 9 (90) | 10 (100) | 10 (100) | 10 (100) | 16 (100) |
The initial cohort was targeted to receive a 500-CFU challenge dose, and the actual dose was 567 CFU (Table 2). As no subject in the initial cohort developed shigellosis, the target dose was increased to 1,000 CFU for the second and third cohorts, and as the disease rate still was below desired levels, it was increased again to a target dose of 1,500 CFU for the final dose-ranging cohort (actual dose received, 1,760 CFU).
TABLE 2.
Frequency of solicited signs and symptoms by cohort
| Characteristic or sign | Value for cohort (n) |
||||
|---|---|---|---|---|---|
| Cohort 1 (10) | Cohort 2 (10) | Cohort 3 (10) | Cohort 4 (10) | Cohort 5 (16) | |
| Target dose (actual dose) (CFU) | 500 (567) | 1,000 (817) | 1,000 (913) | 1,500 (1,760) | 1,500 (1,100) |
| Any diarrhea [n (%)] | 2 (20.0) | 6 (60.0) | 7 (70.0) | 7 (70.0) | 12 (75.0) |
| Moderate or severe diarrhea [n (%)] | 0 (0.0) | 5 (50) | 5 (50) | 7 (70) | 8 (50) |
| Dysentery [n (%)] | 0 (0.0) | 6 (60.0) | 3 (30.0) | 5 (50.0) | 6 (37.5) |
| Vomiting [n (%)] | 0 (0.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) | 3 (18.8) |
| Headache [n (%)] | 5 (50.0) | 7 (70.0) | 8 (80.0) | 7 (70.0) | 10 (62.5) |
| Nausea [n (%)] | 0 (0.0) | 4 (40.0) | 3 (30.0) | 3 (30.0) | 4 (25.0) |
| Abdominal pain [n (%)] | 1 (10.0) | 9 (90.0) | 7 (70.0) | 7 (70.0) | 9 (56.3) |
| Gas [n (%)] | 1 (10.0) | 8 (80.0) | 5 (50.0) | 4 (40.0) | 3 (18.8) |
| Myalgia [n (%)] | 1 (10.0) | 7 (70.0) | 7 (70.0) | 3 (30.0) | 5 (31.3) |
| Malaise [n (%)] | 0 (0.0) | 8 (80.0) | 8 (80.0) | 3 (30.0) | 1 (6.3) |
| Anorexia [n (%)] | 1 (10.0) | 9 (90.0) | 2 (20.0) | 4 (40.0) | 4 (25.0) |
| Arthralgia [n (%)] | 0 (0.0) | 7 (70.0) | 1 (10.0) | 3 (30.0) | 1 (6.3) |
| Fever [n (%)] | 0 (0.0) | 3 (30.0) | 1 (10.0) | 4 (40.0) | 3 (18.8) |
| S. sonnei in stool | 5 (50) | 7 (70) | 10 (100) | 10 (100) | 14 (88) |
| Median (Q1, Q3) time to diarrhea onset (h)a | 108.2 (101.5, 114.9) | 45.2 (39.1, 51.0) | 55.9 (48.5, 76.2) | 35.8 (13.1, 52.1) | 38.6 (31.7, 65.5) |
| Median (Q1, Q3) maximum 24-h no. of loose/liquid stoolsa | 5 (4, 5) | 7 (4, 8) | 4 (3, 9) | 10 (8, 12) | 7 (2, 10) |
| Median (Q1, Q3) maximum 24-h vol (ml) of loose/liquid stoolsa | 454 (319, 588) | 710 (326, 1125) | 616 (342, 1205) | 846 (472, 1092) | 968 (179, 1190) |
| Median (Q1, Q3) total no. of loose/liquid stoolsa | 5 (5, 5) | 14 (7, 20) | 5 (4, 18) | 21 (13, 23) | 14 (3, 26) |
| Median (Q1, Q3) total vol (ml) of loose/liquid stoolsa | 495 (319, 670) | 1419 (598, 1916) | 691 (591, 2562) | 1589 (1020, 2256) | 1425 (211, 2596) |
| Shigellosis per protocol [n (%)]b | 0 (0.0) | 2 (20.0) | 1 (10.0) | 4 (40.0) | 2 (12.5) |
| Alternative endpoint 1 [n (%)]c | 0 (0.0) | 6 (60.0) | 4 (40.0) | 7 (70.0) | 7 (43.8) |
| Alternative endpoint 2 [n (%)]d | 0 (0.0) | 5 (50.0) | 3 (30.0) | 6 (60.0) | 7 (43.8) |
Limited to subjects with diarrhea and excluding subjects with loose stools not meeting the diarrhea definition.
Shedding of S. sonnei in the stool and moderate-severe diarrhea (and/or dysentery) plus either moderate fever or one or more severe intestinal symptoms.
Shedding of S. sonnei in the stool and either (i) severe diarrhea (ii) moderate diarrhea and fever, or (iii) moderate diarrhea with ≥1 moderate constitutional/enteric symptom or dysentery.
Shedding of S. sonnei and either (i) severe diarrhea, (ii) moderate diarrhea and fever, (iii) dysentery and fever, (iv) moderate diarrhea and ≥1 severe constitutional/enteric symptom, or (v) dysentery and ≥1 severe constitutional/enteric symptom.
Not unexpectedly, if subjects developed diarrhea, the time to onset postchallenge (35 to 50 h) was equivalent regardless of the challenge dose. While the difference was not significant (Kruskal-Wallis; all P > 0.1), the median number of loose stools in subjects with diarrhea after receiving approximately a 1,000-CFU challenge dose (cohorts 2, 3, and 5) was 11, compared to 21 loose stools in subjects receiving a 1,760-CFU challenge dose. Additionally, the number of subjects with more severe symptoms appeared to increase as the challenge dose increased from 1,000 CFU to 1,760 CFU (Table 2). Similarly, while the proportion with dysentery remained relatively comparable at doses of ≥1,000 CFU, there was some variability across cohorts at the same target dose. Other solicited signs and symptoms demonstrated similar trends. However, because the highest percentage of subjects meeting the primary endpoint of shigellosis (40%) occurred in cohort 4 (1,760 CFU), a target dose of 1,500 was selected for the dose confirmation cohort (cohort 5). The actual dose administered in cohort 5 was 1,100 CFU, with the corresponding clinical outcomes closely mirroring those in cohorts 2 and 3, further highlighting the dose-sensitive nature of the model.
Due to the relatively low percentage of subjects meeting the primary outcome, post hoc alternative definitions of shigellosis were developed (Table 3). For cohorts 2, 3, and 5, the rate of shigellosis ranged from 40 to 60% using alternative shigellosis definition 1 and 30 to 50% using alternative shigellosis definition 2. In comparison, for cohort 4, the rate of shigellosis was 70% and 60% when alternative definitions 1 and 2, respectively, were used.
TABLE 3.
Classification of disease outcome
| Outcome | Definition | Scale | Source |
|---|---|---|---|
| Diarrhea severity | Divided into 4 categories: (i) no diarrhea; (ii) mild (2 or 3 grade 3–5 stools and <400 g of grade 3–5 stools in 24 h); (iii) moderate (4 or 5 grade 3–5 stools or 400–800 g of grade 3–5 stools in 24 h); (iv) severe: (≥6 grade 3–5 stools or >800 g of grade 3–5 stools in 24 h) | Binary outcome: volunteers were placed in 1 of 2 categories: (i) those with no or mild diarrhea; (ii) those with moderate or severe diarrhea | Defined per protocol (this study) |
| Dysentery | A grade 3–5 stool with gross blood on at least 2 occasions with reportable constitutional/enteric symptoms | Binary outcome: volunteers were grouped by whether they received a dysentery diagnosis during the study | Defined per protocol (this study) |
| Disease severity score | Volunteers are assigned scores across the following categories depending on severity and frequency: (i) objective symptoms (gross blood, oral temperature, vomiting); (ii) subjective symptoms (constitutional/enteric symptoms); (iii) grade 3–5 stool output per 24 h | Ordinal outcome: volunteers’ scores across three categories are added to for a score between 0 and 9 | 12 |
| Shigellosis | One of three definitions must be met: (i) severe diarrhea; (ii) moderate diarrhea with fever ≥38.0°C or ≥1 moderate constitutional/enteric symptom or ≥2 episodes of vomiting in 24 h; (iii) dysentery with fever ≥38.0°C or ≥1 moderate constitutional/enteric symptom or ≥2 episodes of vomiting in 24 h | Binary outcome: volunteers grouped by whether they received a shigellosis diagnosis during the study | 9 |
As the dose of S. sonnei was increased, there was a concomitant increase in the disease severity score, suggesting an association between dose and symptoms experienced by the cohort (Wilcoxon chi-square test; P = 0.02) (Fig. 2).
FIG 2.
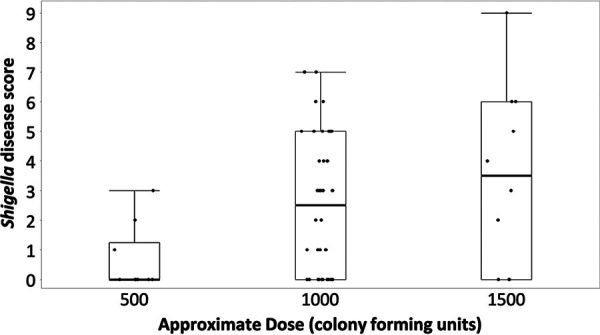
S. sonnei 53G dose and severity score relationship. The shigellosis disease score in challenged volunteers is given by approximate S. sonnei 53G dose. The median is represented by a line, and the box represents the third and first quartiles, while the whiskers represent the third quartile + 1.5*(interquartile range) and the first quartile − 1.5*(interquartile range).
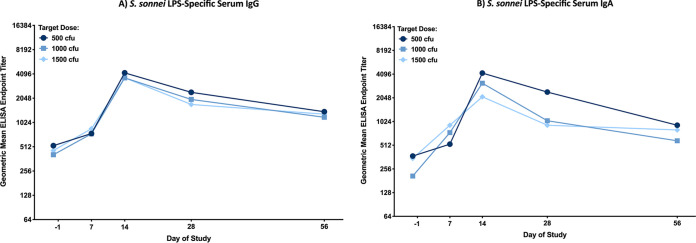
Endpoint titers of serum IgG and IgA directed to S. sonnei lipopolysaccharide (LPS) are shown in Fig. 3A and B. LPS-specific serum IgG and IgA responses peaked on study day 14, and with the exception of the serum IgA responses in cohort 4, the LPS-specific GMT was significantly higher (two-way analysis of variance [ANOVA]; P ≤ 0.009) on day 14 compared to baseline. LPS-specific serum IgG and IgA responses diminished by day 56; however, they remained elevated over baseline. There were no differences in LPS-specific serum IgG or IgA responses across dose groups on any given study day, demonstrating a lack of a dose response in the LPS-specific serum IgG or IgA responses postchallenge.
FIG 3.
S. sonnei LPS-specific serum IgG (A) and IgA (B) geometric mean ELISA endpoint titers with 95% confidence intervals prior to challenge (day −1) and 7, 14, 28, and 56 days postchallenge, grouped by target dose of S. sonnei 53G: 500 CFU (cohort 1), 1,000 CFU (cohorts 2 and 3), and 1,500 CFU (cohorts 4 and 5). There were no significant differences in ELISA titers between any two dose groups at the same time point, as determined by 2-way ANOVA of log-transformed titers with a Bonferroni post hoc test.
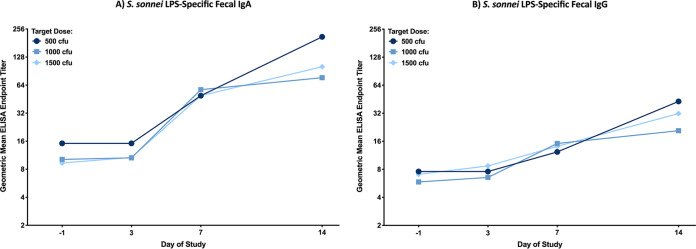
Endpoint titers of fecal IgA and IgG directed to S. sonnei LPS are shown in Fig. 4A and B. LPS-specific fecal IgA and IgG responses were elevated over baseline by day 7 and continued to rise through study day 14 across all dose groups (two-way ANOVA; P = 0.014). The LPS-specific fecal antibody responses followed a similar trend as the LPS-specific serum antibody responses, with no differences observed by dose, as well as no differences observed in the LPS-specific fecal IgG or IgA peak titers or peak fold rises (data not shown).
FIG 4.
S. sonnei LPS-specific fecal IgA (A) and IgG (B) geometric mean ELISA endpoint titers with 95% confidence intervals prior to challenge (day −1) and 3, 7, and 14 days postchallenge, grouped by target dose of S. sonnei 53G: 500 CFU (cohort 1), 1,000 CFU (cohorts 2 and 3), and 1,500 CFU (cohorts 4 and 5). There were no significant differences in ELISA titers between any two dose groups at the same time point, as determined by 2-way ANOVA of log-transformed titers with a Bonferroni post hoc test.
DISCUSSION
A safe and reproducible model of S. sonnei was developed using a lyophilized lot of strain 53G. The proportion of subjects meeting the a priori endpoint of shigellosis was low across all cohorts; however, the safety monitoring committee (SMC) and the adjudication board determined that the per-protocol endpoint was insufficiently sensitive for use in future applications of the model. To improve sensitivity, alternative endpoints of disease were evaluated. These alternative endpoints were considered in earlier Shigella CHIMs and were also highlighted for consideration in a recent paper addressing the standardization of clinical endpoints for Shigella CHIM studies (9). With these alternative post hoc endpoints, the 1,760-CFU dose resulted in a 60 to 70% attack rate (AR) of shigellosis. In an attempt to validate the AR at the approximate target dose of 1,500 CFU, a final cohort of 16 subjects were administered an inoculum targeting this dose. However, the actual dose given in this final cohort was 1,100 CFU, which resulted in a shigellosis rate very similar to those in cohorts 2 and 3, who received approximately 1,000 CFU of S. sonnei. Based on these results and to better ensure doses likely to yield target ARs of ≥60%, the adjudication board members, the investigative team, and the sponsor recommended that 1,500 to 2,000 CFU be the target inoculum in future CHIM studies with this lyophilized strain. Similar recommendations also have been made for the 2457T strain of Shigella flexneri 2a, another commonly used challenge strain of Shigella (9). The reason for the less-than-expected dose for the final cohort is unclear. Prior to dosing participants, repeated practice formulations yielded the target dose of 1,500 CFU Additionally, after the study, subsequent practice dilutions again met the target inoculum dose. Finally, vials of lyophilized 53G are tested yearly with no loss in CFU or L-forming units. However, as the disease spectrum appears to be dose dependent, continued efforts to improve dosing precision are needed.
Overall, the challenge model demonstrated an acceptable safety profile and induced symptoms commonly associated with Shigella sonnei infection. Furthermore, the disease spectrum appears similar to that achieved with comparable doses of the S. flexneri 2457T strain (11, 12). Disease among symptomatic subjects was representative of moderate to severe shigellosis, with no signs of early onset or particularly aggressive disease. However, even at the highest challenge dose, some subjects were asymptomatic, suggesting that, in addition to LPS-specific serum and fecal IgG and IgA, other currently unrecognized host factors, including genetic factors, microbiome, and immune responses to other antigens, may be present.
There was no association between LPS-specific serum IgG or IgA responses and clinical disease. This may have been due to truncation of the infection with antibiotics. An infection not truncated by antibiotics may result in a more prolonged exposure to Shigella antigens, possibly resulting in more robust anti-LPS antibody responses among people with more severe symptoms. It also is possible that a correlation between the disease outcome and immune responses to other Shigella antigens, such as the Ipa proteins, or other immunological responses (such as memory B cells, antibody-secreting cells [ASCs], and antibodies in lymphocyte supernatant [ALS]), may be observed. These immunological analyses are described in the companion article (13).
Five other CHIM studies using fresh plate-grown preparations of this strain have been published and were summarized in a 2013 review of Shigella CHIMs (11). The first study, published by Black et al., utilized 500 CFU S. sonnei in milk to assess efficacy of a candidate vaccine (14). The same dose and inoculum vehicle were utilized by all subsequent studies, with diarrhea and dysentery attack rates ranging between 40 and 70%; however, the endpoint definitions varied. Based on data from an S. flexneri 2a CHIM demonstrating that administration of sodium bicarbonate prior to challenge increases the AR (15), we used sodium bicarbonate as a prechallenge buffer. Also, based on previous studies, we demonstrated that diluting and administering S. sonnei in 0.9% saline instead of sterile water and sodium bicarbonate was the best at preserving viability (M. Venkatesan, personal communication).
An ongoing criticism with the Shigella CHIM is that inoculation is not reflective of natural exposure. Additionally, there have been stated concerns that due to the inoculum size, the high attack rates, and the clinical disease profile, CHIM studies may yield vaccine efficacy estimates that are lower than they would be if the vaccine had been assessed in a field efficacy trial (16). Furthermore, there are clear differences in the primary target population for a Shigella vaccine (i.e., children in LMICs) and the adult population from high-income countries typically utilized for enteric CHIMs. Nonetheless, the CHIM is viewed as a way to shorten the vaccine development pathway (16, 17). For the adult traveler population (including military personnel), the CHIM model is directly applicable and the clinical endpoints appear to be comparable. Similar to the experience with cholera, CHIM may help speed vaccine licensure for a traveler indication (17).
The Shigella vaccine pipeline is expanding with the advancement of several new candidates entering clinical development and others advancing into studies to assess preliminary efficacy. While additional refinements in the model may be needed to increase precision, this study made large strides to improve the Shigella CHIM by using a lyophilized strain of bacteria and applying a consensus disease scoring system. This study and other Shigella CHIMs are likely to play a critical role in future studies assessing vaccine efficacy.
MATERIALS AND METHODS
Study conduct.
The study, designed as a dose-escalating inpatient study to induce shigellosis in approximately 60% of subjects, was conducted on an inpatient unit at the Cincinnati Children’s Hospital Medical Center (CCHMC). A review of the Shigella CHIM literature indicated that shigellosis attack rates in prior S. sonnei studies using the 53G strain tended to range between 50% and 60% (9); consequently, this attack rate was felt to be a reasonable target for the first clinical evaluation of a lyophilized lot of the challenge organism. Attack rates with the lyophilized preparation significantly below this target would not be practical for use in future vaccine efficacy studies evaluating promising vaccine candidates (8).
Based on experience, a target dose of 500 CFU was selected for the initial cohort of 10 subjects (11). Dosing for cohorts 2 to 4 (10 subjects/cohort) was based on the attack rate (AR) of the previous cohort. If the AR was ≤60%, the next cohort was to receive a higher dose, while a lower dose would be used if the AR exceeded 60%. A safety monitoring committee (SMC) reviewed the results for each cohort and suggested the dose for the subsequent cohort. Results from the dose-ranging cohorts were reviewed to select a dose for a fifth “confirmatory” cohort of up to 20 subjects.
Subject recruitment and screening.
Healthy, adult male and nonpregnant, nonbreastfeeding female subjects 18 to 49 years of age (up to the 50th birthday) were eligible. All subjects signed a written informed consent form and passed a test of study comprehension. Subjects with serologic evidence of HIV, hepatitis B or C, or syphilis were excluded, as were those with immunosuppressive illness or IgA deficiency. Due to the potential risk of reactive arthritis (18), subjects with a family history of inflammatory arthritis or who were HLA-B27 positive were excluded. Travel to a country where Shigella is endemic within the past 2 years was exclusionary, as was an endpoint titer of serum IgG to S. sonnei lipopolysaccharide (LPS) of >2,500 (determined by enzyme-linked immunosorbent assay [ELISA]) (19).
Challenge strain.
The strain of S. sonnei (53G) used in the study was isolated in 1954 from a child with diarrhea in Tokyo (20). The seed was maintained at the Center for Vaccine Development (CVD), University of Maryland, and in 1998, a research seed vial was streaked on a Congo red (CR) tryptic soy agar (TSA) plate and transferred to the Walter Reed Army Institute of Research (WRAIR) Pilot Bioproduction Facility (PBF). A master cell bank (MCB) (lot 0593) and production cell bank (PCB) (lot 0599) were manufactured using cGMP and stored at –80°C ± 10°C. In 2013, a vial of the PCB was plated on Congo red, and form I colonies were harvested and grown in a 30-liter fermentor under cGMP conditions. The contents were harvested and lyophilized as 2-ml aliquots in 7.5% dextran T10, 2% sucrose, and 1.5% glycerol in Dulbecco’s phosphate-buffered saline (PBS) at a final concentration of 2 × 109 CFU/ml. This standardized, lyophilized lot of S. sonnei 53G, lot 1794, has been stored at −80°C ± 10°C at the WRAIR PBF. The genomic sequences of the cGMP MCB, PCB, and final released lyophilized S. sonnei 53G preparation were determined to be identical.
Routine viability and stability testing on the product since lot release has confirmed that lot 1794 remains ≥1 × 109 CFU/ml, with ≥80% of colonies retaining the virulence plasmid.
On the day of challenge, a vial of S. sonnei 53G (lot 1794) was placed on ice for 30 min, and then 2 ml of cold sterile water for injection (SWI) was added to the vial. Following an additional 15 min on ice, the colony count of Shigella was estimated (in CFU per milliliter) by reading absorbance at a wavelength of 600 nm. The preparation was then diluted in cold, sterile normal saline to the desired optical density. Subsequently, 1 ml of the diluted challenge solution was added to 30 ml of sterile normal saline and kept on ice until subject administration. To determine the actual dose administered, an aliquot of the challenge material was plated on tryptone soy agar and incubated for 12 h at 37°C; colonies were counted, and antigen phase expression (form I versus form II) was assessed.
Challenge strain administration.
Subjects fasted for 90 min before and after challenge. To neutralize gastric acidity, 5 min before challenge, subjects drank a solution of 2 g of sodium bicarbonate in 120 ml of SWI. The time between rehydration and administration of the inoculum did not exceed 2 h.
Clinical management.
Subjects were directly observed for 90 min after challenge and thereafter evaluated at least daily. All stools were collected, and study staff graded each stool on a scale of 1 to 5, as previously described (21). All grade 3 to 5 stools were weighed and visually assessed for blood. Visual blood was confirmed by a guaiac test.
Ciprofloxacin, 500 mg orally twice per day for 3 days, was initiated 5 days after challenge, with discharge scheduled on the 8th day postchallenge as long as subjects were able to maintain hydration and they had had at least two consecutive stool cultures negative for S. sonnei. Diary cards to record symptoms posthospitalization were given at discharge and reviewed during outpatient clinic visits on days 14, 28, and 56. Assessment of clinical status and collection of additional samples for immunological testing were also performed during the outpatient clinic visits.
Diarrhea severity was assessed as follows: “mild” was defined as two or three grade 3 to 5 stools and <400 g of grade 3 to 5 stool in any 24 h; “moderate” was defined as four or five grade 3 to 5 stools or 400 to 800 g of grade 3 to 5 stool per 24 h; “severe” was defined as six or more grade 3 to 5 stools or >800 g of grade 3 to 5 stool per 24 h. The diarrheal episode was considered resolved when there were no grade 3 to 5 stools for 48 h. Dysentery was defined as grade 3, 4, or 5 stools with gross blood (confirmed by guaiac test) on at least two occasions with reportable constitutional symptoms. Disease severity was also assessed using a previously published disease severity scale (12).
Oral rehydration solutions (ORS) were initiated for any subject with moderate or severe diarrhea. Subjects were offered at least 1.5 ml of ORS for each gram of diarrheal stool and 1.0 ml for each gram of emesis.
The primary objective of the study was to identify the dose of S. sonnei 53G that induced shigellosis in at least 60% of subjects with the per-protocol definition of shigellosis being defined as shedding of S. sonnei in the stool with moderate or severe diarrhea and/or dysentery plus either (i) moderate fever or (ii) one or more severe intestinal symptoms.
An independent adjudication board was utilized to provide an unbiased assessment of the primary (and some secondary) clinical endpoints. Additional secondary endpoints adjudicated included alternative definitions of shigellosis, as follows: option 1 was severe diarrhea, or moderate diarrhea and fever, or moderate diarrhea with ≥1 moderate constitutional/enteric symptom or dysentery; option 2 was severe diarrhea, or moderate diarrhea and fever, or dysentery and fever, or moderate diarrhea and ≥1 severe constitutional/enteric symptom, or dysentery and ≥1 severe constitutional/enteric symptom. These additional endpoints were applied based on recommendations of a recent convening of experienced Shigella CHIM investigators and vaccine developers that focused on further standardizing clinical endpoint for the Shigella CHIM model (9, 22). All clinical endpoints evaluated in this study are outlined in Table 3.
Laboratory. (i) Stool culture.
After the stools were graded and weighed, a portion was streaked on Hektoen enteric agar (HEA) plates and incubated for 18 h at 37°C. Up to two non-lactose-fermenting colonies were picked from the plate and tested with S. sonnei polyvalent group D antiserum to confirm S. sonnei.
(ii) Immunology.
Serum, stool, peripheral blood mononuclear cells (PBMCs), and saliva samples were collected prior to challenge and at various time points postchallenge. Anti-S. sonnei LPS IgG and IgA responses were determined by enzyme-linked immunosorbent assay (ELISA), as previously described with the minor modifications that Immulon 1-B ELISA plates and human-specific secondary antibodies (reserve allophycocyanin [AP]-conjugated goat anti-human IgG or IgA; Seracare) were used (19, 23). Serum samples from study days −1, 7, 14, 28, and 56 and stool samples from study days −1, 3, 7, and 14 were serially diluted starting at a 1:100 (serum) or 1:10 (feces) dilution. Any sample that was negative at the starting dilution was assigned a titer corresponding to half of the starting dilution (1/2 LOD). Endpoint titers were determined for all subjects, and geometric mean titers (GMT) were calculated for each cohort. Immune responders were defined a priori as having a ≥4-fold increase over their baseline titer.
(iii) Statistical analyses.
Nominal data were compared using Pearson’s χ2 or Fisher’s exact test, as appropriate. Comparisons of the frequency and volume of loose stools in a 24-h period across cohorts were made using a Kruskal-Wallis test with appropriate post hoc pairwise comparisons. Time-to-event comparisons were made using rank tests for homogeneity in survival curves. Serologic and fecal antibody titers were log10 transformed prior to analysis, and comparisons across approximate dose delivered were made using a 2-way analysis of variance (ANOVA) with a Bonferroni post hoc test. All statistical tests were interpreted in a two-tailed fashion using an α value of 0.05 in SAS version 9.2 (SAS Institute, Inc., Cary, NC) and JMP.
(iv) Regulatory and ethical review.
This study was conducted under an investigational new drug application (BB-IND-17,015) and was reviewed and approved by the CCHMC institutional review board. The trial was registered with ClinicalTrials.gov (NCT02816346). All subjects had the study explained to them in detail and signed an informed consent document demonstrating their willingness to participate in the study.
Data availability.
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under accession no. JACMSG000000000. The version described in this paper is version JACMSG010000000.
ACKNOWLEDGMENTS
We thank Louis Bourgeois for his critical review and editing of the manuscript and Amanda Nintrup for her technical assistance with manuscript preparation.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the U.S. Government. This is a U.S. Government work. There are no restrictions on its use.
None of the authors have a conflict of interest for any of the materials presented in the manuscript.
This study was funded by the Military Infectious Disease Research Program under award no. D0437_15_NM and contracted to the Cincinnati Children’s Hospital Medical Center from the Natick Contracting Division (W911QY-16-2-0002). The study was also funded by a Collaborative Research and Development Agreement with PATH Enteric Vaccine Solutions, the Walter Reed Army Institute of Research, and the Cincinnati Children’s Hospital Medical Center (ORTA no. 4516).
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/mSphere.00988-19.
REFERENCES
- 1.Lamberti LM, Bourgeois AL, Fischer Walker CL, Black RE, Sack D. 2014. Estimating diarrheal illness and deaths attributable to Shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis 8:e2705. doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr. 2018. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect Dis 18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. 2017. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 35:6783–6789. doi: 10.1016/j.vaccine.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:E564–E575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JDt, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R. 2019. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health 7:E321–E330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani S, Wierzba T, Walker RI. 2016. Status of vaccine research and development for Shigella. Vaccine 34:2887–2894. doi: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan CA, Riddle MS, Chen WH, Talaat KR, Jain V, Bourgeois AL, Frenck RW, Jr, Kotloff KL, Porter C. 2019. Consensus report on Shigella controlled human infection model: clinical endpoints. Clin Infect Dis 69:S591–S595. doi: 10.1093/cid/ciz891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giersing BK, Porter CK, Kotloff K, Neels P, Cravioto A, MacLennan CA. 2019. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine 37:4778–4783. doi: 10.1016/j.vaccine.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Porter CK, Thura N, Ranallo RT, Riddle MS. 2013. The Shigella human challenge model. Epidemiol Infect 141:223–232. doi: 10.1017/S0950268812001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter CK, Lynen A, Riddle MS, Talaat K, Sack D, Gutierrez RL, McKenzie R, DeNearing B, Feijoo B, Kaminski RW, Taylor DN, Kirkpatrick BD, Bourgeois AL. 2018. Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PLoS One 13:e0194325. doi: 10.1371/journal.pone.0194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson KA, Frenck RW, Jr, Dickey M, Suvarnapunya AE, Chandrasekaran L, Weerts HP, Heaney CD, McNeal M, Detizio K, Parker S, Hoeper A, Bourgeois AL, Porter CK, Venkatesan MM, Kaminski RW. 2020. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. mSphere 5:e00988-19. doi: 10.1128/mSphere.00988-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, Formal SB. 1987. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis 155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 16.Porter CK, Louis Bourgeois A, Frenck RW, Prouty M, Maier N, Riddle MS. 2017. Developing and utilizing controlled human models of infection. Vaccine 35:6813–6818. doi: 10.1016/j.vaccine.2017.05.068. [DOI] [PubMed] [Google Scholar]
- 17.Chen WH, Kotloff KL. 2016. Shigella vaccine development: finding the path of least resistance. Clin Vaccine Immunol 23:904–907. doi: 10.1128/CVI.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajene AN, Fischer Walker CL, Black RE. 2013. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr 31:299–307. doi: 10.3329/jhpn.v31i3.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle MS, Kaminski RW, Di Paolo C, Porter CK, Gutierrez RL, Clarkson KA, Weerts HE, Duplessis C, Castellano A, Alaimo C, Paolino K, Gormley R, Gambillara Fonck V. 2016. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. Clin Vaccine Immunol 23:908–917. doi: 10.1128/CVI.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis 159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 21.Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 22.Talaat KR, MacLennan CA, Riddle MS, Chen WH, Jain V, Bourgeois AL, Frenck RW, Jr, Kotloff KL, Porter C. 2019. Consensus report on Shigella controlled human infection model: conduct of studies. Clin Infect Dis 69:S580–S590. doi: 10.1093/cid/ciz892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turbyfill KR, Hartman AB, Oaks EV. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect Immun 68:6624–6632. doi: 10.1128/IAI.68.12.6624-6632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under accession no. JACMSG000000000. The version described in this paper is version JACMSG010000000.