Abstract
Context
US papillary thyroid carcinoma (PTC) incidence recently declined for the first time in decades, for reasons that remain unclear.
Objective
This work aims to evaluate PTC incidence trends, including by histologic subtype and size, and noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).
Design
This descriptive study uses US Surveillance, Epidemiology, and End Results–18 cancer registry data (2000-2017).
Patients
Participants included individuals diagnosed with PTC (2000-2017) or NIFTP (2016-2017).
Results
During 2000 to 2015, PTC incidence increased an average 7.3% per year, (95% CI, 6.9% to 7.8%) during 2000 to 2009, and 3.7% per year (95% CI, 0.2% to 7.3%) during 2009 to 2012, before stabilizing in 2012 to 2015 (annual percentage change [APC] = 1.4% per year, 95% CI, –1.8% to 4.7%) and declining in 2015 to 2017 (APC = –4.6% per year, 95% CI, –7.6% to –1.4%). The recent declines were observed for all sizes of PTC at diagnosis. Incidence of follicular variant of PTC (FVPTC) sharply declined in 2015 to 2017, overall (APC = –21.1% per year; 95% CI, –26.5% to –15.2%) and for all tumor sizes. Observed increases in encapsulated papillary carcinoma (classical PTC subtype) and NIFTP each accounted for 10% of the decline in FVPTC. Classical PTC incidence continuously increased (2000-2009, APC = 8.7% per year, 95% CI, 8.1% to 9.4%; 2009-2017, APC = 1.0% per year, 95% CI, 0.4% to 1.5%), overall and for all sizes except smaller than 1 cm, as did incidence of other PTC variants combined (2000-2017, APC = 5.9% per year, 95% CI, 4.0% to 7.9%).
Conclusion
The reasons underlying PTC incidence trends were multifactorial. Sharp declines in FVPTC incidence during 2015 to 2017 coincided with clinical practice and diagnostic coding changes, including reclassification of noninvasive encapsulated FVPTC from a malignant to in situ neoplasm (NIFTP). Observed increases in NIFTP accounted for 10% of the decline in FVPTC.
Keywords: thyroid cancer, incidence, descriptive trends, epidemiology, NIFTP, overdiagnosis
From the early 1980s to late 2000s, US thyroid cancer incidence rates tripled, rising at a faster rate than any other malignancy (1). Incidence rates peaked in 2015, and from 2015 to 2017 they declined for the first time in more than 3 decades (2, 3). The downturn in thyroid cancer incidence trends appeared to be driven by small, localized papillary thyroid carcinomas (PTCs) (1-4). This reversal in trends for PTC has most likely resulted from an increased awareness of the harms associated with overdiagnosis and overtreatment of indolent disease, and clinical practice recommendations that were recently introduced to minimize these harms (2, 3, 5-8). Specifically, in 2009 and 2015, the American Thyroid Association (ATA) released clinical guidelines recommending against fine-needle aspiration biopsy of small thyroid nodules and those with low-risk imaging characteristics (5, 6). In 2015, the ATA also supported the use of molecular markers for small, indeterminate thyroid nodules, and offered thyroid lobectomy and active surveillance as viable treatment options for the management of nonaggressive, early-stage, or suspected disease (6). Moreover, in 2017, the US Preventive Services Task Force recommended against thyroid screening in the asymptomatic general population (7).
Another recent development was the recommendation by the ATA to change the behavioral classification of noninvasive encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) from a carcinoma to an in situ neoplasm (“noninvasive follicular thyroid neoplasm with papillary-like nuclear features [NIFTP]”) (9). This recommendation followed the results of a 2016 study by Nikiforov et al, which demonstrated a much lower risk of adverse outcomes for noninvasive vs invasive EFVPTCs (10). Nikiforov and colleagues estimated that adoption of the NIFTP terminology could reduce the incidence of total PTC by up to 19% (10). As a result of this recommendation, in January 2017, a substantial modification to the coding system was adopted by the North American Association of Central Cancer Registries (NAACCR) to distinguish infiltrative (nonencapsulated) FVPTC from invasive and noninvasive EFVPTC (11). Specifically, invasive EFVPTCs were to be recoded as encapsulated papillary carcinoma, a type of classical PTC, and noninvasive EFVPTCs were to be recoded as NIFTP. This change also was adopted by the World Health Organization in 2017 (12). These nomenclature changes would be expected to reduce the incidence of FVPTC, specifically. In contrast, changes in clinical practice guidelines related to risk criteria for biopsy, including higher size thresholds for tumors with less-suspicious features by ultrasound, and screening would be expected to affect incidence rates of all histologic subtypes of PTC.
In addition, significant increases in the incidence of less common but aggressive subtypes of PTCs, along with increases in thyroid cancer mortality, have raised concerns about a real increase in the occurrence of aggressive PTC subtypes (1, 4). Thus, other factors (eg, environmental exposures or lifestyle changes) may have contributed to changes in PTC incidence trends independent of the effects of clinical practice changes.
To quantify the relative impact of clinical practices, diagnostic coding changes, and other factors on PTC incidence trends from 2000 to 2017, we used data from the US Surveillance, Epidemiology, and End Results (SEER)-18 cancer registry program to evaluate trends in annual incidence rates for PTC by histologic subtype and tumor size. We also estimated the contribution of the recent nomenclature changes on these trends by evaluating trends in incidence of FVPTC, encapsulated papillary carcinoma, and NIFTP.
Materials and Methods
PTC cases were first primary, microscopically confirmed invasive (malignant) neoplasms of the thyroid gland (International Classification of Diseases for Oncology, third edition [ICD-O-3] topography code C73.9) with histologic codes 8050, 8260, 8340 to 8344, 8350, and 8450 to 8460. Histologic subtypes of PTC (first primary, microscopically confirmed) were further classified according to the following ICD-O-3 histologic codes: FVPTC (8340/3), classical PTC (8050/3, 8260/3, 8341/3, 8343/3), and other PTC variants (eg, oxyphilic cell, tall cell, diffuse sclerosing variant, papillary cystadenoma; 8342/3, 8344/3, 8450/3-8460/3). Beginning January 1, 2017, NAACCR implemented changes to the histologic coding of FVPTCs (8340/3) to distinguish infiltrative (nonencapsulated) FVPTC (8340/3) from invasive EFVPTC/encapsulated papillary carcinoma not otherwise specified (NOS) (8343/3) and noninvasive EFVPTC/NIFTP (8343/2) (11). Because the latter uses an in situ (rather than malignant or invasive) neoplasm behavior code, tumors falling in this category were not included within the total PTC grouping. Before 2017, both noninvasive and invasive EFVPTC should have been coded as FVPTC (8340/3).
Tumor size categories (< 1 cm [excluding no mass/tumor], 1-1.9 cm, 2-3.9 cm, and ≥ 4 cm) were derived using Extent of Disease-10 codes for 1988 to 2003, Collaborative Staging codes for 2004 to 2015, and Extent of Disease Tumor Size Summary codes for 2016 to 2017.
Using the National Cancer Institute’s Joinpoint Regression Analysis program, version 4.2.0, we calculated annual percentage changes (APCs) and 95% CIs to quantify trends in age-adjusted incidence rates (standardized to the 2000 US population). T tests were used to determine whether APCs were significantly different from zero. Log-linear regression models were used to identify joinpoints (calendar years when the APCs changed significantly), allowing for the minimum number of joinpoints necessary to fit the data. Statistical tests were 2-sided (α = .05). In a sensitivity analysis, delay-adjustment factors for total thyroid cancer were applied to incidence rates for each variant and APCs were calculated.
As a post hoc analysis, we calculated the proportion of change in FVPTC incidence rates due to the coding changes formally implemented by NAACCR in 2017 by dividing the difference in incidence rates for noninvasive and invasive EFVPTC by the difference in incidence rates for total FVPTC between 2015 and 2017.
Because our research used publicly available data and involved no direct interaction with patients, institutional review board approval and informed consent were not required.
Results
During 2000 to 2017, a total of 163 387 PTC cases (76.7% women) were identified, including 49 525 FVPTC, 111 058 classical, and 2804 other variants.
Trends for papillary thyroid carcinoma, overall and by histologic subtype
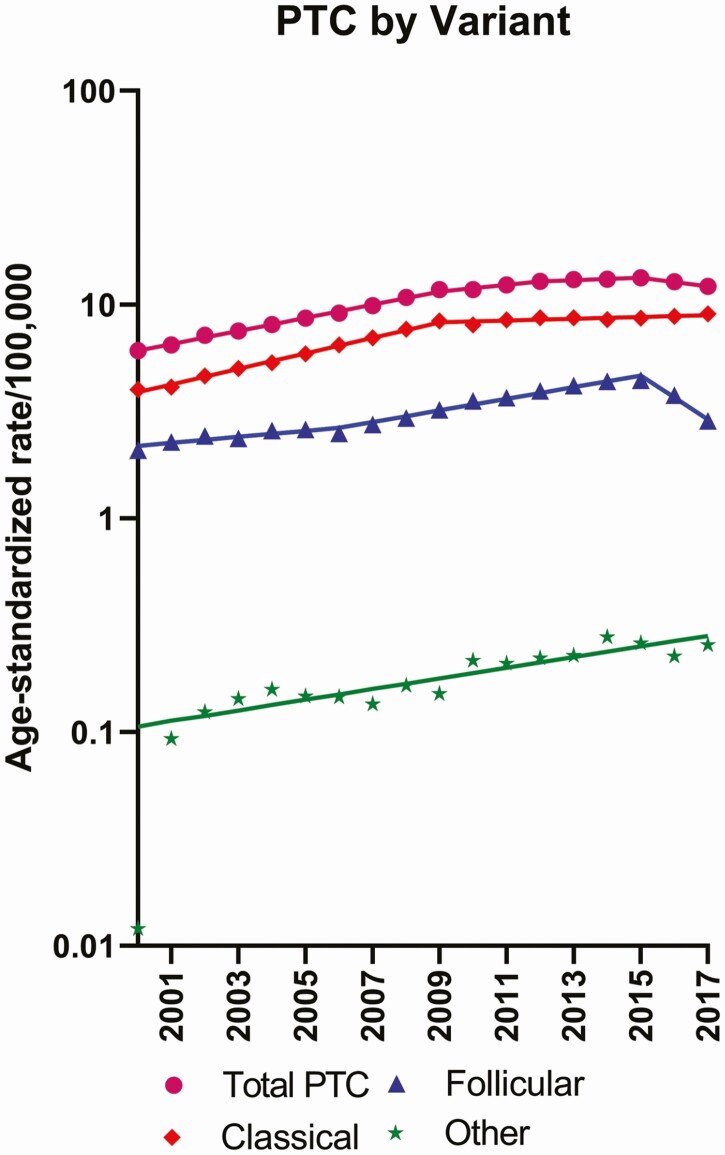
PTC incidence more than doubled during 2000 to 2015 (from 6.10 to 13.32 per 100 000 person-years), increasing an average 7.3% per year (95% CI, 6.9% to 7.8%) during 2000 to 2009 and 3.7% per year (95% CI, 0.2% to 7.3%) during 2009 to 2012, before stabilizing in 2012 to 2015 (1.4% per year, 95% CI, –1.8% to 4.7%). Incidence subsequently declined during 2015 to 2017 by 4.6% per year (95% CI, –7.6% to –1.4%) from 13.32 to 12.15 per 100 000 (Table 1; Fig. 1). When evaluated by histologic subtype, the downturn in overall PTC incidence during 2015 to 2017 appeared to be driven by FVPTC (APC, –21.1% per year; 95% CI, –26.5% to –15.2%). Incidence of classical PTC increased over the entire study period (2000-2009: 8.7% per year, 95% CI, 8.1% to 9.4%; 2009 to 2017: 1.0% per year, 95% CI, 0.4% to 1.5%), as did incidence of other PTC variants (2000-2017: APC, 5.9% per year, 95% CI, 4.0% to 7.9%). When delay-adjustment factors were applied, APCs did not change notably for FVPTC (APC 2015-2017: –20.7% per year; 95% CI, –26.0% to –14.9%), classical PTC (2009-2017: 1.2% per year; 95% CI, 0.6% to 1.7%), or other PTCs (2000-2017: 6.0% per year, 95% CI, 4.0% to 8.0%).
Table 1.
Trends in papillary thyroid carcinoma incidence rates (2000-2017): Surveillance, Epidemiology, and End Results–18 Registry Databasea
| Age-standardized rate, per 100 000 | Segment 1 | Segment 2 | Segment 3 | Segment 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2017 | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | |
| Total PTC, cm (n = 163 387) | 6.10 | 12.15 | 2000-2009 | 7.33 (6.92 to 7.75) | 2009-2012 | 3.69 (0.22 to 7.28) | 2012-2015 | 1.39 (–1.82 to 4.71) | 2015-2017 | –4.55 (–7.62 to –1.39) |
| < 1.0 | 1.64 | 3.90 | 2000-2009 | 10.4 (9.46 to 11.3) | 2009-2013 | 3.84 (0.43 to 7.36) | 2013-2017 | –4.33 (–6.33 to –2.29) | ||
| 1.0-1.9 | 1.46 | 3.76 | 2000-2009 | 10.2 (9.48 to 10.8) | 2009-2013 | 3.34 (0.77 to 5.98) | 2013-2017 | –0.52 (–2.03 to 1.02) | ||
| 2.0-3.9 | 1.60 | 2.90 | 2000-2009 | 5.24 (4.60 to 5.89) | 2009-2015 | 2.91 (1.65 to 4.18) | 2015-2017 | –3.42 (–8.43 to 1.87) | ||
| ≥ 4.0 | 0.58 | 1.16 | 2000-2015 | 5.08 (4.58 to 5.58) | 2015-2017 | –7.08 (–15.3 to 1.93) | ||||
| Unknown | 0.81 | 0.40 | 2000-2008 | –8.31 (–11.9 to –4.61) | 2008-2017 | 0.53 (–3.11 to 4.31) | ||||
| Follicular variant of PTC, cm (n = 49 525) | 2.08 | 2.86 | 2000-2006 | 3.29 (1.74 to 4.86) | 2006-2015 | 6.45 (5.63 to 7.28) | 2015-2017 | –21.1 (–26.5 to –15.2) | ||
| < 1.0 | 0.52 | 0.91 | 2000-2015 | 7.19 (6.40 to 7.99) | 2015-2017 | –23.4 (–34.3 to –10.7) | ||||
| 1.0-1.9 | 0.47 | 0.81 | 2000-2014 | 7.59 (6.76 to 8.43) | 2014-2017 | –12.9 (–18.7 to –6.60) | ||||
| 2.0-3.9 | 0.57 | 0.72 | 2000-2002 | 9.10 (–7.24 to 28.3) | 2002-2006 | –0.23 (–7.46 to 7.56) | 2006-2015 | 6.08 (4.62 to 7.57) | 2015-2017 | –21.4 (–31.0 to –10.4) |
| ≥ 4.0 | 0.25 | 0.38 | 2000-2008 | 2.66 (0.32 to 5.06) | 2008-2015 | 7.63 (4.66 to 10.7) | 2015-2017 | –17.0 (–29.4 to –2.28) | ||
| Unknown | 0.26 | 0.04 | 2000-2008 | –16.4 (–22.0 to –10.5) | 2008-2017 | –0.40 (–8.17 to 8.02) | ||||
| Classical variant of PTC, cm (n = 111 058) | 4.01 | 9.04 | 2000-2009 | 8.71 (8.06 to 9.36) | 2009-2017 | 0.98 (0.43 to 1.53) | ||||
| < 1.0 | 1.11 | 2.95 | 2000-2009 | 11.9 (11.3 to 12.6) | 2009-2013 | 0.94 (–1.37 to 3.30) | 2013-2017 | –2.45 (–3.86 to –1.02) | ||
| 1.0-1.9 | 0.99 | 2.88 | 2000-2009 | 11.0 (9.90 to 12.2) | 2009-2017 | 1.67 (0.79 to 2.56) | ||||
| 2.0-3.9 | 1.03 | 2.11 | 2000-2007 | 6.93 (5.27 to 8.62) | 2007-2017 | 2.34 (1.61 to 3.08) | ||||
| ≥ 4.0 | 0.33 | 0.73 | 2000-2009 | 7.12 (5.69 to 8.56) | 2009-2017 | 1.98 (0.75 to 3.22) | ||||
| Unknown | 0.55 | 0.36 | 2000-2017 | –2.25 (–3.50 to –0.99) | ||||||
| Other PTC variants, cm (n = 2804) | NRa | 0.26 | 2000-2017 | 5.92 (3.95 to 7.93) | ||||||
| < 1.0 | NRa | 0.05 | 2000-2017 | 6.23 (3.88 to 8.63) | ||||||
| 1.0-1.9 | NRa | 0.08 | 2000-2014 | 10.8 (7.66 to 14.1) | 2014-2017 | –7.27 (–24.4 to 13.7) | ||||
| 2.0-3.9 | NRa | 0.08 | 2000-2017 | 6.21 (3.88 to 8.60) | ||||||
| ≥ 4.0 | NRa | 0.05 | 2000-2017 | 4.50 (2.34 to 6.70) | ||||||
| Unknown | NRa | NRa | NR | |||||||
Abbreviations: APC, annual percentage change; NR, not reportable; PTC, papillary thyroid carcinoma.
a Rate suppressed; fewer than 16 cases.
Figure 1.
Trends in age-adjusted incidence rates (cases per 100 000/year) for total papillary thyroid carcinoma (PTC) and PTC subtypes, including follicular variant of PTC, classical PTC, and other PTC variants.
Trends by tumor size
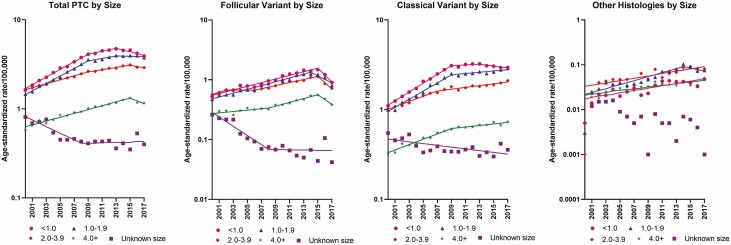
During 2015 to 2017, declines in total PTC were observed for all tumor size categories (Fig. 2A), and relative declines of a similar magnitude (~13%-23% per year) were observed across all FVPTC size categories (Fig. 2B; see Table 1). Continuous increases across the full study period were observed for classical PTCs of all tumor size categories apart from the smallest (< 1 cm), which increased less steeply after 2009 and declined after 2013 (Fig. 2C; see Table 1); these patterns did not change after excluding invasive EFVPTC/encapsulated papillary carcinoma NOS (Supplemental Fig. 1 [14]). Incidence of all other PTC variants increased across the study period for all tumor size categories apart from those 1 to 1.9 cm (Fig. 2D). During 2015 to 2017, the absolute decline in small (< 1 cm) PTCs (0.66/100 000; 586 cases) accounted for 56% of the absolute decline in total PTC (1.17/100 000; 941 cases), with small classical PTCs accounting for 8% of the decline in total PTC (0.10/100 000; 65 cases), and small FVPTCs accounting for 49% (0.57/100 000; 529 cases).
Figure 2.
Trends in age-adjusted incidence rates (cases per 100 000/year) for A, total papillary thyroid carcinoma (PTC) and PTC subtypes, including B, follicular variant of PTC, C, classical PTC, and D, other PTC variants, by tumor size at diagnosis.
Impact of reclassification of encapsulated follicular variant papillary thyroid carcinoma
The absolute decline in FVPTC incidence from 2015 to 2017 was 1.59 per 100 000 (1445 cases), whereas the absolute increases in noninvasive EFVPTC/NIFTP and invasive EFVPTC/encapsulated papillary carcinoma NOS were each 0.16 per 100 000 (156 and 152 cases, respectively) during this 2-year period (Table 2). Thus, the observed increases in noninvasive and invasive EFVPTC together accounted for only 20% of the observed decline in FVPTC. Some EFVPTC cases may have been inadvertently coded as 8340/2 (noninvasive FVPTC); however, we observed only 48 such cases during the full study period and did not evaluate these further.
Table 2.
Case counts and age-adjusted incidence rates by calendar year of diagnosis for total papillary thyroid carcinoma, follicular variant papillary thyroid carcinoma, encapsulated papillary carcinoma not otherwise specified/invasive encapsulated follicular variant papillary thyroid carcinoma (EFVPTC), and noninvasive follicular thyroid neoplasm with papillary-like nuclear features/noninvasive EFVPTC in Surveillance, Epidemiology, and End Results–18 Registries, 2000 to 2017
| ICD-O-3 morphology codes | 8050/3, 8260/3, 8340/3-8344/3, 8350/3, 8450/3-8460/3 | 8340/3 | 8343/3 | 8343/2 | ||||
|---|---|---|---|---|---|---|---|---|
| Classification/term | Total PTC | FVPTCa | Encapsulated papillary carcinoma, NOS/invasive EFVPTCb | NIFTPb/noninvasive EFVPTCb | ||||
| Behavior | Invasive | Invasive | Invasive | Noninvasive | ||||
| Calendar year | Count | Ratec | Count | Ratec | Count | Ratec | Count | Ratec |
| 2000-2014 | 127 528 | 10.03 | 39 142 | 3.07 | 911 | 0.07 | NRd | NRe |
| 2015 | 12 391 | 13.32 | 4173 | 4.45 | 111 | 0.12 | NRd | NRe |
| 2016 | 12 018 | 12.80 | 3558 | 3.77 | 136 | 0.14 | NRd | NRe |
| 2017 | 11 450 | 12.15 | 2728 | 2.86 | 267 | 0.28 | 152 | 0.16 |
| Difference (2017-2015) | –941 | –1.17 | –1445 | –1.59 | 156 | +0.16 | 152 | +0.16 |
Abbreviations: EFVPTC, encapsulated follicular variant papillary thyroid carcinoma; FVPTC, follicular variant papillary thyroid carcinoma; ICD-O-3, International Classification of Diseases for Oncology, third edition; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; NOS, not otherwise specified; NR, not reportable; PTC, papillary thyroid carcinoma.
a Until January 1, 2017, included invasive and noninvasive EFVPTC.
b New code/term implemented by the North American Association of Cancer Registries as of January 1, 2017.
c Rates were age-adjusted to 2000 US standard population and calculated as the number of cases per 100 000 person-years.
d Count suppressed; fewer than 5 cases.
e Rate suppressed; fewer than 16 cases.
Discussion
Multiple factors appear to have contributed to US PTC incidence trends during 2000 to 2017. The slowing down of the increase in incidence of PTC, particularly small (< 1 cm) PTCs, began after 2009, consistent with the 2009 ATA recommendations against biopsy of subcentimeter nodules (6). The greater emphasis on risk stratification for tumor biopsy in the 2015 ATA guidelines, along with recent strong recommendations against screening, are thought to have contributed importantly to the downturn in PTC incidence since the mid-2010s (7, 8). Although we observed a declining incidence of small (< 1 cm) classical PTCs (the most common histologic subtype), the absolute decline in these tumors accounted for only 8% of the decline in total PTC incidence from 2015 to 2017. In contrast, the sharp decline in FVPTCs from 2015 to 2017, with a similar magnitude of decline across tumor size categories, appeared to be primarily responsible for the overall declines in PTC. Incidence rates for other PTC histologic type and size groupings increased continuously over the full study period (2000-2017).
Our results provide a 1-year update to the study by Pereira et al, which similarly evaluated PTC incidence trends by histologic subtype and size using SEER data (3). The results showed declines in small (< 2 cm) but not larger sized FVPTCs from 2014 to 2016. The authors hypothesized that these trends were attributable to 2 factors: 1) the 2015 ATA recommendations for tumor biopsy, with higher size thresholds proposed for less suspicious nodules, including sonographic patterns associated with follicular-pattern lesions, and 2) the change in diagnostic criteria for encapsulated FVPTC, including the introduction of NIFTP (3). Our results provide additional support for an important influence of diagnostic coding changes on FVPTC trends. The introduction of the NIFTP terminology in 2016 and implementation of diagnostic coding changes the following year (9-11) would have specifically affected diagnostic coding of encapsulated FVPTC, with similar effects across tumor sizes.
To our knowledge, ours is the first study to directly evaluate trends in NIFTP incidence rates using SEER cancer registry data. From the observed data, we estimated that 20% of tumors that would have been previously classified as FVPTC were diagnosed or coded as another entity after 2015, with increases in encapsulated papillary carcinoma, a type of classical PTC, and NIFTP each accounting for 10% of the decline in FVPTC. The observed number of NIFTPs recorded in SEER-18 accounted for only 1.3% of the number of total PTCs in 2017, much lower than the estimated proportion predicted by Nikiforov et al (18.6%) based on an expert pathology review of multi-institutional patient data (10). We suspect that the introduction of the new NIFTP terminology may have had a greater impact on FVPTC incidence than could be estimated from observed changes to NIFTP incidence rates in SEER because of incomplete reporting and a broader impact of these discussions on thyroid cancer diagnostic practices. Also, as NAACCR and the World Health Organization formally implemented these new diagnostic criteria only in 2017, it is reasonable to expect a great deal of variability in the acceptance and application of these changes by pathologists at the latter end of the study period, resulting not only in substantial underascertainment but also misclassification (11, 12). Specifically, some classical PTCs may have been misdiagnosed as NIFTP, an observation of the published literature that prompted a revised, stricter definition of this entity in a 2018 report by Nikiforov et al (13). Thus, it will be important to continue monitoring diagnostic reporting of NIFTP to cancer registries in the coming years and the effect that this has on changing trends in PTC at the population level. More widespread adoption of the new NIFTP terminology by pathologists and hospital registrars and increases in molecular testing should contribute to fewer cancer registry records of FVPTC and more records of NIFTP and encapsulated papillary carcinomas over time.
Within our data, we also observed a continuous rise in incidence of larger (> 1 cm) classical PTCs (~2% per year) since 2007 to 2009 even after accounting for delays in cancer registry reporting. Trends in classical PTC were virtually unchanged after excluding cases classified as invasive EFVPTCs/encapsulated papillary carcinoma NOS (8343/3) (Supplemental Fig. 1 [14]), suggesting that the increases in classical PTC incidence were negligibly affected by diagnostic coding changes related to the introduction of NIFTP. Thus, in the absence of any obvious clinical practice or diagnostic changes that could explain these trends, these results seem to add to the growing evidence in support of a true increase in the occurrence of clinically aggressive PTC in the United States (1). Consistent with this hypothesis, the prevalence of BRAFv600E tumor mutations among classical PTC cases has been shown to increase over time, whereas RET/PTC chromosomal aberrations, more commonly observed among radiation-exposed populations, appear to have declined (15). BRAFv600E is the primary genetic alteration associated with classical PTC (10) and is associated with increased risk of recurrence and disease-specific mortality (16, 17). Obesity, one of few known modifiable risk factors (18), has been estimated to account for 15% of the increase in PTC (19), and studies have shown stronger associations between obesity and risk of BRAFv600E mutation–positive vs –negative PTCs (20-22). Excess body weight, central adiposity, and weight gain have been associated more strongly with thyroid cancer death than thyroid cancer incidence (23). Whether obesity may serve as a prognostic indicator for patients with thyroid nodules is currently unclear, but identifying predictors of malignancy and aggressive disease prior to fine-needle aspiration biopsy and thyroidectomy remains an important research priority (24). Additional research is also needed to identify novel risk factors and better understand the putative role of other exposures, including endocrine-disrupting chemicals, iodine supplementation, and declines in cigarette smoking on PTC incidence trends (1).
Invasive EFVPTC (encapsulated papillary carcinoma) and noninvasive EFVPTC (NIFTP), on the other hand, are genetically distinct from infiltrative FVPTC and classical PTC and share more similarities with follicular adenomas and carcinomas (with high prevalence of RAS and absence of BRAF) (10, 25). The declassification of noninvasive EFVPTC from a malignant to in situ neoplasm (NIFTP) should greatly reduce the prevalence of RAS among total thyroid cancer and PTC cases in the United States. Overall, we expect that the various forces that have been concurrently influencing PTC incidence rates will have had a substantial effect on the molecular profile of PTCs in the United States over the last decade and will continue to do so in the coming years.
Major strengths of this study include the large number of patients studied, the 18 years of registry-based data, and the high quality of the cancer registries included. The study’s main limitations include the lack of information on patient exposures, which kept us from directly assessing the impact of environmental, medical, and behavioral/lifestyle factors on PTC incidence trends, and the availability of only 2 years of incidence data for NIFTP.
In summary, our results suggest that the reasons underlying recent US thyroid cancer (specifically PTC) incidence trends have been multifactorial. Diagnostic coding changes, including the declassification of NIFTP from a malignant to in situ neoplasm, appears to have been a factor contributing to the recent decline in total PTC from 2015 to 2017 following decades of rapid increase. Changes in risk criteria for biopsy related to sonographic features and tumor size, continued strong recommendations against thyroid cancer screening, introduction of molecular testing, clinical recommendations supporting lobectomy and active surveillance as viable treatment options for low-risk disease, and general awareness of the harms of thyroid cancer overdiagnosis and overtreatment have likely also contributed to reductions in incidence rates for total PTC, particularly small tumors. Although these factors would be expected to affect the incidence of all histologic subtypes, they are most clearly observed by the decelerating and declining incidence of small (< 1 cm), classical PTC, which, unlike FVPTC, should be minimally influenced by nomenclature changes related to NIFTP. However, we also observed continuous increases in the incidence of larger classical PTCs and other PTC variants over time, suggesting there may also have been a concurrent, true increase in disease due to changes in environmental or lifestyle risk factor prevalence. Therefore, it becomes increasingly important to identify modifiable risk factors that could be targeted in primary prevention efforts. Future descriptive studies of thyroid cancer incidence will need to consider the impact of these concurrent factors by investigating trends by histologic type along with other diagnostic tumor features, as we have demonstrated in the present study.
Glossary
Abbreviations
- APC
annual percentage change
- ATA
American Thyroid Association
- EFVPTC
encapsulated follicular variant papillary thyroid carcinoma
- FVPTC
follicular variant of papillary thyroid carcinoma
- ICD-O-3
International Classification of Diseases for Oncology third edition
- NAACCR
North American Association of Central Cancer Registries
- NIFTP
noninvasive encapsulated follicular variant of papillary thyroid carcinoma
- NOS
not otherwise specified
- PTC
papillary thyroid carcinoma
- SEER
Surveillance Epidemiology and End Results
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Additional Information
Disclosure Summary: J.A.S. is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly. She receives institutional research funding from Exelixis and Eli Lilly. The other authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee M, Powers AE, Morris LGT, Marti JL. Reversal in thyroid cancer incidence trends in the United States, 2000-2017. Thyroid. 2020;30(8):1226-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid. 2020;30(8):1132-1140. [DOI] [PubMed] [Google Scholar]
- 4. Ho AS, Luu M, Barrios L, et al. Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. 2020;6(5):706-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 6. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for thyroid cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(18):1882-1887. [DOI] [PubMed] [Google Scholar]
- 8. Powers AE, Marcadis AR, Lee M, Morris LGT, Marti JL. Changes in trends in thyroid cancer incidence in the United States, 1992 to 2016. JAMA. 2019;322(24):2440-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481-483. [DOI] [PubMed] [Google Scholar]
- 10. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. North American Association of Central Cancer Registries (NAACCR). What you need to know for 2017. https://www.naaccr.org/wp-content/uploads/2017/01/What-You-Need-to-Know-for-2017.pdf. Accessed May 15, 2020.
- 12. Lloyd RF, Osamura RY, Klöppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon, France: IARC; 2017. [Google Scholar]
- 13. Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018;4(8):1125-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitahara CM, Sosa JA, Shiels MS. Influence of nomenclature changes on trends in papillary thyroid cancer incidence in the United States, 2000-2017: Supplemental Figure. OSF. https://osf.io/h35wm/. Deposited August 3, 2020. [DOI] [PMC free article] [PubMed]
- 15. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276-E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enumah S, Fingeret A, Parangi S, Dias-Santagata D, Sadow PM, Lubitz CC. BRAFV600E mutation is associated with an increased risk of papillary thyroid cancer recurrence. World J Surg. 2020;44(8):2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitahara CM, Pfeiffer RM, Sosa JA, Shiels MS. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995-2015). J Natl Cancer Inst. 2020;112(8):810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahman ST, Pandeya N, Neale RE, et al. Obesity is associated with BRAFV600E-mutated thyroid cancer. [Published online ahead of print May 4, 2020.] Thyroid. Doi: 10.1089/thy.2019.0654 [Google Scholar]
- 21. Lee J, Lee CR, Ku CR, et al. Association between obesity and BRAFV600E mutation status in patients with papillary thyroid cancer. Ann Surg Oncol. 2015;22(6 Suppl 3):S683-S690. [DOI] [PubMed] [Google Scholar]
- 22. Shi RL, Qu N, Liao T, et al. Relationship of body mass index with BRAF (V600E) mutation in papillary thyroid cancer. Tumour Biol. 2016;37(6):8383-8390. [DOI] [PubMed] [Google Scholar]
- 23. Kitahara CM, McCullough ML, Franceschi S, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26(2):306-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cappola AR. How to look for thyroid cancer. JAMA. 2017;317(18):1840-1841. [DOI] [PubMed] [Google Scholar]
- 25. Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”