Abstract
Objective
The aim of this study was to explain “obesity paradox” in chronic obstructive pulmonary disease (COPD) by evaluating the effect of body mass index (BMI) on lung function in Chinese patients with COPD.
Methods
A total of 1644 patients diagnosed with COPD were recruited from four Chinese tertiary hospitals and were divided into four groups including underweight, normal weight, overweight and obese according to BMI classification standard. The medical data of these patients were collected and used for the multiple linear regression analyses.
Results
After adjustment for age, sex, educational level, economic status, smoking status, alcohol consumption, duration of COPD history, events of acute exacerbation in previous year, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease and osteoporosis, BMI had a curvilinear correlation with the forced expiratory volume in the first second (FEV1) in patients with Global Initiative for Obstructive Lung Disease (GOLD) 1–2 grade (first-order coefficient β, 0.09; 95% CI, 0.03–0.16; second-order coefficient β, −0.002; 95% CI, −0.003–-0.001; P<0.01). However, BMI had a positive correlation with FEV1 in patients with GOLD 3–4 grade (β, 0.01; 95% CI, 0.008–0.017; P<0.01) when BMI was used as a quantitative variable. When BMI was used as a qualitative variable, only FEV1 in overweight group with GOLD 1–2 grade was significantly higher than that of normal weight group (P<0.01). Interestingly, both overweight and obese groups had higher FEV1 in GOLD 3–4 grade compared with normal weight group (β, 0.06; 95% CI, 0.02–0.11; β, 0.11; 95% CI, 0.04–0.18; P<0.01). The effect of BMI on predicted percentage of FEV1 (FEV1%) was similar to that of FEV1 in different GOLD grades.
Conclusion
Obesity only had a protective effect on lung function in COPD patients with GOLD 3–4 grade rather than GOLD 1–2 grade.
Trial Registry
ClinicalTrials.gov, No.: NCT 03182309, URL: www.clinicaltrials.gov.
Keywords: overweight, obesity
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by restricted airflow, is a common disease in the older population. The prevalence of COPD was 13.7% for people over 40 years of age in China.1 Obesity characterized by excessive accumulation of body fat can be evaluated by body mass index (BMI). Obesity is a common comorbidity of COPD, with estimates ranging from 6% to 54%.2–6 However, the prevalence of obesity in Chinese patients with COPD remains unclear.
The severity of COPD depends on degree of airflow limitation reflected by impaired lung function of patients. Body weight has an independent effect on lung function apart from tobacco exposure.7 Many studies indicated that obesity was associated with decreased lung function in the general population because of its effect on gas exchange, respiratory mechanics, muscular endurance and breath control.8,9 In addition, obesity could also contribute to decreased lung function through chronic low-grade inflammation.10 But less attention has been given to the association between obesity and lung function in patients with different diseases. Obesity is more likely associated with metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease, obstructive sleep apnea and cancer, which all result in an increased risk of death.11,12 Allison et al’s study from a multicenter prospective cohort suggested that obesity in patients with COPD might contribute to worse COPD-related health consequences such as acute exacerbation of COPD and dyspnea scores.13 Therefore, it is believed that obesity in patients with COPD should be treated with weight loss through exercise, which is conducive to improved lung function and reduced complications.14 However, there have been studies that suggested that “overweight” and obesity could prolong survival in patients with chronic heart or kidney diseases.15,16 This phenomenon is referred to as the “obesity paradox”.15 The obesity paradox also existed in COPD patients because the mortality and exacerbation frequency of obese patients with COPD was lower than that of normal weight patients.17 It seems that obesity has a protective effect on the prognosis of COPD. Because of the obesity paradox, whether to treat obesity in COPD remains controversial for clinicians. The weight loss may improve cardiovascular outcomes but at the same time, the deteriorating respiratory outcomes will increase mortality of patients.18 Because the reference standards for obesity are different, it is necessary to explore the relationship between different BMIs and lung function of COPD patients in a Chinese population.
In this study, we tried to explain the obesity paradox by evaluating the effect of BMI on lung function of different Global Initiative for Obstructive Lung Disease (GOLD) grades in Chinese patients with COPD by multiple linear regression analyses.
Methods
Ethics Approval and Data Sharing Statement
This study was part of a national key research and development program about COPD, which was a national clinical registration study initiated in January 2016 (Clinical Trials ID: NCT 03182309). The procedure of this study was reviewed and approved by the Ethics Committee of Renmin Hospital of Wuhan University (No.2017K-C014). This study was performed in accordance with the Declaration of Helsinki. Every patient included in this study was informed about the purpose of the study and written consent was signed. Regarding the data sharing, because this project will last for 5 years and multiple research centers participated, currently this project is not fully completed, our present study is only one part of this project, which focused on studying the effect of BMI on lung function in COPD patients based on current clinical data, so these data cannot be shared until the project is fully completed.
Subjects
All participants were enrolled in the primary cohort from four Chinese tertiary hospitals. All the participants were more than 40 years of age and were not pregnant, and had been diagnosed with COPD using a spirometer. The participants who had a history of bronchial asthma, bronchiectasis, pulmonary fibrosis, tuberculosis, cancer, renal dysfunction, neuro-muscular disease, or mental illness were excluded. The data of the patients who refused to complete lung function test, or if their results were considered as unqualified by the operators because the patients could not fully cooperate with lung function test, were considered as incomplete data and were excluded (n=11). Finally, 1644 participants met the inclusion criteria for the study and were classified into four groups including underweight (BMI<18.5 kg/m2), normal weight (BMI:18.5–23.9 kg/m2), overweight (BMI: 24–27.9 kg/m2) and obese (BMI≥28 kg/m2) according to BMI classification standard for Chinese population.19
Data Collection
A post-bronchodilator FEV1/forced vital capacity (FVC) ratio of <0.7 was diagnosed as COPD according to the GOLD guidelines.20 Lung function parameters, including the FVC, the FEV1, the predicted percentage of FEV1 (FEV1%) and the FEV1/FVC%, were measured using a spirometer (Jaeger MasterScreen Body, Germany) after using bronchodilator.21 The other clinical data of participants including the general information, modified Medical Research Council (mMRC) dyspnea scale comorbidities such as hypertension, diabetes mellitus, cardiovascular disease and cerebrovascular disease, COPD history, events of COPD exacerbation in the previous year were collected using the same standardized protocol from the multicenter study. Data could only be downloaded online by a third party, a special statistical expert who did not participate in the clinical study.
Statistical Analysis
Differences between multiple groups were compared using the Chi-squared test for categorical variables, the one-way ANOVA for normal quantitative variables and the Wilcoxon rank sum test for non-normal quantitative variables. The multiple linear regression models were used to determine the associations between different BMIs and lung function. Age, sex, educational level, economic status, smoking status, alcohol consumption, duration of COPD history, events of acute exacerbation in previous year, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease and osteoporosis were considered as covariates. Due to the curvilinear relation between the BMI and FEV1 during the quantitative data exploration, a second-order term of BMI was added in the model when BMI was used as a quantitative variable in the formal analysis. If the coefficient of the second-order term was not significant, then only the regular first-order term of BMI was retained. Collinearity of independent variables was also assessed and was negative. All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC) and a P<0.05 is considered statistically significant.
Results
Demographic Characteristics
The baseline characteristics of patients in this study are shown in Table 1. Among the 1644 COPD patients included in the study, a total of 198 patients were underweight (12.0%), 757 patients were normal weight (46.0%), 460 patients were overweight (28.0%) and 229 patients were obese (14.0%). There were no significant differences in gender or economic status in these four groups (P>0.05).
Table 1.
Baseline Characteristics of COPD Patients with Different BMIs (n=1644)
| Characteristics | Underweight (n=198) | Normal Weight (n=757) | Overweight (n=460) | Obese (n=229) | P value |
|---|---|---|---|---|---|
| Sex, male, n (%) | 174 (87.9) | 653 (86.3) | 378 (82.2) | 179 (78.2) | 0.13 |
| Age, yr | 67.0±9.2 | 66.4±8.8 | 64.9±8.7 | 62.6±10.2 | 0.01 |
| Neck Circumference, cm | 35.1±2.7 | 37.4±2.6 | 39.5±3.0 | 42.3±4.3 | <0.01 |
| Degree of education, n (%) | 0.03 | ||||
| Under college | 182 (92.0) | 662 (87.5) | 385 (83.7) | 198 (86.5) | |
| Above college | 16 (8.0) | 95 (12.5) | 75 (16.3) | 31 (13.5) | |
| Economic status, n (%) | 0.51 | ||||
| Higher income | 0 (0.0) | 12 (1.6) | 8 (1.7) | 2 (0.9) | |
| Middle income | 19 (9.6) | 74 (9.8) | 48 (10.4) | 18 (7.9) | |
| Lower income | 179 (90.4) | 671 (88.6) | 404 (87.9) | 209 (91.2) | |
| Smoking status, n (%) | <0.01 | ||||
| Never | 35 (17.7) | 134 (17.7) | 105 (22.8) | 51 (22.3) | |
| Former | 50 (25.3) | 188 (24.8) | 130 (28.3) | 75 (32.7) | |
| Current | 113 (57.0) | 435 (57.5) | 225 (48.9) | 103 (45.0) | |
| Alcohol consumption, n (%) | 0.05 | ||||
| Never | 129 (65.2) | 461 (60.9) | 262 (57.0) | 136 (59.4) | |
| Former | 20 (10.1) | 48 (6.3) | 49 (10.6) | 21 (9.2) | |
| Current | 49 (24.7) | 248 (32.8) | 149 (32.4) | 72 (31.4) | |
| FVC, L | 2.35±0.81 | 2.60±0.88 | 2.88±0.97 | 2.80±1.02 | <0.01 |
| FEV1, L | 1.05±0.57 | 1.29±0.62 | 1.56±0.72 | 1.67±0.74 | <0.01 |
| FEV1% Predicted | 41.65±18.71 | 50.75±22.10 | 59.22±23.23 | 62.05±22.20 | <0.01 |
| FEV1/FVC% Predicted | 44.95±12.67 | 49.14±12.73 | 55.75±12.16 | 58.88±9.93 | <0.01 |
| Severity of COPD, n (%) | <0.01 | ||||
| GOLD 1 | 12 (6.1) | 96 (12.7) | 105 (22.8) | 53 (23.1) | |
| GOLD 2 | 38 (19.2) | 241 (31.8) | 172 (37.5) | 103 (45.0) | |
| GOLD 3 | 95 (48.0) | 286 (37.8) | 140 (30.4) | 60 (26.2) | |
| GOLD 4 | 53 (26.7) | 134 (17.7) | 43 (9.3) | 13 (5.7) | |
| mMRC dyspnea scale | 2.02±0.96 | 1.79±0.96 | 1.58±0.97 | 1.55±1.00 | <0.01 |
| Hypertension, n (%) | 43 (21.7) | 257 (33.9) | 218 (47.4) | 147 (64.2) | <0.01 |
| Diabetes mellitus, n (%) | 7 (3.5) | 60 (7.9) | 69 (15.0) | 51 (22.3) | <0.01 |
| Cardiovascular disease, n (%) | 34 (17.2) | 163 (21.5) | 131 (28.5) | 1 (39.7) | <0.01 |
| Cerebrovascular disease, n (%) | 10 (5.1) | 73 (9.6) | 50 (10.9) | 24 (10.5) | 0.12 |
| Osteoporosis, n (%) | 3 (1.5) | 24 (3.2) | 14 (3.0) | 4 (1.7) | 0.45 |
| COPD history, month, median (Q1,Q3) | 60 (24, 120) | 54 (14, 120) | 36 (2, 84) | 12 (0, 60) | <0.01 |
| `Events in previous year | |||||
| Acute exacerbation, n (%) | 142 (71.7) | 441 (58.3) | 250 (54.3) | 101 (44.1) | <0.01 |
| NIV, n (%) | 11 (5.6) | 51 (6.7) | 24 (5.2) | 23 (10.0) | 0.10 |
| Blood cell analysis | |||||
| WBC, ×109/L | 7.35±3.20 | 7.52±2.95 | 7.48±2.48 | 7.39±2.38 | 0.86 |
| RBC, ×1012/L | 4.30±0.56 | 4.50±0.61 | 4.56±0.54 | 4.73±0.59 | <0.01 |
| HB, g | 129.2±16.16 | 136.7±17.5 | 140.2±16.17 | 143.0±17.4 | <0.01 |
| PLT, ×109/L | 218.02±83.16 | 221.50±77.22 | 211.85±69.49 | 216.54±53.92 | 0.07 |
Notes: Continuous variables which are obviously skewed were presented as median (Q1, Q3), other continuous variables were described as means ±SD. Categorical variables were expressed as percentages. P value for the difference between the groups.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; GOLD 1, FEV1 ≥80% predicted; GOLD 2, FEV1=50–79% predicted; GOLD 3, FEV1=30–49% predicted; GOLD 4, FEV1 <30% predicted; mMRC, modified Medical Research Council; NIV, non-invasive ventilation; WBC, white blood cells; RBC, red blood cells; HB, hemoglobin; PLT, platelets.
Clinical Characteristics
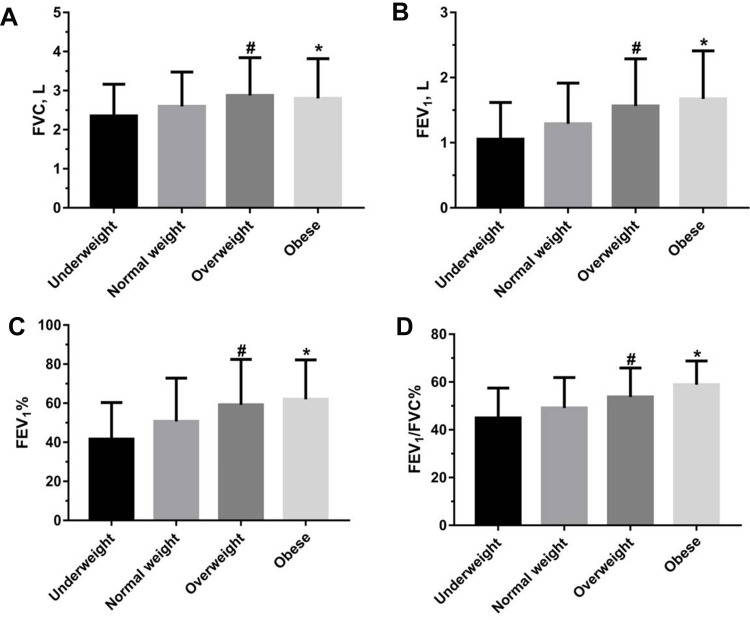
There were significant differences in age, neck circumference, degree of education, smoking status, acute exacerbation of COPD in previous year, mMRC dyspnea scale, complication (hypertension, diabetes mellitus and cardiovascular disease), duration of COPD history, red blood cell count and hemoglobin in the four groups (P<0.05) (Table 1). The FVC, FEV1, FEV1% and FEV1/FVC% of the patients in overweight and obese COPD groups were significantly higher than that of the patients in underweight and normal weight COPD groups (P<0.01) (Figure 1A–D). However, there were no statistical differences in FVC, FEV1, FEV1% of the patients in overweight and obese COPD groups (P>0.05).
Figure 1.
The differences in FVC, FEV1, FEV1% and FEV1/FVC% of the four groups of patients with COPD (A–D). Statistical analysis was performed using one-way ANOVA test. The data were represented as mean values ±SD. #p<0.05 indicating the statistical difference between overweight group with COPD and underweight or normal weight group. *p<0.05 indicating the statistical difference between obese group with COPD and underweight or normal weight group.
Abbreviations: COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1%, predicted percentage of FEV1; FEV1/FVC%, percentage of FEV1 and FVC.
The Effect of BMI on Lung Function of Patients with COPD
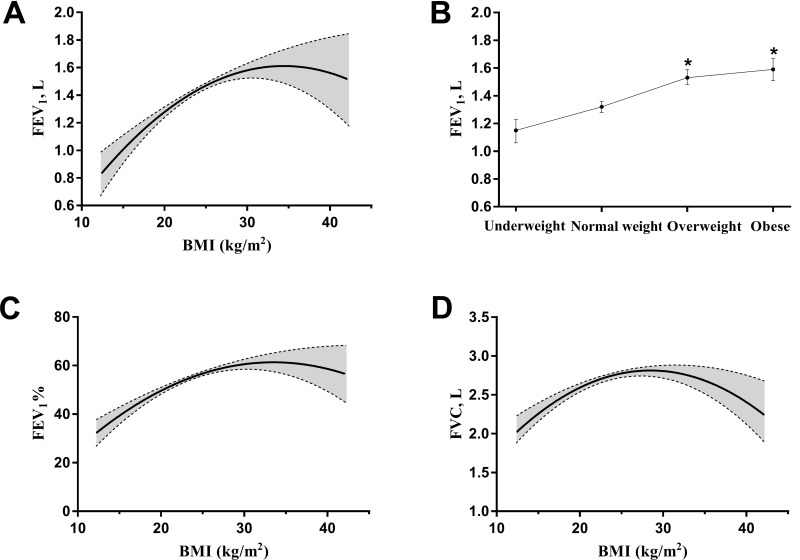
To investigate the effects of BMI on lung function in COPD patients, we analyzed the correlation between BMI and lung function parameters by multiple linear regression. As shown in Figure 2A, when BMI was used as a quantitative variable, BMI had a significantly curvilinear correlation (quadratic polynomial curve) with the FEV1 (first-order coefficient β, 0.11; 95% CI, 0.06–0.16; second-order coefficient β, −0.002; 95% CI, −0.003–-0.001; P<0.01) after adjustment for age, sex, educational level, economic status, smoking status, alcohol consumption, duration of COPD history, events of acute exacerbation in previous year, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease and osteoporosis. When BMI was used as a qualitative variable and the normal weight group was designated as the reference level, both the overweight group (β, 0.22; 95% CI, 0.15–0.28; P<0.01) and the obese group (β, 0.27; 95% CI, 0.18–0.36; P<0.01) had higher FEV1 levels than the normal weight group after adjusting for the previously mentioned covariates (Figure 2B). The effect of BMI on FEV1% or FVC was similar to that of BMI on FEV1 (Figure 2C and D).
Figure 2.
The effect of BMI on lung function in the patients with COPD. Statistical analysis was performed using multiple linear regression analysis. (A and B) BMI used as the quantitative and qualitative variable to assess the relation between BMI and FEV1. (C and D) BMI used as the quantitative variable to assess the relation between BMI and FEV1% or FVC. *p<0.05 vs normal weight group.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; FEV1%, predicted percentage of FEV1; FVC, forced vital capacity.
The Effect of BMI on Lung Function in GOLD Grades
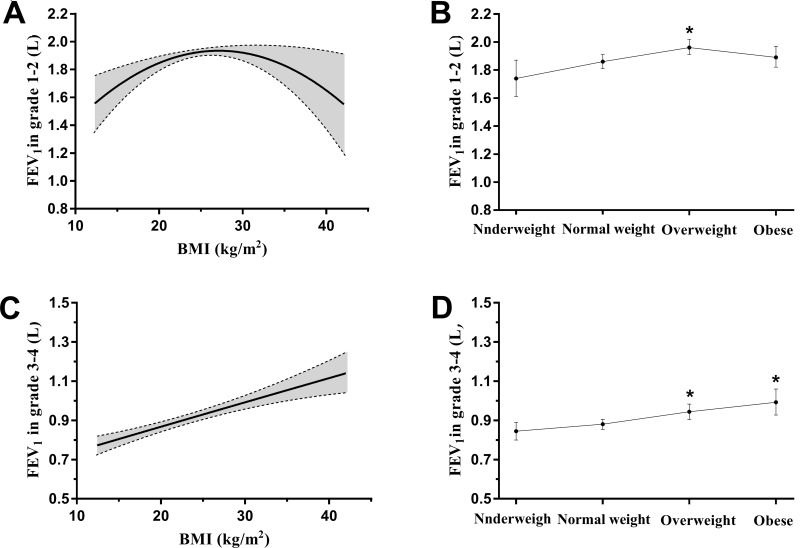
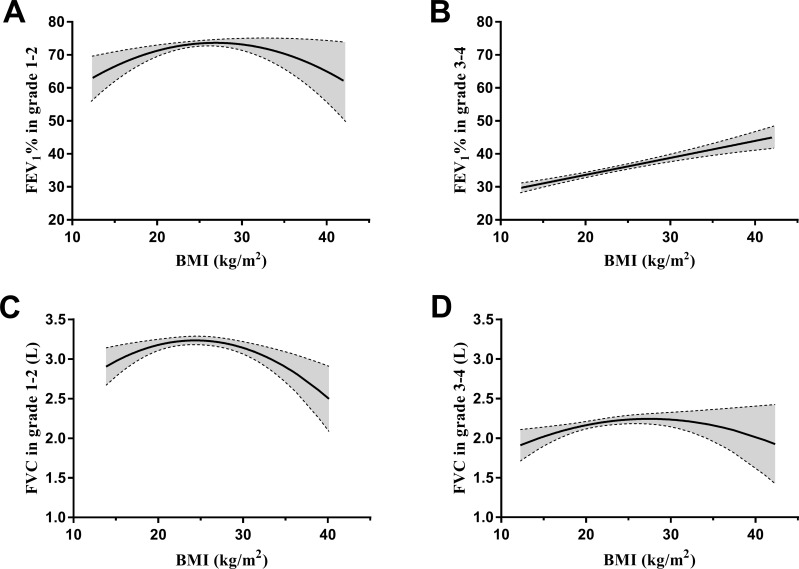
We further investigated whether lung function in GOLD 1–2 grade and GOLD 3–4 grade was affected by BMI using multiple linear regression analysis. As shown in Figure 3A, when BMI was used as a quantitative variable, BMI had a significantly curvilinear correlation with FEV1 in GOLD 1–2 grade (first-order coefficient β, 0.09; 95% CI, 0.03–0.16; second-order coefficient β, −0.002; 95% CI, −0.003–-0.001; P<0.01) after adjustment for age, sex, educational level, economic status, smoking status, alcohol consumption, duration of COPD history, events of acute exacerbation in previous year, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease and osteoporosis. However, when BMI was used as a qualitative variable, FEV1 in overweight COPD patients was significantly higher than that of normal weight patients after adjustment for previously mentioned covariates (β, 0.10; 95% CI, 0.03–0.18; P<0.01) (Figure 3B). Interestingly, among the GOLD 3–4 grade patients, BMI did not have the quadratic polynomial curvilinear relationship but had straight linear positive correlation (β, 0.01; 95% CI, 0.01–0.02; P<0.01) when BMI was used as a quantitative variable (Figure 3C). Moreover, when BMI was used as a qualitative variable, both overweight and obese had higher FEV1 in GOLD 3–4 grade compared with normal weight after adjustment for previously mentioned concomitant variable (β, 0.06; 95% CI, 0.02–0.11; β, 0.11; 95% CI, 0.04–0.18; P<0.01) (Figure 3D). BMI had similar effect on FEV1% in GOLD grades of COPD patients (Figure 4A and B). When BMI was used as a quantitative variable, BMI had a significantly curvilinear correlation with FVC both in GOLD 1–2 grade (first-order coefficient β, 0.15; 95% CI, 0.06–0.23; second-order coefficient β, −0.003; 95% CI, −0.005–-0.001; P<0.01) and GOLD 3–4 grade (first-order coefficient β, 0.08; 95% CI, 0.01–0.15; second-order coefficient β, −0.001; 95% CI, −0.003–0.000; P<0.05) after adjustment for previously mentioned variable (Figure 4C and D).
Figure 3.
The effect of BMI on FEV1 in GOLD grade of COPD patients. Statistical analysis was performed using multiple linear regression analysis. (A and B) BMI used as the quantitative and qualitative variable to assess the relation of BMI and FEV1 in GOLD 1–2 grade. (C and D) BMI used as the quantitative and qualitative variable to assess the relation between BMI and FEV1 in GOLD 3–4 grade. *p<0.05 indicating the significant difference compared to normal weight group.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; GOLD, global initiative for obstructive lung disease.
Figure 4.
The effect of BMI on FEV1% and FVC in GOLD grade of COPD patients. Statistical analysis was performed using multiple linear regression analysis. The curve and the 95% CI area were obtained from the regression equation by fixing each covariate at a certain level. (A and B) BMI used as the quantitative variable to assess the relation of BMI and FEV1% in GOLD 1–2 grade and GOLD 3–4 grade. (C and D) BMI used as the quantitative variable to assess the relation between BMI and FVC in GOLD 1–2 grade and GOLD 3–4 grade.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1%, predicted percentage of forced expiratory volume in the first second; FVC, forced vital capacity; GOLD, global initiative for obstructive lung disease.
Discussion
Obesity was not only highly prevalent but also occurred more in COPD patients than in the general population.17 Our multicenter study showed that the prevalence of obesity in COPD was 14% in China. The prevalence of obesity in COPD reported by many research groups has variability.2–6 The difference mainly resulted from the difference in definition of obesity and the relatively lower prevalence of obesity in the general population of China. In addition, we found that obesity was most prevalent in COPD patients with GOLD 2 grade (45%) and least prevalent in COPD patients with GOLD 4 grade (5.7%), which were similar to the results reported by Steuten et al.5
In clinical practice, “underweight” is one of the most important concerns in COPD care. Being underweight might increase mortality risk of COPD patients as shown in previous studies.18,22 In our present study, we found that underweight patients with COPD had lower lung function, higher dyspnea scores and acute exacerbation frequency of COPD in previous year than obese patients with COPD. This result is consistent with that of another retrospective study of 774 COPD patients in China.23 The phenomenon was explained by the fact that the underweight patients might have malnutrition, low muscularity and weak resistance against respiratory infection, all of which increased the risk of COPD.24–26 In addition, we found that underweight patients with COPD were older and their disease duration was longer, mainly because these patients had an increased frequency of exacerbation, leading to a faster decline in lung function and a poorer quality of life.27
The most significant finding in our present study was that being overweight only had a positive correlation with lung function (FEV1 and FEV1%) in COPD patients with GOLD 1–2 grade, but both being overweight and obese had a positive correlation with lung function in COPD patients with GOLD 3–4 grade. Our results indicted that obesity had a protective effect on lung function in GOLD 3–4 grade rather than GOLD 1–2 grade, which may partially explain the obesity paradox in COPD. A meta analysis including 62 studies showed that “overweight” or obesity may be detrimental to lung function in adults with or without asthma and the damage in asthma was less severe than in healthy people.28 But the studies included in the meta analysis had mainly focused on subjects with or without asthma. Moreover, COPD is characterized by irreversible airflow obstruction, which is different from asthma. Our study indicated that obesity not only had no protective effect, but that further increased BMI, such as morbid obesity, may worsen lung function in GOLD 1–2 grade, which suggested that in the early stages of COPD, nutritional status was critical to maintaining the rate of decline in lung function, while excessive weight gain may be counter-productive. In addition to BMI, lung function was also influenced by gender, age, history and acute exacerbation frequency of COPD. Studies by others also showed that “overweight” was highly prevalent and had the lowest mortality risk in patients with of COPD,18 which was consistent with our results. The patients in GOLD 3–4 grade were liable to complicate with weight loss, mainly muscle loss, which could result in respiratory hypofunction and further worsen lung function and increase mortality. Obesity was not beneficial to lung function in the general population. Interestingly, our study found that increased BMI had a protective effect on lung function in GOLD 3–4 grade. Obese patients not only have more body fat, but also increased muscle mass. Nutritional status, which is of significant value for prognosis of COPD, played an important role in lung function in later stage COPD patients.18 Sami et al’s cross-sectional study that included 72 male patients with COPD also showed that increased BMI had a positive relation with FEV1 in GOLD 4 grade.29 Landbo et al suggested that the relative risk for mortality seemed decreased in overweight and obese patients with COPD GOLD 3–4 grade, while it was increased in those with GOLD 1–2 grade.18 In addition, we found that the effect of BMI on FVC in patients with COPD had a curvilinear correlation in GOLD 1–2 grade and GOLD 3–4 grade. Our results suggested that increasing BMI to a certain threshold might impair FVC in COPD patients, which might be related to airflow limitation.
Obesity tends to cause many comorbidities such as cardiovascular and metabolic diseases.11 The comorbidities are also relatively common in COPD.30 In our present study we found that in COPD patients who were obese, rates of hypertension, diabetes mellitus and cardiovascular disease were significantly higher than in those with COPD with normal weight, which was in line with previous studies.30,31 The mechanism may be related to increasing BMI, aging, smoking and inflammatory status in obesity. The comorbidities may explain the reason why obese patients with COPD have higher hospitalization rates.32 Therefore early detection and management of co-existing diseases may benefit quality of life and reduce mortality.
Although our study was not a prospective study, we found a low incidence of acute exacerbations in obese COPD patients in previous year, suggesting that obesity held some protective significance to COPD. In a recent study reported by Sun et al, they found that high BMI had a protective effect for rate of FEV1 decline.33 The possible mechanism was that obese patients with COPD might receive medical attention earlier than underweight patients, which was beneficial to maintain the progressive decline of lung function. In terms of physical mechanisms, obese COPD patients may benefit from obesity by reducing lung hyperinflation which may have a beneficial effect on lung function.34 However, in another meta analysis, the results indicated that the survival advantage seems to diminish, when the BMI is above 32 kg/m2.17 Therefore, cut off point of BMI for advantages in different GOLD grades needs to be further elucidated, because of the intricate relation between BMI and lung function in patients with COPD.
We acknowledge that there are some limitations in this study. First, in this study, we used only a single measure method of BMI to assess obesity reflecting the nutritional status of patients, while the distribution of body composition (fat distribution or muscle content) was ignored. For example, fat free mass index which was associated with COPD prognosis might better capture body composition, but the operation is more complicated than BMI.35 Second, because our study was cross-sectional and had a relatively small sample size in subgroups, it was impossible to determine a causal relationship between BMI and lung function in patients with COPD. Finally, the data obtained from multiple centers may have heterogeneity, but it was minimized using standard instruments, trained researchers, and a centralized electronic data acquisition and management system.
Conclusion
Our results in this study indicated that obesity only had a protective effect on lung function in COPD patients with GOLD 3–4 grade rather than GOLD 1–2 grade, which may be used to guide refined management of BMI in COPD patients with different GOLD stages. In future, the pathophysiological mechanism of causal relationship between BMI and lung function of COPD patients needs to be further studied.
Funding Statement
This work was supported by the National Key Research and Development Program of China (project number: 2016YFC1304403).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 2.Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montes de Oca M, Talamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650. doi: 10.1016/j.rmed.2007.12.025 [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140(2):461–468. doi: 10.1378/chest.10-2582 [DOI] [PubMed] [Google Scholar]
- 5.Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91. doi: 10.1016/j.pcrj.2005.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi: 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 7.Sato M, Shibata Y, Abe S, et al. Retrospective analysis of the relationship between decline in FEV(1) and abdominal circumference in male smokers: the Takahata study. Int J Med Sci. 2013;10(1):1–7. doi: 10.7150/ijms.5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrielsen AM, Lund MB, Kongerud J, Viken KE, Roislien J, Hjelmesaeth J. The relationship between anthropometric measures, blood gases, and lung function in morbidly obese white subjects. Obes Surg. 2011;21(4):485–491. doi: 10.1007/s11695-010-0306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63(7):649–654. doi: 10.1136/thx.2007.086801 [DOI] [PubMed] [Google Scholar]
- 10.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. doi: 10.1080/17476348.2018.1506331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643 [DOI] [PubMed] [Google Scholar]
- 13.Lambert AA, Putcha N, Drummond MB, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77. doi: 10.1016/j.chest.2016.08.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James BD, Jones AV, Trethewey RE, Evans RA. Obesity and metabolic syndrome in COPD: is exercise the answer? Chron Respir Dis. 2018;15(2):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891–894. doi: 10.1016/S0002-9149(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. doi: 10.1093/ajcn/81.3.543 [DOI] [PubMed] [Google Scholar]
- 17.McDonald VM, Wood LG, Holland AE, Gibson PG. Obesity in COPD: to treat or not to treat? Expert Rev Respir Med. 2017;11(2):81–83. doi: 10.1080/17476348.2017.1267570 [DOI] [PubMed] [Google Scholar]
- 18.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115 [DOI] [PubMed] [Google Scholar]
- 19.Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 20.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 22.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017 [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10(8):5086–5099. doi: 10.21037/jtd.2018.08.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaheen S, Barker DJ. Early lung growth and chronic airflow obstruction. Thorax. 1994;49(6):533–536. doi: 10.1136/thx.49.6.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park CH, Yi Y, Do JG, Lee YT, Yoon KJ. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore). 2018;97(37):e12281. doi: 10.1097/MD.0000000000012281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect. 2018;24(1):24–28. doi: 10.1016/j.cmi.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 27.Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–1960. doi: 10.1016/j.rmed.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 28.Forno E, Han YY, Mullen J, Celedon JC. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J Allergy Clin Immunol Pract. 2018;6(2):570–581 e510. doi: 10.1016/j.jaip.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sami R, Sadegh R, Esmailzadehha N, Mortazian S, Nazem M, Zohal M. Association of anthropometric indexes with disease severity in male patients with chronic obstructive pulmonary disease in Qazvin, Iran. Am J Mens Health. 2018;12(4):1023–1028. doi: 10.1177/1557988318760053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zewari S, Hadi L, van den Elshout F, Dekhuijzen R, Heijdra Y, Vos P. Obesity in COPD: comorbidities with practical consequences? COPD. 2018;15(5):464–471. doi: 10.1080/15412555.2018.1509951 [DOI] [PubMed] [Google Scholar]
- 31.Divo MJ, Cabrera C, Casanova C, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005 [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. doi: 10.1186/s12931-019-1209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–971. [DOI] [PubMed] [Google Scholar]
- 35.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. [DOI] [PubMed] [Google Scholar]