Abstract
The purpose of this prospective study was to analyze the relationship between ventricular morphology and parameters of cardiac function in two different athletic groups and controls, using feature tracking cardiac magnetic resonance (FT-CMR). Twenty-three professional soccer players (22 ± 4 years), 19 competitive triathletes (28 ± 6 years) and 16 controls (26 ± 3 years) were included in the study. CMR was performed using a 1.5 T scanner. Cardiac chamber volumes, mass and biventricular global myocardial strain were obtained and compared. In comparison to the control subjects, athletes were characterized by a higher cardiac volume (p < 0.0001), higher cardiac mass (p < 0.001), reduced longitudinal strain of the left and right ventricle (p < 0.05 and p < 0.01 respectively) and reduced left ventricular radial strain (p < 0.05). Soccer players revealed higher amounts of left ventricular mass (87 ± 15 vs. 75 ± 13 g/m2, p < 0.05) than triathletes. Moreover, they showed a greater decrease in left and right ventricular longitudinal strain (p < 0.05 and p < 0.05) as well as in radial left ventricular strain (p < 0.05) in comparison to triathletes. An increase in left ventricular mass correlated significantly with a decrease in longitudinal (r = 0.47, p < 0.001) and radial (r = − 0.28, p < 0.05) strain. In athletes, attenuation of strain values is associated with cardiac hypertrophy and differ between soccer players and triathletes. Further studies are needed to investigate whether it is an adaptive or maladaptive change of the heart induced by intense athletic training.
Keywords: Athlete´s heart, Cardiac hypertrophy, Magnetic resonance imaging, CMR feature tracking, Myocardial strain
Introduction
Morphological changes of the heart associated with exercise are well studied by both echocardiography and cardiac magnetic resonance (CMR) imaging [1–5]. However, changes in myocardial mechanics of athlete’s heart are less understood. While some echocardiographic studies reported no change in left ventricular (LV) strain in endurance athletes, others observed a reduction of apical radial strain [6–8]. Myocardial strain describes deformation (relative change of the fiber length) of the myocardium during the cardiac cycle and is a marker of systolic function [9, 10]. Feature tracking CMR (FT-CMR) is an imaging technique that allows quantification of myocardial strain as well as strain rates using short and long-axis cine images [9, 11]. The level of cardiac hypertrophy and dilatation of the heart chambers may vary depending on the type and extent of the sport activity [1–5]. To understand the changes of myocardial strain in correlation with the degree of exercise-induced myocardial hypertrophy is important. Excessive myocardial hypertrophy due to a high level of athletic activity was previously linked to focal myocardial fibrosis and higher blood pressure response during exercise. However, left ventricular ejection fraction (LVEF) of athletes with focal myocardial fibrosis was preserved [12]. Myocardial strain is an early marker of systolic dysfunction, which precedes a decline in LVEF [9] and might therefore be an important parameter when evaluating athlete´s heart. We hypothesized that myocardial deformation might be influenced by the different types of sports activity. The purpose of this prospective study was to assess ventricular morphology and parameters of systolic function between two athletic groups, professional soccer players and amateur triathletes using FT-CMR.
Materials and methods
Subjects
The ethics committee of the general medical council approved the study. The subjects were recruited between 2014 and 2017 and gave their written informed consent. Twenty-three male professional soccer players (22 ± 4 years, range 18–32 years) were recruited during their competitive season in the center for athletic medicine. Nineteen male amateur triathletes (28 ± 6 years, range 18–36 years) were contacted through advertisement at local triathlon clubs and were included if they had participated in at least 10 h of weekly training and regular participation in triathlon competitions in the past 3 years. Sixteen male control subjects (26 ± 3 years, range 20–31 years) with < 3 h exercise per week were recruited through local advertisement. Study exclusion criteria were contraindications for CMR or any disease. All subjects were instructed to arrive rested with no exercise in the preceding 72 h. Food and caffeine intake were restricted in the preceding 3 h.
CMR protocol
Non-contrast CMR was performed using a 1.5T Achieva scanner equipped with a 5-channel cardiac phased array receiver coil (Philips Healthcare, Best, The Netherlands). The protocol included ECG-triggered steady-state free-precession cine CMR in short axis and 2-, 3- and 4-chamber views with the following imaging parameters: acquired voxel size (AVS) 1.98 × 1.80 × 6 mm3, reconstructed voxel size (RVS) 1.36 × 1.36 × 6 mm3, gap 4 mm, 9–10 slices for full LV coverage, echo time = 1.67 ms, repetition time = 3.34 ms, flip angle = 60°, parallel acquisition technique = SENSE.
CMR data analysis
Two investigators independently and blindly analyzed each CMR using cvi42 software (Circle Cardiovascular Imaging Inc, Calgary, Alberta, Canada). CMR parameters were indexed to body surface area (BSA) and are given as the mean of two observers. Evaluation of ventricular volumes and LV mass was performed in standard fashion on short axis cine images [13]. Left (LA) and right atrial (RA) volumes and ejection fraction (EF) were quantified using the biplane area-length method, excluding pulmonary veins and atrial appendage [14]. The interventricular septum (IVS) thickness and lateral wall thickness of LV in end-diastole were measured on a basal short axis slice immediately basal to the tips of the papillary muscles in end-diastole [13]. Relative wall mass ratio was calculated as a ratio of LV mass index/LV end-diastolic volume index (LVEDVi) [15]. Septal/lateral wall thickness ratio was calculated as a ratio of wall thickness of septal and lateral segments [15]. LV cavity diameter was measured in 3-chamber view at the mitral chordae level basal to the tips of papillary muscles [13]. Peak systolic LV and right ventricular (RV) strain as well as diastolic strain rate were analyzed with cine CMR images using Segment feature tracking software version 2.1.R.6108 (Medviso, Lund, Sweden). This software analyzes myocardial strain and diastolic strain rate by computing interframe deformation fields using an endocardial tracking strategy based on non-rigid image registration [9, 16]. LV global peak systolic radial, longitudinal and circumferential strain were measured on three long and three short axis cine slices by manual delineation of the endo- and epicardial contours in end-diastole. RV global peak systolic longitudinal and circumferential strain and diastolic strain rate were measured on a single 4-chamber and three short axis cine slices by manual delineation of the endocardial contours in end-diastole. Contours were then automatically propagated by the software throughout the cardiac cycle generating myocardial strain [9]. Diastolic function was assessed with a dedicated software (CMRtools®, Cambs, UK) [17]. Briefly, LV volumetry was performed using manual delineation of the LV endocardial borders in end-diastolic, end-systolic and mid-diastolic short axis views, trabeculae and papillary muscles were excluded from the LV cavity and time-volume-curves from all time frames of the cardiac cycle were calculated [17, 18]. The differentiated time-volume-curve is characterized by two diastolic peaks including the early peak-filling rate (EPFR) and atrial peak-filling rate (APFR), which represent the maximum speed of passive LV filling and the maximum speed of LV filling secondary to atrial contraction. The peak filling rate ratio (PFRR) describes the contribution of each in diastolic LV filling and is calculated as EPFR/APFR [17, 19].
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA, USA) and MedCalc for Windows, version 13.3.3.0 (MedCalc Software, Ostend, Belgium). Continuous data are presented as mean ± standard deviation (SD) and categorical data are presented as absolute numbers and percentages. Continuous data were compared using the two-sided Student's t-test and categorical variables were compared using the χ2 test or Fischer’s exact test as appropriate. Statistical significance was defined as p < 0.05.
Results
Demographics of athletes and controls
There were no significant differences in age, weight, height and in BSA between athletes and controls (Table 1). However, athletes had a lower body mass index (BMI) compared to controls (p < 0.05). The soccer players were younger than the triathletes (p < 0.05), but weight, height, BMI and BSA did not differ (Table 2).
Table 1.
Demographics and CMR parameters of athletes (soccer players and triathletes) and sedentary controls
| Athletes (n = 42) | Controls (n = 16) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 25 ± 5 | 26 ± 3 | 0.291 |
| Weight (kg) | 76 ± 9 | 80 ± 14 | 0.173 |
| Height (m) | 1.83 ± 0.06 | 1.82 ± 0.08 | 0.437 |
| BMI (kg/m2) | 23 ± 2 | 24 ± 3 | < 0.05 |
| BSA (m2) | 1.97 ± 0.13 | 2.00 ± 0.21 | 0.495 |
| CMR left heart | |||
| LV ejection fraction (%) | 58 ± 3 | 59 ± 3 | 0.239 |
| IVS thickness in ED (mm) | 11.1 ± 1.5 | 9.4 ± 1.0 | < 0.001 |
| LV lateral wall thickness in ED (mm) | 10.2 ± 1.6 | 8.7 ± 1.0 | < 0.01 |
| Septal/lateral wall thickness ratio | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.643 |
| LV diameter in ED (mm) | 5.6 ± 0.3 | 5.4 ± 0.4 | < 0.05 |
| LVMi (g/m2) | 82 ± 15 | 67 ± 7 | < 0.001 |
| LVEDVi (ml/m2) | 105 ± 11 | 91 ± 10 | < 0.0001 |
| LVESVi (ml/m2) | 44 ± 5 | 37 ± 6 | < 0.0001 |
| LVSVi (ml/m2) | 61 ± 8 | 54 ± 6 | < 0.01 |
| Relative wall mass ratio (LVMi/LVEDVi) | 0.78 ± 0.11 | 0.74 ± 0.08 | 0.195 |
| LAEDVi (ml/m2) | 19 ± 6 | 14 ± 5 | < 0.01 |
| CMR right heart | |||
| RV ejection fraction (%) | 57 ± 5 | 59 ± 5 | 0.503 |
| RVEDVi (ml/m2) | 103 ± 13 | 92 ± 12 | < 0.01 |
| RVESVi (ml/m2) | 43 ± 8 | 38 ± 8 | < 0.05 |
| RVSVi (ml/m2) | 60 ± 9 | 54 ± 7 | < 0.05 |
| RAEDVi (ml/m2) | 26 ± 8 | 19 ± 6 | < 0.001 |
| Strain parameters | |||
| LV radial (%) | 40 ± 8 | 45 ± 9 | < 0.05 |
| LV longitudinal (%) | − 16 ± 2 | − 18 ± 1 | < 0.05 |
| LV circumferential (%) | − 15 ± 3 | − 16 ± 3 | 0.141 |
| RV longitudinal (%) | − 19 ± 3 | − 22 ± 2 | < 0.01 |
| RV circumferential (%) | − 9 ± 3 | − 9 ± 4 | 0.899 |
Values are mean ± SD for continuous data. Bold characters indicate statistically significant values (p < 0.05)
BMI body mass index, BSA body surface area, ED end-diastole, IVS interventricular septum, LAEDVi left atrial end-diastolic volume index, LVEDVi left ventricular end-diastolic volume index, LV left ventricular, LVESVi left ventricular end-systolic volume index, LVMi left ventricular mass index, LVSVi left ventricular stroke volume index, RAEDVi right atrial end-diastolic volume index, RV right ventricular, RVEF right ventricular ejection fraction, RVEDVi right ventricular end-diastolic volume index, RVESVi right ventricular end-systolic volume index, RVSVi right ventricular stroke volume index
Table 2.
Demographics and CMR parameters of professional soccer players and competitive triathletes
| Soccer players (n = 23) | Triathletes (n = 19) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 22 ± 4 | 28 ± 6 | < 0.001 |
| Weight (kg) | 77 ± 9 | 74 ± 8 | 0.290 |
| Height (m) | 1.83 ± 0.07 | 1.83 ± 0.06 | 0.855 |
| BMI (kg/m2) | 23 ± 2 | 22 ± 2 | 0.116 |
| BSA (m2) | 1.99 ± 0.14 | 1.96 ± 0.13 | 0.491 |
| CMR—left heart | |||
| LV ejection fraction (%) | 58 ± 4 | 59 ± 3 | 0.328 |
| Heart rate (beats/min) | 53 ± 9 | 56 ± 10 | 0.391 |
| IVS thickness in ED (mm) | 11.5 ± 1.7 | 10.5 ± 1.1 | < 0.05 |
| LV lateral wall thickness in ED (mm) | 10.9 ± 1.5 | 9.3 ± 1.4 | < 0.01 |
| Septal/lateral wall thickness ratio | 1.07 ± 0.2 | 1.13 ± 0.1 | 0.183 |
| LV diameter in ED (mm) | 5.6 ± 0.3 | 5.6 ± 0.3 | 0.890 |
| LVMi (g/m2) | 87 ± 15 | 75 ± 13 | < 0.05 |
| LVEDVi (ml/m2) | 107 ± 10 | 102 ± 12 | 0.168 |
| LVESVi (ml/m2) | 45 ± 5 | 42 ± 5 | 0.071 |
| LVSVi (ml/m2) | 62 ± 8 | 60 ± 8 | 0.467 |
| Relative wall mass ratio (LVMi/LVEDVi) | 0.82 ± 0.12 | 0.74 ± 0.10 | < 0.05 |
| LAEDVi (ml/m2) | 20 ± 6 | 17 ± 5 | 0.272 |
| CMR—right heart | |||
| RV ejection fraction (%) | 57 ± 5 | 59 ± 5 | 0.222 |
| RVEDVi (ml/m2) | 104 ± 13 | 102 ± 14 | 0.510 |
| RVESVi (ml/m2) | 45 ± 8 | 42 ± 9 | 0.242 |
| RVSVi (ml/m22) | 60 ± 9 | 60 ± 9 | 0.991 |
| RAEDVi (ml/m2) | 28 ± 8 | 23 ± 7 | < 0.05 |
| Strain parameters | |||
| LV radial (%) | 38 ± 8 | 43 ± 7 | < 0.05 |
| LV longitudinal (%) | − 16 ± 2 | − 17 ± 2 | < 0.05 |
| LV circumferential (%) | − 15 ± 3 | − 15 ± 2 | 0.634 |
| RV longitudinal (%) | − 18 ± 2 | − 20 ± 3 | < 0.05 |
| RV circumferential (%) | − 9 ± 3 | − 8 ± 2 | 0.101 |
Values are mean ± SD for continuous data. Bold characters indicate statistically significant values (p < 0.05)
BMI body mass index, BSA body surface area, ED end-diastole, IVS interventricular septum, LAEDVi left atrial end-diastolic volume index, LVEDVi left ventricular end-diastolic volume index, LV left ventricular, LVESVi left ventricular end-systolic volume index, LVMi left ventricular mass index, LVSVi left ventricular stroke volume index, RAEDVi right atrial end-diastolic volume index, RV right ventricular, RVEF right ventricular ejection fraction, RVEDVi right ventricular end-diastolic volume index, RVESVi right ventricular end-systolic volume index, RVSVi right ventricular stroke volume index
Cardiac function, volumes and mass assessed by CMR
While IVS thickness in athletes was increased compared to controls (11.1 ± 1.5 mm vs. 9.4 ± 1 mm, p < 0.001) septal/lateral wall thickness ratio did not differ between groups (p = 0.643 Table 1). The LV mass index was markedly higher in athletes with 82 ± 15 g/m2 than in controls with 67 ± 7 g/m2 (p < 0.001). The indexed end-diastolic LV volume (LVEDVi) was significantly higher in athletes than in controls (105 ± 11 vs. 91 ± 10 ml/m2, p < 0.0001), which was also true for the indexed end-diastolic RV volume (103 ± 13 vs. 92 ± 12 ml/m2, p < 0.01, Table 1). The relative wall mass ratio did not differ between the athletes and controls (p = 0.195, Table 1).
Soccer players had a higher LV mass index with 87 ± 15 g/m2 than triathletes with 75 ± 13 g/m2 (p < 0.05, Table 2). Also, they had a higher relative wall mass ratio compared to triathletes (0.82 ± 0.12, 0.74 ± 0.10, p < 0.05). Furthermore, IVS thickness of soccer players was higher compared to triathletes (p = 11.5 ± 1.7 vs. 10.5 ± 1.1 mm, p < 0.05, Table 2). Septal/lateral wall thickness ratio did not differ between the groups (p = 0.183, Table 2). Indexed end-diastolic LV and RV volumes did not differ between the athletic groups (p = 0.168 and p = 0.510, Table 2).
Strain and strain rate analysis by feature tracking CMR
LV global radial and longitudinal strain were lower in athletes with 40 ± 8% and − 16 ± 2%, compared to the sedentary controls with 45 ± 9% (p < 0.05) and − 18 ± 1% (p < 0.05) respectively (Table 1). Similarly, RV global longitudinal strain was lower in athletes with − 19 ± 3% compared to the controls with − 22 ± 2% (p < 0.01). Global circumferential LV (p = 0.141) and RV (p = 0.899) strain values were similar between groups (Table 1). LV global radial and longitudinal strain were lower in soccer players with 38 ± 8% and − 16 ± 2% compared to triathletes with 43 ± 7% (p < 0.05) and − 17 ± 2% (p < 0.05), respectively. Soccer players had a lower RV global longitudinal strain with − 18 ± 2% than triathletes with − 20 ± 3% (p < 0.05, Table 2). Global circumferential LV (p = 0.634) and RV (p = 0.101) strain values were similar between groups (Table 2).
LV (59 ± 15 vs. 71 ± 17%/s, p < 0.01) and RV (74 ± 25 vs. 100 ± 25%/s, p < 0.01) diastolic longitudinal strain rates were lower in athletes compared to controls. LV and RV diastolic circumferential strain rates showed a tendency for lower values in athletes compared to controls (Table 3). LV diastolic radial strain rate (p = 0.221) did not differ. LV diastolic longitudinal strain rate (54 ± 11 vs. 64 ± 18%/s, p < 0.05) was lower in soccer players compared to triathletes. Concordantly, RV diastolic longitudinal strain rate (p = 0.065) showed a tendency for lower values in soccer players, but other diastolic strain rates values did not differ (Table 4).
Table 3.
CMR-Derived diastolic function in athletes (soccer players and triathletes) and sedentary controls
| Diastolic function | Athletes (n = 42) | Controls (n = 16) | p value |
|---|---|---|---|
| EPFRi (ml/s/m2) | 242 ± 71 | 234 ± 68 | 0.759 |
| APFRi (ml/s/m2) | 101 ± 44 | 165 ± 72 | 0.0001 |
| PFRR (EPFRi/APFRi) | 2.6 ± 1.2 | 1.6 ± 0.6 | < 0.01 |
| LV diastolic radial strain rate (%/s) | − 214 ± 62 | − 237 ± 64 | 0.221 |
| LV diastolic longitudinal strain rate (%/s) | 59 ± 15 | 71 ± 17 | < 0.01 |
| LV diastolic circumferential strain rate (%/s) | 67 ± 16 | 76 ± 22 | 0.088 |
| RV diastolic longitudinal strain rate (%/s) | 74 ± 25 | 100 ± 25 | < 0.01 |
| RV diastolic circumferential strain rate (%/s) | 41 ± 12 | 49 ± 22 | 0.073 |
Values are mean ± SD for continuous data. Bold characters indicate statistically significant values (p < 0.05)
LV left ventricular, RV right ventricular, EPFRi early peak filling rate index, APFRi atrial peak filling rate index, PFRR peak filling rate ratio
Table 4.
CMR-derived diastolic function in soccer players and triathletes
| Diastolic function | Soccer players (n = 23) | Triathletes (n = 19) | p value |
|---|---|---|---|
| EPFRi (ml/s/m2) | 242 ± 71 | 237 ± 83 | 0.835 |
| APFRi (ml/s/m2) | 101 ± 44 | 105 ± 41 | 0.756 |
| PFRR (EPFRi/APFRi) | 2.8 ± 1.3 | 2.5 ± 1.1 | 0.504 |
| LV diastolic radial strain rate (%/s) | − 206 ± 60 | − 224 ± 65 | 0.364 |
| LV diastolic longitudinal strain rate (%/s) | 54 ± 11 | 64 ± 18 | < 0.05 |
| LV diastolic circumferential strain rate (%/s) | 70 ± 15 | 63 ± 17 | 0.158 |
| RV diastolic longitudinal strain rate (%/s) | 67 ± 22 | 82 ± 27 | 0.065 |
| RV diastolic circumferential strain rate (%/s) | 39 ± 11 | 43 ± 13 | 0.338 |
Values are mean ± SD for continuous data. Bold characters indicate statistically significant values (p < 0.05)
LV left ventricular, RV right ventricular, EPFRi early peak filling rate index, APFRi atrial peak filling rate index, PFRR peak filling rate ratio
LV diastolic function assessed by CMR
Passive LV filling was similar between athletes and controls with comparable EPFRi values (p = 0.759, Table 3). However, APFRi was lower with 101 ± 44 ml/s/m2 in athletes than in sedentary controls 165 ± 72 ml/s/m2 (p = 0.0001) leading to higher PFRR in athletes compared to the controls (2.6 ± 1.2 vs. 1.6 ± 0.6, p < 0.01). No differences in EPFRi (p = 0.835), APFRi (p = 0.756) and PFRR (p = 0.504) were found between soccer players and triathletes as shown in Table 4.
Correlation of LV morphology and systolic function with strain parameters
An increase in LV mass index (Fig. 1) correlated with a decrease in radial (r = − 0.28, p < 0.05, Fig. 2a) and longitudinal strain (r = 0.47, p < 0.001, Fig. 2b), but not with circumferential strain (r = 0.10, p = 0.448; Fig. 2c). An increase of LVEDVi was associated with a decrease in radial (r = − 0.26, p < 0.05; Fig. 3a) and longitudinal strain (r = 0.36, p < 0.01; Fig. 3b), but not with circumferential strain (r = 0.25, p = 0.062; Fig. 3c). LVEF was associated with radial (r = 0.26, p < 0.05) and circumferential strain (r = − 0.39, p < 0.01), but not with longitudinal strain (r = − 0.15, p = 0.272; Fig. 4a–c).
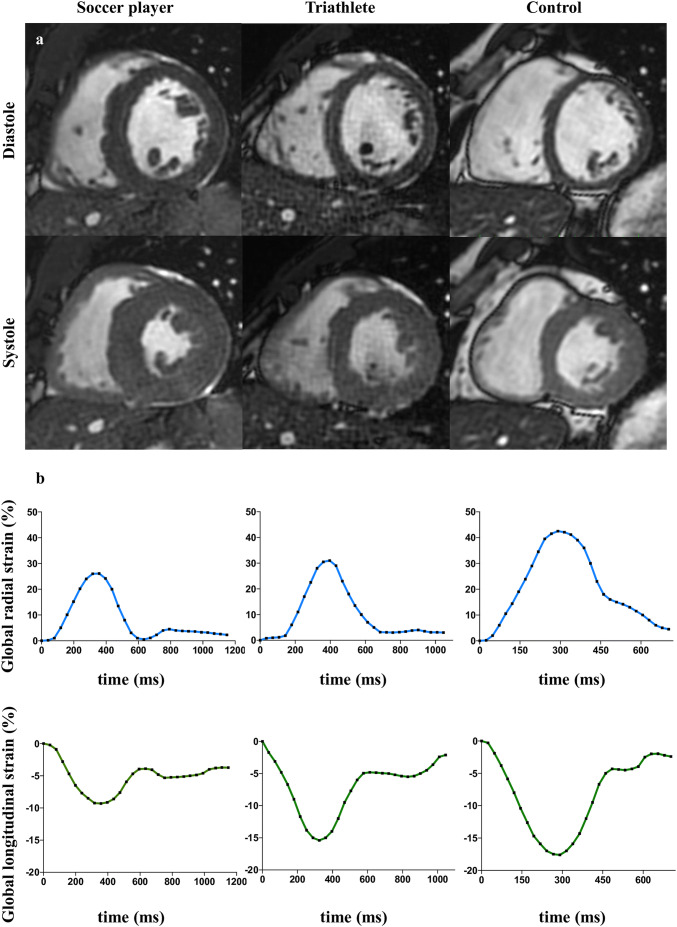
Fig. 1.
Left ventricular mass and myocardial strain. Representative short axis cine CMR images at papillary muscle level in systole and diastole (a). In comparison to controls, both athlete groups were characterized by higher myocardial mass. This was pronounced in professional soccer players. Increase in left ventricular mass correlated with a decrease in radial and longitudinal strain (b)
Fig. 2.
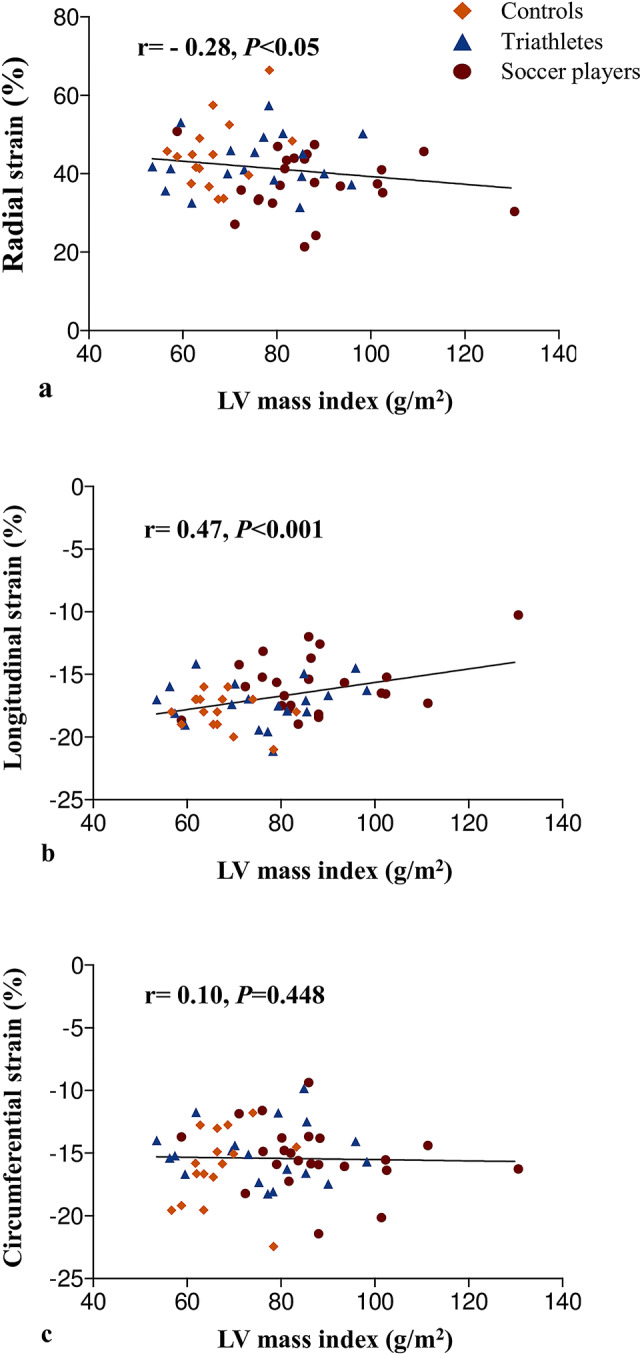
Correlations between left ventricular mass index and strain parameters. Increase in left ventricular mass index was associated with decrease in radial (a) and longitudinal strain (b), but not circumferential (c) strain
Fig. 3.
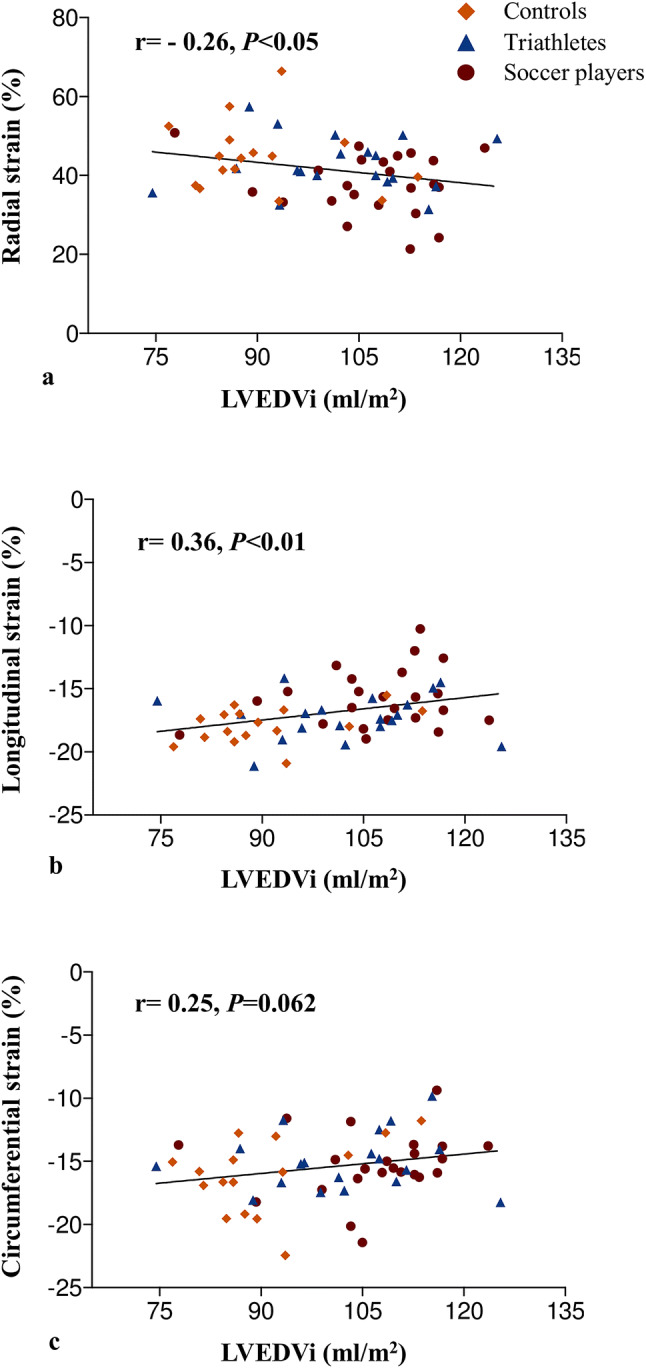
Correlations between indexed end-diastolic left ventricular volume and strain parameters. An increase in left ventricular volume was associated with a decrease in radial (a), longitudinal (b), but not significantly with circumferential (c) strain
Fig. 4.
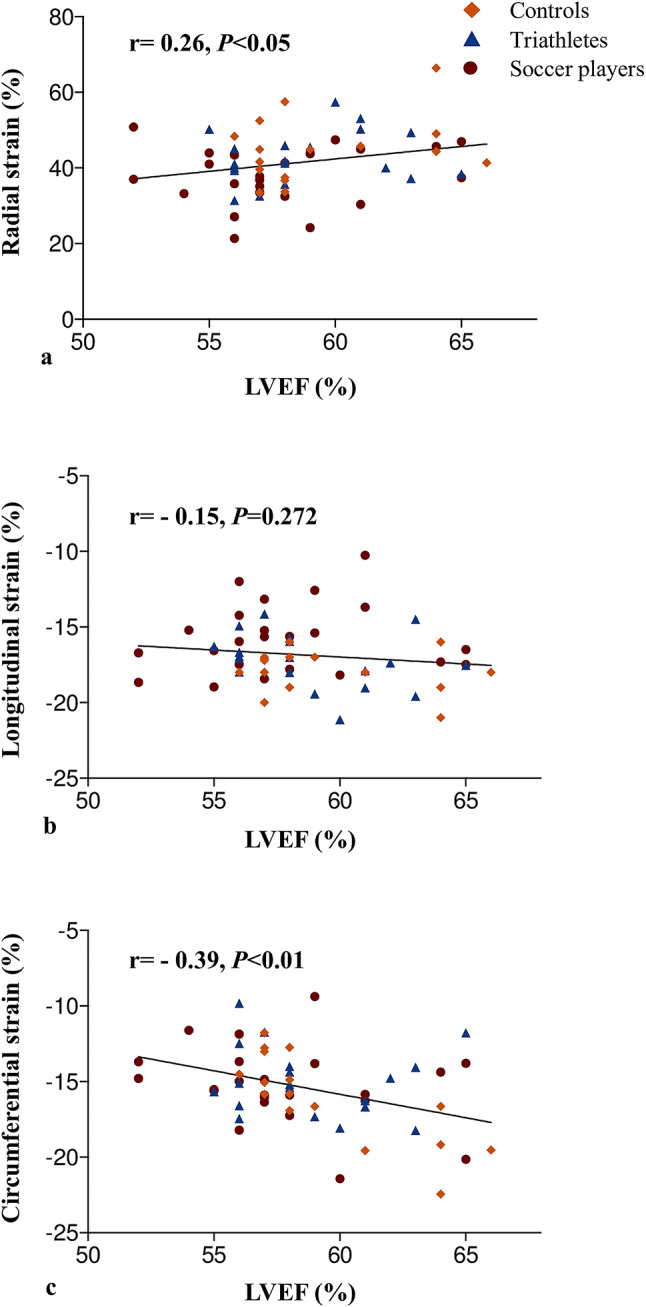
Correlations between left ventricular ejection fraction and strain parameters. An increase in left ventricular ejection fraction was associated with an increase in radial (a) and circumferential (c) strain, but not longitudinal (b) strain
Discussion
This study analyzed parameters of systolic and diastolic function in correlation with exercise-induced changes in cardiac morphology, between professional soccer players and triathletes in comparison to sedentary controls using CMR. The major findings were: (1) athletes had significantly reduced global radial and longitudinal strain compared to controls, while global circumferential strain did not differ, (2) professional soccer players had significantly higher LV mass and reduced biventricular global myocardial strain values in comparison to triathletes, (3) an increase in LV mass correlated with a decrease in radial and longitudinal strain.
In line with findings of previous studies, we observed larger ventricular dimensions in professional soccer players and competitive triathletes than in sedentary controls [4, 20]. Both athlete groups were characterized by eccentric hypertrophy [15], which was pronounced in soccer players. Scharf et al. reported on professional soccer players and triathletes in two separate studies using CMR and observed larger ventricular cavities, an increase of the myocardial mass and an unchanged LVEF in comparison to controls [2, 3]. Interestingly, a direct comparison of BSA-matched soccer players and triathletes in our study showed a significantly higher LV mass in the former, while parameters of ventricular dimension did not differ. Furthermore, soccer players were characterized by higher relative wall mass ratio and greater LV wall thickness in end-diastole. One explanation for this observation might be the different type of exercise. While triathlon primarily consists of high dynamic training, soccer requires high dynamic, but also strength training [21, 22]. Thus, one can assume that the strength component of training might be a contributor to the greater LV hypertrophy in soccer players. So far characterization of myocardial strain in athletes was primarily done by echocardiography. No differences in LV strain were observed between power athletes and top-level rowers compared to controls [7, 23]. However, cyclists and professional soccer players have been characterized by decreased LV apical radial and longitudinal strain [6, 24]. Swoboda et al. reported lower LV circumferential strain in endurance athletes as assessed with CMR tissue tagging, whereas peak LV longitudinal strain by FT-CMR was similar to controls [25]. Why different athletic activities have varying contribution to attenuation of myocardial strain remains elusive. Our findings support the concept that LV hypertrophy and ventricular enlargement intrinsic to athletes including top-level soccer players are the main contributors to the decrease in biventricular strain parameters [22]. Accordingly, we found a significant negative correlation of radial and longitudinal LV strain with LV mass and end-diastolic volume, but no correlations with circumferential strain. Longitudinal strain is believed to primarily represent the contraction of the subendocardial fibers, whereas contraction of the subepicardial fibers contributes to circumferential shortening, and both aspects contribute to radial thickening [26]. Subclinical myocardial dysfunction caused by hypertension is reported to cause pathology at the subendocardial level affecting longitudinal strain first and inducing a compensatory increase of circumferential strain through hypertrophy of the subepicardial fibers [27]. Soccer players in our study showed lower longitudinal and radial strain values, preserved circumferential strain and higher level of LV hypertrophy in comparison to both triathletes and controls. Thus, it can be postulated that chronic pressure and volume overload, intrinsic to extreme athletic activity, might lead to a gradual change of the subendocardial and subepicardial myocardial fibers characterized by attenuation of longitudinal and radial strain and sustained circumferential strain to guarantee preserved LVEF. This notion could be supported by the positive correlation of LV circumferential strain with LVEF in our study, which is also in line with previous studies [28]. Hinojar et al. studied 74 HCM patients by FT-CMR. All patients showed attenuation of all strain values of the LV. Furthermore, this study demonstrated that the degree of LV hypertrophy and the amount of LV fibrosis were independent predictors of the impairment of LV mechanics, especially LV mass seemed to be the most important factor affecting myocardial strain [29]. Presence of myocardial fibrosis in athletes has been reported to occur in up to 50% of asymptomatic athletes [12, 30]. Therefore, these changes might also represent true impairment in LV strain. However, attenuation of strain values as an adaptive change is possible. Lower EF of athletes at rest as a result of a higher chamber volumes is overall accepted as an adaptive effect demonstrating increased effectiveness of athletes heart [31]. Similarly, less myocardial deformation might be needed to eject a similar stroke volume. In an echocardiographic study, high level athletes with higher chamber- and stroke volumes showed lower longitudinal strain values in comparison to low level athletes [32]. In our study athletes had similar chamber volumes, while differing in myocardial mass and strain. This suggests that myocardial mass rather than ventricular volume determines strain reduction. Notably, in pathological hypertrophy (HCM or Hypertensive heart disease) reduced strain is associated with a normal or increased EF [23, 29]. In fact, patients with hypertension were reported to have lower global longitudinal strain values in comparison to athletes [7]. In our population, the changes in strain were moderate and differentiating athletes from non-athletes might deem difficult. To fully understand the impact of exercise induced cardiac hypertrophy associated with strain attenuation in athletes, further studies are needed.
Analysis of diastolic parameters showed significant increase of PFRR in athletes compared to controls. This was driven primarily by lower APFR, which is in line with previously described decrease in A-wave peak velocity in echocardiographic studies [33–35]. EPFR did not differ between groups, confirming echocardiographic data on constant E-wave peak velocities [33–35]. In an echocardiographic study by Santoro et al. a significantly higher E/A ratio in endurance athletes was observed, caused by a decrease of the late (atrial) peak diastolic filling velocity [33]. It seems that endurance training leads to increased relaxation and elasticity of the LV at the end of diastole with secondary effects on peak atrial diastolic filling [36]. While increased PFRR suggests improved diastolic function, diastolic strain rates were decreased in our athletes.
Some limitations need to be addressed. Currently, no longitudinal data is available to study the prognostic implications of the observed decreased LV longitudinal and radial strain parameters in subjects involved in professional or competitive sports. We cannot exclude presence of the myocardial fibrosis in our study cohort and its potential influence on LV strain parameters. Our cohort included male subjects only. Therefore, our findings can only be extended to healthy male athletes [37]. The numeric strain values might vary using a different software [38]. Further, evaluation of diastolic function in echocardiography comprises of many parameters and using CMR only evaluation of EPFR, APFR, and PFRR is feasible. Additional echocardiographic parameters such as E/e′, measurement of tricuspid regurgitation velocity, e′ velocity and pulmonary vein doppler velocity is missing and would be required to fully understand changes of diastolic function in CMR [39]. Furthermore, LA volume as an important parameter of diastolic function is only of limited use since all athletes have enlarged heart chambers. This study is also limited by the small number of subjects.
In conclusion, our study revealed that attenuation of longitudinal and radial strain values is associated with the level of sport induced myocardial hypertrophy in athletes and differ between soccer players and triathletes. Further studies are needed to investigate the impact of increased hypertrophy in athletes on cardiac function.
Acknowledgements
Open Access funding provided by Projekt DEAL. We thank Benedikt Scherz for his assistance during the data acquisition.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Compliance with ethical standards
Conflict of interest
All authors and co-authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee of the general medical council for the city of Hamburg (Ärztekammer Hamburg; PV4764) approved the study.
Informed consent
All subjects gave their written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pelliccia A, Maron BJ, Spataro A, et al. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324(5):295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 2.Scharf M, Brem MH, Wilhelm M, et al. Atrial and ventricular functional and structural adaptations of the heart in elite triathletes assessed with cardiac MR imaging. Radiology. 2010;257:71–79. doi: 10.1148/radiol.10092377. [DOI] [PubMed] [Google Scholar]
- 3.Scharf M, Brem MH, Wilhelm M, et al. Cardiac magnetic resonance assessment of left and right ventricular morphologic and functional adaptations in professional soccer players. Am Heart J. 2010;159:911–918. doi: 10.1016/j.ahj.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Utomi V, Oxborough D, Whyte GP, et al. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart. 2013;99:1727–1733. doi: 10.1136/heartjnl-2012-303465. [DOI] [PubMed] [Google Scholar]
- 5.D’Ascenzi F, Anselmi F, Piu P, et al. Cardiac magnetic resonance normal reference values of biventricular size and function in male athlete’s heart. JACC Cardiovasc Imaging. 2019;12:1755–1765. doi: 10.1016/j.jcmg.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Nottin S, Doucende G, Schuster-Beck I, et al. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete’s heart: left ventricular regional strains in athletes. J Physiol. 2008;586:4721–4733. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galderisi M, Lomoriello VS, Santoro A, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23:1190–1198. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea A, Cocchia R, Riegler L, et al. Left ventricular myocardial velocities and deformation indexes in top-level athletes. J Am Soc Echocardiogr. 2010;23:1281–1288. doi: 10.1016/j.echo.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Morais P, Marchi A, Bogaert JA, et al. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson. 2017;19:24. doi: 10.1186/s12968-017-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RJ, Moody WE, Umar F, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871–881. doi: 10.1093/ehjci/jev006. [DOI] [PubMed] [Google Scholar]
- 11.Moody WE, Taylor RJ, Edwards NC, et al. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis: CMR-feature tracking versus tagging. J Magn Reson Imaging. 2015;41:1000–1012. doi: 10.1002/jmri.24623. [DOI] [PubMed] [Google Scholar]
- 12.Tahir E, Starekova J, Muellerleile K, et al. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC Cardiovasc Imaging. 2018;11:1260–1270. doi: 10.1016/j.jcmg.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le T-T, Tan RS, De Deyn M, et al. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson. 2016;18:21. doi: 10.1186/s12968-016-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulton N, Rajiah P. Utility of magnetic resonance imaging in the evaluation of left ventricular thickening. Insights Imaging. 2017;8:279–293. doi: 10.1007/s13244-017-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyde B, Bouchez S, Thieren S, et al. Elastic image registration to quantify 3-D regional myocardial deformation from volumetric ultrasound: experimental validation in an animal model. Ultrasound Med Biol. 2013;39:1688–1697. doi: 10.1016/j.ultrasmedbio.2013.02.463. [DOI] [PubMed] [Google Scholar]
- 17.Schoennagel BP, Fischer R, Grosse R, et al. Peak filling rates assessed by CMR imaging indicate diastolic dysfunction from myocardial iron toxicity. JACC Cardiovasc Imaging. 2016;9:1353–1354. doi: 10.1016/j.jcmg.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Maceira A, Prasad S, Khan M, Pennell D. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 19.Aquaro GD, Pizzino F, Terrizzi A, et al. Diastolic dysfunction evaluated by cardiac magnetic resonance: the value of the combined assessment of atrial and ventricular function. Eur Radiol. 2019;29:1555–1564. doi: 10.1007/s00330-018-5571-3. [DOI] [PubMed] [Google Scholar]
- 20.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart: a meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.CIR.101.3.336. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JH, Haskell W, Snell P, Van Camp SP. Task force 8: classification of sports. J Am Coll Cardiol. 2005;45:1364–1367. doi: 10.1016/j.jacc.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Helgerud J, Rodas G, Kemi OJ, Hoff J. Strength and endurance in elite football players. Int J Sports Med. 2011;32:677–682. doi: 10.1055/s-0031-1275742. [DOI] [PubMed] [Google Scholar]
- 23.Saghir M, Areces M, Makan M. Strain Rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete’s heart) J Am Soc Echocardiogr. 2007;20:151–157. doi: 10.1016/j.echo.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Richand V, Lafitte S, Reant P, et al. An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:128–132. doi: 10.1016/j.amjcard.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 25.Swoboda PP, Erhayiem B, McDiarmid AK, et al. Relationship between cardiac deformation parameters measured by cardiovascular magnetic resonance and aerobic fitness in endurance athletes. J Cardiovasc Magn Reson. 2016;18:48. doi: 10.1186/s12968-016-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claus P, Omar AMS, Pedrizzetti G, et al. Tissue tracking technology for assessing cardiac mechanics. JACC Cardiovasc Imaging. 2015;8:1444–1460. doi: 10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin. 2008;4:379–382. doi: 10.1016/j.hfc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J. Myocardial energetics in cardiac hypertrophy. Clin Exp Pharmacol Physiol. 2002;29:351–359. doi: 10.1046/j.1440-1681.2002.03657.x. [DOI] [PubMed] [Google Scholar]
- 29.Hinojar R, Fernández-Golfín C, González-Gómez A, et al. Prognostic implications of global myocardial mechanics in hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking. Relations to left ventricular hypertrophy and fibrosis. Int J Cardiol. 2017;249:467–472. doi: 10.1016/j.ijcard.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 30.Wilson M, O’Hanlon R, Prasad S, et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol. 2011;110:5. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisman EZ, Frank AG, Ben-Ari E, et al. Altered left ventricular volume and ejection fraction responses to supine dynamic exercise in athletes. J Am Coll Cardiol. 1990;15:582–588. doi: 10.1016/0735-1097(90)90630-8. [DOI] [PubMed] [Google Scholar]
- 32.Dores H, Mendes L, Dinis P, et al. Myocardial deformation and volume of exercise: a new overlap between pathology and athlete’s heart? Int J Cardiovasc Imaging. 2018;34:1869–1875. doi: 10.1007/s10554-018-1412-3. [DOI] [PubMed] [Google Scholar]
- 33.Santoro A, Alvino F, Antonelli G, et al. Endurance and strength athlete’s heart: analysis of myocardial deformation by speckle tracking echocardiography. J Cardiovasc Ultrasound. 2014;22:196. doi: 10.4250/jcu.2014.22.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caselli S, Di Paolo FM, Pisicchio C, et al. Patterns of left ventricular diastolic function in olympic athletes. J Am Soc Echocardiogr. 2015;28:236–244. doi: 10.1016/j.echo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Major Z, Csajági E, Kováts T, et al. Comparison of left and right ventricular adaptation in endurance-trained male athletes. Acta Physiol Hung. 2015;102(1):23–33. doi: 10.1556/APhysiol.102.2015.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Claessens PJM, Claessens CWF. Supernormal left ventricular diastolic function in triathletes. Tex Heart Inst J. 2001;28:9. [PMC free article] [PubMed] [Google Scholar]
- 37.Andre F, Steen H, Matheis P, et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barreiro-Pérez M, Curione D, Symons R, et al. Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking algorithms. Eur Radiol. 2018;28:5137–5147. doi: 10.1007/s00330-018-5538-4. [DOI] [PubMed] [Google Scholar]
- 39.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]