Abstract
Background
Inflammatory autoimmune diseases are chronic diseases that often affect women of childbearing age. Therefore, detailed knowledge of the safety profile of medications used for management of inflammatory autoimmune diseases during pregnancy is important. However, in many cases the potential harmful effects of medications (especially biologics) during pregnancy (and lactation) on mother and child have not been fully identified.
Objective
Our aim was to update the data on the occurrence of miscarriages and (major) congenital malformations when using biologics during pregnancy based on newly published articles. Additionally, we selected several different secondary outcomes that may be of interest for clinicians, especially information on adverse events in the use of a specific biologic during pregnancy.
Material and Methods
A search was conducted from 1 January 2015 until 4 July 2019 in Embase.com, Medline Ovid, Web of Science, Cochrane CENTRAL, and Google Scholar with specific search terms for each database. Selection of publications was based on title/abstract and followed by full text (double blinded, two researchers). An overview was made based on outcomes of interest. References of the included publications were reviewed to include and minimize the missing publications.
Results
A total of 143 publications were included. The total number of cases ranged from nine for canakinumab to 4276 for infliximab. The rates of miscarriages and major congenital malformations did not show relevant differences from those rates in the general population.
Conclusion
Despite limitations to our study, no major safety issues were reported and no trend could be identified in the reported malformations.
Electronic supplementary material
The online version of this article (10.1007/s40265-020-01376-y) contains supplementary material, which is available to authorized users.
Key Points
| The rates of miscarriages and major congenital malformations after exposure to biologics during pregnancy do not deviate from these rates in the general population. |
| It is likely that use of adalimumab, certolizumab pegol, and etanercept is safe during pregnancy. |
| Data on risks of using abatacept, anakinra, canakinumab, golimumab, rituximab, tocilizumab, ustekinumab, and vedolizumab are scarce. |
Introduction
Chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), inflammatory bowel disease (IBD), and psoriasis, are common in women of childbearing age [1–3].
Active disease may not only be harmful for the mother, but also carries a risk for the fetus. There is an overlap between biologics used for the management of rheumatic, gastroenteric, and dermatologic autoimmune diseases. Even though biologics have shown efficacy in keeping the disease under control, their safety profile in pregnant women is still uncertain. Based on the mode of action, biologics are classified into tumor necrosis factor (TNF)-α inhibitors and non-TNF biologics. At present, pregnancy registry data have become available for only four TNF-α inhibitors (adalimumab, certolizumab pegol, etanercept, and infliximab) [4–7]. For other biologics (non-TNF-α inhibitors) and biosimilars used during pregnancy in autoimmune diseases there is still insufficient information on the occurrence of adverse events such as congenital malformations (CMs). As a precaution, the use of such medications during pregnancy is discouraged before acquisition of reassuring results from a pregnancy registry. In addition, unintentional exposure to medication(s) may also occur in cases of unplanned pregnancies. Accordingly, knowledge on the safety of medication during pregnancy is of great importance [8–10].
In the pre-authorization period, pregnant women are excluded from randomized clinical trials (RCTs) for ethical reasons. After authorization, the main available data are collected prospectively by different registries or retrospectively from healthcare databases, and in some circumstances published as case reports. Summarizing the available data in a systematic review can be helpful to gain a better perspective on the use of different biologics during pregnancy and lactation.
The European League Against Rheumatism (EULAR) have published a systematic review on this topic (search period 2008–2015) [8]. However, it is important to provide up-to-date knowledge based on published data after this period. In this regard, a meta-analysis that included data from 24 studies was published by Tsao et al., in which the authors pooled the available evidence to assess the impact of biologic therapy during pregnancy [11]. This current systematic review aimed to update the data on the impact of maternal exposure to biological therapy by focusing specifically on miscarriages and (major) CMs. Furthermore, in this systematic review, each biologic is considered separately and the pattern of reported CMs is documented. Other clinically relevant outcomes such as vaccination response, detectable drug levels during different stages of pregnancy, infections, and stillbirths are also investigated.
Thus the aim of this systematic review was to update the data on miscarriages and (major) CMs separately for each biologic used in inflammatory autoimmune diseases, based on newly published articles since 2015. Due to overlap in indications of biologics between rheumatic diseases, IBD, and psoriasis, studies on the use of biologics in IBD and psoriasis were also included.
Materials and Methods
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [11] guideline was followed in the design of this study.
Protocol and Registration
This systematic review was registered retrospectively at PROSPERO under the code CRD42019135316. The recorded protocol was not provided prospectively in PROSPERO [12].
Information Sources and Search Strategy
A systematic search was conducted in the following databases: Embase.com, Medline Ovid, Web of Science, Cochrane CENTRAL, and Google Scholar. The search strategy was designed and conducted by an experienced librarian with input from the study’s main investigator (NG). The Medical Subject Heading (MeSH) terms used for each database are detailed in the Electronic Supplementary Material (ESM). The search only included articles published from 1 January 2015 until 4 July 2019.
Study Selection and Data Collection Process
All relevant citations were saved in a bibliographic reference manager (Endnote × 9 version, Thomson Reuters, Philadelphia, PA, USA) and duplicates were removed. Titles and abstracts were analyzed according to inclusion and exclusion criteria (see Box 1). Additional citations were identified from the reference list of all previously selected articles. The selection process was conducted by two researchers (NG and IC) with a medical background, by reviewing the titles and the abstracts, independently. Discrepancies were resolved through discussions at consensus meetings.
Data Items, Outcomes, and Prioritization
Primary outcomes were defined as:
Miscarriages Spontaneous loss of pregnancy before 24 weeks of gestation, also known as spontaneous abortion [13];
Major CMs Conditions caused by failure of a particular body site or body system to develop correctly during the antenatal period, which had medical implications, required surgical repair, or were life threatening. (The list of malformations considered as major can be found in Supplementary Table 1) [14].
Other variables for which data were sought were categorized in two groups:
- Maternal-related outcomes:
- Ectopic pregnancies Any condition characterized by implantation of the embryo outside the endometrium and endometrial cavity during pregnancy;
- Induced abortions The removal of an embryo or fetus from the uterus at a stage of pregnancy when it is deemed incapable of independent survival; at any time between conception and the 24th week of gestation (GW);
- Pregnancy-related hypertension Gestational hypertension, pre-eclampsia, and eclampsia;
- Premature rupture of membranes Spontaneous rupture of fetal membranes before the onset of labor;
- Emergent caesarian sections Unplanned caesarian section (C-section) due to maternal/fetal condition; other types/uncategorized C-sections were not considered in the results;
- Flare-up during pregnancy/post-partum Active disease during pregnancy/during 6 months after delivery;
- Detectable drug concentration in breast milk
- Child-related outcomes:
- Minor CMs Conditions caused by failure of a particular body site or body system to develop correctly during the antenatal period, which pose no significant health problem in the neonatal period and tend to have limited social or cosmetic consequences for the affected individual, were considered minor CMs;
- Live births The complete expulsion or extraction from the mother of a baby, irrespective of the duration of the pregnancy, which, after such separation, breathes or shows any other evidence of life;
- Still birth A baby born with no signs of life at or after 24 weeks of gestation;
- Neonatal death Death during the first 28 days of life;
- Low birth weight When the infant was born weighing less than 2500 g;
- Pre-term birth When the infant was born before 37 weeks of gestation;
- Small for gestation age (SGA) When birth weight was below − 2 standard deviations of the mean or below the 10th percentile according to local intrauterine growth charts;
- Adequate vaccination response Measured by laboratory findings on immune response after vaccination;
-
(i)Adverse drug reactions (ADRs) related to vaccinations;
-
(j)Allergies;
-
(k)Eczema;
-
(l)Serious/opportunistic infections Infections that required medical intervention/hospitalization;
-
(m)Anti-drug antibodies at birth in the newborn;
-
(n)Detectable drug levels
-
(i)in cord blood
-
(ii)in infant’s blood at birth
-
(iii)during first 6, 9, and 12 months of life;
-
(i)
-
(o)Abnormal development Physical/psychological developmental delay in children.
-
(i)
ther variables for which data were sought were categorized in two groups.
Data that did not meet mentioned definitions were not considered in the final results.
Risk of Bias in Individual Studies
The quality of the studies was scored by awarding points in each domain following the guidelines of the Newcastle–Ottawa Scale (NOS) for either cohort studies or case–control studies. The NOS assigns up to a maximum of nine points for the least risk of bias in three domains: (1) selection of study groups (four points); (2) comparability of groups (two points); and (3) exposure or outcome (three points) for case–control and cohort studies (see Supplementary Table 2, ESM) [15].
Summary Measures
Numbers of cases occurred for each specific outcome and with exposure to each specific biologic were extracted from each article and entered into the chart.
Risk of Bias Across Studies
Due to heterogeneity of the data the calculated percentages are up to clinical interpretations and comparison of the outcomes should be performed with caution.
Data Synthesis
All included articles were considered in data synthesis if they had reported at least one outcome for a specific biologic. Percentage of occurrence for each outcome was calculated as cumulative number of cases divided by the population, for which that specific outcome was reported in the included publications.
Confidence in Cumulative Evidence
The cumulative evidence of the studies was assessed by GRADE scoring (The Grading of Recommendations Assessment, Development and Evaluation Working Group; [retrieved 17 September 2019]) and the score of the Oxford Centre for Evidence-Based Medicine (CEBM) [retrieved 17 September 2019] [16–18].
The GRADE approach results in an assessment of the quality of evidence on four levels: (1) high (++++), (2) moderate (+++), (3) low (++), and (4) very low (+) based on all publications and their limitations. First a primary ranking of ‘high’ or ‘low’ was assigned to RCTs or observational studies (if included for the specific group of publications for one specific biologic). Then the initial ranking was downgraded or upgraded based on among others risk of bias, inconsistency, imprecision, indirectness, confounding in cumulative evidence based on all included publications for the specific biologic. It should be mentioned that the final score is not an average of individual scores, but a cumulative assessment for all the publications together, as explained above [16, 18].
The strength of cumulative evidence was graded using a 1–5 ordinal scale for CEBM, in which the lower the risk of confounding (bias), the further to the left the type of evidence will lie in the scale. Steps were taken as for GRADE score and final score was assigned to the cumulative evidence from all publications included for one biologic at a time [17].
For both the GRADE scoring and the CEBM calculations the included studies and case reports were weighted considering case–control studies are recommended studies for identifying congenital anomalies [19] followed by cohort studies and case reports, as they have the lowest weight. Abstracts scored lower than full articles, because the methods section often does not sufficiently describe the control group and the adjusting for confounders. The GRADE and CEBM scores based on these considerations are presented in Table 1.
Table 1.
Summary of the references and GRADE scores (confidence in cumulative evidence)
| Drug | Number and type of publications | References | GRADE | CEBM |
|---|---|---|---|---|
| Abatacept | 1 cohort, 2 register data (2 abstracts), 2 case reports/series (1 abstracts) | [9, 20–23] | ++ | 3–4 |
| Adalimumab | 19 cohorts (9 abstract), 14 register data (6 abstract), 6 case controls (1 abstract), 11 case reports/series (3 abstract) | [1, 20–69] | +++ | 3 |
| Anakinra | 4 cohorts (1 abstract), 2 case reports/series | [20, 57, 70–73] | + | 4 |
| Canakinumab | 1 cohort, 1 case report (1 abstract) | [72, 74] | + | 4 |
| Certolizumab pegol | 14 cohorts (6 abstract), 10 register data (1 abstract), 4 case controls (1 abstract), 4 case reports/series (3 abstract) | [1, 20, 22, 23, 31, 33, 37, 39, 40, 42, 44, 48, 54, 55, 57, 58, 63, 64, 66, 68, 75–88] | ++ | 3–4 |
| Etanercept | 12 cohorts (5 abstract), 6 register data (2 abstract), 2 case controls (1 abstract), 6 case reports/series (2 abstract) | [1, 20–23, 27, 33, 35, 40, 41, 44, 45, 47, 53–57, 60, 63, 66, 68, 87, 89–91] | +++ | 3 |
| Golimumab | 9 cohorts (2 abstract), 3 register data (1 abstract), 2 case controls (1 abstract), 2 case reports/series (1 abstract) | [20, 22, 23, 33, 35, 40, 44, 48, 53–55, 58, 64, 66, 68, 80] | + | 4 |
| Infliximab | 23 cohorts (11 abstract), 13 register data (2 abstract), 3 case controls, 20 case reports/series (10 abstract) | [1, 20–25, 28, 31, 34–38, 40, 42–44, 46–48, 51, 53, 55, 57, 58, 60–62, 64–68, 76, 77, 79, 80, 92–112] | ++ | 3–4 |
| Rituximab | 5 cohorts (2 abstract), 3 register data (2 abstract), 1 case reports/series (1 abstract) | [20, 22, 44, 45, 56, 57, 63, 66, 113] | + | 4 |
| Tocilizumab | 1 cohort, 1 register data (1 abstract), 8 case reports/series (3 abstract) | [22, 23, 41, 45, 114–119] | ++ | 3–4 |
| Ustekinumab | 4 cohorts, 3 register data (2 abstract), 15 case reports/series (3 abstract) | [27, 37, 44, 53, 59, 60, 64, 66, 67, 77, 80, 120–130] | + | 4 |
| Vedolizumab | 2 cohorts (1 abstract), 1 register data, 2 case controls (1 abstract), 2 case reports/series (1 abstract) | [64, 77, 131–135] | + | 4 |
Results
Study Selection
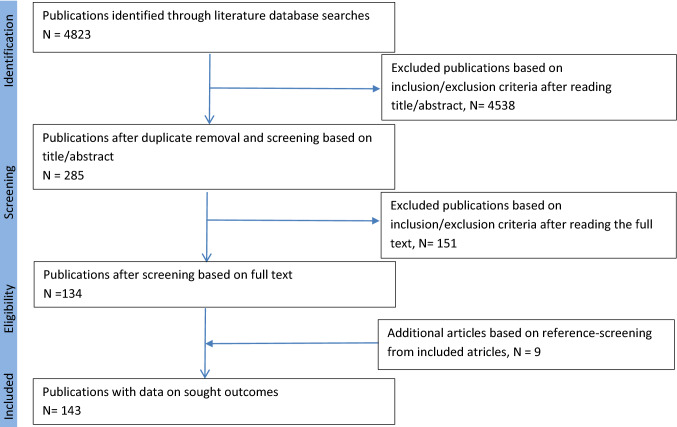
A total of 4823 articles were primarily extracted from the mentioned databases up to 4 July 2019. Selection of publications was based on title/abstract and followed by full text (double blinded, two researchers). 151 articles were excluded after reading the full text based on the following reasons: duplicate articles (including abstracts/posters that had published full texts) (n = 69), information on the outcome of the pregnancies exposed to biologics not provided (n = 64), studies with the same population source as another included article (n = 13), in vitro studies (n = 3), only paternal exposure was investigated (n = 1), studies on diseases other than intended for this article (n = 1). References of the included articles were screened for additional inclusion. Nine additional articles were added to the dataset sheets based on reference-screening of included articles. A total of 143 articles were included. The steps taken are shown in Fig. 1.
Fig. 1.
Flow diagram of databases searched according to the PRISMA guidelines
Study Characteristics
In total 143 publications were included. The number of publications for each biologic ranged from two (canakinumab) to 59 (infliximab). The references, type of publications, and GRADE and Oxford CEBM scores for each biologic are presented in Table 1.
Risk of Bias Within the Studies
The authors acknowledge the risk of information bias in some studies that evaluated pooled data on several biologics, without reporting the numbers of outcomes for each biologic separately. The results of these studies, however, are described in this article as two additional categories under the subtitles of “TNF alpha blocker (in general, not specified in the full text)”and “Biologics (in general, not specified in the full text)”.
Results of Individual Studies and Synthesis of the Results
The key outcomes for each biologic are discussed below. To gain a better perspective regarding overall possible confounders, data on maternal characteristics such as disease type, co-morbidities, concomitant medication exposure, presence of anti-drug antibodies in maternal serum, exposure during the first and all three trimesters, and rate of breastfeeding after delivery were calculated, if possible (Table 2). Outcome measures are presented in Tables 3 and 4 for maternal- and child-related outcomes, respectively. It should be noted that due to heterogeneity of the data the calculated percentages cannot be compared.
Table 2.
Maternal characteristics of pregnancies exposed to different biologic drugs
| Drug | Abatacept | Adalimumab | Anakinra | Canakinumab | Certolizumab pegol | Etanercept | Golimumab | Infliximab | Rituximab | Tocilizumab | Ustekinumab | Vedolizumab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indications | ||||||||||||
| Rheumatoid diseases | 4/157 (2.5) | 118/2,027 (5.8) | 7/35 (20) | 9/9 (100)* | 318/883 (36) | 458/1145 (40) | 9/31 (29) | 34/4276 (0.8) | 42/42 (100) | 340/373 (91.2) | 1/54 (1.9) | – |
| Inflammatory bowel disease | – | 1182/2027 (58.3) | – | – | 328/883 (37.2) | – | 5/31 (16.1) | 2539/4276 (59.4) | – | – | 23/54 (42.6) | 147/147 (100) |
| Psoriasis | – | 12/2027 (0.6) | 3/35 (8.6) | – | – | 97/1145 (8.5) | – | 10/4276 (0.2) | – | – | 26/54 (48.1) | – |
| Not defined/other | 153/157 (97.4) | 715/2027 (35.2) | 25/35 (71.4) | – | 237/883 (26.8) | 591/1145 (51.6) | 17/31 (54.8) | 1693/4276 (39.6) | – | 33/373 (8.8) | 5/54 (9.3) | – |
| Pre-pregnancy existing co-morbidities | ||||||||||||
| APS | 0/1 (0) | 0/19 (0) | 0/1 (0) | – | 1/12 (8.3) | 0/74 (0) | 0/4 (0) | 0/20 (0) | 0/2 (0) | – | – | – |
| Diabetes | 5/151 (3.3) | – | – | – | – | 36/337 (10.7) | – | – | – | – | – | – |
| Hospitalization/surgery | – | 1/5 (20) | – | – | – | – | – | 34/260 (13.1) | – | –- | – | 1/5 (20) |
| Hypertension† | – | – | 1/5 (20) | – | – | 74/337 (22) | – | 2/16 (12.5) | – | 1/16 (6.2) | – | – |
| Mental disorders | – | – | – | – | – | 126/337 (37.4) | – | 0/14 (0) | – | 1/16 (6.2) | – | – |
| Smoker | – | 8/63 (12.7) | 1/5 (20) | – | 0/6 (0) | – | – | 53/333 (15.9) | – | 1/14 (7.1) | – | – |
| Alcohol | – | – | 1/5 (20) | – | – | – | – | 0/16 (0) | – | 0/14 (0) | – | – |
| Previous miscarriages | – | 0/4 (0) | 1/4 (25) | 1/8 (12.5) | – | – | –- | 3/19 (15.8) | – | 33/304 (10.9) | – | – |
| Previous unfertility | – | 5/58 (8.6) | 1/5 (20) | – | – | – | – | 3/73 (4.1) | – | – | – | – |
| Perianal complications | – | 12/58 (20.6) | – | – | 2/27 (7.4) | – | – | 3/115 (26.1) | – | – | – | 6/39 (15.4) |
| Thyroid disfunction | – | – | – | – | – | 38/337 (11.3) | – | 0/14 (0) | – | 1/16 (6.2) | – | – |
| Other | – | – | – | – | – | – | – | – | – | 1/16 (6.2)+ | – | 1/20 (5)++ |
| Concomitant exposure (any) | – | 71/278 (25.5) | 4/5 (80) | – | 402/553 (72.7) | 70/353 (19.8) | – | 85/461 (42.9) | 5/23 (21.7) | 54/210 (25.7) | – | 6/34 (17.6) |
| Corticosteroid | – | 11/61 (18.1) | – | – | 14/30 (46.7) | – | – | 90/450 (20) | 15/27 (55.6) | 0/2 (0) | 3/35 (8.6) | |
| Cyclosporine | – | – | – | – | 0/4 (0) | 0/5 (0) | – | 2/228 (0.9) | 2/23 (8.7) | – | 1/2 (50) | – |
| Hydroxychloroquine | – | – | – | – | 6/21 (28.6) | – | – | – | 3/22 (13.6) | 1/16 (6.2) | – | – |
| Leflunomide | – | 1/9 (11.1) | – | – | 0/6 (0) | 0/31 (0) | – | – | 4/23 (17.4) | 2/17 (11.7) | – | – |
| Mesalazine | – | 5/58 (8.6) | – | – | 0/3 (0) | – | – | 124/348 (35.6) | – | – | – | 4/9 (44.4) |
| MTX | – | 6/80 (7.5) | 0/5 (0) | – | 0/25 (0) | 59/343 (17.2) | – | 36/221 (16.3) | 5/23 (21.7) | 40/208 (25.6) | – | 0/10 (0) |
| NSAID | – | – | 1/5 (20) | – | 5/13 (38.5) | – | – | – | – | – | – | – |
| Sulfasalazine | – | – | – | – | 5/21 (23.8) | – | – | – | 5/22 (22.7) | 1/16 (6.2) | – | 1/24 (4.2) |
| Thiopurines | – | 33/136 (24.3) | – | – | – | – | – | 116/296 (39.2) | 2/22 (9.1) | – | – | 2/10 (20) |
| Any non-biologic DMARDs | – | – | – | – | – | 23/337 (6.9) | – | 5/57 (8.8) | – | – | – | – |
| Another biologic | – | 23/803 (2.9) | – | 2/8 (25) | 3/17 (17.6) | 23/337 (6.8) | – | 21/819 (2.6) | 2/22 (9.1) | 1/4 (25) | – | |
| Other | – | – | 1/4 (25) Colchicine | – |
394/541 (72.8) Any drugs |
46/337 (13.6) Teratogenic DMARDs | – |
63/83 (75.9) Folic acid |
– | – | – | – |
| Anti-drug antibodies | – | 2/16 (12.5) | – | – | 0/21 (0) | – | – | 0/2 (0) | – | – | – | – |
| Exposure period | ||||||||||||
| First trimester | – | 529/534 (99) | 29/34 (85.3) | 9/9 (100) | 424/528 (80.3) | 252/268 (94) | 10/10 (100) | 510/675 (75.6) | 9/25 (36) | 341/355 (96.1) | 22/25 (88) | 38/47 (80.8) |
| All three trimesters | 0/2 (0) | 19/78 (24.3) | 25/33 (75.8) | 4/9 (44.4) | 9/41 (21.9) | 19/256 (7.4) | 0/10 (0) | 20/94 (21.3) | 3/25 (12) | 10/355 (2.8) | 12/31 (38.7) | 35/47 (74.4) |
| Breastfeeding | – | 19/78 (24.3) | 14/25 (56) | – | 9/41 (21.9) | 5/27 (18.5) | – | 20/94 (21.3) | – | 5/39 (12.8) | 1/4 (25) | 12/75 (16) |
Values are given as number (%)
NSAID non-steroidal anti-inflammatory drugs, MTX methotrexate, HCQ hydroxychloroquine, ADRs adverse drug reactions, RA rheumatoid arthritis, JIA juvenile rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis, FMF familial Mediterranean fever, APS anti-phospholipid syndrome, DMARDs disease-modifying antirheumatic drugs
†Not related to pregnancy
*4/9 CAPS (cryopyrin-associated periodic fever syndromes), 1/9 Cogan syndrome, and 1/9 not defined
+Previous pregnancy complications
++Obesity
Table 3.
Outcomes of pregnancy exposure related to different biologic drugs
| Drug | Abatacept | Adalimumab | Anakinra | Canakinumab | Certolizumab pegol | Etanercept | Golimumab | Infliximab | Rituximab | Tocilizumab | Ustekinumab | Vedolizumab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of pregnancies | 157 | 2027 | 35 | 9 | 883 | 1145 | 31 | 4276 | 42 | 368 | 54 | 147 |
| Prospective | 99 (63.1) | 898 (44.3) | 8 (22.9) | 1 (11.1) | 641 (72.6) | 657 (57.4) | 15 (48.4) | 2453 (57.4) | 35 (83.3) | 204 (55.4) | 36 (66.7) | 58 (39.5) |
| Retrospective | 58 (36.9) | 1129 (55.7) | 27 (77.1) | 8 (88.9) | 242 (27.4) | 488 (42.6) | 16 (51.6) | 1823 (42.6) | 7 (16.7) | 164 (44.6) | 18 (33.3) | 89 (60.5) |
| Pregnancy related outcomes | ||||||||||||
| Misscarriages (total) | 40/153 (26.1) | 16/180 (8.9) | 1/33 (3.1) | 1/9 (11.1) | 78/679 (11.5) | 69/359 (19.2) | – | 170/1584 (10.7) | 1/24 (4.2) | 84/361 (23.3) | 3/27 (11.1) | 19/72 (26.4) |
| Prospective | 17/70 (24.3) | 11/121 (9.1) | 1/27 (3.70) | 0/1 (0) | 47/556 (8.5) | 0/4 (0) | – | 169/1551 (10.9) | 1/19 (5.3) | 43/199 (21.6) | 1/17 (5.9) | 9/57 (15.8) |
| Retrospective | 23/83 (27.7)€ | 5/59 (8.4) | 0/6 (0) | 1/8 (12.5) | 31/123 (25.2) | 69/355 (19.4) | – | 1/33 (3) | 0/5 (0) | 41/162 (25.3) | 2/10 (20) | 10/15 (66.7) |
| Ectopic pregnancy | – | – | 0/6 (0) | – | 0/3 (0) | – | – | 9/1363 (0.7) | – | 0/16 (0) | – | – |
| Induced abortion | 20/153 (13.1) | 3/86 (3.5) | 0/5 (0) | – | 37/679 (5.4) | 30/338 (8.9) | – | 90/1552 (5.8) | 1/19 (5.3) | 6/69 (8.7) | 1/26 (3.8) | 7/57 (12.3) |
| Diseases during pregnancy | ||||||||||||
| Placental abnormalities | – | 0/6 (0) | – | – | 1/23 (4.3) | – | – | 1/6 (16.7) | – | – | – | 2/4 (50) |
| Pregnancy-related hypertension | – | 1/5 (20) | – | – | 5/454 (1.1) | 74/362 (20.4) | – | – | 1/19 (5.3) | 1/20 (5) | – | 1/21 (4.8) |
| Gestational diabetes | 5/148 (3.4) | 1/53 (1.8) | – | – | 6/468 (1.3) | – | – | – | – | – | – | – |
| Serious infections | – | 1/6 (16.6) | 1/9 (11.1) | – | 25/543 (4.6) | 33/363 (9.1) | – | 2/8 (25) | – | – | – | – |
| Other | – | – | – | – | – | 40/337 (11.9)∞ | – | – | – | – | – | – |
| Delivery | ||||||||||||
| Early membrane rupture | 1/86 (1.2) | 0/5 (0) | 1/5 (20) | – | 2/17 (11.8) | 1/25 (4) | – | 1/7 (14.3) | – | 53/288 (18.4) | – | – |
| Emergent C-section | 1/86 (1.2) | 0/4 (0) | 2/4 (50) | – | 1/16 (6.2) | – | – | 6/1372 (0.4) | – | 2/5 (40) | – | 2/33 (6.1) |
| Dose range (N)† | – | 40 mg/2w (14), 40 mg/w (6) | 50 mgD (2), 100 mgD (21), 200–300 mgD (1) | 120 mg once (1), 150 mg/8w (4), 300 mg/8w (1), 150 mg/4w (2) | 200 mg/2w (36), 400 mg/4w (3) | – | – | 5 mg/kg/8w (37)††, 5 mg/kg/6w (14), 7.5 mg/kg/8w (1), 5 mg/kg/4w (8), 10 mg/kg/8w (2), 10 mg/kg/6w (4), 10 mg/kg/4w (1) | – | 4 mg/kg/4w (13), 8 mg/kg/4w (147) | 45 mg/12w (5), 90 mg/12w (3), 90 mg/8w (1), 45 mg/2w (1), 90 mg/4w (1) | 300 mg/8w (13) |
| Detectable in breast milk | – | 2/21 (9.5) | – | – | 13/25 (52) | – | 0/1 (0) | 19/29 (65.5) | – | 4/4 (100) | 4/6 (66.7) | 5/5 (100) |
Values are given as number (%)
W week, D daily
†Data on dose were not retractable in all the cases, the number of patients, for whom dose was reported, is mentioned in bracket instead of percentages. Doses are mentioned from lowest to the highest in order
††In two cases infliximab was used at dose of 5 mg/kg one to three times during the third trimester, the rest of the cases were administered during all the trimesters
€The rest of the prospective and retrospective data were not distinguished
∞Hemorrhage
Table 4.
Child-related outcomes of pregnancy exposure related to different biologic drugs
| Drug | Abatacept | Adalimumab | Anakinra | Canakinumab | Certolizumab pegol | Etanercept | Golimumab | Infliximab | Rituximab | Tocilizumab | Ustekinumab | Vedolizumab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth-related outcomes | ||||||||||||
| Live birth | 88/152 (57.8) | 200/222 (90.0) | 32/33 (96.9) | 8/9 (88.9) | 582/702 (82.9) | 270/372 (72.6) | 6/6 (100) | 1740/2017 (86.3) | 19/24 (79.2) | 218/362 (60.2) | 26/30 (86.7) | 39/69 (56.5) |
| Major CMs (total) | 7/88 (7.9) | 46/1008 (4.6) | 1/34 (2.9) | 0/8 (0) | 10/535 (1.9) | 47/815 (5.8) | 0/13 (0) | 51/1222 (4.2) | 0/20 (0) | 11/345 (3.2) | 0/28 (0) | 2/110 (1.8) |
| Prospective | 0/36 (0.0) | 25/543 (4.6) | 1/27 (3.7) | 0/1 (0) | 10/507 (2) | 30/466 (6.4) | 0/8 (0) | 28/751 (3.7) | 0/20 (0) | 8/194 (4.1) | 0/18 (0) | 2/40 (5) |
| Retrospective | 7/52 (13.4)€ | 21/465 (4.5) | 0/7 (0) | 0/7 (0) | 0/28 (0) | 17/349 (4.9) | 0/5 (0) | 23/471 (4.9) | – | 3/151 (2) | 0/10 (0) | 0/70 (0) |
| Minor CMs | – | 2/68 (2.9) | 1/9 (11.1) | – | – | 0/20 (0) | 0/2 (0) | 2/94 (2.1) | – | 0/4 (0) | 0/19 (0) | 1/5 (20) |
| Low birth weight (< 2500 g) | 0/1 (0) | 11/123 (8.9) | 6/19 (31.6) | 0/6 (0) | 25/243 (10.3) | 31/253 (12.2) | 0/2 (0) | 65/584 (11.1) | 0/6 (0) | 27/118 (22.9) | 2/24 (8.3) | 1/9 (11.1) |
| Pre-term births (< 37 weeks) | – | 4/146 (2.7) | 6/28 (21.4) | 0/8 (0) | 42/380 (11.1) | 40/269 (14.9) | 0/2 (0) | 104/1,754 (5.9) | 0/19 (0) | 32/177 (18.1) | 3/20 (15) | 16/94 (17) |
| SGA | – | 1/87 (1.1) | – | – | 5/463 (1.1) | 0/48 (0) | 0/5 (0) | 3/106 (2.8) | 0/6 (0) | 3/39 (7.7) | – | – |
| Still birth/intrauterine death (≥ 20 weeks) | 4/151 (2.6) | 2/258 (0.8) | 0/32 (0) | – | 5/570 (0.9) | 0/30 (0) | – | 2/2,146 (0.1) | 2/30 (6.7) | 1/202 (0.5) | 0/23 (0) | 1/28 (3.6) |
| Neonatal death | – | – | 0/32 (0) | – | 2/543 (0.4) | 0/29 (0) | – | 0/435 (0) | 0/30 (0) | 0/3 (0) | 0/14 (0) | – |
| Immune system-related outcomes | ||||||||||||
| Adequate vaccination response | – | 17/17 (100) | – | – | 9/9 (100) | 25/26 (96.2) | 2/2 (100) | 12/12 (100) | – | – | 1/1 (100) | – |
| ADRs related to vaccination | – | 1/102 (0.9) | – | – | 0/26 (0) | 0/12δ (0) | – | 6/197 (3) | – | – | 0/3 (0) | 0/1 (0) |
| Allergies | – | 5/103 (4.9) | – | – | – | – | – | 8/183 (4.4) | – | – | – | – |
| Eczema | – | 6/44 (13.6) | – | – | – | – | – | 15/61 (24.6) | – | – | – | – |
| (Serious/opportunistic) infectionsΨ | 0/17 (0) | 13/229 (5.7) | 0/30 (0) | 0/8 (0) | 2/41 (4.9) | 0/62 (0) | 0/4 (0) | 212/786 (27) | 0/13 (0) | 0/3 (0) | 0/7 (0) | 5/110 (4.6) |
| Drug-related outcomes | ||||||||||||
| Anti-drug antibodies at birth | – | – | – | – | 0/16 (0) | – | – | 4/51 (7.8) | – | – | – | – |
| Detectable drug levels | ||||||||||||
| Cord blood | – | 50/50 (100) | – | – | 6/26 (23.1) | – | – | – | – | – | – | – |
| At birth | – | 28/36 (77.8) | – | – | 1/14 (7.1) | – | – | 51/99 (51.5) | – | – | – | – |
| During first 6 months | – | – | – | – | 0/14 (0) | – | – | – | – | – | – | 0/5 (0) |
| At 9 months of age | – | 1/37 (27) | – | – | – | – | –- | 7/46 (15.2) | – | – | – | – |
| At 12 months of age | – | 0/36 (0) | – | – | – | – | – | 3/46 (6.5) | – | – | – | – |
| Abnormal development | – | 1/61 (1.6) | 1/32 (3.1) | 0/8 (0) | 0/8 (0) | – | – | 33/533 (6.2) | – | 0/3 (0) | 0/4 (0) | 0/5 (0) |
Values are given as number (%)
SGA (small for gestational age): below 10th centile for weight at birth, CM congenital malformation.
ΨSerious infections defined as antibiotic therapy or hospitalization because of the infection
δVaccinations including polio, hepatitis B, diphtheria, pertussis, tetanus, Haemophilus influenza, and pneumococcus vaccines at 3, 6, and 12 months
Abatacept
In total 157 maternal pregnancies were investigated in five articles. Outcomes regarding miscarriages were investigated for 153 of these pregnancies, in which 40 miscarriages were reported (26.1%).
Seven major CMs were reported in 88 pregnancies (7.9%), which included:
Cleft palate (n = 1)
Congenital aortic-anomaly (n = 1)
Meningocele (n = 1)
Pyloric stenosis (n = 1)
Skull malformation (unspecified) (n = 1)
Ventricular septal defect (VSD) (n = 1)
Congenital arterial malformation (n = 1) [9].
The percentage and variety of co-medication use (including methotrexate (MTX) exposure) could not be extracted for the reported miscarriages from the presented data.
Adalimumab
A total of 2027 maternal pregnancies were described in 50 articles. Outcomes regarding miscarriages were reported for 180 pregnancies, in which 16 miscarriages occurred (8.9%). Forty-six (n = 46) major CMs were reported in 1008 pregnancies (4.6%). Thirteen major CMs were described as follows (the other cases did not describe explicitly the major CM or the major CM could not be linked to this specific biologic):
Cleft palate (n = 1).
Cleft palate, micrognathia, myopia, glaucoma and esophageal motility disorder (n = 1).
Congenital diaphragmatic hernia and obstructive mega ureter (n = 1).
Polydactyly (n = 1).
Hexadactyly (n = 1).
Hexadactyly both feet and atrial septal defect (ASD) (n = 1).
Esophageal atresia with tracheo-esophageal fistula, VSD, syndactyly in both feet, peripheral pulmonary stenosis, persistent foramen ovale (PFO) (n = 1).
ASD with left–right shunt and aneurysm, cavum septum pellucidum on both sides, hemangioma at left flank (n = 1).
VSD and hip dysplasia (n = 1).
Hemangioma right temple, umbilical hernia (n = 1).
Imperforate anus (n = 1).
Amniotic band sequence: talipes and amputation of four fingers of the right hand (n = 1).
Cystic adenomatoid malformation of the right lung, incomplete right bundle branch block, pericardial effusion, PFO, persistent ductus arteriosus (PDA) and slight persistent pulmonary hypertension (co-medication: etanercept) (n = 1) [1, 20, 68].
Data on overall concomitant medication was available for 278 cases. Use of one or more co-medications was reported for 71 of these patients (25.5%). Data on exposure to MTX were available for 80 cases, of which six patients (7.5%) were exposed to MTX.
Growth was investigated in 61 children exposed to adalimumab during pregnancy, of which only one primary growth failure due to cystic fibrosis was reported (1.6%) [43].
Adalimumab was detectable in breast milk of two patients from the total 21 investigated cases (9.5%), with a maximum concentration of 0.71 μg/mL seen between 12 and 24 h after injection. In seven cases breast milk was examined after 7 days from the injection and it was not detectable in any of these cases [80].
Anakinra
In total 35 maternal pregnancies were investigated in six articles. Outcomes regarding miscarriages were investigated for 33 of these pregnancies, in which only one miscarriage was reported (3.1%, concomitant therapy was not reported in this case) [72]. One major CM was reported (2.9%, unilateral renal agenesis and ectopic neurohypophysis, which also had abnormal growth due to growth hormone deficiency) [72]. One minor CM (a case of tied tongue after exposure to anakinra during pregnancy [73]) was reported among nine cases that were investigated for such an outcome. Data on overall concomitant medication was available for five cases. Use of one or more co-medications was reported for four of these patients (80.0%). MTX exposure was not reported for any of these patients.
Emergent C-sections were reported in two cases. One case because of a pathological cardiotocography (CTG) and premature rupture of the membranes and umbilical cord entanglement. The other case was a secondary emergent C-section due to uterine rupture under spontaneous delivery [70].
Canakinumab
In total nine pregnancies were investigated in two articles [72, 74]. Outcomes regarding miscarriages were investigated for all of these pregnancies, in which only one miscarriage was reported (11.1%, at 6 weeks of pregnancy to a mother with refractory Cogan syndrome). Concomitant medication was not reported in this case [72]. No CMs were reported in eight live births. Data on overall concomitant medication (including MTX exposure) were not available.
Certolizumab Pegol
In total 883 maternal pregnancies were investigated in 32 articles. Outcomes regarding miscarriages were investigated for 679 of these pregnancies, in which 78 miscarriages were reported (11.5%). Ten major CMs were reported from a total of 535 investigated cases (1.9%). In the article by Broms et al. the described major CMs were not categorized based on the kind of biologic used during pregnancy. Therefore, the one reported case of major CM for certolizumab pegol in this study could not be associated with the described cases [40]. Nine described cases in other articles could be linked to certolizumab pegol and were as follows (the rest of the cases were not described explicitly or could not be linked to this specific biologic):
Clubfeet and Hirschsprung’s disease (n = 1)
Anal fistula (n = 1)
Talipes (n = 1)
Vesicoureteric reflux (n = 1)
Cerebral ventricle dilatation (n = 1)
Hydronephrosis (n = 1)
Congenital heart disease (n = 1)
Accessory auricle (n = 1)
Data on overall concomitant medication were available for 553 cases. Use of one or more co-medications was reported for 402 of these patients (72.7%). Data on exposure to MTX were available for 25 cases, of which none were exposed to MTX.
From the two reported cases of early membrane ruptures, one was due to infection [75]. The other one occurred at the 35th week in a mother exposed to certolizumab pegol during the first trimester [1].
Reported infections in mothers were mainly urinary tract infections and upper respiratory tract infections. In one case pyelonephritis with septicemia at 30th GW was reported in a patient who was on certolizumab pegol combination therapy with hydroxychloroquine, sulfasalazine, and prednisone [75].
Two cases of neonatal deaths occurred in a twin pregnancy; one of the twins was born at 25th GW with brain damage and pneumo-peritoneum, the other twin was born at 27th GW with heart defects and passed away due to a gastrointestinal infection at the 8th week of age [78].
Concentration in breast milk was investigated in 13 cases. Certolizumab pegol was detectable in three of these cases with a maximum concentration of 0.29 μg/mL peak. In two cases breast milk was examined after 7 days following the injection, and it was not detectable in either case [80].
Etanercept
In total 1145 maternal pregnancies were investigated in 26 articles. Outcomes regarding miscarriages were investigated for 359 of these pregnancies, in which 69 miscarriages were reported (19.2%). From 815 exposed cases during pregnancy, 47 major CMs were reported (5.8%). Six described cases are as follows (the rest of the cases were not described explicitly or could not be linked to this specific biologic):
Hypoplastic left heart and hypospadias (n = 1)
Agenesis of left kidney (n = 1)
Bilateral hydronephrosis (n = 1)
ASD (n = 1)
Wolf–Parkinson–White syndrome with heart failure (n = 1)
Data on overall concomitant medication were available for 353 cases. Use of one or more co-medications was reported for 70 of these patients (19.8%). Data on exposure to MTX were available for 343 cases, in which 59 patients (17.2%) were exposed to MTX.
Golimumab
In total 31 maternal pregnancies were investigated in 16 publications. No data regarding miscarriages could be extracted from the presented information in these articles. No major CMs were reported in 13 exposures during pregnancy for golimumab. Data on overall concomitant therapy (including MTX exposure) were not available.
Infliximab
In total 4276 maternal pregnancies were investigated in 59 articles. The largest amount of prospective data was reported from a Janssen biologics database, which reported pregnancy results of 1362 reported spontaneous cases, clinical studies, and registries [101]. Outcomes regarding miscarriages were investigated for 1584 of these pregnancies, in which 170 miscarriages were reported (10.7%).
In total 51 major CMs were reported in 1222 pregnancies (4.2%). Fourteen described major CMs are as follows (the rest of the cases were not described explicitly or could not be linked to this specific biologic):
-
1–3.
VSD (n = 3).
-
4, 5.
Cleft palate (n = 2).
-
6, 7.
Facial hemangiomata (n = 2).
-
8.
ASD (n = 1)
-
9.
Ectrodactyly (n = 1)
-
10.
Polydactyly (n = 1)
-
11.
Hydronephrosis and obstructive megaureter (n = 1)
-
12.
Pelviureteric junction obstruction (n = 1)
-
13.
Small aortic-pulmonary collateral, hypospadias, hepatic cyst (n = 1)
-
14.
Megacystis plus bilateral talipes (n = 1) [43, 68, 107–109].
Two cases of minor CMs were reported in 94 patients (2.1%). This included one case of duplex kidney and one case of aberrant subclavian artery [136]. Data on overall concomitant medication were available for 461 cases. Use of one or more co-medications was reported for 85 of these patients (42.9%). Data on exposure to MTX were available for 221 cases, of which 36 patients (16.3%) were exposed to MTX.
Five hundred and forty-seven patients from a total of 1939 (28.2%) were exposed to infliximab during the entire pregnancy (all three trimesters). The data on elimination of infliximab during pregnancy were available only from a pharmacokinetics (PK) study, which compared drug levels and its clearance during different trimesters in 22 pregnancies. It was reported in this study that infliximab clearance decreased by 12% in the second and third trimesters [112].
The median ratio of infliximab in cord blood to maternal level was 2.63 (95% confidence interval (CI), 1.67–4.03) in 52 patients [median duration of therapy until 23 GW (range 21–32)] [43] and 3.25 in two patients in another study (therapy continued until last week of gestation) [28]. In one case report in a patient under infliximab treatment with the dose of 10 mg/kg every 6 weeks until the 32th GW, the cord blood concentration was 110.1 μg/mL. Plasma infliximab level in the mother at the time of delivery was 59.7 µg/mL [93]. The median ratio of infant to maternal drug concentration at birth was 1.97 (95% CI 1.50–2.43) in 44 patients (18 patients and 26 patients received last infusion before and after the 30th GW, respectively) [136]. In another study the median concentration was 7.8 μg/mL in patients who used infliximab until the 18th–36th weeks of gestation [51].
Infliximab was detectable in 19 patients’ breast milk out of a total of 29 patients, with a maximum range of 0.15–0.74 μg/mL 24 h after infusion, detectable in 17 samples from a total of 29 after 48 h, and detectable in five samples from a total of eight after 168 h (no concentrations were reported for samples in the last two groups). Weight-adjusted dose was not calculated in this study [80].
Six cases from a total of 1372 (0.4%) required emergency C-section. One of these cases had undergone an emergency C-section because of placental dysfunction, in which a very low-birth-weight preterm infant was born [99].
There was a case report of an infant diagnosed with vertical transmission of disseminated histoplasmosis from mother at the time of delivery and through the placenta (authors’ conclusion: probable involvement of the central nervous system). The mother was taking infliximab (IBD indication) until the 32th GW (delivery at 35th GW) [93].
A follow-up of 533 children exposed to infliximab showed abnormal development in 33 of these children (6.2%). Descriptions of abnormal development were provided in three of these cases: two cases reported deviations in weight or height percentiles and one case of late gross motor function development, which was resolved by 20 months of age [43, 94, 109].
Regarding vaccination (one or more vaccinations with Bacillus Calmette-Guérin (BCG), rotavirus, Haemophilus influenzae type b (Hib), tetanus, or hepatitis B), in total 197 cases were investigated in different publications [1, 43, 51, 58, 62, 77, 94, 100, 109, 137]. Twenty-nine cases were investigated regarding response to tetanus and hepatitis B vaccination, of which 28 had adequate response [28, 51, 62, 77, 100]. Adverse drug reactions were reported in six cases with mild reactions to rotavirus vaccination (fever, n = 5 and diarrhea, n = 1) in children exposed to infliximab during pregnancy [77].
Rituximab
In total 42 maternal pregnancies were investigated in nine articles. Outcomes regarding miscarriages were investigated for 24 of these pregnancies, of which only one (4.2%) miscarriage was reported at 12th GW in a case exposed to rituximab 1 month prior to conception and MTX at the time of conception [113].
No major CMs were reported for 20 pregnancy cases expose to rituximab. Data on exposure to MTX were available for 23 cases. It was reported for five of these patients (21.7%).
Two cases of stillbirth were reported, which occurred in the same patient, with rituximab exposure at 9 and 18 months prior to conception [113].
Tocilizumab
In total 368 maternal pregnancies were investigated in ten articles. Outcomes regarding miscarriages were investigated for 361 of these pregnancies, in which 84 miscarriages were reported (23.3%). Data on concomitant exposure to MTX for reported miscarriages were not available.
Eleven major CMs were reported in a total of 345 investigated pregnancies with tocilizumab (3.2%). Eight of these cases were described as follows:
Pyelectasia (n = 1)
Multicystic dysplasia of right kidney (n = 1)
Esophageal fistula (n = 1)
Coarctation of the aorta, VSD, and inguinal hernia (n = 1)
Absence of one cardiac cavity (n = 1)
Bilateral hip dysplasia (n = 1)
Polydactyly (n = 1)
Marked thoracic scoliosis, myelomeningocele, missing sacrum (caudal regression syndrome) (n = 1) [117].
Data on overall concomitant medication were available for 210 cases. Use of one or more co-medications was reported for 54 of these patients (25.7%). Data on exposure to MTX were available for 203 cases, in which 40 patients (19.7%) were exposed to MTX. The results of these pregnancies were reported for just two cases (both live births, in one of them tocilizumab, MTX, and hydroxychloroquine till the sixth week of gestation). One case of stillbirth occurred in a pregnancy also exposed to MTX.
Concentration in breast milk was investigated in one study, in which drug concentrations of between 6 and 60,000 ng/mL were measured up to 28 days after administration of 400 mg tocilizumab, with a peak concentration before day 7. The time taken to reach the maximum concentration (Tmax) in breast milk was calculated as 3.2 days. Milk to serum concentration ratios were: 0.0015, 0.00082 and 0.0014 for three patients [119].
Ustekinumab
In total 54 maternal pregnancies were investigated in 22 articles. Outcomes regarding miscarriages were investigated for 27 of these pregnancies, in which three miscarriages were reported (11.1%). In one case of miscarriages the treatment dose of ustekinumab was higher than average due to refractory Crohn’s disease [122, 124].
In 28 pregnancies investigated with regard to CMs, no cases were reported. Data on overall concomitant medication (including MTX exposure) were not available.
In one case with the last administered dose at 33 GW, concentration in cord blood at birth was measured (8 µg/mL), which was higher than the drug concentration in the mother’s blood at the same point (4.3 µg/mL) [123].
Concentrations in breast milk were detectable in four cases from a total of six samples. Peak concentrations were between 12 and 72 h after injection (range 0.72–1.57 μg/mL). In three cases from four samples ustekinumab was still detectable 48 h after injection [80].
Vedolizumab
In total 147 maternal pregnancies were investigated in seven articles. Outcomes regarding miscarriages were investigated for 72 of these pregnancies, in which 19 miscarriages were reported (26.4%).
From 110 pregnancies investigated for major CMs, two cases were reported (1.8%). The descriptions of these CMs were as follows:
One congenital central nervous system anomaly caused by agenesis of the corpus callosum and left frontal polymicrogyria. Estimated time of conception was 79 days after the vedolizumab infusion [132]
One case of congenital hypothyroidism [135].
One minor CM of hip dysplasia was also reported [131]. Data on overall concomitant medication were available for 34 cases. Use of one or more co-medications was reported for six of these patients (17.6%). Data on exposure to MTX were available for ten cases, of which none were exposed to MTX.
From a total of 28 investigated cases regarding stillbirth, one case was reported to the Organization of Teratology Information Services (OTIS) due to fetal growth restriction and decreased amniotic fluid volume-induced labor (26th week) [132].
Drug concentrations were measured in mothers’ serum and breast milk in the study by Lehat et al. [133]. No statistically significant correlation was found in five patients with a serum concentration range of 4200–18,000 ng/mL and milk concentration range of 22–216 ng/mL (p = 0.11). Vedolizumab levels peaked at 3–4 days following infusion to a maximum of 480 ng/mL, and then slowly decreased [133]. Maternal and infants’ serum concentrations of vedolizumab were also measured in five patients [131]. The range of drug concentrations was 1.10–14.40 μg/mL in mothers and 1.00–8.70 μg/mL in infants at the time of delivery [131] (weight-adjusted doses were not reported).
One case of a preterm born child, who developed Kawasaki disease with eosinophilia at 3 months, treated with corticosteroids was reported [138]. In one study in 70 children exposed to vedolizumab during pregnancy there were no malignancies reported during the first year of life [134].
Other Biologics
The general conclusions of the articles, which could not be included in the above-mentioned subcategories, are discussed in this section.
Secukinumab
Only three case reports/series were found in the search between 1 January 2015 until 4 July 2019. In total five patients in these articles were reported, therefore this is not included in the results tables. Three of these cases reported spontaneous abortions and two resulted in live births. No information on CMs could be found [60, 139, 140]. One retrospective research in a Novartis global safety database was found on this subject; however, it was excluded from the final results as this was a research letter. Two congenital malformations from 54 total live births and 26 spontaneous abortions from 238 maternal pregnancies are reported. The type of CMs in maternal exposure was not specified with paternal exposure [141].
Belimumab
There were two case reports on using belimumab during pregnancy, which were excluded as they were published as research letters. Therefore, this is not discussed in the results tables. One Ebstein anomaly, one miscarriage, and one healthy birth were reported in a patient with systemic lupus erythematosus who was under belimumab treatment during three pregnancies. The mother was 41 years old at the time of the miscarriage [142]. The other case report was of a healthy birth after exposure to belimumab up to the 26th week of gestation. However, marked B-cell depletion and reduced T-cell subsets were reported in the baby at birth. B cells were in the normal range at 4 months of age. Rotavirus vaccination 6 weeks after birth and diphtheria–tetanus–pertussis, haemophilus, and pneumococcus vaccinations at 3 and 5 months of age resulted in satisfactory responses [143].
TNFα Blocker (in General, Not Specified)
Some of the publications discussed TNF-α blockers in general and did not mention separate data for each biologic. As the outcomes could not be related to one specific biologic with reported data, they were not discussed in the above subcategories [144–154]. In general, these studies have shown a higher chance of flare in patients who discontinue TNF-α blockers during pregnancy [144, 146]. Furthermore, a higher risk of pregnancy complications and severe acute respiratory infections in the first year of life in intrauterine-exposed infants were seen (specially in combination therapy with thiopurines and the chronic use of conventional steroids) [150].
Demyelinating disorders in children exposed to TNF-α blockers during pregnancy were investigated in a nested case–control study in 399 rheumatoid arthritis patients, which did not report any cases of demyelinating disorders in these children during the follow-up period [152].
Biologics (in General, Not Specified)
In some of the included publications the type of biologic used was not specified for the outcomes. In general, these studies did not show any abnormal outcomes in children exposed to biologics during pregnancy/lactation [155–167].
Risk of Bias Across Studies
The authors acknowledge that the risk of bias in total is high as the data are heterogenic and confounding factors could not be retrieved in all of the cases and from all of the studies. Biologics used in different dosages and with different protocols for different indications were considered together in this article. However, it should be remembered that this will not affect the recognition of causality—if any—for CMs and miscarriages (aims of this study).
Additional Analysis
Additional data to the above-mentioned information have been acquired. As different publications had different primary and secondary outcomes and because this additional information is scarce but still of importance, the authors see the necessity to summarize and present this data.
These further details for each biologic can be found in Tables 2, 3, and 4.
Comparison of the results regarding the rates of major CMs from this study with the rates in the systematic review of EULAR, are provided in ESM, Table 3.
Discussion
Available data extracted from the most recent published literature shows that the rates of miscarriages and major CMs in investigated biologics do not differ drastically from those rates in the general population (estimated as 10–20% and 2–5.5%, respectively). In some cases the information on other outcomes (such as ADRs related to vaccination, pre-term births, etc.) was not available. Despite this fact and the heterogeneity of the data, no new safety concerns were identified from a total of 143 investigated publications regarding the use of biologics during pregnancy.
Regarding the association of CMs, preterm birth, and birth weight with biologic use during pregnancy, a recent meta-analysis by Tsao et al. also showed no increased odds ratios [11]. Nonetheless, it should be considered that the methods in the two studies are different. We have investigated a broader range of studies and have summed up data on miscarriages (co-primary outcome) and additional secondary outcomes such as vaccination response and detectable drug levels during different periods. We have also described the pattern of reported CMs. In our systematic review, each biologic was considered separately. This can be of additional value if one specific biologic is the matter of interest. The study of Tsao et al. showed that the underlying conditions are important factors to be considered as a potential confounder for the pregnancy outcomes. In accordance with this conclusion, we provided detailed information on maternal disease baseline characteristics.
According to EMA guidelines, in order to exclude a ten- or twofold risk of congenital malformations for medicine use during pregnancy there is a need for at least 300 or 1000 prospectively collected sets of data on pregnancies, respectively. This information should be collected from exposed pregnancies to that particular pharmaceutical product during at least the first trimester [168, 169]. Based on summed up data in our study combined with the data from the EULAR study, it can be concluded that the use of adalimumab, certolizumab pegol, and infliximab during pregnancy does not carry a twofold increased risk of combined major CMs for the offspring, compared to this risk in the general population (estimated as 2–5.5%) [170]. The rates of major CMs from this study were comparable with the rates in the systematic review of EULAR for most of the investigated biologics, with some minor differences [8]. The use of adalimumab, certolizumab pegol, infliximab, and etanercept during pregnancy has been conditionally approved by the EMA recently, based on the results of their pregnancy registries conducted by pharmaceutical companies [4–7].
For several reasons the impact of medication on the incidence rate of miscarriages is difficult to determine. Often in prospective studies women are only included when they are pregnant and not in the pre-conception period. In these studies early miscarriages are missed. In retrospective studies there is a chance of publication bias, as only women with an abnormal pregnancy are reported. Reliable data would only be available from prospective studies if women are included from the moment they actively try to become pregnant. Furthermore, confounding by indication (some diseases cause more miscarriages) and confounding by concomitant medication (like MTX) can also affect the results. The results on miscarriages and malformations are comparable for adalimumab, certolizumab, and etanercept, although the transplacental passage of adalimumab is extremely high.
In our study, only abatacept, tocilizumab, and vedolizumab show slightly higher rates for miscarriage compared to the incidence rate of miscarriages in the general population (estimated as 10–20%) [90]. It should be considered that the numbers of pregnancies investigated for miscarriages are limited for abatacept [153] and vedolizumab [72]. Due to limited numbers, it cannot be concluded that there is a causal relationship between a slightly higher rate of miscarriages and the use of abatacept or vedolizumab. Regarding tocilizumab, high percentages of miscarriages can be due to publication bias, as this percentage is higher in retrospective data compared to prospective data. For tocilizumab, the high rate of MTX use compared to other biologics can also explain the higher total rate of miscarriages. Comparing the results of miscarriages from this study with the systematic review of EULAR, the rates were comparable for most of the investigated biologics, with some minor differences as follows: rates of miscarriages for anakinra and rituximab were lower in our study versus the EULAR study (1/33 (3.1%) vs. 4/40 (10.0%) and 1/24 (4.2%) vs. 48/210 (22.9%), respectively). However, because of the small number of cases, these findings remain inconclusive [8].
From the secondary outcomes with acceptable reported sample sizes, the results were often as expected. Still there were some increased incidences detected, such as early membrane rupture, low birth weight, and pre-term birth rates with tocilizumab therapy during pregnancy. Increased risk of all these three events have been described with the use of corticosteroids [171–173]. It should be noted that the rate of exposure to corticosteroids in the reviewed publications for tocilizumab was high (Table 2).
Further, serious infections rates in children exposed to infliximab during pregnancy were higher than expected (212/786 (27%)). This highlights the need for continuing research evaluating the safety of infliximab on various maternal infections and infections in offspring. Different studies have shown that combination therapy with anti-TNF α and thiopurines during pregnancy is associated with a higher rate of infections in offspring when compared to monotherapy with anti-TNF α [42, 136]. The overall rate of exposure to thiopurines for infliximab in our systematic review was 39.2%, higher than other biologics, which can explain the higher rates of serious infections in this group. Additionally, most of the reported infections were from the study by Truta et al., which reported acute respiratory infections, without differentiating the serious infections from non-serious infections. In this study, data acquisition was done retrospectively, which increases the chance of publication bias. Furthermore, in most of the pregnancies in this study infliximab was discontinued 90 days or less before delivery, therefore it is more likely that an immunoglobulin with a high affinity for the neonatal Fc receptor (in this case infliximab) transfers through the placenta and reaches the fetus [111, 174].
From the available data on other secondary outcomes some subcategories may give the impression of an increased incidence compared to the normal population such as placental abnormality rate for infliximab and vedolizumab (1/6 (16.7%) and 2/4 (50.0%), respectively), low birth-weight rate for anakinra (6/19 (31.6%)), anti-drug antibodies at birth (4/51 (7.8%)), and detectable drug levels at the 9th (7/46 (15.2%)) and 12th months of age (3/46 (6.5%)), in children exposed to infliximab during pregnancy. However, conclusions should be drawn with caution regarding the above-mentioned increased incidences.
Limitations
The protocol of this study was recorded retrospectively in PROSPERO (after conducting the first search in the databases). Another limitation of this study is that the small sample sizes and small numbers of reports involved in some outcomes (especially secondary outcomes) make interpretations based on acquired results uncertain. Furthermore, type of disease, disease activity during pregnancy, extent of systemic inflammation, and organ involvement, co-morbidities, and concomitant drug therapy could also have contributed to negative outcomes. In this study all types of rheumatologic, gastroenteric, and dermatologic biologics were considered together as the types of biologics used in the management of these diseases overlap. All the increased incidences should be further investigated and confirmed in future studies, aiming to collect prospective data.
Conclusions
Based on the results of our study it is likely that adalimumab, certolizumab pegol, and etanercept conceivably can be prescribed safely during pregnancy, especially considering the negative effects of active disease on mothers, pregnancy outcomes, and the children. Regarding infliximab, high rates of infections in children have been detected, mainly from one study [111], and a more conservative approach, especially in combination therapy with thiopurines, is recommended. Discontinuation before the third trimester may decrease the chance of infections in offspring. Further, the risks of using abatacept, anakinra, canakinumab, golimumab, rituximab, tocilizumab, ustekinumab, and vedolizumab are not well known and available data are scarce in this regard. This study confirms the previous literature reviews carried out on use of biologics during pregnancy in autoimmune diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Declarations
Funding
This study was part of N. Ghalandari’s PhD project. The entire PhD project is funded by Dutch Medicines Evaluation Board (CBG-MEB).
Conflicts of interest
Dr. R.J.E.M. Dolhain received an unrestricted grant from UCB Pharma B.V. and the Dutch Arthritis Association. The other authors have no conflicts to declare.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files). All included articles are listed as references.
Code availability
All articles were extracted from the following databases: Embase.com, Medline Ovid, Web of science, Cochrane CENTRAL, and Google scholar.
Author contributions
NG: conceptualization, methodology, investigation, resources, data curation, writing the original draft, visualization. RJEMD: conceptualization, validation, writing—review and editing, supervision. JMWH: calidation, writing—review and editing, supervision. EPvanP: validation, writing—review and editing, supervision. MK: resources, data curation, visualization. HJMJC: methodology, investigation, resources, data curation, validation, writing—review and editing, project administration.
References
- 1.Hoxha A, Calligaro A, Di Poi E, Peccatori S, Favaro M, Del Ross T, et al. Pregnancy and foetal outcomes following anti-tumor necrosis factor alpha therapy: a prospective multicentre study. Jt Bone Spine. 2017;84(2):169–173. doi: 10.1016/j.jbspin.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Marchioni RM, Lichtenstein GR. Tumor necrosis factor-alpha inhibitor therapy and fetal risk: a systematic literature review. World J Gastroenterol. 2013;19(17):2591–2602. doi: 10.3748/wjg.v19.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauscher AE, Fleischer AB, Phelps KC, Feldman SR. Psoriasis and pregnancy. JCMS. 2002;6(6):561–570. doi: 10.1007/s10227-001-0147-1. [DOI] [PubMed] [Google Scholar]
- 4.EuropeanMedicinesAgency. Summary of Product Characteristics (SmPC) Etanercept (Enbrel) European Medicines Agency 2019; https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdf. Assessed July 2019.
- 5.EuropeanMedicinesAgency. Summary of Product Characteristics (SmPC) Infliximab (Remicade) European Medicines Agency 2019; https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf. Assessed July 2019.
- 6.EuropeanMedicinesAgency. Summary of Product Characteristics (SmPC) Certolizumab pegol (Cimzia). European Medicines Agency 2019; https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf. Assessed July 2019.
- 7.EuropeanMedicinesAgency. Summary of Product Characteristics (SmPC) Adalimumab (Humira) European Medicines Agency 2018; https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf. Assessed July 2019.
- 8.Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Ray L, Vemuri S, Simon TA. Pregnancy outcomes following exposure to abatacept during pregnancy. Semin Arthritis Rheum. 2015;45(3):351–356. doi: 10.1016/j.semarthrit.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Ostensen M, Forger F. Management of RA medications in pregnant patients. Nat Rev Rheumatol. 2009;5(7):382–390. doi: 10.1038/nrrheum.2009.103. [DOI] [PubMed] [Google Scholar]
- 11.Tsao NW, Rebic N, Lynd LD, De Vera MA. Maternal and neonatal outcomes associated with biologic exposure before and during pregnancy in women with inflammatory systemic diseases: a systematic review and meta-analysis of observational studies. Rheumatology. 2020;59:1808–1817. doi: 10.1093/rheumatology/keaa064. [DOI] [PubMed] [Google Scholar]
- 12.Ghalandari N, Dolhain RJEM, Hazes JMW, et al. The pre- and post-authorisation data published by the European medicines agency on the use of biologics during pregnancy and lactation. Br J Clin Pharmacol. 2020;86(3):580–590. doi: 10.1111/bcp.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewhurst J. Dewhurst's textbook of obstetrics and gynaecology. New York: Wiley; 2012. [Google Scholar]
- 14.Bacino CA. Birth defects: Epidemiology, types, and patterns. Table 2. UpToDate Waltham, MA: UpToDate Inc. https://www.uptodate.com/contents/birth-defects-epidemiology-types-and-patterns?search=congenital%20malformations&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed 27 Feb 2019.
- 15.Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013; https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Assessed July 2019.
- 16.Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. 2013;6(1):50–54. doi: 10.1111/jebm.12018. [DOI] [PubMed] [Google Scholar]
- 17.Jeremy H, Iain CGP. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) table of evidence.Background document. https://www.cebm.net/index.aspx?o=5653.
- 18.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AA. Systematic identification of drugs that cause birth defects—a new opportunity. N Engl J Med. 2003;349(26):2556–2559. doi: 10.1056/NEJMsb031395. [DOI] [PubMed] [Google Scholar]
- 20.Bazzani C, Scrivo R, Andreoli L, Baldissera E, Biggioggero M, Canti V, et al. Prospectively-followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre study. Clin Exp Rheumatol. 2015;33(5):688–693. [PubMed] [Google Scholar]
- 21.Jarosova K, Andelova K, Hejduk K, Uher M, Vencovsky J. Pregnancy outcomes in adult juvenile idiopathic arthritis patients treated with biologic agents. Arthritis Rheumatol. 2015;67:583. [Google Scholar]
- 22.Strangfeld A, Pattloch D, Spilka M, Manger B, Krummel-Lorenz B, Grassler A, et al. Pregnancies in patients with long-standing rheumatoid arthritis and biologic DMARD treatment: course of disease during pregnancy and pregnancy outcomes. Arthritis Rheumatol. 2015;67(suppl 10):3050–3051. [Google Scholar]
- 23.Yoshida T, Akiyama Y, Katsuyama N. Study of 31 pregnancies during rheumatoid arthritis treatment: Treatment course, condition of newborns, and problems. Int J Rheum Dis. 2019;22:221–222. [Google Scholar]
- 24.Allsopp S, Daveson AJ. Pregnancy outcomes in patients with inflammatory bowel disease in a regional clinic. J Gastroenterol Hepatol. 2015;30:124. [Google Scholar]
- 25.Bernardes C, Loureiro R, Carvalho D, Borges V, Russo P, Saiote J, et al. Anti-tumour necrosis-alpha therapy during pregnancy in patients with inflammatory bowel disease: safety in women and children. J Crohns Colitis. 2017;11:S344. [Google Scholar]
- 26.Burmester GR, Landewé R, Genovese MC, Friedman AW, Pfeifer ND, Varothai NA, et al. Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(2):414–417. doi: 10.1136/annrheumdis-2016-209322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echeverría-García B, Nuño-González A, Dauden E, Vanaclocha F, Torrado R, Belinchón I, et al. A case series of patients with psoriasis exposed to biologic therapy during pregnancy: the BIOBADADERM register and a review of the literature. Actas Dermo Sifiliogr. 2017;108(2):168–170. doi: 10.1016/j.ad.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Esteve-Sole A, Deya-Martinez A, Teixido I, Ricart E, Gompertz M, Torradeflot M, et al. Immunological changes in blood of newborns exposed to anti-TNF-alpha during pregnancy. Front Immunol. 2017;8:1123. doi: 10.3389/fimmu.2017.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujikawa K, Endo Y, Mizokami A, Takahashi K, Tabuchi M, Ohba K, et al. Successful treatment with adalimumab for intestinal Behcet’s disease during pregnancy. Intern Med. 2016;55(10):1375–1378. doi: 10.2169/internalmedicine.55.6590. [DOI] [PubMed] [Google Scholar]
- 30.Iranzo González-Cruz I, Gil Borrás R, Martin Arranz MD, García Corbalán MJ, Jaen RM. The use of adalimumab during pregnancy in Crohn's disease. Enferm Inflamm Intest Dia. 2017;16(3):158–162. [Google Scholar]
- 31.Kammerlander H, Nielsen J, Knudsen T, Kjeldsen J, Friedman S, Norgard BM. Anti-TNF-alpha use during the third trimester of pregnancy in women with moderate-severe inflammatory bowel disease and the risk of preterm birth and low birth weight. Inflamm Bowel Dis. 2017;23(11):1916–1923. doi: 10.1097/MIB.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CD, Johnson DL, Xu R, Luo Y, Adam MP, Braddock SR, et al. Birth outcomes following pregnancy exposure to adalimumab: the OTIS autoimmune diseases in pregnancy project. Pharmacoepidemiol Drug Saf. 2017;26:218–219. [Google Scholar]
- 33.Dall'ara F, Reggia R, Bazzani C, Andreoli L, Agosti M, Mazza G, et al. Safety of anti-TNF alfa agents during pregancy and breastfeeding: longterm follow up of exposed children in a case-series of mothers with chronic arthritides. Ann Rheum Dis. 2016;75:493. [Google Scholar]
- 34.Kiely CJ, Subramaniam K, Platten J, Pavli P. Safe and effective: Anti-tumour necrosis factor therapy use in pregnant patients with Crohn disease and ulcerative colitis. Intern Med J. 2016;46(5):616–619. doi: 10.1111/imj.13057. [DOI] [PubMed] [Google Scholar]
- 35.Kimyon G, Zengin O, Kisacik B, Onat AM. Anti-TNF drugs may result to elevated abortion rates in late pregnancy. Ann Rheum Dis. 2015;74:1276. [Google Scholar]
- 36.Komoto S, Motoya S, Nishiwaki Y, Matsui T, Kunisaki R, Matsuoka K, et al. Pregnancy outcome in women with inflammatory bowel disease treated with anti-tumor necrosis factor and/or thiopurine therapy: a multicenter study from Japan. Intestinal Res. 2016;14(2):139–145. doi: 10.5217/ir.2016.14.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahadevan U, Martin C, Kane SV, Dubinsky M, Sands BE, Sandborn W. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? Results from the PIANO Registry. Gastroenterology. 2016;150(4):S91–S92. [Google Scholar]
- 38.Seow CH, Leung Y, Vande Casteele N, Ehteshami Afshar E, Tanyingoh D, Bindra G, et al. The effects of pregnancy on the pharmacokinetics of infliximab and adalimumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1329–1338. doi: 10.1111/apt.14040. [DOI] [PubMed] [Google Scholar]
- 39.Truta B, Canner J, Efron J, Safar B. The long-term effect of biologics in newborns. J Crohn's Colitis. 2016;10:S234. [Google Scholar]
- 40.Broms G, Granath F, Ekbom A, Hellgren K, Pedersen L, Sorensen HT, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14(2):234. doi: 10.1016/j.cgh.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Tan BE, Lim AL, Kan SL, Lim CH, Tsang EEL, Ch’ng SS, et al. Real-world clinical experience of biological disease modifying anti-rheumatic drugs in Malaysia rheumatoid arthritis patients. Rheumatol Int. 2017;37(10):1719–1725. doi: 10.1007/s00296-017-3772-8. [DOI] [PubMed] [Google Scholar]
- 42.Chaparro M, Verreth A, Lobaton T, Gravito-Soares E, Julsgaard M, Savarino E, et al. Long-term safety of in utero exposure to anti-TNFalpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol. 2018;113(3):396–403. doi: 10.1038/ajg.2017.501. [DOI] [PubMed] [Google Scholar]
- 43.Kanis SL, de Lima A, van der Ent C, Rizopoulos D, van der Woude CJ. Anti-TNF levels in cord blood at birth are associated with anti-TNF type. J Crohns Colitis. 2018;12:939–947. doi: 10.1093/ecco-jcc/jjy058. [DOI] [PubMed] [Google Scholar]
- 44.Tsao NW, Sayre EC, Hanley G, Sadatsafavi M, Lynd LD, Marra CA, et al. Risk of preterm delivery and small-for-gestational-age births in women with autoimmune disease using biologics before or during pregnancy: a population-based cohort study. Ann Rheum Dis. 2018;77(6):869–874. doi: 10.1136/annrheumdis-2018-213023. [DOI] [PubMed] [Google Scholar]
- 45.Smith R, Kilding R, Kuet KP, Fairlie F, Jokhi R, Bonnett T, et al. A review of the outcomes of women with rheumatoid arthritis (RA) treated with biologic agents attending the sheffield combined obstetrics and rheumatology clinic 2002 to 2013. Ann Rheum Dis. 2018;77:1393. [Google Scholar]
- 46.Silva EFDC, Baima JP, De Barros JR, Renosto FL, De Sibia CDF, Saad-Hossne R, et al. Anti-TNF exposure during pregnancy in Crohn's disease patients. Case Rep Gastroenterol. 2018;12:608–616. doi: 10.1159/000493921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pola CS, Carreras ID, Ferrer MB, Carpio D, Martín CA. Outcomes of pregnancies exposed to biological drugs. Basic Clin Pharmacol Toxicol. 2018;123:38–39. [Google Scholar]
- 48.Luu M, Benzenine E, Doret M, et al. Continuous anti-TNFα use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the French National Health Insurance Database (EVASION) Am J Gastroenterol. 2018;113(11):1669–1677. doi: 10.1038/s41395-018-0176-7. [DOI] [PubMed] [Google Scholar]
- 49.Labetoulle R, Roblin X, Paul S. Prolonged persistence of adalimumab transferred from mother to infant during pregnancy. Ann Intern Med. 2018;169(1):60–61. doi: 10.7326/L17-0629. [DOI] [PubMed] [Google Scholar]
- 50.Klein A, Becker I, Minden K, Foeldvari I, Haas JP, Horneff G. Adalimumab versus adalimumab and methotrexate for the treatment of juvenile idiopathic arthritis: long-term data from the German BIKER registry. Scand J Rheumatol. 2019;48(2):95–104. doi: 10.1080/03009742.2018.1488182. [DOI] [PubMed] [Google Scholar]
- 51.Duricova D, Dvorakova E, Hradsky O, et al. Safety of anti-TNF-alpha therapy during pregnancy on long-term outcome of exposed children: a controlled, multicenter observation. Inflamm Bowel Dis. 2019;25(4):789–796. doi: 10.1093/ibd/izy294. [DOI] [PubMed] [Google Scholar]
- 52.Garufi S, Maida M, Fiorentini T, Speciale A, Taci A, Camilleri S, et al. Safety profile of adalimumab therapy in pregnancy: a single center experience. Dig Liver Dis. 2018;50(2):e142–e143. [Google Scholar]
- 53.Berman M, Zisman D, Wollman J, et al. The effect of pregnancy on disease activity in patients with psoriatic arthritis. J Rheumatol. 2018;45(12):1651–1655. doi: 10.3899/jrheum.171218. [DOI] [PubMed] [Google Scholar]
- 54.Andreoli L, Gerardi MC, Bazzani C, Filippini M, Fredi M, Gorla R, Lazzaroni MG, Nalli C, Taglietti M, Lojacono A, Zatti S, Motta M, Tincani A. Long-term outcome of children born to mothers with chronic arthritis and exposed to TNF-inhibitors during pregnancy: a case-control study [abstract]. Arthritis Rheumatol. 2018;70(suppl 10).
- 55.Genest G, Spitzer KA, Laskin CA. Maternal and fetal outcomes in a cohort of patients exposed to tumor necrosis factor inhibitors throughout pregnancy. J Rheumatol. 2018;45(8):1109–1115. doi: 10.3899/jrheum.171152. [DOI] [PubMed] [Google Scholar]
- 56.Bobirca A, Bobirca F, Ancuta I, Mihai C, Tataru C, Comsa C, et al. Pregnancy in rheumatoid arthritis—a Romanian cohort. Ann Rheum Dis. 2017;76:1151–1152. [Google Scholar]
- 57.De Stefani E, Padovan M, Bortoluzzi A, Capucci R, Govoni M. Exposition to biological therapy during pregnancy: a single-centre study of pregnancy outcome in mothers with rheumatic diseases. Ann Rheum Dis. 2017;76:828–829. [Google Scholar]
- 58.Bendaoud S, Nahon S, Gornet JM, Pariente B, Beaugerie L, Abitbol V, et al. Live-vaccines and lactation in newborn exposed in utero to anti-TNF: a multi-centre French experience in inflammatory bowel disease. J Crohn's Colitis. 2018;12:S527. [Google Scholar]
- 59.Mugheddu C, Atzori L, Lappi A, Murgia S, Rongioletti F. Biologics exposure during pregnancy and breastfeeding in a psoriasis patient. Dermatol Ther. 2019;32(3):e12895. doi: 10.1111/dth.12895. [DOI] [PubMed] [Google Scholar]
- 60.Odorici G, Di Lernia V, Bardazzi F, Magnano M, Di Nuzzo S, Cortelazzi C, et al. Psoriasis and pregnancy outcomes in biological therapies: a real-life, multi-center experience. J Eur Acad Dermatol Venereol. 2019;33(10):e374–377. doi: 10.1111/jdv.15671. [DOI] [PubMed] [Google Scholar]
- 61.Julsgaard M, Hvas CL, Gearry RB, Gibson PR, Fallingborg J, Sparrow MP, et al. Anti-TNF therapy in pregnant women with inflammatory bowel disease: effects of therapeutic strategies on disease behavior and birth outcomes. Inflamm Bowel Dis. 2019;26(1):93–102. doi: 10.1093/ibd/izz110. [DOI] [PubMed] [Google Scholar]
- 62.Lee KE, Jung SA, Park SH, Moon CM, Shim SY, Kim ES, et al. Influence of anti-tumor necrosis factor-alpha therapy to pregnant inflammatory bowel disease women and their children's immunity. Intestinal Res. 2019;17(2):237–243. doi: 10.5217/ir.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mills BS, Dao KH, Tecson KM, Fishman E, Tate R, Cush JJ. Perceptions of pregnancy and lactation from the Pregnancy and Lactation Autoimmune Network (PLAN) Registry. J Rheumatol. 2019;47(1):149–154. doi: 10.3899/jrheum.181067. [DOI] [PubMed] [Google Scholar]
- 64.Bennett AL, Mamunes A, Kim M, Garrett A, Duley C, Annis K, et al. The importance of monitoring the postpartum period in moderate to severe Crohn's disease. Gastroenterology. 2019;156(6):S869. doi: 10.1093/ibd/izab104. [DOI] [PubMed] [Google Scholar]
- 65.Flanagan E, Gibson PR, Ross A, Rosella O, Bell SJ. Stability of serum concentrations of infliximab and adalimumab across pregnancy in IBD. J Crohn's Colitis. 2019;13:S449–S450. [Google Scholar]
- 66.Tsao N, Hanley G, Sadatsafavi M, Lynd L, Marra C, De Vera M. Risk of major congenital malformations associated with exposure to biologics before or during pregnancy: a population-based cohort study. J Rheumatol. 2018;45(7):970. [Google Scholar]
- 67.Lund T, Thomsen SF. Use of TNF-inhibitors and ustekinumab for psoriasis during pregnancy: a patient series. Dermatol Ther. 2017;30(3):e12454. doi: 10.1111/dth.12454. [DOI] [PubMed] [Google Scholar]
- 68.Weber-Schoendorfer C, Oppermann M, Wacker E, Bernard N, Beghin D, Cuppers-Maarschalkerweerd B, et al. Pregnancy outcome after TNF-α inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol. 2015;80(4):727–739. doi: 10.1111/bcp.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawai Y, Tsuchiya T, Aoki S. Pregnancy outcomes of patients exposed to adalimumab in Japan. Dig Dis. 2018;37(2):123–130. doi: 10.1159/000493462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venhoff N, Voll RE, Glaser C, Thiel J. IL-1-blockade with Anakinra during pregnancy: retrospective analysis of efficacy and safety in female patients with familial Mediterranean fever (IL-1-Blockade mit Anakinra in der Schwangerschaft: Retrospektive Wirksamkeits- und Sicherheitsanalyse bei Patientinnen mit familiarem Mittelmeerfieber). Z Rheumatol. 2017. [DOI] [PubMed]
- 71.Ilgen U, Kucuksahin O. Anakinra use during pregnancy: report of a case with Familial Mediterranean Fever and infertility. Eur J Rheumatol. 2017;4(1):66–67. doi: 10.5152/eurjrheum.2017.16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Youngstein T, Hoffmann P, Gul A, Lane T, Williams R, Rowczenio DM, et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology. 2017;56(12):2102–2108. doi: 10.1093/rheumatology/kex305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith CJF, Chambers CD. Five successful pregnancies with antenatal anakinra exposure. Rheumatology. 2018;57(7):1271–1275. doi: 10.1093/rheumatology/key093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunn JL, Waller R, O'Neil L, David J. Canakinumab use during pregnancy for the treatment of systemic onset juvenile idiopathic arthritis and refractory macrophage activation syndrome. Rheumatology. 2018;57:iii50. [Google Scholar]
- 75.Forger F, Zbinden A, Villiger PM. Certolizumab treatment during late pregnancy in patients with rheumatic diseases: low drug levels in cord blood but possible risk for maternal infections. A case series of 13 patients. Jt Bone Spine. 2016;83(3):341–343. doi: 10.1016/j.jbspin.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Taleban S, Gundogan F, Chien EK, Degli-Esposti S, Saha S. Placental inflammation is not increased in inflammatory bowel disease. Ann Gastroenterol. 2015;28(4):457–463. [PMC free article] [PubMed] [Google Scholar]
- 77.Beaulieu DB, Ananthakrishnan AN, Martin C, Cohen RD, Kane SV, Mahadevan U. Use of Biologic Therapy by Pregnant Women With Inflammatory Bowel Disease Does Not Affect Infant Response to Vaccines. Clin Gastroenterol Hepatol. 2018;16(1):99–105. doi: 10.1016/j.cgh.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clowse MEB, Scheuerle AE, Chambers C, Afzali A, Kimball AB, Cush JJ, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70(9):1399–1407. doi: 10.1002/art.40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kattah MG, Milush JM, Burt T, McCabe RP, Whang MI, Ma A, et al. Anti-TNF and thiopurine therapy in pregnant IBD patients does not significantly alter a panel of B-cell and T-cell subsets in 1-year-old infants. Clin Transl Gastroenterol. 2018;9(4):143. doi: 10.1038/s41424-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology. 2018;155(3):696–704. doi: 10.1053/j.gastro.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 81.Mariette X, Forger F, Abraham B, Flynn AD, Molto A, Flipo RM, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228–233. doi: 10.1136/annrheumdis-2017-212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clowse ME, Wolf DC, Forger F, Cush JJ, Golembesky A, Shaughnessy L, et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol. 2015;42(12):2270–2278. doi: 10.3899/jrheum.140189. [DOI] [PubMed] [Google Scholar]
- 83.Ferrer Bradley I, Marín I, Maroto N, Mora M, Hinojosa E, López B, et al. Efficacy and safety of certolizumab pegol in Crohn's disease patients with loss of response or intolerance to previous anti-TNF. J Crohn's Colitis. 2016;10:S276. [Google Scholar]
- 84.Meroni M, Ramoni VL, Brucato AL, Limonta M, Ostensen M, Cutolo M. Child outcome after certolizumab pegol exposure in rheumatoid arthritis pregnancies: a case series report. Ann Rheum Dis. 2016;75:711. [Google Scholar]
- 85.Truta B, Canner J, Efron J, Safar B. Life virus vaccines in infants with intrauterine exposure to biologics. J Crohn's Colitis. 2016;10:S221. [Google Scholar]
- 86.Chaparro M, Verreth A, Lobaton T, Gravito-Soares E, Julsgaard M, Savarino E, et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am J Gastroenterol. 2018;113(3):396–403. doi: 10.1038/ajg.2017.501. [DOI] [PubMed] [Google Scholar]
- 87.Shimada H, Kameda T, Kanenishi K, Miyatake N, Nakashima S, Wakiya R, et al. Effect of biologic disease-modifying anti-rheumatic drugs for patients with rheumatoid arthritis who hope to become mothers. Clin Rheumatol. 2019;38(5):1453–1458. doi: 10.1007/s10067-019-04450-3. [DOI] [PubMed] [Google Scholar]
- 88.Kimball A, Clowse M, Wolf DC, Golembesky A, Shaughnessy L, De Cuyper D, et al. Characteristics and outcomes of prospectively reported pregnancies exposed to certolizumab pegol from a safety database. J Am Acad Dermatol. 2016;74(5):31. [Google Scholar]
- 89.Carman WJ, Accortt NA, Anthony MS, Iles J, Enger C. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26(9):1109–1118. doi: 10.1002/pds.4261. [DOI] [PubMed] [Google Scholar]
- 90.Ohyama A, Tsuboi H, Noma H, et al. Associations between maternal clinical features and fetal outcomes in pregnancies of mothers with connective tissue diseases. Mod Rheumatol. 2019;29(2):344–350. doi: 10.1080/14397595.2018.1452352. [DOI] [PubMed] [Google Scholar]
- 91.Onalan G, Tohma YA, Zeyneloglu HB. Effect of etanercept on the success of assisted reproductive technology in patients with endometrioma. Gynecol Obstet Investig. 2018;83(4):358–364. doi: 10.1159/000484895. [DOI] [PubMed] [Google Scholar]
- 92.Adachi A, Komine M, Hirano T, Tsuda H, Karakawa M, Murata S, et al. Case of generalized pustular psoriasis exacerbated during pregnancy, successfully treated with infliximab. J Dermatol. 2016;43(12):1439–1440. doi: 10.1111/1346-8138.13429. [DOI] [PubMed] [Google Scholar]
- 93.Carlucci JG, Halasa N, Buddy Creech C, Dulek DE, Gómez-Duarte OG, Nelson GE, et al. Vertical transmission of histoplasmosis associated with anti-tumor necrosis factor therapy. J Pediatric Infect Dis Soc. 2016;5(2):e9–e12. doi: 10.1093/jpids/piw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Lima A, Zelinkova Z, Van Der Ent C, Steegers EAP, Van Der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut. 2016;65(8):1261–1268. doi: 10.1136/gutjnl-2015-309321. [DOI] [PubMed] [Google Scholar]
- 95.Forgan-Smith KR, Roche JE. Letter: a case supporting the use of rescue infliximab therapy for fulminant ulcerative colitis in pregnancy. Aliment Pharmacol Ther. 2015;41(3):325. doi: 10.1111/apt.13037. [DOI] [PubMed] [Google Scholar]
- 96.Gao J, Liao W, Wang L, Hao J, Wang G. Successful treatment of generalized pustular psoriasis in a pregnant Chinese woman with single-dose infliximab. J Eur Acad Dermatol Venereol. 2016;30:29. [Google Scholar]
- 97.García Lagunar MH, Chica Marchal A, Ferris Villanueva E, Guerrero Bautista R, Gutiérrez Cívicos MDR, Muñoz García I, et al. Use of infliximab in a pregnant woman with ankylosing spondylitis. Eur J Hosp Pharm. 2015;22:A13–A14. [Google Scholar]
- 98.Jamieson C, Morosan M, Cameron M. Crohn’s disease: first diagnosis in pregnancy and management. Obstet Med. 2017;10(2):85–87. doi: 10.1177/1753495X16671231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minami N, Matsuura M, Koshikawa Y, Yamada S, Honzawa Y, Yamamoto S, et al. Maternal and fetal outcomes in pregnant Japanese women with inflammatory bowel disease: our experience with a series of 23 cases. Intest Res. 2017;15(1):90–96. doi: 10.5217/ir.2017.15.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheibani S, Cohen R, Kane S, Dubinsky M, Church JA, Mahadevan U. The effect of maternal peripartum anti-TNFα use on infant immune response. Dig Dis Sci. 2016;61(6):1622–1627. doi: 10.1007/s10620-015-3992-2. [DOI] [PubMed] [Google Scholar]
- 101.Slater J, Phillips J, Guo CY, Clark M, Geldhof A, Gearhart N, et al. Pregnancy outcomes in women exposed to infliximab (innovator) Ann Rheum Dis. 2016;75:496. [Google Scholar]
- 102.Stiegler JD, Lucas CT, Sami N. Pyoderma gangrenosum in pregnancy successfully treated with infliximab and prednisone. JAAD Case Rep. 2017;3(5):387–389. doi: 10.1016/j.jdcr.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carlon DV, Perez EP, Camba AH, Ramos L. Outcomes of pregnancies among women with inflammatory bowel disease: results from a single-center cohort [Poster] J Perinat Med. 2015;43:125. [Google Scholar]
- 104.Gao JM, Ferguson L, Roberts N. Management of psoriasis during pregnancy: two cases of infliximab use and a review of safety evidence of systemic medications. Br J Dermatol. 2018;179:62. [Google Scholar]
- 105.Charre C, Ramière C, Dumortier J, Abravanel F, Lhomme S, Gincul R, et al. Chronic genotype 3 hepatitis E in pregnant woman receiving infliximab and azathioprine. Emerg Infect Dis. 2018;24(5):941–943. doi: 10.3201/eid2405.171845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Oliveira Andrade S, Pignatari JF, Ogawa GMT, De Paula Bellini P, Bonfim LRT, De Miranda Moura Cortês M, et al. Twin pregnancy in patients with Takayasu arteritis: a case report [abstract]. Adv Rheumatol. 2018;58(Supplement 1).
- 107.Kolar M, Bortlik M, Duricova D, Lukas M, Hruba V, Machkova N, et al. Pregnancy outcomes in women with inflammatory bowel disease treated with biosimilar infliximab. Gastroenterol Hepatol. 2018;72(1):20–26. [Google Scholar]
- 108.Lichtenstein GR, Feagan BG, Mahadevan U, Salzberg BA, Langholff W, Morgan GJ, et al. Pregnancy outcomes reported during the 13-year TREAT Registry: a descriptive report. Am J Gastroenterol. 2018;113(11):1678–1688. doi: 10.1038/s41395-018-0202-9. [DOI] [PubMed] [Google Scholar]
- 109.Vestergaard T, Kammerlander H, Brock B, Julsgaard M. Immunoglobulin and infliximab concentrations in dichorionic twins exposed to infliximab in utero. J Crohn's Colitis. 2017;11(9):1152–1153. doi: 10.1093/ecco-jcc/jjx034. [DOI] [PubMed] [Google Scholar]
- 110.Kleinmann JF, Arnaud L, Sauleau E, Gouyette N, Tajima R, Sibilia J. Efficacy and safety of infliximab originator in patients with Takayasu arteritis within the RTU (temporary recommendation of use) in France. Ann Rheum Dis. 2017;76:611–612. [Google Scholar]
- 111.Truta B, Bayless T, Canner J, Bashar S. Child outcome in IBD pregnancy: early vs late discontinuation of IFX therapy. J Crohn's Colitis. 2019;13:S428. [Google Scholar]
- 112.Grisic AM, Rasmussen M, Ungar B, Ilvemark JF, Huisinga W, Bolstad N, et al. Infliximab clearance is decreased during 2nd and 3rd trimesters of pregnancy in inflammatory bowel disease. Gastroenterology. 2019;156(6):S18. doi: 10.1177/2050640620964619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Birmingham L, Watson K, Kearsley-Fleet L, King Y, Pattinson U, Symmons D, et al. Pregnancy outcomes in patients with rheumatoid arthritis with a history of treatment with rituximab: results from the BSRBR-RA. Rheumatology. 2015;54:i184–i185. [Google Scholar]
- 114.Kaneko K, Sugitani M, Goto M, Murashima A. Tocilizumab and pregnancy: four cases of pregnancy in young women with rheumatoid arthritis refractory to anti-TNF biologics with exposure to tocilizumab. Mod Rheumatol. 2016;26(5):672–675. doi: 10.3109/14397595.2016.1140256. [DOI] [PubMed] [Google Scholar]
- 115.Nakajima K, Watanabe O, Mochizuki M, Nakasone A, Ishizuka N, Murashima A. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod Rheumatol. 2016;26(5):667–671. doi: 10.3109/14397595.2016.1147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weber-Schoendorfer C, Schaefer C. Pregnancy outcome after tocilizumab therapy in early pregnancy—a case series from the German Embryotox Pharmacovigilance Center. Reprod Toxicol. 2016;60:29–32. doi: 10.1016/j.reprotox.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Hoeltzenbein M, Beck E, Rajwanshi R, Gøtestam Skorpen C, Berber E, Schaefer C, et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. 2016;46(2):238–245. doi: 10.1016/j.semarthrit.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 118.Salonga A, Chay J, Nicholls D. A case series of patients with rheumatoid arthritis exposed to tocilizumab during pregnancy. Intern Med J. 2018;48:26–27. [Google Scholar]
- 119.Saito J, Yakuwa N, Kaneko K, Nakajima K, Takai C, Goto M, et al. Clinical application of the dried milk spot method for measuring tocilizumab concentrations in the breast milk of patients with rheumatoid arthritis. Int J Rheum Dis. 2019;22(6):1130–1137. doi: 10.1111/1756-185X.13557. [DOI] [PubMed] [Google Scholar]
- 120.Galluzzo M, D'Adamio S, Bianchi L, Talamonti M. Psoriasis in pregnancy: case series and literature review of data concerning exposure during pregnancy to ustekinumab. J Dermatolog Treat. 2019;30(1):40–44. doi: 10.1080/09546634.2018.1468066. [DOI] [PubMed] [Google Scholar]
- 121.Vu M, Dolianitis C, Braue A, Varigos G, Nicolopoulos J. Outcomes of ustekinumab exposure during pregnancy: a case series of six chronic plaque psoriasis patients and literature review. Australas J Dermatol. 2018;59:5. [Google Scholar]
- 122.Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn's disease: a Canadian tertiary care centre experience. Scand J Gastroenterol. 2017;52(12):1354–1359. doi: 10.1080/00365521.2017.1373847. [DOI] [PubMed] [Google Scholar]
- 123.Rowan CR, Cullen G, Mulcahy HE, Keegan D, Byrne K, Murphy DJ, et al. Ustekinumab drug levels in maternal and cord blood in a woman with crohn's disease treated until 33 weeks of gestation. J Crohns Colitis. 2018;12(3):376–378. doi: 10.1093/ecco-jcc/jjx141. [DOI] [PubMed] [Google Scholar]
- 124.Venturin C, Nancey S, Danion P, Uzzan M, Chauvenet M, Bergoin C, et al. Fetal death in utero and miscarriage in a patient with Crohn's disease under therapy with ustekinumab: case-report and review of the literature. BMC Gastroenterol. 2017;17(1):80. doi: 10.1186/s12876-017-0633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cortes X, Borrás-Blasco J, Antequera B, Fernandez-Martinez S, Casterá E, Martin S, et al. Ustekinumab therapy for Crohn’s disease during pregnancy: a case report and review of the literature. J Clin Pharm Ther. 2017;42(2):234–236. doi: 10.1111/jcpt.12492. [DOI] [PubMed] [Google Scholar]
- 126.Fierens H, Baeck M. Psoriasis and pregnancy in the era of biologics. Case report of ustekinumab treatment during pregnancy. Louvain Med. 2016;135(4):231–235. [Google Scholar]
- 127.Rocha K, Piccinin MC, Kalache LF, Reichert-Faria A. Pregnancy during ustekinumab treatment for severe psoriasis. Dermatology. 2015;231(2):103–104. doi: 10.1159/000380880. [DOI] [PubMed] [Google Scholar]
- 128.Alsenaid A, Prinz JC. Inadvertent pregnancy during ustekinumab therapy in a patient with plaque psoriasis and impetigo herpetiformis. J Eur Acad Dermatol Venereol. 2016;30(3):488–490. doi: 10.1111/jdv.12872. [DOI] [PubMed] [Google Scholar]
- 129.Galli-Novak E, Mook SC, Buning J, Schmidt E, Zillikens D, Thaci D, et al. Successful pregnancy outcome under prolonged ustekinumab treatment in a patient with Crohn's disease and paradoxical psoriasis. J Eur Acad Dermatol Venereol. 2016;30(12):e191–e192. doi: 10.1111/jdv.13499. [DOI] [PubMed] [Google Scholar]
- 130.Nedbalová L, Šerclová Z, Lukešová L. Treatment of a pregnant Crohn’s disease patient with ustekinumab: a case report. Gastroenterol Hepatol. 2019;73(1):43–45. [Google Scholar]
- 131.Flanagan E, Gibson PR, Begun J, Ghaly S, Garg M, Andrews JM, et al. Letter: vedolizumab drug concentrations in neonates following intrauterine exposure. Aliment Pharmacol Ther. 2018;48(11–12):1328–1330. doi: 10.1111/apt.15027. [DOI] [PubMed] [Google Scholar]
- 132.Mahadevan U, Vermeire S, Lasch K, Abhyankar B, Bhayat F, Blake A, et al. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(7):941–950. doi: 10.1111/apt.13960. [DOI] [PubMed] [Google Scholar]
- 133.Lahat A, Shitrit AB, Naftali T, Milgrom Y, Elyakim R, Goldin E, et al. Vedolizumab levels in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2018;12(1):120–123. doi: 10.1093/ecco-jcc/jjx120. [DOI] [PubMed] [Google Scholar]
- 134.Moens A, Van Der Woude CJ, Julsgaard M, Sebastian S, Arebi N, Alzinaty M, et al. Outcomes of pregnancy in ibd patietns treated with vedolizumab, anti-tnf or conventional therapy. Gastroenterology. 2019;156(6):S633. [Google Scholar]
- 135.Bar-Gil Shitrit A, Ben Yaʼacov A, Livovsky DM, Cuker T, Farkash R, Hoyda A, et al. Exposure to vedolizumab in IBD pregnant women appears of low risk for mother and neonate: a first prospective comparison study. Am J Gastroenterol. 2019;114(7):1172–1175. doi: 10.14309/ajg.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 136.Julsgaard M, Christensen LA, Gibson PR, Gearry RB, Fallingborg J, Hvas CL, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151(1):110–119. doi: 10.1053/j.gastro.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Esteve-Solé A, Deyà-Martínez À, Teixidó I, Ricart E, Gompertz M, Torradeflot M, et al. Immunological changes in blood of newborns exposed to anti-TNF-α during pregnancy. Front Immunol. 2017;8(SEP):1123. doi: 10.3389/fimmu.2017.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bar-Gil Shitrit A, Koslowsky B, Milgrom Y, Lahat A, Goldin E, Granovsky-Grisaru S. Vedolizumab is safe for use in pregnant patients with IBD; report of our preliminary data. J Crohn's Colitis. 2018;12:S242. [Google Scholar]
- 139.Griffin L, Ramsay B, Hackett C, Ahmad K, Boggs J, Lynch M. Practical experience of secukinumab in the treatment of psoriasis: experience from a single centre. Br J Dermatol. 2018;179:66–67. doi: 10.1007/s11845-020-02336-x. [DOI] [PubMed] [Google Scholar]
- 140.Nardin C, Colas M, Curie V, Pelletier F, Puzenat E, Aubin F. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther. 2018;8(2):323–326. doi: 10.1007/s13555-018-0232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Warren RB, Reich K, Langley RG, Strober B, Gladman D, Deodhar A, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179(5):1205–1207. doi: 10.1111/bjd.16901. [DOI] [PubMed] [Google Scholar]
- 142.Kumthekar A, Danve A, Deodhar A. Use of belimumab throughout 2 consecutive pregnancies in a patient with systemic lupus erythematosus. J Rheumatol. 2017;44(9):1416. doi: 10.3899/jrheum.170327. [DOI] [PubMed] [Google Scholar]
- 143.Bitter H, Bendvold AN, Østensen ME. Lymphocyte changes and vaccination response in a child exposed to belimumab during pregnancy. Ann Rheum Dis. 2018;77(11):1692. doi: 10.1136/annrheumdis-2018-213004. [DOI] [PubMed] [Google Scholar]
- 144.Berman M, Paran D, Wolman Y, Elkayam O. The effect of pregnancy on disease activity outcomes in psoriatic arthritis patients. Arthritis Rheum. 2016;68:3679. [Google Scholar]
- 145.Bröms G, Granath F, Stephansson O, Kieler H. Preterm birth in women with inflammatory bowel disease—the association with disease activity and drug treatment. Scand J Gastroenterol. 2016;51(12):1462–1469. doi: 10.1080/00365521.2016.1208269. [DOI] [PubMed] [Google Scholar]
- 146.Fischer-Betz R, Sander O, Specker C, Brinks R, Schneider M. High risk of flares during pregnancy in women with rheumatoid arthritis who discontinue treatment with TNF-inhibitors at conception. Ann Rheum Dis. 2015;74:715. [Google Scholar]
- 147.Picardo S, So K, Venugopal K. Pregnancy outcomes in inflammatory bowel disease: experiences from a Western Australian tertiary center. J Gastroenterol Hepatol. 2017;32:141–142. [Google Scholar]
- 148.van den Brandt S, Zbinden A, Baeten D, Villiger PM, Østensen M, Förger F. Risk factors for flare and treatment of disease flares during pregnancy in rheumatoid arthritis and axial spondyloarthritis patients. Arthritis Res Ther. 2017;19(1):64. doi: 10.1186/s13075-017-1269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Desai RJ, Bateman BT, Huybrechts KF, Patorno E, Hernandez-Diaz S, Park Y, et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ. 2017;356:j895. doi: 10.1136/bmj.j895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Truta B, Althumairi A, Canner J, Safar B, Efron J. Potential risks of immunosuppressant drugs to the pregnant patient ACG/Radhika Srinivasan gender-based research award. Am J Gastroenterol. 2015;110:S966. [Google Scholar]
- 151.Förger F, Bandoli G, Luo Y, Robinson L, Johnson DL, Chambers CD. No association of discontinuing tumor necrosis factor inhibitors before gestational week twenty in well-controlled rheumatoid arthritis and juvenile idiopathic arthritis with a disease worsening in late pregnancy. Arthritis Rheum. 2019;71(6):901–907. doi: 10.1002/art.40821. [DOI] [PubMed] [Google Scholar]
- 152.Vinet E, Scott S, Moura C, Pineau C, Curtis J, Bernatsky S. Demyelinating disorders in RA offspring exposed to TNF inhibitors. J Rheumatol Conf. 2018;45(7):1057. [Google Scholar]
- 153.Fishman E, Dao KH, Cush JJ. Effects of disease activity and drug exposure on pregnancy outcomes with inflammatory arthritis. Arthritis Rheum. 2016;68:3223–3224. [Google Scholar]
- 154.Kanis SL, De Lima A, Zelinkova Z, Dijkstra G, West R, Ouwendijk RJ, et al. Long-term outcome of children born to IBD mothers preliminary result from a multicenter retrospective study in the Netherlands. Gastroenterology. 2015;148(4):S378. [Google Scholar]
- 155.Dissanayake T, Maksymowych W, Keeling S. Peripartum issues in the inflammatory arthritis (IA) patient: a survey of the rapport registry. J Rheumatol. 2017;44(6):926–927. doi: 10.1038/s41598-020-60451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roy A, Chambers CD, Martin C, Kane SV, Dubinsky M, Sandborn WJ, et al. Exposure to biologic therapy and childhood development among offspring of women with inflammatory bowel disease: results from the PIANO registry. Gastroenterology. 2017;152(5):S85–S86. [Google Scholar]
- 157.Sato R, Ikuma M, Takagi K, et al. Exposure of drugs for hypertension, diabetes, and autoimmune disease during pregnancy and perinatal outcomes: an investigation of the regulator in Japan. Medicine. 2015;94(1):e386. doi: 10.1097/MD.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ursin K, Lydersen S, Skomsvoll JF, Wallenius M. Psoriatic Arthritis Disease Activity During and After Pregnancy: A Prospective Multicenter Study. Arthritis Care Res (Hoboken). 2019;71(8):1092–1100. doi: 10.1002/acr.23747. [DOI] [PubMed] [Google Scholar]
- 159.Meng X, Dunsmore G, Koleva P, et al. The profile of human milk metabolome, cytokines, and antibodies in inflammatory bowel diseases versus healthy mothers, and potential impact on the newborn. J Crohns Colitis. 2019;13(4):431–441. doi: 10.1093/ecco-jcc/jjy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vinet É, De Moura C, Pineau CA, Abrahamowicz M, Curtis JR, Bernatsky S. Serious infections in rheumatoid arthritis offspring exposed to tumor necrosis factor inhibitors: a cohort study. Arthritis Rheum. 2018;70(10):1565–1571. doi: 10.1002/art.40536. [DOI] [PubMed] [Google Scholar]
- 161.Berard A, Sheehy O, Girard S, Zhao JP, Bernatsky S. Risk of preterm birth following late pregnancy exposure to NSAIDs or COX-2 inhibitors. Pain. 2018;159(5):948–955. doi: 10.1097/j.pain.0000000000001163. [DOI] [PubMed] [Google Scholar]
- 162.Soares E, Gravito-Soares M, Mendes S, Ferreira M, Portela F, Sofia C. Biological therapy and pregnancy in inflamatory bowel disease: the long-term safety of in Utero exposure in a terciary center. United Eur Gastroenterol J. 2016;4(5):A641. [Google Scholar]
- 163.Takei H, Kaneko Y, Takeuchi T. Pregnancy outcome and its relevant factors in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 2017;76:1227. [Google Scholar]
- 164.Bond S, Young L, Poulsen K, Barrett H, Taylor A, Barrett C. Vaccination decisions and incidence of neonatal infections in mothers exposed to biological during pregnancy. Intern Med J. 2018;48:5–6. [Google Scholar]
- 165.Chambers C, Jones KL, Johnson DL, Xu R, Luo Y. No increase in serious or opportunistic infections in infants prenatally exposed to biologics. Pharmacoepidemiol Drug Saf. 2018;27:236–237. [Google Scholar]
- 166.Azulay-Abulafia L, Felix PAO, Souza C, Carvalho A, Levy R, Valenzuela F, et al. Immunobiologics in pregnancy: case reports from Latin America. J Eur Acad Dermatol Venereol. 2019;33:18–19. [Google Scholar]
- 167.Wang S, Pena-Sanchez JN, Winquist B, Li W, Jones JL, Fowler SA. Characterization of women with inflammatory bowel disease and exposure to biologics or immunomodulators during pregnancy. Gastroenterology. 2019;156(6):S406. [Google Scholar]
- 168.(EMA) EMA. Committee for medicinal products for human use (CHMP) Guideline on risk assessment of medicinal products on human reproduction and lactation: from data to labelling. 2008. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-assessment-medicinal-products-human-reproduction-lactation-data-labelling_en.pdf. Accessed Jan 2020.
- 169.Strom BL, Kimmel SE, Hennessy S. Pharmacoepidemiology. Hoboken, NJ: Wiley-Blackwell; 2020. Available from: 10.1002/9781119413431. https://onlinelibrary.wiley.com/book/10.1002/9781119413431. Accessed Mar 2020.
- 170.Egbe A, Uppu S, Lee S, Stroustrup A, Ho D, Srivastava S. Congenital malformations in the newborn population: a population study and analysis of the effect of sex and prematurity. Pediatr Neonatol. 2015;56(1):25–30. doi: 10.1016/j.pedneo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 171.Palmsten K, Bandoli G, Vazquez-Benitez G, Xi M, Johnson DL, Xu R, et al. Oral corticosteroid use during pregnancy and risk of preterm birth. Rheumatology. 2019;59(6):1262–1271. doi: 10.1093/rheumatology/kez405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28(6):399–415. doi: 10.1016/j.tem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Man YA, Bakker-Jonges LE, Goorbergh CM, Tillemans SP, Hooijkaas H, Hazes JM, et al. Women with rheumatoid arthritis negative for anti-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Ann Rheum Dis. 2010;69(2):420–423. doi: 10.1136/ard.2008.104331. [DOI] [PubMed] [Google Scholar]
- 174.Mahadevan U, Wolf DC, Dubinsky M, Cortot A, Lee SD, Siegel CA, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(3):286–e24. doi: 10.1016/j.cgh.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.