Abstract
Purpose
This analysis evaluated the relationship between treatment-free interval (TFI, in PALOMA-2)/disease-free interval (DFI, in PALOMA-3) and progression-free survival (PFS) and overall survival (OS, in PALOMA-3), treatment effect in patients with bone-only disease, and whether intrinsic subtype affects PFS in patients receiving palbociclib.
Methods
Data were from phase 3, randomized PALOMA-2 and PALOMA-3 clinical studies of hormone receptor‒positive/human epidermal growth factor receptor 2‒negative (HR+ /HER2−) advanced breast cancer (ABC) patients receiving endocrine therapy plus palbociclib or placebo. Subpopulation treatment effect pattern plot (STEPP) analysis evaluated the association between DFI and PFS and OS. PFS by luminal subtype and cyclin-dependent kinase (CDK) 4/6 or endocrine pathway gene expression levels were evaluated in patients with bone-only disease; median PFS and OS were estimated by the Kaplan–Meier method.
Results
Median durations of TFI were 37.1 and 30.9 months (PALOMA-2) and DFI were 49.2 and 52.0 months (PALOMA-3) in the palbociclib and placebo groups, respectively. Among the PALOMA-2 biomarker population (n = 454), 23% had bone-only disease; median PFS was longer with palbociclib versus placebo (31.3 vs 11.2 months; hazard ratio, 0.41; 95% CI 0.25‒0.69). The interaction effect of bone-only versus visceral disease subgroups on median PFS with palbociclib was not significant (P = 0.262). Among the PALOMA-3 biomarker population (n = 302), 27% had bone-only disease. STEPP analyses showed that palbociclib PFS benefit was not affected by DFI, and that palbociclib OS effect may be smaller in patients with short DFIs. Among patients who provided metastatic tumor tissues (n = 142), regardless of luminal A (hazard ratio, 0.23; 95% CI 0.11‒0.47; P = 0.0000158) or luminal B (hazard ratio, 0.26; 95% CI 0.12‒0.56; P = 0.000269) subtype, palbociclib improved PFS versus placebo.
Conclusions
These findings support palbociclib plus endocrine therapy as standard of care for HR+ /HER2− ABC patients, regardless of baseline TFI/DFI or intrinsic molecular subtype, including patients with bone-only disease.
Trial registration
Pfizer (clinicaltrials.gov:NCT01740427, NCT01942135).
Keywords: Bone-only disease, Disease-free interval, Intrinsic subtype, Palbociclib, Treatment-free interval
Background
Selecting the optimal treatment approach in patients with advanced breast cancer (ABC) is challenging because of the lack of valid predictors of long-term survival [1]. Many patients with hormone receptor‒positive/human epidermal growth factor receptor 2‒negative (HR+ /HER2−) ABC respond to endocrine therapy (ET), a mainstay treatment for these cancers [2, 3]. Both biomarkers and clinical parameters have been evaluated as potential predictors of ET benefit [4]. Estrogen and progesterone receptor and HER2 status are still the most important biomarkers predictive of benefit from ET [5–7]. More recently, various intrinsic molecular subtypes of breast cancer have been characterized (luminal A, luminal B, HER2-enriched, basal-like, normal-like, among others) to assess potential differences in patient prognosis and treatment response [8, 9]. Luminal molecular subtype tumors comprise the majority of breast cancers (83%) [10] and are associated with substantially better outcomes than other molecular subtypes [8, 11]. Additionally, luminal tumors are associated with longer disease-free intervals (DFIs), a predictor of better prognosis in patients with de novo and recurrent ABC [11, 12].
With regard to clinical parameters for patients with HR+ ABC, bone-only metastases are usually associated with a prolonged natural history compared with those with visceral disease [13], and these patients are frequently considered candidates for single-agent ET [14]. Patients with HR+/HER2− ABC presenting with visceral metastases typically have a worse prognosis than patients without visceral metastases [15] and are often initially treated with chemotherapy in spite of their hormonal receptor status [14] and regardless of guidelines suggesting the importance of endocrine-based therapy for this group of patients [3]. In addition, patients with de novo metastatic disease or patients with a longer DFI have a better prognosis than those with a shorter DFI or recurrent metastatic breast cancer [16, 17]. Nevertheless, many patients receiving systemic therapies, including ET or chemotherapy, relapse or eventually develop resistance [18, 19].
Palbociclib is a first-in-class cyclin-dependent kinase (CDK) 4/6 inhibitor that blocks cell cycle progression from G1 to S phase and has shown synergistic activity with antiestrogens [20, 21]. In the PALOMA-2 and PALOMA-3 clinical trials, palbociclib plus ET significantly improved progression-free survival (PFS) versus ET alone in patients with HR+ /HER2− ABC [22, 23]. In PALOMA-2, median PFS was 27.6 months with palbociclib plus letrozole as first-line ABC therapy versus 14.5 months with placebo plus letrozole (hazard ratio, 0.56; P < 0.0001; data cutoff, May 31, 2017) [24]. In PALOMA-3, median PFS was 11.2 months with palbociclib plus fulvestrant versus 4.6 months with placebo plus fulvestrant in patients with ABC whose disease had progressed following ET (hazard ratio, 0.50; P < 0.0001; data cutoff, October 23, 2015) [25]. Additionally, median overall survival (OS) was 34.9 months with palbociclib plus fulvestrant compared with 28.0 months with placebo plus fulvestrant (hazard ratio, 0.81; P = 0.09; data cutoff, April 13, 2018) [26].
Identifying subgroups of patients and intrinsic molecular subtypes of breast cancer that are sensitive to or resistant to palbociclib treatment is important to optimize patients’ therapy selection. It is also important to characterize subgroups of patients who benefit from ET alone and might not require combination treatment options. This analysis evaluated the relationship between initial treatment-free interval (TFI, in PALOMA-2) or DFI (in PALOMA-3) and PFS and OS outcomes (in PALOMA-3). A biomarker analysis of CDK4/6 and endocrine pathways was performed to examine palbociclib treatment effect in patients with bone-only versus visceral disease. Additionally, the effect of luminal subtypes of breast cancer on palbociclib treatment benefit was assessed.
Methods
Study design and patients
This analysis included data from the phase 3, double-blind, placebo-controlled, randomized PALOMA-2 (NCT01740427) and PALOMA-3 (NCT01942135) clinical studies [22, 23]. PALOMA-2 included postmenopausal women (n = 666) with previously untreated estrogen receptor‒positive/HER2‒ ABC who were randomized 2:1 to receive palbociclib (125 mg/day, 3 weeks on/1 week off schedule) plus letrozole (2.5 mg/day) or placebo plus letrozole [23]. In PALOMA-3, women (n = 521) of any menopausal status with HR+/HER2− ABC whose disease had progressed after previous ET were randomized 2:1 to receive palbociclib (125 mg/day, 3/1 schedule) plus fulvestrant (500 mg on days 1 and 15 of cycle 1 and on day 1 of each subsequent cycle) or placebo plus fulvestrant [22]. Microarray data from the PALOMA-2 and PALOMA-3 studies were previously deposited in Gene Expression Omnibus (accession numbers GSE133394 and GSE128500, respectively); 568 baseline tumor tissues from either primary or metastatic biopsies were collected from patients in PALOMA-2 [27] and 462 tumor samples were collected, including 302 evaluable samples (53% archival primary samples and 47% metastatic biopsy samples), from PALOMA-3 [28].
In the PALOMA-2 study, visceral metastases referred to any lung (including pleura) or liver involvement. Bone-only disease was defined as bone lesions confirmed by computed tomography (CT), magnetic resonance imaging (MRI), or bone X-ray, and nonvisceral disease was defined as the absence of lung (including pleura) or liver involvement with the exclusion of any patient with bone-only disease. In the PALOMA-3 study, visceral metastases referred to lung, liver, brain, pleural, and peritoneal involvement. Bone-only disease was defined as lytic or mixed lytic-blastic lesions that could be accurately assessed by CT or MRI, and nonvisceral disease was defined as absence of lung, liver, brain, pleural, or peritoneal involvement with the exclusion of any patient with bone-only disease.
Analysis populations
The intent-to-treat (ITT) population included all patients who were randomized, with study treatment assignment designated according to initial randomization. The biomarker population in PALOMA-2 was defined as a subset of the safety population who had a baseline value for ≥ 1 biomarker. In PALOMA-3, the biomarker population comprised a subset of the safety population who had both baseline and ≥ 1 follow-up value for ≥ 1 biomarker. An analysis of PFS by luminal subtype was also performed among patients from PALOMA-3 who had provided metastatic disease tumor tissues. The TFI analysis was performed in patients who received adjuvant therapy in PALOMA-2 and the DFI and subpopulation treatment effect pattern plot (STEPP) analyses were performed in patients who received adjuvant therapy in PALOMA-3. STEPP analyses of TFI data have been previously published [24].
Treatment-free interval/disease-free interval analysis
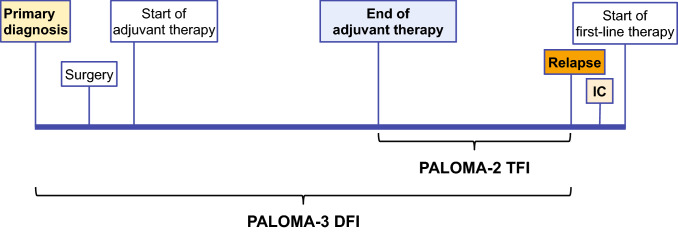
Treatment-free interval was defined as the time between the end of any (neo)adjuvant therapy and relapse, and DFI was defined as the time between the first diagnosis of breast cancer and disease recurrence (Fig. 1). An analysis based on DFI versus TFI was performed for PALOMA-3 to correspond with the baseline DFI patient characteristics presented in the initial PALOMA-3 primary manuscript [29].
Fig. 1.
TFI and DFI definitions. DFI disease-free interval, IC informed consent, TFI treatment-free interval
The exploratory STEPP [30] is a statistical method to explore treatment by covariate interactions from two treatment arms. The method is based on constructing overlapping subpopulations of patients with respect to a covariate of interest and observing the pattern of the treatment effects estimated across subpopulations. STEPP analyses were conducted in the ITT population using data from patients who received (neo)adjuvant therapy to evaluate the effect of DFI in PALOMA-3 assessed as continuous variables on PFS and OS outcomes. STEPP analyses evaluating PFS effect in patients with visceral versus nonvisceral (excluding bone-only) metastases were also conducted.
Luminal subtype analyses
In PALOMA-2, patients provided freshly biopsied formalin-fixed paraffin-embedded (FFPE) tissue from metastatic or recurrent tumor lesions whenever possible; however if a tissue sample was unavailable, the study investigator could recommend a de novo fresh biopsy [27, 31]. In PALOMA-3, all patients provided FFPE tissue from metastatic disease, except for patients with bone-only disease or relapse while on adjuvant therapy and who had surgery within 3 years who could provide archival primary tissue [28].
Luminal subtypes were determined by gene expression profiles from FFPE tumor tissue [27, 28, 31]. The EdgeSeq Oncology Biomarker Panel (HTG Molecular Diagnostics; Tucson, AZ, USA) was used for gene expression profiling [27, 28]. The intrinsic molecular subtypes were determined using the absolute intrinsic molecular subtyping algorithm through a set of binary rules that compared expression measurements for pairs of genes from each individual patient [27, 28, 31, 32].
The Kaplan–Meier method was used to estimate median PFS by luminal subtype. To assess the effect of luminal subtype in metastatic disease, PFS by luminal subtype only among patients who provided metastatic disease tumor tissues was evaluated in PALOMA-3. Intrinsic subtype analysis using data from PALOMA-2 have been previously reported [27]. Hazard ratios and 95% confidence intervals (CIs) were computed using the Brookmeyer and Crowley method.
Analyses by bone-only, nonvisceral (excluding bone-only), and visceral disease
Median PFS for each treatment arm was estimated and biomarker analyses of CDK4/6 and endocrine pathways were compared in patients with bone-only disease, nonvisceral disease (excluding bone-only), or visceral disease. Cox proportional hazards models were performed to evaluate the interaction between disease site at baseline (bone only, visceral, or nonvisceral that excluded bone only) and treatment effect, as well as the interaction between prespecified baseline gene expression (eg, cyclin-dependent kinase 4 [CDK4], estrogen receptor 1 [ESR1], cyclin-dependent kinase 6 [CDK6], cyclin D1 [CCND1], cyclin D3 [CCND3], cyclin E1 [CCNE1]) and treatment effect in patients with bone-only, nonvisceral, and visceral disease, respectively; in the biomarker population, the model included interaction terms of treatment by gene expression with main effect terms “treatment” and “gene expression.” Gene expression data were quantile normalized and log2 transformed. Interaction P values were reported. No adjustments for multiple comparisons were made because of the exploratory nature of the analyses. Statistical tests were two-sided with P < 0.05 considered significant.
Results
PALOMA-2
In total, 418 patients (62.8%) received adjuvant therapy and were included in the TFI analysis (data cutoff, May 31, 2017; Table 1) and 454 patients were included in the biomarker population. The median duration of TFI was 37.1 months in the palbociclib plus letrozole group and 30.9 months in the placebo plus letrozole group. Overall, 35.4% and 34.0% of patients in the palbociclib and placebo groups, respectively, had a TFI of ≤ 1 year and 32.5% and 32.6% had a TFI of > 5 years, respectively. Previously published STEPP analyses indicated that TFI did not affect palbociclib treatment outcomes (PFS) in the overall population or in patients with visceral or nonvisceral metastases who received adjuvant therapy [24].
Table 1.
Summary of baseline TFI/DFI
| PALOMA-2 | Palbociclib + letrozole
(n = 277) |
Placebo + letrozole (n = 141) |
|---|---|---|
| TFI, n (%) | ||
| ≤ 1 year | 98 (35.4) | 48 (34.0) |
| > 1‒2 years | 25 (9.0) | 16 (11.3) |
| > 2‒5 years | 64 (23.1) | 31 (22.0) |
| > 5 years | 90 (32.5) | 46 (32.6) |
| Duration of TFI, months | ||
| Mean (SD) | 50.2 (59.9) | 55.6 (69.9) |
| Median (range) | 37.1 (− 10.7 to 337.5) | 30.9 (− 3.6 to 332.7) |
| PALOMA-3 | Palbociclib + fulvestrant (n = 232) |
Placebo + fulvestrant (n = 123) |
|---|---|---|
| DFI, n (%) | ||
| ≤ 1 year | 10 (4.3) | 3 (2.4) |
| > 1‒2 years | 30 (12.9) | 19 (15.4) |
| > 2‒5 years | 101 (43.5) | 49 (39.8) |
| > 5‒10 years | 53 (22.8) | 34 (27.6) |
| > 10 years | 37 (15.9) | 18 (14.6) |
| Duration of DFI, months | ||
| Mean (SD) | 65.9 (54.0) | 69.6 (57.4) |
| Median (range) | 49.2 (0.03‒277.9) | 52.0 (2.8‒326.4) |
TFI was defined as the time between the end of any (neo)adjuvant therapy and relapse in PALOMA-2, and DFI was defined as the time between the first diagnosis of breast cancer and disease recurrence in PALOMA-3
DFI disease-free interval, TFI treatment-free interval
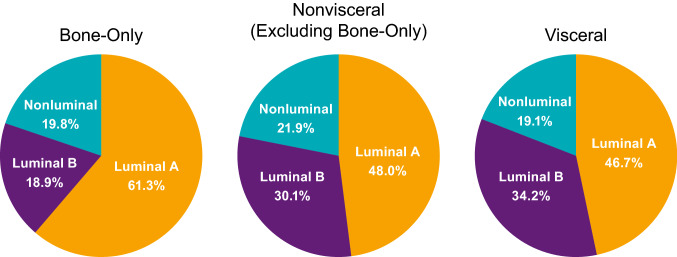
Of the 454 patients included in the biomarker population, 303 received palbociclib plus letrozole and 151 received placebo plus letrozole. Most patients with bone-only disease in the biomarker population had luminal A tumors (61.3%), 18.9% had luminal B tumors, and 19.8% had nonluminal tumors (Fig. 2). Luminal A disease was also more common than luminal B and nonluminal disease in patients with visceral disease.
Fig. 2.
PALOMA-2: breast cancer subtype distribution by bone-only, nonvisceral (excluding bone-only), and visceral diseasea. aBiomarker population; n = 454
In the ITT population, bone-only disease was reported in 23% (n = 150) of patients. Median PFS was prolonged with palbociclib plus letrozole compared with placebo plus letrozole in patients with bone-only, visceral disease, and nonvisceral (excluding bone-only) disease, respectively, but the interaction effect was not statistically significant (P = 0.240; Table 2). In the biomarker population, the improvement in median PFS with the addition of palbociclib to letrozole appeared to be more profound in patients with bone-only disease compared with those with visceral disease or nonvisceral disease, but the interaction effect was not statistically significant (P = 0.262).
Table 2.
Median PFS in patients with bone-only, nonvisceral (excluding bone-only), and visceral disease
| PALOMA-2 | PALOMA-3 | |||||
|---|---|---|---|---|---|---|
| Palbociclib + letrozole | Placebo + letrozole | Interaction P value | Palbociclib + fulvestrant | Placebo + fulvestrant | Interaction P value | |
| ITT population | ||||||
| Bone-only, n | 102 | 48 | 0.240 | 86 | 38 | 0.471 |
| Median PFS (95% CI), months | 36.2 (27.6–NE) | 11.2 (8.2–22.0) | 14.3 (11.2–NE) | 9.2 (4.8–20.0) | ||
| Hazard ratio (95% CI) | 0.40 (0.26–0.62) | 0.40 (0.26–0.62) | 0.64 (0.38–1.06) | 0.64 (0.38–1.06) | ||
| Nonvisceral (excluding bone-only), n | 128 | 64 | 61 | 32 | ||
| Median PFS (95% CI), months | 33.4 (27.6–NE) | 23.5 (13.8–30.6) | 16.6 (11.1–NE) | 5.6 (3.5–9.3) | ||
| Hazard ratio (95% CI) | 0.60 (0.40–0.89) | 0.60 (0.40–0.89) | 0.40 (0.23–0.73) | 0.40 (0.23–0.73) | ||
| Visceral, n | 214 | 110 | 200 | 104 | ||
| Median PFS (95% CI), months | 19.3 (16.4–24.2) | 12.3 (8.4–16.4) | 9.2 (7.5–11.1) | 3.4 (1.9–5.1) | ||
| Hazard ratio (95% CI) | 0.61 (0.46–0.80) | 0.61 (0.46–0.80) | 0.47 (0.36–0.62) | 0.47 (0.36–0.62) | ||
| Biomarker population | ||||||
| Bone-only, n | 72 | 34 | 0.262 | 54 | 27 | 0.363 |
| Median PFS (95% CI), months | 31.3 (23.9–NE) | 11.2 (5.5–22.0) | 16.6 (11.2–NE) | 11.2 (4.8–20.0) | ||
| Hazard ratio (95% CI) | 0.41 (0.25–0.69) | 0.41 (0.25–0.69) | 0.79 (0.41–1.60) | 0.79 (0.41–1.60) | ||
| Nonvisceral (excluding bone-only), n | 83 | 40 | 32 | 21 | ||
| Median PFS (95% CI), months | 27.7 (21.9–NE) | 21.9 (13.8–30.6) | NE (9.1–NE) | 5.5 (1.9–NE) | ||
| Hazard ratio (95% CI) | 0.63 (0.39–1.03) | 0.63 (0.39–1.03) | 0.44 (0.20–0.96) | 0.44 (0.20–0.96) | ||
| Visceral, n | 148 | 77 | 108 | 60 | ||
| Median PFS (95% CI), months | 19.2 (14.0–24.2) | 11.3 (8.3–16.6) | 9.5 (7.5–12.1) | 2.2 (1.9–4.2) | ||
| Hazard ratio (95% CI) | 0.67 (0.49–0.93) | 0.67 (0.49–0.93) | 0.46 (0.32–0.67) | 0.46 (0.32–0.67) | ||
CI confidence interval, ITT intent-to-treat, NE not estimable, PFS progression-free survival
In the biomarker population, mRNA levels at baseline of CDK4, ESR1, CDK6, CCND1, CCND3, and CCNE1 were similar between patients with bone-only disease and visceral disease (Table 3). In patients with visceral disease, CDK4 gene expression was associated with letrozole resistance (P = 0.010; Table 3). No statistically significant treatment interactions were observed for any other gene in any of the patient subgroups.
Table 3.
Gene expression levels at baseline and treatment effect interaction
| PALOMA-2 | PALOMA-3 | |||||
|---|---|---|---|---|---|---|
| Bone-only (n = 106) |
Nonvisceral (excluding bone-only) (n = 123) |
Visceral (n = 225) |
Bone-only (n = 81) |
Nonvisceral (excluding bone-only) (n = 53) |
Visceral (n = 168) |
|
| Baseline gene mRNA expression levels, median (minimum, maximum) | ||||||
| CDK4 | 10.3 (8.6, 11.2) | 10.2 (8.5, 11.4) | 10.4 (8.3, 11.9) | 11.3 (9.4, 14.2) | 11.4 (10.1, 12.9) | 11.3 (9.7, 13.2) |
| ESR1 | 12.7 (8.6, 16.0) | 13.4 (8.7, 16.0) | 13.1 (7.5, 16.7) | 13.2 (8.8, 16.5) | 12.8 (9.3, 17.3) | 13.4 (8.6, 16.5) |
| CDK6 | 8.7 (6.3, 10.6) | 8.7 (4.2, 10.6) | 8.7 (3.9, 10.5) | 9.9 (8.7, 12.7) | 9.9 (8.8, 12.2) | 9.9 (8.1, 12.1) |
| CCND1 | 12.4 (8.8, 16.7) | 12.7 (8.5, 16.7) | 12.6 (8.6, 16.7) | 13.0 (9.1, 16.5) | 12.9 (8.4, 17.3) | 12.9 (9.0, 17.3) |
| CCND3 | 10.1 (8.2, 11.6) | 10.1 (8.3, 12.2) | 10.1 (8.2, 11.6) | 10.6 (8.1, 12.5) | 10.4 (9.0, 12.0) | 10.3 (8.6, 12.2) |
| CCNE1 | 6.7 (3.7, 9.3) | 6.8 (2.2, 8.7) | 7.0 (3.2, 11.5) | 6.6 (1.9, 9.6) | 7.2 (4.1, 9.7) | 6.9 (1.9, 9.9) |
| Treatment interaction, P value | ||||||
| CDK4 | 0.601 | 0.518 | 0.010 | 0.161 | 0.223 | 0.735 |
| ESR1 | 0.745 | 0.079 | 0.643 | 0.468 | 0.530 | 0.287 |
| CDK6 | 0.718 | 0.526 | 0.338 | 0.008 | 0.818 | 0.654 |
| CCND1 | 0.493 | 0.562 | 0.823 | 0.479 | 0.555 | 0.311 |
| CCND3 | 0.946 | 0.153 | 0.626 | 0.101 | 0.341 | 0.239 |
| CCNE1 | 0.891 | 0.578 | 0.196 | 0.114 | 0.036 | 0.004 |
CCND1 cyclin D1, CCND3 cyclin D3, CCNE1 cyclin E1, CDK4 cyclin-dependent kinase 4, CDK6 cyclin-dependent kinase 6, ESR1 estrogen receptor 1
PALOMA-3
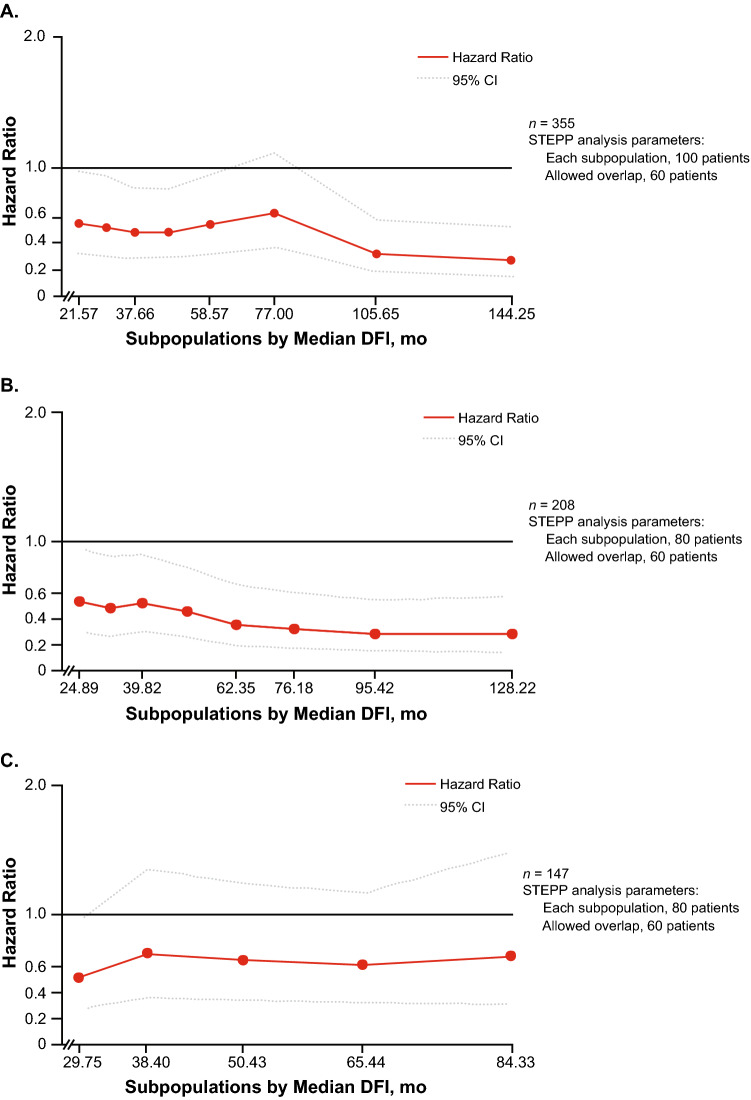
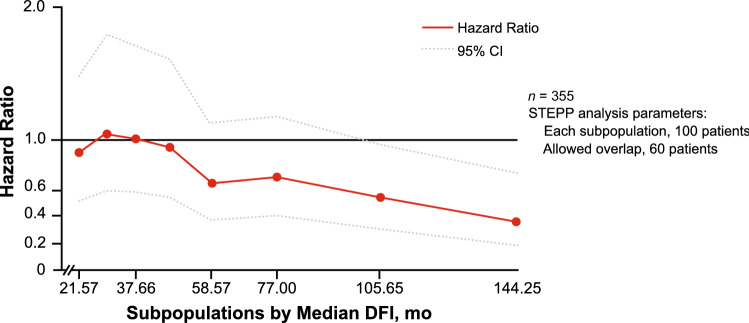
A total of 355 patients (68.1%) received previous (neo)adjuvant therapy and were included in the DFI analysis (data cutoff, October 23, 2015 for PFS and May 24, 2018 for OS; Table 1) and a total of 302 patients were included in the biomarker population. The median duration of DFI was similar between the palbociclib plus fulvestrant group (49.2 months) and the placebo plus fulvestrant group (52.0 months). Most patients in either treatment arm had a DFI of > 2 years. STEPP analyses of the PFS treatment effect of palbociclib indicated that PFS benefit was not affected by DFI in all patients who had received adjuvant therapy, regardless of whether they had visceral or nonvisceral disease (Fig. 3). In contrast, STEPP analyses of the OS treatment effect of palbociclib suggested that the OS benefit may be smaller in patients with short DFIs (Fig. 4).
Fig. 3.
PALOMA-3: STEPP Analyses Evaluating PFS Effect. a Patients who received adjuvant therapy, b patients who received adjuvant therapy and had visceral metastases, and c patients who received adjuvant therapy and had nonvisceral metastasesa. CI confidence interval, DFI disease-free interval, PFS progression-free survival, STEPP subpopulation treatment effect pattern plot. aDFI was defined as the time between the first diagnosis of breast cancer and disease recurrence in PALOMA-3
Fig. 4.
PALOMA-3: STEPP analyses evaluating OS effect in patients who received adjuvant therapya. CI confidence interval, DFI disease-free interval, OS overall survival, STEPP subpopulation treatment effect pattern plot. aDFI was defined as the time between the first diagnosis of breast cancer and disease recurrence in PALOMA-3
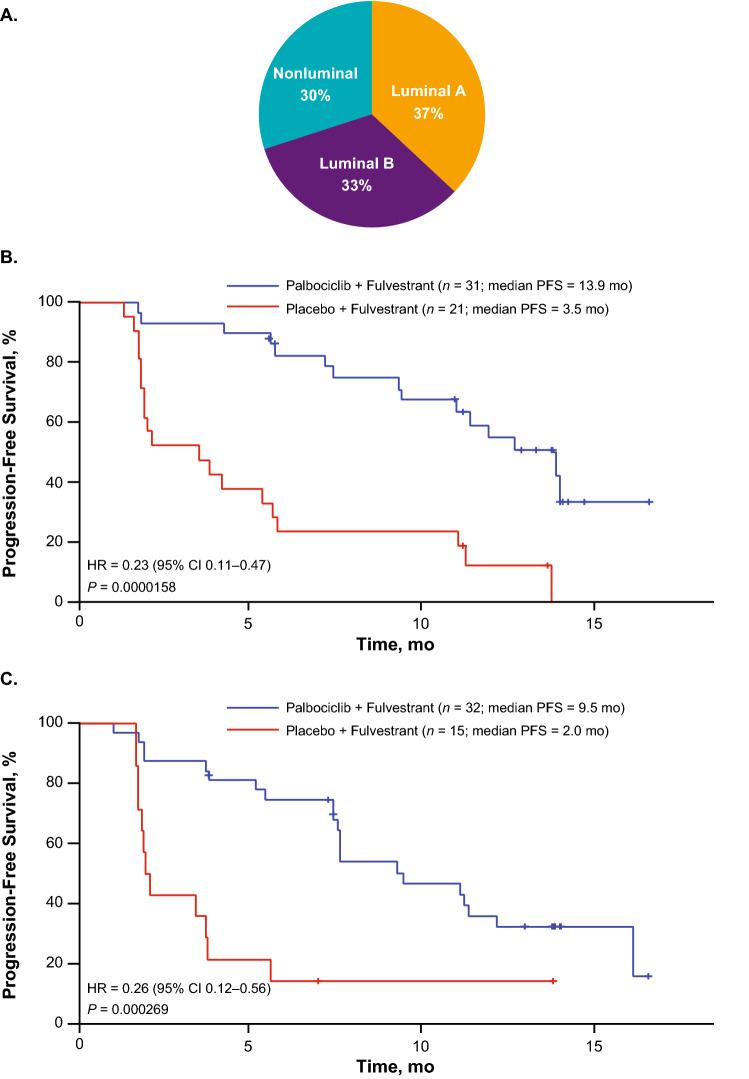
Of the 462 tumor samples analyzed (from 521 patients), 142 (47%) were metastatic biopsy samples. Among the patients who provided metastatic disease tumor tissues, 37% were luminal A subtype, 33% were luminal B subtype, and 30% had nonluminal tumor type (Fig. 5a). Among patients who provided metastatic disease tumor tissues, palbociclib plus fulvestrant improved PFS compared with placebo plus fulvestrant, regardless of luminal A or luminal B tumor subtype (Fig. 5b, c). In patients with luminal A tumors, the median PFS was 13.9 months in the palbociclib plus fulvestrant group versus 3.5 months in the placebo plus fulvestrant group (hazard ratio, 0.23; 95% CI 0.11‒0.47; P = 0.0000158). Among patients with luminal B tumors, the median PFS was 9.5 months in the palbociclib plus fulvestrant group versus 2.0 months in the placebo plus fulvestrant group (hazard ratio, 0.26; 95% CI 0.12‒0.56; P = 0.000269).
Fig. 5.
PALOMA-3: PFS by Subtype Distribution. a Intrinsic subtype distribution of tumors and PFS by b luminal A or c luminal B subtype among patients who provided metastatic disease tumor tissues (n = 142). CI confidence interval, HR hazard ratio, PFS progression-free survival
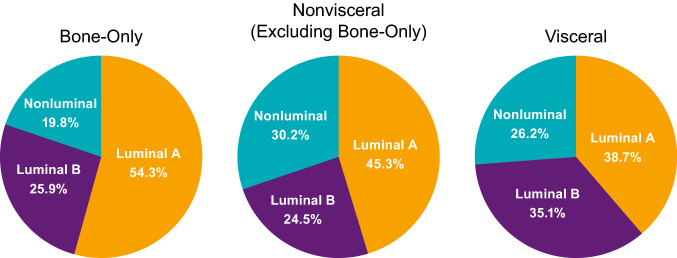
Of the 302 patients included in the biomarker population, 194 patients were included in the palbociclib plus fulvestrant group and 108 in the placebo plus fulvestrant group. Luminal A subtype was more prominent in patients with bone-only disease (54.3% vs 25.9%) and similar in those with visceral disease compared with patients with luminal B subtype (38.7% vs 35.1%; Fig. 6). In patients with bone-only disease, 19.8% had nonluminal tumor subtype; in those with visceral disease, 26.2% had nonluminal tumor types.
Fig. 6.
PALOMA-3: breast cancer subtype distribution by bone-only, nonvisceral (excluding bone-only), and visceral disease (n = 302)
Bone-only disease was reported in 24% (n = 124) of patients in the ITT population. Median PFS was longer in the palbociclib plus fulvestrant group compared with the placebo plus fulvestrant group in patients with bone-only, visceral disease, and nonvisceral (excluding bone-only), respectively (Table 2). In the biomarker population, 27% (n = 81) of patients had bone-only disease. Similar to findings in the ITT population, median PFS was longer with palbociclib plus fulvestrant compared with placebo plus fulvestrant in both the bone-only and visceral subgroups. Median PFS was not reached in the palbociclib plus fulvestrant group in patients with nonvisceral (excluding bone-only) disease. Among patients with bone-only disease, the median PFS was 16.6 months in the palbociclib plus fulvestrant group compared with 11.2 months in the placebo plus fulvestrant group (hazard ratio, 0.79; 95% CI 0.41‒1.60; Table 2).
Baseline mRNA levels of CDK4, ESR1, CDK6, CCND1 CCND3, and CCNE1 were similar between patients with bone-only, nonvisceral (excluding bone-only), and visceral disease in the biomarker population (Table 3). Statistically significant treatment interactions were observed for CDK6 in the bone-only disease subgroup and for CCNE1 in the visceral subgroup (Table 3).
Discussion
The introduction of CDK4/6 inhibitors for the treatment of HR+/HER2− ABC has made a substantial impact on patients’ outcomes [23, 33–35]. The current analyses suggest that palbociclib plus ET improved PFS compared with ET plus placebo in patients who received adjuvant therapy and developed disease recurrence as well as in patients with bone-only disease and visceral disease. The degree of benefit observed was consistent across different lengths of TFI (PALOMA-2) or DFI (PALOMA-3), regardless of whether patients had visceral or nonvisceral metastases [24].
Previous findings have shown that patients with luminal subtype disease benefit from the combination of palbociclib plus letrozole, regardless of luminal A or luminal B subtype [27, 31]. In patients with luminal A tumors, the median PFS was 30.4 months with palbociclib plus letrozole compared with 17.0 months with placebo plus letrozole (hazard ratio, 0.55; 95% CI 0.39‒0.77; P = 0.000547) [27, 31]. Patients with luminal B tumors had a median PFS of 19.6 months in the palbociclib plus letrozole group compared with 11.0 months in the placebo plus letrozole group (hazard ratio, 0.51; 95% CI 0.34‒0.77; P = 0.00109) [27, 31]. Additionally, a previous analysis of patients from the PALOMA-3 study who provided primary or metastatic samples demonstrated that patients with luminal A or luminal B subtypes both benefited from palbociclib plus fulvestrant (luminal A: median PFS was 16.6 months with palbociclib plus fulvestrant versus 4.8 months with placebo plus fulvestrant [hazard ratio, 0.41; 95% CI 0.25‒0.66]; luminal B: median PFS was 9.2 months with palbociclib plus fulvestrant versus 3.5 months with placebo plus fulvestrant [hazard ratio, 0.64; 95% CI 0.38‒1.09]) [28]. Together with present results by luminal subtype in patients who provided metastatic disease tumor tissues and received palbociclib plus fulvestrant, these findings highlight that, despite clear differences in prognosis, the magnitude of palbociclib plus ET effect was similar in luminal A and luminal B tumor subtypes.
The present analysis adds to the current body of literature evaluating clinical subgroups or tumor biomarkers that may identify patients who benefit from palbociclib combination treatment. Patients with ABC who present with visceral metastases generally have a worse prognosis than patients without visceral metastases [15]. However, consistent with the current findings, previous subgroup analyses of patients with and without visceral metastases or with bone-only disease showed significant improvements in median PFS with palbociclib plus ET compared with placebo plus ET [24, 36]. In a previous biomarker analysis of tumor tissues from PALOMA-2, no predictive biomarker was associated with lack of benefit from palbociclib plus letrozole treatment, but higher CDK4 expression was identified as a marker of resistance to treatment with letrozole alone [27, 31]. A similar biomarker analysis of patients in PALOMA-3 showed that palbociclib plus fulvestrant was effective in all biomarker groups assessed, but high CCNE1 mRNA expression was associated with relative resistance to palbociclib plus fulvestrant [28].
Longer DFIs are associated with significantly longer survival rates in patients with breast cancer [16]. STEPP analyses from both the PALOMA-2 and PALOMA-3 studies suggested that the PFS treatment effect of palbociclib was not affected by the length of TFI or DFI in all patients who had received adjuvant therapy, regardless of whether they had visceral or nonvisceral metastases [24]. Similarly, findings from MONALEESA-2, a phase 3, double-blind, randomized study of postmenopausal women with HR+/HER2− ABC, demonstrated that the PFS benefit associated with ribociclib plus letrozole compared with placebo plus letrozole was consistent in patients with a TFI of ≤ 24 months versus > 24 months, ≤ 36 months versus > 36 months, and ≤ 48 months versus > 48 months [37]. In contrast, the MONARCH-3 study, a phase 3, double-blind, randomized study of patients with HR+/HER2− ABC, showed that PFS benefit for abemaciclib plus a nonsteroidal aromatase inhibitor versus placebo plus a nonsteroidal aromatase inhibitor was lower in subgroups of patients with a baseline TFI < 36 months compared with ≥ 36 months [38]. However, the MONARCH-3 study had a shorter median duration of follow-up (17.8 months) [38] than the PALOMA-2 and PALOMA-3 analyses. In contrast to the PFS treatment effect results, the STEPP analyses from PALOMA-3 suggested that the OS treatment effect of palbociclib may be smaller in patients with short DFIs. These findings are consistent with previously published OS results from PALOMA-3 showing that patients with a DFI of > 24 months derived greater benefit from palbociclib plus fulvestrant than patients with a DFI of ≤ 24 months [26].
Regardless of whether patients had luminal A or luminal B subtype tumors, palbociclib plus ET improved PFS compared with placebo plus ET [27, 31]. HR+ status is the common driver in luminal disease [10]. These data support the investigation of palbociclib in early stage HR+ breast cancer, regardless of luminal A or B status. Further research is warranted to evaluate the effect of palbociclib plus ET on nonluminal tumor subtypes as previous findings suggest that ET may be less beneficial in patients with nonluminal breast cancer compared with those with luminal cancers [39].
One of the most common sites for metastatic breast cancer is bone, with bone metastases present in approximately 80% of patients with metastatic breast cancer [40]. Supporting the present findings, a previous retrospective study found that various breast cancer subtypes are associated with different sites of metastases [41]. Compared with the HER2 and triple-negative subtypes, luminal A and luminal B subtypes were significantly associated with bone relapse, with 51% of patients with bone relapse having bone-only metastases [41]. Additionally, a significantly higher proportion of patients with luminal A subtype had bone-only metastases [41]. These preferential differences in metastatic sites may be explained by differentially expressed genes in patients with luminal subtype A or B versus other subtypes that dictate metastatic disease tropisms [41, 42].
In the present analysis of patients with bone-only disease, median PFS was prolonged with palbociclib plus ET compared with placebo plus ET in both PALOMA-2 and PALOMA-3. These findings are consistent with results from a previous analysis of PALOMA-2 and PALOMA-3 data that demonstrated improvement in PFS with palbociclib plus ET versus placebo plus ET in patients with bone-only disease (median PFS, PALOMA-2: NR vs 11.2 months; hazard ratio, 0.36; 95% CI 0.22‒0.59; P < 0.0001; data cutoff, February 26, 2016; PALOMA-3: 14.3 vs 9.2 months; hazard ratio, 0.63; 95% CI 0.38‒1.06; P < 0.05; data cutoff, October 23, 2015) [36].
Conclusions
This analysis reinforces the benefit of palbociclib plus ET as a standard of care for patients with HR+/HER2− ABC, regardless of baseline TFI/DFI or intrinsic molecular subtype at the time of initial diagnosis or at the time of disease recurrence, including patients with bone-only disease, and suggests that according to both the published literature and currently available data, palbociclib plus ET should also be considered as an upfront treatment option in patients with more indolent disease. Additionally, CDK4 gene expression was similar between patients with bone-only, nonvisceral (excluding bone-only), and visceral disease, but may be associated with resistance to single-agent letrozole. Further studies focusing on therapeutic strategies related to palbociclib sensitivity and development of resistance might clarify a more appropriate treatment sequence.
Acknowledgements
Medical writing support was provided by Anny Wu, PharmD, of ICON plc (North Wales, PA, USA), and was funded by Pfizer Inc.
Abbreviations
- ABC
Advanced breast cancer
- CCND1
Cyclin D1
- CCND3
Cyclin D3
- CCNE1
Cyclin E1
- CDK
Cyclin-dependent kinase
- CDK4
Cyclin-dependent kinase 4
- CDK6
Cyclin-dependent kinase 6
- CI
Confidence interval
- CT
Computed tomography
- DFI
Disease-free interval
- ESR1
Estrogen receptor 1
- ET
Endocrine therapy
- FFPE
Formalin-fixed paraffin-embedded
- HER2−
Human epidermal growth factor receptor 2–negative
- HR
Hazard ratio
- HR+
Hormone receptor–positive
- IC
Informed consent
- ITT
Intent-to-treat
- MRI
Magnetic resonance imaging
- LumA
Luminal A
- LumB
Luminal B
- NE
Not estimable
- Non-Lum
Nonluminal
- OS
Overall survival
- PFS
Progression-free survival
- STEPP
Subpopulation treatment effect pattern plot
- TFI
Treatment-free interval
Author contributions
All authors were involved in the design of this analysis. RSF, MC, JE, KAG, MC, CG, EG, YL, CHB, DJS, NCT, and HSR participated in the acquisition, analysis, and interpretation of data. DRL and ZZ were involved in the statistical analysis and interpretation of data. All authors were involved in drafting or revising the manuscript critically for important intellectual content, approving the final version to be published, and agreed to be accountable for all aspects of the work.
Funding
This analysis was sponsored by Pfizer Inc.
Data availability
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Compliance with ethical standards
Conflict of interest
RS Finn has received consulting fees from Pfizer Inc, AstraZeneca, Bayer, Novartis, Bristol-Myers Squibb, Eisai, Eli Lilly, Merck, and Roche, as well as other research funding from Pfizer Inc, and honoraria from Bayer, Pfizer Inc, Bristol-Myers Squibb, Novartis, Eisai, and Eli Lilly. M Cristofanilli has received honoraria from Pfizer Inc and has been a consultant or advisor for Dompé, Agendia, and Vortex. J Ettl has received consulting fees from Eli Lilly, Pfizer Inc, Novartis Pharma KK, Eisai, and Roche; honoraria from AstraZeneca, Pfizer Inc, Eli Lilly, Novartis Pharma KK, and Roche; and travel support from AstraZeneca, Pfizer Inc, Novartis, and Eli Lilly. KA Gelmon has received consulting/advisory fees from Pfizer Inc, Novartis, AstraZeneca, NanoString Technologies, and Merck. M Colleoni has received honoraria from Novartis and has been a consultant or advisor for Pierre Fabre, Pfizer Inc, OBI Pharma, Puma Biotechnology, Celldex, and AstraZeneca. E Gauthier, Y Liu, DR Lu, and Z Zhang are employees of and may own stock in Pfizer Inc. C Giorgetti and C Huang Bartlett are former employees of and may own stock in Pfizer Inc. DJ Slamon has received consulting fees from Pfizer Inc, Eli Lilly, and Novartis; has performed contracted research for Pfizer Inc and Novartis; is a Pfizer Inc stockholder; has received travel accommodation/expenses from Pfizer Inc and BioMarin; and has a leadership role in BioMarin. NC Turner has received honoraria from Pfizer Inc, has been a consultant or advisor for Pfizer Inc, and has received research funding from Pfizer Inc, Eli Lilly, and Novartis. HS Rugo’s institution received research funding from Plexxikon, Macrogenetics, OBI Pharma, Eisai, Pfizer Inc, Novartis, Eli Lilly, Roche, and Merck.
Ethical approval and consent to participate
The PALOMA-2 and PALOMA-3 studies were approved by an institutional review board and/or an independent ethics committee at each participating site. All patients provided written informed consent before study enrollment. The studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Carla Giorgetti and Cynthia Huang Bartlett: at the time of the study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee ES, Jung SY, Kim JY, Kim JJ, Yoo TK, Kim YG, Lee KS, Lee ES, Kim EK, Min JW, Han W, Noh DY, Moon HG. Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann Oncol. 2016;27:828–833. doi: 10.1093/annonc/mdw036. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 3.Rugo H, Rumble B, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield LA, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian J, Muss HB, Burstein HJ. Endocrine therapy for hormone receptor—positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 4.Patani N, Martin LA, Dowsett M. Biomarkers for the clinical management of breast cancer: international perspective. Int J Cancer. 2013;133:1–13. doi: 10.1002/ijc.27997. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, Senkus E, Cardoso F. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM) Eur J Cancer. 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Mosly D, Turnbull A, Sims A, Ward C, Langdon S. Predictive markers of endocrine response in breast cancer. World J Exp Med. 2018;8:1–7. doi: 10.5493/wjem.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 8.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, Díez M, Viladot M, Arance A, Muñoz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 10.American Cancer Society (2017) Breast cancer facts and figures 2017–2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Accessed 4 Sep 2018
- 11.Brouckaert O, Laenen A, Vanderhaegen J, Wildiers H, Leunen K, Amant F, Berteloot P, Smeets A, Paridaens R, Christiaens MR, Floris G, Moerman P, Van Limbergen E, Peeters S, Weltens C, Vergote I, Neven P. Applying the 2011 St Gallen panel of prognostic markers on a large single hospital cohort of consecutively treated primary operable breast cancers. Ann Oncol. 2012;23:2578–2584. doi: 10.1093/annonc/mds062. [DOI] [PubMed] [Google Scholar]
- 12.Yamamura J, Kamigaki S, Fujita J, Osato H, Komoike Y. The difference in prognostic outcomes between de novo stage IV and recurrent metastatic patients with hormone receptor-positive, HER2-negative breast cancer. In Vivo. 2018;32:353–358. doi: 10.21873/invivo.11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Park S, Ahn HK, Yi JH, Cho EY, Sun JM, Lee JE, Nam SJ, Yang J-H, Park YH, Ahn JS, Im Y-H. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat. 2011;43:89–95. doi: 10.4143/crt.2011.43.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25:S131–S141. doi: 10.3747/co.25.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harb WA. Management of patients with hormone receptor-positive breast cancer with visceral disease: challenges and treatment options. Cancer Manag Res. 2015;7:37–46. doi: 10.2147/cmar.s72592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco G, Holli K, Heikkinen M, Kallioniemi OP, Taskinen P. Prognostic factors in recurrent breast cancer: relationships to site of recurrence, disease-free interval, female sex steroid receptors, ploidy and histological malignancy grading. Br J Cancer. 1990;62:142–146. doi: 10.1038/bjc.1990.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ, Peters FP, van Riel JM, Peters NA, de Boer M, Peer PG, Tjan-Heijnen VC. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112:1445–1451. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner NC, Neven P, Loibl S, Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2016;389:2403–2414. doi: 10.1016/S0140-6736(16)32419-9. [DOI] [PubMed] [Google Scholar]
- 19.Rivera E, Gomez H. Chemotherapy resistance in metastatic breast cancer: the evolving role of ixabepilone. Breast Cancer Res. 2010;12(Suppl 2):S2. doi: 10.1186/bcr2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 21.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Huang Bartlett C, Koehler M, Slamon DJ. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 23.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 24.Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, Mori A, Gauthier ER, Bartlett CH, Gelmon KA, Slamon DJ. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner NC, André F, Cristofanilli M, Verma S, Iwata H, Loi S, Harbeck N, Ro J, Colleoni M, Zhang K, Bartlett CH, Giorgetti C, Slamon D (2016) Treatment postprogression in women with endocrine-resistant HR+ HER2− advanced breast cancer who received palbociclib plus fulvestrant in PALOMA-3. Poster presented at 39th annual San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, December 6–10, 2016.
- 26.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 27.Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Dieras V, Im SA, Gelmon KA, Harbeck N, Lu DR, Gauthier E, Huang Bartlett C, Slamon DJ. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 28.Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, DeMichele A, Harbeck N, Andre F, Bayar MA, Michiels S, Zhang Z, Giorgetti C, Arnedos M, Bartlett CH, Cristofanilli M. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C, Randolph S, Koehler M, Cristofanilli M, PALOMA3 Study Group Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 30.Bonetti M, Zahrieh D, Cole BF, Gelber RD. A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med. 2009;28:1255–1268. doi: 10.1002/sim.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Dieras V, Im SA, Gelmon KA, Harbeck N, Lu DR, Gauthier E, Huang Bartlett C, Slamon DJ. Biomarker analyses of response to cyclin dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 32.Paquet ER, Hallett MT. Absolute assignment of breast cancer intrinsic molecular subtype. J Natl Cancer Inst. 2015;107:357. doi: 10.1093/jnci/dju357. [DOI] [PubMed] [Google Scholar]
- 33.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 35.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2 − advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/jco.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 36.Turner NC, Finn RS, Martin M, Im SA, DeMichele A, Ettl J, Dieras V, Moulder S, Lipatov O, Colleoni M, Cristofanilli M, Lu DR, Mori A, Giorgetti C, Iyer S, Bartlett CH, Gelmon KA. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669–680. doi: 10.1093/annonc/mdx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janni W, Alba E, Bachelot T, Diab S, Gil-Gil M, Beck TJ, Ryvo L, Lopez R, Tsai M, Esteva FJ, Aunon PZ, Kral Z, Ward P, Richards P, Pluard TJ, Sutradhar S, Miller M, Campone M. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2 − advanced breast cancer: tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat. 2018;169:469–479. doi: 10.1007/s10549-017-4658-x. [DOI] [PubMed] [Google Scholar]
- 38.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 39.Prat A, Cheang MC, Galvan P, Nuciforo P, Pare L, Adamo B, Munoz M, Viladot M, Press MF, Gagnon R, Ellis C, Johnston S. Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol. 2016;2:1287–1294. doi: 10.1001/jamaoncol.2016.0922. [DOI] [PubMed] [Google Scholar]
- 40.Yanae M, Fujimoto S, Tane K, Tanioka M, Fujiwara K, Tsubaki M, Yamazoe Y, Morishima Y, Chiba Y, Takao S, Komoike Y, Tsurutani J, Nakagawa K, Nishida S. Increased risk of SSEs in bone-only metastatic breast cancer patients treated with zoledronic acid. J Bone Oncol. 2017;8:18–22. doi: 10.1016/j.jbo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143:471–478. doi: 10.1309/AJCPYO5FSV3UPEXS. [DOI] [PubMed] [Google Scholar]
- 42.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.