Abstract
Purpose
Peripheral T cell lymphomas (PTCLs) have an overall poor prognosis. Indeed, registry data in elderly patients show that the median progression-free survival (mPFS) following first- and second-line therapies are only 6.7 and 3.1 months, respectively. The aim of the study is to show the activity of metronomic chemotherapy, a regular administration of low chemotherapeutic drug doses allowing a favourable toxicity profile, on elderly PTCL patients.
Methods
We report a series of 17 PTCL patients, treated with the all-oral metronomic schedule DEVEC (prednisolone–etoposide–vinorelbine–cyclophosphamide) in four Italian centres. Patients 5/17 (29.4%) were treatment-naïve (naïve) and 12/17 (70.6%) were relapsed-refractory (RR), respectively. The median age was 83 years (range 71–87) and 71.5 years (range 56–85) for naïve and RR, respectively. In vitro activity of metronomic vinorelbine (VNR), etoposide (ETO) and their concomitant combination on HH, a PTCL cell line, was also assessed.
Results
Histology: PTCL-not-otherwise-specified = 12; angioimmunoblastic = 2; NK/T nasal type = 1; adult-type leukaemia lymphoma = 1, transformed Mycosis Fungoides = 1. The overall response rate was 80 and 58% in naïve and RR, respectively; whereas the PFS was 20 in naïve (95% CI 0–43) and 11 months (95% CI 4.2–17.8) in RR. The occurrence of relevant adverse events was 23.5%, which was managed with ETO dose reduction. In vitro experiments showed that both metronomic VNR and ETO caused a significant inhibitory activity on HH cells and a strong synergism when administered concomitantly.
Conclusion
All-oral DEVEC showed an encouraging activity and acceptable toxicity. This schedule deserves further studies in elderly PTCL also for assessing combinations with targeted drugs.
Electronic supplementary material
The online version of this article (10.1007/s00280-020-04172-3) contains supplementary material, which is available to authorized users.
Keywords: Metronomic chemotherapy, Low dose, Peripheral T cell lymphoma, Vinorelbine, Etoposide, Cyclophosphamide, Prednisolone, Prognosis
Introduction
Peripheral T cell lymphomas (PTCLs) are rare and heterogeneous entities, derived from mature T cells, with an overall poor prognosis [1]. The T cell registry project, recently reported data on 311 PTCL-not-otherwise-specified (PTCL-NOS), which is the most common subtype. The median overall (OS) and the progression‐free survival (PFS) were 20 and 10 months, respectively [2]. However, older, non-fit or relapsed/refractory (RR) patients who cannot be treated with transplant have even a worse prognosis. In fact, the study by Mak and colleagues reported that in such subsets [3], the median PFS after first-line therapy was only 6.7 months, while following second line, the median PFS and OS were only 3.1–5.5 months, respectively. Presently, there is no standard therapy for elderly naïve and RR-PTCL (RR-PTCL) as most patients become chemo-refractory and do not benefit from following salvage chemotherapies [3]. Therefore, truly effective and less toxic treatments are surely more requested and needed. Indeed, targeted drugs such as brentuximab, pralatrexate, histone deacetylase inhibitors, demethylating agents and various tyrosine-kinase inhibitors have already shown promising efficacy in PTCL, while active combinations are paving their way [1].
Metronomic chemotherapy (mCHEMO), has become in the last decade, an promising therapeutic approach in solid tumours, but has rarely been experimented, preclinically and clinically, in aggressive lymphomas [4, 5]. mCHEMO can be defined as a regular administration of chemotherapy that is able to sustain low, prolonged, and active plasma levels of drugs causing a favourable tolerability [6]. mCHEMO has complex pharmacodynamics including the anti-angiogenic and immune-mediated effects that lead to tumour dormancy [7], but also the direct impact on cancer cell proliferation [8]. Recently, we demonstrated that the all-oral metronomic schedule DEVEC [Deltacortene® (prednisone), etoposide, vinorelbine, cyclophosphamide] could be successfully administered in diffuse large B cell lymphoma (DLBCL) patients [9]. The aim of the present study is to report on a series of elderly PTCL subjects, considered not fit for intravenous chemotherapy schedules, who were treated with DEVEC in four Italian centres.
Materials and methods
Patients and therapeutic schedule
Four Italian clinical centres prospectively collected data on PTCL patients treated with the DEVEC schedule (Ethical Approval no 4640). The mCHEMO has been planned with an induction and a deescalated maintenance phase, both being composed of six cycles going on 28 days, as already described [9]. All along the first cycle, patients were controlled weekly with medical examination and blood test. In case of adverse reactions, chemotherapy was held until recovery and the next cycle begun at decreased doses of etoposide [9]. In subjects achieving less than complete response (CR), maintenance cycles were delivered alternating: (1) cyclophosphamide (CTX) 50 mg for 14 days/etoposide (ETO) 50 mg for 7 days and (2) CTX 50 mg for 14 days/vinorelbine (VNR) 30 mg thrice a week 3 weeks on/1 week off, until progression or excessive toxicity. Low molecular weight heparin and low-dose acetylsalicylic acid were given to patients with high and low medium risk of thrombosis, respectively. Adverse drug reactions were recorded based on CATCAE v4.03. DEVEC-treated patients were as follows: (1) treatment-naïve, frail by CGA [10] and ≥ 65 years, or unfit and ≥ 85 years; or (2) R/R ≥ 55 years, recognized not fitting for MTD-CHT. Patients with a malabsorption syndrome, swallow dysfunctions, HIV positive or with central nervous system involvement were excluded from the trial. Caregivers were required for very old or frail patients to guarantee the proper administration of DEVEC. An interim restaging by computerized-tomography (CT) scan was carried out between the second and third induction cycles and on completion of the induction phase by FDG positron-emission CT-scan (CT-PET) [11]and every 6 months thereafter. Patients were all re-staged with CT-PET scan when they finished the therapy, based on the revised Cheson’s criteria (2007). All the data were retrieved as of 10 January 2020.
In vitro experiments
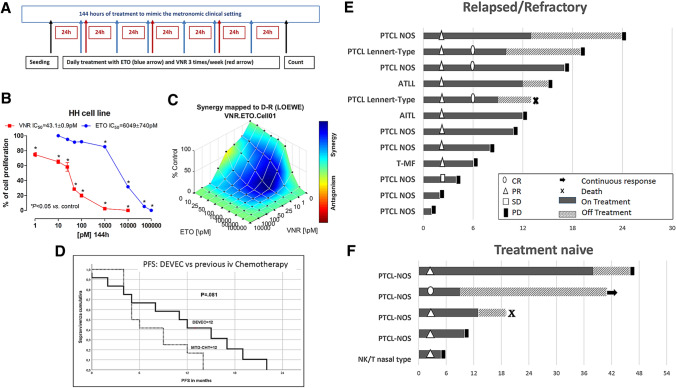
L-glutamine, antibiotics, RPMI-1640 medium and fetal bovine serum (FBS) were bought from Sigma Aldrich SRL (Milan, Italy). VNR and ETO were purchased from Selleckchem (DBA Italia, Milan, Italy). In vitro studies were carried out with drugs diluted from a 10-mM stock solution (in 100% dimethylsulfoxide). Vehicle-treated controls received the same concentration of dimethylsulfoxide in the media as cells of the highest concentration of VNR and ETO. Sterile plastics for cell culture were purchased by Costar (Cambridge, MA, USA). The human PTCL cell line HH (ATCC® CRL-2105™) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in RPMI-1640 medium, supplemented with 20% heat-inactivated FBS, antibiotics and l-glutamine 2 mM. The cells were routinely maintained in tissue culture flasks and kept in a humidified atmosphere of 5% CO2 at 37 °C. HH cells (2 × 104 cells/well) were cultured into 24-well sterile plastic plates and allowed to replicate overnight. Cells were treated with VNR (0.001–10 nM) and ETO (0.025–100 nM), or with vehicle alone for 144 h, as previously described [12, 12]. VNR or ETO were added every 48 h or 24 h, respectively, to mimic the clinical schedules, such as the thrice a week metronomic VNR treatment or the daily metronomic ETO administration (Fig. 1a). At the end of the treatment, the viable cells were counted with a hemocytometer. Cell viability was assessed by trypan blue dye exclusion. The number of treated cells was expressed as a percentage of control ones (vehicle alone). The drug concentrations that decreased cell proliferation by 50% (IC50) compared to vehicle-treated cells were calculated by a nonlinear regression fit of the obtained mean values.
Fig. 1.
a 144 h treatment schedules of metronomic etoposide (ETO; blue arrow) and metronomic vinorelbine (VNR; red arrows) and etoposide, mimicking the clinical setting. b Antiproliferative activity of metronomic vinorelbine (144 h) and metronomic etoposide (144 h) on HH cells. Symbols and bars, mean values ± SEM. *, P < 0.05 vs. control. c The 3-dimensional landscape of the dose matrix of combination responses for VNR and ETO based on the Loewe model, where blue reflects evidence of synergy and red represents evidence of antagonism. The model supported synergy of the combination in reducing HH cell line viability. Cell viability was plotted as % control. d Progression-free survival in PTCL following DEVEC and following the previous line of IV chemotherapy. e, f Swimmer plot of relapsed/refractory and treatment-naïve PTCL patients following DEVEC
Furthermore, the concomitant combination of VNR and ETO was performed at different concentrations with a fixed molar ratio of 1:100, respectively. The synergistic, additive and antagonistic effect of the drug combination was mapped with the Loewe additivity model, using the Combenefit software (v.2.021; https://sourceforge.net/projects/combenefit/files/Combenefit%202.02%20WIN_64%20%28PREFERRED%29, last access 04-07-2020). All experiments were repeated, independently, three times with at least nine samples for each concentration.
Statistical analysis
The data (mean ± SEM) of all the in vitro experiments were analyzed with ANOVA, followed by the Student–Newman–Keuls test. The level of significance was set at P < 0.05. Statistical analyses were performed using the GraphPad Prism software package version 5.0 (GraphPad Software, Inc, San Diego, CA).
The main endpoint of the clinical study was the effect of mCHEMO in terms of overall (OS), progression free (PFS) and duration of response (DOR). OS was measured from the date of treatment start until death from any cause or date of last known contact for living patients. PFS was measured from the date of treatment start to either the last follow-up or the occurrence of one of the following events: progression, relapse or death from any cause. DOR was measured from date of response to the date of progression. Continuous variables were reported as the median and range. Survival functions were estimated with the Kaplan–Meier method. Statistical comparisons between curves were performed with the log-rank test and the effect of covariate was estimated by means of the Cox proportional hazard (PH) regression analysis, with a confidence interval at 95% (95% CI). The comparative statistical tests were considered as significant if the two-sided p value was less than 0.05. The statistical analysis was performed with Stata 14.2 software (StataCorp LLC, College Station, USA).
Results
In vitro experiments showed that both metronomic VNR and daily metronomic ETO caused a significant, direct, concentration-dependent inhibitory activity on HH cell proliferation with an experimental IC50 of 43.1 ± 0.9 pM and 6.05 ± 0.74 nM (Fig. 1b), respectively. Computational quantification of the drug combination responses, plotted as a 3-dimensional synergy map over the dose matrix, showed that the combination of VNR with ETO in HH cells was highly synergistic using the Loewe additivity model (Fig. 1c).
From September 2012 to August 2018, 17 subjects started the DEVEC schedule. Five out of 17 (29.4%) were treatment-naïve (naïve) and 12/17 (70.6%) were RR patients, respectively. Histology: PTCL-NOS = 12 (2 of Lennert type); angioimmunoblastic PTCL (AITL) = 2; NK/T nasal type = 1; nodal adult-type leukaemia lymphoma (ATLL) = 1, transformed Mycosis Fungoides (tMF) = 1. The median age of the naïve patients was 83 years (range 71–87) and they were all classified as frail [10] (Table 1). The median age of RR patients was 71.5 years (range 56–85), who had received ≥ 1 lines of chemotherapy (median 1, range 1–4, supplementary Table 1) and 7/12 (58%) were refractory [13] (Table 1). The median and total number of DEVEC cycles administered were 9 (range 1–38) and 180, respectively (Table 1). Haematological treatment-related adverse events (TRAE) were recorded in 8/17 patients, the most frequent was G3 neutropenia in 6/17 (35%). Four G4 neutropenia lasting for more than 6 days, occurred in 3/17 (17.6%) patients who were heavily pre-treated or with bone marrow-involvement. Five non-haematological TRAE of grade ≥ 3 were recorded in 4/17 patients (23.5%): 1 bacterial meningitis, 2 pneumonia, 2 neutropenic sepsis. No treatment-related deaths occurred. Two patients discontinued at cycle 9 and 13, respectively (Fig. 1e, f) following TRAEs. These two patients died, respectively, 4 and 5 months after mCHEMO discontinuation, one of myocardial infarction and the other of stroke. Overall, 8/17 (47%) had ETO dose reductions. The dose intensity during induction cycles for ETO, CTX and VNR were 55%, 100% and 100%, respectively. The direct cost of drugs included into the oral DEVEC schedule was estimated 930 and 817 Euro (year 2017) for a single induction and maintenance cycle, respectively.
Table 1.
Features of 17 peripheral T cell lymphoma (PTCL) patients treated with DEVEC
| Naive | Relapsed/refractory | |
|---|---|---|
| Patients | ||
| Median age (range) | 83 years (70–87) | 71.5 years (56–85) |
| Male sex | 4/5 (80%) | 6/12 (50%) |
| Diagnosis | ||
| PTCL-NOS | 4 | 7 |
| PTCL-NOS Lennert type | 2 | |
| AITL | 1 | |
| NK/T nasal type | 1 | |
| ATLL/nodal | 1 | |
| tMF | 1 | |
| Stage III–IV | 5/5 | 11/12 |
| IPI 3–5 | 5/5 (100%) | 9/12 |
| PIT 3–4 | 5/5 (100%) | 8/12 |
| Previous chemo (median, range) | – | 1–3 (1) |
| DEVEC cycles (median, range) | 10 (7–38) | 8.5 (1–13) |
| ORR (%) | 4/5 (80%) | 8/12 (66%) |
| CR (%) | 1/5 (20%) | 3/12 (25%) |
PTCL-NOS not-otherwise-specified, AITL angioimmunoblastic T cell lymphoma, ATLL adult type T cell leukaemia lymphoma, tMF transformed mycosis fungoides, IPI international prognostic index, PIT prognostic index for T cell lymphoma
The median follow-up, from treatment beginning, was 45 months (range 14–72). At the time of analysis, 16/17 (94%) patients died: 15/17 (88%) for disease progression and 2/17 (12%) for non-TRAE. Tumour shrinkage was recorded in 5/5 (100%), naïve (95% CI 55–100%) and in 9/12 (75%), RR (95% CI 43–95%) (Fig. 1e, f). At the end of the induction phase, the ORR was 80 and 58% in naïve and RR, respectively. CR was observed in 1/5 (20%) naive and 3/12 (25%) RR, respectively. Median PFS and OS for naïve was 20 (95% CI 0–43) and 46 months, respectively (Supplementary Figure 1). While in RR, the OS was 13 months (95% CI 11.3–14.6) and the median PFS was 11 months (95% CI 4.2–17.8). Worthy of note the median PFS of the RR subset, to the treatment done before the DEVEC had been only 5 months (95% CI 3.3–6.7) (Fig. 1c).
Discussion
This short communication shows that the all-oral metronomic DEVEC chemotherapy caused an objective response in most of naïve and RR patients, allowing a durable remission in a considerable proportion of subjects. Worthy of note, in the RR group, the median PFS after DEVEC was longer than the mPFS achieved after previous intravenous chemotherapy (Fig. 1c, supplementary Table 1). Indeed, PTCL following first line tend to become chemotherapy refractory [3]. Hence, this observation underlies the efficacy of this mCHEMO schedule in elderly PTCL which may challenge IV schedules. Conversely, mPFS did not appear to differ between subjects who achieved CR or PR (Supplementary Figure 1). Interestingly, in the latter group, lymphoma lesions showed a waxing and waning pattern on progress CT-PET (data not shown).
We believe our experience, in this difficult-to-treat lymphoma subset, may be useful in clinical practice. In fact, the all-oral administration, and the possibility to tailor doses on individual toxicity may be convenient for the elderly. Furthermore, with the COVID-19 pandemic, it is becoming a priority for clinical oncologists to limit the admission of vulnerable elderly subjects to hospitals as well as to reduce the intensity of immune suppressive chemotherapies.
As far as we know, there has been only limited and anecdotal data published on mCHEMO in PTCL, which have already suggested the potential activity of this approach [14–16]. Indeed, our experimental data on the human PTCL cell line HH clearly show that at least two of the drugs, included in the DEVEC schedule, directly and significantly affected the PTCL cell proliferation. Both VNR and ETO given in vitro metronomically were active at very low concentrations (pM and nM, respectively) maintained for a protracted time. Furthermore, their concomitant metronomic combination revealed to be highly synergistic in HH cells, suggesting that the interaction between the two drugs could be more effective that the use of single drug schedules. Moreover, these effective concentrations are fully compatible with the drug plasma levels found in patients treated with metronomic 30 mg VNR schedule [17] and with daily 50 mg ETO [18], the same doses adopted by the DEVEC schedule.
However, our findings might seem at odds with the results of a recent series of elderly PTCL. In fact, it was reported that patients who receive low-intensity treatment performed worse than those who are treated with standard dose IV schedules. However, the “lower intensity” group received either the cyclophosphamide–vincristine–prednisolone (CVP) intravenous schedule or a single-agent chemotherapy with palliative intent [19]. We acknowledge our study on this rare disease has a small sample size, which limits the relevance of our findings and, thus, no definitive conclusions can be drawn. In addition, this series reports on patients with heterogeneous entities of mature T cell lymphomas. Indeed, as recently underlined by Zain [20], there are over 27 different subtypes of peripheral T cell lymphoma and scientists are just beginning to understand the differences between the various subtypes beyond histologic variations. Thus, the clinical behaviours of PTCLs included into our study may be different. Nonetheless, it gathered only subjects with very poor prognosis due to: (1) the histologic subtypes of PTCL (Table 1); (2) the high rate of patients with poor prognostic score (Table 1); (3) the prevalence of RR, and (4) the advanced age. Furthermore, as data were prospectively collected and the participating centres from the beginning agreed on both the protocol and the dose reduction schedule, we believe this partly overcome the bias of being a retrospective study. Conversely, the occurrence of relevant TRAE was not negligible and appeared related to > 2 previous lines of systemic therapy and bone marrow involvement. Indeed, ETO which required dose reduction in about 50% may cause mucositis, infections, and myelosuppression, even at metronomic doses. In conclusion, the DEVEC schedule showed encouraging activity and acceptable toxicity in vulnerable elderly PTCL patients both at disease onset and as second-line treatment. Notwithstanding, the small sample size and the heterogeneity of histologic subtypes do not allow definitive deductions. Further trials are necessary for assessing the clinical efficacy of DEVEC in PTCL. Besides, pharmacological studies investigating the potential synergies of mCHEMO combinations with targeted drugs are also eagerly awaited in T cell lymphomas [1].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was supported by a grant from the Azienda Ospedaliera Sant’Andrea to MCC and from the University of Pisa to GB.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
It was waived by the local Ethics Committee of Sapienza University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Christina Cox, Email: chrisscox@gmail.com.
Guido Bocci, Email: guido.bocci@med.unipi.it.
References
- 1.Marchi E, O’Connor OA. The rapidly changing landscape in mature T-cell lymphoma (MTCL) biology and management. CA Cancer J Clin. 2020;70:47–70. doi: 10.3322/caac.21589. [DOI] [PubMed] [Google Scholar]
- 2.Federico M, Bellei M, Marcheselli L, Schwartz M, Manni M, Tarantino V, et al. Peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). A new prognostic model developed by the International T cell Project Network. Br J Haematol. 2018;181:760–769. doi: 10.1111/bjh.15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 4.Wu K, Sun XQ, Wang CQ, Gao TX, Sun P, Wang Y, et al. Metronomic combination chemotherapy using everolimus and etoposide for the treatment of non-Hodgkin lymphoma. Cancer Med. 2019;8:4688–4698. doi: 10.1002/cam4.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schelker RC, Herr W, Reichle A, Vogelhuber M. Low-dose trofosfamide plus rituximab is an effective and safe treatment for diffuse large B-cell lymphoma of the elderly: a single center experience. BMC Cancer. 2018;18:1–8. doi: 10.1186/s12885-018-4885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol. 2016;13:659–673. doi: 10.1038/nrclinonc.2016.64. [DOI] [PubMed] [Google Scholar]
- 7.Natale G, Bocci G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett. 2018;432:28–37. doi: 10.1016/j.canlet.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Orlandi P, Di Desidero T, Salvia G, Muscatello B, Francia G, Bocci G. Metronomic vinorelbine is directly active on non small cell lung cancer cells and sensitizes the EGFRL858R/T790M cells to reversible EGFR tyrosine kinase inhibitors. Biochem Pharmacol. 2018;152:327–337. doi: 10.1016/j.bcp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Cox MC, Pelliccia S, Marcheselli L, Battistini R, Arcari A, Borza PA, et al. The metronomic all-oral DEVEC is an effective schedule in elderly patients with diffuse large b-cell lymphoma. Invest New Drugs. 2019;37:548–558. doi: 10.1007/s10637-019-00769-5. [DOI] [PubMed] [Google Scholar]
- 10.Merli F, Luminari S, Rossi G, Mammi C, Marcheselli L, Ferrari A, et al. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by comprehensive geriatric assessment: results from a study of the fondazione italiana linfomi. Leuk Lymphoma. 2014;55:38–43. doi: 10.3109/10428194.2013.788176. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 13.Carson KR, Horwitz SM, Pinter-Brown LC, Rosen ST, Pro B, Hsi ED, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017;123:1174–1183. doi: 10.1002/cncr.30416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucci A, Cerqui E, Ungari M, Ferrari S, Baushi L, Facchetti F, et al. Continuous oral cyclophosphamide and prednisolone as a valuable treatment option for peripheral T cell lymphoma. Br J Haematol. 2011;152:113–116. doi: 10.1111/j.1365-2141.2010.08367.x. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S, Yu J, Haverkos B, Su H. Remission of an extensively pre-treated relapsing/refractory ALK-positive anaplastic large cell lymphoma following metronomic therapy. Leuk Lymphoma. 2016;57:1194–1196. doi: 10.3109/10428194.2015.1055484. [DOI] [PubMed] [Google Scholar]
- 16.Coleman M, Martin P, Ruan J, Furman R, Niesvizky R, Elstrom R, et al. Prednisone, etoposide, procarbazine, and cyclophosphamide (PEP-C) oral combination chemotherapy regimen for recurring/refractory lymphoma: Low-dose metronomic, multidrug therapy. Cancer. 2008;112:2228–2232. doi: 10.1002/cncr.23422. [DOI] [PubMed] [Google Scholar]
- 17.Di Desidero T, Derosa L, Galli L, Orlandi P, Fontana A, Fioravanti A, et al. Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients. Invest New Drugs. 2016;34(6):760–770. doi: 10.1007/s10637-016-0385-0. [DOI] [PubMed] [Google Scholar]
- 18.Peng Yong W, Desai AA, Innocenti F, Ramirez J, Shepard D, Kobayashi K, et al. Pharmacokinetic modulation of oral etoposide by ketoconazole in patients with advanced cancer. Cancer Chemother Pharmacol. 2007;60:811–819. doi: 10.1007/s00280-007-0428-5. [DOI] [PubMed] [Google Scholar]
- 19.Wudhikarn K, Bunworasate U, Julamanee J, Lekhakula A, Ekwattanakit S, Khuhapinant A, et al. Characteristics, treatment patterns, prognostic determinants and outcome of peripheral T cell lymphoma and natural killer/T cell non-Hodgkin lymphoma in older patients: the result of the nationwide multi-institutional registry Thai lymphoma study group. J Geriatr Oncol. 2020;11:62–68. doi: 10.1016/j.jgo.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Zain JM. Aggressive T-cell lymphomas: 2019 updates on diagnosis, risk stratification, and management. Am J Hematol. 2019;94(8):929–946. doi: 10.1002/ajh.25513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.