Abstract
The coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is still wreaking havoc in many parts of the world and poses a great burden to healthcare systems worldwide. Mitigation and suppression strategies have been implemented globally but the disease has proven to be difficult to contain. Initially many elective gastrointestinal endoscopies were cancelled to reduce the risk of infection and conserve personal protective equipment, but many endoscopy units are now faced with the dilemma of resuming endoscopy service during the pandemic as indefinitely postponing diagnostic procedures may lead to a delay in the diagnosis and treatment of malignancies. Further concerns are surfacing as COVID-19 is now known to affect the gastrointestinal tract and may potentially be spread via the fecal-oral route. Until more effective drugs and vaccines are available, it is unlikely that the pandemic will wind down in the near future. Maintaining a balance between protecting healthcare workers and patients from being infected on the one hand and providing timely and effective clinical care on the other will become increasingly important as the pandemic persists. In this narrative review, the risk of COVID-19 infection for healthcare workers and patients undergoing endoscopy, and recommendations on maintaining safe, high-quality endoscopy practice will be discussed.
Keywords: SARS-CoV-2, Reorganization, Infection prevention and control, Personal protective equipment
Introduction
Since being declared a global pandemic by the World Health Organization (WHO) in March 2020, COVID-19 has infected more than 35 million individuals and caused more than 1 million deaths as of October 6, 2020.1 The virus is highly contagious2 and spreads via respiratory droplets and direct contact, with recent studies even suggesting the potential for airborne spread in some circumstances.3 , 4 In addition, though primarily considered as a respiratory disease, it is now well-known that COVID-19 can affect various organ systems including the digestive system.5 In a systematic review and meta-analysis, 17.6%6 of patients with COVID-19 were found to have gastrointestinal (GI) symptoms such as diarrhea, poor appetite, nausea, vomiting, and abdominal pain. This may have prognostic implications as well as patients with diarrhea were more likely to have cytokine storms and develop multiorgan damage.7 Given that studies have identified SARS‐CoV‐2 RNA in rectal swabs and stool specimens of COVID‐19 patients even after the clearance of the virus from the upper respiratory tract,8 mounting evidence suggests that there is a potential for fecal-oral transmission9 , 10 which poses as a considerable hazard to endoscopists.
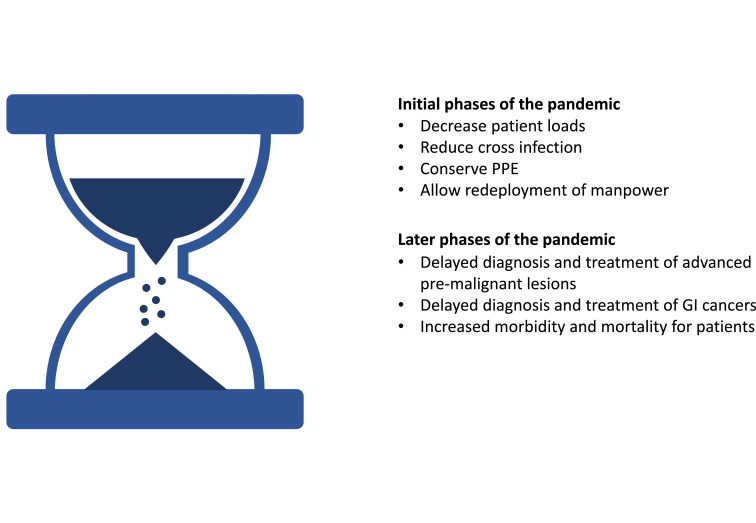
Initially many elective GI endoscopies were cancelled to reduce the risk of infection and conserve personal protective equipment (PPE) in line with international consensus statements and guidelines.11, 12, 13, 14 Only urgent, life-saving procedures or time sensitive procedures such as those performed in cancer patients were continued. Reports from around the globe have shown drastic reductions in endoscopy volumes due to COVID-19.15, 16, 17 However, many endoscopy units are now faced with the dilemma that indefinitely postponing diagnostic procedures may lead to a delay in the diagnosis and treatment of advanced premalignant lesions18 and malignancies.19 A modeling study from England suggests that the COVID-19 pandemic may lead to an increase of 16% and 6% of colorectal cancer and esophageal cancer deaths within five years respectively, due to delays in diagnosis.20 Maintaining a suitable balance between protecting healthcare workers (HCWs) and patients on the one hand, and providing a timely and effective clinical service on the other will become increasingly important as the pandemic persists (Figure 1 ). Modeling studies project that COVID-19 related social distancing may need to be kept in place till 202221 suggesting that we are in this for the long haul. In this narrative review, the risk of COVID-19 infection for HCWs and patients during endoscopy, and recommendations on maintaining safe, high-quality endoscopy practice will be discussed.
Figure 1.
Deferring nonurgent endoscopy. A balance between benefit and risk. Abbreviations: PPE personal protective equipment.
Risk of COVID-19 for Patients and Healthcare Workers Undergoing Endoscopy
In a survey from Northern Italy, Repici et al conducted a telephone survey at two weeks follow up after endoscopy and found that only one out of 802 patients that responded turned out to be COVID-19 positive.22 Reassuringly, the SCOTS (Symptoms, infectious Contacts, Occupational risk, Travel risk, Shielding status) project group also reported similar results with no patients developing COVID-19 after endoscopy during the recovery phase of the pandemic at 7 and 14 days telephone follow-up23 where a COVID-minimized pathway was instituted.24 The SCOTS criteria are now recommended in British Society of Gastroenterology (BSG) guidance.
With regard to HCWs, a recent proof of concept study utilizing laser particle counters showed that esophago-gastro-duodenoscopy (EGD) was associated with increased levels of aerosol-sized particles,25 providing evidence to support the prevailing consensus that upper endoscopy is an aerosol generating procedure (AGP).26, 27, 28 Whether colonoscopy is also an AGP is still debated but the American Gastroenterological Association (AGA) extends their recommendations to lower GI procedures as well.13 The previously mentioned study from Northern Italy also surveyed hospitals and found that only 4.3% of HCWs tested positive for COVID-19.22 Despite this, our guard should not be let down as the risk of COVID-19 to frontline staff is still substantial. A large, prospective cohort study utilizing self-reported data from a smartphone application estimated that frontline HCWs have a 10-fold increased risk of contracting the virus when compared to the general populace and that having inadequate access to, reusing, and exposure to positive COVID-19 patients with or without adequate PPE all increased the risk of contracting the virus.29
Taken together, emerging evidence suggests that GI endoscopy appears to be relatively safe for patients and HCWs if strict infection prevention and control measures are taken.
Safe Endoscopy Practices
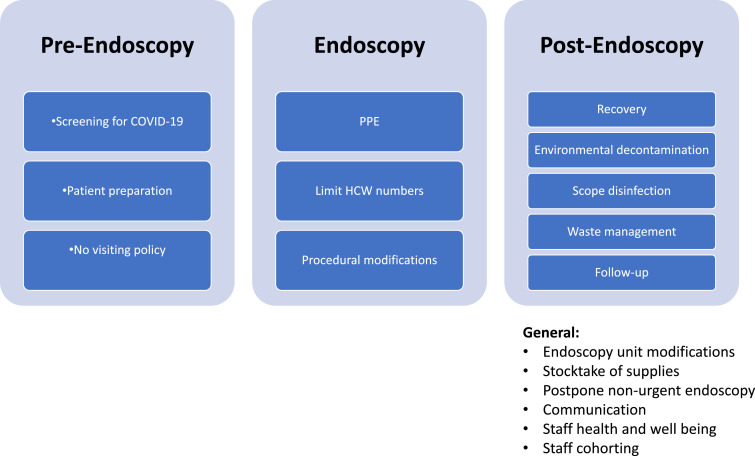
Several guidelines and expert advice on performing endoscopy during COVID-19 have been published.11, 12, 13, 14 , 30, 31, 32, 33 To ensure that endoscopy is safe for both patients and HCWs, operational reorganization of endoscopy units is necessary during the pandemic34 (Figure 2 ).
Figure 2.
Considerations for safe endoscopy. Abbreviations: COVID-19 coronavirus disease 2019, HCW healthcare workers; PPE personal protective equipment.
General Measures
Modifications to the endoscopy unit
The establishment of clearly segregated clean and contaminated zones for both waiting areas and recovery rooms, designated one-way flows for equipment and patients, and separate gown-up and gown-down areas are necessary to minimize cross-contamination. Refuse bins should be lidded for the disposal of used PPE.11 Checkpoints should be established at the entrances of endoscopy units for the screening and triage of patients.32 In addition, designated toileting facilities should be provided for suspected or confirmed cases. Hygiene measures after toileting such as closing the lid before flushing to reduce bioaerosol formation35 and handwashing must be emphasized.
Stocktake of essential supplies
The pandemic has led to global supply chain disruptions worldwide with limited PPE stocks expected for the foreseeable future.36 Maintaining adequate PPE levels is essential to protect both patients and HCWs. A stocktake of current levels of PPE,37 and establishing the anticipated supply and usage rates are essential when discussing with hospital administrators to plan ahead for endoscopy service provision.
Postpone endoscopy for patients with nonurgent indications
There has been a broad consensus for the policy of deferring nonurgent, elective cases to decrease the patient load of hospitals, reduce the risk of cross-infection, minimize potential exposure for HCWs and allow redeployment of manpower for essential COVID-19 related services.11 Essentially, all endoscopies except those done for potentially life-threatening conditions such as gastrointestinal bleeding, acute cholangitis, biliary pancreatitis and foreign body retrieval, together with selected cancer patients with time-sensitive indications should be postponed at the peak of the outbreak.11 The rationale and finalized lists of prioritized procedures is beyond the discussion of this review. Differing recommendations with regard to screening colonoscopy in fecal immunochemical test positive patients12 , 13 , 38 and patients requiring variceal screening39, 40, 41, 42 are a testament that this decision process in real-world settings is complex, and that a certain degree of discretion should be allowed for endoscopists when making such decisions. Some experts opine that fecal immunochemical test during COVID-19 could be used as a triage tool to guide timing and help prioritize investigations due to limited capacity, but at the end of the day these triage tests cannot entirely replace confirmatory tests such as colonoscopy.43 The European Society of Gastrointestinal Endoscopy/European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGE/ESGENA) guidance advocates to include an evaluation of gastrointestinal disease‐related morbidity/mortality for elective cases as well.12 It is advisable that units systematically keep track of all patients that had procedures deferred and prioritize access to regular endoscopic services once this is resumed.44
Alternative investigations
In appropriate clinical scenarios, alternative investigations such as computed tomography (CT) colonography or colon capsule endoscopy may be considered. The procedural profile of CT colonography may be advantageous during the COVID-19 pandemic as this modality favors social distancing and preserves healthcare supplies such as PPE.45 As for colon capsule endoscopy, it is emerging as a safe and innovative alternative for investigating the colon and can also serve as an effective triage test. The procedure can be carried out by one HCW only minimizing the risk of exposure and the capsule itself is disposable. An additional benefit for capsule endoscopy is that it can be carried out in the community.46
Communication
Regular communication between frontline HCWs, endoscopy leadership and hospital administrators is required so that views from all stakeholders are taken into account. A regular daily huddle with endoscopy leadership has been advocated to review policies regularly and make changes rapidly given the fluid situation.47
Staff health and well being
Personal hygiene measures such as frequent handwashing, keeping hair short and facial hair clean-shaven, wearing facemasks48 , 49 using hospital-issued scrubs, wearing dedicated endoscopy shoes,37 taking a shower before leaving and using eye protection49 should be enforced.
Physical and social distancing49 , 50 measures are also important. Examples include changing all meetings to virtual platforms, using designated chairs, computers, and phones for work,51 and only allowing unidirectional sitting arrangements for staff in lounge/canteens to minimize infection from face-to-face interactions,37 and maintaining travel restrictions.52
In addition, HCWs are under immense physical and psychological stress during this pandemic and their well-being needs to be addressed. Maintaining a healthy lifestyle is essential to tackle these issues. Emotional and psychological tools should also be made easily accessible for HCWs and spouses.11
Staff cohorting
A dedicated clinical team to take care of confirmed or potentially infectious patients, and other teams to help manage lower risk patients and provide support should be considered if there is sufficient manpower.11
Screening for COVID-19
Symptomatology and FTOCC
All patients entering the endoscopy unit should be screened for COVID-19. This should include a mandatory questionnaire for respiratory symptoms such as cough, runny nose, and/or shortness of breath; and also gastrointestinal symptoms such as nausea, vomiting and diarrhea as these may be the only symptoms present in some cases.53 This should be coupled together with questionnaires on fever, travel history, occupation, contact, and clustering (FTOCC). Some experts also recommend including screening questions on alterations of taste54 and smell.55 , 56 Endoscopy will only proceed for patients who screen negative unless for life-threatening indications.
Polymerase chain reaction testing
The most widely advocated method of pre-endoscopy testing for COVID-19 is by reverse transcriptase-polymerase chain reaction (RT-PCR).24 , 57, 58, 59 An economic analysis has shown that performing nucleic acid amplification tests for SARS-CoV-2 on all patients is an effective strategy, though this is subject to the availability of local resources.60 Recently, recommendations from the AGA suggest that a pre-testing strategy will likely work best for regions with intermediate prevalence of asymptomatic carriers (0.5%-2%).61 Real world data from the United Kingdom during the recovery phase of the pandemic found that only 0.11% of asymptomatic patients tested positive for SARS-CoV-2 on nasopharyngeal swab testing pre-endoscopy.23
Serology
Some authors have recommended testing for immunoglobulins against SARS-CoV-262 as a screening test. However, this strategy may only indicate a past infection and has no role in diagnosing active COVID-19 in asymptomatic individuals prior to endoscopy.61
CT of the chest
Chest CT has also been suggested as a potential screening strategy where it was widely utilized by centers in China.63 , 64 It is thought that CT findings may precede a positive RT-PCR SARS-CoV-2 test,65 though later studies showed that almost 18% of nonsevere COVID-19 patients displayed no radiographic or CT abnormality limiting its usefulness.66 In Mainland China, a negative chest CT together with a negative nucleic acid amplification tests for SARS-CoV-2 are usually required prior to proceeding with endoscopy to improve safety for both patients and healthcare staff during the COVID-19 pandemic. However, this strategy may be difficult to be implemented more widely due to issues relating to generalizability, accessibility and cost.11
Point-of-care testing
The rationale behind point-of-care testing is that these rapid and easy-to-use devices can provide COVID-19 testing outside of laboratory settings using various techniques and allow for more accurate risk stratification.51 However, despite some promising results many of these tests have yet to be validated. At present, the WHO advises that these should only be used in research settings until further evidence is made available.67
Pre-endoscopy
Patient preparation
All patients planning for endoscopy will be required to wear surgical masks, perform hand hygiene with alcohol‐based hand rub, and/or wear gloves. Close communication with wards should be encouraged to restrict the number of patients in the waiting areas at any given time to avoid overcrowding and minimize waiting times.11 While waiting, patients should be spaced apart to avoid close contact in the endoscopy unit with a distance of at least 1-1.8 meters.37 , 49 , 68
“No Visiting” policy
A strict no visiting policy should be enforced to minimize the risk of cross‐infection.12 , 69 In case of exceptional circumstances such as on compassionate grounds, need of specific assistance or translational services, the same risk assessment for patients should be applied to the caregivers.
Endoscopy
PPPE
Patients who have suspected or confirmed COVID-19 should have endoscopy performed in negative pressure facilities where available.11 HCWs involved in this setting must wear the highest level of PPE available including N95 respirators or equivalent, water resistant gowns, hair nets, and face shields/goggles. The practice of double gloving has also been recommended by the AGA.13 Faced with limited availability of respirators, the ESGE/ESGENA suggests that extended use of N95 for up to 4 hours is acceptable.12 The Centers for Disease Control and Prevention has also issued recommendations allowing for extended use and limited reuse of respirators.70 These practices should be avoided if PPE stocks are sufficient as reusing N95 respirators has been shown to increase the risk of contracting the virus, though these findings were before disinfection protocols were widely available.29 Some experts also suggest that if comprehensive screening strategies are in place, this will allow lower levels of PPE to be worn for lower risk procedures and increase room throughput for endoscopy, while conserving PPE and maintaining patient and staff safety.59
In addition, HCWs should have a respirator fit test for N95 respirators or equivalent equipment performed if this was not done recently, or if significant changes in facial contour or fluctuations of body weight have occurred.71 All HCWs must also receive training and education related to the use of PPE to minimize the infection risk during donning and doffing.72
Limit healthcare worker numbers and contact
The number of HCWs involved for endoscopic procedures should be limited to the absolute minimum required for patient care and safety.73 Endoscopy should be performed by independent endoscopists with all on‐hands training suspended. Unsurprisingly these restrictions will have tangible effects on training with an international online survey by Pawlak et al showing that COVID-19 has led to high rates of anxiety and burnout among endoscopy trainees worldwide.74 Specific measures by training programs to address this will be needed as the pandemic continues.
HCWs involved in endoscopy should also stay in the endoscopy room and avoid encountering other staff. Communication can be facilitated by radio devices.11 Other staff in the clean area can help complete the procedure report to avoid contamination and conserve PPE.37
Procedural modifications
Some experts have advised against the use of local anesthetic sprays due to their potential to generate aerosols.33 Adequate sedation is also important to minimize patient discomfort and retching during the procedure.11 Use of a dental sucker in the oral cavity for continuous suction during upper endoscopy was shown to significantly decrease the particle counts of all sizes including aerosols and may be a useful adjunct.25
Postendoscopy
Recovery
Designated recovery rooms to separate suspected and/or confirmed COVID‐19 patients and other patients are needed to minimize the risk of cross‐infection.11
Early reversal of sedation in suspected or confirmed COVID‐19 should be considered to avoid prolonged sedative effects and respiratory failure, which may require high flow oxygen therapy and in more serious circumstances resuscitation as these are known AGPs.75
Specific cardiopulmonary resuscitation care pathways tailored for patients with suspected or confirmed COVID‐19 need to be implemented with sufficient training and drilling provided for frontline HCWs. The guidance promulgated by the Resuscitation Council of the United Kingdom requires staff to wear PPE that protects against AGPs during resuscitation, restrict the number of HCWs present, avoid clinical examinations that require placing ears and cheeks close to the patient's mouth, avoid mouth‐to‐mouth ventilation, start compression‐only cardiopulmonary resuscitation, with early airway interventions to be performed only by experienced staff such as anesthetists.76
Environmental decontamination
There is a risk of fomite transmission as viable SARS‐CoV‐2 has been shown to be present on plastic and stainless steel surfaces for up to 3 days.77 Standard disinfection measures using hydrogen peroxide,78 alcohol- and chlorine‐based chemicals are reported to be effective.79 Disinfection should be performed after each case especially for surfaces frequently in contact with patients such as bed rails, bedside tables, furniture, and the floor.69 At the end of each endoscopy list, or if gross contamination has occurred, an in‐depth cleansing process followed by disinfection is required.11 In addition, ultraviolet irradiation and ozone treatment may potentially have a role to clean and sterilize the air, endoscopic equipment, tables, and environs of endoscopy units.80
Scope disinfection
Reprocessing of endoscopes and accessories should be performed according to existing guidelines.81 , 82 Studies have shown that SARS-CoV-2 is readily inactivated by commonly used disinfectants suggesting that current reprocessing protocols should be sufficient to prevent COVID-19 infection.78 When HCWs handle equipment for a suspected or confirmed COVID-19 patient further precautions such as using N95 or equivalent respirators should be strongly considered.12
Waste management
Medical waste from suspected or confirmed COVID‐19 patients or high risk cases should be packaged appropriately. Some experts advocate using double medical waste bags clearly marked as COVID-19 waste.31 These should be handled with care and disposed according to the relevant local regulations.12
Follow‐up
If manpower allows, contacting patients by phone for a follow-up at day 7 and 14 to inquire if they have developed any COVID-19 related symptoms or have been diagnosed to have COVID-19 can help with contact tracing and minimize the risk of nosocomial outbreaks.12 , 69
Conclusion
In contrast to the Severe Acute Respiratory Syndrome (SARS) outbreak in 2003 which was contained within 8 months,83 the COVID-19 pandemic is exhibiting a vastly different epidemic trajectory. Despite encouraging studies showing that some drugs such as interferon-based combination therapy,84 remdesivir,85 , 86 and steroids87 may be effective against SARS-CoV-2, the future will likely depend on the development of safe and effective vaccines.88, 89, 90 In the meantime, strict adherence to personal hygiene measures and social distancing will be of utmost importance. As COVID-19 rages on further issues relating to complacency38 and response fatigue91 will also need to be addressed.
The safety of endoscopy for both patients and healthcare workers is paramount as endoscopy units adapt to the ongoing outbreak and start resuming regular service.92 Stringent measures taken before, during, and after endoscopy can hopefully mitigate the risk of infection. The silver lining is that this is an opportunity to introduce new care models and enhance our preparedness for future pandemics.
Footnotes
Conflicts of interest The author declares no conflicts of interests.
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at:https://covid19.who.int/?gclid=CjwKCAjw1K75BRAEEiwAd41h1JvfQ25izFkCqaU2_jZQhNc8oQYdW9LjKGZVvT1dSD1yWoeeecNfWhoCex0QAvD_BwE [Accessed October 7, 2020].
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa939. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 5.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong Cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Han C, Zhang S, et al. Diarrhea and altered inflammatory cytokine pattern in severe coronavirus disease 2019: impact on disease course and in-hospital mortality. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15166. Available at: [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding S, Liang TJ. Is SARS-CoV-2 Also an enteric pathogen with potential fecal-oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.052. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Costa AF, Mera RM, et al. SARS-CoV-2 in rural Latin America. A population-based study in coastal Ecuador. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1055. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lui RN, Wong SH, Sánchez-Luna SA, et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35:749–759. doi: 10.1111/jgh.15053. [DOI] [PubMed] [Google Scholar]
- 12.Gralnek IM, Hassan C, Beilenhoff U, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020 doi: 10.1055/a-1155-6229. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultan S, Lim JK, Altayar O, et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.072. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu PWY, Ng SC, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repici A, Pace F, Gabbiadini R, et al. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.003. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes N, Smith ZL, Spitzer RL, et al. Changes in gastroenterology and endoscopy practices in response to the COVID-19 pandemic: results from a North American Survey. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.071. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parasa S, Reddy N, Faigel DO, et al. Global impact of the COVID-19 pandemic on endoscopy: an international survey of 252 centers from 55 countries. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.06.009. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralnek IM, Hassan C, Dinis-Ribeiro M. COVID-19 and endoscopy: implications for healthcare and digestive cancer screening. Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1038/s41575-020-0312-x. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30388-0. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repici A, Aragona G, Cengia G, et al. Low risk of covid-19 transmission in GI endoscopy. Gut. 2020 doi: 10.1136/gutjnl-2020-321341. Available at: [DOI] [PubMed] [Google Scholar]
- 23.Hayee B, The SCOTS project group. East J, et al. Multicentre prospective study of COVID-19 transmission following outpatient GI endoscopy in the UK. Gut. 2020 doi: 10.1136/gutjnl-2020-322730. Published Online First: 14 September. [DOI] [PubMed] [Google Scholar]
- 24.British Society of Gastroenterology guidance on recommencing GI endoscopy in the deceleration & early recovery phases of the COVID-19 pandemic. 2020. Available at:https://www.bsg.org.uk/covid-19-advice/bsg-guidance-on-recommencing-gi-endoscopy-in-the-deceleration-early-recovery-phases-of-the-covid-19-pandemic/ [Accessed August 4, 2020]. [DOI] [PMC free article] [PubMed]
- 25.Chan SM, Ma TW, Ka-Chun Chong M, et al. A proof of concept study: esophagogastroduodenoscopy is an aerosol-generating procedure and continuous oral suction during the procedure reduces the amount of aerosol generated. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.002. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.British Society of Gastroenterology rationale around current advice to all endoscopy Units. 2020. Available at:https://www.bsg.org.uk/covid-19-advice/bsg-rationale-around-current-advice-to-all-endoscopy-units/ [Accessed August 6, 2020].
- 27.Brewster DJ, Chrimes N, Do TBT, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID ‐19 adult patient group. Med J Aust. 2020;212:472–481. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handbook_of_COVID-19_Prevention_and_Treatment.pdf. Available at: https://esge.org/documents/Handbook_of_COVID-19_Prevention_and_Treatment.pdf.
- 29.Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30164-X. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse F, Borgaonkar M, Leontiadis GI. COVID-19: Advice from the Canadian Association of Gastroenterology for Endoscopy facilities, as of March 16, 2020. J Can Assoc Gastroenterol. 2020;3:147–149. doi: 10.1093/jcag/gwaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai N, Mei Z, Zhang W, et al. Endoscopy works during the pandemic of coronavirus COVID-19: recommendations by the Chinese Society of Digestive Endoscopy. United European Gastroenterol J. 2020;8:798–803. doi: 10.1177/2050640620930632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furfaro F, Vuitton L, Fiorino G, et al. SFED recommendations for IBD endoscopy during COVID-19 pandemic: Italian and French experience. Nat Rev Gastroenterol Hepatol. 2020;17:507–516. doi: 10.1038/s41575-020-0319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irisawa A, Furuta T, Matsumoto T, et al. Gastrointestinal endoscopy in the era of the acute pandemic of COVID‐19: Recommendations by Japan Gastroenterological Endoscopy Society (Issued on April 9 th, 2020.) Dig Endosc. 2020 doi: 10.1111/den.13703. https://onlinelibrary.wiley.com/doi/abs/10.1111/den.13703 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danese S, Ran ZH, Repici A, et al. Gastroenterology department operational reorganisation at the time of covid-19 outbreak: an Italian and Chinese experience. Gut. 2020 doi: 10.1136/gutjnl-2020-321143. Available at: [DOI] [PubMed] [Google Scholar]
- 35.Knowlton SD, Boles CL, Perencevich EN, et al. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob Resist Infect Control. 2018;7:16. doi: 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . World Health Organization; 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 February 2020.https://apps.who.int/iris/handle/10665/331215 Available at: [Accessed August 4, 2020] [Google Scholar]
- 37.Soetikno R, Teoh AYB, Kaltenbach T, et al. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176–183. doi: 10.1016/j.gie.2020.03.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lui RN, Tang RS, Chiu PW. Striving to protect patients and healthcare professionals in endoscopy units during pandemics: from SARS to COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.002. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bollipo S, Kapuria D, Rabiee A, et al. One world, one pandemic, many guidelines: management of liver diseases during COVID-19. Gut. 2020;69:1369–1372. doi: 10.1136/gutjnl-2020-321553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong GL-H, Wong VW-S, Thompson A, et al. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5:776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arasaradnam RP, Bhala N, Evans C, et al. Faecal immunochemical testing in the COVID-19 era: balancing risk and costs. Lancet Gastroenterol Hepatol. 2020;5:717–719. doi: 10.1016/S2468-1253(20)30185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapuria D, Bollipo S, Rabiee A, et al. Roadmap to resuming care for liver diseases after COVID-19. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15178. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno CC, Yee J, Ahmed FS, et al. CT colonography's role in the COVID-19 pandemic: a safe(r), socially distanced total colon examination. Abdom Radiol (NY) 2020 doi: 10.1007/s00261-020-02674-5. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacLeod C, Wilson P, Watson AJM. Colon capsule endoscopy: an innovative method for detecting colorectal pathology during the COVID-19 pandemic? Colorectal Dis. 2020;22:621–624. doi: 10.1111/codi.15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson CC, Shen L, Lee LS. COVID-19 in endoscopy: Time to do more? Gastrointest Endosc. 2020;92:435–439. doi: 10.1016/j.gie.2020.03.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwok KO, Lai FYL, Wei VWI, et al. Comparing the impact of various interventions to control the spread of COVID-19 in twelve countries. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.06.029. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Shahidi N, Gilroy N, et al. Proposal for the return to routine endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.04.050. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aziz M, Perisetti A, Lee-Smith WM, et al. Taste changes (dysgeusia) in COVID-19: A systematic review and metaanalysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.003. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung TW-H, Sridhar S, Zhang AJ, et al. Olfactory dysfunction in coronavirus disease 2019 patients: observational cohort study and systematic review. Open Forum Infect Dis. 2020;7:ofaa199. doi: 10.1093/ofid/ofaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ENT UK. Loss of sense of smell as marker of COVID-19 infection. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7319208/.
- 57.Joint AGA/DHPA Guidance: Recommendations for Resumption of Elective Endoscopy During the COVID-19 Pandemic. 2020. Available at:https://www.dhpassociation.org/2020/04/27/aga-dhpa-resume-endoscopy-covid19/ [Accessed August 4, 2020].
- 58.Hennessy B, Vicari J, Bernstein B, et al. Guidance for resuming GI endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.05.006. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rees CJ, East JE, Oppong K, et al. Restarting gastrointestinal endoscopy in the deceleration and early recovery phases of COVID-19 pandemic: Guidance from the British Society of Gastroenterology. Clin Med. 2020;20:352–358. doi: 10.7861/clinmed.2020-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corral JE, Hoogenboom SA, Kröner PT, et al. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.04.049. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sultan S, Siddique SM, Altayar O, et al. AGA institute rapid review and recommendations on the role of pre-procedure SARS-CoV2 testing and endoscopy. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.043. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425–427. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Q, Liu G, Wang J, et al. Control measures to prevent Coronavirus disease 2019 (COVID‐19) pandemic in endoscopy centers: a multi‐centre study. Dig Endosc. 2020 doi: 10.1111/den.13755. https://onlinelibrary.wiley.com/doi/abs/10.1111/den.13755 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han J, Wang Y, Zhu L, et al. Preventing the spread of COVID-19 in digestive endoscopy during the resuming period: meticulous execution of screening procedures. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.3855. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. Available at:https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 [Accessed August 6, 2020].
- 68.ACG News Team. Joint GI Society Message on COVID-19. 2020. Available at: https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/ [Accessed August 6, 2020].
- 69.Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.019. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. 2020. Available at: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html [Accessed August 6, 2020].
- 71.Zhuang Z, Bergman M, Brochu E, et al. Temporal changes in filtering-facepiece respirator fit. J Occup Environ Hyg. 2016;13:265–274. doi: 10.1080/15459624.2015.1116692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suen LKP, Guo YP, Tong DWK, et al. Self-contamination during doffing of personal protective equipment by healthcare workers to prevent Ebola transmission. Antimicrob Resist Infect Control. 2018;7:157. doi: 10.1186/s13756-018-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. Available at:https://apps.who.int/iris/bitstream/handle/10665/330674/9789240000919-eng.pdf?sequence=1&isAllowed=y.
- 74.Pawlak KM, Kral J, Khan R, et al. Impact of COVID-19 on endoscopy trainees: an international survey. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.06.010. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Public Health England. COVID-19 infection prevention and control guidance. Available at:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/893320/COVID-19_Infection_prevention_and_control_guidance_complete.pdf.
- 76.Resuscitation Council UK. Resuscitation Council UK Statement on COVID-19 in relation to CPR and resuscitation in first aid and community settings. Available at:https://www.resus.org.uk/covid-19-resources/covid-19-resources-general-public/resuscitation-council-uk-statement-covid-19 [Accessed August 6, 2020].
- 77.Doremalen N van, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iacucci M, Cannatelli R, Labarile N, et al. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol. 2020;5:598–606. doi: 10.1016/S2468-1253(20)30119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ASGE Quality Assurance in Endoscopy Committee. Calderwood AH, Day LW, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Update 2018. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 83.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20:e102–e107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. Available at: [DOI] [PubMed] [Google Scholar]
- 86.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2024671. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020 doi: 10.1126/science.abc6284. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Statement on the fourth meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of coronavirus disease (COVID-19). Available at:https://www.who.int/news-room/detail/01-08-2020-statement-on-the-fourth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-coronavirus-disease-(covid-19) [Accessed August 4, 2020].
- 92.Holtmann G, Quigley EM, Shah A, et al. “It ain't over … till it's over!” Risk-mitigation strategies for patients with gastrointestinal diseases in the aftermath of the COVID-19 pandemic. J Gastroenterol Hepatol. 2020;35:1117–1123. doi: 10.1111/jgh.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]