Abstract
Interactions of genes in intersecting signaling pathways, as well as environmental influences, are required for the development of psoriasis. Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor and transcription factor which inhibits the expression of many proinflammatory genes. We tested the hypothesis that low levels of PPARγ expression promote the development of psoriatic lesions. We combined experimental results and network functional analysis to reconstruct the model of PPARγ-downregulated signaling in psoriasis. We hypothesize that the expression of IL17, STAT3, FOXP3, and RORC and FOSL1 genes in psoriatic skin is correlated with the level of PPARγ expression, and they belong to the same signaling pathway that regulates the development of psoriasis lesion.
1. Introduction
Psoriasis is an example of chronic inflammatory skin disorder with a complex multifactorial origin. Multiple genes cause heterogeneous heredity of psoriasis [1, 2]. Interactions of predisposing genes, as well as environmental influences, are required for the development of the disease.
Family genotyping supports the hypothesis that different phenotypes or manifestations of psoriasis are determined by different genetic loci [3]. These loci are associated with psoriasis and located at least on 13 different chromosomes and are named PSORS (psoriasis susceptibility), PSORS1-PSORS13 ([4]). Each PSORS contains a list with several revealed genes candidates [5].
Peroxisome proliferator-activated receptors (PPARs) do not get on lists of gene candidates for psoriasis; however, the important role of PPARs in antiinflammatory and immunomodulatory cellular signaling pathways has been established. Recently, association of proline12/alanine gene polymorphism (rs1801282) in peroxisome proliferator-activated receptor gamma (PPARγ, NCBI Gene ID: 5468) was found to be associated with psoriasis and obesity in Egyptian patients [6].
PPARs perform function primarily as ligand-dependent transcription factors which activate genes with PPAR-responsive elements (PPREs) in their promoter. PPARγ is detected mostly in well-differentiated suprabasal keratinocytes within the human epidermis [7]. Human hair follicle epithelial stem cells also express PPARγ which maintains their survival in normal conditions [8]. Skin adipocytes and sebocytes are the next large PPARγ depositions [9, 10], and the protein is vital for their differentiation [11, 12]. The PPARγ expression was reported to be downregulated in the psoriatic skin of mice, and DDH1 dose dependently could restore the gene expression [13]. In vitro experimental models of psoriasis showed that the expression of other PPARs (PPARa) was also decreased in the skin, while PPARb and PPARd expressions were increased [14]. Mice model of inflammatory skin diseases revealed that the expression of PPARγ and PPARa was decreased in the skin due to the absence of the Dlx3 gene [15]. The medical suppression of PPARγ improved the health of the mice model of atopic dermatitis [16]. Wang et al. reported that gene PPARγ had high level of expression in the skin of IMQ-induced psoriasis mice, and а PPARγ-selective antagonist GSK3787 was able to decrease the inflammation in the skin [17]. Finally, another animal model research showed that mutant mice with deleted PPARγ did not have sebaceous glands and normal hair follicles (HF) and developed scarring alopecia and skin inflammation [18]. There is no experimental evidence about PPARγ activity level in human skin of patients with psoriasis to our knowledge.
PPARγ signaling in psoriasis has been studied at a good level, but conflicting experimental results do not allow describing a clear picture of protein-protein interactions and pathological changes in cell pathways leading to the development of psoriasis (read below section “Pathway Model of PPARγ Signaling in Psoriasis”).
In this work, we tested a hypothesis that low levels of PPARγ may change the activity of cellular signaling pathways in the skin and facilitate the chronic inflammatory and immune response in psoriatic lesion in humans. Based on the literature-based protein-protein interactome (PPI) and pathway analysis, we proposed that low PPARγ activity promotes the development of psoriatic lesions due to changes in the inflammatory signaling pathways regulated by STAT3, RORC, FOXP3, FOSL1, and IL17A. To check the hypothesis, we measured the expression of these genes altogether with PPARγ on the mRNA level in the skin of patients with psoriasis before and after low-intensity laser treatment.
2. Materials and Methods
2.1. Protein-Protein Interactome (PPI) Analysis and Pathway Model Reconstruction
To reconstruct the PPARγ-psoriasis interactome, we used the literature-based database PSD (Resnet-2020 ®, Elsevier Pathway Studio database). PSD is a mammal-centered database where relationships between biological terms and molecules extracted from published papers with natural language processing (NLP) technology. Data from public databases with experimental types of connections are also present in PSD. Resnet-2020 contains over one million objects and more than 12 million relationships with more than 55 million supporting sentences ([19], http://www.pathwaystudio.com/).
For PPI analysis, we used SQL language and ran queries to filter PSD connections and found inhibited by PPARγ expression targets that simultaneously have positive relationships with psoriasis (see “PPARγ targets and regulators” file, list 1 in supplemental materials). To find PPARγ regulators, we selected genes that negatively regulate expression of PPARγ and simultaneously negatively regulate PPARγ expression targets (see “PPARγ targets and regulators” file, list 2 in supplemental materials). To focus only on gene expression signaling and exclude other molecular types of interaction, we considered only two types of relationships in PSD that indexed sentences about the changing of mRNA or gene expression (“Expression” and “PromoterBinding”). Queries and other parameters of network filtering are available by a request.
We used the Pathway Studio software to reconstruct the model of PPARγ signaling. Models are interactive networks which describe connections between molecules and related phenotype or biological processes. Models are kept in RNEF format, connected with PSD, and included different annotations of molecules and relationships (synonymes, identificators, references, sentences, effects, mechanism of actions, and more). All files can be found in supplemental materials (see below).
2.2. Pathway Functional Analysis
List of proteins that we had identified in the PPI analysis was set up with subnetwork enrichment analysis (SNEA, Pathways Studio), Fisher's exact test, Enrichr tool [20], and KEGG mapping tool [21]. SNEA was used to find cell processes statistically enriched with genes from list 1 and 2. SNEA is the modification of gene set enrichment analysis that accounts for relationships between genes in the network [22]. Fisher's test was used to find associated Pathway Studio pathways and Gene Ontology (GO) functional gene groups [23]. We found the associated KEGG pathways with the KEGG mapping tool, and we found the other associations with the Enrichr tool.
Cell processes were selected if more than 5 genes from the list 3 (combined genes from list 1 and list 2) were overlapped with total genes associated with the pathway and if more than 5% genes from the lists 1 and 2 were overlapped with a subnetwork or GO group. We selected top subnetworks and KEGG pathways filtered by rank and top PS pathways and GO groups filtered by Jaccard index. For the comparison of methods, we selected top 50 subnetworks, 50 pathways, and 50 GO groups after manual filtering off unrelated diseases (such as cancer), viral, and bacterial KEGG pathways. See supplemental materials for results of pathway functional analysis (“PPARG network analysis” file and “PPARG Enrichr analysis” file).
2.3. Microarray In Silico Analysis
Public microarray data (GEO, GSE13355) was used to verify the reconstructed model of PPARγ signaling in psoriasis. GSE13355 contains data about the expression of the human genome in skin samples of 58 patients with psoriasis [24]. DEs (differentially expressed genes) were identified with a two-class unpaired t-test between samples of lesional skin of each patient (PP samples) and nonlesional skin uninvolved samples (PN samples). Multiple probes were averaged by the best p value or maximum magnitude. The Pathway Studio software was used for calculation of DE and pathway analysis.
2.4. Supplemental Materials
All supplemental materials are available to download from ResearchGate resource by the link https://www.researchgate.net/publication/340427568_Supplemental_Materials_The_role_of_PPARg_downregulated_signaling_in_psoriasis [25]. All pathway models and their annotations are available for browsing and can be downloaded at http://www.transgene.ru/ppar-pathways.
3. Results and Discussion
3.1. Reconstruction of Downregulated PPARγ Pathway Model Associated with Psoriasis
For testing the hypothesis that low levels of PPARγ trigger inflammatory signaling pathways in the skin, we analysed the protein-protein interaction literature-based network (PSD, Elsevier Pathway Studio) and several public ontologies and databases (Gene Ontology, Human Protein Atlas, KEGG, Reactome).
First, in the PSD network, we identified PPARγ downstream expression targets and upstream regulators (inhibitors) of PPARγ expression. For researching the downstream targets, we looked for the genes and proteins which were reported to be inhibited by PPARγ and simultaneously were positive biomarkers for psoriasis. 146 associated with psoriasis genes and gene families whose expressions are repressed by PPARγ had been found. For researching the upstream of PPARγ signaling, we focused on the transcriptional factors which can inhibit both the expression of PPARγ and his direct targets. 99 associated with psoriasis unique negative regulators of PPARγ had been identified. Then we combine regulators with targets to obtain 182 names of unique genes forming the PPARγ-downregulated subnetwork associated with psoriasis (see supplemental materials, “PPARG regulators and targets” file, list 3) (Figure 1).
Figure 1.
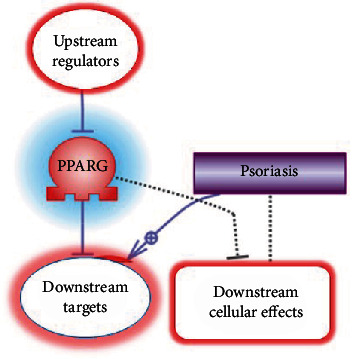
The logic of discovering the members of PPARγ-downregulated subnetwork associated with psoriasis.
3.2. Comparative Pathway Analysis of PPARγ-Downregulated Signaling Associated with Psoriasis
Several methods of pathway analysis were performed to explore the functional roles of 182 targets, and regulators of PPARγ revealed in PPI analysis. Methods of pathway functional analysis are widely used for discovering cellular processes and signalings that are statistically associated with the list of genes or proteins [26].
We compared results from pathway functional analysis with three resources: Gene Ontology, Elsevier Pathways, and KEGG Pathways. Gene Ontology is the source of groups of proteins or genes manually assigned by their different functional roles. Elsevier Pathways and KEGG Pathways are manually reconstructed schemas or models of interactions between proteins describing molecular mechanisms of one or several biological processes. Gene Set Enrichment Analysis (GSEA) is a well-known method to analyse predefined and manually created collections of gene groups and pathways [27]. Besides GSEA, we used SNEA method which allows finding associated cellular processes based on literature-based PPI network. SNEA does not use predefined groups of genes or pathways and is considered less biased [22, 26].
According to the results of comparative pathway analysis, PPARγ-downregulated signaling is associated with adipogenesis, activation of myeloid proinflammatory cells (with a predominance of mast cells and dendritic cells), and activation of overall immune system response (with a predominance of Th17 cells). Also, fibrogenesis, cell-to-cell contacts, vascular-related processes, and universal cell processes, such as cell proliferation or cell death, were identified (Figure 2). Cellular possesses directly associated with psoriasis were present in results from each source that we compared (Table 1).
Figure 2.
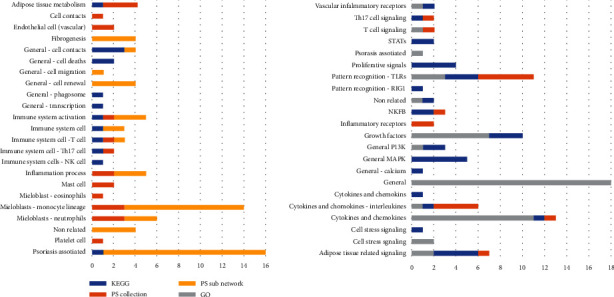
Cellular processes associated with members of PPARγ-downregulated signaling associated with psoriasis. Numbers are for the sum of pathways, subnetworks, or GO groups in each category. Different sources are highlighted with blue (KEGG database), orange (Pathway Studio Pathway Collection), light orange (Resnet-2020 database), and grey (GO). For the complete list of results, see supplemental materials (“PPARG network analysis” file).
Table 1.
Cell processes directly related to psoriasis and enriched with genes and proteins from the PPARγ-downregulated signaling. See complete results with additional statistics in supplemental materials, “PPRAG network analysis” file.
| Name of the process or signaling | Source | Rank or Jaccard similarity (the closer to 10%, the more similarity) | Category |
|---|---|---|---|
| Keratinocyte activation in psoriatic arthritis | PS Collection | 9.13% | Disease |
| Lesion size | PS subnetwork | 46 | Targets neighbors |
| Keratinocyte proliferation | PS subnetwork | 100 | Targets neighbors |
| Skin fibrosis | PS Collection | 9.09% | Disease |
| T cell differentiation block in psoriasis | PS Collection | 8.88% | Disease |
| Th17 cell and Th1 immune response in psoriatic arthritis | PS Collection | 8.82% | Disease |
| Dendritic cell dysfunction in psoriatic arthritis | PS Collection | 8.44% | Disease |
| T cell cytotoxic response against melanocytes in vitiligo | PS Collection | 6.20% | Disease |
| Synovial fibroblast activation in psoriatic arthritis | PS Collection | 8.18% | Disease |
| Inflammatory reaction in acne vulgaris | PS Collection | 5.70% | Disease |
| Apoptotic keratinocyte clearance recession in systemic lupus erythematosus | PS Collection | 5.69% | Disease |
| Atopic dermatitis | PS Collection | 5.62% | Disease |
| Hair follicle keratinocyte apoptosis | PS Collection | 5.56% | Disease |
| Vitiligo | PS Collection | 5.07% | Disease |
| Melanogenesis—Homo sapiens | KEGG | n/a | Pathway |
| Positive regulation of timing of anagen | GO | 1.10% | GO: biological_process |
| Glycosaminoglycan binding | GO | 4.24% | GO: molecular_function |
Top subnetworks from SNEA were neighbors of adipogenesis and adipocyte differentiation, followed by the immune response, and T-development. The subnetworks “neighbours of monocyte recruitment or differentiation” and “macrophage differentiation” had the most percent (9%) of overlapped genes from PPARγ-downregulated signaling.
GSEA analysis of PS Pathway Collection and KEGG pathways resulted in many cancer-related processes. The disease taxonomy filtering with PS pathways about skin and immune system identified processes related to adipokines and IL17 signaling (Table 2). The signaling of aryl hydrocarbon receptor (AHR) in Th17 cells was the pathway with the biggest percent (48%) of overlapped genes from PPARγ-downregulated signaling.
Table 2.
List of PS pathways associated with members of PPARγ-downregulated signaling associated with psoriasis. See complete results with additional statistics in supplemental materials, “PPRAG network analysis” file.
| Pathway name | # of entities | Overlap | Percent overlap | p value | Jaccard similarity | Pathway taxonomy top category |
|---|---|---|---|---|---|---|
| EGFR—expression targets in skin | 98 | 26 | 26 | 4.68E-11 | 10.28% | Biomarkers |
| GPCRs family—expression targets in lymphoid system and blood | 89 | 24 | 26 | 1.97E-10 | 9.76% | Biomarkers |
| Adipokine production by adipocyte | 58 | 20 | 34 | 4.8E-16 | 9.13% | Biological process |
| Skin fibrosis | 83 | 22 | 26 | 3.29E-17 | 9.09% | Disease |
| Scavenger receptor OLR1 in inflammation-related endothelial dysfunction | 73 | 21 | 28 | 2.8E-17 | 9.01% | Disease |
| T cell differentiation block in psoriasis | 52 | 19 | 36 | 5.87E-18 | 8.88% | Disease |
| Adipokine production by adipocyte impaired in obesity | 56 | 19 | 33 | 2.99E-17 | 8.72% | Disease |
| CD40—expression targets in thymus | 58 | 19 | 32 | 4.67E-10 | 8.64% | Biomarkers |
| Lymphocyte-mediated myocardial injury in myocarditis | 84 | 21 | 25 | 6.67E-16 | 8.61% | Disease |
| IL17 signaling in psoriasis | 49 | 18 | 36 | 4.08E-17 | 8.49% | Disease |
| Th17 cell differentiation | 73 | 19 | 26 | 8.94E-13 | 8.09% | Biological process |
Among the top KEGG pathways enriched with our gene list, we identified general MAPK and PI3K signaling and cancer-related pathways (for example, “hsa05200 Pathways in cancer - Homo sapiens”). TNF signaling pathway (hsa04668), as well as Th17 cell (hsa04659) and IL17 pathway (hsa04657), was also in the top 10 results. The cytokine-cytokine receptor interaction (hsa05200) and PI3K-Akt signaling (hsa04151) had the highest number of overlapped entities (48 and 34).
The list of revealed in pathway analysis molecular cascades completes the lists of cell processes.
There was no surprise that activation of general cellular flows like ERK/MAPK, RAS/ACT1, and adipose cells related with AMPK, MTOR, and cAMP cascades was associated with the list of PPARγ targets and regulators. Also, among the top of associated molecular signalings, there were well predictable inflammatory cascades like Toll-like receptors, interleukins, and interleukin receptors signaling (IL17, IL1B, IL6, and IL1R1) altogether with all-purpose cytokines and cytokine receptors signaling (CXCR3, CCR1, and TNF). Signalings related to transcription factors NFKB and STATs also were significantly associated with the analysed list. GO functional group “GO: glycosaminoglycan binding”; “IL1R1 signaling in Pneumocytes” from PS Pathway Collection; and “ErbB signaling pathway” (hsa04012) from KEGG had the maximum rank (see complete results with additional statistics in supplemental materials, “PPRAG network analysis” file). Glycosaminoglycans are essential for skin functioning. IL1R1 is a receptor commonly activated in any nonspecific inflammatory processes. Finally, the ERbB/EGFR family is involved in cell proliferation and tumor development.
Additional comparison of pathway analysis results with other pathways databases (WikiPathways, Reactome, and Biocarta analysed with Enrichr tool) confirmed results obtained with PS Pathway Collection (Figure 3). Pathways from all sources revealed skin inflammatory processes, TLRs, and interleukin-related cascades. However, the list of molecules was different compared with PS and KEGG results presenting IL10 and IL22R and no IL17 associations. In addition, analysis with DisGeNET [28] confirmed that the PPARγ regulators and targets are connected with psoriasis since top diseases associated with the list 3 were as follows: psoriasis, epithelial hyperplasia of skin, and inflammatory dermatosis. Allergic reaction, neutrophilia, and vascular diseases were also in the top 10 results (see supplemental materials, file “PPARG Enrichr analysis” file).
Figure 3.
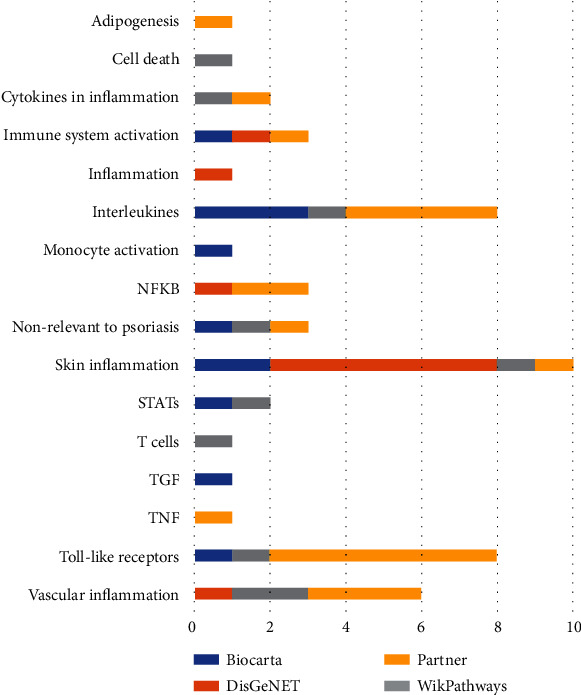
Comparison of results of pathway analysis (GSEA) with different sources for PPARγ-downregulated signaling associated with psoriasis. Results were calculated with the Enrichr tool. Numbers are for the sum of pathways in each category. Different sources are highlighted with blue (Biocarta database), grey (Partner database), orange (DisGeNET), and yellow (WikiPathways).
3.3. Pathway Model of PPARγ Signaling in Psoriasis
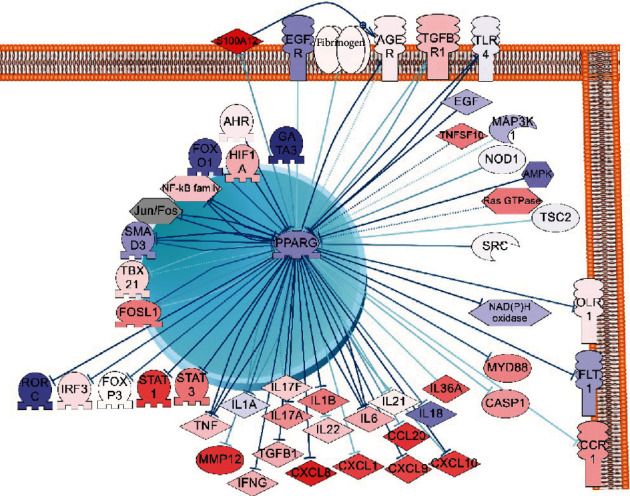
Considering results of PPI network and functional pathway analysis, we build a hypothetical model that describes cellular molecular mechanisms of involvement of PPARγ in the maintenance of chronic inflammatory and immune response in human psoriatic skin. Literature-based network (PSD) was used to build the model. Figure 4 described the adopted for the publication-simplified version of the downregulated PPARγ pathway model. See supplemental materials for the completed version of the pathway model.
Figure 4.
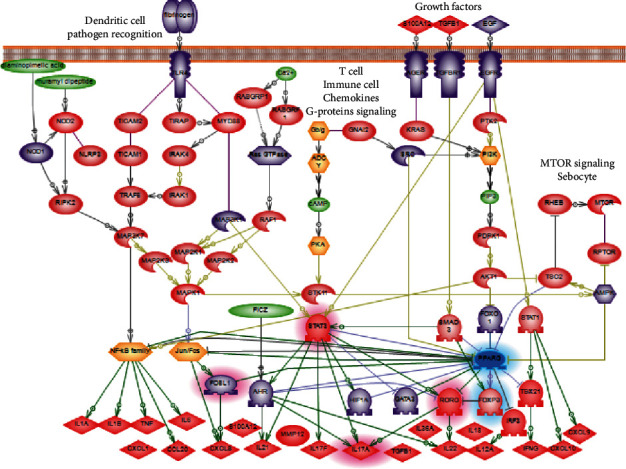
Model of downregulated PPARγ signaling in psoriasis. PPARγ which expression is downregulated in psoriasis is colored blue. Regulators that may inhibit the expression of PPARγ (data based on PPI and pathway functional analysis) are colored in violet. Targets which may be overexpressed in psoriasis due to the decrease of the negative impact of PPARγ are colored in bright red. FOXP3, STAT3, IL17A, RORC, and FOSL1 are highlighted according to own experiments (read section “Preliminary analysis of PPARγ signaling in human psoriatic skin”). Downexpressed genes (PPARγ and FOXP3) are highlighted in blue, and overexpressed genes are highlighted in red.
Based on the model, reducing the level of the PPARγ gene expression may be a result of the overregulation of several cascades. Pattern recognition receptors (TLRs, NOD1, NOD2, and CLEC7A) that sensor pathogens and highly expressed in keratinocytes and monocytes during the infection may be one of such cascades. All-purpose cellular cascades like growth factor signaling, G-proteins, and MTOR signaling also were reported to be inhibitors of PPARγ expression in literature and revealed in our analysis. Moreover, transcription factors including NF-kBs, JUN-FOS, AHR, GATA3, HIF1A, FOXO1, and FOSL1 can directly inhibit PPARγ expression. All these transcription factors are overstimulated in the inflammatory and immune response. For example, it is reported that NF-kBs are stimulated in systemic inflammatory processes in general and in psoriasis as well [29, 30].
In healthy skin, PPARγ inhibits mentioned transcriptional factors in a feedback regulation loop. PPARγ may directly bind and suppress transcriptional factors STAT3 and RORC, by thus blocking the synthesis of proinflammatory cytokines including IL17. Less quantity of expressed cytokines decreases the Th17 cell proliferation, minimises chemotaxis of neutrophils and monocytes, and results in the reduction of inflammation in psoriatic lesions.
IL17 which is produced mostly by TH17 cells plays the central role in the development of psoriasis because it stimulates keratinocytes to secrete proinflammatory cytokines and antibacterial peptides [31]. IL17 pathway and Th17 cells had a strong association with PPARγ-downregulated signaling confirmed by our network and functional analysis.
Th17 cells need robust activity of STAT3 gene for their function and differentiation. Also, STAT3 is described as an important linkage between keratinocytes and immune cells [32]. Previously, the expression of STAT3 was shown to be repressed due to PPARγ activation [33]. STAT3 may also act as a regulator of PPARγ expression; however, it is not clear whether with positive or negative effect [34].
As a transcription factor, STAT3 is reported to be a strong activator of RORC (RORγ) and, probably, IL17 gene expression. From the other side, gene RORC is the major inductor of the expression of IL17 cytokine family [35]. PPARγ was shown to bind the RORC promoter and suppress its expression altogether with RORC-mediated Th17 cell differentiation [36].
Transcription factor FOXP3 is closely associated with psoriasis and the diminishing of Treg cell number [37, 38]. It was shown that activated PPARγ induces the stable FOXP3 expression by strong inhibiting effect on DNA methyltransferases. The activating effect of PPARγ on FOXP3 results in the proliferation of iTreg cells [39].
FOSL1 is the transcriptional factor which plays an important role in many processes related to cell differentiation and tissue remodeling ([40–42]). FOSL1 (FOS-like antigen 1) is expressed in low level in healthy tissues; however, its expression rises due to presence of mitogens or toxins. The accumulation of the FOSL1 protein in the skin depends on the stage of the keratinocyte differentiation [43]. Markers of stratum corneum differentiation like gene IVL are the main expression targets of FOSL1 [44].
The degree of the pathogenicity of downregulated PPARγ in psoriatic lesion depends on the cell type. It is known that PPARγ is expressed in Th17 cells as well as in keratinocytes, sebocytes, and other cells of the psoriatic lesion [7, 8, 10–12]. Functional and network analysis supported the association of PPARγ-downregulated signaling with keratinocytes, vascular endothelium, vascular smooth muscle cells, macrophages, fibroblasts and adipocytes, and monocytes lineage (particular with CD33+ and CD14+ monocytes) (Figure 5). However, we did not attempt to separate the PPARγ pathway model by appropriate cell types which is a disadvantage of this work. There is no reliable way to take in account cell specificity in our modeling paradigm. Moreover, we expect that most of the revealed from the literature network analysis cascades will be equal for different human cells due to insufficient experimental studies.
Figure 5.
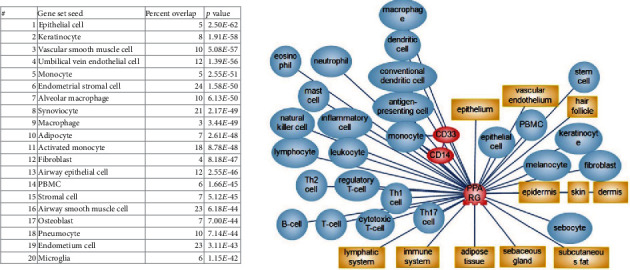
Cells associated with downregulated PPARγ signaling. SNEA method and Pathway Studio were used to calculate the results. See the complete list with statistics in supplemental materials, “PPARG and cell” file.
For additional evaluation of the reconstructed model, we analysed the public microarray data (GEO:GSE13355). In that experiment, biopsies from 58 psoriatic patients were run on Affymetrix microarrays contain more than 50000 gene probes [45]. We uploaded raw data from GEO and calculated differentially expressed genes (DEs) between samples of psoriatic skin and unaltered samples for all patients. Then, we used the pathway analysis to explore the difference in the expression for genes of the PPARγ model we build (Figure 6).
Figure 6.
Evaluation of PPARγ-downregulated subnetwork (selected PPARγ regulators and targets) associated with psoriasis using microarray data analysis (results of differential expression analysis of psoriatic lesions vs. unaltered lesions). The saturation in blue indicates the degree of gene downexpression in psoriatic samples in comparison with unaltered samples. The saturation in red indicates the degree of gene overexpression. The list of targets and regulators can be seen in supplemental materials, “PPARG regulators and targets,” list 3.
PPARγ gene was slightly downregulated in psoriatic lesions comparing to nonaltered lesions in GSE13355 microarray data (Figure 6, PPARγ expression diagram).
We assumed that regulators of PPARγ signaling should have higher expression in psoriatic lesion than in normal skin. Only S100A12 (S100 calcium-binding protein A12) had a significantly higher level of expression in analysed microarray data comparing with all regulators of PPARγ that we selected for the model (Figures 6 and 7). S100A12 binds to the AGER receptor which belongs to the immunoglobulin superfamily and is involved in many processes of inflammation and immune response. S100A12 is thought to be the most prominent biomarker of psoriasis [46]. Also, polymorphisms in AGER receptor were found to be associated with psoriasis [47].
Figure 7.
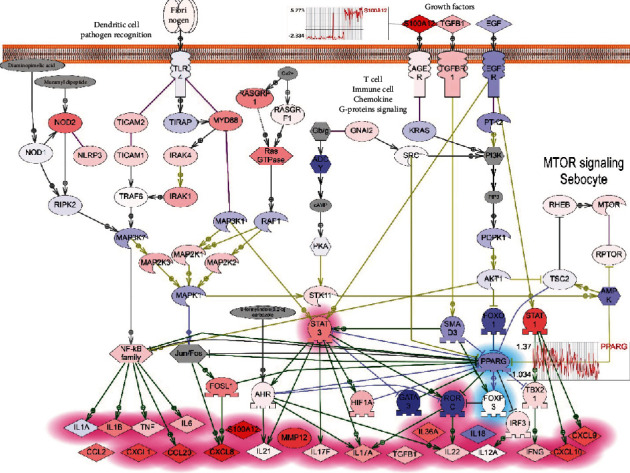
Evaluation of PPARγ-downregulated model associated with psoriasis using microarray data analysis (results of differential expression analysis of psoriatic lesions vs. unaltered lesions from GEO:GSE13355). The saturation in blue indicates the degree of gene downexpression in psoriatic samples in comparison with unaltered samples. The saturation in red indicates the degree of gene overexpression. The plots of expression pattern in psoriatic lesions compared with healthy skins samples are shown for PPARγ and S100A12 genes.
The EGFR signaling almost completely was downexpressed in this microarray data including the FOXO1 expression which is one of the direct inhibitors of PPARγ. Therefore, EGFR/FOXO1 signaling probably does not play an important role in the regulation of PPARγ in psoriasis (Figure 7).
4. Conclusion
In our previous work, we reviewed the recent progress in psoriasis pathways and published two pathway models. The first pathway model described the shift to TH17 cell production during the differentiation of psoriatic T cells. The primary cause of the shift of the T cell differentiation is supposed to be genetic mutations, for example, in IL23R receptors. The second model showed how elevated levels of IL17 and IL22 may activate keratinocytes to release different cytokines and chemokines for attracting neutrophils and other inflammatory cells in the psoriatic lesion [19].
In this work, we tested the hypothesis that PPARγ signaling when downregulated may promote psoriasis. We built the model of PPARγ-dependent pathways involved in the development of the psoriatic lesions. However, we used a different approach for reconstructing the pathway model and selected key members with bioinformatic analysis. We included in the pathway model the top statistically significant regulators of PPARγ gene expression and PPARγ-depended expression targets. Then, we included significant molecular cascades and cell processes from results of the functional analysis (IL17 signaling, TLRs signaling, activation of STAT3 or NFKB transcription factors, and others). We tested the model with analysis of published microarray data.
While the prominent role of RORC in psoriasis as the major controller of Th17 cell differentiation is well described, however, the evidence of RORC expression in psoriasis is controversial and supported by work where mice T cells and dendritic cells had increased STAT3/RORC expression [48]; still, patients with psoriasis had elevated level of RORC (RORG-t isoform) [49]. In analysed published microarray data, the level of expression of RORC was downregulated in most of 58 patients.
We detected downregulation of PPARγ gene expression in human psoriatic skin from 23 patients with real-time PCR method (data not shown). Our results are similar to the data from microarray on 58 patients where average PPARγ gene expression also is slightly downregulated in psoriatic lesions [45]. Our results do not confirm the work of Westergaard et al. which described the slightly higher level of the PPARγ expression in human psoriatic skin compared to normal skin. However, the level of PPARγ mRNA was close to the detection limit in their research [39]. This difference may be due the detection of different isoforms of PPARγ which all have different patterns of the expression [50]. More research on protein level is needed to conclude whether PPARγ gene expression is downregulated in psoriatic lesions.
Within the framework of the model validation, we hypothesize that signaling related to repressed PPARγ activity is correlated with the development of psoriasis. IL17A, STATS3, and RORC (RORg) are statistically significant PPARγ negative targets, and we detected higher levels of their mRNA in psoriatic lesion of 23 patients and moreover, the decrease of their expression levels after laser treatment (preliminary results not shown). The alignment of our preliminary experimental results with microarray data and PPI network analysis shows that the reconstructed model of PPARγ-downregulated signaling in psoriasis can be useful for further research.
Acknowledgments
We thank the “University Diagnostic Laboratory” LLC for funding along with Elsevier Inc. and Pathway Studio® for software and the database.
Data Availability
All supplemental materials (four excel files) are available to download from ResearchGate resource by the link https://www.researchgate.net/publication/340427568_Supplemental_Materials_The_role_of_PPARg_downregulated_signaling_in_psoriasis. All pathways models and their annotations are available for browsing and can be downloaded at http://www.transgene.ru/ppar-pathways.” Preprint has posted on bioRxiv: https://biorxiv.org/cgi/content/short/2020.09.01.274753v1.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary materials include 3 files with results of network and pathways analysis in Pathway Studio software, one file with results of GSEA in Enrichr tool, and one file with preliminary results of experimental validations (real-time gene expression analysis) of the model.
References
- 1.Nickoloff B. J., Nestle F. O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. The Journal of Clinical Investigation. 2004;113(12):1664–1675. doi: 10.1172/JCI200422147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters B. P., Weissman F. G., Gill M. A. Pathophysiology and treatment of psoriasis. American Journal of Health-System Pharmacy. 2000;57(7):645–659. doi: 10.1093/ajhp/57.7.645. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson L., Enlund F., Torinsson Å., et al. A genome-wide search for genes predisposing to familial psoriasis by using a stratification approach. Human Genetics. 1999;105(6):523–529. doi: 10.1007/s004399900182. [DOI] [PubMed] [Google Scholar]
- 4.Merve H. M. Psoriasis and genetics. In: Sevilay K., editor. Büyük Başak (Tran.), Psoriasis. Rijeka: IntechOpen; 2017. p. p. 1. [DOI] [Google Scholar]
- 5.Barker J. N. W. N. Genetic aspects of psoriasis. Clinical and Experimental Dermatology. 2001;26(4):321–325. doi: 10.1046/j.1365-2230.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 6.Seleit I., Bakry O., Abd El Gayed E., Ghanem M. Peroxisome proliferator-activated receptor-γ gene polymorphism in psoriasis and its relation to obesity, metabolic syndrome, and narrowband ultraviolet B response: a case–control study in Egyptian patients. Indian Journal of Dermatology. 2019;64(3):192–200. doi: 10.4103/ijd.IJD_114_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Icre G., Wahli W., Michalik L. Functions of the peroxisome proliferator-activated receptor (PPAR) α and β in skin homeostasis, epithelial repair, and morphogenesis. Journal of Investigative Dermatology Symposium Proceedings. 2006;11(1):30–35. doi: 10.1038/sj.jidsymp.5650007. [DOI] [PubMed] [Google Scholar]
- 8.Billoni N., Buan B., Gauti B. Expression of peroxisome proliferator activated receptors (PPARs) in human hair follicles and PPARα involvement in hair growth. Acta Dermato-Venereologica. 2000;80(5):329–334. doi: 10.1080/000155500459240. [DOI] [PubMed] [Google Scholar]
- 9.Alestas T., Ganceviciene R., Fimmel S., Müller-Decker K., Zouboulis C. C. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. Journal of Molecular Medicine. 2006;84(1):75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T., Miki Y., Kakuo S., et al. Expression of steroidogenic enzymes in human sebaceous glands. Journal of Endocrinology. 2014;222(3):301–312. doi: 10.1530/JOE-14-0323. [DOI] [PubMed] [Google Scholar]
- 11.Nehrenheim K., Meyer I., Brenden H., Vielhaber G., Krutmann J., Grether-Beck S. Dihydrodehydrodiisoeugenol enhances adipocyte differentiation and decreases lipolysis in murine and human cells. Experimental Dermatology. 2013;22(10):638–643. doi: 10.1111/exd.12218. [DOI] [PubMed] [Google Scholar]
- 12.Paus R., Klein J., Permana P. A., et al. What are subcutaneous adipocytes really good for…? Experimental Dermatology. 2007;16(1):45–47. doi: 10.1111/j.1600-0625.2006.00519_1.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitahata K., Matsuo K., Hara Y., et al. Ascorbic acid derivative DDH-1 ameliorates psoriasis-like skin lesions in mice by suppressing inflammatory cytokine expression. Journal of Pharmacological Sciences. 2018;138(4):284–288. doi: 10.1016/j.jphs.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Friedmann P., Cooper H., Healy E. Peroxisome proliferator-activated receptors and their relevance to dermatology. Acta Dermato-Venereologica. 2005;85(3):194–202. doi: 10.1080/00015550510030104. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J., Kita R., Kwon H.-S., et al. Epidermal ablation of Dlx3 is linked to IL-17-associated skin inflammation. Proceedings of the National Academy of Sciences. 2011;108(28):11566–11571. doi: 10.1073/pnas.1019658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung K., Tanaka A., Fujita H., et al. Peroxisome proliferator–activated receptor γ–mediated suppression of dendritic cell function prevents the onset of atopic dermatitis in NC/Tnd mice. Journal of Allergy and Clinical Immunology. 2011;127(2):420–429.e6. doi: 10.1016/j.jaci.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Hao Y., Wang X., et al. A PPARδ-selective antagonist ameliorates IMQ-induced psoriasis-like inflammation in mice. International Immunopharmacology. 2016;40:73–78. doi: 10.1016/j.intimp.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Sardella C., Winkler C., Quignodon L., et al. Delayed hair follicle morphogenesis and hair follicle dystrophy in a lipoatrophy mouse model of Pparg total deletion. Journal of Investigative Dermatology. 2018;138(3):500–510. doi: 10.1016/j.jid.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesterova A. P., Yuryev A., Klimov E. A., et al. Disease Pathways: An Atlas of Human Disease Signaling Pathways. 1st ed. Waltham: Elsevier; 2019. [Google Scholar]
- 20.Chen E. Y., Tan C. M., Kou Y., et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14(1):p. 128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehisa M., Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Science. 2019;29(1):28–35. doi: 10.1002/pro.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotelnikova E., Yuryev A., Mazo I., Daraselia N. Computational approaches for drug repositioning and combination therapy design. Journal of Bioinformatics and Computational Biology. 2011;8(3):593–606. doi: 10.1142/s0219720010004732. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner M., Ball C. A., Blake J. A., et al. Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding J., Gudjonsson J. E., Liang L., et al. Gene expression in skin and lymphoblastoid cells: refined statistical method reveals extensive overlap in cis-eQTL signals. American Journal of Human Genetics. 2010;87(6):779–789. doi: 10.1016/j.ajhg.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobolev V. Supplemental Materials [WWW Document] Research Gate; 2020. URL https://www.researchgate.net/publication/340427568_Supplemental_Materials_The_role_of_PPARg_downregulated_signaling_in_psoriasis. [Google Scholar]
- 26.Nesterova A. P., Klimov E. A., Zharkova M., et al. Chapter 14- applications of disease pathways in biology and medicine. In: Nesterova A. P., Klimov E. A., Zharkova M., et al., editors. Disease Pathways. Elsevier; 2020. pp. 629–668. [DOI] [Google Scholar]
- 27.Subramanian A., Tamayo P., Mootha V. K., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings. National Academy of Sciences. United States of America. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piñero J., Bravo À., Queralt-Rosinach N., et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Research. 2017;45(D1):D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T., Zhang J., Yin J., et al. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. The Journal of Biological Chemistry. 2010;285(7):4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X., He M., Liu T., Zeng Y., Zhang W. Effect of salusin-ß on peroxisome proliferator-activated receptor gamma gene expression in vascular smooth muscle cells and its possible mechanism. Cellular Physiology and Biochemistry. 2015;36(6):2466–2479. doi: 10.1159/000430207. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A., Nikamo P., Lohcharoenkal W., et al. MicroRNA-146a suppresses IL-17–mediated skin inflammation and is genetically associated with psoriasis. Journal of Allergy and Clinical Immunology. 2017;139(2):550–561. doi: 10.1016/j.jaci.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhari S., Saini N. Hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. Journal of Cellular Physiology. 2014;229(11):1630–1638. doi: 10.1002/jcp.24608. [DOI] [PubMed] [Google Scholar]
- 33.Hsu H.-T., Sung M.-T., Lee C.-C., et al. Peroxisome proliferator-activated receptor γ expression is inversely associated with macroscopic vascular invasion in human hepatocellular carcinoma. International Journal of Molecular Sciences. 2016;17(8):p. 1226. doi: 10.3390/ijms17081226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuna H., Avdiushko R. G., Sindhava V. J., et al. Regulation of the mucosal phenotype in dendritic cells by PPARγ: role of tissue microenvironment. Journal of Leukocyte Biology. 2014;95(3):471–485. doi: 10.1189/jlb.0713408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaishi M., Ishizaki M., Suzuki K., Isobe T., Shimozato T., Sano S. Oral administration of a novel RORγt antagonist attenuates psoriasis-like skin lesion of two independent mouse models through neutralization of IL-17. Journal of Dermatological Science. 2017;85(1):12–19. doi: 10.1016/j.jdermsci.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Hermann-Kleiter N., Meisel M., Fresser F., et al. Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORγt binding to the Il17a promoter. Journal of Autoimmunity. 2012;39(4):428–440. doi: 10.1016/j.jaut.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorn Bovenschen H., van de Kerkhof P. C., van Erp P. E., Woestenenk R., Joosten I., Koenen H. J. P. M. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. Journal of Investigative Dermatology. 2011;131(9):1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 38.Shu Y., Hu Q., Long H., Chang C., Lu Q., Xiao R. Epigenetic variability of CD4+CD25+ Tregs contributes to the pathogenesis of autoimmune diseases. Clinical Reviews in Allergy & Immunology. 2017;52(2):260–272. doi: 10.1007/s12016-016-8590-3. [DOI] [PubMed] [Google Scholar]
- 39.Lei J., Hasegawa H., Matsumoto T., Yasukawa M. Peroxisome proliferator-activated receptor α and γ agonists together with TGF-β convert human CD4 + CD25 − T cells into functional Foxp3 + regulatory T cells. The Journal of Immunology. 2010;185(12):7186–7198. doi: 10.4049/jimmunol.1001437. [DOI] [PubMed] [Google Scholar]
- 40.Sobolev V. V., Zolotorenko A. D., Soboleva A. G., et al. Effects of expression of transcriptional factor AP-1 FOSL1 gene on psoriatic process. Bulletin of Experimental Biology and Medicine. 2011;150(5):632–634. doi: 10.1007/s10517-011-1208-0. [DOI] [PubMed] [Google Scholar]
- 41.Sobolev V. V., Zolotarenko A. D., Soboleva A. G., et al. Expression of the FOSL1. Genetika. 2010;46(1):104–110. [PubMed] [Google Scholar]
- 42.Young M. R., Colburn N. H. Fra-1 a target for cancer prevention or intervention. Gene. 2006;379:1–11. doi: 10.1016/j.gene.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Mehic D., Bakiri L., Ghannadan M., Wagner E. F., Tschachler E. Fos and Jun proteins are specifically expressed during differentiation of human keratinocytes. Journal of Investigative Dermatology. 2005;124(1):212–220. doi: 10.1111/j.0022-202X.2004.23558.x. [DOI] [PubMed] [Google Scholar]
- 44.Adhikary G., Crish J., Lass J., Eckert R. L. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Investigative Ophthalmology & Visual Science. 2004;45(4):p. 1080. doi: 10.1167/iovs.03-1180. [DOI] [PubMed] [Google Scholar]
- 45.Nair R. P., Duffin K. C., Helms C., et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nature Genetics. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilsmann-Theis D., Wagenpfeil J., Holzinger D., et al. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. Journal of the European Academy of Dermatology and Venereology. 2016;30(7):1165–1170. doi: 10.1111/jdv.13269. [DOI] [PubMed] [Google Scholar]
- 47.Puig L., López-Ferrer A. The AGEs of psoriasis: a biomarker for severity and a pathogenetic link to comorbidities. Acta Dermato-Venereologica. 2017;97(7):p. 775. doi: 10.2340/00015555-2716. [DOI] [PubMed] [Google Scholar]
- 48.Nadeem A., Al-Harbi N. O., Ansari M. A., et al. Psoriatic inflammation enhances allergic airway inflammation through IL-23/STAT3 signaling in a murine model. Biochemical Pharmacology. 2017;124:69–82. doi: 10.1016/j.bcp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Mendoza G. J., Almeida O., Steinfeld L. Intermittent fetal bradycardia induced by midpregnancy fetal ultrasonographic study. American Journal of Obstetrics and Gynecology. 1989;160(5):1038–1040. doi: 10.1016/0002-9378(89)90155-5. [DOI] [PubMed] [Google Scholar]
- 50.Meirhaeghe A., Amouyel P. Impact of genetic variation of PPARγ in humans. Molecular Genetics and Metabolism. 2004;83(1-2):93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Avci P., Gupta A., Sadasivam M., et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Y., Richman L., Morehouse C., et al. Type I interferon: potential therapeutic target for psoriasis? PLoS One. 2008;3(7, article e2737) doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials include 3 files with results of network and pathways analysis in Pathway Studio software, one file with results of GSEA in Enrichr tool, and one file with preliminary results of experimental validations (real-time gene expression analysis) of the model.
Data Availability Statement
All supplemental materials (four excel files) are available to download from ResearchGate resource by the link https://www.researchgate.net/publication/340427568_Supplemental_Materials_The_role_of_PPARg_downregulated_signaling_in_psoriasis. All pathways models and their annotations are available for browsing and can be downloaded at http://www.transgene.ru/ppar-pathways.” Preprint has posted on bioRxiv: https://biorxiv.org/cgi/content/short/2020.09.01.274753v1.


