Abstract
Objectives:
To test the effect of perceived sleep duration on cognitive performance.
Methods:
Sixteen healthy individuals [8F; mean age (± SD): 24.2 ± 3.0 years)] received an 8-hour sleep opportunity followed by a 5-hour opportunity on two consecutive nights. Upon waking, they were randomized to being informed that they received either an 8-h or 5-h sleep opportunity, via a clock that ran either fast, slow or normally. Cognitive performance was assessed using 10-min auditory psychomotor vigilance tests and subjective sleepiness ratings. Homeostatic and circadian sleep drive was assessed using waking electroencephalography (EEG).
Results:
Reaction time was significantly quicker when individuals thought that they had slept for 8 hours but given a 5-hour sleep opportunity. Conversely, reaction times were significantly slower when individuals thought they had 5 hours of sleep but given an 8-hour sleep opportunity. EEG delta power (1.0–4.5 Hz) during wake increased significantly when sleep was restricted to 5 hours, and individuals thought they slept for 5 hours, but this increase was attenuated with a perceived sleep duration of 8 hours following a 5-hour opportunity. EEG delta power did not increase, however, with perceived sleep restriction. EEG high-alpha activity (10.5–11.5 Hz) was consistently higher when participants thought that they had an 8-hour sleep opportunity, regardless of the actual duration.
Conclusions:
These results suggest that perceived sleep duration may modulate psychosomatic responses. Additional studies with predefined outcomes and analyses are necessary to confirm these findings, which may have important implications for understanding how sleep affects cognition and psychosomatic responses.
Keywords: Alertness, Cognition, False-clock paradigm, Perceived time, Sleep
INTRODUCTION
The age-old “mind/body” problem continues to challenge philosophers and scientists alike - how can something non-material, such as a thought, affect the material body? There is a growing placebo literature demonstrating that this effect can be real and often potent. Studies of this phenomenon have been derived from Langer’s mind/body unity theory first tested in 19791–3 and the most recent test of this idea, and the one most relevant to the present study, concerns time perception. In a recent study, patients with Type 2 diabetes were given clocks that ran either twice as fast, twice as slow or normally timed while patients played computer games for 90 minutes. There was a ‘dose-dependent effect of time perception on blood glucose levels and hunger with those exposed to the faster clock, who thought that 3 hours had passed, having the lowest blood glucose levels and highest hunger. Blood sugar levels appeared to follow perceived, not actual, time.4
In the current study, we examined whether perceived sleep duration can affect cognitive performance. Cognitive performance is strongly predicted by prior actual sleep duration, with total5, 6 or partial sleep restriction7, 8 having a negative effect. Sustained attention, the ability to detect inconspicuous signals over prolonged periods of time,9 in particular, is significantly impaired following sleep loss.6, 10, 11 A recent meta-analysis examining 61 studies from 71 different populations confirmed that sleep restriction negatively affects multiple domains of neurocognitive functioning including sustained attention, executive functioning and long-term memory.12 Sustained attention is required for a wide range of cognitive processes ranging from driving13 to learning and memory.14 Failure of sustained attention due to sleep loss can have devastating consequences for drowsy driving risk, workplace accidents and injuries. Therefore, understanding the impact of perceived sleep duration on fundamental cognitive domains is important both scientifically and operationally.
One study has shown that perceived sleep quality can influence cognitive performance,15 but the lack of objective measures of sleep-wake regulatory processes in that study prevent characterization of the mechanisms mediating this response. In another study, patients with insomnia who received negative sham feedback regarding their overnight sleep efficiency had impaired daytime function, lower positive mood and alert cognition as compared to insomnia patients who received positive sham feedback about their overnight sleep efficiency.16 In the current study, we evaluated the association between sustained attention and perceived sleep duration in healthy individuals without any sleep disorders, and then explored whether neurophysiologic sleep-wake regulatory mechanisms, namely homeostatic sleep drive and the circadian wake drive, are modulated by perceived sleep duration that can explain changes in the association between sustained attention and perceived sleep duration.
MATERIALS AND METHODS
Participants
Sixteen healthy participants [8 females; mean age (± SD): 24.2 ± 3.0 years)] were studied in the Intensive Physiological Monitoring Unit in the Center for Clinical Investigation at Brigham and Women’s Hospital. The participants were recruited as part of a 6-day inpatient study, which included two experiments, the first of which examined the effects of evening light exposure on sleep, alertness and melatonin suppression (Clinical Trial Registration Number: NCT01586039). The primary outcomes from the registered trial are presented in a prior publication.17 The second trial was not registered as the intervention was not health-related and was exploratory. Participants completed all study procedures related to the 4-day light-exposure trial during the first four days of the inpatient visit and then completed procedures related to the outcomes reported here during the next two days. All participants were screened, consented and enrolled for a single 6-day inpatient study, which included both the 4-day light exposure trial and the 3-day study on the effects of perceived sleep duration on neurobehavioral performance. Day 4 of the evening light-exposure trial was also Day 1 of the sleep-perception study. The study was approved by the Partners Human Research Committee, and participants provided written informed consent. All participants were recruited from the local population using online and print advertisements targeted to recruit healthy participants between the ages of 18–30 years old. Recruitment advertisements were run approximately daily until all participants completed the study. Approximately 54 participants were enrolled and screened to complete the study in 16 participants. All 16 participants who began the 6-day inpatient trial completed it. All had comprehensive but unremarkable physical, psychological and ophthalmologic exams, including a negative Ishihara color blindness test. For at least 3 weeks prior to admission, participants maintained a self-selected, constant 8-h sleep/rest/dark schedule confirmed via a time- and date-stamped voicemail at bedtime and wake time and with actigraphy for at least 1 week prior to admission. Participants were asked to refrain from prescription and nonprescription medications, supplements, recreational drugs, caffeine, alcohol, and nicotine. Compliance was verified by urine and blood toxicology during screening and urine toxicology upon admission.
Protocol Design
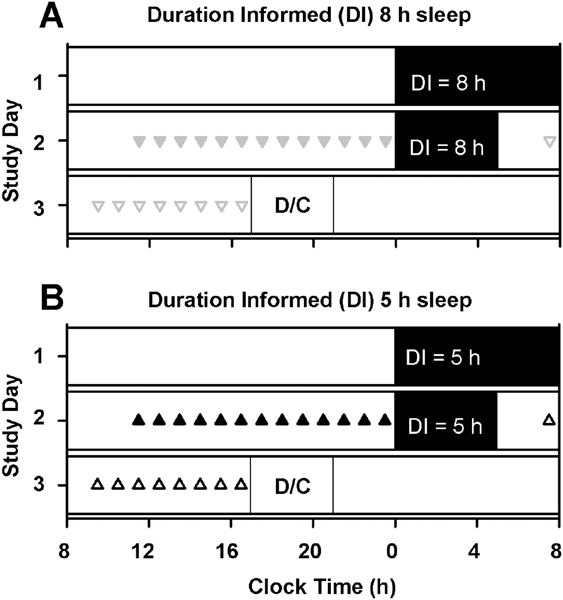
Participants were studied in an environment free of time cues for 6 days, the first 4 of which have been described previously.17 All participants completed an 8-h sleep opportunity on Day 1 (day 4 of the 6-day inpatient visit) and a 5-h sleep opportunity on Day 2 (day 5 of the 6-day inpatient visit) of the sleep-perception study. Participants were ‘informed’ that they slept for either eight or five hours on both nights by being provided a custom-designed clock that either sped up or slowed down displayed time, as appropriate starting at wake on Day 2 of the perceived-sleep study. Participants were randomized to the duration-informed condition at admission to the 6-day inpatient visit but were kept in a time-free environment during the first four days of the 6-day inpatient visit. There were therefore two sleep duration conditions (actual sleep 8 hours on Night 1 and 5 hours on Night 2) and within each of those conditions there were two levels (informed sleep 8 hours or 5 hours). All participants slept 8 hours on Night 1 and 5 hours on Night 2. The randomization of what they were informed resulted in two study groups (Figure 1): (1) Night 1 – slept 8 hours, informed 8 hours; Night 2 – slept 5 hours, informed 8 hours (n=8; Fig 1A); (2) Night – slept 8 hours, informed 5 hours; Night 2 – slept 5 hours, informed 5 hours (n=8; Fig 1B). Day and 3 of the perceived-sleep study included 16- and 12-hour wake episodes, respectively. Participants were discharged on Day 3 of the perceived-sleep study (day 6 of the inpatient visit).
Fig. 1. Study protocol.
All individuals completed a 6-hour light exposure prior to sleep on Day 1 as part of an unrelated study. All participants slept 8 hours on Night 1 and 5 hours on Night 2. The randomization of what they were informed resulted in two study groups: (1) Night 1 – slept 8 hours, duration informed (DI) 8 hours; Night 2 – slept 5 hours, DI 8 hours (n=8; Fig 1A); (2) Night 1 – slept 8 hours, DI 5 hours; Night 2 – slept 5 hours, DI 5 hours (n=8; Fig 1B). Cognitive batteries were completed hourly during the day after the 8-hour sleep opportunity (on ▼DI = 8 h group; ▲ DI=5 h group) or after the 5-hour sleep opportunity (on ᐁDI = 8 h group;Δ DI=5 h group). Symbols are maintained across all plots. (D/C = Discharge).
Participants were informed about their sleep duration using a clock custom-developed for the study (Harvard University Electronic Instrument Design Laboratory, Cambridge, MA, USA). The clock is identical to standard commercially available digital clocks but had the interval time-keeping circuitry modified to slow down or speed up time. The clock was placed in the same location of the room for all participants at the start of the wake episode on Day 2 and kept in the room in the same location until the end of the study.
Participants were not told by study staff how long they slept but rather the staff stated, “last night you went to bed at X”, where X was the participant’s actual bedtime. The participants were not instructed further. Participants were not informed at any point during screening or during the inpatient data collection phase that the effects of sleep-duration misperception on cognitive performance were being assessed. All participants were made aware, however, during screening that sleep loss affects performance as part of our standard consent procedure.
Study Lighting Conditions
Participants slept in darkness. During each wake episode, maximum ambient light was ~190 lux (48 μW/cm2) when measured in the horizontal plane at 187 cm and ~88 lux (23 μW/cm2) in the vertical plane at 137 cm. Ambient lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/HO/EW, 95W; F32T8/ADV841/A, 32W; F25T8/TL841, 25W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA, USA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA, USA).
Sleepiness and Performance Assessments
Subjective sleepiness was assessed using an auditory version of the Karolinska Sleepiness Scale (KSS), a 9-point scale from 1-“very alert” to 9-“very sleepy, fighting sleep.”18 Objective performance was measured with a 10-minute auditory psychomotor vigilance task (PVT-10A), during which the participant was instructed to respond via button press as soon as possible to an auditory signal presented at random intervals (1–9 seconds).19, 20 EEG based correlates of alertness were measured using the Karolinska Drowsiness test (KDT) with 3 minutes eyes open 0 minutes eyes closed.19 During the KDT, participants were instructed to relax and fixate on a 5-cm black dot 1-m away attached to a computer screen for 3 minutes with their eyes open. Subjective performance and effort rating was assessed using the Performance, Effort, and Evaluation Rating Scale (PEERS).21, 22 The test battery was administered every 60 min starting 2.5 h after wake.19
Sleep and Waking EEG Recordings
Polysomnography [electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and a 2-lead electrocardiogram (ECG)] was recorded continuously during scheduled sleep using a portable, modular, battery-operated, ambulatory, digital polysomnographic recorder (Vitaport-3 digital recorder, TEMEC Instruments B.V., Kerkrade, The Netherlands) using the International 10–20 System for electrode placement with linked mastoid references (Mx). Only data from C3-Mx (sleep) and Cz-Mx (wake) are presented. All EEG signals were high-pass filtered (time constant: 1.0 seconds), low-pass filtered (−6 dB at 30 Hz, 12 dB/octave), and digitized (resolution: 12-bit, sampling rate: 256 Hz, storage rate: 256 Hz). The raw signals were stored on a Flash RAM Card (SanDisk, Sunnyvale, CA, USA) and downloaded off-line. Electrode impedances were checked using an OhmMate impedance meter (TEMEC Instruments B.V., Kerkrade, The Netherlands) at the beginning and end of each sleep episode. Electrode impedances were documented, and applications repeated until the impedances were all <10 kΩ.
Data Analysis and Statistics
All results are presented as mean ± SEM unless specified otherwise. Significance was set to p<0.05. All analyses and graphical presentations were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA) and SigmaPlot for Windows Version 11.0 (Systat Software Inc., San Jose, CA, USA), respectively. Associations between self-reported sleep opportunity duration (outcome) and either sleep duration informed by clock (predictor) or actual sleep opportunity duration (predictor) was tested in two ways. First, generalized linear mixed models with a gamma distribution model was used to test the association between the outcome as a continuous variable and categorical predictors and subject level random effects with participants nested within the condition of duration informed. Second, self-reported sleep duration was dichotomized as matching or not matching the duration informed by the clock or time in bed and the probability of having a matching response was compared using a binomial probability test stratified by day in the study (Day 1 following 8 h time in bed or Day 2 following 5 h time in bed). Time-course data for sleepiness, performance and EEG power density data were analyzed using repeated-measures restricted maximum likelihood estimation linear regression with group (duration informed 8 hours or 5 hours) and time (hours since wake) as main effects and their interaction, stratified by study day.19
EEG signals derived from Cz-Mx during wake were processed and analyzed as described previously.19 Absolute power density was calculated for delta band (1.0–4.5 Hz) and relative power density was calculated for theta (5.0–7.5 Hz), alpha (8.0–11.5 Hz) and Hi-Alpha (10.5–11.5 Hz). Relative power density was calculated by expressing the power density values for each participant and condition as a percentage of power density during the last six hours of a 16 h wake episode in dim light (<3 lux) completed during the first day of the 6-day study.
RESULTS
Association between perceived sleep duration, clock-informed sleep duration, and actual sleep opportunity
The participants’ reported sleep durations, as a continuous outcome, were strongly associated to the duration informed by the clock (p=0.0002) but not to the actual sleep opportunity (p=0.19). As a categorical outcome, a majority (~81%) of the individuals’ reported sleep duration matched the duration informed by the clock (p=0.01 both days), whereas self-reported sleep duration matching time in bed was no better than chance (~50% for both study days, p=1.0).
Effects of overnight sleep restriction on cognitive performance impairment
We first verified that the 5-hour sleep opportunity caused the expected cognitive impairment in our sample population that can be expected from the imposed sleep restriction.8, 11, 23 Participants completed hourly cognitive performance and subjective sleepiness tests during the scheduled wake episode (Fig. 1A and B). We compared cognitive outcomes between the 8-hour and 5-hour sleep opportunity conditions with the correct clock information provided (Night 1 for 8-hour group and Night 2 for 5-hour group). We found significantly higher subjective sleepiness (p=0.008, Fig. S1A), reaction time (p=0.001, Fig. S1B), and EEG delta power during wake (p=0.04, Fig. S1D), but no significant differences in attentional failures (Fig. S1B), confirming the expected cognitive impairment induced by sleep restriction.
Effects of perceived sleep duration on subjective sleepiness and cognitive performance
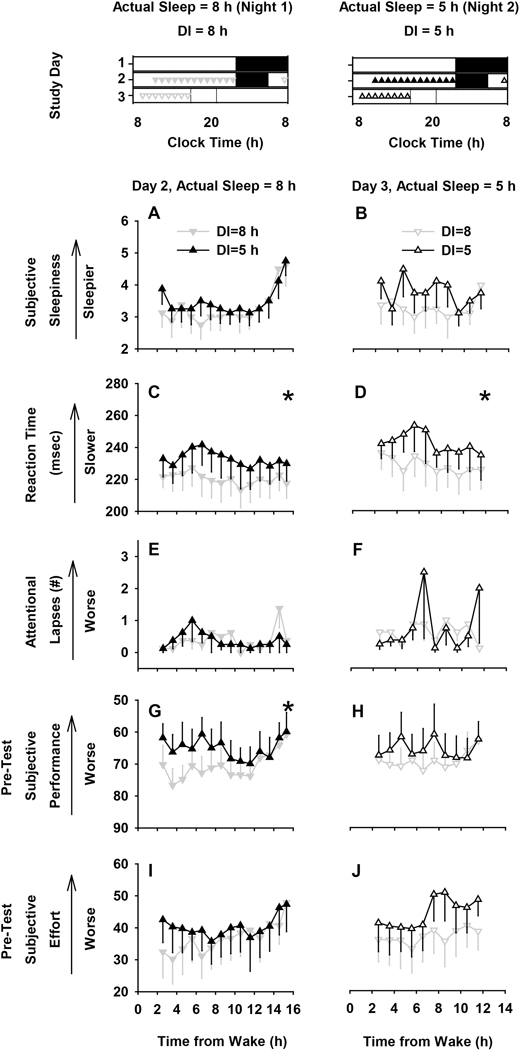
We did not find a significant difference in subjective sleepiness (Fig. 2A and B). In contrast to subjective sleepiness, reaction time on a 10-min auditory psychomotor vigilance test (PVT-10A)19, 20 was significantly changed by perceived sleep duration perception. In between-subject analyses, reaction times were significantly higher when participants were informed indirectly that they had slept for 5 hours as compared to when they were informed that they had slept for 8 hours, regardless of whether they were given an 8-hour (Night 1, p=0.01) or 5-hour (Night 2, p=0.02) sleep opportunity (Fig. 2C and D). We did not find a difference in attentional failures (responses >500 msec), however (Fig. 2E and F). On average, reaction time was up to ~22 msec slower when individuals perceived less sleep than they got.
Fig. 2. Effects of perceived sleep duration on neurobehavioral outcome measures.
Time course (mean±SEM) of neurobehavioral variables after an 8-hour sleep opportunity (left panels) and duration informed (DI) 8 h (▼) or 5 h (▲) and after a 5-hour sleep opportunity (right panels) and duration informed 8 h (ᐁ) and 5 h (Δ). Study protocol plotted in the first row illustrates the timing of the measures compared in the lower panels. Neurobehavioral variables included subjective sleepiness (A and B), reaction time (C and D), attentional failures (reaction time > 500 msec) (E and F), pre-test subjective performance rating (G and H) and pre-test subjective effort rating (I and J). * Signifies main effect of group p<0.05; repeated measures REML linear regression (main and interaction effects of group x time). There was a significant effect of time (p<0.05) only on subjective sleepiness in the 8 h actual sleep opportunity condition.
Effects of perceived sleep duration on subjective effort and motivation
Since motivation can be a significant mediator of cognitive performance,24 we also assessed whether subjective ratings of performance and effort were affected by perceived sleep duration immediately before and after each PVT-10A test using the Performance Evaluation & Effort Rating Scales (PEERS).21, 22 Immediately before starting the PVT-10A test, participants predicted that their performance levels would be low when they were told that they slept for 5 hours, despite having an 8-hour sleep opportunity (Fig. 2G), and this effect was statistically significant (p=0.02). Conversely, they predicted that their performance level would be high when they were informed that they slept 8 hours, despite having only 5 hours of time in bed (Fig. 2H), but this effect did not reach statistical significance. Perceived sleep duration was not associated with significant differences in post-test subjective performance rating (Fig. S2A and B) nor were there any significant differences in self-reported ratings of the level of effort required to complete the PVT-10A test before (Fig. 2I and J) or after (Fig. S2C and D) the objective test was administered.
Effects of perceived sleep duration on sleep-wake mechanisms
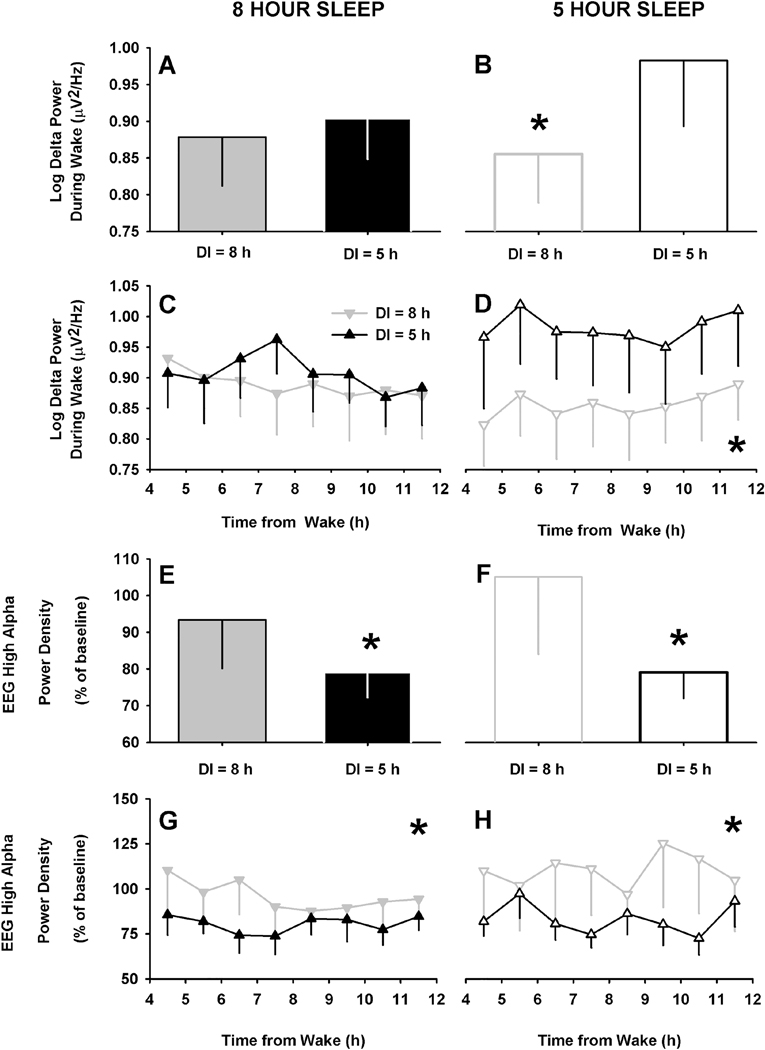
To explore potential mechanisms further, we assessed the effects of perceived sleep duration on objective sleep-wake regulatory processes25, 26 including the homeostatic sleep drive which increases with time awake, defined as delta power (1.0–4.5 Hz)27–29 in the waking EEG, and circadian wake drive (waking EEG high alpha power, 10.5–11.5 Hz)28, 29. As expected,28 we found that EEG delta power during wake was significantly increased in the daytime following the 5-hour sleep restriction condition (Night 2) compared to the 8-hour sleep condition (Night 1) (p=0.04, Fig. S1D). When participants were informed that they had slept longer than they did (i.e., slept 5 hours, but indirectly informed 8 hours), however, there was a significant relative reduction in delta power during wake (p=0.0004, Fig. 3B). These differences were sustained throughout the day (p=0.002 after adjusting for time of day), as shown in Fig. 3D. In contrast, when individuals were informed that they had slept less than they did (i.e., slept 8 hours, but indirectly informed 5 hours) there was no relative increase in delta power on average (Fig. 3A) or across the day (Fig. 3C).
Fig. 3. Effects of perceived sleep duration on electroencephalographic markers of arousal.
Group mean (±SEM) (A, B, E and F) and time course (mean ±SEM) (C, D, G and H) of EEG markers of arousal in the 8 hour (filled gray bars and ▼) and 5 hour (filled black bars and ▲) duration informed (DI) groups under actual 8-hour sleep opportunity (left panel) or in the 8-hour (unfilled gray bars and ᐁ) and 5-hour (unfilled black bars and Δ) DI groups under actual 5-hour sleep opportunity. Arousal measures included absolute power in the delta band (1.0–4.5 Hz) (A-D) and relative power density in the high-alpha band (10.5–11.5 Hz) (E-H). * Signifies main effect of group p<0.05; repeated measures REML linear regression [with either only group (A, B, E and F) or main and interaction effects of group x time (C, D, G and H)]. There was no significant effect of time or interaction on any of the measures.
We also assessed changes in waking EEG high alpha (10.5–11.5 Hz) power density, a sensitive neural correlate of the circadian wake drive28, 29 and found it significantly lower when individuals were informed that they had slept for 5 hours, and significantly higher when told that they had slept for 8 hours, irrespective of whether they received an actual 8-hour (p=0.03) or 5-hour (p=0.01) sleep opportunity (Fig. 3E-H). As expected, high alpha was not different between the actual 5 hour and 8 hours sleep conditions with correct durations informed, validating that the high alpha frequency, unlike delta power, is not modulated by sleep pressure (Fig. S3).28, 29
DISCUSSION
Our results show an interesting and previously unrecognized association between someone’s perception about how much they slept the previous night and their cognitive performance the next day. We found that when people perceived that they had slept only 5 hours, having actually received 8 hours time in bed, their cognitive performance was significantly worse than those who slept 8 hours and were ‘informed’ that it was 8 hours. Consistent with this finding, we also found that those who slept 5 hours but perceived that it was 8 hours performed significantly better than those who slept 5 hours and thought it was 5 hours. We found a substantial ~20-msec difference between the fastest and slowest mean reaction times due to perceived sleep duration which is equivalent to the performance deterioration over four nights with only 5 h of sleep or two nights with only 3 h of sleep,11 a change that is functionally relevant. Moreover, those who perceived that they had slept for 8 hours when the actual sleep opportunity was 5 hours, showed significantly lower levels of delta activity in their waking EEG, a well-established objective physiological marker of sleep pressure, suggesting a direct psychological impact on an objective biological marker of sleep regulation.
Importantly, comparisons of data relative to actual sleep showed that the observed physiology was normal, further confirming the robust effects of psychological processes on cognition and sleep-wake regulatory mechanisms. Moreover, our results show that in the absence of external cues, individuals can reliably assess their overnight sleep duration when timed on their habitual schedule, but rely heavily, when available, on external cues such as clock time, even when the information is false.
In contrast to objective measures, we did not find a consistent role of subjective effort or motivation in mediating the effects of perceived sleep duration on cognitive performance, nor of perceived sleep duration on subjective ratings of sleepiness. This lack of agreement between daytime subjective sleepiness and objective performance is consistent with several previous studies on the effects of placebo,30 light wavelength19 or chronic sleep loss23 comparing subjective and objective measures, Furthermore, the lack of any consistent changes in subjective measures of sleepiness, performance and effort ratings, reinforce the critical inconsistency between objective measures of cognitive performance and subjective ratings of individuals’ sleepiness or self-assessments of how well they will perform.19, 23, 31
There are several limitations of the current study, however. We only studied a relatively simple test of attention and, although objective EEG data also changed with sleep duration perception, future studies should examine its impact on higher order cognitive processes. This study followed directly on from a prior study, which may have affected alertness levels and prior sleep, albeit minimally. The combination of the two studies may, however, have reduced possible priming effects by minimizing pre-conceived ideas about the sleep perception component. Participants received the clock partway through the study. While this may have raised suspicions regarding the purpose of the clock, most participants perceived the sleep duration as inferred by the clock. Moreover, all participants completed the 8-hour sleep first, followed by the shorter 5-hour sleep, which may have introduced possible order effects into the analyses. A longer within-subject cross-over design study should be undertaken to replicate these findings. Finally, these results should be interpreted with caution due to the preliminary and exploratory nature of these analyses, and further careful validation is required.
Overall, we have demonstrated that sustained attention is sensitive to an individuals’ perception of their sleep duration. Even when participants are given sufficiently long sleep opportunities (8 hours) but think that they are sleep deprived (indirectly told 5 hours), their performance is impaired. Additional studies are required, however, to determine whether this effect can be sustained across multiple days. Moreover, we found that effect was mostly unidirectional i.e., perceived short sleep impairs performance, but it is not clear that perceived long sleep can improve performance. Clinically, this ‘wake state misperception’ may have a similar basis to the sleep state misperception (SSM) described by about a third of insomnia patients; indeed SSM is associated with abnormalities in high frequency EEG activity (10–30 Hz) and reduced delta (0.5–3.5 Hz) activity during sleep,32, 33 bands in which we also see changes during ‘wake state misperception’. Additionally, with the increasing use of wearable technology that provide feedback on sleep patterns, quality and structure, often with limited validity and or reliability,34, 35 our results suggest that such feedback may have deleterious effects on cognitive performance in users. Further research in this area may have important operational and clinical implications.
Supplementary Material
HIGHLIGHTS.
Cognitive performance changed based on perceived sleep duration
Attention was better when participants thought they had more sleep than they had
Perceived sleep duration modulated waking EEG correlates of sleep-wake regulation
Perceived sleep duration was not correlated to time spent in bed
Acknowledgments
We thank Alicia Foote, Wendy Chan, research staff, and research participants at the Division of Sleep and Circadian Disorders at the Brigham and Women’s Hospital; the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at the Brigham and Women’s Hospital; Jonathan Williams M.D. for medical supervision; Sleep Core staff (BWH) for EEG scoring; Jim MacArthur, Paul A Johnson School of Engineering and Applied Sciences, Harvard University for developing the clocks.
Funding: This work was supported in-part by an investigator-initiated grant from Biological Illuminations LLC, a subsidiary of Lighting Science Group Corporation (LSGC). SAR was supported in part by NIH/NHLBI T32-HL007901. The project described was supported by Grant Number 1 UL1 TR 001102 and Grant Number 8 UL1 TR000170–05, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health.
Footnotes
Conflicts of Interest: None of the authors report financial interests associated with the work presented. holds patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in subjects exposed to light at night. SAR owns equity in Melcort Inc. SAR has provided paid consulting services to Sultan & Knight Limited, Bambu Vault LLC. SAR has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., Seoul Semiconductor Co. LTD. In the last 3 years, SWL reports commercial interests from the last 3 years (2015–2018), unrelated to the work, that are listed below. Dr. Lockley has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Delos Living LLC, Noble Insights, OpTerra Energy Services Inc., Pegasus Capital Advisors LP, Serrado Capital, Slingshot Insights and Team C Racing. He has current consulting contracts with Akili Interactive, Apex 2100 Ltd, BHP Billiton, Consumer Sleep Solutions, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, McCullough Hill Leary PS, Paul, Weiss, Rifkind, Wharton & Garrison LLP, PlanLED, Six Senses, Stantec and Wyle Integrated Science and Engineering. Dr. Lockley has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation and F.LUX Software LLC; has equity in iSLEEP, Pty; royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Estee Lauder, Informa Exhibitions (USGBC), and Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from IES, Lightfair, USGBC, DIN and SLTBR. Dr. Lockley has completed investigator-initiated research grants from Biological Illumination LLC and has an ongoing investigator-initiated grant from F. Lux Software LLC; He is a Program Leader for the non-profit CRC for Alertness, Safety and Productivity, Australia, through an adjunct faculty position at Monash University and unpaid Member of the Scientific Advisory Board for the non-profit Midwest Lighting Institute. Dr. Lockley holds a process patent for ‘Systems and methods for determining and/or controlling sleep quality’, which is assigned to the Brigham and Women’s Hospital per Hospital policy. Dr. Lockley has also served as a paid expert in legal proceedings related to light and health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer E, Djikic M, Pirson M, Madenci A, Donohue R. Believing is seeing: using mindlessness (mindfully) to improve visual acuity. Psychol Sci. 2010; 21 (5): 661–666. [DOI] [PubMed] [Google Scholar]
- 2.Langer EJ. Mindfulness: Addison-Wesley/Addison Wesley Longman, 1989. [Google Scholar]
- 3.Langer EJ, Chanowitz B, Palmerino M, Jacobs S, Rhodes M, Thayer P. Higher stages of human development: Perspectives on adult growth. New York, NY, US: Oxford University Press, 1990. [Google Scholar]
- 4.Park C, Pagnini F, Reece A, Phillips D, Langer E. Blood sugar level follows perceived time rather than actual time in people with type 2 diabetes. Proc Natl Acad Sci U S A. 2016; 113 (29): 8168–8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 2005; 25 (1): 117–129. [DOI] [PubMed] [Google Scholar]
- 6.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010; 136 (3): 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007; 3 (5): 519–28. [PMC free article] [PubMed] [Google Scholar]
- 8.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997; 20 (4): 267–277. [PubMed] [Google Scholar]
- 9.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001; 35 (2): 146–160. [DOI] [PubMed] [Google Scholar]
- 10.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001; 139 (3): 253–267. [PubMed] [Google Scholar]
- 11.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003; 12: 1–12. [DOI] [PubMed] [Google Scholar]
- 12.Lowe CJ, Safati A, Hall PA. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci Biobehav Rev. 2017; 80: 586–604. [DOI] [PubMed] [Google Scholar]
- 13.Jongen S, Perrier J, Vuurman EF, Ramaekers JG, Vermeeren A. Sensitivity and validity of psychometric tests for assessing driving impairment: effects of sleep deprivation. PLoS ONE 2015; 10 (2): e0117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology 1987; 19 (1): 1–32. [Google Scholar]
- 15.Draganich C, Erdal K. Placebo sleep affects cognitive functioning. J Exp Psychol Learn Mem Cogn. 2014; 40 (3): 857–864. [DOI] [PubMed] [Google Scholar]
- 16.Gavriloff D, Sheaves B, Juss A, Espie CA, Miller CB, Kyle SD. Sham sleep feedback delivered via actigraphy biases daytime symptom reports in people with insomnia: Implications for insomnia disorder and wearable devices. J Sleep Res. 2018; 27 (6): e12726. [DOI] [PubMed] [Google Scholar]
- 17.Rahman SA, St Hilaire MA, Lockley SW. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol Behav. 2017; 177: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990; 52 (1–2): 29–37. [DOI] [PubMed] [Google Scholar]
- 19.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014; 37 (2): 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorrian J, Rogers NL, Dinges DF. Psychomotor Vigilance Performance: Neurocognitive Assay Sensitive to Sleep Loss In: Kushida CA, ed. Sleep Deprivation. Clinical Issues, Pharmacology, and Sleep Loss Effects. New York: Marcel Dekker, 2005:39–70. [Google Scholar]
- 21.Dinges DF, Kribbs NB, Steinberg KN, Powell JW. Do we lose the willingness to perform during sleep deprivation? Sleep Res. 1992; 21: 318. [Google Scholar]
- 22.Dijk DJ, Neri DF, Wyatt JK, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol Regul Integr Comp Physiol. 2001; 281: R1647–R1664. [DOI] [PubMed] [Google Scholar]
- 23.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26 (2):117–126. [DOI] [PubMed] [Google Scholar]
- 24.Hull JT, Wright Jr. KP, Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003; 18 (4): 329–338. [DOI] [PubMed] [Google Scholar]
- 25.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994; 166 (1): 63–68. [DOI] [PubMed] [Google Scholar]
- 26.Borbély AA. A two process model of sleep regulation. Human Neurobiol. 1982; 1: 195–204. [PubMed] [Google Scholar]
- 27.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neurosci. 2002; 114 (4): 1047–1060. [DOI] [PubMed] [Google Scholar]
- 28.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997; 239 (2–3): 121–124. [DOI] [PubMed] [Google Scholar]
- 29.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol 1999; 277 (6 Pt 2): R1771–R1779. [DOI] [PubMed] [Google Scholar]
- 30.Anderson C, Horne JA. Placebo response to caffeine improves reaction time performance in sleepy people. Hum Psychopharmacol. 2008; 23 (4): 333–336. [DOI] [PubMed] [Google Scholar]
- 31.St Hilaire MA, Ruger M, Fratelli F, Hull JT, Phillips AJ, Lockley SW. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017; 40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002; 25 (6): 630–640. [PubMed] [Google Scholar]
- 33.Schneider-Helmert D, Kumar A. Sleep, its subjective perception, and daytime performance in insomniacs with a pattern of alpha sleep. Biol Psychiatry. 1995; 37 (2): 99–105. [DOI] [PubMed] [Google Scholar]
- 34.Ko PR, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer Sleep Technologies: A Review of the Landscape. J Clin Sleep Med. 2015; 11 (12): 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behar J, Roebuck A, Domingos JS, Gederi E, Clifford GD. A review of current sleep screening applications for smartphones. Physiol Meas. 2013; 34 (7): R29–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.