Abstract
The extracellular matrix (ECM) is a complex and dynamic structural scaffold for cells within tissues, and also plays an important role in regulating cell function. Recently it has become appreciated that the ECM contains bioactive motifs that can directly modulate immune responses. In this review, we describe strategies for engineering immunomodulatory biomaterials that utilize natural ECM-derived molecules, and have the potential to harness the immune system for applications ranging from tissue regeneration to drug delivery. A top-down approach utilizes full-length ECM proteins and can include collagen, fibrin, or hyaluronic acid-based materials, as well as matrices derived from decellularized tissue. These materials have the benefit of maintaining natural conformation and structure, but are often heterogeneous and encumbers precise control. Instead, a bottom-up approach leverages immunomodulatory domains, such as RGD, matrix metalloproteinase (MMP)-sensitive peptides, or leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) ligands, which can be incorporated into synthetic materials. These materials have tunable control over immune cell functions, and also allow for combinatorial approaches by incorporating multiple peptides. However, the synthetic approach lacks the full natural context of the original ECM protein. Thus, incorporation of full-length ECM proteins, ECM-derived peptides, or a mixture of both, into biomaterials provides a broad range of engineering techniques for immunomodulation through material interactions. Here, we describe the immunomodulatory effects of these biomaterial engineering strategies, and highlight potential future directions in this field.
Keywords: extracellular matrix, tissue engineering, biomaterials, immunomodulation, cytokines, inflammation, wound repair
Graphical Abstract
In this review, we describe strategies for engineering immunomodulatory biomaterials that utilize natural ECM-derived molecules, and have the potential to harness the immune system for applications ranging from tissue regeneration to drug delivery. The incorporation of full-length ECM proteins, ECM-derived peptides, or a mixture of both, into biomaterials provides a broad range of engineering tools for immunomodulation through material interactions.

1. Introduction
The extracellular matrix (ECM) is a complex and dynamic structural support for cells within tissues, and is comprised of proteins, glycoproteins, and polysaccharides. Each type of tissue in the body has ECM with unique and varied protein content, which not only provides an appropriate structural scaffold for cells to adhere and organize, but also regulates many different cellular functions to maintain homeostasis.[1] ECMs contain bioactive sequences, which are recognized by specific cell surface receptors that are often expressed on multiple cell types, and thus facilitate a wide range of cellular activities (Figure 1).[2] In addition, cells themselves manipulate their ECM microenvironment by synthesizing new matrix and modifying and digesting existing matrix, thereby altering its modulatory effects.[1] ECM remodeling can expose previously hidden bioactive sequences or degrade intact bioactive sequences.[3] Furthermore, digested soluble ECM fragments often modulate cell behavior in a manner that is distinct from the insoluble scaffold. The interplay between cells and their environment makes the ECM a dynamic nexus of homeostatic control, critical for the healthy function of cells.
Figure 1.
Schematic representation of ECM-immune cell interactions. Interactions include LAIR1-collagen interaction that inhibit inflammatory signaling, MMPs that drive matrix degradation at cleavage motifs, and RGD that facilitates cellular adhesion to ECM via integrin binding.
Given the role of the ECM in structural support of tissues, there has been significant effort in developing ECM-based scaffolds for tissue engineering and regenerative medicine.[3–5] For these applications, a biomaterial scaffold is often used to encourage the adhesion and infiltration of cells, and their differentiation and organization into functional tissues. These engineered tissues are designed with the ultimate goal of replacing damaged or diseased tissue. However, as with all materials implanted into the body, the immune response significantly influences the ability of engineered tissues to integrate and functionally interact with the host.[1,5] The initial inflammatory response to implantation is dominated by activation of innate immune cells, including neutrophils and macrophages, followed by the later arrival of dendritic cells (DCs). These myeloid effectors then bridge to the adaptive immune system through macrophage and antigen presentation by DCs to T and B cells in secondary lymphoid organs, mediating long-term immune recognition of implanted materials. The dynamic macrophage response to injury and implanted materials is characterized by an initial inflammatory phase, characterized in mice by the secretion of TNFα or other inflammatory cytokines, expression of iNOS, and CD86; also referred to as M1). This is followed by a pro-healing phase, marked by macrophage secretion of IL-10 and expression of arginase and CD206; also referred to as M2).[6–8] TGFβ, VEGF, and other factors may also be secreted, and several subtypes of macrophage phenotype have been characterized during the wound healing process.[9] Over time, persistent activation of immune cells by foreign materials can lead to chronic inflammation.[10] Thus, an emerging strategy in tissue engineering is to design materials that can directly control the host immune response.[2] Furthermore, it has recently become appreciated that many ECM components in fact have natural immunomodulatory domains that bind to receptors found on immune cells, enabling their adhesion and regulating their function.[5] Full length ECM proteins and/or ECM-modeled peptides can be used in biomaterial scaffolds to mimic the natural regulatory role of the matrix on the immune system and generate desired immune cell functions to aid in the longevity and functionality of implants.
In this review, we will provide an overview of engineering approaches to create ECM-based materials for immunomodulation, primarily in the context of regenerative medicine. We will first discuss top-down approaches, in which full length ECM molecules or native tissues are used as building blocks for biomaterials. Topics will include naturally-occurring ECM proteins, their immunomodulatory effects, and engineering methods to manipulate these effects. We will then discuss bottom-up approaches, in which specific bioactive or immunomodulatory domains found in ECMs are synthesized as peptides and engineered into different materials to provide control over interacting immune cell responses. Finally, we will describe materials that incorporate multiple ECM-derived domains to more closely mimic the multifaceted immunomodulatory functions of ECM in the body. These approaches all strive to achieve materials with ability to control immune responses and provide better tissue integration and functionality. This review will then conclude with discussion of the future direction and perspectives of these approaches in developing immunomodulatory ECM-based biomaterials.
2. Immunomodulation by naturally derived ECMs
Matrix materials can be fabricated using a top-down approach, whereby tissues or blood are processed to isolate matrix components, which are then assembled in vitro to create a biomaterial. This technique often relies on self-assembly of ECM components into hydrogels, but engineering methods can also be used to generate materials with different physical characteristics, for example fibril architecture, stiffness, or matrix pore size. Furthermore, the processing techniques used to digest tissues for matrix isolation often expose or mask native functional groups found within the ECM, altering its bioactivity. Thus, the immunomodulatory features of fabricated ECM materials may be engineered by controlling the macroscopic, microscopic, and molecular properties.
2.1. Collagen
Collagen is the most abundant ECM component in the body, and comprises a large family of proteins with distinct functions in the ECM architecture.[11] There are 29 different types of collagen, all of which form a characteristic right-handed triple helix structure.[12] One collagen triple helix is comprised of three polypeptide chains, each with approximately 300 [X-Y-Gly] units, where X and Y can be any amino acid but with a preference towards proline and hydroxyproline in the X and Y positions, respectively. Homo-trimeric and heterotrimeric collagen triple helices pack together to form fibers and meshes in every tissue in the body. Collagens used most often in biomaterials applications, Types I-III, V, and XI, have fibrillar quaternary structures. Immune cells express a number of receptors that bind directly to collagen, including integrins, discoidin domain receptors DDR1 and 2, and leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) of the leukocyte receptor complex.[13,14] Collagen I, II, III, and XVII are high-affinity ligands for LAIR-1 receptors on immune cells, where binding prevents degranulation of peripheral basophils, and more generally suppressed immune cell activity [15,16]. Macrophage scavenger receptors (MSR) selectively bind exposed ligands on denatured collagen, facilitating conformation-specific effects.[17,18] The effects of collagen fibril structure on macrophage-like U937 cell production of remodeling enzyme matrix metalloproteinase-9 (MMP-9) has also been explored.[18] Intact fibrils promoted the greatest MMP-9 production, whereas fibrils degraded via mechanical loading promoted an intermediate induction of MMP-9, compared to polystyrene culture surface; this result suggests that the structure of collagen and the damage induced during injury is recognized by macrophages, and regulates their differential response.
Modification of collagen or denatured collagen (gelatin) may be used to alter the mechanical integrity and/or biochemical structure of the matrix to control cellular response. For example, chemical crosslinking method has been shown to affect matrix structure and macrophage function.[19] Collagen crosslinked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide (EDC/NHS) adopted a porous structure, and elicited the lowest activation of both inflammatory TNFα and anti-inflammatory CCL22 cytokines in human primary macrophages, compared to formaldehyde and glutaraldehyde crosslinked collagen. However, formaldehyde crosslinking induced a greater CD163 (remodeling-associated) phenotype, indicating further complexity in relationship between crosslinking and macrophage phenotype modulation for wound healing. In a separate study, gelatin crosslinked with varying amounts of glutaraldehyde were implanted subcutaneously in mice, and showed no differences in fibrous capsule thickness and F4/80 macrophage recruitment.[20] This study also revealed significant temporal variation; inflammatory iNOS expression remained constant between 3 and 21 days post implant, but pro-healing markers, including arginase and CD163, increased over time, along with the capsule thickness.
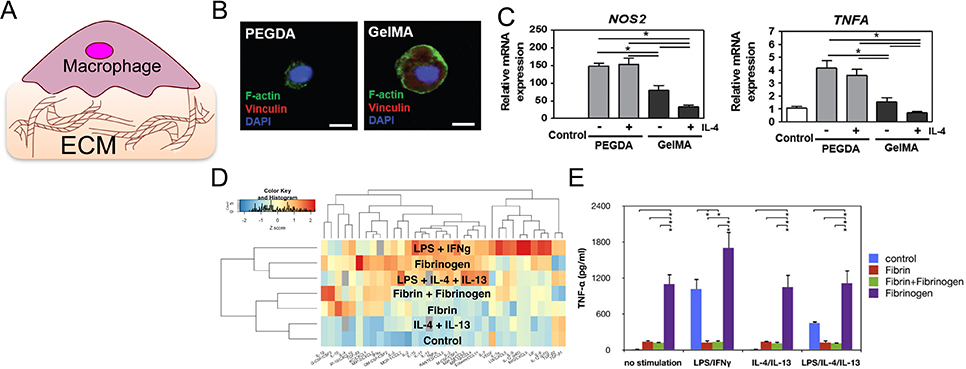
Crosslinking of gelatin can be achieved by adding a photosensitive methacryloyl group to gelatin molecules (gelatin methacryloyl, GelMA), and offers the ability to tune gelatin crosslinking with light. Using this method, the Donaldson et. al. created collagen-based materials with varied stiffness and topological patterns, and explored the mechanism underlying their effects on monocyte/macrophage behavior.[21] Human peripheral monocytes cultured on hydrogels made of GelMA exhibited lower inflammatory Tnfα gene expression 30 minutes and 4 hours after LPS stimulation, when compared to cells cultured on tissue culture plastic. The hydrogels were also found to serve as a TNFα “sink,” reducing soluble TNFα in supernatant, as measured by ELISA. Cha et. al. found that THP-1 immortal human monocytes encapsulated in softer hydrogels (2.95 kPa) showed significantly greater cell survival compared to cells in stiffer hydrogels (15 kPa and 25 kPa gels).[22] Macrophages cultured on GelMA, with or without IL-4 stimulation, showed higher gene expression of anti-inflammatory Il10 and Il1ra, lower inflammatory Inos and Tnfa, and better adherance compared to cells cultured on polyethylene glycol diacrylate (PEGDA) hydrogels (Figure 2B, 2C). PEGDA lacks integrin binding sites, and indeed the expression of integrins, including α2β1, were suppressed in cells cultured on PEGDA. Furthermore, the prevention of integrin adhesion in cells cultured on GelMA, by using a blocking antibody, led to an inflammatory phenotype; this suggests that integrin engagement in collagen-based hydrogels may play a role in negative regulation of inflammation.
Figure 2.
Collagen- and fibrin-based materials modulate the macrophage inflammatory response. (A) Schematic of immune cell interaction with ECM-based materials. (B) Confocal images of THP-1 macrophage expression of adhesion molecule vinculin on nonadhesive polyethylene glycol diacrylate hydrogel (PEGDA, left) and adhesive gelatin hydrogel (GelMA, right). Scale bar is 10 μm. (C) mRNA expression levels of inflammatory markers NOS2 and TNFA in macrophages cultured on PEGDA vs. GelMA with or without IL-4 treatment. (B) and (C) are adapted from Cha et al., 2017.[22] (D) Z-score heatmap of inflammatory cytokines secreted by macrophages cultured on fibrin or polystyrene and stimulated with LPS, IFNγ, IL-4, IL-13, and/or fibrinogen. Red indicates relatively high levels of secretion and blue indicates low levels of secretion. (E) TNFα secretion by BMDM cultured on control polystyrene (blue and purple), fibrin (red and green), and/or fibrinogen (green and purple) in the indicated stimulation conditions. (D) and (E) are adapted from Hsieh et al. 2017.[37]
Collagen-based matrices can modulate more than the innate immune compartment. Lymphoid follicle organoids for germinal center B-cell formation were engineered by encapsulating naive B cells in gelatin hydrogels ionically crosslinked with 0–2% silica nanoparticles, and found to induce robust germinal center (GC) formation, maturation, and class switching to IgG and IgE upon activation.[23] It is thought that additional RGD sites are exposed during the denaturation of collagen I to form gelatin, providing adhesion sites and signaling for upregulation of αvβ3 integrin in GC B cells.[24] Furthermore, inhibition of αvβ3 binding with the small molecule inhibitor cilengitide prevented GC formation, and organoids generated using non-adhesive PEG-based materials functionalized with adhesive peptides RGD or REDV demonstrated that αvβ3 (RGD binding), and not α4β1 (REDV binding), is essential to GC B-cell differentiation.[24]
In summary, collagen-based materials appear to have significant effects on immune cells, including macrophages and B cells, with hydrogel mechanics and adhesion domains comprising significant control parameters for immunomodulation. Given the dependence of cellular interactions with collagen on its structure and exposed ligands, future studies engineering the tertiary and quaternary structure of collagens may provide further control over immune responses using these biomaterials. In addition, the design of cell interaction sites and non-native cysteines into recombinant, full-length human collagen III has been an approach for examining their ability to induce neural stem/progenitor cell differentiation and myofibroblast growth.[25–27] Similarly, a potential route for future studies could be the use of such recombinant strategies to specifically prescribe non-native functionality (e.g., non-native cell interaction sites, degradation kinetics, mechanical properties) into collagen; this approach could then examine the impact of specific molecular modifications of collagen on immune cells and enable the tuning of collagen’s immunomodulatory effects, as part of the larger wound-healing response.
2.2. Fibrin
Fibrin is a primary component of the hemostatic clot, and plays an important role as the provisional matrix in the natural wound healing process after injury.[28] Fibrin is formed by the polymerization of its circulating precursor, fibrinogen, which is cleaved by activated thrombin in the terminal step of the coagulation cascade. Polymerization is thought to expose integrin binding sites including RGD, P1 and P2 peptides, which promote the adhesion of immune cells via αmβ2.[29–32] Platelets also bind to fibrin through αiibβ3 (GP2b3a), ɑvβ1, and P-selectin,[33] further stabilizing the clot. As healing progresses, fibrin degradation is mediated primarily by cleavage of lysine-X-Y motifs, by activated plasmin, as well as matrix metalloproteinases (MMPs) [34].
To study the effects of fibrin on immune cell function, fibrin matrices were generated in vitro through the mixing of purified fibrinogen with activated thrombin. Early work showed that macrophage motility through a fibrin matrix was inhibited as fibrinogen or Factor-XIII crosslinker concentration was increased, whereas fibroblasts migrated more effectively through Factor-XIII crosslinked fibrin gels.[35,36] More recently, our laboratory has examined the effects of fibrin on macrophage functional activation).[37] We found that, when primary murine bone marrow derived macrophages (BMDM) were cultured on fibrin hydrogels, soluble inflammatory TNFα cytokine secretion in response to LPS/IFNɣ was significantly reduced compared to cells cultured on tissue culture plastic (Figure 2D, 2E). In fact, fibrin clusters with anti-inflammatory cytokines based on the cytokines secreted in response to stimulation. In contrast, the precursor fibrinogen delivered in the soluble form potentiated inflammatory activation. These results are consistent with studies described above on GelMA, where culture on an adhesive hydrogel inhibitions macrophage inflammatory activation, although the results may also be due to sequestration of TNFα in the fibrin gel. Furthermore, when, fibrin-based hydrogels were applied to porcine burn wounds, contraction and macrophage and neutrophil recruitment was reduced,[38] showing that pro-healing effects of fibrin have been extended to in vivo injury models.
In most injury settings, infiltrating leukocytes will likely be exposed not only to a fibrin matrix, but a microenvironment rich with platelets and proteins found in plasma. Interestingly, the incorporation of leukocytes in a clot generated from platelet-rich plasma increased their release of inflammatory IL1β, TNFα, and IL-6, which was not found in the gelled matrix alone.[39,40] Conditioned media obtained from leukocyte-incorporated matrix cultures also suppressed proliferation and promoted NFκB in both fibroblasts and osteoblasts. Moreover, fibrin is observed in the brain of humans and mouse models of Alzheimer’s and multiple sclerosis, causing inflammation and neurotoxicity by activating microglia and macrophages.[41,42] Fibrin degradation products generated by plasmin treatment are also found to promote leukocyte recruitment and increase inflammatory IL-1β and IL-6 production from monocytes, but decrease activities associated with bacterial killing in neutrophils including superoxide production and phagocytosis.[43–46] Together, these data suggest a complex interplay between matrix remodeling and immune regulation.
Finally, there is evidence that fibrin and the hemostasis cascade influence the adaptive immune activity through activation of dendritic cell trafficking and sequestration of lymphangiogenic growth factors.[47] Activated thrombin, which regulates fibrinogen conversion to fibrin, also activates PAR-1, one of the four known protease-activated receptors and is expressed by monocytes, macrophages, and circulating T cells.[48] PAR-1 has been found to be required for DC trafficking and presentation for T-cell activation.[49] Moreover, fibrin can bind VEGF-C during wound healing, and this sequestration is required for lymphangiogenesis, or the formation of new lymphatic vessels, which is key to maintaining communication between antigen presenting cells and the adaptive immune compartment.[50] Although fibrin also contains adhesion binding sites such as RGD, which were described above to influence B cell maturation, it remains unknown whether fibrin has any direct effects on the adaptive immune compartment. In summary, fibrin-based materials have been found to elicit both inflammatory and anti-inflammatory effects on the innate injury response, and to facilitate communication with the adaptive compartment. It is possible that fibrin and immune cell source may play a role observed in varied in vitro investigations, as the effects of fibrin are likely highly complex, dependent on the presence of other cell types or proteins in the microenvironment. There is evidence that these effects are dynamic as well, perhaps enhancing the initial inflammatory response while hastening the transition to the later proliferative and remodeling phases of wound healing.[51,52] In addition, as with the studies in collagen described above, matrix mechanics and architecture may also play a role. It is unclear what specific factors determine the direction of fibrin immunoregulation, and further studies are needed to fully characterize and understand the interactions between the provisional matrix and the immune system.
2.3. Hyaluronic acid
The glycosaminoglycan hyaluronic acid (HA) was initially discovered in 1934 as a primary component of the vitreous humor of the eye, and has since been used in a myriad of applications, from cosmetics to drug delivery.[53] Commercial production of HA was initially optimized by extraction from avian rooster comb, but is now more commonly achieved through bacterial fermentation, where molecular weight (MW) can be tuned by culture conditions.[54,55] HA is a non-branched polyanionic polysaccharide with MWs ranging from 1 kDa to 2 MDa and is primarily localized to skin and musculoskeletal tissue, where it associates with proteoglycan aggrecan to form aggregates.[56] In the same family as chondroitin and heparin sulfates, HA is notably not sulfated, and undergoes rapid turnover in the body, particularly during wound healing. While hydrated HA self-associates to form a hydrogel, it can also be engineered to form thin films, hydrogels, and nanoparticles.[56]
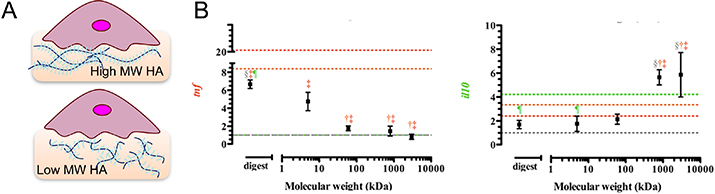
Structural and biochemical effects of HA on immune cells are dependent on MW; high MW HA tends to be inert or immunosuppressive, while lower MW HA, formed by degradation or damage, provokes an inflammatory response (Figure 3).[57] In macrophages, soluble high MW HA induced an anti-inflammatory polarization with high Arg1 gene expression and IL-10 secretion, in comparison to low MW HA, which induced an inflammatory macrophage phenotype with high Tnfa gene and TNFα cytokine expressions.[53] The response of macrophages cultured on HA nanofibers in the presence of LPS mimicked that of macrophages on high MW HA, with increased secretion of IL-10, IL-2, and VEGF compared to cells on polystyrene.[58] IL-2 regulates the powerful IL-6 mediated inflammatory response, making it an important immunomodulatory lever controlled by ECM.[59,60] VEGF promotes angiogenesis, and conditioned media of macrophages cultured on PLA-based scaffolds with immobilized high MW HA stimulated tube formation in human umbilical vascular endothelial cell cultures.[61] These effects are mediated primarily through cellular adhesion to HA via CD44 receptors, which are expressed on both innate and adaptive immune cells and can be crosslinked by binding high MW HA, but not low MW HA.[53,62] Crosslinking CD44 with Fab antibody fragments resulted in increased phagocytosis and M2-polarization of murine BMDM, supporting differential immune response to HA mediated by CD44 binding.[63] Interestingly, activation of monocytes appears to modulate their interactions with HA, as stimulation with TNFα appears to promote HA binding, while IL-4 negatively regulates this interaction.[64] These data suggest potential crosstalk between activation state and cell adhesion to the matrix environment.
Figure 3.
Effect of HA molecular weight on macrophage polarization. (A) Schematic of immune cell cultured on HA hydrogels of varied molecular weights. (B) Gene expression of Il10 and Tnfα in mouse sarcoma macrophages J774A.1 stimulated with IL-4 and HA of different molecular weights for 24 h. Adapted from Rayahin et al., 2015.[53]
CD44-HA interactions may be also exploited for delivery to immune cells. Binding of low MW HA nanoparticles to THP-1 cells is associated with higher CD44 expression and has been correlated positively to binding of soluble and (siRNA-loaded) HA nanoparticles, but negatively with HA internalization.[65] Because of their biocompatibility, HA particles have been used extensively in the development of vehicles for cancer therapeutics, delivering nucleic acids, peptides, and other applications [66]. Conjugation and surface modification is mainly facilitated by carboxylic acid coupling chemistry. HA particles also hold promise for regenerative medicine; HA microparticles conjugated with engineered BMP-2 peptide promoted chondrogenesis of mesenchymal stem cells in vitro.[67] While HA particles has potential for multiple therapeutic applications, further studies are needed to evaluate their impact on the immune system to improve translation of these therapies to the clinic.
With respect to other immune cells, culture of DCs on thin films of high MW HA inhibited their maturation, as measured by expression of CD40, CD80, and CD86, and MHC class II molecules (e.g. HLA-DQ and HLA-DR), whereas cells cultured on chitosan and PLGA films had higher expression of these unique maturation markers after incubation with immature DCs.[68] Mature DCs also expressed lower surface CD44 on HA films compared to immature DCs and DCs on the other materials,[62] which could potentially be explained by endocytosis of surface CD44 upon HA binding. In the adaptive immune system, memory T cell differentiation into an IL-10 producing Treg phenotype can be induced by high MW HA, but not low MW HA, and this effect was abrogated with the addition of CD44 competitive inhibitor osteopontin.[69] Treg differentiation was not observed with other ECM hydrogels including fibrin or Matrigel. Together, these data further support the idea that that high MW HA dampens the innate immune response and inflammation, but the lower MW form induces inflammatory activation. Since different MW HAs are fairly straightforward to synthesize by hyaluronidase digestion, this feature could potentially provide a direct way to engineer materials with defined immunomodulatory properties.
2.4. Decellularized matrices
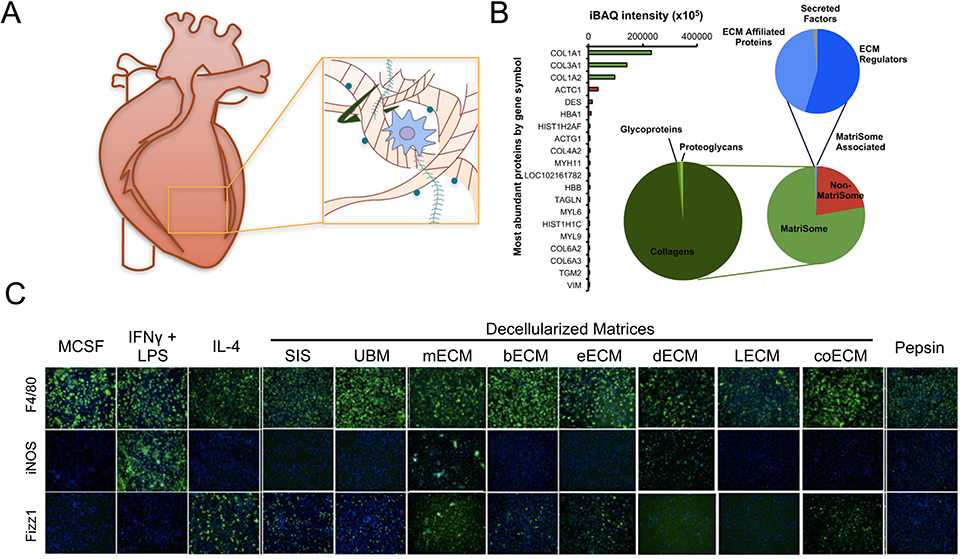
Decellularized matrices are created through removal of cells from tissues via detergents or other cell lysis procedures, and can also include antigen removal, lyophilization, and further digestion. Clinically, these materials have been used to support healing of tendon, bone, muscle, skin, breast, heart and vascular tissue, among others.[70] The composition of these materials is highly dependent on the sources of tissue and processing method. For example, commercial urinary bladder matrix protein (Micro Matrix™) is composed of 78% matrix and matrix-associated proteins, 98% of which are collagens by mass spectrometry (Figure 4). Non-matrix proteins include actin, desmin, and hemoglobin, which are present in high abundance in bladder tissue.[71] The unique physical and biochemical properties of decellularized matrices define their immunomodulatory effects.
Figure 4.
Tissue source affects composition and immunomodulatory effects of decellularized matrices. (A) Schematic of extraction of decellularized matrix. (B) Composition of commercial porcine urinary bladder matrix (MicroMatrix™) as determined by mass spectrometry. Adapted from Sadtler et al., 2017.[71] (C) Immunofluorescence images of F4/80 (pan macrophage), iNOS (M1 marker) and Fizz1 (M2 marker) in macrophages cultured on polystyrene with cytokines or pepsin, or solubilized decellularized ECM derived from various tissues: SIS (small intestinal submucosa), UBM (urinary bladder matrix), mECM (skeletal muscle ECM), bECM (brain ECM), eECM (esophageal ECM), dECM (dermal ECM), lECM (liver ECM), coECM (colonic ECM). Adapted from Dziki et al., 2017.[78]
Early work from the Badylak group showed that decellularized urinary bladder matrix allografts promote anti-inflammatory macrophage polarization, as measured by a lower CCR7:CD163 ratio, and reduces fibrotic response to abdominal wall implants in rats, compared to cellular autografts (Figure 4).[72,73] More recently, they have found that the method of decellularization, as well as post-processing techniques, are also major factors in the immune response. Brown et al. compared autologous cell delivery in decellularized allografts to the matrix alone, and found that decellularized matrix promoted more moderate and anti-inflammatory macrophage polarization, measured by CD163/arginase (M2) and iNOS/CCR7 (M1) via immunohistochemistry and gene expression.[72] Similarly, work by Wong et al. showed that removal of both lipophilic and hydrophilic antigens dramatically reduced presentation of the most prevalent antigens, MHCI and alpha-gal, in decellularized bovine pericardium[74]. Compared to soluble antigen removal alone, this treatment led to an increase in anti-inflammatory macrophages, reduced CD3+ T cell and CD79+ B cell responses, as well as reduced fibrous capsule thickness when implanted as a carotid artery matrix patch in pigs, suggesting suppression of the humoral response.[74] To manipulate the structure of decellularized matrices, urinary bladder matrix hydrogel was rendered porous by mixing with soluble mannitol beads and thermally responsive poly(NIPAAm-co-VP-co-MAPLA), and found to enhance macrophage chemotaxis and the ratio of CD206+ to CD86+ macrophages recruited to the rat hind limb injection site, compared to non-porous hydrogel.[75] In addition, crosslinking of decellularized bovine pericardium using EDC has been found to reduce secretion of MMP-2 and MMP-9 degradation enzymes, while minimally changing cytokine secretion by U937 macrophage-like cells in vitro, suggesting that the strategy could potentially extend the life of this putative artificial heart valve material.[76] These studies show that the processing method and resulting microstructure regulate both the adaptive and innate immune response to decellularized ECM.
The source of tissue is also an important determinant of the effect of decellularized matrix products on the immune response. For example, brain-derived ECM promoted higher TNFα, nitric oxide, and arginase production in primary rat macrophages, but lower prostaglandin E2 compared to urinary bladder-ECM.[77] The latter was found to contain greater high MW HA content, and hyaluronidase treatment reduced prostaglandin E2 (PGE2) production to the levels elicited by brain-derived ECM. In another study, pepsin solubilized ECM derived from porcine small intestinal submucosa, esophagus, and colon induced CD206 and Fizz1 protein expression in BMDM, whereas dermal ECM elicited iNOS expression, and liver and muscle-derived ECMs did not induce significant macrophage polarization.[78] The Elisseeff group studied decellularized tissue from an array of different organs and found significantly lower myeloid recruitment to cardiac ECM compared to bone, lung, and liver ECMs at 3 weeks after subcutaneous implantation.[79] Their studies showed a mixed population of M1/M2 macrophages in response to all ECMs, but significantly more CD4+ T helper (Th) and CD4+FoxP3+ regulatory T (Treg) cells recruited to bone-derived ECM, and greater CD8+ cytotoxic T cells recruited to cardiac-derived ECM. Furthermore, studies in a volumetric muscle loss model using WT and Rag−/− (T and B-cell deficient) showed that anti-inflammatory and MHCII-expressing subsets were dependent on the adaptive immune compartment.[80] These findings suggest a role for the adaptive immune system in matrix-enhanced anti-inflammatory immune response. Finally, the age of tissue may be another factor, as a recent study showed that small intestinal submucosa (SIS) ECM from 12-week pigs generates a higher inflammatory iNOS and lower anti-inflammatory Fizz1 response compared to SIS ECM from 52-week pigs.[81] Source-dependent responses may be due to tissue-specific decellularization agents used, and/or unique composition of the matrices. Regardless of the mechanism, the distinct responses elicited by ECMs derived from different tissue sources may provide a potential tool to tailor immune response to the application, in vivo.
While ongoing research probes the immunomodulatory effects of diverse decellularized ECMs, there are also many commercially available decellularized matrix wound dressings, which have been studied in the context of their elicited immune response. Integra (bovine tendon-derived, Type I collagen and chondroitin-6-sulfate) was found to reduce inflammation, with higher CD163 and lower TNFα protein expression in both primary human and THP-1 macrophages, when compared to PriMatrix (fetal bovine dermis-derived, Type I and III collagen), AlloMend (human dermis-derived collagen and elastin matrix), and Oasis (porcine small intestine submucosa, collagen I, II, III, elastin, glycosaminoglycans, proteoglycans, glycoproteins).[19] However, trends in gene expression markers were not consistent between the two cell sources tested, and dynamic responses were observed across 6 days of culture. Using a murine full thickness skin wound model, El Masry et. al. evaluated immunomodulatory effects of commercial equine pericardial collagen matrix (sPCM) on macrophages.[82] sPCM showed greater macrophage recruitment, along with increased early inflammatory Il1b/Inos/Tnfa gene expression, and prolonged late anti-inflammatory Arginase1/Vegf/Il10 gene expression, compared to polycarbonate mesh control. Additionally, sPCM promoted apoptotic cell uptake (efferocytosis) in post-implant day 3 murine wound macrophages, a key functional role of macrophages in early wound healing. These findings provide evidence for pro-healing matrix-mediated modulation of macrophages in vivo. Following up on their earlier studies, Sadtler et. al. characterized both the innate and adaptive immune response to commercial urinary bladder matrix protein (UBM, Micro Matrix™), which is primarily composed of type I collagen.[71] Upon implantation, over 40 percent of cells recruited to UBM were F4/80+ macrophages at 1 week post-implant, with upregulated CD206 and downregulated CD86 compared to saline-treated controls. Further, while MHCII expressing macrophages typically express M1 markers, MHCII+ cells from UBM-treated mice were over 90% anti-inflammatory (CD206+), indicating a material-directed shift towards anti-inflammatory signaling between the innate and adaptive immune compartments. This work again suggests the integrated effects of decellularized ECM on the immune system.
In summary, antigen removal processing, microstructure, tissue source, and age, all affect the immunomodulatory properties of decellularized matrices. While xenogeneic and allogeneic tissues are readily available, it remains difficult to control the precise molecular composition of these scaffolds, as well as contaminants in materials derived from these sources, such as adsorbed growth factors, chemokines, and antigenic proteins. To obtain better control over the matrix composition, we turn to synthetic hydrogel systems to build from the bottom-up.
3. Engineered ECM peptide-mimetic materials
In the top-down approach, full length ECM proteins were used as design components to elicit desired immune responses. However, this approach is constrained in engineering versatility and precise control due to the structural and conformational nature of these native proteins, and due to the challenges of independently tuning different parameters; for example, the moduli of the material usually cannot be decoupled from its concentration, and hence the number of cell interaction sites. To overcome these limitations, a bottom-up approach to designing immunomodulatory materials has been explored. The ability of ECM proteins to tune immune cell behaviors is facilitated by bioactive amino acid sequences that encode specific cellular cues. These domains can be functionalized into biomaterials to regulate specific immune cell functions. This section will focus on engineering ECM-derived peptides into various materials for immunomodulatory applications.
3.1. RGD
The bioactive motif with the amino acid sequence Arg-Gly-Asp (RGD) was first identified on fibronectin as the minimum cellular binding motif [83]. RGD-containing domains have since been recognized in other various extracellular matrix (ECM) proteins such as fibrinogen, vitronectin, laminin, and collagens.[84,85] Cellular integrins, including αvβ3, αMβ2 and others, are capable of binding to the RGD domain on ECM proteins.[86] RGD bioactive domains have been isolated and functionalized as ECM-mimetic peptides in biomaterials engineering. In recent years, it has been identified that these bioactive regions are multifaceted in their cellular signaling functionality. Specifically, here we will describe immunomodulatory effects of the ECM mimetic domain RGD, which exhibits various immunomodulatory effects on both innate and adaptive immune cells.
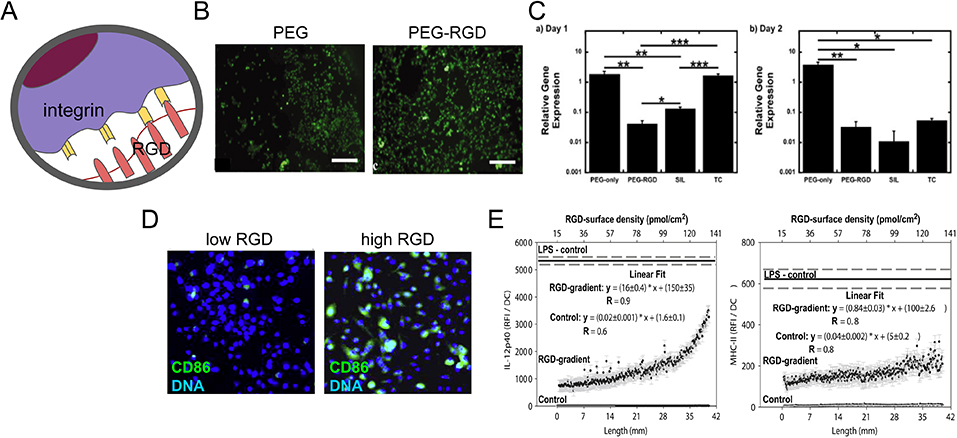
In the innate immune system, RGD plays an important role in adhesion of myeloid cells including neutrophils, macrophages, and DCs. Both soluble and surface functionalized RGD peptides bind myeloid cells via integrins. A synthetic peptide derived from the RGD adhesion domain of the basement membrane glycoprotein entactin facilitated increased neutrophil adhesion comparable to full recombinant entactin.[87] As with neutrophils, RGD-engineered materials increased cellular adhesion in macrophages (Figure 5B).[88] It has also been demonstrated that an increased RGD surface density yields an increase in DC surface adhesion, relative to carboxyl gradient controls (Figure 5D).[89] Thus, RGD peptides increase myeloid cell adhesion when functionalized onto surfaces or engineered into cellular scaffolds, enhancing their interactions with materials.
Figure 5.
RGD facilitates cellular adhesion and differentially modulates the activation of myeloid cells. (A) Schematic representation of cellular adhesion facilitated by RGD domains in the ECM and cellular integrins. (B) Immunofluorescence images of calcein AM-stained BMDMs on PEG only or RGD-PEG surfaces. Scale bar is 200 μm. (C) Relative gene expression of Tnfa in BMDMs at days one (left) and two (right), seeded onto PEG-only, PEG-RGD, medical grade silicon (SIL) and tissue culture polystyrene (TC) surfaces. Gene expression was normalized to housekeeping gene L32. (B) and (C) are adapted from Lynn et al., 2010.[88] (D) Immunofluorescence image of CD86 immunostained (green) and dendritic cell nuclei stained with DAPI (blue) DCs on low and RGD densities. (E) Expression of DC production of IL-12p40 and stimulatory molecule MHC-II when cultured for 24 h a gradient hydrogel with indicated RGD peptide surface density. The solid line represents the background-corrected relative fluorescence intensity of DCs cultured in the presence of LPS with the dashed line indicating the standard error. Data sets were linear curve fit and equations with the obtained parameters are shown. (D) and (E) are adapted from Acharya et al., 2010.[89]
RGD peptides are capable of modulating more than just adhesion to a material; they also have an inhibitory effect on neutrophil chemotactic activity and phagocytic functionality.[87] Senior et al. investigated the effects of pretreatment with an RGD peptide (SIGFRGDQTC) on neutrophil chemotaxis. The RGD peptides inhibited chemotactic activity of neutrophils on the basement membrane protein when compared to the negative peptide control. This suggests that naturally-occurring RGD motifs in ECM proteins can facilitate chemotactic functionality of neutrophils. The effect of these RGD peptides on neutrophil phagocytic functionality was also quantified. Neutrophils demonstrated fibrinogen-stimulated phagocytosis of EIgG proteins, but pre-incubation with RGD peptide reduced the phagocytic index (i.e. the number of EIgG ingested/ 100 neutrophil cells) to 20% of the maximum. This study highlights the ability of RGD peptide binding to reduce phagocytic and chemotactic functionalities in neutrophils.
Other myeloid immune cells, such as macrophages, also showed altered immunomodulatory effects due to RGD interactions; these changes include phenotype, cytokine production, and phagocytic functionality. Zaveri et al. investigated the role of RGD and the αMβ2 integrin in macrophage activation.[90] RGD peptides elicited an anti-inflammatory effect and reduced both phagocytosis and pro-inflammatory cytokine production in stimulated and unstimulated macrophages. Using soluble RGD peptides to block binding by integrins, a substantial decrease and nearly complete inhibition was observed in TNFα and IL-6 production. The study also demonstrated a significant decrease in microparticle uptake at two and 24 hours compared to the negative control. These results suggest that RGD peptides alone had an anti-inflammatory effect on macrophage cytokine production and phagocytosis.
In the context of synthetic ECM-mimetic hydrogels, RGD has been reported to elicit anti-inflammatory effects from macrophages as well as increased cellular adhesion when compared to the unmodified polymer meshes.[88,91] The Bryant group compared macrophage response to PEG hydrogels, PEG tethered to RGD (PEG-RGD), medical grade silicone, and tissue culture polystyrene surfaces. The in vitro gene expressions of both Tnfα and Il1b by macrophages were approximately 100-fold less on PEG-RGD compared to expression on PEG (Figure 5C). Longer-term viability studies of implanted PEG-RGD hydrogels in vivo yielded an inflammatory cell layer thickness of about 20–40 μm, compared to PEG-only hydrogels which had a 100–200 μm pro-inflammatory layer. Response to RGD-PEG hydrogels exhibited a typical foreign body reaction that was similar to silicone controls, but a strong inflammatory reaction was observed towards PEG-only hydrogels. More detailed investigations were performed to examine a range of cytokine gene expression (e.g., Tnfa, Il1b, Il10, Il12b, Arginase, Inos, Vegfa) over time, with and without lipopolysaccharide stimulation. Overall, results indicated a decrease in inflammatory effect (but not elimination) due to RGD incorporated into a synthetic polymer matrix. Macrophages on the different surfaces were found to yield similar phenotype changes, but the magnitude of these responses and their temporal shifts varied depending on the material. The temporal function of cytokine production could be an important relationship to consider in the context of wound healing; one could envision that timing of macrophage responses could be engineered to achieve shorter healing timelines. Although more studies are needed, the temporal effects due to RGD could be utilized as a tuning parameter for engineering biomaterials to modulate the immune response in response to injury and to promote healing.
In macrophages, RGD peptides do not only affect the production of cytokines, but have been observed to increase the production of certain enzymes, in particular matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).[92,93] Jones et al. examined whether MMPs and their inhibitors (TIMPs) were involved in macrophage adhesion and fusion, as part of the foreign body reaction to biomaterials.[92] Different materials, including those with surface-bound RGD peptides, were tested for their effects on MMP and TIMP enzyme expression by human monocytes/macrophages. Surfaces with RGD peptides strongly elicited MMP-9 and TIMP-1/TIMP-2 production, while weakly yielding MMP-10 and MMP-8. These studies highlight the potential for RGD not only to enhance adhesion of cells to the materials, but also to alter the potential of macrophages to degrade and remodel their surrounding matrix environment.
The immunosuppressive effects of RGD peptides may not be conserved in all innate immune cell types. The interaction of DCs with RGD peptides was investigated by Acharya et al.[89] This study quantified the immunomodulatory effects of RGD on DC phenotype by examining DC expression of activation markers and cytokines at increasing RGD surface densities. They observed that the expression of DC activation markers CD86 and MHC II were upregulated at increasing RGD peptide density (Figure 5E). Furthermore, the production of cytokines IL-12p40 and IL-10 were both increased, with IL-12p40 being the most sensitive to RGD density (Figure 5E). Thus, generally RGD was observed to activate DCs.
RGD peptides have also been shown to alter T cells cytokine secretion under certain conditions. Bollyky et al.[69] examined the role of the ECM in peripheral immune tolerance and described a phenomena in which hyaluronic acid (HA) promoted induction of FoxP3/IL-10–producing regulatory T cells (Treg) from T cell precursors. Osteopontin, a matrix glycoprotein found in chronic inflammation, which binds to CD44 and αvβ3 integrin, was used to investigate the role of binding integrins in osteopontin-mediated IL-10 inhibition.[94–97] Preincubation of anti-CD3/CD28 activated T cells with RGD peptides (but not RGE negative control peptides) rescued the suppression of IL-10 by osteopontin.
3.2. Enzyme-Sensitive Peptides
ECM degradation is mediated primarily by matrix metalloproteinases (MMPs), a family of proteases whose functions include selective degradation of a wide variety of ECM proteins and interactions with bioactive molecules, some of which mediate immunomodulatory effects.[98,99] MMPs were initially described in observations of enzymatic degradation of collagen during tadpole tail metamorphosis; this enzyme was named interstitial collagenase (i.e., MMP-1).[100] In terms of the immune system, MMPs can directly impact the function of various chemokines and cytokines. For example, MMP-3 can inactivate monocyte chemoattractant protein (MCP) 1, 2 and 3, while MMP-7 and MMP-12 can release latent TNFα activating this inflammatory cytokine.[99,101–103] In the context of the natural ECM, an extensive range of amino acid sequences are susceptible to cleavage by different MMPs; one study found 4300 peptides sensitive to nine human MMPs,[104] suggesting a diverse array of possible moieties to use in engineering enzyme-sensitive materials.
MMP-sensitive peptides are incorporated into synthetic hydrogels to increase the extent of matrix remodeling, natural integration of the engineered material into native tissues, and extent of immune cell invasion of the implanted materials. Work performed by West and Hubbell pioneered a new approach in biomimetic hydrogels by incorporating peptides that were susceptible to degradation by either fibroblast collagenase (MMP-1) or plasmin (fibrinolytic protease) into a PEG hydrogel.[93,105] Patterson et al. found that enzyme-sensitive materials degrade at different rates depending on the type of MMP, with faster rates at higher MMP concentrations[106]. Degradation was also dependent on the peptide substrate’s sequence, revealing an engineering strategy for controlled degradation. Here, we will focus on the few studies which have investigated the effects that these peptides have on immune cells.
Macrophages are known to produce MMPs,[107,108] and studies have examined the ability of this expression for materials degradation and macrophage infiltration. However, the extent of degradation can be material dependent; for example, synthetic polymer hydrogels crosslinked with MMP-sensitive peptides show limited degradation by macrophages alone.[93] Amer et al. utilized PEG crosslinked with either MMP-sensitive peptide (CVPLS↓LYSGC) or MMP-insensitive crosslinker to form hydrogels and test the in vitro effects of RAW 264.7 macrophage cells and in vivo implantation on hydrogel degradation.[93] No significant degradation in hydrogel integrity was observed when the material was incubated in macrophage-conditioned media or after in vivo implantation of the material, and it was hypothesized that either the concentrations of MMP-2/9 were too low to result in substantial hydrogel degradation, or that PEG allowed for the nonspecific adsorption of MMP inhibitor proteins (e.g., alpha-2-macroglobulin, murinoglobulin-1, factor Xa, thrombin). Another explanation could be that material-induced release of macrophage TIMPs inhibited the functionality of secreted MMPs, arresting the degradation of the engineered PEG hydrogel. However, further research is need to support these hypotheses. In contrast, in vitro studies using MMP enzymes alone showed degradation of material as expected, and it was concluded that the inability of macrophages to degrade and infiltrate hydrogels in this investigation was due to insufficient levels of MMPs.
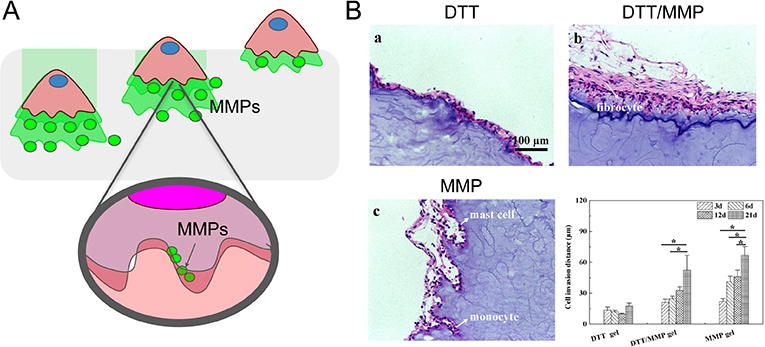
One recent study by Yu et al. exemplified the synergistic effects of both co-culture and natural biomaterial scaffolds on the extent of cell invasion.[109] To mimic the migration ability of vascular cells responding to chemokines that are expressed by inflammatory cells, human vascular smooth muscle cells (SMCs) were co-cultured with macrophages using U937 macrophage cells. Hyaluronic Acid (HA) hydrogels were crosslinked with either MMP-sensitive peptides (GCRD-GPQG↓-IWGQ-DRCG-NH2) or non-degradable dithiothreitol (DTT). Chemoattractants (e.g., IL-1β, IL-6) produced by U937 cells in the lower chamber of a transwell system promoted the migration of SMC cells grown on the gels in the upper chamber. A larger migration distance through the gel was observed in MMP-sensitive gels relative to DTT control gels, and in the presence of macrophages. Migration distance was further increased when MMP gels were pre-treated with U937 cells, suggesting that U937 cells could additionally be releasing MMP enzymes that aid in matrix degradation. Cell infiltration depths in materials implanted in vivo supported these same trends, with greatest infiltration in MMP-sensitive gels compared to DTT gels (Figure 6B).
Figure 6.
MMP sensitive peptides facilitate immune-mediated matrix degradation. (A) Schematic representation of immune cell infiltration facilitated by matrix degradation via MMPs. (B) H&E stained sections of dithiothreitol (DTT) crosslinked hydrogels with increasing the MMP sensitive peptide crosslinker content (from 0 to 100%) implanted subcutaneously in mice for a total of 21 days and quantification (bottom right) of cell invasion distance in the different materials at 3, 6, 12, and 21 days. Adapted from Yu et al., 2018.[131]
Immunogenicity of MMP-sensitive peptides is a concern for the development of ECM-mimetic hydrogel scaffolds. In a study by Fonseca et al., the activation of human monocyte-derived immature DCs by a MMP sensitive peptide (PVGLIG) was examined.[110] Immature DCs were incubated with soluble PVGLIG, soluble PVGLIG-alginate conjugates, or LPS as a positive maturation control. Activation markers (CD83, CD86) expressed by DCs treated with MMP-cleavable peptides (over all concentrations tested) were significantly lower than LPS-stimulated control groups and were expressed at similar levels to both the unstimulated DCs (negative control) and unmodified alginate control groups. These results showed that PVGLIG peptides and peptide-alginate conjugates did not up-regulate the expression of DC activation markers.
In addition to eliciting engineered responses from the innate immune system, MMP-cleavable peptides have also been utilized as a component for the controlled release of antigens in adaptive CD8+ T cell response. In a recent study by Dong et al., an MMP2/9 sensitive peptide (PLGLAG) was used to increase the delivery of an antigen epitope directly to MHC I complexes of DCs.[111] Although CTL-epitope peptides have been used as vaccines against cancer and infection, the efficacy of these conventional peptide formulations is less than optimal due to short plasma half-life of peptides in vivo and the dependence on intracellular DC/APC processing.[112–116] Directly loading antigenic epitopes onto MHC I of DCs bypassed intra-DC processing, and the antigen release that allowed for direct epitope loading was facilitated by the MMP-9 sensitive peptide that connected the CTL epitope peptide to an immune-tolerant elastin-like polypeptide (iTEP) macromolecule carrier. Cleavage by MMP-9 secreted by DCs enabled epitope peptide delivery, enhancing epitope-specific CD8+ T cell response by as high as 9.6-fold compared to the MMP-insensitive control vaccine. Future studies in vivo will be needed to elucidate whether such a strategy is viable for vaccine development, since in vivo environments could potentially include other MMP-secreting cells and exhibit lower DC concentrations.
3.3. LAIR-1 Binding Peptide
Full-length ECM proteins have a multitude of bioactive sequences that elicit a variety of immune cell behaviors. Some of these bioactive regions play a role in mediating natural immune cell activation and inflammatory responses. This regulatory functionality comes from binding and activating inhibitory immune cell surface receptors. One such surface receptor is known as leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1 and/or CD305), expressed in both mice and humans on a majority of immune cell types, including natural killer cells, T cells, B cells, monocytes/macrophages, DCs, eosinophils, basophils, and mast cells.[117] Collagen, the most abundant ECM proteins in the human body, is a natural ligand for LAIR-1, with varying degrees of binding affinity depending on the collagen type and specific binding domain.[15] Interestingly, tumor cells can overexpress collagen and inhibit NK cell toxicity through this interaction with LAIR-1,[118] and chronic autoimmune inflammation has also been linked to decreased LAIR-1 expression.[119] Bioactive regions of LAIR-1 binding on collagen II and III have been identified, isolated, and categorized by cell adhesion and induction of immune activity.[16] It has been hypothesized that the amount or density of collagen may contribute towards setting the specific thresholds for activation and recruitment of immune cells in various tissues.[117]
Isolated collagen peptide domains, with high affinity for LAIR-1, have been synthetically produced to explore their potential as bioengineered materials for immunomodulation. Work from our own groups have showcased the effect of immobilizing a specific collagen LAIR-1 binding domain on macrophage phenotype and reduction of inflammatory responses.[120] In our study, we used a peptide first identified by Farndale and Meyaard,[16] which showed the greatest level of inhibition of CD3-induced T cell activation as well as significant inhibition of FcεR1-induced degranulation of mast cells. An N-terminal cysteine that was added to the peptide enabled surface functionalization. The resulting LAIR-1 binding peptide (LAIR1p) was conjugated to the surface of maleimide-functionalized surfaces.
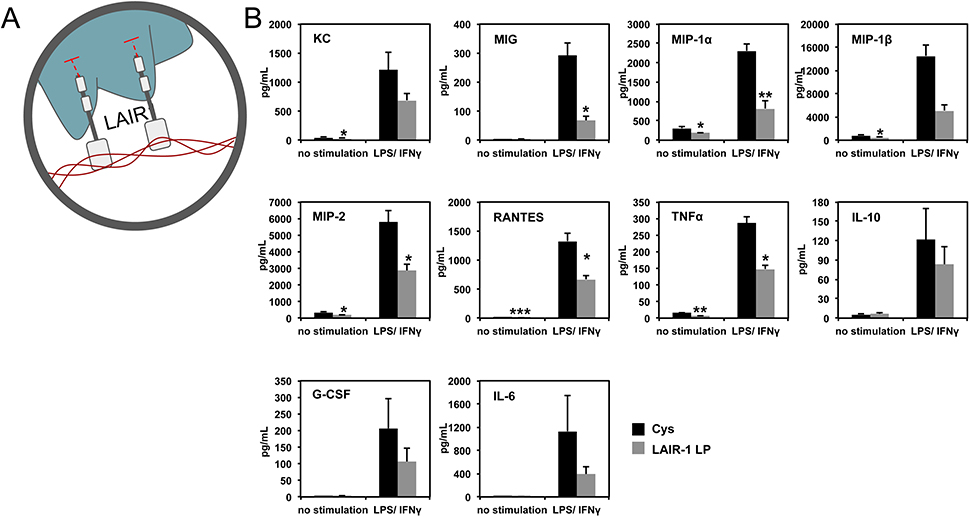
To investigate the immunomodulatory function of LAIR1p, murine BMDM were stimulated with LPS and IFN-γ (to mimic an inflammatory response due to injury) and cultured on LAIR1p saturated surfaces. Results showed a 60% reduction of the pro-inflammatory cytokine TNFα secreted by BMDM cultured on LAIR1p, compared to growth on non-LAIR1p control surfaces (Figure 7).[120] These results were recapitulated for human monocyte derived macrophages. This reduction was not observed for LAIR1p delivered in soluble form (not attached to surface), and no statistically significant effect on IL-10 production was observed. Surface conjugated LAIR1p also significantly inhibited the production of other pro-inflammatory cytokines/chemokines (Figure 7), such as MIG (monokine induced by interferon-γ; CXCL-9), MIP-1α (macrophage inflammatory protein-1α; CCL-3), MIP-1β (CCL-4), MIP-2 (CXCL-2) and RANTES (regulated upon activation, normal T-cell expressed, and secreted; CCL-5). These results show the potential of engineering LAIR-1 binding ligands into biomaterials for suppression of inflammatory macrophage activation. Further work is needed to elucidate the effects of LAIR1p on other immune cell types to assess its global immunomodulatory effects.
Figure 7.
LAIR1 ligands in collagen modulate inflammatory cytokine production. (A) Schematic representation of LAIR-1 binding to collagen. (B) Levels of secreted cytokines from BMDM cultured on cysteine (control) or LAIR-1 ligand peptide (LP) coated surfaces, with or without LPS/IFNγ stimulation. Adapted from Kim et al., 2017.[120]
3.4. Multidomain Peptides and Hybrid Hydrogels
The natural ECM has a variety of molecules, each of which contain specific structures and sequences that encode for a myriad of cellular cues that can individually and combinatorially modulate (and be modulated by) a wide spectrum of cell types. In a bottom-up approach, the incorporation of multiple-ECM inspired domains or larger ECM-derived components into engineered matrices can potentially elicit multifaceted and synergistic immune responses. For example, fibrin gamma chain was polymerized with PEG polymer, and shown to have similar coiled-coil conformation as native fibrin.[121] The composite hydrogel did not elicit IL-2 or IFN-γ immune responses in mice, suggesting limited T-cell response, but did elicit greater antibody production when compared to fibrin alone.
Multi-domain peptide (MDP) hydrogels are injectable ECM-mimetic materials, engineered to form self-assembling meshes that often utilize peptides to control cellular behavior. MDP hydrogel self-assembly is driven by its A-B-A building block motif; positively charged lysine residues (A blocks) flank the B block, which is comprised of alternating hydrophilic and hydrophobic amino acids that form a facial amphiphile (Figure 8A). Self-assembled structural features can be tuned by molecular chemistry, assembly environment, and assembly kinetics, yielding geometries that range from micelles to meshes.[122] Multiple different ECM-derived peptides can also be incorporated into the central and terminal regions of the A-B-A block motif. The assembly and material properties of MDPs have been extensively reviewed by others.[122,123]
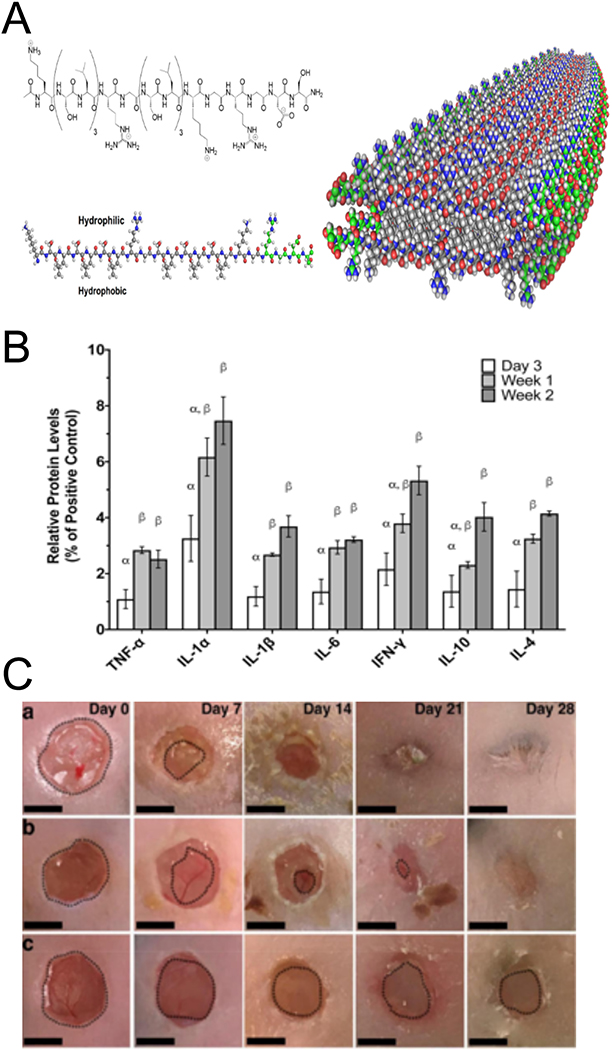
Figure 8.
MDPs are self-assembled molecules that allow for the incorporation of multiple ECM derived peptides. (A) Schematic depiction of self assembly of A-B-A block motif peptides into the basic fiber building block of MDPs, adapted from Kumar et al, 2015.[140] B) Relative levels of pro- and anti-inflammatory cytokines present in dorsal tissues after MDP subcutaneous injection into rats at different time points.[139] (C) Gross morphometry images of skin wounds were treated with MDP hydrogel (top), IntraSite (middle), and buffer control (bottom). Scale bars are 5mm Adapted from Carrejo et al., 2018.[126]
Interestingly, MDPs alone, without bioactive or ECM-mimetic peptides or drugs, have been shown to be biocompatible and have significant pro-healing effects. Xu et al. characterized MDP hydrogels for their cytotoxicity on BMDMs.[124] Peptides with the general sequence Kx(QL)yKz and their resulting hydrogels, were tested, showing that higher peptide concentrations (>1 μM) resulted in reduced cytotoxicity, which was attributed to the formation of ECM-like supramolecular structures at the higher concentrations. Moore et al. demonstrated the in vivo pro-healing effects of these MPDs in a rat subcutaneous implant model (Figure 8B).[125] An MDP with sequence K2(SL)6K2 implanted in vivo showed cellular infiltration, matrix degradation, angiogenesis/vascularization, and innervation. A cytokine array analysis performed on these extracted hydrogels showed the presence of several chemokines involved in immune and inflammatory cell trafficking and recruitment. MDP hydrogels elicited an initial acute proinflammatory response that included the infiltration of neutrophils and macrophages, which then progressed to a pro-resolution response, suggesting the host’s ability to successfully resolve the material-induced inflammatory response. These responses to this material promoted wound-healing in a diabetic mouse model (Figure 8C).[126] The positive results were somewhat surprising, given that the peptide sequence did not contain known bioactive sequences and that prior in vitro results did not support strong bioactivity of this peptide sequence.
Immunomodulatory effects can also be elicited by the incorporation of engineered ECM mimetic peptides. Kumar et al. showed that MDP hydrogel with a sequence containing adhesive RGD and enzyme sensitive LRG (K(SL)2(SLRG)(SL)3K(GRGDS) [“SLac”]) had increased in vitro hydrogel degradation as well as increased cellular adhesion of human monocyte cell line THP-1 when compared with hydrogels fabricated with (K2(SL)6K2).[127] Implanting the material subcutaneously in a rat model showed that SLac gels promoted macrophage invasion and matrix degradation.
Multiple ECM mimetic peptides have also been engineered into hydrogel platforms to increase the viability of the biomaterial in vivo. For example, Tian et al. used PEG hydrogels engineered with multiple bioactive peptides to evaluate the interdependent effects of ECM cues on the clustering of follicular DCs during the formation of malignant B and T cell lymphoma in an organoid model.[128] Functionalized PEG was conjugated to integrin-binding adhesion peptides RGD (NH2-GRGDSPC-COOH) (for αvβ3 integrins) or REDV (NH2-GREDVGC-COOH) (for α4β1 integrins), together with either MMP-sensitive peptide VPM (NH2-GCRDVPMSMRGGDRCG-COOH) or non-degradable dithiothreitol (DTT). In non-degradable PEG hydrogels, DCs were distributed as individual cells throughout the hydrogel, independent of RGD, and formed only small clusters with the incorporation of REDV. However, in degradable peptide crosslinked hydrogels, the presence of RGD led to large cluster formation, which was further enhanced with REDV. The immunomodulatory effects of adhesive peptides on follicular DC cluster size were regulated by the presence of the MMP-9 sensitive peptide, highlighting the combinatorial effects that multiple ECM-mimetic peptides have on cell behavior.
4. Conclusion and Future Directions
This review highlights the potential of ECM-derived hydrogels for modulation of the immune system in biomedical applications. Both naturally derived (top-down) and synthetic (bottom-up) engineering approaches have shown promise, each with their own strengths and weaknesses. However, more comprehensive investigations of ECM immunomodulation is needed to inform translation to useful clinical therapeutics. While analysis of hallmark markers of inflammatory (e.g. TNFα, IL-1β) and anti-inflammatory (e.g. IL-10, TGFβ) responses is valuable, particularly for screening many different materials, a panel of markers better captures the complexity of cellular interactions with ECM-derived materials, particularly in addressing the combinatorial effects of innate and adaptive immune compartment. Additionally, the temporal dynamics play a key role in cytokine secretion, adhesion, and other effects, making time another key parameter in need of thorough examination. Consideration of immune cell phenotypes, such as phagocytosis, antigen presentation, and activation are also important readouts of biomaterial-immune cell interactions.
With respect to the ECM-derived hydrogel-immune interaction, the above behaviors have been largely explored in macrophages, which are clearly a major player in the immune response to implanted materials. However, probing the role of other immune cells in both the innate and adaptive compartments is needed to truly understand the effects of these biomaterials on the immune system. Studies describing the effects of ECM-based materials on T and B cells have been particularly sparse,[47,129,130] as is work on neutrophils and the interface between the innate and adaptive immune system via DCs and macrophages.[49,68,129] Some investigators have moved beyond studying the response of individual immune cell types, developing co-culture models to analyze cell-matrix-cell crosstalk and more closely mimic the physiologic response to ECM.[131] Co-culturing two or more cell types on biomaterials has been shown to highlight otherwise concealed immunomodulatory properties,[131] providing a high-throughput platform that holds promise to help reconcile disparities between in vitro and in vivo studies. Choice of cells in all of these studies is critical. The many cancer-derived cell lines and variable primary sources used, without context, make the comparison of any findings to physiologic immune response difficult to parse. For example, U937 and THP-1 monocytes are both isolated from leukemia patients and widely used as macrophage-like cells to characterize the biomaterial response; however, their response to ECM-derived materials may or may not be aligned with that of healthy macrophages in vivo.[18,19,22,65,76,131] Additionally, there is little work on disease or tissue-specific immune response to ECM-derived biomaterials. For example, the diversity of ECM in the brain, and microglial response to biomaterials, are still not well understood.[41,42,58]
Studies of interactions between immune cells and ECM-based scaffolds may provide important mechanistic insight, but translating these materials into the clinic ultimately requires analysis of response in vivo. Furthermore, local environments have a profound effect on cellular behavior, often causing discrepancies between in vitro and in vivo data.[132] For example, pro-healing effects of MDP hydrogels were observed in implant studies in vivo,[125] which were unexpected based on in vitro observations of minimal enhancement of cell viability, adhesion, and morphology compared to MDPs engineered with ECM-derived peptides [133,134]. This example highlights the need for better models and caution when translating in vitro-in vivo data. Nonetheless, in vivo experiments testing immune response to ECM-derived materials are not without their own challenges, often relying on histologic analysis and may not be straightforward to quantify. It is important that future research prioritize a robust quantitative and temporal analysis of cytokine production, immune cell phenotype, and matrix remodeling, in vivo as well as in vitro, when investigating the impacts of ECM-derived materials on the immune system. Additionally, the development and standardization of relevant disease models will be important for clinical translation.
This review has outlined two main approaches for immunomodulatory ECM engineering: top-down naturally-derived ECMs and bottom-up synthetic hydrogels. Applications of these engineered materials are diverse, not only to modulate the immune system for tissue regeneration, but also to deliver drugs, bioactive molecules (such as cytokines, chemokines and growth factors), and cells.[82,127,134–138] In each engineering approach, certain materials show notable potential. Decellularized matrices have been commercialized for clinical use,[78] and show unique immunomodulatory effects based on source tissue and physical characteristics, but these materials remain heterogeneous and subject to contamination with cell-products, effectively limiting engineering methods to finely control the immune response. Single ECM materials, on the other hand, have more precise composition and thus consistent cellular responses, but require sophisticated molecular engineering tools to tune their biochemistry and immunomodulatory effects.[25–27]
Bottom-up synthetic biomimetic materials enable an approach arguably more amenable to biomaterials engineering, and provide the ability for modular design. However, this synthetic approach lacks the full natural context of the original ECM protein, and may yield different or less efficacious responses. In this category, MDPs in particular have shown great promise; they are biodegradable and modular, with examples that demonstrate support of a pro-resolution environment.[126,139] Combining these synthetic materials with naturally-derived ECM proteins that provide further control of bioactivity, may generate hybrid materials that leverage the advantages of both approaches, allowing for more precise tuning of the immune response.
It is clear that the ECM is a dynamic and complex effector for maintaining homeostasis (Figure 9), but the exact mechanisms and bioactive sequences that facilitate these complex, dynamic interactions are not well understood. It is important, therefore, not only to continue to engineer materials for biomedical applications, but also to probe the impact of biological context on how these signals are interpreted. Understanding the impact of structures, from local molecular conformation to cell- and tissue-level interactions, will facilitate greater control over immune responses. The variety of molecules present in the natural ECM and the multitude of cellular cues encoded into each, combined with the capabilities of molecular and biomaterials engineering, allows for a combinatorial design approach that holds the potential for precise immunomodulatory control for biomedical applications.
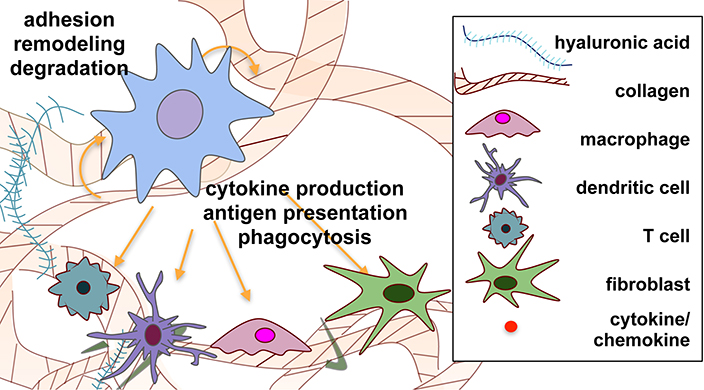
Figure 9.
Schematic representation of dynamic cell-matrix crosstalk during immune cell-matrix interactions. Material-cell crosstalk is represented through degradation of matrix by enzymes produced by cells, the production of ECM proteins by cells, and the immunomodulatory domains in the ECM described in this review. Cellular crosstalk is depicted via the production of, and interaction with, cytokines and chemokines by the various cell types.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Disease (NIAID) Grant R21AI128519-01 and National Institute of Biomedical Imaging and Bioengineering Grant R21EB022240.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67(2):149–159. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Peppas NA. Hydrogels and Scaffolds for Immunomodulation. Advanced Materials. 2014;26(38):6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dziki JL, Huleihel L, Scarritt ME, Badylak SF. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Engineering Part A. 2017;23(19–20):1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. The International Journal of Biochemistry & Cell Biology. 2004;36(6):1031–1037. [DOI] [PubMed] [Google Scholar]
- 5.Taraballi F, Sushnitha M, Tsao C, et al. Biomimetic Tissue Engineering: Tuning the Immune and Inflammatory Response to Implantable Biomaterials. Advanced Healthcare Materials. 2018;7(17):1800490. [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambayashi T, Jacob CO, Strassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell Immunol. 1996;171(1):153–158. [DOI] [PubMed] [Google Scholar]
- 8.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. [DOI] [PubMed] [Google Scholar]
- 10.Vishwakarma A, Bhise NS, Evangelista MB, et al. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends in Biotechnology. 2016;34(6):470–482. [DOI] [PubMed] [Google Scholar]
- 11.Burgeson RE, Nimni ME. Collagen types. Molecular structure and tissue distribution. Clinical orthopaedics and related research. 1992(282):250–272. [PubMed] [Google Scholar]
- 12.Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boraschi-Diaz I, Wang J, Mort JS, Komarova SV. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Frontiers in Physics. 2017;5.29170738 [Google Scholar]
- 14.Orgel JP, San Antonio JD, Antipova O. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res. 2011;52(1):2–17. [DOI] [PubMed] [Google Scholar]
- 15.Lebbink RJ, de Ruiter T, Adelmeijer J, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203(6):1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebbink RJ, Raynal N, de Ruiter T, Bihan DG, Farndale RW, Meyaard L. Identification of multiple potent binding sites for human leukocyte associated Ig-like receptor LAIR on collagens II and III. Matrix Biology: Journal of the International Society for Matrix Biology. 2009;28(4):202–210. [DOI] [PubMed] [Google Scholar]
- 17.Gowen BB, Borg TK, Ghaffar A, Mayer EP. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biology. 2000;19(1):61–71. [DOI] [PubMed] [Google Scholar]
- 18.Veres SP, Brennan-Pierce EP, Lee JM. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. Journal of Biomedical Materials Research Part A. 2015;103(1):397–408. [DOI] [PubMed] [Google Scholar]
- 19.Witherel CE, Graney PL, Freytes DO, Weingarten MS, Spiller KL. Response of human macrophages to wound matrices in vitro. Wound Repair and Regeneration. 2016;24(3):514–524. [DOI] [PubMed] [Google Scholar]
- 20.Yu T, Wang W, Nassiri S, et al. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. Journal of Biomaterials Science, Polymer Edition. 2016;27(8):721–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson AR, Tanase CE, Awuah D, et al. Photocrosslinkable Gelatin Hydrogels Modulate the Production of the Major Pro-inflammatory Cytokine, TNF-α, by Human Mononuclear Cells. Frontiers in Bioengineering and Biotechnology. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha B-H, Shin SR, Leijten J, et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Advanced Healthcare Materials. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purwada A, Jaiswal MK, Ahn H, et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat Protoc. 2017;12(1):168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Que R, Mohraz A, Da Silva NA, Wang SW. Expanding Functionality of Recombinant Human Collagen Through Engineered Non-Native Cysteines. Biomacromolecules. 2014;15(10):3540–3549. [DOI] [PubMed] [Google Scholar]
- 26.Que RA, Arulmoli J, Da Silva NA, Flanagan LA, Wang SW. Recombinant collagen scaffolds as substrates for human neural stem/progenitor cells. Journal of Biomedical Materials Research Part A. 2018;106(5):1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Que RA, Chan SW, Jabaiah AM, Lathrop RH, Da Silva NA, Wang SW. Tuning cellular response by modular design of bioactive domains in collagen. Biomaterials. 2015;53:309–317. [DOI] [PubMed] [Google Scholar]
- 28.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–367. [DOI] [PubMed] [Google Scholar]
- 29.Ugarova TP, Yakubenko VP. Recognition of Fibrinogen by Leukocyte Integrins. Annals of the New York Academy of Sciences. 2001;936(1):368–385. [DOI] [PubMed] [Google Scholar]
- 30.Yakovlev S, Zhang L, Ugarova T, Medved L. Interaction of Fibrin(ogen) with Leukocyte Receptor αMβ2 (Mac-1): Further Characterization and Identification of a Novel Binding Region within the Central Domain of the Fibrinogen γ-Module. Biochemistry. 2005;44(2):617–626. [DOI] [PubMed] [Google Scholar]
- 31.Kuijper PHM, Torres HIG, Lammers J-WJ, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet and Fibrin Deposition at the Damaged Vessel Wall: Cooperative Substrates for Neutrophil Adhesion Under Flow Conditions. Blood. 1997;89(1):166–175. [PubMed] [Google Scholar]
- 32.Laurens N, Koolwijk P, Maat MPMD. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis. 2006;4(5):932–939. [DOI] [PubMed] [Google Scholar]
- 33.Podolnikova NP, Yakubenko VP, Volkov GL, Plow EF, Ugarova TP. Identification of a Novel Binding Site for Platelet Integrins αIIbβ3 (GPIIbIIIa) and α5β1 in the γC-domain of Fibrinogen. J Biol Chem. 2003;278(34):32251–32258. [DOI] [PubMed] [Google Scholar]
- 34.Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Sub-cellular biochemistry. 2017;82:405–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown LF, Lanir N, McDonagh J, Tognazzi K, Dvorak AM, Dvorak HF. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142(1):273–283. [PMC free article] [PubMed] [Google Scholar]
- 36.Lanir N, Ciano PS, Water LVd, McDonagh J, Dvorak AM, Dvorak HF. Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibronectin, and glycosaminoglycan content on cell migration. The Journal of Immunology. 1988;140(7):2340–2349. [PubMed] [Google Scholar]
- 37.Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomaterialia. 2017;47:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burmeister DM, Roy DC, Becerra SC, Natesan S, Christy RJ. In Situ Delivery of Fibrin-Based Hydrogels Prevents Contraction and Reduces Inflammation. Journal of Burn Care & Research. 2018;39(1):40–53. [DOI] [PubMed] [Google Scholar]
- 39.Anitua E, Zalduendo MM, Prado R, Alkhraisat MH, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: Evaluation of the effect of leukocyte inclusion. Journal of Biomedical Materials Research Part A. 2015;103(3):1011–1020. [DOI] [PubMed] [Google Scholar]
- 40.Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLOS ONE. 2015;10(3):e0121713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin R Targeting fibrin in neurodegeneration. Nature Immunology. 2018:1. [DOI] [PubMed] [Google Scholar]
- 42.Ryu JK, Rafalski VA, Meyer-Franke A, et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat Immunol. 2018;19(11):1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1β, IL-6 and plasminogen activator inhibitors from monocytes in vitro. British Journal of Haematology. 1994;86(2):322–326. [DOI] [PubMed] [Google Scholar]
- 44.Kazura JW, Wenger JD, Salata RA, Budzynski AZ, Goldsmith GH. Modulation of polymorphonuclear leukocyte microbicidal activity and oxidative metabolism by fibrinogen degradation products D and E. J Clin Invest. 1989;83(6):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakovlev S, Medved L. Effect of fibrinogen, fibrin, and fibrin degradation products on transendothelial migration of leukocytes. Thrombosis Research. 2018;162:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross TJ, Leavell KJ, Peterson MW. CD11b/CD18 mediates the neutrophil chemotactic activity of fibrin degradation product D domain. Thrombosis and Haemostasis. 1997;77(5):894–900. [PubMed] [Google Scholar]
- 47.Qu Z, Chaikof EL. Interface between hemostasis and adaptive immunity. Current Opinion in Immunology. 2010;22(5):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83(6):1309–1322. [DOI] [PubMed] [Google Scholar]
- 49.Ramelli G, Fuertes S, Narayan S, Busso N, Acha-Orbea H, So A. Protease-activated receptor 2 signalling promotes dendritic cell antigen transport and T-cell activation in vivo. Immunology. 2010;129(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Güç E, Briquez PS, Foretay D, et al. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials. 2017;131:160–175. [DOI] [PubMed] [Google Scholar]
- 51.Mokarram N, Bellamkonda RV. A Perspective on Immunomodulation and Tissue Repair. Ann Biomed Eng. 2013;42(2):338–351. [DOI] [PubMed] [Google Scholar]
- 52.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. [DOI] [PubMed] [Google Scholar]
- 53.Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater Sci Eng 2015;1(7):481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater 2013;9(7):7081–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Du G, Chen J, Zhu Y, Wang M, Sun J. Microbial production of low molecular weight hyaluronic acid by adding hydrogen peroxide and ascorbate in batch culture of Streptococcus zooepidemicus. Bioresour Technol. 2009;100(1):362–367. [DOI] [PubMed] [Google Scholar]
- 56.Knopf-Marques H, Pravda M, Wolfova L, et al. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Advanced Healthcare Materials. 2016;5(22):2841–2855. [DOI] [PubMed] [Google Scholar]
- 57.Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing: assessment of clinical evidence. Am J Clin Dermatol. 2005;6(6):393–402. [DOI] [PubMed] [Google Scholar]
- 58.Wrobel MR, Sundararaghavan HG. Biomaterial Cues to Direct a Pro-regenerative Phenotype in Macrophages and Schwann Cells. Neuroscience. 2018;376:172–187. [DOI] [PubMed] [Google Scholar]
- 59.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. [DOI] [PubMed] [Google Scholar]
- 60.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kudryavtseva V, Stankevich K, Gudima A, et al. Atmospheric pressure plasma assisted immobilization of hyaluronic acid on tissue engineering PLA-based scaffolds and its effect on primary human macrophages. Mater Design. 2017;127:261–271. [Google Scholar]
- 62.Park J, Babensee JE. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomaterialia. 2012;8(10):3606–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hart SP, Rossi AG, Haslett C, Dransfield I. Characterization of the effects of cross-linking of macrophage CD44 associated with increased phagocytosis of apoptotic PMN. PLOS ONE. 2012;7(3):e33142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levesque MC, Haynes BF. TNFalpha and IL-4 regulation of hyaluronan binding to monocyte CD44 involves posttranslational modification of CD44. Cell Immunol. 1999;193(2):209–218. [DOI] [PubMed] [Google Scholar]
- 65.Rios de la Rosa JM, Tirella A, Gennari A, Stratford IJ, Tirelli N The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv Healthc Mater. 2017;6(4). [DOI] [PubMed] [Google Scholar]
- 66.Huang GL, Huang HL. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv 2018;25(1):766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jha AK, Yang WD, Kirn-Safran CB, Farach-Carson MC, Jia XQ. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30(36):6964–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. Journal of Biomedical Materials Research Part A. 2005;74A(4):503–510. [DOI] [PubMed] [Google Scholar]
- 69.Bollyky PL, Wu RP, Falk BA, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):7938–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parmaksiz M, Dogan A, Odabas S, Elcin AE, Elcin YM. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater. 2016;11(2):022003. [DOI] [PubMed] [Google Scholar]
- 71.Sadtler K, Sommerfeld SD, Wolf MT, et al. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin Immunol. 2017;29:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35(30):8605–8612. [DOI] [PubMed] [Google Scholar]
- 74.Wong ML, Wong JL, Vapniarsky N, Griffiths LG. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials. 2016;92:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Hideyoshi S, Jiang H, et al. Injectable, porous, biohybrid hydrogels incorporating decellularized tissue components for soft tissue applications. Acta Biomaterialia. 2018;73:112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDade JK, Brennan-Pierce EP, Ariganello MB, Labow RS, Lee JM. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomaterialia. 2013;9(7):7191–7199. [DOI] [PubMed] [Google Scholar]
- 77.Meng FW, Slivka PF, Dearth CL, Badylak SF. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials. 2015;46:131–140. [DOI] [PubMed] [Google Scholar]
- 78.Dziki JL, Wang DS, Pineda C, Sicari BM, Rausch T, Badylak SF. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. Journal of Biomedical Materials Research Part A. 2017;105(1):138–147. [DOI] [PubMed] [Google Scholar]
- 79.Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, Elisseeff JH. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nature Reviews Materials. 2016;1(7):16040. [Google Scholar]
- 80.Sadtler K, Estrellas K, Allen BW, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LoPresti ST, Brown BN. Effect of source animal age upon macrophage response to extracellular matrix biomaterials. Journal of Immunology and Regenerative Medicine. 2018;1:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El Masry MS, Chaffee S, Das Ghatak P, et al. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2018:fj.201800352R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–33. [DOI] [PubMed] [Google Scholar]
- 84.Rgd Ruoslahti E. and other recognition sequences for integrins. Annual Review of Cell and Developmental Biology. 1996;12(1):697–715. [DOI] [PubMed] [Google Scholar]
- 85.Aukhil I Biology of wound healing. Periodontol 2000 2000;22:44–50. [DOI] [PubMed] [Google Scholar]
- 86.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. [DOI] [PubMed] [Google Scholar]
- 87.Senior RM, Gresham HD, Griffin GL, Brown EJ, Chung AE. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. 1992. [DOI] [PMC free article] [PubMed]
- 88.Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. Journal of Biomedical Materials Research Part A 2010;93A(3):941–953. [DOI] [PubMed] [Google Scholar]
- 89.Acharya AP, Dolgova NV, Moore NM, et al. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials. 2010;31(29):7444–7454. [DOI] [PubMed] [Google Scholar]
- 90.Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynn AD, Bryant SJ. Phenotypic changes in bone marrow-derived murine macrophages cultured on PEG-based hydrogels activated or not by lipopolysaccharide. Acta Biomaterialia. 2011;7(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones JA, McNally AK, Chang DT, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. Journal of Biomedical Materials Research Part A. 2008;84A(1):158–166. [DOI] [PubMed] [Google Scholar]
- 93.Amer LD, Bryant SJ. The In Vitro and In Vivo Response to MMP-Sensitive Poly(Ethylene Glycol) Hydrogels. Ann Biomed Eng. 2016;44(6):1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9(2):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature Immunology. 2007;8(1):74–83. [DOI] [PubMed] [Google Scholar]
- 96.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. [DOI] [PubMed] [Google Scholar]
- 97.Heilmann K, Hoffmann U, Witte E, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2009;13(6):1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sternlicht MD, Werb Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annual Review of Cell and Developmental Biology. 2001;17(1):463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Seminars in Cell & Developmental Biology. 2008;19(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gross J, Lapiere CM. Collagenolytic Activity in Amphibian Tissues - a Tissue Culture Assay. Proceedings of the National Academy of Sciences of the United States of America. 1962;48(6):1014-&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Westermann D, Savvatis K, Schultheiss H-P, Tschöpe C. Immunomodulation and matrix metalloproteinases in viral myocarditis. Journal of Molecular and Cellular Cardiology. 2010;48(3):468–473. [DOI] [PubMed] [Google Scholar]
- 102.Churg A, Wang RD, Tai H, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167(8):1083–1089. [DOI] [PubMed] [Google Scholar]
- 103.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eckhard U, Huesgen PF, Schilling O, et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biology. 2016;49:37–60. [DOI] [PubMed] [Google Scholar]
- 105.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32(1):241–244. [Google Scholar]
- 106.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31(30):7836–7845. [DOI] [PubMed] [Google Scholar]
- 107.Newby AC. Metalloproteinase Expression in Monocytes and Macrophages and its Relationship to Atherosclerotic Plaque Instability. Arterioscl Throm Vas 2008;28(12):2108–U2120. [DOI] [PubMed] [Google Scholar]
- 108.Sindhu S, Al-Roub A, Koshy M, Thomas R, Ahmad R. Palmitate-Induced MMP-9 Expression in the Human Monocytic Cells is Mediated through the TLR4-MyD88 Dependent Mechanism. Cell Physiol Biochem. 2016;39(3):889–900. [DOI] [PubMed] [Google Scholar]
- 109.Yu S, Duan YY, Zuo XG, Chen XY, Mao ZW, Gao CY. Mediating the invasion of smooth muscle cells into a cell-responsive hydrogel under the existence of immune cells. Biomaterials. 2018;180:193–205. [DOI] [PubMed] [Google Scholar]
- 110.Fonseca KB, Gomes DB, Lee K, et al. Injectable MMP-Sensitive Alginate Hydrogels as hMSC Delivery Systems. Biomacromolecules. 2014;15(1):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong S, Wang P, Zhao P, Chen M. Direct Loading of iTEP-Delivered CTL Epitope onto MHC Class I Complexes on the Dendritic Cell Surface. Molecular Pharmaceutics. 2017;14(10):3312–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239(1):27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinto LA, Edwards J, Castle PE, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188(2):327–338. [DOI] [PubMed] [Google Scholar]
- 114.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. [DOI] [PubMed] [Google Scholar]
- 115.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. [DOI] [PubMed] [Google Scholar]
- 116.Dong SY, Xu TF, Zhao P, Parent KN, Chen MN. A Comparison Study of iTEP Nanoparticle-Based CTL Vaccine Carriers Revealed a Surprise Relationship between the Stability and Efficiency of the Carriers. Theranostics. 2016;6(5):666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meyaard L The inhibitory collagen receptor LAIR-1 (CD305). J Leukoc Biol. 2008;83(4):799–803. [DOI] [PubMed] [Google Scholar]
- 118.Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. 2011;49(1–2):402–406. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Lv K, Zhang CM, Jin BQ, Zhuang R, Ding Y. The role of LAIR-1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cellular Immunology. 2014;287(1):46–52. [DOI] [PubMed] [Google Scholar]
- 120.Kim YK, Chu SH, Hsieh JY, et al. Incorporation of a Ligand Peptide for Immune Inhibitory Receptor LAIR-1 on Biomaterial Surfaces Inhibits Macrophage Inflammatory Responses. Adv Healthc Mater. 2017;6(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudra JS, Tripathi PK, Hildeman DA, Jung JP, Collier JH. Immune responses to coiled coil supramolecular biomaterials. Biomaterials. 2010;31(32):8475–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cui HG, Webber MJ, Stupp SI. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Biopolymers. 2010;94(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moore AN, Hartgerink JD. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Accounts of Chemical Research. 2017;50(4):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu DW, Jiang LH, Singh A, et al. Designed supramolecular filamentous peptides: balance of nanostructure, cytotoxicity and antimicrobial activity. Chem Commun. 2015;51(7):1289–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore AN, Silva TLL, Carrejo NC, Marmolejo CAO, Li IC, Hartgerink JD. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials. 2018;161:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carrejo NC, Moore AN, Lopez Silva TL, et al. Multidomain Peptide Hydrogel Accelerates Healing of Full-Thickness Wounds in Diabetic Mice. ACS Biomater Sci Eng 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar VA, Taylor NL, Shi SY, Wickremasinghe NC, D’Souza RN, Hartgerink JD. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian YF, Ahn H, Schneider RS, et al. Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells. Biomaterials. 2015;73:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736–4750. [DOI] [PubMed] [Google Scholar]
- 130.Sadtler K, Allen BW, Estrellas K, Housseau F, Pardoll DM, Elisseeff JH. The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Nontraumatic Applications<sup/>. Tissue Eng Part A. 2017;23(19–20):1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu S, Duan Y, Zuo X, Chen X, Mao Z, Gao C. Mediating the invasion of smooth muscle cells into a cell-responsive hydrogel under the existence of immune cells. Biomaterials. 2018;180:193–205. [DOI] [PubMed] [Google Scholar]
- 132.Artzi N, Oliva N, Puron C, et al. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging (vol 10, pg 704, 2011). Nature Materials. 2011;10(11):896–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD. Self-Assembling Multidomain Peptide Hydrogels: Designed Susceptibility to Enzymatic Cleavage Allows Enhanced Cell Migration and Spreading. J Am Chem Soc. 2010;132(9):3217–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang MK, Colombo JS, D’Souza RN, Hartgerink JD. Sequence Effects of Self-Assembling MultiDomain Peptide Hydrogels on Encapsulated SHED Cells. Biomacromolecules. 2014;15(6):2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumar VA, Liu Q, Wickremasinghe NC, et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials. 2016;98:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Engineering Part A. 2012;18(1–2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Webber MJ, Tongers J, Newcomb CJ, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):13438–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deegan DB, Zimmerman C, Skardal A, Atala A, Shupe TD. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. Journal of the Mechanical Behavior of Biomedical Materials. 2016;55:87–103. [DOI] [PubMed] [Google Scholar]
- 139.Moore AN, Lopez Silva TL, Carrejo NC, Origel Marmolejo CA, Li I-C, Hartgerink JD. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials. 2018;161:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D’Souza RN, Hartgerink JD. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]