Abstract
Angiotensin-converting enzyme 2 (ACE2) has been recognized as a potential entry receptor for SARS-CoV-2 infection. Binding of SARS-CoV-2 to ACE2 allows engagement with pulmonary epithelial cells and pulmonary infection with the virus. ACE2 is an essential component of renin-angiotensin system (RAS), and involved in promoting protective effects to counter-regulate angiotensin (Ang) II-induced pathogenesis. The use of angiotensin receptor blockers (ARBs) and ACE inhibitors (ACEIs) was implicitly negated during the early phase of COVID-19 pandemic, considering the role of these antihypertensive agents in enhancing ACE2 expression thereby promoting the susceptibility to SARS-CoV-2. However, no clinical data has supported this assumption, but indeed evidence demonstrates that ACEIs and ARBs, besides their cardioprotective effects in COVID-19 patients with cardiovascular diseases, might also be beneficial in acute lung injuries by preserving the ACE2 function and switching the balance from deleterious ACE/Ang II/AT1 receptor axis towards a protective ACE2/Ang (1–7)/Mas receptor axis.
Keywords: SARS-CoV-2, RAS, ACE2, ACEIs, ARBs and COVID-19
Highlights
-
•
Scientists are rushing to beat COVID-19, however so far there is no convincing evidence of any treatment effectiveness from clinical research.
-
•
Downregulation of ACE2 expression by SARS-CoV-2 infection contributes to loss of ACE2-induced protective effects and stimulation of counter-regulatory Ang II-induced pathogenesis.
-
•
RAS blockade by ACEIs and ARBs, besides promoting cardioprotective effects in COVID-19 patients with cardiovascular diseases, might also prevent SARS-CoV-2-induced inflammatory storm.
-
•
More studies are required to understand the role of ACE2 and therapeutic effects of ACEIs and ARBs on the susceptibility and progression of SARS-CoV-2 infection.
1. Introduction
In December 2019 in Wuhan, China, an ongoing rapidly spreading worldwide pandemic of a novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) also termed as COVID-19, was detected (Chen et al., 2020; WHO, 2020). In contrast to SARS-CoV which caused the 2002 outbreak, SARS-CoV-2 exhibits a higher risk of transmission as evident from the rapid global rise in the number of COVID-19 cases. As indicated in reported cases SARS-CoV-2 is transmitted from one individual to the other mainly through respiratory droplets (WHO, 2003). COVID-19 exhibits either asymptomatic upper respiratory infection or potentially fatal atypical pneumonia associated with acute respiratory distress syndrome (ARDS) (Guan and Zhong, 2020; Han et al., 2020) that can cause death in certain individuals (Guan and Zhong, 2020; Li et al., 2020; Wu and McGoogan, 2020). ARDS is an extreme form of acute lung injury with a high mortality rate (30–60%), marked by a rapid rise in inflammatory cells pulmonary edema, severe hypoxia and respiratory failure (Pfeifer, 2010). According to various sources including the Center for Disease Control and Prevention (CDC), while SARS-CoV-2 can infect anyone, however patients with pre-existing comorbidities such as hypertension, type 2 diabetes mellitus, cardiovascular diseases, and chronic obstructive pulmonary disease (COPD) are more likely to display a severe course and to have higher mortality rates (Emami et al., 2020; Team, 2020).
Angiotensin converting enzyme 2 (ACE2) has been identified as a functional receptor for SARS-CoV-2 pulmonary infection (Gheblawi et al., 2020). ACE2 exists in two forms, a membrane bound and a soluble form and SARS-CoV-2 entry into the host cell is mediated by binding of viral spike protein to the membrane bound form (Verdecchia et al., 2020). ACE2 is a key modulator of the renin-angiotensin system (RAS) (Ocaranza and Jalil, 2012), an intricate interlinked system that regulates physiological and pathological functions of cardiovascular, renal and pulmonary system. Abnormal activation of the Ang II/AT1 receptor component of RAS has been implicated in several pathologic conditions, including high blood pressure and contributes to the development of end organ damage through the activation of pro-inflammatory, and pro-fibrotic cascades (Lee et al., 1993; Luft, 2002; Weir, 2007; Gul et al., 2008, 2009, 2012a, 2015; Gul et al., 2009; Muñoz-Durango et al., 2016). ACE2 negatively regulates the Ang II/AT1 component of RAS by enhancing anti-proliferative and anti-apoptotic effects via the activation of ACE2/Ang (1–7)/Mas receptor signaling (Oudit et al., 2003; Zisman et al., 2003a; Patel et al., 2016). In experimental models of lung injury and ARDS, activation of AT1 receptor -mediated signaling drives severe lung failure by elevating inflammatory responses. This is blunted by ACE2 via its antagonistic actions on ACE/Ang II/AT1 receptor axis (Imai et al., 2005; Kuba et al., 2006; Hamming et al., 2007). Indeed, SARS- induced decrease in ACE2 is assumed to induce a more severe acute lung injury during infection by downregulating ACE2/Ang (1–7)/Mas receptor signaling and augmenting the activation of counter-regulatory ACE/Ang II/AT1 receptor axis (Kuba et al., 2006).
Considering the role of angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEIs) in amplifying ACE2 expression and activity in vivo studies (Ishiyama et al., 2004; Ferrario et al., 2005; Igase et al., 2005; Wang et al., 2016), concerns were raised in the early phase of COVID-19 outbreak, on whether these RAS blockers would intensify SARS-CoV-2 infection and severity of disease in patients using these drugs for the management of cardiovascular diseases and hypertension (Guo et al., 2020; Sommerstein, 2020). However, there is no clinical evidence at present demonstrating that these RAS blockers could either increase the susceptibility or aggravate the severity SARS-CoV-2 infection. Contrarily, recent studies have shown that RAS inhibitors are beneficial rather than harmful in patients with lung injury (Vaduganathan et al., 2020). Furthermore, ARBs and ACEIs are critical for the clinical management of high risk cardiovascular patients suffering from COVID-19 (Zhang et al., 2020). Additionally, given the anti-inflammatory effects of ACE2/Ang (1–7)/Mas receptor activation, we assume that ACE2 upregulation by RAS blockers might be helpful in dealing with the deregulated system inflicted by SARS-CoV-2 rather than harmful in patients with lung injury. Here we review the involvement of ACE2 as a receptor for SARS-CoV-2 infection and provide the evidence supporting that RAS blockers are not only beneficial for COVID-19 patient's suffering from cardiovascular diseases, but also in acute lung injuries by preserving the ACE2 function and countering the systemic dysregulation caused by SARS-CoV-2 infection. We also review some RAS based therapies that besides preserving ACE2 functions might also blunt the cytokine storm elicited by SARS-CoV-2 infection.
1.1. Severe acute respiratory syndrome coronavirus (SARS-CoV)
SARS-CoV belongs to a group of enveloped coronavirus in the coronaviridae family which are single-stranded RNA viruses that cause respiratory and intestinal infections in animals and humans (Cui et al., 2019). Coronavirus got its name as it resembles the corona of the sun seen during the solar eclipse due to presence spike proteins that attach with host receptors. Coronaviruses are distinct amongst the enveloped viruses as they emanate from the Endoplasmic Reticulum and Golgi apparatus (ERGIC), from which they attain their membrane envelope. From ERGIC the mature virions are transported to the host secretory pathway to be released from the infected cell (Westerbeck JW, 2015; Schoeman and Fielding, 2019). Coronavirus cause either minor upper respiratory tract infection resembling common cold or lower respiratory tract infections like pneumonia and bronchitis (Yang et al., 2020). However, in past few decades coronavirus infections have led to outbreak of fatal respiratory illnesses called Severe Acute Respiratory Syndrome (Ksiazek et al., 2003; Kao et al., 2004; Zaki et al., 2012; Zhang et al., 2020). The outbreak of SARS-CoV (2002) and current major pandemic SARS-CoV-2 has demonstrated the lethality of coronaviruses. Infection with SARS-CoV produces either mild or severe symptoms, but in extreme cases lung injury leads to gradual respiratory failure causing death of an individual (Gu and Korteweg, 2007; Schoeman and Fielding, 2019).
1.1.1. SARS-CoV-2 structure
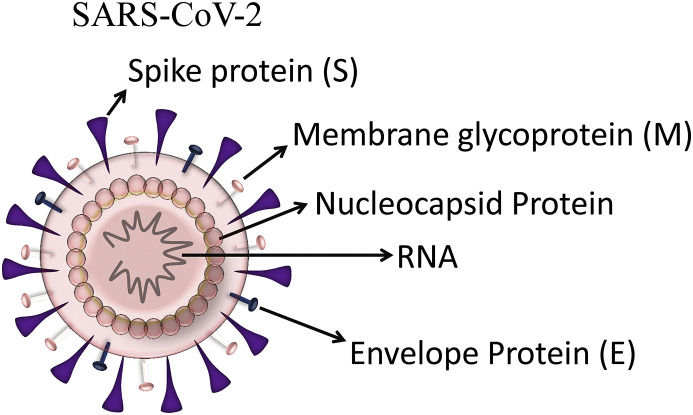
The coronavirus is composed of a positive-sense RNA genome and four major structural proteins termed spike proteins (S), membrane proteins (M), envelope proteins (E) and nucleocaspid proteins (N) and these proteins are requisite to produce mature virus and are important for coronavirus infectivity (Fig. 1 ) (Schoeman and Fielding, 2019) The S protein is a type I membrane glycoprotein that facilitates the attachment of coronavirus to the cell receptors and its subsequent entry into host cell (Song et al., 2004; Kirchdoerfer et al., 2016). The S protein is composed of a short intracellular tail, a transmembrane anchor, and a large ectodomain that comprises of two subdomains, S1 subdomain, that harbors receptor binding domain (RBD) which binds with the receptor of host cell and S2 subdomain, facilitates fusion between virus and host cell membrane (Li, 2016). The most abundant of the four structural proteins of coronavirus is M protein that spans the membrane thrice and it plays a predominant role in intracellular development of the virus. This protein is considered as a basic component of viral assembly and morphogenesis, involved in regulation of replication and packing the genomic RNA into viral particles (Narayanan et al., 2000). The foremost function of the N protein is to package the genomic RNA to form nucleocapsid (de Haan and Rottier, 2005). It may likely be involved in replication process and the host cellular response to viral infection (McBride et al., 2014). The E protein is the minor component of the coronavirus envelope, but it is highly expressed in the ER and Golgi-complex of the infected cell where it helps in assembly, budding and trafficking of infectious virions (Schoeman and Fielding, 2019).
Fig. 1.
Structure of SARS-CoV-2: The structure of SARS-CoV-2 comprises of a positive-sense RNA genome and four different structural proteins: spike(S), membrane (M), envelope (E), and nucleocapsid (N) proteins.
1.1.2. SARS-CoV-2 entry and replication
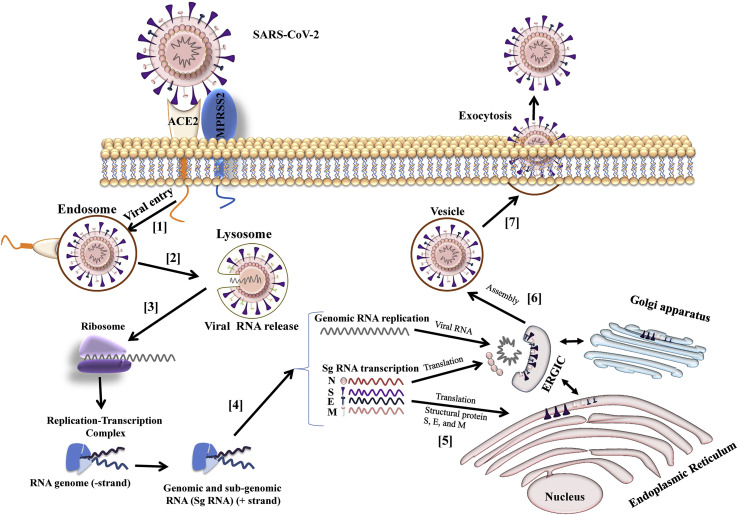
SARS-CoV-2 infects by binding to ACE2 via its S protein and enters the host cells (Gheblawi et al., 2020). To facilitate the fusion and entry of the viral particle into the host cell the spike is primarily activated by a serine protease present on host cell surface called TMPRSS2, to complete this process (Hoffmann et al., 2020). The spike protein S1 subunit binds to the ACE2, and TMPRSS2 cleaves and activates the spike. TMPRSS2 also causes an irreversible conformational change by activating S2 subunit of spike protein to facilitate fusion of the virus to cell membrane and allows its entry to the host cell (Simmons et al., 2013). Following the entry of SARS-CoV-2 positive-stranded RNA is released into the cytoplasm of the host cell and polyproteins are translated. The viral replicase gene encode for two large open reading frames (ORFs), known as ORF1a and ORF1b which are then translated into two polyproteins pp1a and pp1b. These polyproteins are proteolytically cleaved to form 16 non-structural proteins which assemble to form RNA replicase-transcriptase complex (RTC). This complex is used to replicate viral RNA via both replication and transcription. Replication of viral RNA is followed by the transcription of a sub-genomic mRNA including those encoding for the structural proteins by discontinuous transcription. The structural proteins S, E, and M are translated and enveloped in the endoplasmic reticulum and then transported for assembly to the budding ERGIC. Nucleocapsids are formed by assembling genomic RNA and N protein and then transported to ERGIC to be incorporated into virion. Following assembly, mature virions are transported to the cell surface through small vesicles and released from the infected cell via exocytosis to bind a new host cell (Fig. 2 ) (Fehr and Perlman, 2015; Walls et al., 2020).
Fig. 2.
Replication of coronaviruses: [1] The Spike protein on the SARS-CoV-2 binds to ACE2, a protease TMPRSS2 promotes fusion and entry of the virion into the host cell. [2] The virus releases positive-stranded RNA into the cytoplasm [3] RNA is translated in viral replicase complex to synthesize more copies of RNA. [4] The polymerase produces many subgenomic RNA by discontinuous transcription and some of these encode for structural proteins N, S, E and M. [5] The structural proteins S, E, and M are translated and inserted into endoplasm reticulum. [6] The viral structural protein, genomic RNA and N protein are assembled into virions by budding into the lumen of the ERGIC (endoplasmic reticulum (ER)–Golgi intermediate compartment). [7] Following the assembly, the virions are transported to the cell surface in vesicles and released via exocytosis.
1.1.3. Comparing SARS-CoV-2 with SARS-CoV
SARS-CoV that originated in the Guangdong Province of southern China was identified as the cause of severe acute respiratory syndrome (Ksiazek et al., 2003) outbreak in 2002. According to the World Health Organization (WHO), SARS-CoV, spread quickly over 29 countries in Southeast Asia where it effected about 8096 individuals, and caused 774 deaths (WHO, 2003; Fehr and Perlman, 2015; de Wit et al., 2016). SARS was however ultimately controlled in July 2003 by interrupting person to person transmission, following a strict quarantine of infected patients and suspected individuals having symptoms, screening and surveillance of travelers from affected regions and strict implementation of isolation of all contacts. SAR-CoV shows striking similarities with SARS-CoV-2 responsible for the recent outbreak of widespread severe acute respiratory disease that originated in Wuhan, Hubei province, China. SARS-CoV-2 shares less than 80% amino acid sequence identity with SARS-CoV (Lu et al., 2020; Zhou et al., 2020). The RBD of spike protein helps in binding of virus to the host cell is composed of two subdomains, a core structure and a receptor binding motif (RBM). As a result of prominent sequence identity in spike protein it is anticipated that both the coronavirus strains employ the same receptor i.e. ACE2 for the host cell entry. Recognition of the host receptor is a key step for determining the infectivity and transmissibility of the coronavirus; however, it may also be influenced by other factors of host and viral particle which may come into play only when virus is fused to the receptor. Though there are prominent similarities between the two strains, there is a considerable difference in SARS-CoV-2 infectious transmission, severity of symptoms, and degree of community spread. These variations in the virus characteristics are possibly due to the mutations in RBM region of the spike protein of SARS-CoV-2 that intensifies its binding affinity by 10- to 20-fold to human ACE2 compared to SARS-CoV, thereby increasing its infectivity and transmission from person to person (Meng et al., 2020; Wrapp et al., 2020).
1.2. Angiotensin converting Enzyme2 (ACE2) at the core of SARS-CoV-2 infection
1.2.1. ACE2
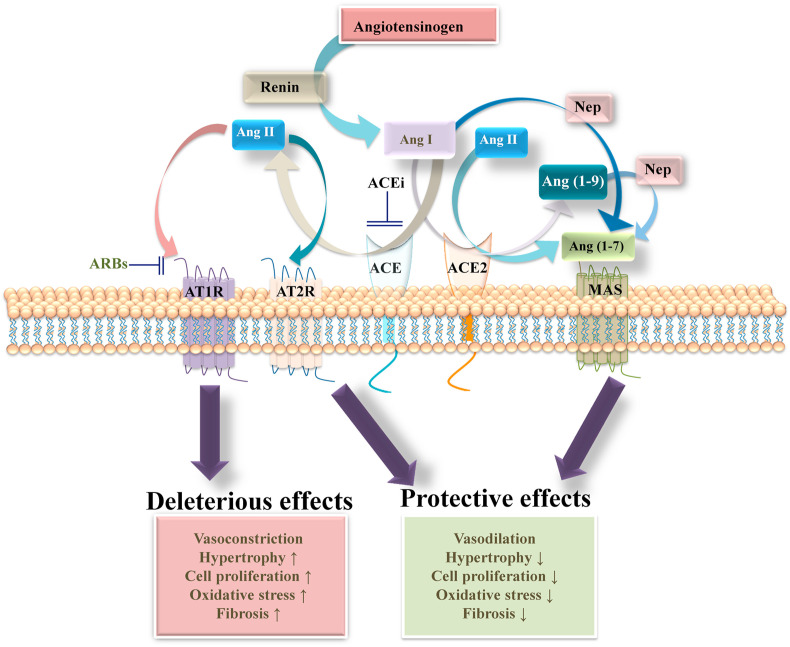
Angiotensinogen is the only precursor of angiotensin peptides, which is hydrolyzed by renin to generate an inactive peptide angiotensin (Ang) I. Angiotensin converting enzyme (ACE) an enzyme synthesized by pulmonary and renal endothelial cells hydrolyses the inactive peptide Ang I to form a biologically active peptide Ang II (Erdös, 1976). Ang II is the biologically active mediator of effects of the RAS whose functions are controlled by two G protein-coupled receptors (GPCR), AT1 receptor and AT2 receptor. Binding of Ang II to the AT1 receptor mediates hemodynamic and renal effects such as vasoconstriction, hypertension, renal tubular salt reabsorption and eliciting aldosterone synthesis (Gul et al., 2012a; Forrester et al., 2018). However, disorders in the RAS contributes to pathological condition such as cardiac hypertrophy, fibrosis, increased inflammatory responses, and oxidative stress (Lee et al., 1993; Luft, 2002; Weir, 2007; Gul et al., 2008, 2009, 2012a, 2012b, 2015; Gul et al., 2009; Muñoz-Durango et al., 2016). In contrast, AT2 receptor stimulation by Ang II mediates vasodilation and opposes the actions of AT1 receptor (Padia and Carey, 2013). The identification of ACE2, an enzyme that hydrolyses Ang II to Ang (1–7), a biologically active peptide of the RAS that promotes vasorelaxation and negatively regulates the actions stimulated by Ang II/AT1 receptor, unraveled the existence of an alternative metabolic pathway for Ang II (Fig. 3 ) (Ferrario, 2010; Ferreira et al., 2010; Oudit et al., 2010).
Fig. 3.
RAS: A classic model of RAS showing deleterious and protective effects. The precursor peptide AGT is cleaved by renin to form the Ang I, which is then cleaved by ACE to form Ang II. Ang II is the main peptide of RAS and exerts it effects by binding to two GPCRs AT1 receptor and AT2 receptor. Binding of Ang II to AT1 receptor stimulates vasoconstrictive/pro-inflammatory pathways, whereas its binding to AT2 receptor activates the vasodilative/anti-inflammatory signaling. Ang II is further cleaved by ACE2 to Ang (1–7), which binds to Mas receptor and promotes vasodilative/anti-inflammatory effects. Ang (1–7) can also be generated directly from Ang I by the action of neprilysin (Nep) or by the action of ACE2 on Ang I to form Ang (1–9), which is subsequently cleaved to generate Ang (1–7) through Nep.
ACE2 is a type I transmembrane protein with homology to ACE, an enzyme responsible for converting Ang I to Ang II, a major effector peptide of the RAS (Riordan, 2003). ACE2 was discovered by two independent research groups in 2000 and it emerged as a potent negative regulator of the RAS counter-balancing the multiple functions of ACE in the cardiovascular system (Donoghue et al., 2000; Tipnis et al., 2000). ACE2 is ubiquitous and widely expressed in human heart, vessels, kidney, brain, gastrointestinal system, lung alveolar epithelial cells and other tissue cells (Oudit et al., 2003). It is composed of 805 amino acids, with an active extracellular carboxypeptide domain, a transmembrane domain and a small cytoplasmic domain. The extracellular carboxypeptide domain is cleaved from the enzyme by ADAM17 (a disintegrin and metalloprotease 17) and released as soluble protein into the blood stream and finally excreted into urine. Ang II is the main substrate for ACE2 and it cleaves the last amino acid (Phe 8), in Ang II to generate Ang (1–7) which upon binding to G-protein coupled Mas receptor antagonizes the Ang II/AT1 receptor axis of the RAS. Alternatively, ACE2 can covert Ang I to Ang (1–9), which is then converted to Ang (1–7) by the action of neprilysin. (Jiang et al., 2014; Marquez et al., 2020). Additionally, Ang I can also be directly hydrolyzed into Ang (1–7) by the actions of neprilysin (Ferrario, 2011; Chappell, 2016). However, the affinity of ACE2 for Ang II is higher than for any other peptide, thus conversion of Ang II to Ang (1–7) is considered as its prime function (Vickers et al., 2002). Evidence demonstrates that an alternative mechanism within RAS may contribute to the positive effects of chronic RAS blockade. The chronic inhibition of ACE decrease Ang II expression, but significantly increases circulating Ang (1–7) levels through the action of neprilysin (Domenig et al., 2016). Similarly, AT1 receptor blockade also enhance Ang (1–7) via ACE2 and diverts Ang II to protective AT2 receptor pathway (Carey and Padia, 2013; Sumners et al., 2015). Besides ACE2 can also hydrolyze other vasoactive peptides such as apelin-13, apelin-17, apelin-36, neurotensin, kinestensin, dynorphin, and bradykinin (BK) fragments, [des-Arg9]-BK and [Lys-des-Arg9]-BK12 (Ocaranza and Jalil, 2012), thereby playing a critical role in regulating cardiac function, blood pressure and vascular reactivity. Studies have demonstrated that under pathological conditions, apelin disrupts Ang II signaling and stimulates antioxidant and anti-inflammatory effects by activating the apelin receptor (Sato et al., 2013; Zhou et al., 2016). Cleavage of apelin by ACE2 inactivates it and attenuates these beneficial effects. Contrarily, apelin is a positive regulator of ACE2 and decrease in apelin also downregulates ACE2 expression suggesting an interplay between ACE2 and apelin (Sato et al., 2013; Than et al., 2014). Degradation of the BK metabolite [des-Arg9]-BK by ACE2 reduces bradykinin B1 receptor (BKB1R)-mediated inflammatory signaling in lungs (Sodhi et al., 2018). A decrease in pulmonary ACE2 activity leads to impaired inhibition of [des-Arg9]-BK/BKHBIR signaling, thus causing the onset of a cytokine storm and severe lung inflammation leading to ARDS (Sodhi et al., 2018). Thus, ACE2 is a multifunctional enzyme that exerts several physiological functions by cleaving a variety of biologically active peptides.
1.2.2. Role of ACE2 in the cardiovascular system
Mounting evidence indicates that ACE2 exhibits protective functions in cardiovascular system and its altered expression is linked to major pathophysiological effects. ACE2 is considered as an endogenous modulator of RAS through its effects on Ang II degradation (Tesanovic et al., 2010). Contrarily, conversion of Ang II to Ang (1–7), by ACE2 shifts the balance towards cardioprotection by activating ACE2/Ang (1–7)/Mas receptor axis, a vasodilatory arm of the RAS with effects opposite to those produced by ACE/Ang II/AT1 receptor (Shah et al., 2010; Patel et al., 2016). Activation of ACE2/Ang (1–7)/Mas receptor axis antagonizes the pathophysiological effects of Ang II in myocardium and enhances vasodilation abilities, attenuates cardiac hypertrophy, fibrosis, cell proliferation, and oxidative stress. These protective effects are due in part to a decrease in Ang II levels as a result of its degradation to Ang (1–7) by ACE2 (Varagic et al., 2014). Thus, ACE2 plays a key role in maintaining a balance between ACE/Ang II/AT1 receptor vasoconstrictory axis and ACE2/Ang (1–7)/Mas receptor vasodilatory axis. Studies have revealed that deficiency of ACE2 leads to reduction in plasma Ang II metabolism thereby raising its levels both in plasma and myocardium (Kassiri et al., 2009; Zhong et al., 2010). Moreover, loss of ACE2 has been found to speed up cardiac failure, while ACE2 overexpression reverses the effect. Mice deficient in ACE2 have been shown to have decreased myocardial contractility, with no changes in blood pressure, cardiac hypertrophy or fibrosis. ACE2 overexpression attenuated pathological cardiac remodeling after myocardial infarction by blocking ACE activity, decreasing Ang II levels, and enhancing Ang (1–7) expression (Zhao et al., 2010). On contrary, ACE2 null mice show an exaggerated accumulation of Ang II in kidney due to its impaired metabolism thereby causing enhanced susceptibility to Ang II-induced hypertension (Liu et al., 2017). However, ACE2 was not found to regulate cardiac structure and functions in ACE2 null mice. Blocking of Ang II generation by ACEIs or inhibition of its binding to AT1 receptor by ARBs have been shown to increase ACE2 expression and activity in animal models of myocardial infarction-induced cardiac hypertrophy (Ocaranza et al., 2006). These observations collectively suggest that decrease in ACE2 is linked to impaired cardiac function whereas excess of ACE2 would be protective to hypertension-induced cardiac dysfunction. In contrast myocardial ACE2 expression was found to be significantly elevated after myocardial infarction in the rat and failing human hearts (Burrell et al., 2005). Moreover, circulating ACE2 levels are usually low in healthy subjects but increase during cardiovascular diseases (Rice et al., 2006; Epelman et al., 2008; Lew et al., 2008). Upregulation of ACE2 was found in cardiac tissue of patients with ischemic cardiomyopathy and idiopathic dilated cardiomyopathy or primary pulmonary hypertension (Zisman et al., 2003a; Burrell et al., 2005). These observations suggest that ACE2 upregulation is a compensatory response to protect heart and plays an important role in the negative regulation of RAS by counter-balancing the effects of Ang II after cardiac injury.
1.2.3. ACE2 is an entry receptor for SARS-CoV-2
SARS-CoV entry into host cells was primarily thought to be accomplished by direct fusion between the host cell membrane and the virus (Simmons et al., 2004). Proteolytic cleavage of SARS-CoV protein at S2 region of spike protein mediate membrane fusion and viral infectivity (Belouzard et al., 2009). Studies have demonstrated that SARS-CoV entry into the host cells is mediated by the binding of the spike protein (S) of virus to the ACE2 of host cell (Li et al., 2003). The susceptibility to SARS-CoV is significantly increased in mice with transgenic overexpression of ACE2 (Yang et al., 2007). It was demonstrated that an antibody against ACE2 but not ACE, block viral replication on Vero E6 cells, thus designating ACE2 as a functional receptor for SARS-CoV (Li et al., 2003). The pathological alterations in lungs induced by experimental SARS-CoV infection were reduced in ACE2-knock out mice compared to wild type mice, indicating ACE2 is a critical SARS receptor required for effective replication of infectious SARS-CoV (Kuba et al., 2005).. Zhou et al. were the first ones to establish SARS-CoV-2 use ACE2 as an entry receptor as SARS-CoV (Zhou et al., 2020). The observation suggested that ACE2 is probably the receptor for SARS-CoV-2 because that it is able to utilize ACE2 from humans, Chinese horseshoe bats, civet cats, and pigs as an entry receptor into ACE2-expressing cells but not in ACE non-expressing cells (Zhou et al., 2020). It was followed by other work (Hoffmann et al., 2020; Walls et al., 2020) which provided clear evidence suggesting that SARS-CoV-2 just like SARS-CoV gain accesses to host cells via ACE2 due to commonalities between the sequences of SARS-CoV-2 and SARS-CoV. Moreover, Hoffmann et al. showed that SARS-CoV-2 entry could be blocked by TMPRSS2 inhibitor and suggests that sera from patients recovering from SARS-CoV could partially protect against SARS-CoV-2 infection (Hoffmann et al., 2020). Though SARS-CoV-2 shares a close link with SARS-CoV, the former binds to ACE2 with higher affinity due to differences in RBM of spike region. This tight association between the SARS-CoV-2 and ACE2 could partly explain the reason for the higher pathogenicity of SARS-CoV-2 in humans compared to SAR-CoV.
Others demonstrated that pulmonary infection of mice with SARS-CoV displayed a marked decrease in ACE2 expression affirming an important role of ACE2 in mediating SARS-CoV infection in the heart (Oudit et al., 2009). Another group found that SARS-CoV infection results in downregulation of ACE2 expression in lung tissue that contributed to the severity of lung pathologies (Kuba et al., 2005). Additionally, they demonstrated that injection of SARS-CoV spike into mice induces acute lung failure that can be reduced by inhibiting the RAS using ACEIs and ARBs. Thus, positive correlation exists between susceptibility to SARS-CoV driven infection and ACE2 expression in vitro (Hofmann et al., 2004); however, the association of ACE2 expression and the susceptibly of SARS-CoV-2 infection is unknown. Another group has reported that human ACE2 expression levels and patterns in different tissues might be critical for the susceptibility, symptoms, and outcome of SARS-CoV-2 infection (Cao et al., 2020).
Li et al. performed a retrospective study and analyzed 425 SARS-CoV-2 positive cases and observed a reduced number of positive cases in children which could be attributed to reduced ACE2 expression in children compared to adults (Li et al., 2020). It was followed by another retrospective analysis comparing the ACE2 expression in nasal passages in a cohort of 305 individuals aged 4 to 60, as the nasal passage is often the first point of contact for SARS-CoV-2. ACE2 expression was found to be age dependent, with the lowest expression levels found in younger children and increasing with age into adulthood (Bunyavanich et al., 2020). Down regulation of ACE2 expression and its activity is associated with acute lung injury, increased accumulation of inflammatory cytokines, exacerbated pulmonary edema that ultimately lead to ARDS. These symptoms are attenuated by repleting with exogenous ACE2, GSK2586881, a recombinant form of human ACE2 (Khan et al., 2017; Zhang and Baker, 2017). RAS activation and downregulation of ACE2 expression have also been associated with pathogenesis of lung injury after SARS-CoV infection (Kuba et al., 2005). It turned out that Ang II levels are upregulated in COVID-19 patients and displays a linear positive correlation to viral load and lung injury (Liu et al., 2020). Activation of RAS is known to cause the wide range of injurious effects in multiple organs such as heart, lungs and kidney, thus RAS blockers might probably relieve of the pathological symptoms of COVID-19 on lung and unconditionally decrease heart and renal damage resulting from the RAS activation.
1.2.4. Role of ACEIs and ARBs in SARS-CoV-2
ACEIs and ARBs are generally used for the management of cardiovascular diseases. ACEIs block the conversion of Ang I to Ang II by targeting ACE and are used in patients with hypertension and type 2 diabetes mellitus for regulating blood pressure, treating cardiac failure and dysfunction, preventing strokes and treating diabetic nephropathy. While on the other hand, ARBs prevent binding of Ang II by blocking AT1 receptor and are used alone or in combination with other drugs for controlling hypertension. They also are used for treating congestive heart failure, preventing diabetes or high blood pressure-related kidney failure, and reducing the risk of stroke in patients with hypertension and an enlarged heart. Nearly all patients with cardiovascular comorbidities qualify for the use of ACEIs and ARBs (Messerli et al., 2018). Chronic treatment with ACEIs or ARBs has been shown to elevate ACE2 activity and expression in several organs which may be beneficial for the cardiovascular system (Zisman et al., 2003b; Ferrario et al., 2005; South et al., 2020). The ability of ACEIs/ARBs to upregulate ACE2 expression is in addition to their main clinical effect to inhibit ACE or block AT1 receptor. Investigators have reported that both ACEIs and ARBs could significantly enhance mRNA expression of cardiac ACE2 (Ferrario et al., 2005). Inhibition of the Ang II binding to AT1 receptor, by losartan, an ARB increased simultaneously both cardiac gene expression and activity of ACE2, while blockade of Ang II synthesis by lisinopril, and ACEIs increased cardiac ACE2 gene expression levels but not its activity in normal Lewis rats. However, combined treatment with lisinopril and losartan abolished the increase in gene expression but significantly increased ACE2 activity. These results reveal a lack of direct correlation between ACE2 mRNA levels and ACE2 activity indicating the existence of the complex ACEI and ARB signaling mechanism by that regulate kinetics of angiotensin peptide formation and metabolism (Ferrario et al., 2005; South et al., 2020). Similarly, Ishiyama et al. revealed that inhibition with ARBs, losartan and olmesartan increased ACE2 mRNA levels which were unaffected by co-administration with PD123319, an AT2 receptor antagonist in the heart of rats after myocardial infarction, suggesting that increase of ACE2 mRNA levels by ARBs could be due to direct inhibition of AT1 receptor (Ishiyama et al., 2004). In summary these evidence suggest a protective role of both ACEIs and ARBs in cardiovascular system via increasing ACE2 expression. However, till date there is no data on the effect of ACEIs and ARBs on ACE2 expression in lungs either in animal models or in humans. Given the role of ACE2 as the point of entry for SARS-CoV-2, there is a continuous debate in the scientific community about the role of ACEIs/ARBs in COVID-19. Some researchers initially suggested that patients should discontinue ACEIs/ARBs as it might predispose them to increased risk of SARS-CoV-2 and could make COVID-19 symptoms worse by increasing ACE2 expression. The argument was based on the assumption that RAS blockers increase ACE2 expression which might increase the susceptibility of SARS-CoV-2 infection (Guo et al., 2020). However, the view of discontinuing ACEIs/ARBs was not supported by numerous American and European cardiology societies who dispelled this misinformation and recommended patients not to stop ACEIs/ARBs, unless advised by the physician. Besides there are no clinical evidence to date suggesting that ACEIs/ARBs make patients more susceptible to the SARS-CoV-2. We highlight that discontinuation of RAS antagonists might exacerbate the clinical course and increase the death rate in COVID-19 patients suffering from hypertension or ischemic heart disease due to their acute risk. To support this argument, a very well organized Chinese study revealed that hospitalized hypertensive COVID-19 patients, treated with ACEIs/ARBs for hypertension showed lesser risk of all-cause mortality compared with non-users of ACE inhibitor/ARB during a 28 days of follow-up (Zhang et al., 2020). Furthermore, there are compelling evidences supporting the use of ACEIs and ARBs assertively because blockade of RAS may protect the COVID-19 patient form severity of lung damage and potentially from cardiovascular abnormalities caused by infection. ACE2 is functionally opposite to ACE and under physiological conditions; ACE2 displays vasodilator activity by inactivating Ang II whereas ACE acts as vasoconstrictor by increasing Ang II levels, to maintain the blood pressure homeostasis. Binding of the SAR-CoV-2 to ACE2 downregulates ACE2, thus causing an imbalance ACE/ACE2 ratio (Pagliaro and Penna, 2020), and shifting it towards ACE and increased Ang II generation. Increase in ACE/Ang II/AT1 receptor axis stimulation is known to enhance pulmonary vascular permeability and acute lung injury (Imai et al., 2005). Consequently, upregulation of ACE2 by RAS blockers could be beneficial in lung damage by not only inhibiting Ang II production and but also by degrading Ang II to vasodilatory peptide Ang (1–7), which appears to be vital in protecting against lung inflammation and fibrosis.
1.3. RAS based COVID-19 therapies
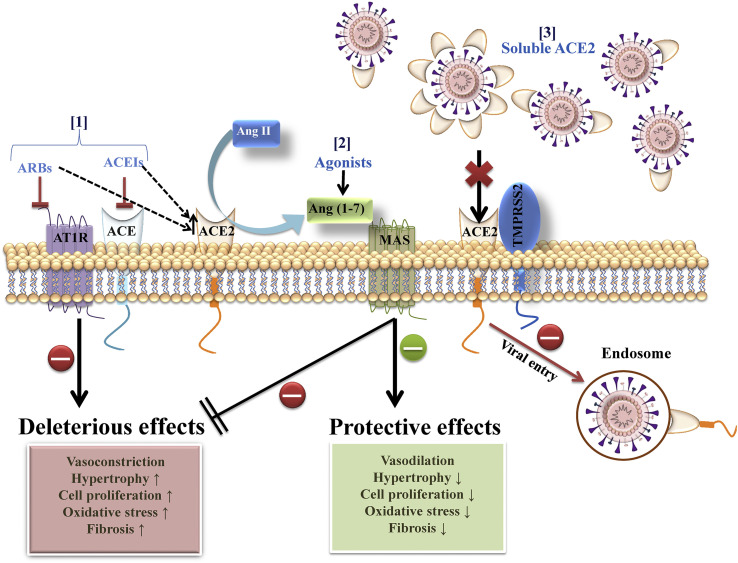
To date no specific drugs and therapeutics are approved by any food and drug association to prevent or treat SARS-CoV-2 infection. The best clinical strategy for the treatment of COVID-19 positive patients is purely supportive care that includes supplemental oxygen and mechanical ventilator support if required and control measures to prevent infection (Wax and Christian, 2020). However, there is a race at several fronts to develop effective therapies for COVID-19. In our opinion use of pharmacological agents that activate ACE2/Ang (1–7)/Mas receptor axis to promote the protective effects and RAS blockers to mitigate the detrimental actions of ACE/Ang II/AT1 receptor axis activation will not only preserve ACE2 functions but also blunt the cytokine storm elicited by SARS-CoV-2 infection (Fig. 4 ).
Fig. 4.
RAS based COVID-19 therapies. [1] ACEIs and ARBs inhibit Ang II/AT1 receptor activation and increase ACE2 expression. [2] Ang (1–7) to counter-balance the deleterious effects of Ang II/AT1 receptor activation. [3] Soluble ACE, block the viral entry.
1.3.1. Ang (1–7)/Mas receptor activation
Significance of Ang (1–7) as a cardioprotective peptide was highlighted with the discovery of ACE2, which hydrolyzes Ang II into Ang (1–7) that binds to a G-protein coupled Mas receptor to counter-regulate the deleterious effects elicited by ACE/Ang II/AT1 receptor axis (Shah et al., 2010; Patel et al., 2016). Besides diminishing the deleterious effects of Ang II in cardiovascular diseases, Ang (1–7)/Mas receptor axis has emerged as a new potential tool for the treatment of ARDS in animal studies (Imai et al., 2005; Kuba et al., 2006; Hamming et al., 2007). Studies have demonstrated an upregulation of ACE/Ang II/AT1 receptor signaling in the pathogenesis of acute lung injury, while ACE2/Ang (1–7)/Mas receptor activation protect against lung injuries (Li et al., 2008). Activation of Ang (1–7)/Mas receptor axis has been shown to attenuate pulmonary fibrosis by downregulating the profibrotic effects of Ang II (Meng et al., 2020). Indeed treatment with exogenous rhACE2 stimulates Ang (1–7) activity and attenuates acute lung failure in ACE2 knockout as well as in wild-type mice (Imai et al., 2005). Treatment with Ang (1–7) agonist AVE0991 is known to exert pulmonary and cardiovascular protective effects (Rodrigues-Machado et al., 2013; Jia, 2016). Therefore, activation of ACE2/Ang (1–7)/Mas receptor axis to restrain SARS-CoV-2 infection by recombinant ACE2 or Ang (1–7) agonists might have therapeutic effects in acute lung injury (Annweiler et al., 2020). In patients with preexisting medical conditions such as cardiovascular disease, diabetes mellitus, hypertension, and obesity activation of ACE2/Ang (1–7)/Mas receptor axis may significantly reduce the progression of comorbidities associated with COVID-19 infection. Aging associated activation of the Ang II/AT1 receptor axis and inhibition of Mas receptor (Yoon et al., 2016), (Yoon and Choi, 2014), (Musso and Jauregui, 2014), could be confounding factor in increased SARS-CoV-2 susceptibility seen in older population, independent of other adverse prognoses related to age. Ang (1–7) has been demonstrated to reduce the activation of proinflammatory and profibrotic cytokines thereby shielding against lung injury and acute respiratory distress (Santos et al., 2018). Along these lines, a double-blinded, placebo-control, randomized clinical trial will be assessing whether TXA127, a pharmaceutical formulation of peptide, Ang (1–7) administration prevents acute kidney injury and deterioration into multi-organ failure in patients with moderate to severe COVID-19 (NCT04401423). Thus, it is conceivable that activation of Mas receptor or infusion with Mas receptor agonist or Ang (1–7) can be an additive measure against COVID-19-induced cytokine storm triggered by SARS-CoV-2 infection that consecutively overwhelms inflammatory response (Peiró and Moncada, 2020; Shete, 2020).
1.3.2. ACE/ang II/AT1 receptor inhibition
Inappropriately, activated RAS is linked to the lung pathophysiology/ARDS as a result of dysregulated inflammatory cytokine production by over activation of ACE/Ang II/AT1 receptor signaling (Imai et al., 2005). However, in contrast activation of counter-regulatory arm of the RAS with effects opposite to those produced by ACE/Ang II/AT1 receptor plays a protective role by inhibiting pro-inflammatory responses during lung injury and ARDS (Imai et al., 2005; Kuba et al., 2006; Hamming et al., 2007). Interestingly, SARS-CoV-2 induced decrease of ACE2 is assumed to trigger inflammatory cascade that in turn induces severe acute lung injury by eliciting ACE/Ang II/AT1 receptor response (Kuba et al., 2006). Interruption of ACE/Ang II/AT1 receptor axis by ACEIs and ARBs might work as an effective treatment option to treat SARS-CoV-2-induced lung injuries as RAS inhibitors have been shown to be beneficial rather than harmful in patients with lung injury (Vaduganathan et al., 2020). Moreover, downregulation of ACE2 by SARS-CoV-2, cause an imbalance in ACE/ACE2 ratio (Pagliaro and Penna, 2020), and the disruption of this balance is prevented by inhibition of ACE/Ang II activation by RAS blockers as they enhance ACE2 activation which in turn would impede exacerbation of acute severe pneumonia. Multiple experimental animal studies suggest that inhibition of ACE/Ang II/AT1 receptor axis by ACEIs and ARBs can mitigate lung edema and microvascular permeability associated with ARDS (Yao et al., 2008; Shen et al., 2009; Asmussen et al., 2011; Chen et al., 2014). At present there is no strong clinical evidence to support role of RAS blockers in relieving ARDS symptoms, except for some observational studies evaluating the association between RAS-inhibition and ARDS (Mortensen et al., 2007; Kim et al., 2017; Hsieh et al., 2020). A recent study has revealed that the treatment of hospitalized hypertensive COVID-19 patients with ACEIs/ARBs show a reduced mortality compared to non-users during a 28 days follow up program (Zhang et al., 2020). Additionally, multiple clinical trials and observational studies are ongoing worldwide to investigate the effects of ACEIs/ARBs on COVID-19 patients. A cohort study (BIRCOV) will monitor the effects of ACEIs and ARBs in hypertensive patients, who are infected or have clinical manifestations of COVID-19 NCT04364984. Likewise, an observational study (COVHYP) will analyze the associations between COVID-19 and hypertension, and treatments with ACEIs and ARBs. The main hypothesis of the study is to assess the effects of ACEIs and ARBs on COVID-19 infection or severity (NCT04374695). Another observational clinical trial will include ACEIs and ARB with influenza vaccination to assess the admitted patients in the evolution of SARS-CoV-2 infection NCT04367883. The effects of telmisartan, an ARB on Ang II-induced proinflammatory cytokine release and eventually ARDS in COVID-19 patients will be assessed by an open-label randomized phase II clinical trial (NCT04355936). Similarly, another open label interventional clinical trial will evaluate the safety of losartan for worsening respiratory illness due to COVID-19 (NCT04335123). An interventional randomized trial will investigate the effects of ACEIs for the treatment of COVID-19 (NCT04345406). The addition of an ARB to the standard of care treatment is considered helpful in reducing the acute lung injury during the early stages of SAR-CoV-2-induced hypoxia. Therefore, a clinical trial has been launched to test whether or not an ARB have an impact on inhibiting respiratory failure in COVID-19 patients requiring mechanical ventilation due to mild to moderate hypoxia (NCT04340557). Moreover, two randomized controlled trial assessing the use of losartan in patients with COVID-19 for outcomes in outpatient (not requiring hospitalization) and inpatient (requiring hospitalization) (NCT04311177, NCT04312009). For losartan two additional trails (COVIDMED) and (CRASH-19) will compare outcome in hospitalized COVID-19 patients treated with losartan, lopinavir/ritonavir, hydroxychloroquine and placebo (NCT04328012) and effects of aspirin, losartan and simvastatin in patients with COVID-19 infection NCT04343001. To improve the clinical outcomes a randomized, doubled-blind and placebo-controlled (TITAN) will evaluate the efficacy of the early use of ivermectin plus losartan for prophylaxis of severe events in cancer patients with recent diagnosis of COVID-19 (NCT04447235). Thus, RAS blockers might work as effective therapeutics for reducing the severity of SARS-CoV-2 virus infections and rising mortality rates, besides being critical for the clinical management of cardiovascular disease in COVID-19 patients (Gurwitz, 2020; Kickbusch and Leung, 2020; Sun et al., 2020).
1.3.3. Soluble recombinant ACE2 therapy
Based on recent the reports, soluble recombinant ACE2 could be a potential treatment for the SARS-CoV-2 infection related underlying comorbidities (Pang et al., 2020). The SARS-CoV-2 has higher affinity for ACE2 compared to SARS-CoV (Shang et al., 2020), thus delivering a soluble form of recombinant ACE2 protein might actually be beneficial therapeutic to combat the spread of viral infection. Studies performed with SARS-CoV have revealed that soluble analogs of theACE2 might be a promising approach for treatment of viral infection. Moore et al. have demonstrated that catalytically inactive form of soluble ACE2 can potently block the infection caused by SARS-CoV from infecting cells in vitro, indicating soluble ACE2 protein has therapeutic potential (Moore et al., 2004). The soluble form of the ACE2 circulates in the blood as it does not possess membrane anchor. Exogenous administration of soluble ACE2 might help in treating SARS-CoV-2 by acting as a competitive interceptor of virus and thus abating the attachment of virus to full length ACE2 at cell surface. The beneficial effects of enhancing ACE2 activity by exogenous administration of ACE2 were tested in a clinical trial (NCT00886353) examined the pharmacokinetic and pharmacodynamic of a soluble recombinant human ACE2 preparation in healthy subjects. Exposure to human recombinant ACE2 reduced Ang II plasma levels, whereas Ang1-7 plasma levels increased only transiently due to rapid conversion to Ang1-5; however, blood pressure and heart rates were not affected suggesting the presence of effective compensatory mechanisms in healthy volunteers (Haschke et al., 2013). Indeed, a recent in vitro study has revealed that a fusion protein of recombinant ACE2 with Fc fragment of human immunoglobulin IgG1 potentially neutralizes the SARS-CoV-2 and exhibits a higher affinity for the RBD of spike protein of SAR-CoV-2 (Lei et al., 2020). Moreover, Monteil et al. demonstrated in SARS-CoV-2 infected African green monkey kidney cell line, Vero-E6, addition of human recombinant soluble ACE2 to the culture milieu blocked the replication of virus (Monteil et al., 2020). Thus, binding of soluble ACE2 to virus will not only slow the viral entry into the host cell but also at the same time rescue cell bound ACE2 to negatively regulate RAS. In a recent phase II clinical trial (NCT01597635), treatment of ARDS patients with a human recombinant ACE2 (GSK2586881) decreased Ang II levels whereas Ang (1–7) and surfactant protein D level increased (Khan et al., 2017). An additional small pilot clinical trial (NCT04382950) will be assessing combination of recombinant bacterial ACE2 -like enzyme of B38-CAP and isotretinoin as a preventive drug for lung injury in COVID-19 positive cases. In experimental animal models recombinant ACE2 administration has been shown to protect against severe lung injury (Imai et al., 2005; Zhou et al., 2020) and Ang II-induced cardiovascular maladaptations. Thus, delivery of soluble recombinant ACE2 might work as an effective treatment for SARS-CoV-2 infection, particularly for those suffering from cardiovascular diseases.
2. Conclusion
ACE2 is a key negative regulator of RAS, however, binding of SARS-CoV-2 to cell bound ACE2, downregulates its expression thereby disrupting the subtle balance between ACE2 and ACE leading to deregulation. This imbalance in RAS by SARS-CoV-2 infection blunts ACE2 mediated protective effects and upregulates counter-regulatory Ang II-induced pathogenesis. Based on our review, we postulate that inhibition of ACE/Ang II/AT1 receptor axis by using ACEIs and ARBs besides being critical for the clinical management of cardiovascular disease in COVID-19 patients, might improve acute lung injuries by switching the balance from deleterious ACE/Ang II/AT1 receptor axis towards protective ACE2/Ang (1–7)/Mas receptor axis. However, more studies are required to understand the role of ACE2 and therapeutic effects of ACEIs and ARBs on the susceptibility and progression of SARS-CoV-2 infection.
Funding
This project was funded by National Plan for Science, Technology and Innovation (MAARIFAH), King Abdul-Aziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-MED3221-02).
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgement
The authors would like to thank Dr. Chansu Park (Chonbuk National University) and Dr. James Sowers (University of Missouri) for critical reading of the manuscript.
References
- Annweiler C., Cao Z., Wu Y., Faucon E., Mouhat S., Kovacic H., Sabatier J.M. Counter-regulatory 'renin-angiotensin' system-based candidate drugs to treat COVID-19 diseases in SARS-CoV-2-infected patients. Infect. Disord. - Drug Targets. 2020 doi: 10.2174/1871526520666200518073329. [DOI] [PubMed] [Google Scholar]
- Asmussen S., Bartha E., Olah G., Sbrana E., Rehberg S.W., Yamamoto Y., Enkhbaatar P., Hawkins H.K., Ito H., Cox R.A., Traber L.D., Traber D.L., Szabo C. The Angiotensin-converting enzyme inhibitor captopril inhibits poly(adp-ribose) polymerase activation and exerts beneficial effects in an ovine model of burn and smoke injury. Shock. 2011;36:402–409. doi: 10.1097/SHK.0b013e318228f614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. Jama. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. discussion 322-364. 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R.M., Padia S.H. Role of angiotensin AT(2) receptors in natriuresis: intrarenal mechanisms and therapeutic potential. Clin. Exp. Pharmacol. Physiol. 2013;40:527–534. doi: 10.1111/1440-1681.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang Z., Li Z., Zhang F., Peng M., Chen Y., Wang Y. Losartan attenuates microvascular permeability in mechanical ventilator-induced lung injury in diabetic mice. Mol. Biol. Rep. 2014;41:809–814. doi: 10.1007/s11033-013-2920-9. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/s0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenig O., Manzel A., Grobe N., Königshausen E., Kaltenecker C.C., Kovarik J.J., Stegbauer J., Gurley S.B., van Oyen D., Antlanger M., Bader M., Motta-Santos D., Santos R.A., Elased K.M., Säemann M.D., Linker R.A., Poglitsch M. Neprilysin is a mediator of alternative renin-angiotensin-system Activation in the murine and human kidney. Sci. Rep. 2016;6:33678. doi: 10.1038/srep33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E.G. Conversion of angiotensin I to angiotensin II. Am. J. Med. 1976;60:749–759. doi: 10.1016/0002-9343(76)90889-5. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/hypertensionaha.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M. ACE2: more of Ang-(1-7) or less Ang II? Curr. Opin. Nephrol. Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/circulationaha.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferreira A.J., Santos R.A., Bradford C.N., Mecca A.P., Sumners C., Katovich M.J., Raizada M.K. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/hypertensionaha.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/circresaha.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Zhong N.S. Clinical characteristics of covid-19 in China. Reply. N. Engl. J. Med. 2020;382:1861–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- Gul R., Kim S.Y., Park K.H., Kim B.J., Kim S.J., Im M.J., Kim U.H. A novel signaling pathway of ADP-ribosyl cyclase activation by angiotensin II in adult rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H77–H88. doi: 10.1152/ajpheart.01355.2007. [DOI] [PubMed] [Google Scholar]
- Gul R., Mahmood A., Luck C., Lum-Naihe K., Alfadda A.A., Speth R.C., Pulakat L. Regulation of cardiac miR-208a, an inducer of obesity, by rapamycin and nebivolol. Obesity. 2015;23:2251–2259. doi: 10.1002/oby.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul R., Park J.H., Kim S.Y., Jang K.Y., Chae J.K., Ko J.K., Kim U.H. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc. Res. 2009;81:582–591. doi: 10.1093/cvr/cvn232. [DOI] [PubMed] [Google Scholar]
- Gul R., Ramdas M., Mandavia C.H., Sowers J.R., Pulakat L. RAS-mediated adaptive mechanisms in cardiovascular tissues: confounding factors of RAS blockade therapy and alternative approaches. Cardiorenal Med. 2012;2:268–280. doi: 10.1159/000343456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul R., Shawl A.I., Kim S.H., Kim U.H. Cooperative interaction between reactive oxygen species and Ca2+ signals contributes to angiotensin II-induced hypertrophy in adult rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H901–H909. doi: 10.1152/ajpheart.00250.2011. [DOI] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9 doi: 10.1161/jaha.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D., Timens W., Turner A.J., Navis G., van Goor H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pöhlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.S., How C.K., Hsieh V.C., Chen P.C. Preadmission antihypertensive drug use and sepsis outcome: impact of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) Shock. 2020;53:407–415. doi: 10.1097/shk.0000000000001382. [DOI] [PubMed] [Google Scholar]
- Igase M., Strawn W.B., Gallagher P.E., Geary R.L., Ferrario C.M. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/shk.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Kao R.Y., Tsui W.H., Lee T.S., Tanner J.A., Watt R.M., Huang J.D., Hu L., Chen G., Chen Z., Zhang L., He T., Chan K.H., Tse H., To A.P., Ng L.W., Wong B.C., Tsoi H.W., Yang D., Ho D.D., Yuen K.Y. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem. Biol. 2004;11:1293–1299. doi: 10.1016/j.chembiol.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., Scholey J.W., Penninger J.M., Oudit G.Y. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/circheartfailure.108.840124. [DOI] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickbusch I., Leung G. Response to the emerging novel coronavirus outbreak. Bmj. 2020;368:m406. doi: 10.1136/bmj.m406. [DOI] [PubMed] [Google Scholar]
- Kim J., Choi S.M., Lee J., Park Y.S., Lee C.H., Yim J.J., Yoo C.G., Kim Y.W., Han S.K., Lee S.M. Effect of renin-angiotensin system blockage in patients with acute respiratory distress syndrome: a retrospective case control study. Korean J Crit Care Med. 2017;32:154–163. doi: 10.4266/kjccm.2016.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., Group S.W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Jiang C., Penninger J.M. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J. Mol. Med. (Berl). 2006;84:814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.A., Böhm M., Paul M., Ganten D. Tissue renin-angiotensin systems. Their role in cardiovascular disease. Circulation. 1993;87:Iv7–13. [PubMed] [Google Scholar]
- Lei C., Fu W., Qian K., Li T., Zhang S., Ding M., Hu S. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. bioRxiv. 2020 doi: 10.1101/2020.02.01.929976. 2020.2002.2001.929976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R.A., Warner F.J., Hanchapola I., Yarski M.A., Ramchand J., Burrell L.M., Smith A.I. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008;93:685–693. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. An Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Molina-Molina M., Abdul-Hafez A., Uhal V., Xaubet A., Uhal B.D. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Huang X.R., Chen H.Y., Fung E., Liu J., Lan H.Y. Deletion of angiotensin-converting enzyme-2 promotes hypertensive nephropathy by targeting Smad 7 for ubiquitin degradation. Hypertension. 2017;70:822–830. doi: 10.1161/hypertensionaha.117.09600. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F.C. Tissue renin-angiotensin system and end-organ damage. J. Mol. Med. (Berl). 2002;80:325–326. doi: 10.1007/s00109-002-0334-6. [DOI] [PubMed] [Google Scholar]
- McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microb. Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli F.H., Bangalore S., Bavishi C., Rimoldi S.F. Angiotensin-converting enzyme inhibitors in hypertension. To Use or Not to Use? 2018;71:1474–1482. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.J., Dorfman T., Li W., Wong S.K., Li Y., Kuhn J.H., Coderre J., Vasilieva N., Han Z., Greenough T.C., Farzan M., Choe H. Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J. Virol. 2004;78:10628–10635. doi: 10.1128/jvi.78.19.10628-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen E.M., Restrepo M.I., Copeland L.A., Pugh J.A., Anzueto A., Cornell J.E., Pugh M.J. Impact of previous statin and angiotensin II receptor blocker use on mortality in patients hospitalized with sepsis. Pharmacotherapy. 2007;27:1619–1626. doi: 10.1592/phco.27.12.1619. [DOI] [PubMed] [Google Scholar]
- Muñoz-Durango N., Fuentes C.A., Castillo A.E., González-Gómez L.M., Vecchiola A., Fardella C.E., Kalergis A.M. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso C.G., Jauregui J.R. Renin-angiotensin-aldosterone system and the aging kidney. Expet Rev. Endocrinol. Metabol. 2014;9:543–546. doi: 10.1586/17446651.2014.956723. [DOI] [PubMed] [Google Scholar]
- Narayanan K., Maeda A., Maeda J., Makino S. Characterization of the coronavirus M protein and nucleocapsid interaction in infected cells. J. Virol. 2000;74:8127–8134. doi: 10.1128/jvi.74.17.8127-8134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaranza M.P., Godoy I., Jalil J.E., Varas M., Collantes P., Pinto M., Roman M., Ramirez C., Copaja M., Diaz-Araya G., Castro P., Lavandero S. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.Hyp.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- Ocaranza M.P., Jalil J.E. Protective role of the ACE2/ang-(1-9) Axis in cardiovascular remodeling. Int. J. Hypertens. 2012;2012:594361. doi: 10.1155/2012/594361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc. Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Liu G.C., Zhong J., Basu R., Chow F.L., Zhou J., Loibner H., Janzek E., Schuster M., Penninger J.M., Herzenberg A.M., Kassiri Z., Scholey J.W. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padia S.H., Carey R.M. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflügers Archiv. 2013;465:99–110. doi: 10.1007/s00424-012-1146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaro P., Penna C. ACE/ACE2 ratio: a key also in 2019 coronavirus disease (Covid-19)? Front. Med. 2020;7:335. doi: 10.3389/fmed.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Cui Y., Zhu Y. Recombinant human ACE2: potential therapeutics of SARS-CoV-2 infection and its complication. Acta Pharmacol. Sin. 2020;41:1255–1257. doi: 10.1038/s41401-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/circresaha.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró C., Moncada S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation. 2020;141:1665–1666. doi: 10.1161/circulationaha.120.047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M. [Acute respiratory insufficiency due to severe lung injury - ARDS and ALI] Pneumologie. 2010;64:590–594. doi: 10.1055/s-0030-1255680. [DOI] [PubMed] [Google Scholar]
- Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.Hyp.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- Riordan J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4:225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Machado M.G., Magalhães G.S., Cardoso J.A., Kangussu L.M., Murari A., Caliari M.V., Oliveira M.L., Cara D.C., Noviello M.L., Marques F.D., Pereira J.M., Lautner R.Q., Santos R.A., Campagnole-Santos M.J. AVE 0991, a non-peptide mimic of angiotensin-(1-7) effects, attenuates pulmonary remodelling in a model of chronic asthma. Br. J. Pharmacol. 2013;170:835–846. doi: 10.1111/bph.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M., Campagnole-Santos M.J. The ACE2/angiotensin-(1-7)/MAS Axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Suzuki T., Watanabe H., Kadowaki A., Fukamizu A., Liu P.P., Kimura A., Ito H., Penninger J.M., Imai Y., Kuba K. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 2013;123:5203–5211. doi: 10.1172/jci69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Gul R., Yuan K., Gao S., Oh Y.B., Kim U.H., Kim S.H. Angiotensin-(1-7) stimulates high atrial pacing-induced ANP secretion via Mas/PI3-kinase/Akt axis and Na+/H+ exchanger. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1365–H1374. doi: 10.1152/ajpheart.00608.2009. [DOI] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Mo H., Cai L., Kong T., Zheng W., Ye J., Qi J., Xiao Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock. 2009;31:500–506. doi: 10.1097/SHK.0b013e318189017a. [DOI] [PubMed] [Google Scholar]
- Shete A. Urgent need for evaluating agonists of angiotensin-(1-7)/Mas receptor axis for treating patients with COVID-19. Int. J. Infect. Dis. 2020;96:348–351. doi: 10.1016/j.ijid.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Zmora P., Gierer S., Heurich A., Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Jr., Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L17–l31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerstein R.G.C. Rapid response: preventing a Covid-19 pandemic: ACE inhibitors as a potential risk factor for fatal Covid-19. BMJ. 2020;368 m810 doi: 10.1136/bmj.m810. [DOI] [Google Scholar]
- Song H.C., Seo M.Y., Stadler K., Yoo B.J., Choo Q.L., Coates S.R., Uematsu Y., Harada T., Greer C.E., Polo J.M., Pileri P., Eickmann M., Rappuoli R., Abrignani S., Houghton M., Han J.H. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2004;78:10328–10335. doi: 10.1128/jvi.78.19.10328-10335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H1084–h1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumners C., de Kloet A.D., Krause E.G., Unger T., Steckelings U.M. Angiotensin type 2 receptors: blood pressure regulation and end organ damage. Curr. Opin. Pharmacol. 2015;21:115–121. doi: 10.1016/j.coph.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.L., Yang J.M., Sun Y.P., Su G.H. [Inhibitors of RAS might Be a good choice for the therapy of COVID-19 pneumonia] Zhonghua Jiehe He Huxi Zazhi. 2020;43:219–222. doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Team, T.N.C.P.E.R.E. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Tesanovic S., Vinh A., Gaspari T.A., Casley D., Widdop R.E. Vasoprotective and atheroprotective effects of angiotensin (1-7) in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:1606–1613. doi: 10.1161/atvbaha.110.204453. [DOI] [PubMed] [Google Scholar]
- Than A., Zhang X., Leow M.K., Poh C.L., Chong S.K., Chen P. Apelin attenuates oxidative stress in human adipocytes. J. Biol. Chem. 2014;289:3763–3774. doi: 10.1074/jbc.M113.526210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagic J., Ahmad S., Nagata S., Ferrario C.M. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr. Hypertens. Rep. 2014;16:420. doi: 10.1007/s11906-014-0420-5. -420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020 doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q., Zhang W., Li Y., Ge J., Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M.R. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin. Therapeut. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Westerbeck JW M.C. A coronavirus E protein is present in two distinct pools with different effects on assembly and the secretory pathway. J. Virol. 2015:9313–9323. doi: 10.1128/JVI.01237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2003. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31. July 2003. [Google Scholar]
- WHO Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Jama. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., Xu Y.F., Ding M.X., Deng H.K., Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007;57:450–459. [PubMed] [Google Scholar]
- Yang Y., Peng F., Wang R., Yange M., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S., Feng D., Wu Q., Li K., Wang L. Losartan attenuates ventilator-induced lung injury. J. Surg. Res. 2008;145:25–32. doi: 10.1016/j.jss.2007.03.075. [DOI] [PubMed] [Google Scholar]
- Yoon H.E., Choi B.S. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29:291–295. doi: 10.3904/kjim.2014.29.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.E., Kim E.N., Kim M.Y., Lim J.H., Jang I.A., Ban T.H., Shin S.J., Park C.W., Chang Y.S., Choi B.S. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang H., Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit. Care. 2017;21:305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang X., Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020 doi: 10.1161/circresaha.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.X., Yin H.Q., Yu Q.T., Qiao Y., Dai H.Y., Zhang M.X., Zhang L., Liu Y.F., Wang L.C., Liu D.S., Deng B.P., Zhang Y.H., Pan C.M., Song H.D., Qu X., Jiang H., Liu C.X., Lu X.T., Liu B., Gao F., Dong B. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum. Gene Ther. 2010;21:1545–1554. doi: 10.1089/hum.2009.160. [DOI] [PubMed] [Google Scholar]
- Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M., Loibner H., Wang X.H., Penninger J.M., Kassiri Z., Oudit G.Y. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/circulationaha.110.955369. 718 pp. following 728. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Cao J., Chen L. Apelin/APJ system: a novel therapeutic target for oxidative stress-related inflammatory diseases (Review) Int. J. Mol. Med. 2016;37:1159–1169. doi: 10.3892/ijmm.2016.2544. [DOI] [PubMed] [Google Scholar]
- Zisman L.S., Keller R.S., Weaver B., Lin Q., Speth R., Bristow M.R., Canver C.C. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.Cir.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- Zisman L.S., Meixell G.E., Bristow M.R., Canver C.C. Angiotensin-(1-7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation. 2003;108:1679–1681. doi: 10.1161/01.Cir.0000094733.61689.D4. [DOI] [PubMed] [Google Scholar]