Abstract
Caesarean section can result in an indentation of the myometrium at the site of the Caesarean scar, called a niche. Niches can cause symptoms of abnormal uterine blood loss, dysmenorrhoea, chronic pelvic pain and dyspareunia and are possibly related to subfertility. Various other explanations for the cause of subfertility after Caesarean section have been proposed in the literature, such as uterine pathology, intra-abdominal adhesions and women’s reproductive choices. Not all niches cause symptoms and the relation with subfertility and a niche in the uterine scar still needs further study since direct evidence is lacking so far. Based on the limited available evidence, and in combination with observations made during sonographic hysteroscopic evaluations and laparoscopic niche repair, we propose and discuss three hypothetical mechanisms: (i) the environment for sperm penetration and implantation may be detrimental; (ii) there could be a physical barrier to embryo transfer and implantation; and (iii) psychogenic factors may reduce the likelihood of pregnancy. Several innovative surgical treatments have been developed and are being implemented for niche-related problems. Promising results are reported, but more evidence is needed before further implementation in daily practice. The additional value of niche resections should be compared to expectant management or fertility therapies, such as ART, in randomized controlled trials. Therefore, our suggested hypotheses should, for the time being, not be used for justification of any specific procedures outside clinical trials.
Keywords: Caesarean section, Niche, Istmocele, fertility, assited reproductive technique
Introduction
Rates of Caesarean section have kept rising in recent decades. The mean worldwide rate for this procedure increased from 6.7% in 1990 to 19.1% in 2014. The Dominican Republic, Brazil and China have the highest rates so far, at 56.4%, 55.6% and 47%, respectively (Betran et al., 2016). The increase is mainly due to the expansion of indications to perform a primary Caesarean section and a sharp decline in vaginal birth rates after a previous Caesarean section. Some of the indications could be prevented, such as maternal request without medical indication in some Western countries (Nilstun et al., 2008; Wortman and Alexander, 2013; Mylonas and Friese, 2015). The World Health Organization estimates the optimal Caesarean rate to be 15%. There is no evidence that Caesarean rates higher than 10% are associated with reductions in maternal and foetal morbidity (Gibbons et al., 2012).
There is increasing interest in the potential long-term morbidity of Caesarean section scars (Diaz et al., 2002; Silver, 2010; Clark and Silver, 2011). The association between a niche after a Caesarean section and gynaecological symptoms has been recently reported (Osser et al., 2009; Bij de Vaate et al., 2011; van der Voet et al., 2014a; Bij de Vaate et al., 2014b). A niche is formally defined by the European Niche Taskforce as an indentation of the uterine myometrium of at least 2 mm at the site of the Caesarean scar, assessed by transvaginal ultrasound (Jordans et al., 2019). Niches are observed in 50–60% of women after a Caesarean section. Prospective cohort studies reported spotting in 30% of women with a niche at 6–12 months after Caesarean section compared with 15% of women without a niche. Postmenstrual spotting is correlated to niche volume and inversely correlated to residual myometrium thickness (van der Voet et al., 2014a; Bij de Vaate et al., 2014b). The detection rate of a niche depends on the population and the diagnostic method used. This can be 2D or 3D transvaginal sonography, sonohysterography or MRI (Bij de Vaate et al., 2011; Bij de Vaate et al., 2014a,b; Antila-Langsjo et al., 2018; Ludwin et al., 2019). Based on the international recommendations on evaluation and reporting system of niches among experts, it was considered that preferably saline or gel should be used in absence of intra-uterine fluid (Jordans et al., 2019). This improves the visibility of niches (Bij de Vaate et al., 2011; Jordans et al., 2019).
In addition to the gynaecological symptoms, in theory, niches may impair subsequent fertility. It has been reported that a niche can reduce the chances of embryo implantation and may lead to spontaneous miscarriages if the implantation is close to or in the niche (Hemminki, 1986; Hemminki, 1996; Naji et al., 2013). A meta-analysis reported that a Caesarean section reduces the probability of subsequent pregnancy by 10% [relative risk (RR) 0.91; 95% 0.87–0.95] on average, compared with a previous vaginal delivery (Gurol-Urganci et al., 2013). Most of the 16 included studies found reduced fertility after a Caesarean section. The size of the effect depended on the type and indication for caesarean section. Studies which controlled for maternal age or specifically analysed primary elective Caesarean section for breech delivery reported smaller effects. However, none of the studies included in the meta-analysis evaluated the relation between subsequent fertility and the presence of a niche. Various explanations for the cause of post-Caesarean subfertility have been proposed, ranging from placental bed disruption and pelvic adhesions influencing tubal oocyte pick-up (Murphy et al., 2002) to women’s reproductive choices (Porter et al., 2003; Oral and Elter, 2007). An increasing variety of therapies to treat niche-related symptoms and improve reproductive outcomes has also been developed and implemented in recent years (van der Voet et al., 2014b; Nikkels et al., 2017; Vervoort et al., 2018a; Vissers et al., 2020). A recent review including various case series and single-arm cohort studies reported improvement of gynaecological symptoms after (laparoscopic) niche resections. Despite the promising outcomes, so far there is no definite proof of the beneficial effects on reproductive outcomes of any intervention over expectant management (Vitale et al., 2020). The outcomes of the studies were biased with a high risk of overestimating the effect of the applied interventions. Because niche-related symptoms and applied therapies lead to more medical consultations and higher costs, it is important to evaluate diagnostic, therapeutic and preventive strategies for niche development (Vervoort et al., 2018b). The beneficial effect of applied therapies for fertility outcomes needs to be proven in randomized controlled trials. Currently, one study has been registered in the trial register, the LAPRESS trial (Dutch trial register number NTR 6534). The results of this study will provide essential information for future implementation of this intervention in daily practice. Apart from repair of a defect, it is also important to prevent niche development. The closure technique of the uterine Caesarean scar has been proposed as an independent factor for niche development (Bij de Vaate et al., 2011). Closing techniques have changed over the years and there is no uniform standard for Caesarean procedures or uterine closures. Two meta-analyses reported low-to-moderate evidence that single- and double-layer closure of the uterine incision is associated on ultrasound with similar incidences of niches (Di Spiezio Sardo et al., 2017; Stegwee et al., 2018). One large study has been registered in the trial register to evaluate the effect of double layer closure versus single layer closure in patients undergoing their first Caesarean section. (Dutch Trial Register (NTR5480).
So far the most intriguing questions are: does a niche impair fertility and what underlying mechanism has been studied insufficiently? Based on the limited available evidence in the recent literature reviewed, combined with our observations during sonographic hysteroscopic evaluation and laparoscopic niche repair, we postulate further hypotheses to explain the association between subfertility and the presence of a niche, and to define the knowledge gaps for future research perspectives. Table I presents an overview of hypotheses on the intermediate role of niches on fertility outcomes.
Table I.
Overview of hypotheses on the intermediate role of the niche on fertility outcomes.
| Hypothesis |
|---|
| Detrimental environment for sperm penetration and implantation |
|
|
|
|
| Physical barrier for embryo transfer and implantation |
|
| Psychogenic causes that reduce the likelihood of pregnancy |
|
Detrimental environment for sperm penetration and implantation
Hypothesis 1: niche-related accumulation of intrauterine fluid impairing implantation
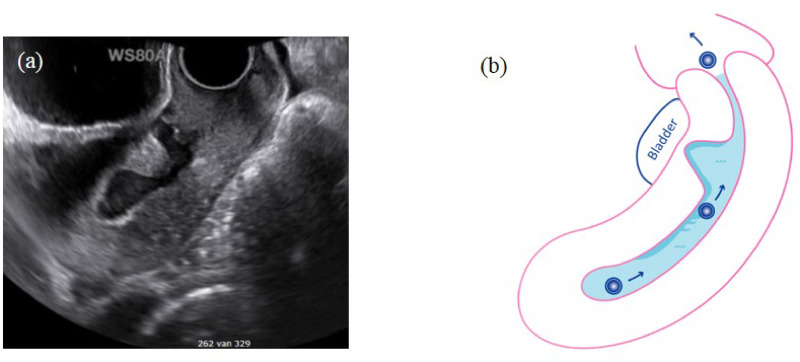
We hypothesize that the accumulation of intracavitary fluid related to the niche may impair embryo implantation, which is in line with the known negative effect of intrauterine fluid in women with hydrosalpinx (Mansour et al., 1991; Sharara and McClamrock, 1997; Sharara and Prough, 1999; Levi et al., 2001; Akman et al., 2005; Strandell et al., 2001). Implantation within the normal endometrium is a precisely timed and complex process that could be affected by interference of intrauterine fluid film covering the endometrium (Fig. 1). This fluid may play an important role in luminal closure to ‘lock’ the embryos in the right locations (Eytan et al., 2001; Yaniv et al., 2003; Eytan et al., 2004).
Figure 1.
Intrauterine fluid accumulation in the uterus and the niche. (a) Image of niche using transvaginal ultrasound in mid-sagittal plane with high fluid accumulation in the uterus and the large niche. (b) Schematic figure of an embryo that is separated from the endometrium on a film of fluid. The intracavitary fluid in relation with the accumulation of fluid in the niche may impact embryo implantation.
The impact of intrauterine fluid on implantation in association with a niche has not yet been studied (Vervoort et al., 2018b). Two retrospective studies showed a detrimental effect of a Caesarean section on implantation. The largest study, comprising 1317 women with one previous Caesarean or vaginal delivery undergoing IVF/ICSI treatment, showed impaired pregnancy outcomes. Live birth rates were lower in women with a previous Caesarean section, at 15.9% versus 23.3% for women with a previous vaginal delivery (odds ratio 0.63 95% CI 0.45–0.87). Of the women in the study, 77% received a single embryo transfer and the quality of embryos did not differ between the two groups (Vissers et al., 2020). The lower clinical pregnancy rate per embryo transfer indicates that implantation is particularly hampered after a Caesarean section. Another retrospective study on 310 IVF patients (Wang et al., 2017) reported a lower clinical pregnancy rate in women with a previous Caesarean section (40.3%), especially if a post-Caesarean scar defect (niche) in combination with endometrial fluid (12.5%) was present, compared to women with a previous vaginal delivery (54.8%) (P ≤ 0.05). The majority of these patients (97%) received a double embryo transfer. In theory, intrauterine accumulation of either blood or fluid may cause a hostile environment for implantation. Some authors have postulated that accumulation of blood may lead to degradation of haemoglobin in the uterine cavity, resulting in a higher iron exposure, which is known to be embryotoxic (Van Langendonckt et al., 2002; Lousse et al., 2009). Unknown embryotoxic factors may also be present in niche-related intrauterine fluid. Besides possible direct embryotoxicity, a continuous flow of intrauterine fluid may also interfere with implantation due to mechanical principles.
Some studies underline the higher prevalence of intrauterine fluid in association with a niche. In a prospective cohort study of 225 women with a previous Caesarean section, intrauterine fluid was seen in 13.3% of the women with a niche versus 6.3% of those without a niche (Bij de Vaate et al., 2011). In a retrospective questionnaire survey among IVF centres in Japan (n = 189), intrauterine accumulation of fluid (in 75% in the ovulatory phase) and postmenstrual spotting (in 48%) were the most commonly reported symptoms in women with niches; however, by definition this resembles a selected population (Tsuji et al., 2015).
Several other cohort studies report promising fertility outcomes after a surgical niche intervention. In a cohort study of 159 women with large niches and who underwent a laparoscopic niche repair because of symptoms, intrauterine fluid was originally observed in 40% of subjects but this number dropped to 7.5% 6 months after the intervention. This points to the niche as the causal factor for fluid accumulation. Pregnancy rates were also very promising in that study in which 40.2% of subjects had failed previous IVF therapy; at 2 years of follow-up, 52.0% conceived naturally with a median time to pregnancy of 3.0 months after discontinuation of contraceptives (Vissers et al., 2020). Two other cohort studies on laparoscopic niche repair in 22 and 38 subfertile patients reported high pregnancy rates (55.6% and 44.0%, respectively) in most patients after more than 1-year follow-up (Tanimura et al., 2015; Donnez et al., 2017). These studies indicate that the niche and related intrauterine fluid may play an important intermediate role in subfertility after Caesarean section (Vervoort et al., 2018b). The beneficial effect of laparoscopic niche resection on reproductive outcomes needs to be proven in randomized controlled trials.
One prospective study of 41 secondary subfertility patients with niches reported a 100% pregnancy rate after hysteroscopic niche resection (Gubbini et al., 2011), yet did not report on the number of women who were lost to follow-up. A hysteroscopic niche resection with dissection of the distal rim of the niche facilitates the outflow of fluid and coagulation of the surface of the niche, while resection of subendometrial glands may, in theory, reduce fluid production. A reduction of intrauterine fluid was also observed after hysteroscopic niche resection in a randomized controlled trial comparing hysteroscopic niche resection with expectant management, although the number of women seeking pregnancy was very limited (Vervoort et al., 2018a). The 6-month follow-up results were promising in terms of spotting and intrauterine fluid, but the long-term outcome is unknown so far. Even a temporary reduction in intrauterine fluid accumulation may result in a window of opportunity for embryo implantation.
In conclusion, accumulation of fluid is seen in approximately 42% of patients with a large niche (Vissers et al., 2020). This may impair pregnancy outcomes due to reduced implantation yet may also be embryotoxic. Future studies are needed to assess the effect on implantation of intrauterine fluid related to a niche and the possible embryotoxicity of the intrauterine fluid, as well as the additional value of surgical interventions on fertility outcomes. Whether removal of the fluid immediately before embryo transfer in women with a niche affects pregnancy outcomes is not known either.
Hypothesis 2: altered immunobiology and/or increased inflammation when a niche is present
A prospective cohort study evaluating the site of implantation using transvaginal ultrasound during early pregnancy in 380 women after a Caesarean section concluded that the site of implantation is affected by the presence of a niche (Naji et al., 2013). The distance between implantation site and scar was related to the risk of spontaneous miscarriage. Implantation very close to or in the niche resulted in miscarriage in seven out of eight pregnancies. Implantation was also seen more frequently in the posterior part of the uterus. It appears that a niche is not a favourable site for implantation. Alternatively, differences in angiogenesis or inflammatory response of the endometrium influenced by regeneration of the uterine wound may play a role. This has not been studied yet in relation to niches or after a Caesarean section. One study evaluating immunology using histology found changes and noted that the most significant alteration at the scar site were fewer leucocytes and less vascularization than in the endometrium of the unscarred uterus (Ben-Nagi et al., 2009). This underlines our hypothesis but future studies are needed to evaluate this topic. Theoretically, the accumulation of fluid and mucus may also facilitate bacterial growth, reducing the chances of successful IVF (Moreno et al., 2016). We are not aware of any studies examining the microbial composition of niches or the angiogenic or inflammatory processes of the endometrium in women with a niche or previous Caesarean section.
In one study, the prevalence of first-trimester Caesarean scar pregnancies was higher than in previously reported in literature. This may be explained by having performed an early scan in order to assess the location of the pregnancy, plus a higher prevalence may be expected in referral centres for Caesarean scar pregnancies. This study reported a spontaneous first‐trimester miscarriage rate of 44%, yet Caesarean scar pregnancies may occur more frequently than we assume since the majority of such pregnancies end up in miscarriage before they are diagnosed (Jurkovic et al., 2003).
Hypothesis 3: distorted contractility of the uterus caused by fibrosis or interruption of the myometrial layer at the site of the niche
We hypothesize that implantation is impaired after Caesarean section due to uncoordinated or impaired uterine contractions.
The non-pregnant uterus shows myometrial contractile activity throughout the menstrual cycle. These contractions originate in the subendometrial myometrium and are controlled by steroids. It has been previously reported that throughout the menstrual cycle endometrial wavelike activity patterns of the uterus with adequate wave patterns appear to be related to successful reproduction in natural cycles and assisted reproduction (Bulletti and de Ziegler, 2006). Contractile waves from fundus to cervix are predominantly seen in the follicular phase and they disappear after ovulation or hCG administration. The pattern is essentially reversed in the luteal phase. It recurs in a similar fashion from cycle to cycle (Abramowicz and Archer, 1990; Lyons et al., 1991; van Gestel et al., 2003; Sammali et al., 2019). One ultrasound study showed that the persistence of follicular contractions until hCG administration predicts a favourable IVF outcome (van Gestel et al., 2007).
A niche involves a discontinuity at the site of a previous Caesarean scar. The uterine incision during a Caesarean section is generally made transversely in the lower uterine segment and could lead to poor contractility of the uterine muscle around the scar and the accumulation of fluid in the defect (Thurmond et al., 1999). This is in line with the reduced thickness of the residual myometrium that is observed in the majority of patients after a Caesarean section (Osser et al., 2009; Bij de Vaate et al., 2011; van der Voet et al., 2014a; Bij de Vaate et al., 2014b). An observational prospective study reported a significant decrease in muscular density in the myometrium covering the defect compared with the adjacent myometrium on histological exams after laparoscopic repair (Donnez et al., 2017).
It is possible that in women with a niche, normal wave patterns are disturbed by uncoordinated or impaired uterine contractions during the menstrual cycle, which could lead to a lower implantation rate.
Hypothesis 4: accumulation of mucus and old blood in the niche, which may impair sperm penetration
We hypothesize that low incision during Caesarean section through cervical tissue containing mucus-producing glands may lead to niches that contain mucus due to local mucus formation (Fig. 2).
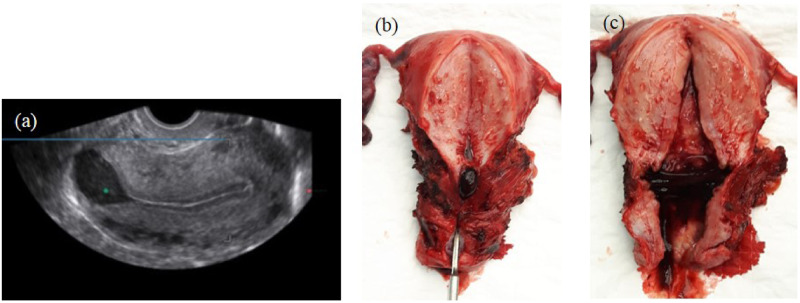
Figure 2.
Accumulation of fluid/blood in the niche. (a) Image of niche using transvaginal ultrasound in mid-sagittal plane with intrauterine fluid accumulation in the large niche. (b and c) Macroscopic image of a uterus with a niche, removed by laparoscopy because of abnormal uterine bleeding and dysmenorrhoea. Clear accumulation of mucus and blood in the niche can be recognized.
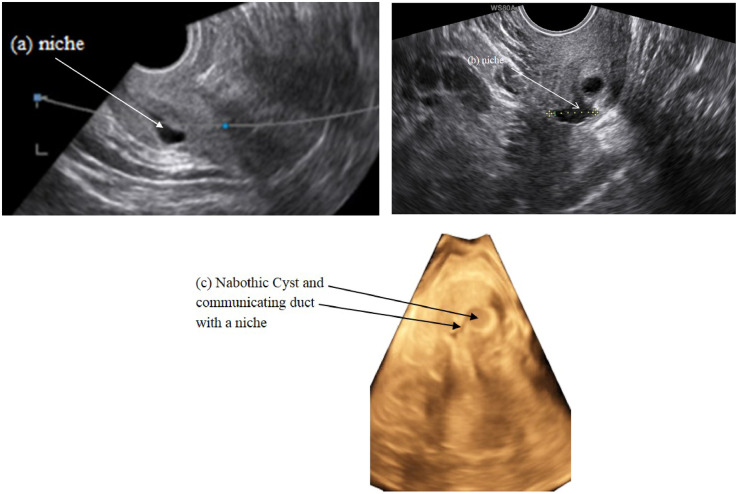
During combined hysteroscopic and sonographic evaluation it became apparent that many niches have an association with nabothian cysts, especially in low locations (Fig. 3).
Figure 3.
Mucus accumulation appears from the niche in the sagittal plane (a) and communicating nabothian cysts with the niche in the transversal plane in 2D ultrasound (b) and 3D ultrasound (c).
The mucus and fluid accumulation in these large niches and uterus may impair penetration of sperm cells and thus embryo implantation. The presence of blood in the cervix may cause impaired sperm‐mucus interaction for immunological reasons. This cervical hostility was defined in terms of an abnormal or negative postcoital test (Glazener and Hull, 1987; Check and Spirito, 1995; Steures et al., 2007) due to absence of forward-progressing spermatozoa. The composition of the fluid and mucus accumulation has not yet been studied.
This factor may be less relevant in case of IUI or IVF if the barrier has already been overcome by the intrauterine catheter. Even in these situations, pregnancy outcomes remain lower, indicating the impairment of implantation to be an independent factor.
Physical barrier for embryo transfer and implantation
Hypothesis 5: a large niche in combination with a strongly retroflexed uterus impairs accessibility for an eventual embryo transfer due to a distorted anatomy
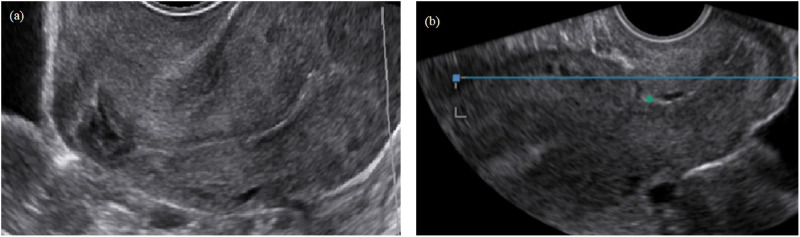
Assisted reproductive techniques are widely used for the treatment of subfertility. A few women have been referred to our department due to the inability to achieve an embryo transfer because of a large niche and a distorted anatomy. Sometimes a niche in combination with a strongly retroflexed uterus and/or a complex niche may hamper the insertion of an insemination or embryo transfer catheter. In rare cases, even under ultrasound guidance, it is impossible to enter the uterine cavity. In such cases, a laparoscopic niche resection to restore the anatomy for embryo transfer could be considered (Vervoort et al., 2018b). During our hysteroscopic niche evaluations, we were not always able to enter the uterine cavity, especially in case of complex niches with many branches (Fig. 4). It may be difficult to determine which endocervical opening has the shortest route to the intrauterine cavity. Combined transrectal evaluation and hysteroscopy may help identify the optimal route. Several patients with these problems had previously failed IVF procedures, and whether embryo transfer had taken place at the correct location is not entirely certain. A systematic review and meta-analysis (including five studies) reported lower clinical pregnancy rates following a difficult embryo transfer (RR 0.75; 95% CI 0.66–0.86) (Phillips et al., 2013).
Figure 4.
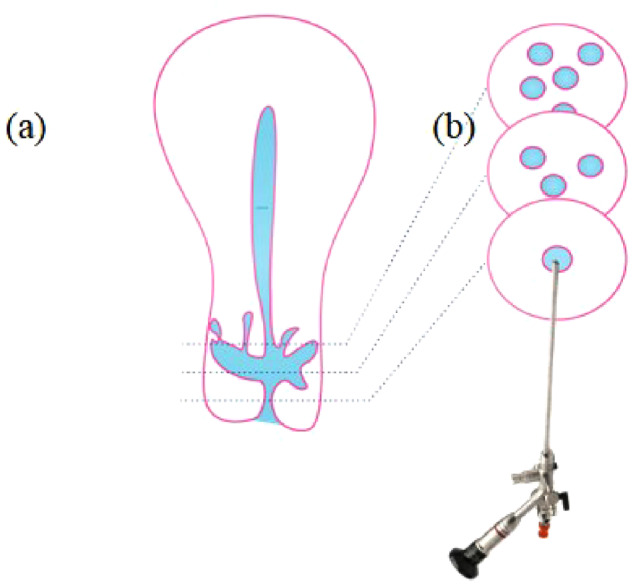
Image of a complex niche with various branches. (a) Schematic diagram of a niche with various branches and (b) schematic diagram of hysteroscopic entrance of the cervix.
It was noted that a large niche is more commonly observed in a retroflexed than an anteflexed uterus. The chances of a retroflexed uterus having an incompletely healed scar are more than double those of a woman with an anteflexed uterus, and these incompletely healed scars are located lower in the uterus than are those which have healed fully (Hayakawa et al., 2006; Osser et al., 2009; Bij de Vaate et al., 2014a). During our laparoscopic repair of large symptomatic niches, we observed that the majority of patients had a strongly retroflexed uterus prior to surgery (Fig. 5a) that reversed into a stretched or anteverted position after completion of the procedure (Fig. 5b).
Figure 5.
A large niche before (a) and after (b) laparoscopic niche repair. The position of the uterus changed from retroverted (a) to anteverted (b).
Where a large uncorrected niche is present, extensive manipulation of the catheter will often be needed in order to enter the uterine cavity. Such extensive manipulation may contribute to uterine irritation, with potential negative effects on embryo implantation. A complex embryo transfer procedure may be responsible for 30% of all IVF failures (Cohen, 1998).
Embryo transfer can become problematic due to a distorted anatomy resulting from a large niche. Even under ultrasound guidance, the catheter may be placed into the niche more easily than into the uterine cavity. Two studies reported that embryo transfer in women with a history of a prior Caesarean section, compared to women with a prior vaginal delivery, took longer and was more likely to have blood or mucus in the catheter (Alvero et al., 2003; Patounakis et al., 2016). Blood in the catheter is one of the criteria for a difficult transfer, which may lead to a lower pregnancy rate (Tomas et al., 2002). Two retrospective studies rated difficulty of embryo transfers in patients with a previous Caesarean section compared with a previous vaginal delivery (Wang et al., 2017; Vissers et al., 2020). In the study of Wang et al. (2017), a difficult embryo transfer ratio (9/144 vs. 0/166, P = 0.001) was seen between patients with a history of Caesarean versus only vaginal delivery. In our study (Vissers et al., 2020), difficulty with embryo transfer was reported more frequently after a previous Caesarean section (9.3%) than after a previous vaginal delivery (1.0%). In a recent prospective cohort study, one reason for a laparoscopic niche resection was severe difficulty or inability to perform an embryo transfer due to extreme retroflexion of the uterus in combination with a large niche in 6.4% of the women. After surgery, the extreme retroflexion was corrected to an anteverted or stretched position in six of the seven women. Five of these women conceived after laparoscopic niche repair (Vervoort et al., 2018b).
Psychogenic causes that reduce the likelihood of pregnancy
Hypothesis 6: niche-related gynaecological symptoms may interfere with sexual intercourse, and focused therapies for niche complaints may interfere with opportunities to conceive during the convalescence period
Approximately 60% of women have a niche after Caesarean section, and approximately 30% of them experience postmenstrual spotting (Osser et al., 2009; Bij de Vaate et al., 2011). The spotting can occur unpredictably during the menstrual cycle. We have conducted interviews and focus group discussions among symptomatic women with a niche (Stegwee et al., 2020, accepted Quality of Life Research Journal). The most commonly reported factor is that the lack of predictability of bleeding/spotting after normal menstruation makes these women feel insecure and gives them a sense of uncleanliness, frequently associated with shame and failure, which affects their sexual behaviour and arousal.
Some studies report a negative association between abnormal uterine bleeding or spotting and the sexual behaviour of both men and women. The presence of vaginal bleeding or spotting is associated with a decrease in genital sexual behaviours (Barnhart et al., 1995; Davis et al., 2002). The majority of men and women (n = 287) (60% of the men and 54% of the women) avoid sexual relations during vaginal spotting, reducing the chances of conception. In this group, 20% considered therapy because of the impact on their wellbeing (18%) or sexual functioning (21%) (Barnhart et al., 1995).
Various other conservative and surgical therapies are applied in order to treat niche-related gynaecological symptoms, such as abnormal bleeding patterns, dysmenorrhoea, chronic pelvic pain and dyspareunia. A very recent large prospective cohort study (n = 146) showed that laparoscopic niche resection improved most subdomains of a quality of life questionnaire, including the physical, mental and pain domains, all of which may impact sexual functioning (Stegwee et al., 2020).
Still, most treatments aiming to reduce symptoms, such as hormonal therapies, endometrial ablation or hysterectomies, interfere with future reproductive potential. In addition, with a laparoscopic niche resection both the waiting time for surgery and the time needed for uterine scar healing (generally 6 months after repair is advised) cause a delay in the resumption of reproductive activities (Huirne et al., 2007, tutorial).
Summary
Subfertility after Caesarean section may be related to the presence of a niche but can also be caused by other factors. It is important to realize that not all niches cause symptoms and that the relation between subfertility and a niche in the uterine scar still needs to be fully proved. Niches without symptoms should, in our opinion, not be treated. The pros and cons of the presented hypotheses are postulated in Table II.
Table II.
Pros and cons of the presented hypotheses.
|
Detrimental environment for sperm penetration and implantation | |
|
|
|
|
| |
|
|
|
| |
| Pros | Cons |
|
| |
|
|
|
| |
| Physical barrier for embryo transfer and implantation | |
|
| |
| |
|
| |
| Pros | Cons |
|
| |
|
|
|
| |
| Psychogenic causes that reduce the likelihood of pregnancy | |
|
| |
| |
|
| |
| Pros | Cons |
|
| |
|
|
Caesarean section, •••; QoL, quality of life.
Conclusion
The lower fertility reported after Caesarean section may have various causes. We suggest that a niche in the uterine scar may play an intermediate role, and with the limited available evidence, we have postulated several hypotheses on the underlying mechanisms. Future research is needed to confirm or refute our ideas. A combination of factors may also likely contribute. More information on the underlying mechanisms is pivotal to the development of selective therapies and the identification of patients who may benefit from additional therapies.
Acknowledgements
The authors thank M.M. van Sitteren for his help with drawing the figures.
Authors’ roles
All authors approve the publication of this version. They all participated in the conception and drafting of the manuscript, including the interpretation of data from the literature.
Funding
This study was not funded.
Conflict of interest
The authors declare that they have no competing interests.
References
- Abramowicz JS, Archer DF.. Uterine endometrial peristalsis–a transvaginal ultrasound study. Fertil Steril 1990;54:451–454. [PubMed] [Google Scholar]
- Akman MA, Erden HF, Bahceci M.. Endometrial fluid visualized through ultrasonography during ovarian stimulation in IVF cycles impairs the outcome in tubal factor, but not PCOS, patients. Hum Reprod 2005;20:906–909. [DOI] [PubMed] [Google Scholar]
- Alvero R, Hearns-Stokes RM, Catherino WH, Leondires MP, Segars JH.. The presence of blood in the transfer catheter negatively influences outcome at embryo transfer. Hum Reprod 2003;18:1848–1852. [DOI] [PubMed] [Google Scholar]
- Antila-Langsjo R, Maenpaa JU, Huhtala H, Tomas E, Staff S.. Comparison of transvaginal ultrasound and saline contrast sonohysterography in evaluation of cesarean scar defect: a prospective cohort study. Acta Obstet Gynecol Scand 2018;97:1130–1136. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Furman I, Devoto L.. Attitudes and practice of couples regarding sexual relations during the menses and spotting. Contraception 1995;51: 93–98. [DOI] [PubMed] [Google Scholar]
- Ben-Nagi J, Walker A, Jurkovic D, Yazbek J, Aplin JD.. Effect of cesarean delivery on the endometrium. Int J Gynaecol Obstet 2009;106:30–34. [DOI] [PubMed] [Google Scholar]
- Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR.. The increasing trend in Caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bij de Vaate AJ, Brolmann HA, van der Voet LF, van der Slikke JW, Veersema S, Huirne JA.. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol 2011;37:93–99. [DOI] [PubMed] [Google Scholar]
- Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brolmann HA, Bourne T, Huirne JA.. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol 2014. a;43:372–382. [DOI] [PubMed] [Google Scholar]
- Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brolmann HA, Bourne T,, Huirne JA.. Reply: Niche risk factor for uterine rupture? Ultrasound Obstet Gynecol 2014. b;44:371–372. [DOI] [PubMed] [Google Scholar]
- Bulletti C,, de Ziegler D.. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol 2006;18:473–484. [DOI] [PubMed] [Google Scholar]
- Check JH,, Spirito P.. Higher pregnancy rates following treatment of cervical factor with intrauterine insemination without superovulation versus intercourse: the importance of a well-timed postcoital test for infertility. Arch Androl 1995;35:71–77. [DOI] [PubMed] [Google Scholar]
- Clark EA, Silver RM.. Long-term maternal morbidity associated with repeat cesarean delivery. Am J Obstet Gynecol 2011;205:S2–S10. [DOI] [PubMed] [Google Scholar]
- Cohen J. Embryo Replacement Technology. San Francisco 31 Annual Postgraduate Course. Birmingham, AL: ASRM, 1998. [Google Scholar]
- Davis AR, Nowygrod S, Shabsigh R, Westhoff C.. The influence of vaginal bleeding on the sexual behavior of urban, Hispanic women and men. Contraception 2002;65:351–355. [DOI] [PubMed] [Google Scholar]
- Di Spiezio Sardo A, Saccone G, McCurdy R, Bujold E, Bifulco G, Berghella V.. Risk of Cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2017;50:578–583. [DOI] [PubMed] [Google Scholar]
- Diaz SD, Jones JE, Seryakov M, Mann WJ.. Uterine rupture and dehiscence: ten-year review and case-control study. South Med J 2002;95:431–435. [PubMed] [Google Scholar]
- Donnez O, Donnez J, Orellana R, Dolmans MM.. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril 2017;107:289–296.e2. [DOI] [PubMed] [Google Scholar]
- Eytan O, Azem F, Gull I, Wolman I, Elad D, Jaffa AJ.. The mechanism of hydrosalpinx in embryo implantation. Hum Reprod 2001;16:2662–2667. [DOI] [PubMed] [Google Scholar]
- Eytan O, Elad D, Zaretsky U,, Jaffa AJ.. A glance into the uterus during in vitro simulation of embryo transfer. Hum Reprod 2004;19:562–569. [DOI] [PubMed] [Google Scholar]
- Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F.. Inequities in the use of cesarean section deliveries in the world. Am J Obstet Gynecol 2012;206:331.e1–19. [DOI] [PubMed] [Google Scholar]
- Glazener C M,, Hull MG.. The sperm-mucus interface: patterns of disorder in the diagnosis of specific causes of penetration failure causing infertility. Hum Reprod 1987;2:673–677. [DOI] [PubMed] [Google Scholar]
- Gubbini G, Centini G, Nascetti D, Marra E, Moncini I, Bruni L, Petraglia F, Florio P.. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: prospective study. J Minim Invasive Gynecol 2011;18:234–237. [DOI] [PubMed] [Google Scholar]
- Gurol-Urganci I, Bou-Antoun S, Lim CP, Cromwell DA, Mahmood TA, Templeton A, van der Meulen JH.. Impact of Caesarean section on subsequent fertility: a systematic review and meta-analysis. Hum Reprod 2013;28:1943–1952. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Itakura A, Mitsui T, Okada M, Suzuki M, Tamakoshi K, Kikkawa F.. Methods for myometrium closure and other factors impacting effects on cesarean section scars of the uterine segment detected by the ultrasonography. Acta Obstet Gynecol Scand 2006;85:429–434. [DOI] [PubMed] [Google Scholar]
- Hemminki E. Effects of cesarean section on fertility and abortions. J Reprod Med 1986;31:620–624. [PubMed] [Google Scholar]
- Hemminki E. Impact of Caesarean section on future pregnancy–a review of cohort studies. Paediatr Perinat Epidemiol 1996;10:366–379. [DOI] [PubMed] [Google Scholar]
- Huirne JA, Vervoort AJ, de Leeuw RA, Brölmann HA, Hehenkamp WJ. Laparoscopic niche resection, a step-by-step tutorial. Eur J Obstet Gynecol Reprod Biol 2017;219:106–112. [DOI] [PubMed] [Google Scholar]
- Jordans IPM, de Leeuw RA, Stegwee SI, Amso NN, Barri-Soldevila PN, van den Bosch T, Bourne T, Brolmann HAM, Donnez O, Dueholm M, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol 2019;53:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ.. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220–227. [DOI] [PubMed] [Google Scholar]
- Levi AJ, Drews MR, Bergh PA, Miller BT, Scott RT Jr.. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril 2001;76:670–674. [DOI] [PubMed] [Google Scholar]
- Lousse JC, Defrere S, Van Langendonckt A, Gras J, Gonzalez-Ramos R, Colette S, Donnez J.. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil Steril 2009;91:1668–1675. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Martins WP, Ludwin I.. Evaluation of uterine niche by three-dimensional sonohysterography and volumetric quantification: techniques and scoring classification system. Ultrasound Obstet Gynecol 2019;53:139–143. [DOI] [PubMed] [Google Scholar]
- Lyons EA, Taylor PJ, Zheng XH, Ballard G, Levi CS, Kredentser JV.. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil Steril 1991;55:771–774. [DOI] [PubMed] [Google Scholar]
- Mansour RT, Aboulghar MA, Serour GI, Riad R.. Fluid accumulation of the uterine cavity before embryo transfer: a possible hindrance for implantation. J In Vitro Fert Embryo Transf 1991;8:157–159. [DOI] [PubMed] [Google Scholar]
- Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, Alonso R, Alama P, Remohi J, Pellicer A, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 2016;215:684–703. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Stirrat GM, Heron J.. The relationship between Caesarean section and subfertility in a population-based sample of 14 541 pregnancies. Hum Reprod 2002;17:1914–1917. [DOI] [PubMed] [Google Scholar]
- Mylonas I,, Friese K.. Indications for and risks of elective cesarean section. Dtsch Arztebl Int 2015;112:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naji O, Wynants L, Smith A, Abdallah Y, Saso S, Stalder C, Van Huffel S, Ghaem-Maghami S, Van Calster B, Timmerman D, et al. Does the presence of a Caesarean section scar affect implantation site and early pregnancy outcome in women attending an early pregnancy assessment unit? Hum Reprod 2013;28:1489–1496. [DOI] [PubMed] [Google Scholar]
- Nikkels C, Vervoort A, Mol BW, Hehenkamp WJK, Huirne JAF, Brolmann HAM.. IDEAL framework in surgical innovation applied on laparoscopic niche repair. Eur J Obstet Gynecol Reprod Biol 2017;215:247–253. [DOI] [PubMed] [Google Scholar]
- Nilstun T, Habiba M, Lingman G, Saracci R, Da Fre M, Cuttini M.. Cesarean delivery on maternal request: can the ethical problem be solved by the principlist approach? BMC Med Ethics 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E,, Elter K.. The impact of cesarean birth on subsequent fertility. Curr Opin Obstet Gynecol 2007;19:238–243. [DOI] [PubMed] [Google Scholar]
- Osser OV, Jokubkiene L, Valentin L.. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet Gynecol 2009;34:90–97. [DOI] [PubMed] [Google Scholar]
- Patounakis G, Ozcan MC, Chason RJ, Norian JM, Payson M, DeCherney AH, Yauger BJ.. Impact of a prior cesarean delivery on embryo transfer: a prospective study. Fertil Steril 2016;106:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JA, Martins WP, Nastri CO, Raine-Fenning NJ.. Difficult embryo transfers or blood on catheter and assisted reproductive outcomes: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013;168:121–128. [DOI] [PubMed] [Google Scholar]
- Porter M, Bhattacharya S, van Teijlingen E, Templeton A.. Does Caesarean section cause infertility? Hum Reprod 2003;18:1983–1986. [DOI] [PubMed] [Google Scholar]
- Sammali F, Kuijsters NPM, Huang Y, Blank C, Rabotti C, Schoot BC, Mischi M.. Dedicated ultrasound speckle tracking for quantitative analysis of uterine motion outside pregnancy. IEEE Trans Ultrason Ferroelectr Freq Control 2019;66:581–590. [DOI] [PubMed] [Google Scholar]
- Sharara FI,, McClamrock HD.. Endometrial fluid collection in women with hydrosalpinx after human chorionic gonadotrophin administration: a report of two cases and implications for management. Hum Reprod 1997;12:2816–2819. [DOI] [PubMed] [Google Scholar]
- Sharara FI,, Prough SG.. Endometrial fluid collection in women with PCOS undergoing ovarian stimulation for IVF. A report of four cases. J Reprod Med 1999;44:299–302. [PubMed] [Google Scholar]
- Silver RM. Delivery after previous cesarean: long-term maternal outcomes. Semin Perinatol 2010;34:258–266. [DOI] [PubMed] [Google Scholar]
- Stegwee SI, Hehenkamp WJK, de Leeuw RA, de Groot CJM, Huirne JAF.. Improved health-related quality of life in the first year after laparoscopic niche resection: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol 2020;245:174–180. [DOI] [PubMed] [Google Scholar]
- Stegwee SI, Jordans I, van der Voet LF, van de Ven PM, Ket J, Lambalk CB, de Groot C, Hehenkamp W, Huirne J.. Uterine Caesarean closure techniques affect ultrasound findings and maternal outcomes: a systematic review and meta-analysis. BJOG 2018;125:1097–1108. [DOI] [PubMed] [Google Scholar]
- Steures P, van der Steeg JW, Hompes PG, Bossuyt PM, Habbema JD, Eijkemans MJ, Koks CA, Boudrez P, van der Veen F, Mol BW.. The additional value of ovarian hyperstimulation in intrauterine insemination for couples with an abnormal postcoital test and a poor prognosis: a randomized clinical trial. Fertil Steril 2007;88:1618–1624. [DOI] [PubMed] [Google Scholar]
- Strandell A, Lindhard A, Waldenstrom U, Thorburn J.. Hydrosalpinx and IVF outcome: cumulative results after salpingectomy in a randomized controlled trial. Hum Reprod 2001;16:2403–2410. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Funamoto H, Hosono T, Shitano Y, Nakashima M, Ametani Y, Nakano T.. New diagnostic criteria and operative strategy for cesarean scar syndrome: endoscopic repair for secondary infertility caused by cesarean scar defect. J Obstet Gynaecol Res 2015;41:1363–1369. [DOI] [PubMed] [Google Scholar]
- Thurmond AS, Harvey WJ, Smith SA.. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J Ultrasound Med 1999;18:13–16; quiz 17–18. [DOI] [PubMed] [Google Scholar]
- Tomas C, Tikkinen K, Tuomivaara L, Tapanainen JS, Martikainen H.. The degree of difficulty of embryo transfer is an independent factor for predicting pregnancy. Hum Reprod 2002;17:2632–2635. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Murakami T, Kimura F, Tanimura S, Kudo M, Shozu M, Narahara H,, Sugino N.. Management of secondary infertility following cesarean section: report from the Subcommittee of the Reproductive Endocrinology Committee of the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res 2015;41:1305–1312. [DOI] [PubMed] [Google Scholar]
- van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HA,, Huirne JA.. Long-term complications of Caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG 2014. a;121:236–244. [DOI] [PubMed] [Google Scholar]
- van der Voet LF, Vervoort AJ, Veersema S, BijdeVaate AJ, Brolmann HA, Huirne JA.. Minimally invasive therapy for gynaecological symptoms related to a niche in the Caesarean scar: a systematic review. BJOG 2014. b;121:145–156. [DOI] [PubMed] [Google Scholar]
- van Gestel I, IJland MM, Evers JL, Hoogland HJ.. Complex endometrial wave-patterns in IVF. Fertil Steril 2007;88:612–615. [DOI] [PubMed] [Google Scholar]
- van Gestel I., IJland MM, Hoogland HJ, Evers JL.. Endometrial wave-like activity in the non-pregnant uterus. Hum Reprod Update 2003;9:131–138. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Donnez J.. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002;77:861–870. [DOI] [PubMed] [Google Scholar]
- Vervoort A, van der Voet LF, Hehenkamp W, Thurkow AL, van Kesteren P, Quartero H, Kuchenbecker W, Bongers M, Geomini P, de Vleeschouwer L, et al. Hysteroscopic resection of a uterine Caesarean scar defect (niche) in women with postmenstrual spotting: a randomised controlled trial. BJOG 2018. a;125:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort A, Vissers J, Hehenkamp W, Brolmann H, Huirne J.. The effect of laparoscopic resection of large niches in the uterine Caesarean scar on symptoms, ultrasound findings and quality of life: a prospective cohort study. BJOG 2018. b;125:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers J, Sluckin TC, Repelaer van Driel-Delprat CC, Schats R, de Groot CJM, Lambalk CB, Twisk JWR, Huirne JAF.. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous Caesarean section: a retrospective cohort study. Hum Reprod 2020;35:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale SG, Ludwin A, Vilos GA, Torok P, Tesarik J, Vitagliano A, Lasmar RB, Chiofalo B.. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet 2020;301:33–52. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Yin TL, Xu WM, Qi QR, Wang XC, Yang J.. Reproductive outcomes in women with prior cesarean section undergoing in vitro fertilization: a retrospective case-control study. J Huazhong Univ Sci Technolog Med Sci 2017;37:922–927. [DOI] [PubMed] [Google Scholar]
- Wortman AC,, Alexander JM.. Placenta accreta, increta, and percreta. Obstet Gynecol Clin North Am 2013;40:137–154. [DOI] [PubMed] [Google Scholar]
- Yaniv S, Elad D, Jaffa AJ,, Eytan O.. Biofluid aspects of embryo transfer. Ann Biomed Eng 2003;31:1255–1262. [DOI] [PubMed] [Google Scholar]