Abstract
Background
Advancing age is associated with high incidence of colorectal cancer (CRC) and high rates of postoperative complications (POCs). However, the impact of of POC severity — evaluated by Clavien–Dindo classification (CDC) or comprehensive complication index (CCI) — on long-term overall survival (OS) in elderly patients after radical CRC resection is not clear.
Methods
Elderly patients aged 65 years or more with CRC undergoing radical resection were retrospectively recruited. POC details were collected and evaluated using CDC grades and the CCI, blinded to patients’ other information. Risk factors for CDC grade ≥II POCs were analyzed by multivariate logistic regression. Effects of CDC grade II–IV POCs on long-term OS were analyzed via propensity-score matching (PSM) analysis followed by Kaplan–Meier curve plotting and multivariate Cox proportional-hazard regression adjusted for all potential confounders. The prognostic value of the CCI was also explored and compared with CDC grades.
Results
A total of 614 elderly patients were identified, of which 20, 106, 25, 11, and 13 cases experienced CDC grade I, II, III, IV, and V POCs, respectively. Higher age, female sex, coronary heart diseases, family history of tumors, preoperative anemia, high amount of bleeding during operation, and high positive dissected lymph–node ratio were found to be risk factors for CDC grade II–V POCs. After PSM analyses, CDC grade II–IV POCs were identified to be associated with poor long-term OS, which was also verified in the entire cohort. The CCI was also found to be significantly associated with decreased long-term OS and showed prognostic values similar to CDC grades.
Conclusion
Both CDC grades and the CCI can be used to evaluate POCs and are associated with long-term OS in elderly patients undergoing radical CRC resection.
Keywords: elderly, colorectal cancer, postoperative complications, Clavien–Dindo classification, comprehensive complication index, overall survival
Introduction
Colorectal cancers (CRCs) are the third–most commonly diagnosed cancers and the second-leading cause of cancer-related deaths around the world, according to the GLOBOCAN 2018 estimate.1 In the US, CRC is the fourth–most commonly diagnosed cancer and the second-leading cause of cancer-related deaths.2 In China, CRC is the third–most commonly diagnosed cancer and the fifth-leading cause of cancer-related deaths.3 Incidence of CRC escalates rapidly with age, and the proportion of CRC in elderly is progressively increasing due to lengthening of life expectancy.4 Estimates suggest that about 16% of the world’s population will be aged >65 years by 2050.5 It has been estimated that 54% of CRCs are diagnosed and that 68% CRC deaths will occur in patients aged ≥65 years in 2020 in USA.2
Surgery, the main treatment for CRC,6 presents a relevant rate of postoperative complications (POCs), which increase with advancing age.7–9 POCs after CRC surgery affect both short-term quality of life and long-term oncological outcomes,10,11 and are frequently used as a measure of surgical care.12 In patients undergoing surgery for primary CRC, recent studies have suggested that infectious POCs13,14 and anastomotic leaks15 are associated with poor short-term and long-term prognosis. In patients with colorectal liver–metastasis resection, infectious POCs16,17 have also been observed to be correlated with poor long-term prognosis. As such, it is essential to robustly and reproducibly assess POCs. Clavien–Dindo classification (CDC) is one of the representative methods for the classification of complications.18,19 It divides the severity of complications based on the level of medical intervention required for resolution of the complication. Studies have revealed that major POCs evaluated by CDC adversely affect long-term outcomes of CRC patients after resection.20,21 However, CDC defines the severity of the complication in a single patient according to the most serious complication, and CDC grade alone cannot effectively reflect multiple complications.
In 2013, Slankamenac et al22 develop a novel global scoring system for POCs based on CDC: the comprehensive complication index (CCI). The CCI integrates each single complication weighted by POC severity and ranges morbidity from 0 (no complications) to 100 (death). Both CDC and the CCI have been validated from clinical and economic points of view.23,24 The CCI seems to be more sensitive for complication reporting than CDC. Research has already suggested that the severity of POCs assessed by the CCI is associated with long-term survival after CRC surgery25 or colorectal liver–metastasis resection.26 Currently, the impact of severity of POCs evaluated by CDC grades or the CCI on long-term survival in elderly patients after radical CRC resection is not clear. In the present study, we retrospectively enrolled 614 elderly patients who had undergone radical resection for CRC to explore the risk factors of CDC grade ≥II POCs, identify the associations of CDC grades and the CCI with long-term overall survival (OS), and compare the prognostic value between CDC grades and the CCI.
Methods
Patients
We had previously established a database of patients with pathologically proven CRC who underwent surgical intervention between January 2003 and December 2014 at Beijing Friendship Hospital.27 This database contained patients’ clinicopathological and follow-up data. In the present study, we retrospectively collected clinicopathological and follow-up data of elderly patients (65 years or older) with CRC who had undergone radical laparoscopic or open surgery. Data collected comprised general background (ie, age, sex, history of drinking and smoking), medical history (ie, history of hypertension, coronary heart disease, diabetes, and personal and family history of malignant tumors), early symptoms (ie, changes in bowel habits and abdominal pain), preoperative clinical manifestations (ie, preoperative hemorrhage and anemia and blood CEA levels), surgical parameters (ie, type of operation and amount of bleeding), detailed POCs (ie, lung infection, bowel obstruction, hypoproteinemia, incision or abdominal infection, wound dehiscence/anastomotic fistula), tumor pathology (ie, TNM stage, differentiation, tumor type, neurovascular tumor thrombus, positive dissected lymph-node ratio [LNR], tumor nodules), postoperative chemotherapy treatment, and follow-up information (OS time and vital status). The final follow-up date of the patients in the database was in June 2016. Inclusion criteria were >65 years old, radical surgical resection (laparoscopic or open), and no other malignancies. Exclusion criteria were no POCs or follow-up information and >15% of data variables unavailable.
Ethical approval was obtained from the institutional review board of Beijing Friendship Hospital. The study was performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments. The review board waived the need for informed consent because of the retrospective nature of the study. Patient data analyzed in the present study were anonymous and complied with relevant local data-protection and privacy regulations.
Postoperative Complication Assessment
Detailed POC information within 30 days after surgery (in-hospital POCs until 30th postoperative day), which had been collected from discharge reports previously, were extracted from our database. The severity of POCs in a single patient was defined by the CDC system (grades 0–V). CDC grade 0 meant no occurrence of POCs, whereas CDC grade V meant postoperative deaths. Overall POCs were defined as those of CDC grade II or higher, and complications with CDC grades 0–I were defined as unremarkable recovery. Major POCs were defined as those of CDC grade III or higher, and minor POCs were defined as those with CDC grades 0–II. The CCI was calculated based on the number of POCs and their CDC grades. POC assessment was performed by two researchers independently and blinded to other clinical features and follow-up outcomes of the patient. Postoperative deaths (ie, CDC grade V) were excluded when performing propensity score–matching (PSM) analysis, Kaplan–Meier curve plotting, and Cox proportional hazard–regression analyses.
Statistical Analysis
Enrolled elderly patients after radical CRC resection were divided into groups according to CDC grades. Missing values were compensatedusing multiple imputation method. Clinicopathological data between different groups were firstly compared via χ2 test, Fisher’s exact test, or Mann–Whitney U test. Multivariate stepwise logistic regression analysis was used to identify independent risk factors for overall POCs. To investigate the relationship between POCs and long-term OS in elderly patients after radical CRC resection, PSM was carried out between patients with CDC grade II–IV POCs (overall POCs) and patients with CDC grade 0–I POCs (unremarkable recovery) at a ratio of 1:2 by logistic regression to reduce the effects of confounders and selection bias based on the covariates age, sex, smoking, drinking, hypertension, diabetes, coronary heart disease, history of malignancy, family history of tumors, bowel-habit changes, abdominal pain, obstruction, preoperative anemia, CEA levels, amount of bleeding, surgical procedure, tumor location, tumor size, positive dissected LNR, differentiation, tumor stages, and postoperative chemotherapy. Kaplan–Meier curves with log-rank (Mantel–Cox) tests were utilized to evaluate OS differences between overall POCs and unremarkable-recovery groups. Univariate Cox proportional-hazard and multivariate models adjusted for potential confounders were also used to clarify associations of POCs evaluated by CDC grades and the CCI with OS. We selected confounders based on whether they changed the effect estimate of POCs on OS by >10% or were significantly associated with OS. CDC and CCI models for predicting OS were constructed via multivariate stepwise Cox regression analysis by incorporating CDC grades and the CCI, respectively. Harrell’s concordance indices (C-indices) were calculated and time-dependent receiver-operating curves (ROCs) were plotted to evaluate the discriminative abilities of the two models. All statistical tests were two-sided and P<0.05 considered significant. For multiple testing, Bonferroni-corrected P-value thresholds were used to reduce the family-wise error rate. R software version 3.4.3 was used for all statistical analyses.
Results
Clinical Characteristics
A total of 614 elderly patients who had had radical CRC resections were finally enrolled. Median age was 74 years, 248 cases were female and 366 male, and 364 had colon cancer and 250 rectal cancer. Most of the patients had been diagnosed at stage II (43.35%) and stage III (35.02%), and 59.61% underwent radical open surgery and 40.39% radical laparoscopic surgery. Median follow-up was 50.57 months and median OS 97 months, while 1-, 3-, and 5-year OS of these patients was 91.21%, 70.63%, and 60.83%, respectively.
Postoperative Complication Evaluation
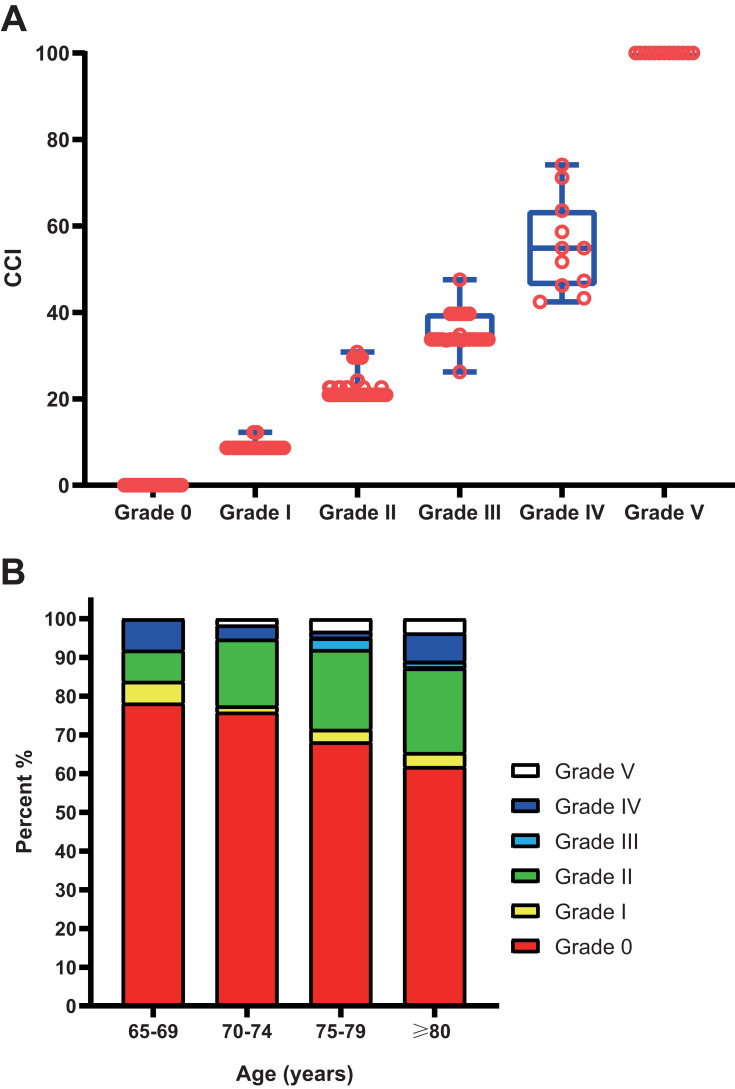
There were 257 POCs in 175 patients (Table S1), and 55 cases had multiple POCs (two or more). Hypoproteinemia, incision or abdominal infection, pulmonary infection, wound dehiscence/anastomotic fistula, and obstruction were the most frequent POCs. Only lung infection was found to be associated with OS in multivariate Cox models (Table S2). POCs were then evaluated by CDC grades and the CCI, respectively. There were 20, 106, 25, 11, and 13 patients experiencing CDC grade I–V complications, respectively. Each CDC grade showed a range of CCI scores, because some patients experienced multiple complications: 8.66–12.25 for grade I, 20.92–30.82 for grade II, 26.22–47.57 for grade III, and 42.43–74.16 for grade IV (Figure 1A). We also divided the patients into different age-groups (each five years), as shown in Figure 1B. POCs increased with age: from 21.77% for 65–69 years to 38.18% for ≥80 years.
Figure 1.
(A) Comprehensive comorbidity index (CCI)–score distribution corresponding to each Clavien–Dindo classification (CDC) grade; (B) CDC-grade distribution corresponding to each age-group in elderly patients after radical colorectal cancer surgery.
Risk Factors for Overall POCs
As shown in Table 1, significant differences in some clinicopathological features between patients with CDC grade 0–I (unremarkable recovery) and grade II–V (overall POCs) were found. In multivariate stepwise logistic regression analysis, advanced age, female sex, coronary heart diseases, family history of tumors, preoperative anemia, high amount of bleeding during operation, and high positive dissected LNR were found to be significantly associated with increased overall POCs (P<0.05, Table 2). When a Bonferroni-corrected threshold of P<0.006 was used, higher age, female sex, and high LNR were still significantly associated with overall POCs.
Table 1.
Comparison of Clinicopathological Characteristics Among Elderly CRC Patients with Overall Postoperative Complications (POCs; CDC grade II–V) and Unremarkable-Recovery POCs (CDC grade 0–I), n=614
| CDC 0–I | CDC II–V | P-value | |
|---|---|---|---|
| n (%) | 459 (100) | 155 (100) | |
| CCI scores | 0 (0–12.25) | 20.92 (20.92–100) | <0.001 |
| Age (years), median (Q1–Q3)] | 74 (70–78) | 76 (72–79) | <0.001 |
| Sex | 0.006 | ||
| Female | 171 (37) | 77 (50) | |
| Male | 288 (63) | 78 (50) | |
| Smoking | 0.517 | ||
| No | 338 (74) | 110 (71) | |
| Yes | 121 (26) | 45 (29) | |
| Drinking | 0.822 | ||
| No | 371 (81) | 124 (80) | |
| Yes | 88 (19) | 31 (20) | |
| Hypertension | 0.054 | ||
| No | 263 (57) | 75 (48) | |
| Yes | 196 (43) | 80 (52) | |
| Diabetes | 0.414 | ||
| No | 381 (83) | 133 (86) | |
| Yes | 78 (17) | 22 (14) | |
| Coronary heart diseases | 0.005 | ||
| No | 419 (91) | 129 (83) | |
| Yes | 40 (9) | 26 (17) | |
| History of malignancy | 0.565 | ||
| No | 435 (95) | 145 (94) | |
| Yes | 24 (5) | 10 (6) | |
| Family history of tumors | 0.050 | ||
| No | 435 (95) | 140 (90) | |
| Yes | 24 (5) | 15 (10) | |
| Bowel-habit changes | 0.766 | ||
| No | 356 (78) | 122 (79) | |
| Yes | 103 (22) | 33 (21) | |
| Abdominal pain | 0.752 | ||
| No | 358 (78) | 119 (77) | |
| Yes | 101 (22) | 36 (23) | |
| Obstruction | 0.153 | ||
| No | 418 (91) | 135 (87) | |
| Yes | 41 (9) | 20 (13) | |
| Preoperative anemia | 0.001 | ||
| No | 270 (59) | 68 (44) | |
| Yes | 189 (41) | 87 (56) | |
| CEA level | 0.868 | ||
| Low | 270 (59) | 90 (58) | |
| High | 189 (41) | 65 (42) | |
| Surgery procedure | 0.003 | ||
| Open | 258 (56) | 108 (70) | |
| Laparoscopic | 201 (44) | 47 (30) | |
| Amount of bleeding | 0.028 | ||
| Low | 208 (45) | 57 (37) | |
| Moderate | 211 (46) | 74 (48) | |
| High | 40 (9) | 24 (15) | |
| Tumor location | 0.061 | ||
| Colon | 282 (61) | 82 (53) | |
| Rectum | 177 (39) | 73 (47) | |
| T stage | 0.506 | ||
| T1 | 17 (4) | 4 (3) | |
| T2 | 57 (12) | 24 (15) | |
| T3 | 232 (51) | 70 (45) | |
| T4 | 153 (33) | 57 (37) | |
| N stage | 0.311 | ||
| N0 | 266 (58) | 97 (63) | |
| N1–N2 | 193 (42) | 58 (37) | |
| M stage | 0.491 | ||
| M0 | 420 (92) | 139 (90) | |
| M1 | 39 (8) | 16 (10) | |
| Tumor size | 0.041 | ||
| Small | 230 (50) | 63 (41) | |
| Large | 229 (50) | 92 (59) | |
| LNR | 0.015 | ||
| Low | 311 (68) | 121 (78) | |
| High | 148 (32) | 34 (22) | |
| Differentiation | 0.987 | ||
| Low | 25 (5) | 8 (5) | |
| Moderate | 255 (56) | 87 (56) | |
| High | 179 (39) | 60 (39) | |
| Neurovascular tumor embolus | 0.534 | ||
| Negative | 411 (90) | 136 (88) | |
| Positive | 48 (10) | 19 (12) | |
| Tumor nodules | 0.984 | ||
| Absent | 355 (77) | 120 (77) | |
| Present | 104 (23) | 35 (23) | |
| Postoperative chemotherapy cycles | 0.003 | ||
| 0–≤2 | 283 (62) | 116 (75) | |
| 3–8 | 176 (38) | 39 (25) |
Abbreviations: CRC, colorectal cancer; CDC, Clavien–Dindo classification; CCI, comprehensive complication index; LNR, (positive dissected) lymph-node ratio; OS, overall survival.
Table 2.
Multivariate Stepwise Logistic Regression Analysis of the Factors Associated with Overall POCs in Elderly Patients that had Undergone Radical CRC Surgery (Entire Cohort, n=614)
| OR | 95% CI | P-value | ||
|---|---|---|---|---|
| Age (continuous) | 1.066 | 1.028 | 1.106 | 0.001 |
| Sex (male vs female) | 0.545 | 0.369 | 0.805 | 0.002 |
| Coronary heart diseases (yes vs no) | 1.822 | 1.031 | 3.220 | 0.039 |
| Family history of tumors (yes vs no) | 2.098 | 1.023 | 4.302 | 0.043 |
| Preoperative anemia (yes vs no) | 1.626 | 1.104 | 2.394 | 0.014 |
| Amount of bleeding (high vs low) | 2.154 | 1.201 | 3.861 | 0.010 |
| Surgery (laparoscopic vs open) | 0.692 | 0.456 | 1.049 | 0.083 |
| LNR (high vs low) | 0.494 | 0.313 | 0.781 | 0.003 |
Abbreviations: CRC, colorectal cancer; LNR, (positive dissected) lymph-node ratio.
POCs and Long-Term Overall Survival
Patients with CDC grade V POCs, ie, those died within 30 days postsurgery, were excluded when investigating the relationship between POCs and long-term OS in elderly patients after radical CRC resection. Given the great difference in baseline characteristics between patients with CDC grade II–IV POCs (overall POCs) and those with CDC grade 0–I POCs (unremarkable recovery), PSM at a ratio of 1:2 was carried out. A total of 309 patients were identified by PSM, of whom 103 had grade II–IV POCs and 206 grade 0–I POCs. No significant difference in clinicopathological characteristics between the two groups existed, including those different before PSM (Table 3 and Table S3).
Table 3.
Comparison of Clinicopathological Characteristics Among Elderly CRC Patients with Overall Postoperative Complications (POCs; CDC Grade II–IV) and Unremarkable-Recovery POCs (CDC Grade 0–I) Before and After Propensity-Score Matching
| Before Propensity-Score Matching (n=601) | After Propensity-Score Matching (n=309) | |||||
|---|---|---|---|---|---|---|
| CDC 0–I | CDC II–IV | P-value | CDC 0–I | CDC II–IV | P-value | |
| n (%) | 459 (100) | 142 (100) | 206 (100) | 103 (100) | ||
| Age (years), median (Q1–Q3) | 74 (70–78) | 76 (72–79) | 0.001 | 75 (71–78) | 75 (71–78) | 0.696 |
| Sex | 0.004 | 0.463 | ||||
| Female | 171 (37) | 72 (51) | 83 (40) | 46 (45) | ||
| Male | 288 (63) | 70 (49) | 123 (60) | 57 (55) | ||
| Coronary heart diseases | 0.003 | 0.885 | ||||
| No | 419 (91) | 117 (82) | 189 (92) | 94 (91) | ||
| Yes | 40 (9) | 25 (18) | 17 (8) | 9 (9) | ||
| Family history of tumors | 0.048 | 0.414 | ||||
| No | 435 (95) | 128 (90) | 191 (93) | 98 (95) | ||
| Yes | 24 (5) | 14 (10) | 15 (7) | 5 (5) | ||
| Preoperative anemia | 0.001 | 0.260 | ||||
| No | 270 (59) | 62 (44) | 112 (54) | 49 (48) | ||
| Yes | 189 (41) | 80 (56) | 94 (46) | 54 (52) | ||
| Surgery procedure | 0.016 | 0.867 | ||||
| Open | 258 (56) | 96 (68) | 130 (63) | 66 (64) | ||
| Laparoscopic | 201 (44) | 46 (32) | 76 (37) | 37 (36) | ||
| Amount of bleeding | 0.050 | 0.728 | ||||
| Low | 208 (45) | 52 (37) | 87 (42) | 43 (42) | ||
| Moderate | 211 (46) | 69 (49) | 99 (48) | 47 (46) | ||
| High | 40 (9) | 21 (15) | 20 (10) | 13 (13) | ||
| LNR | 0.011 | 0.165 | ||||
| Low | 311 (68) | 112 (79) | 149 (72) | 82 (80) | ||
| High | 148 (32) | 30 (21) | 57 (28) | 21 (20) | ||
| Absent | 355 (77) | 111 (78) | 167 (81) | 83 (81) | ||
| Present | 104 (23) | 31 (22) | 39 (19) | 20 (19) | ||
| Postoperative chemotherapy cycles | 0.018 | 0.496 | ||||
| 0–≤2 | 283 (62) | 103 (73) | 134 (65) | 71 (69) | ||
| 3–8 | 176 (38) | 39 (27) | 72 (35) | 32 (31) | ||
Abbreviations: CRC, colorectal cancer; CDC, Clavien–Dindo classification; CCI, comprehensive complication index; LNR, (positive dissected) lymph-node ratio.
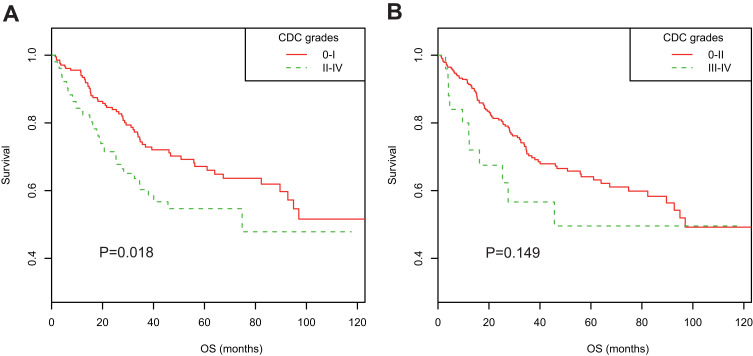
In the PSM cohort, Kaplan–Meier curves suggested overall POCs were associated with decreased OS (3-year OS rate 60.31% vs 73.64%, 5-year OS rate 54.68% vs 67.14%; P=0.018; Figure 2A), whereas major POCs (CDC grade III–IV) were not (Figure 2B). Univariate Cox regression and multivariate analyses adjusted for all potential confounding factors suggested overall POCs were an independent prognostic factor for poor long-term OS (adjusted HR 1.751, 95% CI 1.140–2.691; P=0.011; Table 4). Meanwhile, high CCI scores (per 1-point increase) were also found to be associated with poor OS in both univariate Cox regression analysis and multivariate analysis (adjusted HR 1.017, 95% CI 1.003–1.030; P=0.014). When using a Bonferroni-corrected threshold of P<0.025, both CDC II–IV and the CCI were significantly associated with OS in either univariate or multivariate analyses. These significant associations were also verified in the entire cohort, excluding patients with CDC V POCs (n=601, Table 4).
Figure 2.
Kaplan–Meier survival curves stratified by postoperative complications in propensity score–matched cohorts of elderly patients after radical colorectal cancer surgery. (A) Stratified by CDC grades 0–I and II–IV; (B) stratified by CDC grades 0–II and III–IV.
Table 4.
Associations of Postoperative Complications with OS Analyzed by Multivariate Cox Regression Models in Elderly Patients After Radical CRC Surgery in PSM Cohort and Entire Cohort, Excluding Patients with CDC V POCs
| Postoperative complications | HR | 95% CI | P value | ||
|---|---|---|---|---|---|
| PSM cohort (n=309) | |||||
| Unadjusted | Overall POCs (CDC II–IV vs 0–I) | 1.621 | 1.084 | 2.425 | 0.019 |
| Adjusted§ | Overall POCs (CDC II–IV vs 0–I) | 1.751 | 1.140 | 2.691 | 0.011 |
| Unadjusted | CCI scores (per 1-point increase) | 1.019 | 1.006 | 1.033 | 0.005 |
| Adjusted§ | CCI scores (per 1-point increase) | 1.017 | 1.003 | 1.030 | 0.014 |
| Entire cohort* (n=601) | |||||
| Unadjusted | Overall POCs (CDC II–IV vs 0–I) | 1.520 | 1.097 | 2.107 | 0.012 |
| Adjusted† | Overall POCs (CDC II–IV vs 0–I) | 1.558 | 1.075 | 2.258 | 0.019 |
| Unadjusted | CCI scores (per 1-point increase) | 1.017 | 1.006 | 1.029 | 0.004 |
| Adjusted† | CCI scores (per 1-point increase) | 1.017 | 1.004 | 1.030 | 0.012 |
Notes: §Adjusted for age, smoking, history of malignancy, obstruction, CEA levels, T stage, N stage, tumor size, LNR, tumor nodules, tumor differentiation, and postoperative chemotherapy; †adjusted for age, smoking, history of malignancy, family history of tumors, abdominal pain, obstruction, preoperative anemia, CEA levels, surgery procedure, amount of bleeding, T stage, N stage, tumor size, LNR, tumor nodules, tumor differentiation, and postoperative chemotherapy; *excluding patients with CDC V POCs (n=13).
Abbreviations: CRC, colorectal cancer; CDC, Clavien–Dindo classification; CCI, comprehensive complication index; LNR, (positive dissected) lymph-node ratio; OS, overall survival.
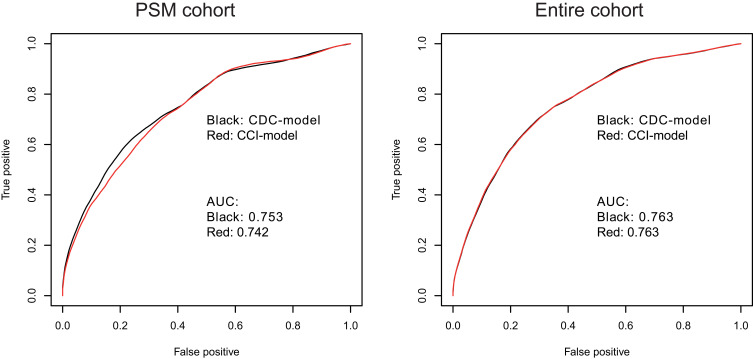
To compare the ability of the CCI with CDC grades in predicting OS of elderly patients undergoing radical CRC resection, CCI and CDC grades were included in multivariate stepwise Cox regression models to construct CDC- and CCI-based prognosis models. In the PSM cohort, the CCI and CDC models were similar, with C-indices of 0.753 and 0.755, respectively, and area under time-dependent ROC (AUC) at 5 years of 0.742 and 0.753, respectively (Table 5 and Figure 3A). In the entire cohort (excluding patients with CDC grade V POCs), the CCI and CDC models were also similar, with C-indices of 0.743 and 0.742, respectively, and AUC at 5 years was 0.763 for both (Figure 3B).
Table 5.
Multivariate Stepwise Cox Proportional Hazard–Regression Models Incorporating CDC or CCI for Predicting OS in Elderly Patients After Radical CRC Surgery (PSM Cohort, n=309)
| HR | 95% CI | P-value | C-index | ||
|---|---|---|---|---|---|
| CDC | 0.7548 | ||||
| Overall POCs (CDC II–IV vs 0–I) | 1.597 | 1.058 | 2.410 | 0.026 | |
| Age (years), continuous | 1.039 | 1.001 | 1.079 | 0.047 | |
| Smoking (yes vs no) | 1.678 | 1.108 | 2.540 | 0.015 | |
| History of malignancy (yes vs no) | 2.251 | 1.158 | 4.377 | 0.017 | |
| CEA levels (high vs low) | 1.763 | 1.157 | 2.686 | 0.008 | |
| N stage (N1–N2 vs N0) | 1.623 | 0.929 | 2.833 | 0.089 | |
| Tumor size (large vs small) | 1.178 | 0.949 | 1.463 | 0.138 | |
| LNR (high vs low) | 1.923 | 1.132 | 3.266 | 0.016 | |
| Tumor nodules (present vs absent) | 1.760 | 1.093 | 2.832 | 0.020 | |
| Postoperative chemotherapy cycles (3–8 vs ≤2) | 0.652 | 0.514 | 0.827 | 0 | |
| CCI | 0.7533 | ||||
| CCI score (continuous) | 1.014 | 1.001 | 1.027 | 0.031 | |
| Age (years), continuous | 1.038 | 1.000 | 1.078 | 0.050 | |
| Smoking (yes vs no) | 1.660 | 1.093 | 2.521 | 0.017 | |
| History of malignancy (yes vs no) | 2.138 | 1.099 | 4.159 | 0.025 | |
| CEA levels (high vs low) | 1.844 | 1.209 | 2.812 | 0.005 | |
| N stage (N1–N2 vs N0) | 1.591 | 0.908 | 2.785 | 0.104 | |
| Tumor size (large vs small) | 1.156 | 0.931 | 1.434 | 0.190 | |
| LNR (high vs low) | 1.904 | 1.124 | 3.228 | 0.017 | |
| Tumor nodules (present vs absent) | 1.736 | 1.080 | 2.789 | 0.023 | |
| Postoperative chemotherapy cycles (3–8 vs ≤2) | 0.656 | 0.516 | 0.834 | 0.001 | |
Abbreviations: CRC, colorectal cancer; CDC, Clavien–Dindo classification; CCI, comprehensive complication index; LNR, (positive dissected) lymph-node ratio; OS, overall survival.
Figure 3.
Time-dependent receiver-operating curves for the multivariate Cox proportional hazard–regression models incorporating CDC grades or CCI to predict 5-year OS in elderly patients undergoing radical CRC surgery. (A) PSM cohort; (B) entire cohort excluding patients with CDC grade V POCs.
Discussion
POCs have been shown always to be associated with longer hospital stays, increased economic cost, and increased postoperative mortality. However, the impact of severity of complications evaluated by CDC grades or the CCI on long-term survival of elderly patients after radical CRC resection is not clear. Odermatt et al20 analyzed 868 cases after CRC resection and found 63 (7%) experienced major POCs (IIIb, IV, and V) and 24 CDC grade V POC. After excluding CDC grade V, they found major POCs (IIIb or IV) were significantly associated with OS. Cienfuegos et al21 examined major POCs (≥CDC grade IIIb) in 950 patients with resected stage I–III colon cancer and identified 51 (5%) cases experienced major POCs. They also found major POCs were associated with long-term OS and disease-free survival (DFS), especially in stage III cancers. Unfortunately, only the association for DFS was significant on multivariate analysis, and they included cases that died with CDC grade V. Durase et al28 performed a study of 2,266 patients with resected CRC and suggested that POCs were associated with long-term OS or DFS, with an increased effect at higher CDC grades, but they did not investigate the severity of POCs indicated by CDC grades in multivariate analyses. Miyamoto et al29 retrospectively analyzed 673 consecutive patients with stage I–III CRC undergoing curative resection and found CDC ≥III POCs were associated with long-term relapse-free survival. In contrast, Slankamenac et al25 did not identify any associations of major POCs (IIIb or IV) with OS in 284 patients with resected CRC.
In elderly patients, Fagard et al30 analyzed 190 patients >70 years old who had had elective CRC surgery and found no significant association of 1-year mortality with CDC ≥II POCs or CDC ≥III POCs. Yamamoto et al31 enrolled 166 elderly patients aged ≥75 years with a histopathological diagnosis of colorectal adenocarcinoma who had undergone curative surgery and found no association of POCs (≥II) with OS. In the present study, we retrospectively enrolled 614 elderly patients undergoing radical resection for CRC and found there were 106, 25, 11, and 13 patients experiencing CDC grade I, II, III, IV, and V complications, respectively. High age, female sex, coronary heart diseases, family history of tumors, preoperative anemia, high amount of bleeding during operation, and high LNR were found to be significantly associated with increased CDC grade II–V complications. Other clinical features, such as CRP:albumin ratio and Geriatric Nutritional Risk Index, have been also identified to be associated with POCs in whole or elderly populations with CRC.32,33 After exclusion patients with CDC grade V complications, overall POCs (CDC grade II–IV) were identified to be associated with decreased OS in the PSM cohort (n=309), a cohort with similar baseline characteristics, and in the entire cohort (n=601). The sample size of 309 cases in the PSM cohort provided 98.5% power with two-sided α=0.05 and 97% power with two-sided α=0.025 for 3-year OS-rate difference between overall POCs and unremarkable groups, as well as provided 93% power with a two-sided α=0.05 and 88% power with two-sided α=0.025 for 5-year OS-rate difference. All power values were higher than the commonly used cutoff of 80%.
Research has shown that adjuvant chemotherapy in patients with CRC is associated with long-term survival benefit after radical resection, and it is recommended that adjuvant chemotherapy be initiated as soon as the patient is medically able, where feasible within 12 weeks.34 POCs may delay adjuvant cancer treatment and decrease the completion rate, which thus portends poor prognosis. Duraes et al recently reported that POCs result in a lower chemotherapy-completion rate for patients with stage III CRC.28 In our study, we found POCs decreased the rate of adjuvant-chemotherapy treatment in elderly patients after radical CRC surgery.
In 2017, for the first time Slankamenac et al25 used the CCI as a composite outcome measure of postoperative morbidity to investigate readmission and long-term survival in 284 patients under surgery for CRC, and found overall combined morbidity assessed by the CCI (treated as a continuous variable) led to more frequent readmission and was associated with poorer long-term survival. Since then, several studies have investigated the clinical value of the CCI in CRC. Carli et al35 used the CCI to measure 30-day POCs as primary outcome to compare effects of multimodal prehabilitation with postoperative rehabilitation in frail patients undergoing CRC resection. Dumitra et al36 explored the relationship of two POC-grading schemas (CDC and CCI) with postoperative quality of life after elective CRC surgery, and found there was a more consistent relationship between the CCI and postoperative health-related quality of life than CDC grades. However, few further studies have validated the associations between POCs evaluated by the CCI and long-term outcomes in patients undergoing resection for primary CRC. In the present study, we, for the first time, determined the associations of POCs evaluated by the CCI with long-term OS and compared the ability of the CCI with CDC in predicting long-term OS of elderly patients undergoing radical CRC surgery. Our results suggested the CCI was significantly associated with long-term OS and exhibited a similar effect with CDC grades in predicting long-term OS in elderly patients undergoing radical CRC surgery.
There are several limitations in our study. Firstly, this was a single-center retrospective study, which might decrease the generalizability of the results. Secondly, this study included a heterogeneous population regarding tumor stage, tumor location, surgery procedure, and treatment, and PSM could not offset all biases. In addition, we collected in-hospital complications only until the 30th postoperative day, so some patients’ complications might not have been recorded because they were discharged before the 30th postoperative day, which also introduced bias. Thirdly, some patients were not followed up to 5 years, which might impact the analysis of the effects of POCs on long-term prognosis. Finally, grade I complications, such as nausea, vomiting, and postoperative pain, were always not regarded as complications in clinical practice and not recorded in our database, resulting in the low rate of grade I complications in our study.
Conclusion
Both CDC grades and the CCI reflect POC burden and are both associated with poor long-term OS in elderly patients undergoing radical resection for CRC. Better strategies to reduce POCs may improve long-term prognosis in elderly patients with CRC.
Acknowledgments
This study was supported by the National Key Technologies R&D Program of China (2015BAI13B09 and 2017YFC0110904), Research Foundation of Beijing Friendship Hospital, Capital Medical University (yyqdkt2018-10), and Wu Jieping Medical Foundation (320.6750.19089-37). Dong Wang and Jinghui Zhang are co–first authors for this study.
Data-Sharing Statement
Data supporting the findings of this study are available from the corresponding author, Yingchi Yang, upon reasonable request.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun. 2020;40(5):205–210. doi: 10.1002/cac2.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aparicio T, Pamoukdjian F, Quero L, Manfredi S, Wind P, Paillaud E. Colorectal cancer care in elderly patients: unsolved issues. Dig Liver Dis. 2016;48(10):1112–1118. doi: 10.1016/j.dld.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 5.Søreide K, Wijnhoven BP. Surgery for an ageing population. Br J Surg. 2016;103(2):e7–e9. doi: 10.1002/bjs.10071 [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 7.Jafari MD, Jafari F, Halabi WJ, et al. Colorectal cancer resections in the aging US population: a trend toward decreasing rates and improved outcomes. JAMA Surg. 2014;149(6):557–564. doi: 10.1001/jamasurg.2013.4930 [DOI] [PubMed] [Google Scholar]
- 8.Zawadzki M, Krzystek-Korpacka M, Rząca M, Czarnecki R, Obuszko Z, Witkiewicz W. Colorectal surgery in elderly population. Pol Przegl Chir. 2018;90(4):29–34. doi: 10.5604/01.3001.0011.8179 [DOI] [PubMed] [Google Scholar]
- 9.Al-Refaie WB, Parsons HM, Habermann EB, et al. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg. 2011;253(5):947–952. doi: 10.1097/SLA.0b013e318216f56e [DOI] [PubMed] [Google Scholar]
- 10.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14(9):2559–2566. [DOI] [PubMed] [Google Scholar]
- 11.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890–899. doi: 10.1097/SLA.0b013e3182128929 [DOI] [PubMed] [Google Scholar]
- 12.Damle A, Alavi K. Objective assessment of quality measurement and improvement. Clin Colon Rectal Surg. 2014;27(1):19–25. doi: 10.1055/s-0034-1366915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497–505. doi: 10.1097/SLA.0000000000000854 [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, et al. Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol. 2018;20(10):1321–1328. doi: 10.1007/s12094-018-1866-8 [DOI] [PubMed] [Google Scholar]
- 15.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259(5):930–938. doi: 10.1097/SLA.0b013e3182a6f2fc [DOI] [PubMed] [Google Scholar]
- 16.Memeo R, de Blasi V, Adam R, et al. Postoperative infectious complications impact long-term survival in patients who underwent hepatectomies for colorectal liver metastases: a propensity score matching analysis. J Gastrointest Surg. 2018;22(12):2045–2054. doi: 10.1007/s11605-018-3854-2 [DOI] [PubMed] [Google Scholar]
- 17.Fernández‐Moreno M-C, Dorcaratto D, Garcés‐Albir M, et al. Impact of type and severity of postoperative complications on long-term outcomes after colorectal liver metastases resection. J Surg Oncol. 2020;122(2):212–225. doi: 10.1002/jso.25946 [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 20.Odermatt M, Miskovic D, Flashman K, et al. Major postoperative complications following elective resection for colorectal cancer decrease long-term survival but not the time to recurrence. Colorectal Dis. 2015;17(2):141–149. doi: 10.1111/codi.12757 [DOI] [PubMed] [Google Scholar]
- 21.Cienfuegos JA, Baixauli J, Beorlegui C, et al. The impact of major postoperative complications on long-term outcomes following curative resection of colon cancer. Int J Surg. 2018;52:303–308. doi: 10.1016/j.ijsu.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 23.de la Plaza Llamas R, Ramia Ángel JM, Bellón JM, et al. Clinical validation of the comprehensive complication index as a measure of postoperative morbidity at a surgical department: a Prospective Study. Ann Surg. 2018;268(5):838–844. doi: 10.1097/SLA.0000000000002839 [DOI] [PubMed] [Google Scholar]
- 24.de la Plaza Llamas R, Hidalgo Vega Á, Latorre Fragua RA, et al. The cost of postoperative complications and economic validation of the comprehensive complication index: prospective Study. Ann Surg. 2019;Publish Ahead of Print. doi: 10.1097/SLA.0000000000003308 [DOI] [PubMed] [Google Scholar]
- 25.Slankamenac K, Slankamenac M, Schlegel A, et al. Impact of postoperative complications on readmission and long-term survival in patients following surgery for colorectal cancer. Int J Colorectal Dis. 2017;32(6):805–811. doi: 10.1007/s00384-017-2811-y [DOI] [PubMed] [Google Scholar]
- 26.Yamashita S, Sheth RA, Niekamp AS, et al. Comprehensive complication index predicts cancer-specific survival after resection of colorectal metastases independent of RAS mutational status. Ann Surg. 2017;266(6):1045–1054. doi: 10.1097/SLA.0000000000002018 [DOI] [PubMed] [Google Scholar]
- 27.Bai Z, Wang J, Wang T, et al. Clinicopathologic parameters associated with postoperative complications and risk factors for tumor recurrence and mortality after tumor resection of patients with colorectal cancer. Clin Transl Oncol. 2018;20(2):176–192. doi: 10.1007/s12094-017-1708-0 [DOI] [PubMed] [Google Scholar]
- 28.Duraes LC, Stocchi L, Steele SR, et al. The relationship between clavien-dindo morbidity classification and oncologic outcomes after colorectal cancer resection. Ann Surg Oncol. 2018;25(1):188–196. doi: 10.1245/s10434-017-6142-6 [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto Y, Hiyoshi Y, Tokunaga R, et al. Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: a propensity-score analysis. J Surg Oncol. 2020;122(2):344–349. doi: 10.1002/jso.25961 [DOI] [PubMed] [Google Scholar]
- 30.Fagard K, Casaer J, Wolthuis A, et al. Postoperative complications in individuals aged 70 and over undergoing elective surgery for colorectal cancer. Colorectal Dis. 2017;19(9):O329–O338. doi: 10.1111/codi.13821 [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Saito H, Uejima C, et al. Estimation of physiological ability and surgical stress score is a useful prognostic indicator for elderly patients with colorectal cancer. Dig Surg. 2020;37(2):145–153. doi: 10.1159/000497455 [DOI] [PubMed] [Google Scholar]
- 32.Man W, Lin H, Liu Z, et al. Usefulness of inflammation-based prognostic scores for predicting the risk of complications after radical resection of colorectal carcinoma. Cancer Manag Res. 2020;12:1029–1038. doi: 10.2147/CMAR.S234448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S, Xie H, Kuang J, Gao F, Gan J, Ou H. The value of geriatric nutritional risk index in evaluating postoperative complication risk and long-term prognosis in Elderly Colorectal cancer patients. Cancer Manag Res. 2020;12:165–175. doi: 10.2147/CMAR.S234688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol. 2019;10(6):1183–1192. doi: 10.21037/jgo.2019.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(3):233. doi: 10.1001/jamasurg.2019.5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumitra TC, Trepanier M, Fiore JF, et al. The relationship of two postoperative complication grading schemas with postoperative quality of life after elective colorectal surgery. Surgery. 2019;166(4):663–669. doi: 10.1016/j.surg.2019.05.058 [DOI] [PubMed] [Google Scholar]